
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 06 September 2022
Sec. RNA
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.973585
This article is part of the Research Topic Non-Coding RNAs and Cancer Chemoresistance View all 4 articles
MicroRNA-135 (miR-135) is a microRNA which is involved in the pathoetiology of several neoplastic and non-neoplastic conditions. Both tumor suppressor and oncogenic roles have been reported for this miRNA. Studies in prostate, renal, gallbladder and nasopharyngeal cancers as well as glioma have shown down-regulation of miR-135 in cancerous tissues compared with controls. These studies have also shown the impact of miR-135 down-regulation on enhancement of cell proliferation and aggressive behavior. Meanwhile, miR-135 has been shown to be up-regulated in bladder, oral, colorectal and liver cancers. Studies in breast, gastric, lung and pancreatic cancers as well as head and neck squamous cell carcinoma have reported dual roles for miR-135. Dysregulation of miR-135 has also been noted in various non-neoplastic conditions such as Alzheimer’s disease, atherosclerosis, depression, diabetes, Parkinson, pulmonary arterial hypertension, nephrotic syndrome, endometriosis, epilepsy and allergic conditions. In the current review, we summarize the role of miR-135 in the carcinogenesis as well as development of other disorders.
MicroRNAs (miRNAs) represent a group of small-sized transcripts with high impact on the regulation of gene expression. For many years the role of miRNA is mostly associated with translation arrest. miRNAs have crucial roles in the developmental processes and have various biological functions (Fu et al., 2013; Ghafouri-Fard et al., 2021a; Hussen et al., 2021). The process of miRNA synthesis contains multiple steps with the final step being incorporation of either the 5p or 3p strands of the mature miRNA duplex into a complex, namely miRNA-induced silencing complex (miRISC). Notably, shuttling of this complex inside the cell has an essential role in the extent of miRNA-mediated modulation of gene expression (O'Brien et al., 2018; Ghafouri-Fard et al., 2021b; Ghafouri-Fard et al., 2021c; Ghafouri-Fard et al., 2021d). miRNAs regulate gene expression in a dynamic manner which results in buffering of expression levels to reach a stable state (O'Brien et al., 2018). Most remarkably, accessibility and relative abundance of miRNAs and their targets can define the genes that are modulated by miRNAs. Moreover, miRNA-mediated inhibition of target transcripts is not universal among different kinds of cells. This effect is modulated by alternative splicing/polyadenylation events and the amounts of cell type-specific factors that change secondary structure of target transcripts (O'Brien et al., 2018).
The impact of several miRNAs in normal developmental and pathological conditions has been investigated unraveling a wide variety of different functions for these transcripts. Most notably, circulating miRNAs have recently attracted attention of researchers for their application in diagnosis of human disorders, particularly cancers (Wang et al., 2018). Although in some cases alterations in the levels of miRNAs in the peripheral blood might be solely a by-products of the underlying condition, many of these miRNAs have been shown to participate in the occurrence and development of cancer through direct or indirect routes. They can be used as tools for subtype classification of tumors, detection of resistance to chemo- or radiotherapy and clinical outcome (Wang et al., 2018). However, there is an urgent need for conduction of large scale studies to enhance the sensitivity, specificity, and applicability of these markers.
miR-135 is an example of miRNAs with diverse roles in both neoplastic and non-neoplastic conditions and possible application as a maker for both kinds of disorders. This miRNA is encoded by three different loci, namely MIR135A1 (3p21.2), MIR135A2 (12q23.1) and MIR135B (1q32.1). While MIR-135A1 and MIR-135A2 are located at different chromosomes in humans, they are transcribed into miR-135a. MIR-135B is the only gene accountable for miR-135b expression in humans.
miR-135b has been found to be expressed in brain, cerebellum, artery, colon, lung, stomach, esophagus, thyroid, salivary gland, breast, ovary, prostate and testis. miR-135a has a broader range of tissue expression. In addition to these tissues, it is expressed in whole blood, spinal cord, tibial nerve, heart, skeletal muscle, small intestine, adipocyte, kidney, liver, lung, spleen and a number of other tissues (GeneCards, 2017).
In the current review, we summarize the role of miR-135 in the carcinogenesis as well as development of other disorders.
In silico analyses using the Cancer Genome Atlas (TCGA) data on gastrointestinal cancers (including those originated from colon, esophagus, liver, pancreas, rectum and stomach) has shown that over-expression of miR-135 in the cancerous tissues is associated with a poor overall survival in these types of cancers. These significant findings were based on the receiver operating characteristic curves and Kaplan-Meier analyses which included 1,488 patients with gastrointestinal cancers whose data on survival and miR-135 expression was available in the TCGA (Chao et al., 2019).
In a pioneer study in this field, Nagel et al. have shown that miR-135a and miR-135b target the 3′ UTR of APC to decrease expression of this tumor suppressor gene and enhance activity of Wnt pathway. Authors have also reported significant over-expression of miR-135a and miR-135b in colorectal adenomas and carcinomas in correlation with down-regulation of APC transcripts (Nagel et al., 2008). Subsequently, Valeri et al. have shown that APC loss induces up-regulation of miR-135b leading to dysregulation of PTEN/PI3K pathway and up-regulation of SRC which in turn promotes cell transformation and progression of colorectal cancer. Over-expression of miR-135b has been found to be a common finding in sporadic and inflammatory bowel disease-associated human colorectal cancers. Moreover, its over-expression is correlated with tumor stage and poor patients’ survival. Suppression of miR-135b in animal models of colorectal cancer could reduce tumor growth through modulation of genes participating in proliferation, invasion, and apoptosis (Valeri et al., 2014).
Bai et al. have shown that the exosome-mediated delivery of miR-135b to gastric cancer cells enhances angiogenic processes in these cells both in vitro and in vivo. Tumor cells-derived miR-135b could suppress expression of FOXO1 transcription factor that regulates gluconeogenesis and glycogenolysis by insulin signaling. This miRNA can also increase development of blood vessels (Bai et al., 2019). Huangfu et al. have shown that up-regulation of miR-135b enhances cell proliferation, migratory aptitude and invasive properties of gastric cancer cells through targeting the tumor suppressor gene CAMK2D which is a serine/threonine protein kinase pertaining to the subfamily of Ca2+/calmodulin-dependent protein kinases. Notably, in vivo administration of miR-135b antagonist has suppressed tumor growth and metastatic potential of tumors in xenograft models (Huangfu et al., 2021). Another study in early gastric cancer has reported down-regulation of miR-135a in about one-third of patients. Notably, these patients have exhibited advanced TNM stage and higher possibility of lymph node metastasis compared with patients having high levels of miR-135a. Functional studies have revealed that miR-135a suppresses viability of cells, EMT, invasive properties, and their migration. The rho-associated, coiled-coil-containing protein kinase 1 ROCK1 has been identified as the target of miR-135a in gastric cancer cells (Shin et al., 2014a). Similar to this report, He et al. have demonstrated down-regulation of miR-135 in gastric cancer tissues compared with adjacent tissues. Down-regulation of this miRNA has been associated with lower overall survival rate of patients. The impact of miR-135 on reduction of proliferation, invasion and migration of gastric cancer cells has also been confirmed in BGC-823 and SGC-7901 cell lines. Moreover, it has been revealed that this miRNA has a regulatory role on expression of the GTP exchange factor SMAD2 (He et al., 2019). On the other hand, Han et al. have found that miR-135b is the mostly up-regulated miRNA in gastric tissues from K19-C2mE and Gan mice. Moreover, expression of this miRNA has been shown to be elevated during the early stages of gastritis-associated carcinogenesis. This miRNA has also been shown to be up-regulated in gastric tumor tissues from gp130 F/F mice and human clinical samples. Interleukin 1 could enhance expression of this miRNA in gastric organoids and immortalized cell lines. Oncogenic effects of miR-135b in gastric cancer have been exerted through targeting FOXN3 and RECK tumor suppressors (Han et al., 2019).
Zhou et al. have shown up-regulation of miR-135b in pancreatic cancer tissues and pancreatic cancer stem cells (CSCs). This miRNA has been shown to target the apoptosis and differentiation gene JADE-1. Up-regulation of miR-135b has increased proliferation, migratory potential, and invasion of pancreatic CSCs, suppressed their apoptosis and surged levels of stemness-related factors. Furthermore, miR-135b has been shown to enhance phosphorylated levels of AKT and mTOR. In vivo studies have also confirmed the impact of miR-135b up-regulation on acceleration of tumor growth (Zhou et al., 2020). Contrary to this study, Zhang et al. have shown down-regulation of miR-135a in pancreatic cancer tissues and cell lines compared with the corresponding controls. Luciferase activity assay has shown the interaction between long non-coding RNA (lncRNA) UCA1 and miR-135a. Notably, miR-135a could reverse the impact of UCA1 on apoptosis and viability of pancreatic cancer cells (Zhang et al., 2017a).
Zhou et al. have found that expression of miR-135a-5p is repeatedly diminished in gallbladder cancer tissues in correlation with histologic grade. Forced over-expression of miR-135a-5p has suppressed proliferation of gallbladder cancer cells in vitro and in vivo. Furthermore, the cell surface protein participating in receptor-mediated endocytosis VLDLR has been identified as a direct target of miR-135a-5p. The p38 MAPK pathway has been found to participate in miR-135a/VLDLR downstream signaling (Zhou et al., 2014). Consistently, miR-135a has been shown to inhibit invasiveness and metastasis of gallbladder cancer cells and induce their apoptosis through regulation of ROCK1, HOXA10 and BCL-2 expression levels. miR-135a-loaded liposomes adapted with Anti-EGFR antibodies could decrease tumor growth in xenograft models (Feng et al., 2020).
Jiang et al. have reported a tumor suppressor role for miR-135 in human breast cancer cell lines as well as mice models. They have reported that forced over-expression of miR-135 suppresses growth, migratory aptitude, invasiveness and epithelial-mesenchymal transition (EMT) of MDA-MB-468 and MCF-7 cell lines. Mechanistically, miR-135 can suppress activity of Wnt/β-catenin pathway (Figure 1) (Jiang et al., 2019).
