- 1College of Computer Science, Chongqing University, Chongqing, China
- 2Chongqing Key Laboratory of Software Theory and Technology, Chongqing, China
- 3Faculty of Science and Engineering, Iwate University, Morioka, Japan
Numerous varieties of life forms have filled the earth throughout evolution. Evolution consists of two processes: self-replication and interaction with the physical environment and other living things around it. Initiated by von Neumann et al. studies on self-replication in cellular automata have attracted much attention, which aim to explore the logical mechanism underlying the replication of living things. In nature, competition is a common and spontaneous resource to drive self-replications, whereas most cellular-automaton-based models merely focus on some self-protection mechanisms that may deprive the rights of other artificial life (loops) to live. Especially, Huang et al. designed a self-adaptive, self-replicating model using a greedy selection mechanism, which can increase the ability of loops to survive through an occasionally abandoning part of their own structural information, for the sake of adapting to the restricted environment. Though this passive adaptation can improve diversity, it is always limited by the loop’s original structure and is unable to evolve or mutate new genes in a way that is consistent with the adaptive evolution of natural life. Furthermore, it is essential to implement more complex self-adaptive evolutionary mechanisms not at the cost of increasing the complexity of cellular automata. To this end, this article proposes new self-adaptive mechanisms, which can change the information of structural genes and actively adapt to the environment when the arm of a self-replicating loop encounters obstacles, thereby increasing the chance of replication. Meanwhile, our mechanisms can also actively add a proper orientation to the current construction arm for the sake of breaking through the deadlock situation. Our new mechanisms enable active self-adaptations in comparison with the passive mechanism in the work of Huang et al. which is achieved by including a few rules without increasing the number of cell states as compared to the latter. Experiments demonstrate that this active self-adaptability can bring more diversity than the previous mechanism, whereby it may facilitate the emergence of various levels in self-replicating structures.
1 Introduction
A cellular automaton (CA) is a discrete dynamical system that consists of a huge number of identical finite-state automata (Abou-Jaoudé et al., 2016; Xiao et al., 2020). Self-replication is a fundamental feature of life in biological resources, and it is a process of biosynthesis in which the original structure is replicated in the exact same structure (Cea et al., 2015; Baris et al., 2022; Gemble et al., 2022). Research of self-replication on CAs was founded by von Neumann (1966) and was viewed as one of the origins of artificial life research (Marchal, 1998; Gindin et al., 2014). In addition to reproducing offsprings with identical structures, attempts at including self-adapting mechanisms into the self-replicating models have been done (Suzuki and Ikegami, 2003; Sayama, 2004; Huang et al., 2013). In particular, Huang et al. (2013) designed a self-adaptive, self-replicating model using a greedy selection mechanism, which can increase the ability of the loops to survive through an occasionally abandoning part of their own structural information, for the sake of adapting to the restricted environment. Although the greedy mechanism is straightforward and sounds natural, it seems too passive. In addition to the self-adaptation which helps organisms survive (Williams and Burt, 1997), evolution and mutation are also inherent abilities of living things for adapting to environments in more active ways (Agrawal, 2001; Wilke et al., 2001; Miles et al., 2020; Moore et al., 2021; Monroe et al., 2022; Sasani et al., 2022), like the RNA virus (Domingo and Holland, 1997).
Likewise, identification of multiple adaptive mutations turns out to be essential for studying adaptation (Aminetzach et al., 2005; Scott, 2013; Lawson et al., 2020; Zuko et al., 2021). And, point mutations including insertions and replacements can help perform edits in human cells, thereby, in principle, correcting up to most of the known genetic variants associated with human diseases (Poduri et al., 2013; Anzalone et al., 2019; Buisson et al., 2019). Especially, changes in the self-replicating structure and behavior are controlled via their genetic memory (Bilotta and Pantano, 2006; Sha et al., 2020). As the living environment becomes more and more hostile, living organisms may have to change their own structures to survive. Self-adaptation through gene mutation, therefore, provides a spontaneous drive for natural life to survive against crucial competition with other living things and evolve into more advanced forms (Bilotta and Pantano, 2006; Sha et al., 2020). Moreover, self-adaptation has gained much attention in other fields such as knowledge architecture discovering (Edwards et al., 2009; Duan, 2019; Lei and Duan, 2021; Li et al., 2021) and edge computing (Xia et al., 2015; Song et al., 2018), due to its promise of more sophisticated and flexible computational paradigms (Duan et al., 2019a,b).
Inspired by the gene mutation-based self-adaptability in nature, this article endows two active mechanisms to the self-replicating loops which can facilitate the dynamical adaption of their structures to limited cellular regions. The new active mechanisms only need to change some rules in the passive model Huang et al. (2013), without increasing the number of cell states. The self-replication progress also contains two stages. In the first stage, the shape-encoding scheme is utilized to generate genetic information (construction signals), and the constructed arm receives the genetic codes to stretch forward, rightward, or leftward. During this period, collisions may occur at any moment and it seems urgently necessary to find a way out of a stalemate. Similar to the gene mutation process, we propose two solutions to resolve the collision. One mechanism generates, rather than waiting , a genetic code which resembles the insert mutation from single point mutation (Bargmann et al., 1986; Shenhav and Zeevi, 2020). Especially, the insertion of a transposable element can increase Drosophila’s resistance to an organophosphate pesticide (Aminetzach et al., 2005), which helps Drosophila to survive. In order to simplify the rules Huang et al. (2013), we randomly change the direction of the construction arms’ head. Another mechanism will choose to change following the genetic code from the mother loop next to the construction arm, which is similar to replace mutation (Vogel, 1972). The method of replacing genetic codes is used in suppression of tumorigenicity of human prostate carcinoma cells (Bookstein et al., 1990). After finishing the first extension stage of the construction arm, the mother loop will send a validation signal to the arm for the sake of confirming whether there is a closed loop or not. If it succeeds, the signal will cut off the link between the child loop and mother loop; otherwise, the construction arm will be drawn back. Finally, several typical and initial configurations are selected for the numerical experiments, which demonstrate that our new active mechanisms can obtain more types of variation loops, thereby increasing the opportunities of the organisms’ survival and expanding biodiversity (Klimentidis, 2012; Becerra-Rodríguez et al., 2021).
This article is organized as follows: Section 2 reviews related works. Section 3 gives an overview of the self-timed cellular automata and describes self-replicating loops with two active mechanisms which are capable of self-adapting their structures when the space is not enough to replicate themselves completely. Detailed comparison experiments are done in Section 4, followed by discussions given in Section 5.