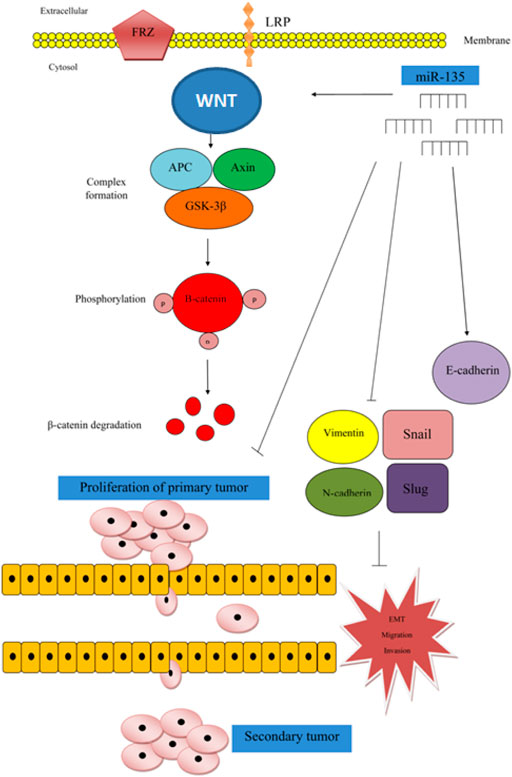
FIGURE 1. In breast cancer, miR-135 is regarded as a tumor suppressor miRNA, since over-expression of miR-135 could suppress cell proliferation, migration, invasion and EMT by inhibition of Wnt/β-catenin axis. In fact, expression of miR-135 has been positively associated with expression of p-GSK3, but inversely associated with expressions of Wnt and β-catenin. Up-regulation of miR-135 has increased p-GSK3 levels (Jiang et al., 2019). Mechanistically, GSK-3β participates in the formation of a complex with APC and Axin which has a role in β-catenin phosphorylation and its degradation (Wu and Pan, 2010).
Similarly, Yang et al. have reported down-regulation of miR-135-5p in breast cancer samples compared to neighboring breast tissues with a more prominent reduction in patients having lymph node involvement. On the other hand, expression of SMAD3 has been shown to be increased in cancerous samples. Functional studies have shown that miR-135-5p inhibits TGF-β-mediated EMT, thus reducing metastatic ability of breast cancer cells in vivo. Besides, SMAD3 silencing has led to a similar phenotype with miR-135-5p up-regulation in breast cancer cells. Additional mechanistical assays have revealed that SMAD3, a fundamental modulator of TGF-β/SMAD signaling is directly targeted by miR-135-5p (Yang et al., 2020a).
In an attempt to find miRNAs regulating expression of the bone-metastasis promoting factor Runx2, Taipaleenmäki et al. have reported the inhibitory role of miR-135 on this factor in breast tissues. While miR-135 has been reported to be highly expressed in normal breast epithelial cells, it has not been expressed in metastatic breast cancer cells and clinical samples with high expression of Runx2. Forced over-expression of miR-135 in metastatic MDA-MB-231-luc cells has decreased expression of Runx2 and levels of pro-metastatic targets of Runx2, namely IL11, MMP-13, and PTHrP. These effects have been accompanied with reduction of tumor growth and bone metastasis in animal models (Taipaleenmäki et al., 2015).
Through a bioinformatics approach, Bertoli et al. have identified miR-135b as one of miRNAs with essential roles in regulation of the reformed functional pathways in basal type of breast cancer. With a degree centrality of 12, miR-135b has been shown to control expression of 12 over 35 genes inside 1 couple pathways (ethanol degradation X and Mismatch repair in eukaryotes). Experiments have confirmed over-expression of miR-135b in BT20 and MDA-MB-231 breast cancer cell lines compared to normal-like cell line of breast, i.e. MCF10A (Bertoli et al., 2021). Consistent with this study, Uva et al. have reported that expression levels of miR-135b are firmly correlated with triple negative breast cancer (TNBC) with basal-like phenotype. Target analyses of miR-135b have shown impact of this miRNA on TGF-β, WNT and ERBB pathways. Moreover, miR-135b expression has been correlated with neoplastic proliferative index (Uva et al., 2018).
Aakula et al. have shown that miR-135b has binding sites for 3′ UTR of androgen receptor (AR), estrogen receptor (ER) and HIF1AN and acts as a regulator of these genes. This miRNA has higher expressions in patients with ER negative breast tumors and in prostate cancer patients with low levels of AR (Aakula et al., 2015). In addition, Tribollet et al. have discovered a negative relationship between ER with miR-135 expression in breast and prostate cancers. miR-135a has an ability to suppress invasion and aggressiveness of these malignant cells (Tribollet et al., 2016).
miR-135 has been shown to be increased in bladder cancer cell lines and clinical samples. Up-regulation of miR-135a has enhanced proliferation of bladder cancer cells, while suppression of miR-135a has reversed this effect. PHLPP2 and FOXO1 have been identified as direct targets of miR-135a whose expressions are decreased by miR-135a. Cumulatively, miR-135a can promote proliferation in bladder cancer cells through decreasing levels of PHLPP2 and FOXO1 (Mao et al., 2015). Consistent with this study, Mao et al. have reported up-regulation of miR-135a, β-catenin, cyclin D1 and vimentin in bladder cancer samples compared with non-cancerous controls. Moreover, they have reported down-regulation of GSK3β and E-cadherin in cancerous samples. Functional studies have shown the role of miR-135a in acceleration of EMT, invasion and migratory potential of bladder cancer cells through enhancing activity of Wnt/β-catenin signaling. These effects are mediated via GSK3β down-regulation (Mao et al., 2018). Another study in bladder cancer cells has shown interaction between miR-135a and lncRNA MBNL1-AS1. In fact, the tumor suppressor role of MBNL1-AS1 is exerted through decreasing miR-135a levels and influencing activity of PHLPP2/FOXO1 axis (Wei et al., 2020).
Tian et al. have shown down-regulation of miR-135a in non-small cell lung cancer (NSCLC) cells compared with normal bronchial epithelium. miR-135a has been found to inhibit proliferation, invasiveness and metastatic ability of NSCLC cells. Notably, miR-135a could suppress expression of several molecules in the RAS signaling pathway via suppression of expression of RAB1B (Tian et al., 2020). On the other hand, Zhao et al. have reported a pro-oncogenic role for miR-135b in NSCLC. They have also reported association between up-regulation of miR-135b and poor prognosis in these patients. The pro-proliferative, pro-angiogenic and anti-apoptotic effects of miR-135b have been verified in animal models as well. miR-135b has been shown to directly target the deubiquitinase CYLD transcript, thus controlling ubiquitination and activation of NF-κB pathway. Expression of this miRNA has been shown to be enhanced by IL-6/STAT3 axis. Cumulatively, IL-6/STAT3/miR-135b/NF-κB constitutes a positive feedback circuit which contributes in the development of NSCLC (Zhao et al., 2021).
Studies in prostate, renal and nasopharyngeal cancers as well as glioma have shown down-regulation of miR-135 in cancerous tissues compared with controls. These studies have also shown the impact of miR-135 down-regulation on enhancement of cell proliferation and aggressive behavior. On the other hand, miR-135 has been shown to be up-regulated in oral, cervical and liver cancers as well as myxoid liposarcoma, multiple myeloma and melanoma. Supplementary Table S1 summarizes the role of miR-135 in diverse types of cancers.
miR-135a has been shown to contribute to paclitaxel resistance in various tumor cells possibly through down-regulation of APC (Holleman et al., 2011). On the other hand, miR-135 has been reported to suppress tumor growth and cell invasion, and increase sensitivity to 5-fluorouracil and doxorubicin drugs through targeting FAK (Golubovskaya et al., 2014). Table 1 shows the effects of miR-135 in regulation of response to chemotherapy, radiotherapy and other therapeutic modalities.
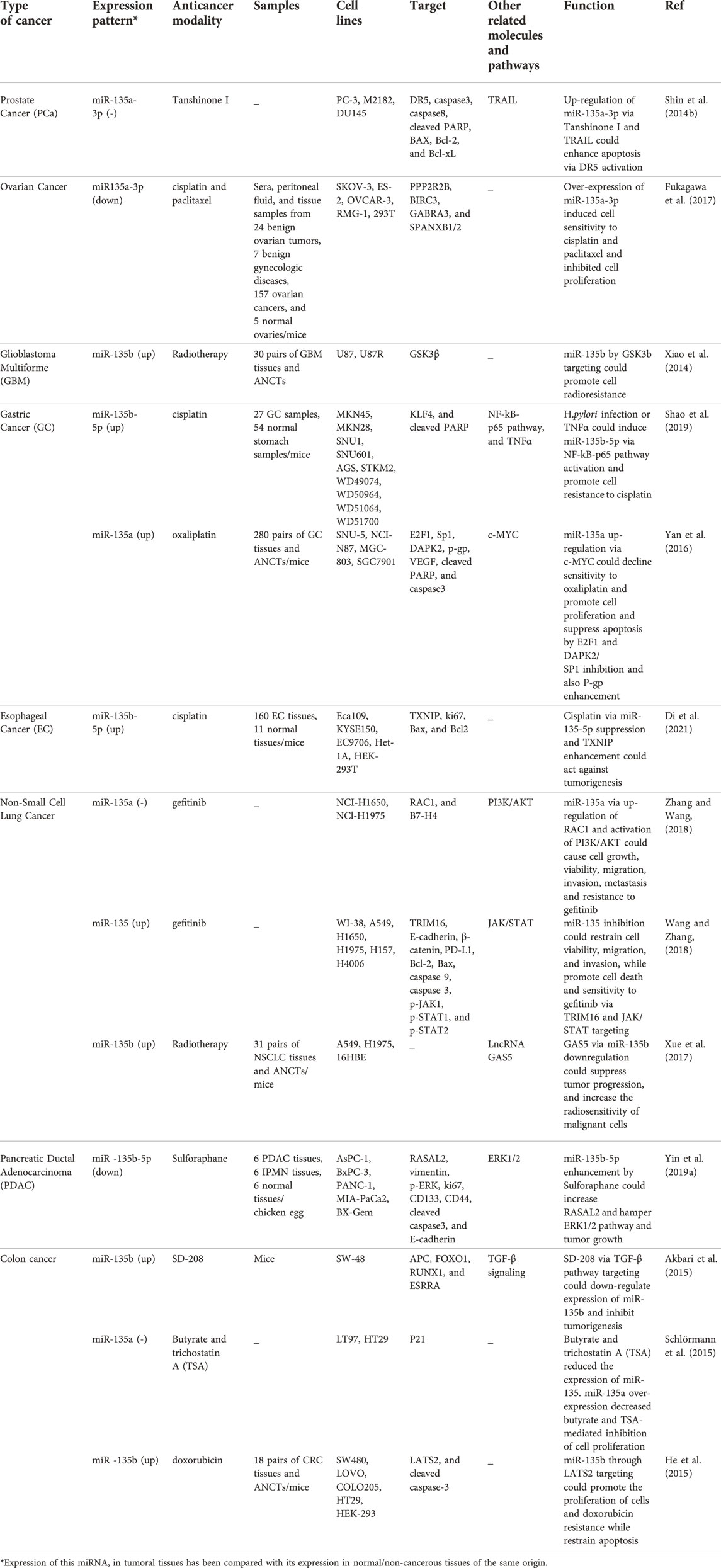
TABLE 1. Effect of miR-135 in the response to chemotherapy, radiotherapy and other therapeutic modalities.
Several studies have verified correlations between serum/tissue levels of miR-135 and patients’ survival (Table 2). In a cohort of breast cancer patients, a positive correlation has been detected between miR-135b expression and ki67 expression, and a negative correlation has been reported between miR-135b expression and age and androgen receptor expression (Ghafouri-Fard et al., 2021b). Other studies have also reported association between expression of this miRNA and tumor size, tumor grade or other clinical data (Table 2).
miR-135 has diverse roles in the pathogenesis of human disorders. miR-135b acts as a neuroprotective miRNA via targeting GSK3β, thus it could neutralize effect of MPP + on proliferation and apoptosis of cells (Zhang et al., 2017b). Moreover, experiments in Zebrafish model have shown that miR-135a shields neural crest cells against alcohol-induced apoptosis and craniofacial deformities through regulation of Siah1/p38/p53 axis (Yuan et al., 2020).
miR-135 can also affect the process of atherosclerosis through different mecahnisms (Figure 2).
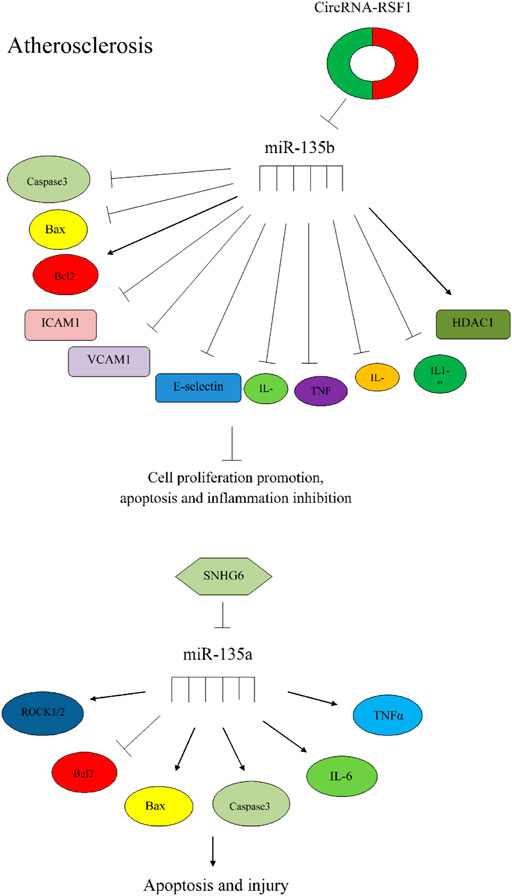
FIGURE 2. The importance of miR-135 in atherosclerosis. Circular RNA-RSF1 could promote proliferation of vascular endothelial cells and prevent apoptosis and inflammation via regulation of miR-135b-5p, HDAC1, ICAM1, VCAM1, caspase3, bcl2, and Bax (Hu et al., 2019). SNHG6 could increase apoptosis and injury via miR-135a-5p sponging and regulation of expression of ROCK1/2 and apoptotic factors (Ren et al., 2015).
Table 3 shows the role of miR-135 in non-cancerous disorders.
According to Kim et al. study in mice models and RBL2H3, B16F1, B16F10 cell lines, miR-135-5p could prevent allergic inflammation through targeting P62 (Shan et al., 2020). Sung et al. have performed a series of assays in Neuro-2a cells and mice model. Their investigation has revealed that ischemic preconditioning as an endogenous neuroprotective process promotes expression of ATP-binding cassette subfamily A member 1 (ABCA1), suppresses miR-135-5p expression, decreases the Bax/Bcl2 proportion and activates caspase-9 and caspase-3, thus protecting neural cells against mitochondria-dependent apoptosis and subsequent brain injuries (Sung et al., 2021). According to Xie et al. study, miR-135b-5p inhibition could protect cells separated from myocardial tissues of mice from apoptosis and reperfusion injury by activating JAK2/STAT3 signaling axis (Xie et al., 2017).
In an interesting study, the relationship between physical exercise and miR-135 expression in old mice was identified. In this survey, authors have reported miR-135a-5p down-regulation via exercise. The consequent cell cycle progression and proliferation of neural precursor cells has led to neurogenesis. Phosphatidylinositol signaling protein IP3 has been recognized as a target of this miRNA, so miR-135a-5p/IP3 axis has been suggested as a potential target for treatment of age-related brain injuries (Pons-Espinal et al., 2019).
According to Honardoost et al. study, miR-135 transfection to C2C12 cell line caused Insr gene down-regulation, glucose uptake reduction and development of insulin resistance phenotype (Honardoost et al., 2016).
miR-135 is a miRNA which is involved in the pathoetiology of several neoplastic and non-neoplastic conditions. Both tumor suppressor and oncogenic roles have been reported for this miRNA. Two studies in animal models of spontaneous carcinogenesis has reported oncogenic roles for miR-135b in colorectal (Valeri et al., 2014) and gastric (He et al., 2019) cancers. Since these two studies have provided the strongest level of evidence, oncogenic function is supported for this miRNA.
Studies in prostate, renal, gallbladder and nasopharyngeal cancers as well as glioma have shown down-regulation of miR-135 in cancerous tissues compared with controls. Meanwhile, miR-135 has been shown to be up-regulated in bladder, oral, colorectal and liver cancers. Studies in breast, gastric, lung and pancreatic cancers as well as head and neck squamous cell carcinoma have reported dual roles for miR-135. These different effects of miR-135 cannot be explained either by tissue-dependent factor or by different genetic loci that encode this miRNA (MIR135A versus MIR135B). Other explanations for these observations are the presence of tissue-dependent elements, abundance of RNA binding proteins in each tissue, impact of alternative splicing/polyadenylation events on miR-135 targets and the amounts of cell type-specific factors that change secondary structure of target transcripts.
Studies aimed at identification of the impact of miR-135 on EMT process have shown extremely contradictory results. For instance, miR-135-5p as a tumor suppressor miRNA inhibits this process (Yang et al., 2020a). On the contrary, in bladder and gastric cancer, miR-135a and miR-135b have been found to increase EMT (Mao et al., 2018; Huangfu et al., 2021).
Circulatory levels of miR-135 can be used as prognostic markers in different types of cancers. For instance, high expression of miR-135a-3p in serum samples of patients with ovarian cancer has been associated with good prognosis (Hu et al., 2014). Moreover, over-expression of miR-135a in blood samples of patients with colon cancer has been associated with good prognosis 83)). On the other hand, high serum levels of miR-135b in patients with multiple myeloma have been correlated with the severity of bone lesions (Hao et al., 2016).
Amplification or deletion of any of three mentioned loci for miR-135, namely MIR135A1 (3p21.2), MIR135A2 (12q23.1) and MIR135B (1q32.1) might be associated with dysregulation of certain members of this family. For instance, frequent deletion of the MIR135A1 locus has been found to be associated with poor prognosis in primary breast cancers. Mechanistically, deletion of this locus and subsequent down-regulation of miR-135a levels enhances progression of ERα+ breast cancers and their resistance to tamoxifen (Zhang et al., 2018).
Studies in different tissues and cell types have identified common pathways (e.g. AKT and WNT) and target genes (such as APC, FOXO1, FOXN1, RECK and some MMPs) for miR-135. Identifying these common functions would also provide insight into the impact of exosomes-delivered miR-135 on function of distal recipient cells/tissues.
miR-135 has been shown to interact with a number of lncRNAs, namely MBNL1-AS1, MALAT1, UCA1, MEG3, DANCR, SMAD5-AS1, NCK1-AS1 and RAET1K. In fact, these lncRNAs exert their impacts on cellular functions through sponging miR-135. In addition, miR-135 has been found to modulate activity of several signaling pathways such as Wnt/β-catenin, TGF-β/SMAD, ERBB, PI3K, p38 MAPK EGFR, FAK, NF-κB, Notch, IL-6/STAT3, AKT/mTOR and Hippo. This extensive mode of action shows complexity of functional network influenced by miR-135.
Differences in pri- and pre-miRNA sequences indicate that diverse phases might be rate-limiting for each precursor, enabling the fruition of extra regulatory mechanisms. Moreover, miR-135 might be subjected to distinctive modes of regulation through certain interactions with different RNA-binding proteins.