2 Related works
Self-reproduction is one of the fundamental features in nature. Von Neumann was able to exhibit a universal Turing machine embedded in a cellular space using 29-states per cell and the 5-cell neighborhood. After that, many studies were done to reduce the complexity of the machine (Codd, 2014), re-mold signal-crossing organs (Buckley and Mukherjee, 2005), and realize self-replicating in the hardware (Merkle, 1992; Pesavento, 1995; Tempesti et al., 1998).
After ignoring the universality in computations, Langton (1984) proposed a simple self-replicating loop based on the periodic emitter (Codd, 2014) in a two-dimensional cellular space. Langton’s loop uses 8-states and 5-cell neighborhood (von Neumann neighborhood). After that, Langton’ loop attracts much attention and various attempts have been done, such as deleting the external sheath (Tempesti, 1995) or the inner sheath (Byl, 1989), producing unsheathed loops with less states (Reggia et al., 1993), and considering self-replication on asynchronous cellular automata (Nehaniv, 2002). Likewise, Ibáñez et al. (1995) introduced the ability of self-inspection, which allows the genome to dynamically construct concomitantly with its interpretation. Making full of the self-inspection ability, Morita and Imai (1996b) proposed a shape-encoding mechanism that depends on genetic codes from the loops’ phenotypical pattern to self-replication. Afterward, there were many studies in two-dimensional (Morita and Imai, 1996a) or three-dimensional reversible cellular space (Imai et al., 2002). In addition to self-replication, interacting between different loops has been conjectured, including self-protection with shielding, deflecting, and poisoning (Sayama, 2004), settling collisions with inroad, counter, defensive, and cancel methods (Suzuki and Ikegami, 2003). Such actions always harm the right of others to live.
All the aforementioned self-replicating models are based on synchronous CAs, in which all the cells are iterated to undergo state transitions simultaneously at every discrete time step. In nature, living systems are characterized by asynchronous timing modes, whereby studying self-replication on asynchronous cellular automata (ACAs) turns out to be crucial for a deeper understanding of the underlying mechanisms Huang et al. (2013). In an ACA, cells are updated at random timings independently from other cells, not needing a central clock signal to be distributed to all cells at any time. On the other hand, the unpredictable updating order of cells tends to bring more difficulty into the construction and self-reproduction on ACAs than on synchronous CAs. Nevertheless, Takada et al. (2007) designed a self-replicating loop based on the self-timed cellular automaton, which can self-reproduce parallelly and cope with the deadlock caused by collisions between self-replicating loops due to the asynchronous updating sequence. Especially, they used a simple mechanism that permits two colliding arms to fall back simultaneously. Huang et al. (2013) endowed a self-adaptive ability to the model, which allows two loops to not retract their arms but continue to accomplish self-replication when a collision occurs on occasion. In this case, the dead head will wait for a construction signal that can move the head into a direction away from the collision. More specifically, the choice of using which signal is made locally at the moment when the end of the constructing arm runs into an obstacle, and hence, such a selection is greedy. As a result, the passive self-adaptation can work in many situations where the normal reproduction of a loop is disturbed by some external constrain, thereby enabling the loop to survive and reproduce in a wide variety of regions (Huang et al., 2013).
3 Materials and methods
3.1 Self-timed cellular automata
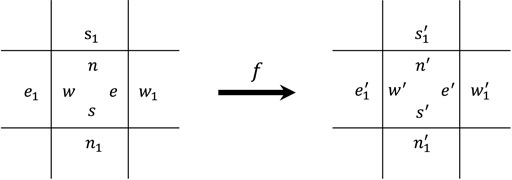
Our self-replicating loops are implemented on a self-timed cellular automaton (Peper et al., 2002; Takada et al., 2007), which comprises of a two-dimensional asynchronous cellular array of identical cells. Each cell is partitioned into four parts in a one-to-one correspondence with its neighboring cells, and each part has a state taken from a finite set of states at a time. Thus, a STCA may be deemed to a partitioned cellular automaton (Imai et al., 2002). Each cell undergoes transitions according to a transition function f that operates on the four parts of the cell and the nearest part of its four neighbors. The transition function f is defined as follows:
where each value in parentheses denotes the new state of a partition after updating (see Figure 1).
Also, transition rules of an STCA are rotation symmetric, such that rotating both the left-hand side and the right-hand side of a rule in a multiple of 90° simultaneously give rise to equivalent rules of the original one. The transitions of cells in an STCA occur randomly and are independent of each other, i.e., an ACA. Because the update of a cell may change the nearest sub-cells of its neighboring cells, to prevent a write–conflict situation from occurring, we assume that all neighboring cells never undergo transitions at the same time. To this end, an effective scheme that can be used to iterate the STCA’s global transition is called random choice, by which at a time, only one cell is randomly selected with uniform probability to undergo a transition.
3.2 Self-replicating loops with active self-adaptability
Different from sheathed self-replicating loops in Suzuki and Ikegami (2003), a self-replicating loop implemented on our STCA model is unsheathed and needs the same number of states as the passive model in Huang et al. (2013). Four-cell states are used for each part of any cell, denoted by ♯, ◦, • and ■, respectively. The state ♯ is often shown blank in the figures for convenience. A cell is quiescent if all of its four sub-cells are in the state ♯. Transition rules are listed in Supplementary Appendix A, excluding the rotational symmetry equivalents.
3.2.1 Normal self-replicating based on shape-encoding mechanism
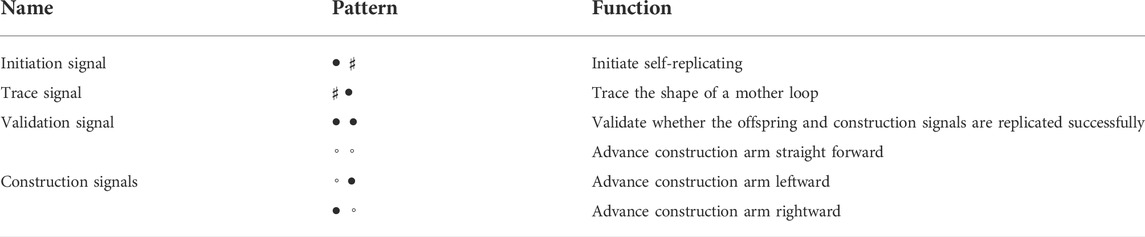
When enough space is left, a loop can normally replicate itself in the cell region. Several signals listed in Table 1 are used to fulfill the self-replication according to the shape-encoding mechanism.
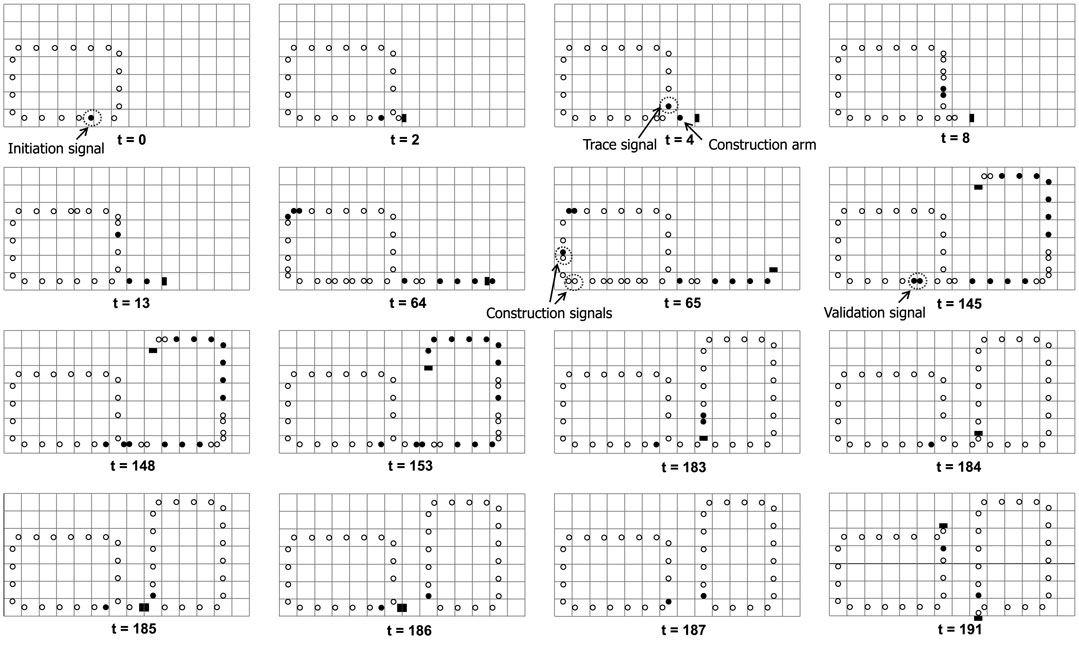
Figure 2 illustrates a typical self-replicating process of a loop, which is similar to Huang et al. (2013). An initiation signal will transmit counterclockwise before the replication starts. When the initiation signal arrives at a left-turn corner of the loop, it generates an initial construct arm stretching out from the corner, as well as an inspection head to trace the shape of the mother loop. The inspection head •• will sequentially encodes each cell into an appropriate construction signals including going straight, turning right, and turning left. The signals from the mother loop are continuously transmitted to the head of the construct arm and are decoded into the corresponding part. Moreover, as soon as the shape-encoding process finishes, a validation signal is generated to verify whether the sub loop is constructed. If self-replicating succeeds, the signal will cut off the umbilical cord between the mother and the child, whereby both loops can start further replications individually.
3.2.2 Adaptive self-replication with mutations
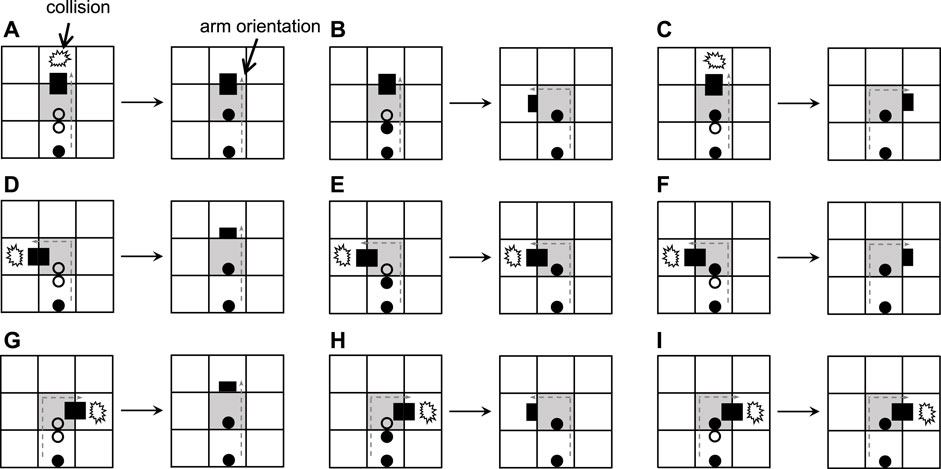
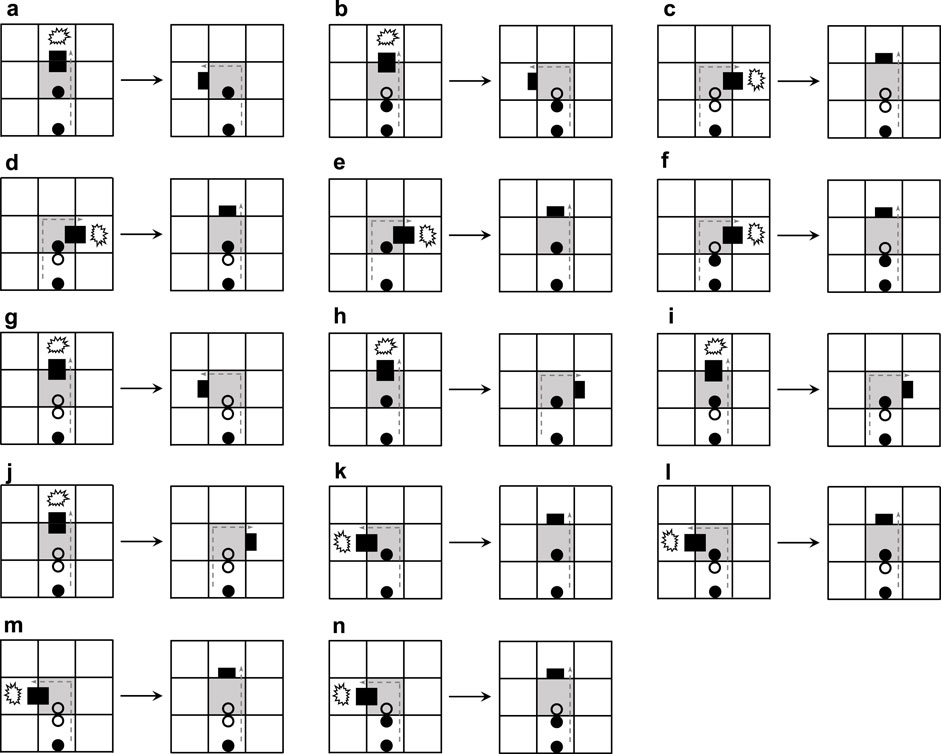
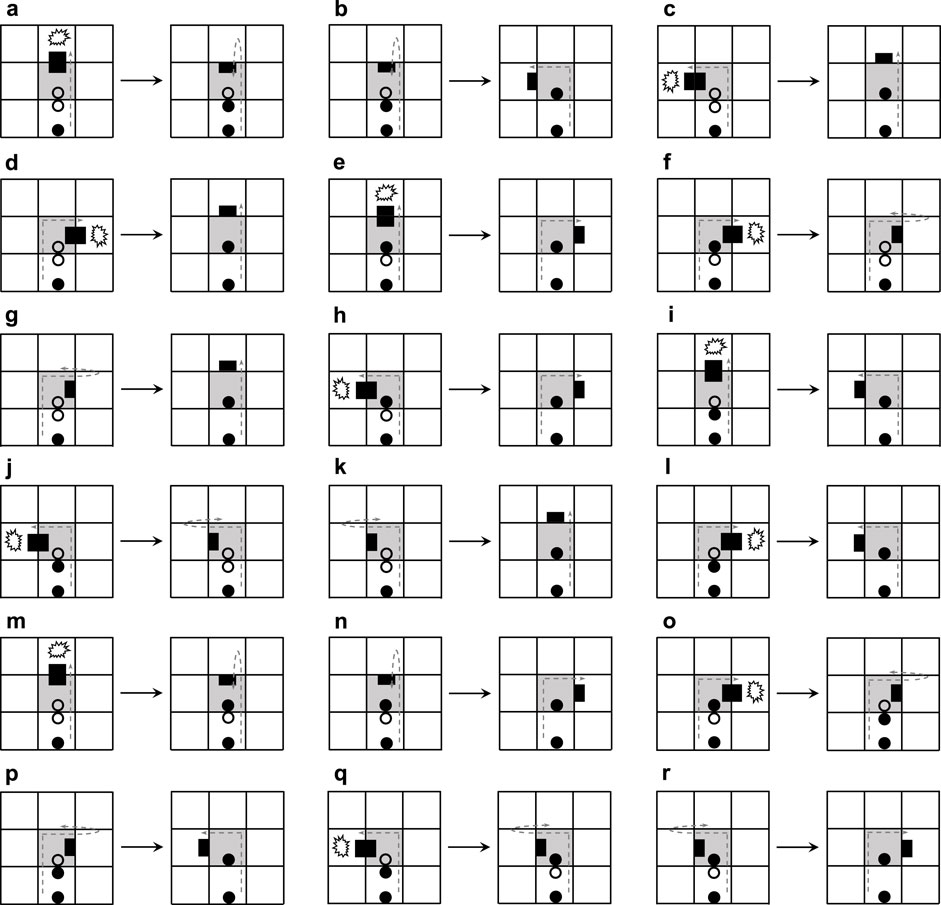
What will happen if there is no extra space for normal self-replication of a loop or if the space is taken up by the arms of other loops? Huang et al. (2013) considered a greedy selection mechanism to deal with the situation, which means only useful information is retained during self-replication. And the details are shown in Figure 3. After a collision occurs, the construction arm’s head becomes a dead head waiting for the construction signals coming from its mother. If the signal can work, then use it and change the direction of the construction arm. Otherwise, simply throw it away. Although such self-adaptation is simple and straightforward, it is passive and weak, resulting in much smaller child loops. In order to increase the adaptability and diversity of self-replicating models, we propose two novel mechanisms for active adaptation as follows:
Adding: add a different construction signal next to the head of the construction arm. For simplicity, the direction is directly changed at random.
Changing: change the construction signal following the head of the construction arm to other construction signals that are selected randomly.