Dysregulation of miR-135 has also been noted in various non-neoplastic conditions such as Alzheimer’s disease, atherosclerosis, depression, diabetes, Parkinson, pulmonary arterial hypertension, nephrotic syndrome, endometriosis, epilepsy and allergic conditions.
Regarding the different roles of miR-135 in different contexts, several issues should be addressed about the possible effects of tissue-dependent elements that affect expression of miR-135 in each tissue. Although manipulation of expression of miR-135 is a possible therapeutic option for cancer, it is not expected that miR-135-targeted therapies enter clinical settings in near future.
SF wrote the draft and revised it. BH designed and supervised the study. SK and SE collected the data and designed the tables and figures. All the authors read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor (WCC) declared a past co-authorship with the author(s) (BMH).
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.973585/full#supplementary-material
Aakula, A., Leivonen, S. K., Hintsanen, P., Aittokallio, T., Ceder, Y., Børresen-Dale, A. L., et al. (2015). MicroRNA-135b regulates ERα, AR and HIF1AN and affects breast and prostate cancer cell growth. Mol. Oncol. 9 (7), 1287–1300. PubMed PMID: 25907805. Pubmed Central PMCID: PMC5528813. Epub 2015/04/25. eng. doi:10.1016/j.molonc.2015.03.001
Akbari, A., Ghahremani, M. H., Mobini, G. R., Abastabar, M., Akhtari, J., Bolhassani, M., et al. (2015). Down-regulation of miR-135b in colon adenocarcinoma induced by a TGF-β receptor I kinase inhibitor (SD-208). Iran. J. Basic Med. Sci. 18 (9), 856–861. PubMed PMID: 26523217. Pubmed Central PMCID: PMC4620183. Epub 2015/11/03. eng.
Ardalan, M., Hejazian, S. M., Sharabiyani, H. F., Farnood, F., Ghafari Aghdam, A., Bastami, M., et al. (2020). Dysregulated levels of glycogen synthase kinase-3β (GSK-3β) and miR-135 in peripheral blood samples of cases with nephrotic syndrome. PeerJ 8, e10377. PubMed PMID: 33362958. Pubmed Central PMCID: PMC7749650. Epub 2020/12/29. eng. doi:10.7717/peerj.10377
Bai, M., Li, J., Yang, H., Zhang, H., Zhou, Z., Deng, T., et al. (2019). miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Mol. Ther. 27 (10), 1772–1783. PubMed PMID: 31416776. Pubmed Central PMCID: PMC6822229. Epub 2019/08/17. eng. doi:10.1016/j.ymthe.2019.06.018
Bertoli, G., Cava, C., Corsi, F., Piccotti, F., Martelli, C., Ottobrini, L., et al. (2021). Triple negative aggressive phenotype controlled by miR-135b and miR-365: New theranostics candidates. Sci. Rep. 11 (1), 6553–6612. doi:10.1038/s41598-021-85746-w
Chao, C., Sang, C., Wang, M., Wang, Z., Li, Y., Luo, G., et al. (2019). Prognostic significance of microRNA-135 in patients with digestive system cancers: A systematic review and meta-analysis. Biosci. Rep. 39 (12), BSR20190845. PubMed PMID: 31803920. Pubmed Central PMCID: PMC6923328. Epub 2019/12/06. eng. doi:10.1042/BSR20190845
Chen, B., Yang, W., Zhao, H., Liu, K., Deng, A., Zhang, G., et al. (2020). Abnormal expression of miR-135b-5p in bone tissue of patients with osteoporosis and its role and mechanism in osteoporosis progression. Exp. Ther. Med. 19 (2), 1042–1050. PubMed PMID: 32010267. Pubmed Central PMCID: PMC6966120. Epub 2020/02/06. eng. doi:10.3892/etm.2019.8278
Chen, C., Mao, X., Cheng, C., Jiao, Y., Zhou, Y., Ren, T., et al. (2021). miR-135a reduces osteosarcoma pulmonary metastasis by targeting both BMI1 and KLF4. Front. Oncol. 11, 620295. PubMed PMID: 33828977. Pubmed Central PMCID: PMC8019936. Epub 2021/04/09. eng. doi:10.3389/fonc.2021.620295
Chen, Y., Zhang, J., Wang, H., Zhao, J., Xu, C., Du, Y., et al. (2012). miRNA-135a promotes breast cancer cell migration and invasion by targeting HOXA10. BMC cancer 12, 111. PubMed PMID: 22439757. Pubmed Central PMCID: PMC3350382. Epub 2012/03/24. eng. doi:10.1186/1471-2407-12-111
Cheng, Z., Liu, F., Zhang, H., Li, X., Li, Y., Li, J., et al. (2017). miR-135a inhibits tumor metastasis and angiogenesis by targeting FAK pathway. Oncotarget 8 (19), 31153–31168. PubMed PMID: 28415713. Pubmed Central PMCID: PMC5458197. Epub 2017/04/19. eng. doi:10.18632/oncotarget.16098
Choi, S. A., Koh, E. J., Kim, R. N., Byun, J. W., Phi, J. H., Yang, J., et al. (2020). Extracellular vesicle-associated miR-135b and -135a regulate stemness in Group 4 medulloblastoma cells by targeting angiomotin-like 2. Cancer Cell. Int. 20 (1), 558. PubMed PMID: 33292274. Pubmed Central PMCID: PMC7678136. Epub 2020/12/10. eng. doi:10.1186/s12935-020-01645-6
Dang, Z., Xu, W. H., Lu, P., Wu, N., Liu, J., Ruan, B., et al. (2014). MicroRNA-135a inhibits cell proliferation by targeting Bmi1 in pancreatic ductal adenocarcinoma. Int. J. Biol. Sci. 10 (7), 733–745. PubMed PMID: 25013381. Pubmed Central PMCID: PMC4081607. Epub 2014/07/12. eng. doi:10.7150/ijbs.8097
Deng, Y. Q., Yang, Y. Q., Wang, S. B., Li, F., Liu, M. Z., Hua, Q. Q., et al. (2015). Intranasal administration of lentiviral miR-135a regulates mast cell and allergen-induced inflammation by targeting GATA-3. PloS one 10 (9), e0139322. PubMed PMID: 26418311. Pubmed Central PMCID: PMC4587974. Epub 2015/09/30. eng. doi:10.1371/journal.pone.0139322
Di, Y., Jiang, Y., Shen, X., Liu, J., Gao, Y., Cai, H., et al. (2021). Downregulation of miR-135b-5p suppresses progression of esophageal cancer and contributes to the effect of cisplatin. Front. Oncol. 11, 679348. PubMed PMID: 34277424. Pubmed Central PMCID: PMC8281352. Epub 2021/07/20. eng. doi:10.3389/fonc.2021.679348
Ding, H., Huang, J., Wu, D., Zhao, J., Huang, J., and Lin, Q. (2020). Silencing of the long non-coding RNA MEG3 suppresses the apoptosis of aortic endothelial cells in mice with chronic intermittent hypoxia via downregulation of HIF-1α by competitively binding to microRNA-135a. J. Thorac. Dis. 12 (5), 1903–1916. PubMed PMID: 32642094. Pubmed Central PMCID: PMC7330306. Epub 2020/07/10. eng. doi:10.21037/jtd-19-2472
Ding, Y., Zhong, M., Qiu, B., Liu, C., Wang, J., and Liang, J. (2021). Abnormal expression of miR-135a in patients with depression and its possible involvement in the pathogenesis of the condition. Exp. Ther. Med. 22 (1), 726. PubMed PMID: 34007335. Pubmed Central PMCID: PMC8120643. Epub 2021/05/20. eng. doi:10.3892/etm.2021.10158
Duan, Q., Sun, W., Yuan, H., and Mu, X. (2018). MicroRNA-135b-5p prevents oxygen-glucose deprivation and reoxygenation-induced neuronal injury through regulation of the GSK-3β/Nrf2/ARE signaling pathway. Arch. Med. Sci. 14 (4), 735–744. PubMed PMID: 30002689. Pubmed Central PMCID: PMC6040137. Epub 2018/07/14. eng. doi:10.5114/aoms.2017.71076
Feng, L., Lin, T., Che, H., and Wang, X. (2020). Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1. Cancer Cell. Int. 20, 53. PubMed PMID: 32099526. Pubmed Central PMCID: PMC7029463. Epub 2020/02/27. eng. doi:10.1186/s12935-020-1123-4
Fu, G., Brkić, J., Hayder, H., and Peng, C. (2013). MicroRNAs in human placental development and pregnancy complications. Int. J. Mol. Sci. 14 (3), 5519–5544. doi:10.3390/ijms14035519
Fukagawa, S., Miyata, K., Yotsumoto, F., Kiyoshima, C., Nam, S. O., Anan, H., et al. (2017). MicroRNA-135a-3p as a promising biomarker and nucleic acid therapeutic agent for ovarian cancer. Cancer Sci. 108 (5), 886–896. PubMed PMID: 28231414. Pubmed Central PMCID: PMC5448652. Epub 2017/02/24. eng. doi:10.1111/cas.13210
Gao, S., Chen, T., Hao, Y., Zhang, F., Tang, X., Wang, D., et al. (2020). Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell. Res. Ther. 11 (1), 56. PubMed PMID: 32054526. Pubmed Central PMCID: PMC7020560. Epub 2020/02/15. eng. doi:10.1186/s13287-020-1570-9
GeneCards, (2017). MIR135A1 gene - MicroRNA 135a-1. Available at: https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIR135A1&keywords=mir-135a.