Collisions are often inevitable due to the unpredictable nature of asynchronous updating. If the construction arm of a self-replicating loop perceives that the space is occupied, then it cannot extend furthermore and the state of the construction arm head will change from ♯■ to ■■ (called dead end). There are many situations when a collision occurs, such as an arm bumping into another loop’s arm or an arm meeting the body of a loop.
Figure 4 elaborates the process of adding mechanisms for active adaptation. When the arm under going straight collides with an obstacle (Figures 4A,H), the current blocking state will be changed by randomly selecting one of the two orientations, namely turning left and turning right. Even a construction signal behind the dead head is a straight-going signal; the mechanism will add a random direction (Figure 4G and Figure 4J). Especially if the construction signal behind the dead head is a left-turning signal, the dead head will turn left and become normal after going straight is blocked (Figure 4B). Similarly, if there is a right-turning signal, the head will turn right (Figure 4I). Whatever a construction signal is behind the dead head, if the head is blocked by turning left or right, then the head will go straight.
The content of the changing mechanism is presented in Figure 5. If an arm going straight meets an obstacle and the construction signal behind the dead head is a straight-going signal, then the straight-going signal will change to a left-turning signal (Figure 5A) or a right-turning signal (Figure 5M) and the head goes back. Such a state is not durable, and after which the arm will turn left (Figure 5B) or turn right (Figure 5N). If the construction signal behind the dead head can mitigate the collision, the original signal remains constant (Figures 5C–E, H, and I). When the arm is blocked to turn left and the construction signal following the dead head is a left-turning signal, the construction signal will randomly mutate to a right-turning signal (Figure 5Q) or straight-going signal (Figure 5J). Similarly, the aforementioned situation also happens on turning right.
We can see from Figure 6 that the greedy selection mechanism, adding mechanism, and changing mechanism can produce different sub-loops from the same initial configuration. Especially, the changing mechanism does not self-replicate at the beginning.
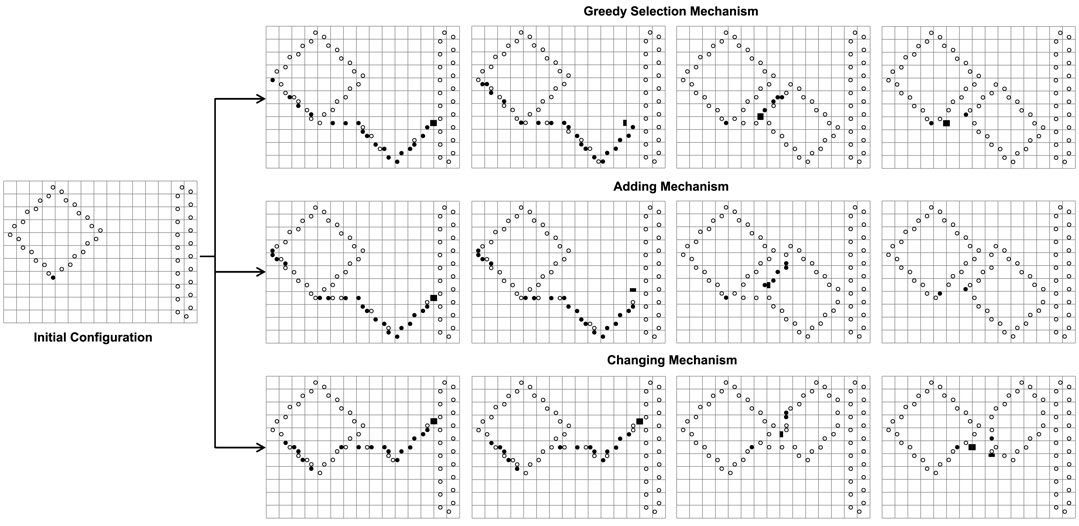
FIGURE 6. The different results of the greedy selection mechanism, adding mechanism, and changing mechanism starting from the same initial configuration where normal replication is limited by space.
4 Experiments
In order to testify that active adaptation can produce more diversity of species than the previous passive adaptation, we set up various initial configurations and different boundary values to conduct the experiments. We used the trait distribution entropy from Sayama (2004) to characterize the diversity of the population, which shows as follows:
where ni is a quantity of loops that are made of i cells and N the number of loops in the current space. Moreover, the value of the trait distribution entropy ranges from 0 to logN and log function takes the logarithm base 10 instead of base e. H = 0 means that the space is filled with the same loop and H = logN can be obtained when each loop in the current space differs from each other (i.e., the value of each ni is 0 or 1 for all i). Especially, loops which posses different manifestations belong to different species even if the loops consist of the identical number of cells.
We use different initial configurations to do experiments as shown in Figure 7, in which, the first three are common shapes and the last two are irregular. For simplicity, all possible final structures of replicated sub-loops starting from the initial configuration in Figure 7A by either self-adaptation mechanism are listed in Figure 8. In addition, the quantities and distributions of each structure in the cellular spaces using greedy selection mechanism, adding mechanism, and changing mechanism are provided in Tables 2, 3, and 4, respectively. As a result, compared with the other two active mechanisms, the greedy selection (passive) mechanism has a highest value of H in 80*65 cellular space, because the space is not filled with one or two identical and abundant small loops. However, on the whole, the adding mechanism and changing mechanism have higher values of H than the greedy selection mechanism.

FIGURE 8. All final loop structures starting from the configuration in Figure 7A by the greedy selection, adding, and changing mechanisms.

TABLE 2. Statistical numbers of the loops with various structures for the greedy selection mechanism on different cellular spaces starting from the initial configuration in Figure 7A.
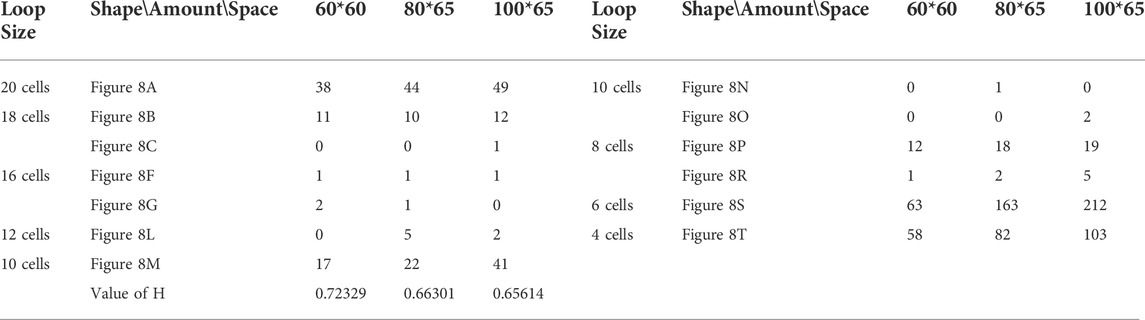
TABLE 3. Statistical numbers of the loops with various structures for the adding mechanism on different cellular spaces starting from the initial configuration in Figure 7A.
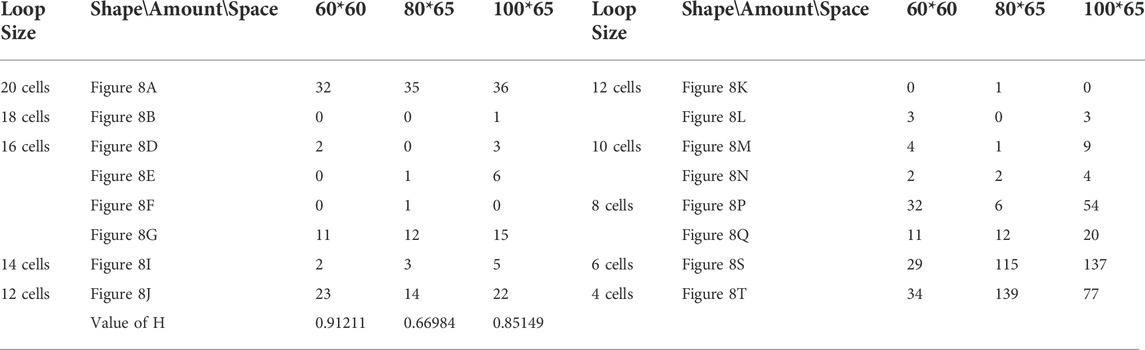
TABLE 4. Statistical numbers of the loops with various structures for the changing mechanism on different cellular spaces starting from the initial configuration in Figure 7A.
Likewise, Tables 5, 6, and 7 provide the self-replication results starting from the initial configuration in Figure 7B, along with all possible final sub-loops given in Figure 9. The value of H of the greedy selection mechanism is lower than that of adding mechanism and changing mechanism, which means that the adding mechanism and the changing mechanism can give rise to more diversity. Moreover, small loops appear later in the changing mechanism than in the adding mechanism, leaving more room for larger loops to self-replicate and bring more kinds of species. In addition, Tables 8, 9, and 10 demonstrate the results from the initial configuration in Figure 7C by each mechanism, in which the greedy selection mechanism can achieve the highest value of H in 100*100 cellular space. All possible loop structures are shown in Figure 10. Though the kinds of loops are the least for greedy selection mechanism, there is the maximum number of loops. Therefore, in the same biological environment, when the kinds of species are relatively small and the population is relatively large, the species also have a high diversity. Especially, the adding mechanism can produce many loops with complete quantity and different sizes.
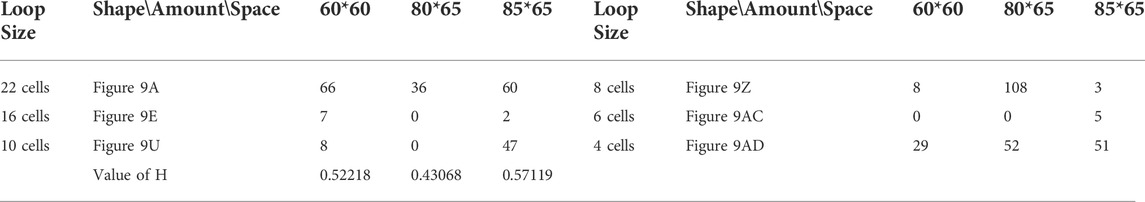
TABLE 5. Statistical numbers of the loops with various structures for the greedy selection mechanism on different cellular spaces starting from the initial configuration in Figure 7B.