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B. M., Kadkhoda, S., Taheri, M., and Tafrishinejad, A. (2021). A review on the role of miR-149-5p in the carcinogenesis. Int. J. Mol. Sci. 30 (1), 415. PubMed PMID: 35008841. Pubmed Central PMCID: PMC8745060. Epub 2022/01/12. eng. doi:10.3390/ijms23010415
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B. M., Taheri, M., and Samadian, M. (2021). A review on the role of miR-1246 in the pathoetiology of different cancers. Front. Mol. Biosci. 8, 771835. PubMed PMID: 35047553. Pubmed Central PMCID: PMC8762223. Epub 2022/01/21. eng. doi:10.3389/fmolb.2021.771835
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B. M., Taheri, M., and Samadian, M. (2021). A review on the role of miR-1290 in cell proliferation, apoptosis and invasion. Front. Mol. Biosci. 8, 763338. PubMed PMID: 35004844. Pubmed Central PMCID: PMC8740132. Epub 2022/01/11. eng. doi:10.3389/fmolb.2021.763338
Ghafouri-Fard, S., Shaterabadi, D., Abak, A., Shoorei, H., Bahroudi, Z., Taheri, M., et al. (2021). An update on the role of miR-379 in human disorders. Biomed. Pharmacother. = Biomedecine Pharmacother. 139, 111553. PubMed PMID: 33845370. Epub 2021/04/13. eng. doi:10.1016/j.biopha.2021.111553
Gheysarzadeh, A., Sadeghifard, N., Afraidooni, L., Pooyan, F., Mofid, M. R., Valadbeigi, H., et al. (2018). Serum-based microRNA biomarkers for major depression: MiR-16, miR-135a, and miR-1202. J. Res. Med. Sci. 23, 69. PubMed PMID: 30181751. Pubmed Central PMCID: PMC6116664. Epub 2018/09/06. eng. doi:10.4103/jrms.JRMS_879_17
Golubovskaya, V. M., Sumbler, B., Ho, B., Yemma, M., and Cance, W. G. (2014). MiR-138 and MiR-135 directly target focal adhesion kinase, inhibit cell invasion, and increase sensitivity to chemotherapy in cancer cells. Anticancer. Agents Med. Chem. 14 (1), 18–28. PubMed PMID: 23438844. Pubmed Central PMCID: PMC3883917. Epub 2013/02/27. eng. doi:10.2174/187152061401140108113435
Gomez Zubieta, D. M., Hamood, M. A., Beydoun, R., Pall, A. E., and Kondapalli, K. C. (2017). MicroRNA-135a regulates NHE9 to inhibit proliferation and migration of glioblastoma cells. Cell. Commun. Signal. 15 (1), 55. PubMed PMID: 29268774. Pubmed Central PMCID: PMC5740897. Epub 2017/12/23. eng. doi:10.1186/s12964-017-0209-7
Guo, L. M., Ding, G. F., Xu, W., Ge, H., Jiang, Y., Chen, X. J., et al. (2018). MiR-135a-5p represses proliferation of HNSCC by targeting HOXA10. Cancer Biol. Ther. 19 (11), 973–983. PubMed PMID: 29580143. Pubmed Central PMCID: PMC6301828. Epub 2018/03/28. eng. doi:10.1080/15384047.2018.1450112
Han, T. S., Voon, D. C., Oshima, H., Nakayama, M., Echizen, K., Sakai, E., et al. (2019). Interleukin 1 up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology 156 (4), 1140–1155. PubMed PMID: 30508510. Epub 2018/12/07. eng. doi:10.1053/j.gastro.2018.11.059
Hao, M., Zang, M., Zhao, L., Deng, S., Xu, Y., Qi, F., et al. (2016). Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis. Oncotarget 7 (15), 19589–19600. PubMed PMID: 26995755. Pubmed Central PMCID: PMC4991403. Epub 2016/03/21. eng. doi:10.18632/oncotarget.7319
He, Y., Wang, J., Wang, J., Yung, V. Y., Hsu, E., Li, A., et al. (2015). MicroRNA-135b regulates apoptosis and chemoresistance in colorectal cancer by targeting large tumor suppressor kinase 2. Am. J. Cancer Res. 5 (4), 1382–1395. PubMed PMID: 26101704. Pubmed Central PMCID: PMC4473317. Epub 2015/06/24. eng.
He, Y., Wu, L., Dai, Y., Li, J., and Liu, S. (2019). MicroRNA-135 inhibits gastric cancer metastasis by targeting SMAD2. Eur. Rev. Med. Pharmacol. Sci. 23 (21), 9436–9444. PubMed PMID: 31773690. Epub 2019/11/28. eng. doi:10.26355/eurrev_201911_19437
Holleman, A., Chung, I., Olsen, R. R., Kwak, B., Mizokami, A., Saijo, N., et al. (2011). miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene 30 (43), 4386–4398. PubMed PMID: 21552288. Pubmed Central PMCID: PMC3572709. Epub 2011/05/10. eng. doi:10.1038/onc.2011.148
Honardoost, M., Arefian, E., Soleimani, M., Soudi, S., and Sarookhani, M. R. (2016). Development of insulin resistance through induction of miRNA-135 in C2C12 cells. Cell. J. 18 (3), 353–361. PubMed PMID: 27602317. Pubmed Central PMCID: PMC5011323. Epub 2016/09/08. eng. doi:10.22074/cellj.2016.4563
Hu, H., Li, H., and Feng, X. (2019). Downregulation of lncRNA NCK1-AS1 inhibits cancer cell migration and invasion in nasopharyngeal carcinoma by upregulating miR-135a. Cancer Manag. Res. 11, 10531–10537. PubMed PMID: 31908525. Pubmed Central PMCID: PMC6925550. Epub 2020/01/08. eng. doi:10.2147/CMAR.S221326
Hu, Z., Yu, D., Gu, Q. H., Yang, Y., Tu, K., Zhu, J., et al. (2014). miR-191 and miR-135 are required for long-lasting spine remodelling associated with synaptic long-term depression. Nat. Commun. 5, 3263. PubMed PMID: 24535612. Pubmed Central PMCID: PMC3951436. Epub 2014/02/19. eng. doi:10.1038/ncomms4263
Huang, K. T., Kuo, I. Y., Tsai, M. C., Wu, C. H., Hsu, L. W., Chen, L. Y., et al. (2017). Factor VII-induced MicroRNA-135a inhibits autophagy and is associated with poor prognosis in hepatocellular carcinoma. Mol. Ther. Nucleic Acids 9, 274–283. PubMed PMID: 29246306. Pubmed Central PMCID: PMC5675721. doi:10.1016/j.omtn.2017.10.002
Huangfu, L., He, Q., Han, J., Shi, J., Li, X., Cheng, X., et al. (2021). MicroRNA-135b/CAMK2D Axis contribute to malignant progression of gastric cancer through EMT process remodeling. Int. J. Biol. Sci. 17 (8), 1940–1952. PubMed PMID: 34131397. Pubmed Central PMCID: PMC8193265. Epub 2021/06/17. eng. doi:10.7150/ijbs.58062
Hussen, B. M., Hidayat, H. J., Salihi, A., Sabir, D. K., Taheri, M., and Ghafouri-Fard, S. (2021). MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 138, 111528. PubMed PMID: 33770669. Epub 2021/03/27. eng. doi:10.1016/j.biopha.2021.111528
Icli, B., Wu, W., Ozdemir, D., Li, H., Haemmig, S., Liu, X., et al. (2019). MicroRNA-135a-3p regulates angiogenesis and tissue repair by targeting p38 signaling in endothelial cells. FASEB J. 33 (4), 5599–5614. PubMed PMID: 30668922. Pubmed Central PMCID: PMC6436660. Epub 2019/01/23. eng. doi:10.1096/fj.201802063RR
Jiang, D., Zhou, B., Xiong, Y., and Cai, H. (2019). miR-135 regulated breast cancer proliferation and epithelial-mesenchymal transition acts by the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 43 (4), 1623–1634. PubMed PMID: 30720046. Pubmed Central PMCID: PMC6414157. Epub 2019/02/06. eng. doi:10.3892/ijmm.2019.4081
Jiang, H., Qian, Y., Shen, Z., Liu, Y., He, Y., Gao, R., et al. (2021). Circulating microRNA-135a-3p in serum extracellular vesicles as a potential biological marker of non-alcoholic fatty liver disease. Mol. Med. Rep. 24 (1), 498. PubMed PMID: 33955511. Pubmed Central PMCID: PMC8127071. Epub 2021/05/07. eng. doi:10.3892/mmr.2021.12137
Jin, H., Luo, S., Wang, Y., Liu, C., Piao, Z., Xu, M., et al. (2017). miR-135b stimulates osteosarcoma recurrence and lung metastasis via Notch and wnt/β-catenin signaling. Mol. Ther. Nucleic Acids 8, 111–122. PubMed PMID: 28918013. Pubmed Central PMCID: PMC5493819. Epub 2017/09/18. eng. doi:10.1016/j.omtn.2017.06.008
Le, H., Wang, X., Zha, Y., Wang, J., Zhu, W., Ye, Z., et al. (2017). Peripheral lung adenocarcinomas harboring epithelial growth factor receptor mutations with microRNA-135b overexpression are more likely to invade visceral pleura. Oncol. Lett. 14 (6), 7931–7940. PubMed PMID: 29250182. Pubmed Central PMCID: PMC5727608. Epub 2017/12/19. eng. doi:10.3892/ol.2017.7195
Lee, H. W., and Park, S. H. (2017). Elevated microRNA-135a is associated with pulmonary arterial hypertension in experimental mouse model. Oncotarget 8 (22), 35609–35618. PubMed PMID: 28415675. Pubmed Central PMCID: PMC5482602. Epub 2017/04/19. eng. doi:10.18632/oncotarget.16011
Li, J., Liang, H., Bai, M., Ning, T., Wang, C., Fan, Q., et al. (2015). miR-135b promotes cancer progression by targeting transforming growth factor beta receptor II (TGFBR2) in colorectal cancer. PloS one 10 (6), e0130194. PubMed PMID: 26061281. Pubmed Central PMCID: PMC4462589. Epub 2015/06/11. eng. doi:10.1371/journal.pone.0130194
Lin, L., He, Y., Xi, B. L., Zheng, H. C., Chen, Q., Li, J., et al. (2016). MiR-135a suppresses calcification in senescent VSMCs by regulating KLF4/STAT3 pathway. Curr. Vasc. Pharmacol. 14 (2), 211–218. PubMed PMID: 26202084. Pubmed Central PMCID: PMC5403971. Epub 2015/07/24. eng. doi:10.2174/1570161113666150722151817
Liu, D., Zhang, X., Yan, C., Li, Y., Tian, X., Zhu, N., et al. (2015). MicroRNA-495 regulates the proliferation and apoptosis of human umbilical vein endothelial cells by targeting chemokine CCL2. Thromb. Res. 135 (1), 146–154. doi:10.1016/j.thromres.2014.10.027
Liu, H. M., Jia, Y., Zhang, Y. X., Yan, J., Liao, N., Li, X. H., et al. (2019). Dysregulation of miR-135a-5p promotes the development of rat pulmonary arterial hypertension in vivo and in vitro. Acta Pharmacol. Sin. 40 (4), 477–485. PubMed PMID: 30038339. Pubmed Central PMCID: PMC6462033. Epub 2018/07/25. eng. doi:10.1038/s41401-018-0076-9
Lopes, C. B., Magalhães, L. L., Teófilo, C. R., Alves, A., Montenegro, R. C., Negrini, M., et al. (2018). Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC cancer 18 (1), 721. PubMed PMID: 29976158. Pubmed Central PMCID: PMC6034275. Epub 2018/07/07. eng. doi:10.1186/s12885-018-4631-z
Luo, X., Yang, R., Bai, Y., Li, L., Lin, N., Sun, L., et al. (2021). Binding of microRNA-135a (miR-135a) to homeobox protein A10 (HOXA10) mRNA in a high-progesterone environment modulates the embryonic implantation factors beta3-integrin (ITGβ3) and empty spiracles homeobox-2 (EMX2). Ann. Transl. Med. 9 (8), 662. PubMed PMID: 33987360. Pubmed Central PMCID: PMC8106024. Epub 2021/05/15. eng. doi:10.21037/atm-21-596
Lv, K., Liu, Y., Zheng, Y., Dai, S., Yin, P., and Miao, H. (2021). Long non-coding RNA MALAT1 regulates cell proliferation and apoptosis via miR-135b-5p/GPNMB axis in Parkinson's disease cell model. Biol. Res. 54 (1), 10. PubMed PMID: 33726823. Pubmed Central PMCID: PMC7968316. Epub 2021/03/18. eng. doi:10.1186/s40659-021-00332-8
Magalhães, L., Quintana, L. G., Lopes, D. C. F., Vidal, A. F., Pereira, A. L., D'Araujo Pinto, L. C., et al. (2018). APC gene is modulated by hsa-miR-135b-5p in both diffuse and intestinal gastric cancer subtypes. BMC cancer 18 (1), 1055. PubMed PMID: 30376837. Pubmed Central PMCID: PMC6208123. Epub 2018/11/01. eng. doi:10.1186/s12885-018-4980-7
Mao, X. P., Zhang, L. S., Huang, B., Zhou, S. Y., Liao, J., Chen, L. W., et al. (2015). Mir-135a enhances cellular proliferation through post-transcriptionally regulating PHLPP2 and FOXO1 in human bladder cancer. J. Transl. Med. 13, 86. PubMed PMID: 25888950. Pubmed Central PMCID: PMC4367980. Epub 2015/04/19. eng. doi:10.1186/s12967-015-0438-8
Mao, X. W., Xiao, J. Q., Li, Z. Y., Zheng, Y. C., and Zhang, N. (2018). Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 50 (1), e429. PubMed PMID: 29350680. Pubmed Central PMCID: PMC5799799. Epub 2018/01/20. eng. doi:10.1038/emm.2017.239
Mirabutalebi, S. H., Karami, N., Montazeri, F., Fesahat, F., Sheikhha, M. H., Hajimaqsoodi, E., et al. (2018). The relationship between the expression levels of miR-135a and HOXA10 gene in the eutopic and ectopic endometrium. Int. J. Reprod. Biomed. 16 (8), 501–506. PubMed PMID: 30288484. Pubmed Central PMCID: PMC6163047. Epub 2018/10/06. eng. doi:10.29252/ijrm.16.8.501
Nagel, R., le Sage, C., Diosdado, B., van der Waal, M., Oude Vrielink, J. A., Bolijn, A., et al. (2008). Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 68 (14), 5795–5802. PubMed PMID: 18632633. Epub 2008/07/18. eng. doi:10.1158/0008-5472.CAN-08-0951
Nezu, Y., Hagiwara, K., Yamamoto, Y., Fujiwara, T., Matsuo, K., Yoshida, A., et al. (2016). miR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene 35 (48), 6177–6188. PubMed PMID: 27157622. Pubmed Central PMCID: PMC5143367. Epub 2016/05/10. eng. doi:10.1038/onc.2016.157
O'Brien, J., Hayder, H., Zayed, Y., and Peng, C. (2018). Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9, 402. doi:10.3389/fendo.2018.00402
O'Reilly, S., Ciechomska, M., Fullard, N., Przyborski, S., and van Laar, J. M. (2016). IL-13 mediates collagen deposition via STAT6 and microRNA-135b: A role for epigenetics. Sci. Rep. 6, 25066. PubMed PMID: 27113293. Pubmed Central PMCID: PMC4844987. Epub 2016/04/27. eng. doi:10.1038/srep25066
Olasz, E. B., Seline, L. N., Schock, A. M., Duncan, N. E., Lopez, A., Lazar, J., et al. (2015). MicroRNA-135b regulates leucine zipper tumor suppressor 1 in cutaneous squamous cell carcinoma. PloS one 10 (5), e0125412. PubMed PMID: 25938461. Pubmed Central PMCID: PMC4418692. Epub 2015/05/06. eng. doi:10.1371/journal.pone.0125412
Petracco, R., Dias, A. C. O., Taylor, H., Petracco, Á., Badalotti, M., Michelon, J. D. R., et al. (2019). Evaluation of miR-135a/b expression in endometriosis lesions. Biomed. Rep. 11 (4), 181–187. PubMed PMID: 31565224. Pubmed Central PMCID: PMC6759580. Epub 2019/10/01. eng. doi:10.3892/br.2019.1237
Petracco, R., Grechukhina, O., Popkhadze, S., Massasa, E., Zhou, Y., and Taylor, H. S. (2011). MicroRNA 135 regulates HOXA10 expression in endometriosis. J. Clin. Endocrinol. Metab. 96 (12), E1925–E1933. PubMed PMID: 21956427. Pubmed Central PMCID: PMC3232619. Epub 2011/10/01. eng. doi:10.1210/jc.2011-1231
Pons-Espinal, M., Gasperini, C., Marzi, M. J., Braccia, C., Armirotti, A., Pötzsch, A., et al. (2019). MiR-135a-5p is critical for exercise-induced adult neurogenesis. Stem Cell. Rep. 12 (6), 1298–1312. PubMed PMID: 31130358. Pubmed Central PMCID: PMC6565832. Epub 2019/05/28. eng. doi:10.1016/j.stemcr.2019.04.020
Ren, J. W., Li, Z. J., and Tu, C. (2015). MiR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation in human malignant melanoma cells. Int. J. Clin. Exp. Pathol. 8 (6), 6356–6366. PubMed PMID: 26261511. Pubmed Central PMCID: PMC4525845. Epub 2015/08/12. eng.