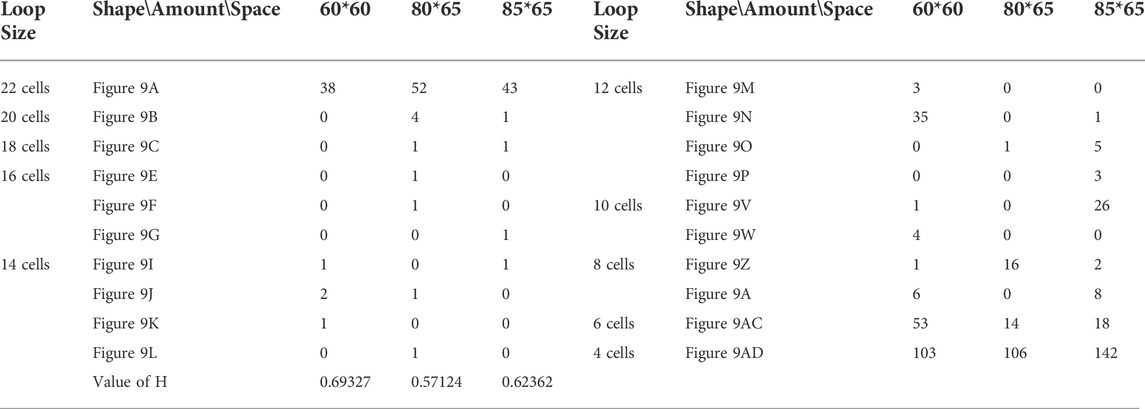
TABLE 6. Statistical numbers of the loops with various structures for the adding mechanism on different cellular spaces starting from the initial configuration in Figure 7B.
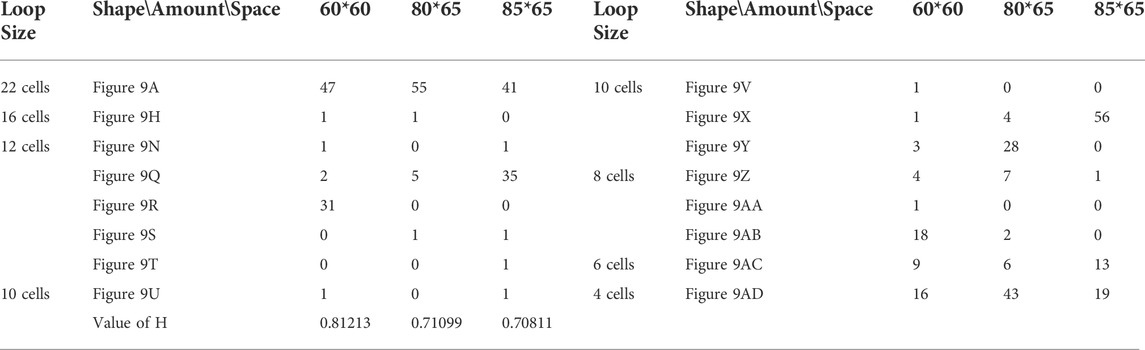
TABLE 7. Statistical numbers of the loops with various structures for the changing mechanism on different cellular spaces starting from the initial configuration in Figure 7B.
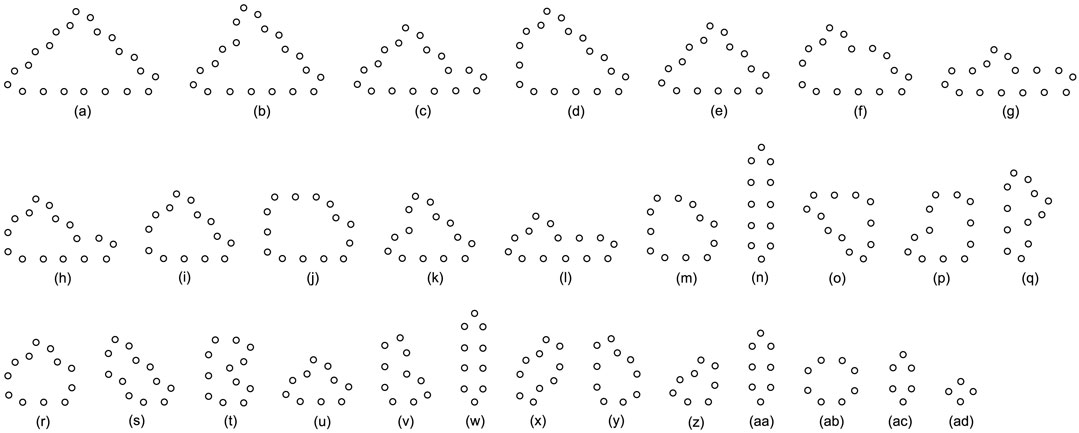
FIGURE 9. All final loop structures starting from the configuration in Figure 7B by the greedy selection, adding, and changing mechanisms.
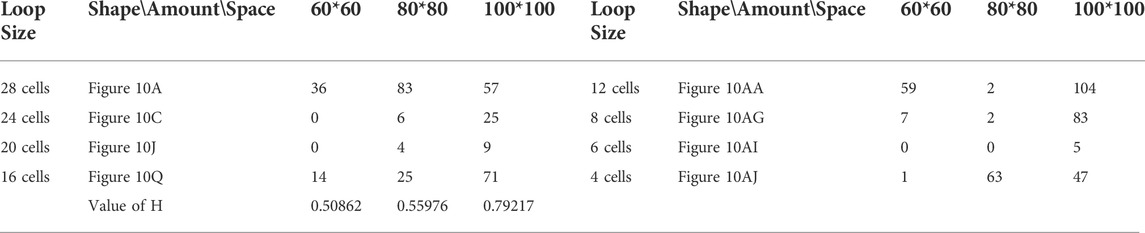
TABLE 8. Statistical numbers of the loops with various structures for the greedy selection mechanism on different cellular spaces starting from the initial configuration in Figure 7C.