Schlörmann, W., Naumann, S., Renner, C., and Glei, M. (2015). Influence of miRNA-106b and miRNA-135a on butyrate-regulated expression of p21 and Cyclin D2 in human colon adenoma cells. Genes. Nutr. 10 (6), 50. PubMed PMID: 26559563. Pubmed Central PMCID: PMC4642459. Epub 2015/11/13. eng. doi:10.1007/s12263-015-0500-4
Shan, H., Guo, D., Zhang, S., Qi, H., Liu, S., Du, Y., et al. (2020). SNHG6 modulates oxidized low-density lipoprotein-induced endothelial cells injury through miR-135a-5p/ROCK in atherosclerosis. Cell. Biosci. 10, 4. PubMed PMID: 31921409. Pubmed Central PMCID: PMC6947907. Epub 2020/01/11. eng. doi:10.1186/s13578-019-0371-2
Shao, L., Chen, Z., Soutto, M., Zhu, S., Lu, H., Romero-Gallo, J., et al. (2019). Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J. 33 (1), 264–274. PubMed PMID: 29985646. Pubmed Central PMCID: PMC6355059. Epub 2018/07/10. eng. doi:10.1096/fj.201701456RR
Shin, E. A., Sohn, E. J., Won, G., Choi, J. U., Jeong, M., Kim, B., et al. (2014). Upregulation of microRNA135a-3p and death receptor 5 plays a critical role in Tanshinone I sensitized prostate cancer cells to TRAIL induced apoptosis. Oncotarget 5 (14), 5624–5636. PubMed PMID: 25015549. Pubmed Central PMCID: PMC4170628. Epub 2014/07/13. eng. doi:10.18632/oncotarget.2152
Shin, J. Y., Kim, Y. I., Cho, S. J., Lee, M. K., Kook, M. C., Lee, J. H., et al. (2014). MicroRNA 135a suppresses lymph node metastasis through down-regulation of ROCK1 in early gastric cancer. PloS one 9 (1), e85205. doi:10.1371/journal.pone.0085205
Sõber, S., Laan, M., and Annilo, T. (2010). MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 391 (1), 727–732. PubMed PMID: 19944075. Pubmed Central PMCID: PMC2806518. Epub 2009/12/01. eng. doi:10.1016/j.bbrc.2009.11.128
Sung, H. Y., Choi, E. N., Han, J., Chae, Y. J., Im, S. W., Kim, H. S., et al. (2021). Protective role of ABCA1 in ischemic preconditioning is mediated by downregulation of miR-33-5p and miR-135-5p. Sci. Rep. 11 (1), 12511. PubMed PMID: 34131232. Pubmed Central PMCID: PMC8206355. Epub 2021/06/17. eng. doi:10.1038/s41598-021-91982-x
Taipaleenmäki, H., Browne, G., Akech, J., Zustin, J., van Wijnen, A. J., Stein, J. L., et al. (2015). Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 75 (7), 1433–1444. PubMed PMID: 25634212. Pubmed Central PMCID: PMC4383679. Epub 2015/01/31. eng. doi:10.1158/0008-5472.CAN-14-1026
Tian, Y., Zhang, L., Yu, Q., Wang, Z., and Yang, X. (2020). MiR-135a inhibits non-small cell lung cancer progression by suppressing RAB1B expression and the RAS pathway. Aging (Albany NY) 12 (14), 14480–14489. PubMed PMID: 32710726. Epub 07/25. eng. doi:10.18632/aging.103494
Tribollet, V., Barenton, B., Kroiss, A., Vincent, S., Zhang, L., Forcet, C., et al. (2016). miR-135a inhibits the invasion of cancer cells via suppression of ERRα. PloS one 11 (5), e0156445. doi:10.1371/journal.pone.0156445
Uva, P., Cossu-Rocca, P., Loi, F., Pira, G., Murgia, L., Orrù, S., et al. (2018). miRNA-135b contributes to triple negative breast cancer molecular heterogeneity: Different expression profile in basal-like versus non-Basal-like phenotypes. Int. J. Med. Sci. 15 (6), 536–548. PubMed PMID: 29725243. eng. doi:10.7150/ijms.23402
Valeri, N., Braconi, C., Gasparini, P., Murgia, C., Lampis, A., Paulus-Hock, V., et al. (2014). MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 25 (4), 469–483. PubMed PMID: 24735923. eng. doi:10.1016/j.ccr.2014.03.006
van Battum, E. Y., Verhagen, M. G., Vangoor, V. R., Fujita, Y., Derijck, A., O'Duibhir, E., et al. (2018). An image-based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting krüppel-like factor 4. J. Neurosci. 38 (3), 613–630. PubMed PMID: 29196317. Pubmed Central PMCID: PMC6596187. Epub 2017/12/03. eng. doi:10.1523/JNEUROSCI.0662-17.2017
Vidal, A. F., Cruz, A. M., Magalhães, L., Pereira, A. L., Anaissi, A. K., Alves, N. C., et al. (2016). hsa-miR-29c and hsa-miR-135b differential expression as potential biomarker of gastric carcinogenesis. World J. Gastroenterol. 22 (6), 2060–2070. PubMed PMID: 26877610. Pubmed Central PMCID: PMC4726678. Epub 2016/02/16. eng. doi:10.3748/wjg.v22.i6.2060
Wang, H., Peng, R., Wang, J., Qin, Z., and Xue, L. (2018). Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 10 (1), 59–10. doi:10.1186/s13148-018-0492-1
Wang, N., Tao, L., Zhong, H., Zhao, S., Yu, Y., Yu, B., et al. (2016). miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol. Lett. 11 (1), 543–550. PubMed PMID: 26870245. Pubmed Central PMCID: PMC4727074. Epub 2016/02/13. eng. doi:10.3892/ol.2015.3970
Wang, N., and Zhang, T. (2018). Downregulation of MicroRNA-135 promotes sensitivity of non-small cell lung cancer to gefitinib by targeting TRIM16. Oncol. Res. 26 (7), 1005–1014. PubMed PMID: 29295721. Pubmed Central PMCID: PMC7844745. Epub 2018/01/04. eng. doi:10.3727/096504017X15144755633680
Wang, Y., Yang, Z., Zhang, K., Wan, Y., Zhou, Y., and Yang, Z. (2021). miR-135a-5p inhibitor protects glial cells against apoptosis via targeting SIRT1 in epilepsy. Exp. Ther. Med. 21 (5), 431. PubMed PMID: 33747170. Pubmed Central PMCID: PMC7967866. Epub 2021/03/23. eng. doi:10.3892/etm.2021.9848
Wei, X., Yang, X., Wang, B., Yang, Y., Fang, Z., Yi, C., et al. (2020). LncRNA MBNL1-AS1 represses cell proliferation and enhances cell apoptosis via targeting miR-135a-5p/PHLPP2/FOXO1 axis in bladder cancer. Cancer Med. 9 (2), 724–736. PubMed PMID: 31769229. Pubmed Central PMCID: PMC6970060. Epub 2019/11/27. eng. doi:10.1002/cam4.2684
Wu, D., and Pan, W. (2010). GSK3: A multifaceted kinase in Wnt signaling. Trends biochem. Sci. 35 (3), 161–168. PubMed PMID: 19884009. Pubmed Central PMCID: PMC2834833. Epub 2009/11/04. eng. doi:10.1016/j.tibs.2009.10.002
Xiao, S., Yang, Z., Lv, R., Zhao, J., Wu, M., Liao, Y., et al. (2014). miR-135b contributes to the radioresistance by targeting GSK3β in human glioblastoma multiforme cells. PloS one 9 (9), e108810. PubMed PMID: 25265336. Pubmed Central PMCID: PMC4181861. Epub 2014/09/30. eng. doi:10.1371/journal.pone.0108810
Xie, B., Lu, C., Chen, C., Zhou, J., and Deng, Z. (2020). miR-135a alleviates silica-induced pulmonary fibrosis by targeting NF-κB/Inflammatory signaling pathway. Mediat. Inflamm. 2020, 1231243. PubMed PMID: 32617074. Pubmed Central PMCID: PMC7317310. Epub 2020/07/04. eng. doi:10.1155/2020/1231243
Xie, X. J., Fan, D. M., Xi, K., Chen, Y. W., Qi, P. W., Li, Q. H., et al. (2017). Suppression of microRNA-135b-5p protects against myocardial ischemia/reperfusion injury by activating JAK2/STAT3 signaling pathway in mice during sevoflurane anesthesia. Biosci. Rep. 37 (3), BSR20170186. PubMed PMID: 28522550. Pubmed Central PMCID: PMC6434087. Epub 2017/05/20. eng. doi:10.1042/BSR20170186
Xie, Y., Li, F., Li, Z., and Shi, Z. (2019). miR-135a suppresses migration of gastric cancer cells by targeting TRAF5-mediated NF-κB activation. Onco. Targets. Ther. 12, 975–984. PubMed PMID: 30774383. Pubmed Central PMCID: PMC6362934. Epub 2019/02/19. eng. doi:10.2147/OTT.S189976
Xu, S., Cecilia Santini, G., De Veirman, K., Vande Broek, I., Leleu, X., De Becker, A., et al. (2013). Upregulation of miR-135b is involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. PloS one 8 (11), e79752. PubMed PMID: 24223191. Pubmed Central PMCID: PMC3819242. Epub 2013/11/14. eng. doi:10.1371/journal.pone.0079752
Xu, X. M., Qian, J. C., Deng, Z. L., Cai, Z., Tang, T., Wang, P., et al. (2012). Expression of miR-21, miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncol. Lett. 4 (2), 339–345. PubMed PMID: 22844381. Pubmed Central PMCID: PMC3402725. Epub 2012/07/31. eng. doi:10.3892/ol.2012.714
Xu, Y., Zhao, S., Cui, M., and Wang, Q. (2015). Down-regulation of microRNA-135b inhibited growth of cervical cancer cells by targeting FOXO1. Int. J. Clin. Exp. Pathol. 8 (9), 10294–10304. PubMed PMID: 26617737. Pubmed Central PMCID: PMC4637552. Epub 2015/12/01. eng.