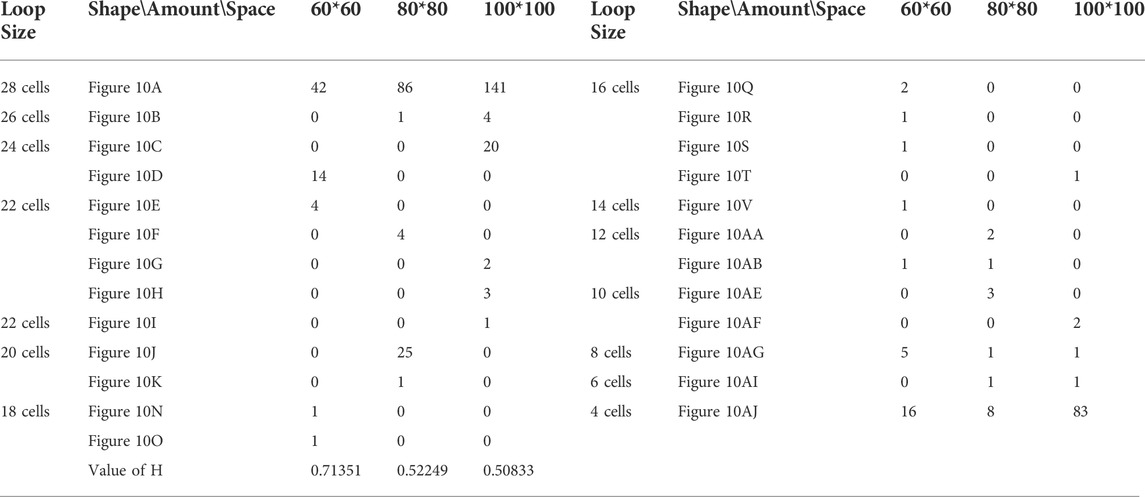
TABLE 9. Statistical numbers of the loops with various structures for the adding mechanism on different cellular spaces starting from the initial configuration in Figure 7C.
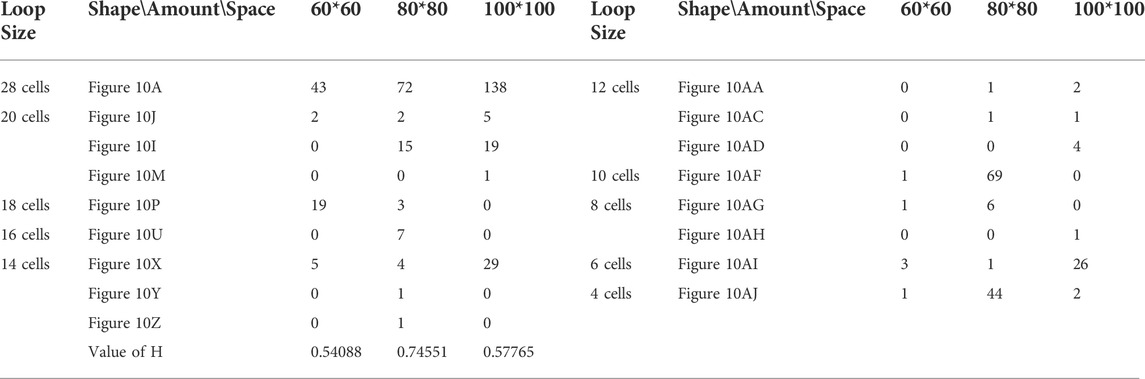
TABLE 10. Statistical numbers of the loops with various structures for the changing mechanism on different cellular spaces starting from the initial configuration in Figure 7C.
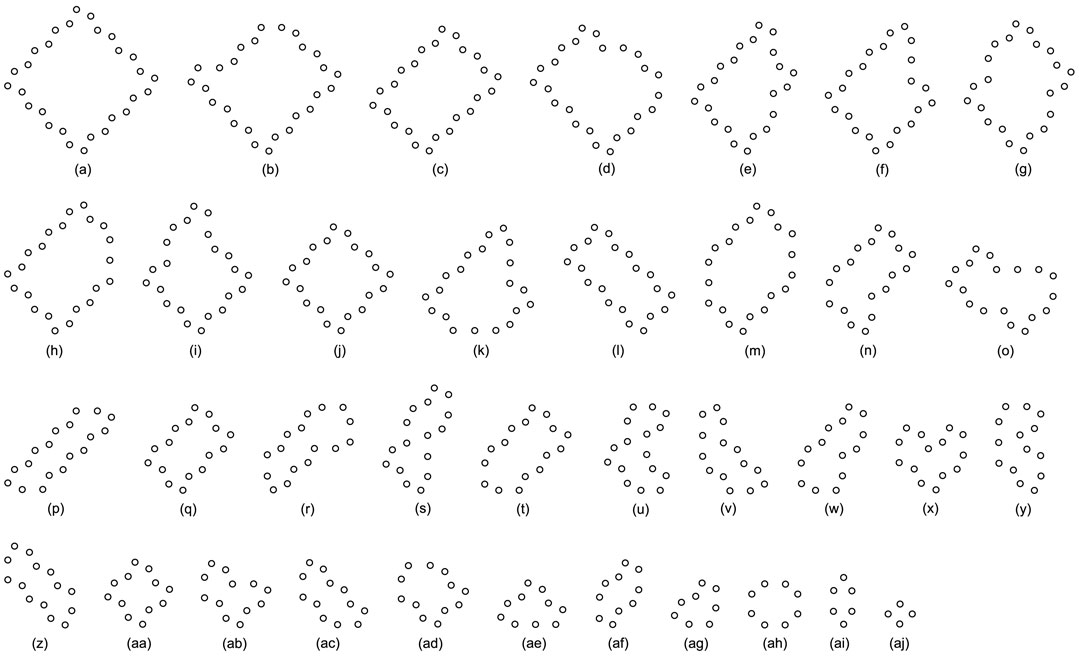
FIGURE 10. All final loop structures starting from the configuration in Figure 7C by the greedy selection, adding, and changing mechanisms.
All replicating results of the loop structures from the configuration in Figure 7D are given in Figure 11. In this case, the values of H using the adding mechanism and the changing mechanism in Tables 12, 13, respectively are obviously higher than that of the greedy selection mechanism in Table 11. Furthermore, self-replications starting from the irregular and symmetric shapes in Figure 7E are elaborated in Tables 14, 15, and 16 with various types of sub-loops shown in Figure 12. It can be verified that the loop that is the same as the initial configuration quickly takes up the entire space, leaving little room for the smaller ones, which creates a smaller population of loops and owns the lowest diversity of species.