Xu, Z., Han, Y., Liu, J., Jiang, F., Hu, H., Wang, Y., et al. (2015). MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci. Rep. 5, 12276. PubMed PMID: 26184978. Pubmed Central PMCID: PMC4505325. Epub 2015/07/18. eng. doi:10.1038/srep12276
Xue, Y., Ni, T., Jiang, Y., and Li, Y. (2017). Long noncoding RNA GAS5 inhibits tumorigenesis and enhances radiosensitivity by suppressing miR-135b expression in non-small cell lung cancer. Oncol. Res. 25 (8), 1305–1316. PubMed PMID: 28117028. Pubmed Central PMCID: PMC7841232. Epub 2017/01/25. eng. doi:10.3727/096504017X14850182723737
Yamada, Y., Hidaka, H., Seki, N., Yoshino, H., Yamasaki, T., Itesako, T., et al. (2013). Tumor-suppressive microRNA-135a inhibits cancer cell proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer Sci. 104 (3), 304–312. PubMed PMID: 23176581. Pubmed Central PMCID: PMC7657112. Epub 2012/11/28. eng. doi:10.1111/cas.12072
Yan, L. H., Chen, Z. N., Li, L., Chen, J., Wei, W. E., Mo, X. W., et al. (2016). miR-135a promotes gastric cancer progression and resistance to oxaliplatin. Oncotarget 7 (43), 70699–70714. PubMed PMID: 27683111. Pubmed Central PMCID: PMC5342584. Epub 2016/09/30. eng. doi:10.18632/oncotarget.12208
Yang, G., and Yin, B. (2017). Therapeutic effects of long-circulating miR-135a-containing cationic immunoliposomes against gallbladder carcinoma. Sci. Rep. 7 (1), 5982. PubMed PMID: 28729631. Pubmed Central PMCID: PMC5519676. Epub 2017/07/22. eng. doi:10.1038/s41598-017-06234-8
Yang, S., Zhan, X., He, M., Wang, J., and Qiu, X. (2020). miR-135b levels in the peripheral blood serve as a marker associated with acute ischemic stroke. Exp. Ther. Med. 19 (6), 3551–3558. PubMed PMID: 32346417. Pubmed Central PMCID: PMC7185079. Epub 2020/04/30. eng. doi:10.3892/etm.2020.8628
Yang, W., Feng, W., Wu, F., Gao, Y., Sun, Q., Hu, N., et al. (2020). MiR-135-5p inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by targeting SMAD3 in breast cancer. J. Cancer 11 (21), 6402–6412. PubMed PMID: 33033523. Pubmed Central PMCID: PMC7532519. Epub 2020/10/10. eng. doi:10.7150/jca.47083
Yang, X., Wang, X., Nie, F., Liu, T., Yu, X., Wang, H., et al. (2015). miR-135 family members mediate podocyte injury through the activation of Wnt/β-catenin signaling. Int. J. Mol. Med. 36 (3), 669–677. PubMed PMID: 26134897. Pubmed Central PMCID: PMC4533775. Epub 2015/07/03. eng. doi:10.3892/ijmm.2015.2259
Yang, X., Wu, D., Du, H., Nie, F., Pang, X., and Xu, Y. (2017). MicroRNA-135a is involved in podocyte injury in a transient receptor potential channel 1-dependent manner. Int. J. Mol. Med. 40 (5), 1511–1519. PubMed PMID: 28949388. Pubmed Central PMCID: PMC5627871. Epub 2017/09/28. eng. doi:10.3892/ijmm.2017.3152
Yang, Y., Ishak Gabra, M. B., Hanse, E. A., Lowman, X. H., Tran, T. Q., Li, H., et al. (2019). MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat. Commun. 10 (1), 809. PubMed PMID: 30778058. Pubmed Central PMCID: PMC6379428. Epub 2019/02/20. eng. doi:10.1038/s41467-019-08759-0
Yin, L., Xiao, X., Georgikou, C., Luo, Y., Liu, L., Gladkich, J., et al. (2019). Sulforaphane induces miR135b-5p and its target gene, RASAL2, thereby inhibiting the progression of pancreatic cancer. Mol. Ther. Oncolytics 14, 74–81. PubMed PMID: 31044154. Pubmed Central PMCID: PMC6479751. Epub 2019/05/03. eng. doi:10.1016/j.omto.2019.03.011
Yin, N., Zhu, L., Ding, L., Yuan, J., Du, L., Pan, M., et al. (2019). MiR-135-5p promotes osteoblast differentiation by targeting HIF1AN in MC3T3-E1 cells. Cell. Mol. Biol. Lett. 24, 51. PubMed PMID: 31410089. Pubmed Central PMCID: PMC6686269. Epub 2019/08/15. eng. doi:10.1186/s11658-019-0177-6
Yuan, F., Yun, Y., Fan, H., Li, Y., Lu, L., Liu, J., et al. (2020). MicroRNA-135a protects against ethanol-induced apoptosis in neural crest cells and craniofacial defects in zebrafish by modulating the siah1/p38/p53 pathway. Front. Cell. Dev. Biol. 8, 583959. PubMed PMID: 33134300. Pubmed Central PMCID: PMC7561719. Epub 2020/11/03. eng. doi:10.3389/fcell.2020.583959
Zhang, J., Liu, W., Wang, Y., Zhao, S., and Chang, N. (2017). miR-135b plays a neuroprotective role by targeting GSK3β in MPP(+)-Intoxicated SH-SY5Y cells. Dis. Markers 2017, 5806146. PubMed PMID: 28484287. Pubmed Central PMCID: PMC5412211. Epub 2017/05/10. eng. doi:10.1155/2017/5806146
Zhang, L., Sun, Z. J., Bian, Y., and Kulkarni, A. B. (2013). MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett. 331 (2), 230–238. PubMed PMID: 23340180. Pubmed Central PMCID: PMC3660136. Epub 2013/01/24. eng. doi:10.1016/j.canlet.2013.01.003
Zhang, S. Y., Huang, S. H., Gao, S. X., Wang, Y. B., Jin, P., and Lu, F. J. (2019). Upregulation of lncRNA RMRP promotes the activation of cardiac fibroblasts by regulating miR-613. Mol. Med. Rep. 20 (4), 3849–3857. doi:10.3892/mmr.2019.10634
Zhang, T., and Wang, N. (2018). miR-135a confers resistance to gefitinib in non-small cell lung cancer cells by upregulation of RAC1. Oncol. Res. 26 (8), 1191–1200. PubMed PMID: 29386087. Pubmed Central PMCID: PMC7844633. Epub 2018/02/02. eng. doi:10.3727/096504018X15166204902353
Zhang, W., Wu, M., Chong, Q-Y., Zhang, M., Zhang, X., Hu, L., et al. (2018). Loss of estrogen-regulated MIR135A1 at 3p21.1 promotes tamoxifen resistance in breast cancer. Cancer Res. 78 (17), 4915–4928. doi:10.1158/0008-5472.CAN-18-0069
Zhang, X., Gao, F., Zhou, L., Wang, H., Shi, G., and Tan, X. (2017). UCA1 regulates the growth and metastasis of pancreatic cancer by sponging miR-135a. Oncol. Res. 25 (9), 1529–1541. PubMed PMID: 28315290. Pubmed Central PMCID: PMC7841060. Epub 2017/03/21. eng. doi:10.3727/096504017X14888987683152
Zhang, X., Lu, J., Zhang, Q., Luo, Q., and Liu, B. (2021). CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 54 (1), 11. PubMed PMID: 33757583. Pubmed Central PMCID: PMC7986494. Epub 2021/03/25. eng. doi:10.1186/s40659-021-00335-5
Zhang, X., You, J. M., Dong, X. J., and Wu, Y. (2020). Administration of mircoRNA-135b-reinforced exosomes derived from MSCs ameliorates glucocorticoid-induced osteonecrosis of femoral head (ONFH) in rats. J. Cell. Mol. Med. 24 (23), 13973–13983. PubMed PMID: 33089961. Pubmed Central PMCID: PMC7754047. Epub 2020/10/23. eng. doi:10.1111/jcmm.16006
Zhang, Y., Zhang, Z., Yi, Y., Wang, Y., and Fu, J. (2020). CircNOL10 acts as a sponge of miR-135a/b-5p in suppressing colorectal cancer progression via regulating KLF9. Onco. Targets. Ther. 13, 5165–5176. PubMed PMID: 32606737. Pubmed Central PMCID: PMC7292486. Epub 2020/07/02. eng. doi:10.2147/OTT.S242001
Zhao, C-C., Jiao, Y., Zhang, Y-Y., Ning, J., Zhang, Y-R., Xu, J., et al. (2019). Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/β-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell. Death Dis. 10 (4), 252–315. doi:10.1038/s41419-019-1479-3
Zhao, J., Wang, X., Mi, Z., Jiang, X., Sun, L., Zheng, B., et al. (2021). STAT3/miR-135b/NF-κB axis confers aggressiveness and unfavorable prognosis in non-small-cell lung cancer. Cell. Death Dis. 12 (5), 1–16. doi:10.1038/s41419-021-03773-x
Zhao, Z., Lin, X., Tong, Y., and Li, W. (2019). Silencing lncRNA ZFAS1 or elevated microRNA-135a represses proliferation, migration, invasion and resistance to apoptosis of osteosarcoma cells. Cancer Cell. Int. 19, 326. PubMed PMID: 31827400. Pubmed Central PMCID: PMC6892223. Epub 2019/12/13. eng. doi:10.1186/s12935-019-1049-x
Zheng, C., Li, X., Ren, Y., Yin, Z., and Zhou, B. (2019). Long noncoding RNA RAET1K enhances CCNE1 expression and cell cycle arrest of lung adenocarcinoma cell by sponging miRNA-135a-5p. Front. Genet. 10, 1348. PubMed PMID: 32010197. Pubmed Central PMCID: PMC6979007. Epub 2020/02/06. eng. doi:10.3389/fgene.2019.01348
Zheng, K., Hu, F., Zhou, Y., Zhang, J., Zheng, J., Lai, C., et al. (2021). miR-135a-5p mediates memory and synaptic impairments via the Rock2/Adducin1 signaling pathway in a mouse model of Alzheimer's disease. Nat. Commun. 12 (1), 1903. PubMed PMID: 33771994. doi:10.1038/s41467-021-22196-y
Zheng, Y., Zheng, B., Meng, X., Yan, Y., He, J., and Liu, Y. (2019). LncRNA DANCR promotes the proliferation, migration, and invasion of tongue squamous cell carcinoma cells through miR-135a-5p/KLF8 axis. Cancer Cell. Int. 19, 302. PubMed PMID: 31827393. Pubmed Central PMCID: PMC6862788. Epub 2019/12/13. eng. doi:10.1186/s12935-019-1016-6
Zhou, H., Guo, W., Zhao, Y., Wang, Y., Zha, R., Ding, J., et al. (2014). MicroRNA-135a acts as a putative tumor suppressor by directly targeting very low density lipoprotein receptor in human gallbladder cancer. Cancer Sci. 105 (8), 956–965. PubMed PMID: 24903309. Pubmed Central PMCID: PMC4317855. Epub 2014/06/07. eng. doi:10.1111/cas.12463
Zhou, J., Wang, H., Che, J., Xu, L., Yang, W., Li, Y., et al. (2020). Silencing of microRNA-135b inhibits invasion, migration, and stemness of CD24(+)CD44(+) pancreatic cancer stem cells through JADE-1-dependent AKT/mTOR pathway. Cancer Cell. Int. 20, 134. PubMed PMID: 32351328. Pubmed Central PMCID: PMC7183669. Epub 2020/05/01. eng. doi:10.1186/s12935-020-01210-1
Keywords: miRNA, miR-135, cancer, expression, biomarker
Citation: Kadkhoda S, Eslami S, Mahmud Hussen B and Ghafouri-Fard S (2022) A review on the importance of miRNA-135 in human diseases. Front. Genet. 13:973585. doi: 10.3389/fgene.2022.973585
Received: 20 June 2022; Accepted: 04 August 2022;
Published: 06 September 2022.
Edited by:
William C. Cho, QEH, Hong Kong SAR, ChinaReviewed by:
Kristina Snipaitiene, Vilnius University, LithuaniaCopyright © 2022 Kadkhoda, Eslami, Mahmud Hussen and Ghafouri-Fard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soudeh Ghafouri-Fard, cy5naGFmb3VyaWZhcmRAc2JtdS5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.