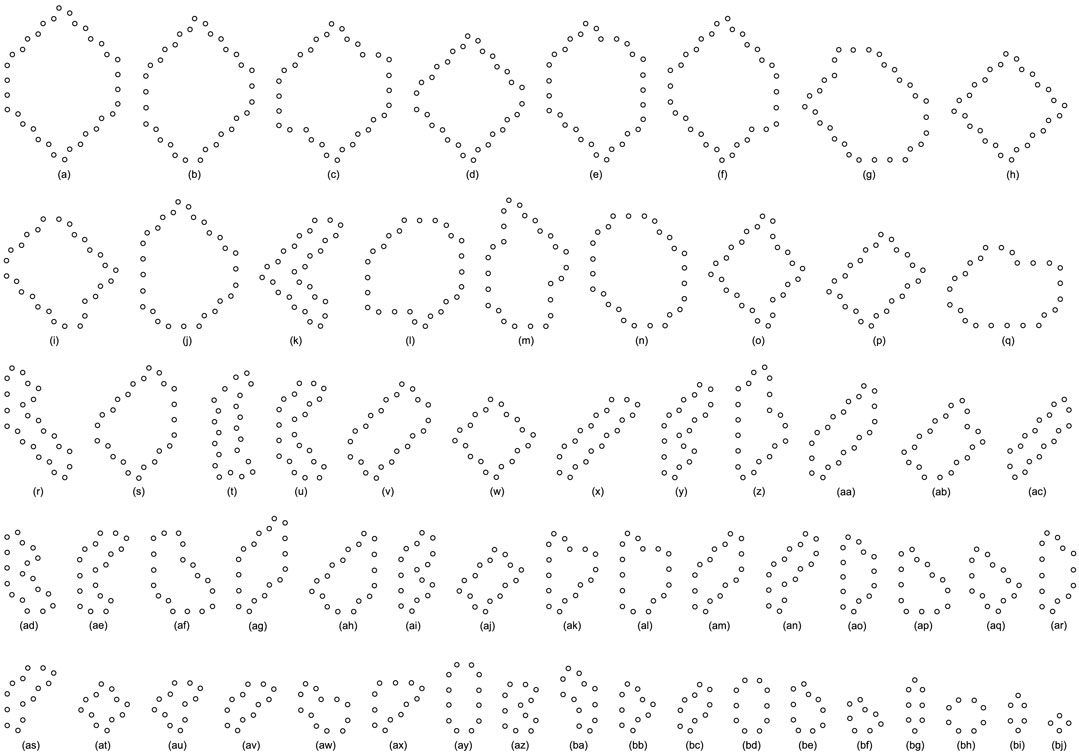
FIGURE 11. All final loop structures starting from the configuration in Figure 7D by the greedy selection, adding, and changing mechanisms.
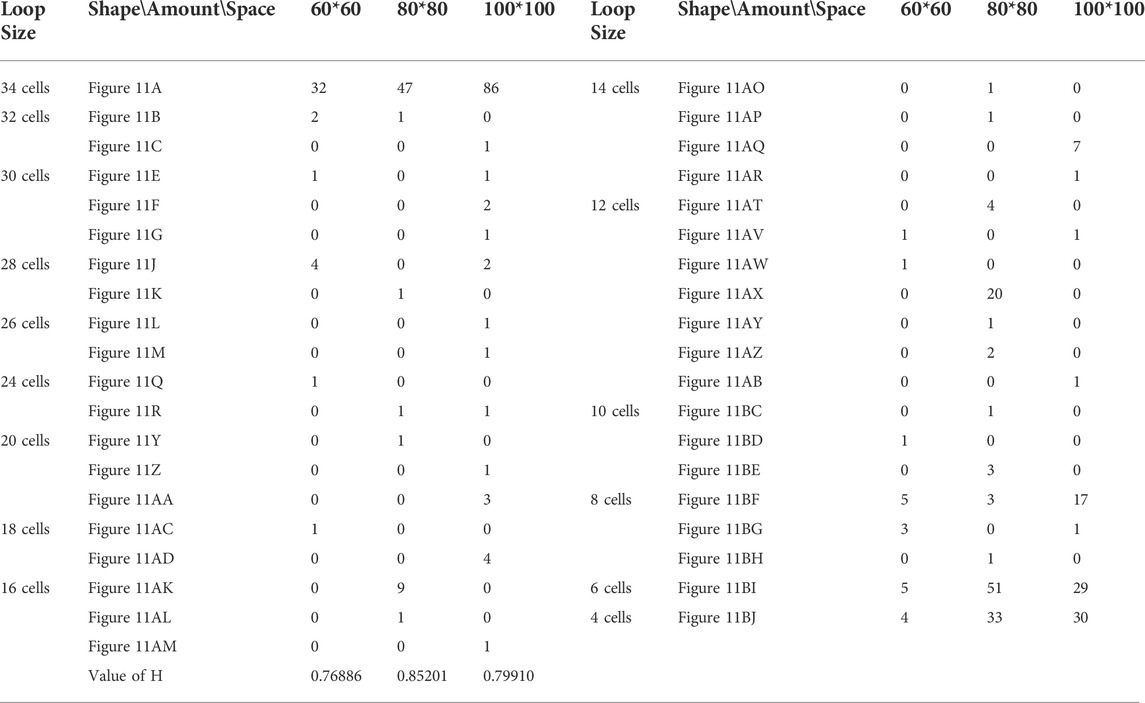
TABLE 12. Statistical numbers of the loops with various structures for the adding mechanism on different cellular spaces starting from the initial configuration in Figure 7D.
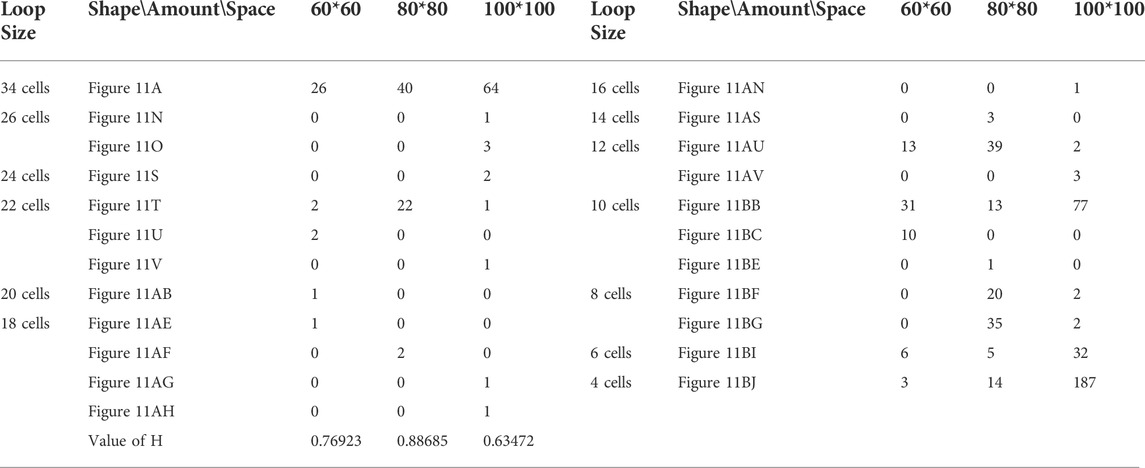
TABLE 13. Statistical numbers of the loops with various structures for the changing mechanism on different cellular spaces starting from the initial configuration in Figure 7D.
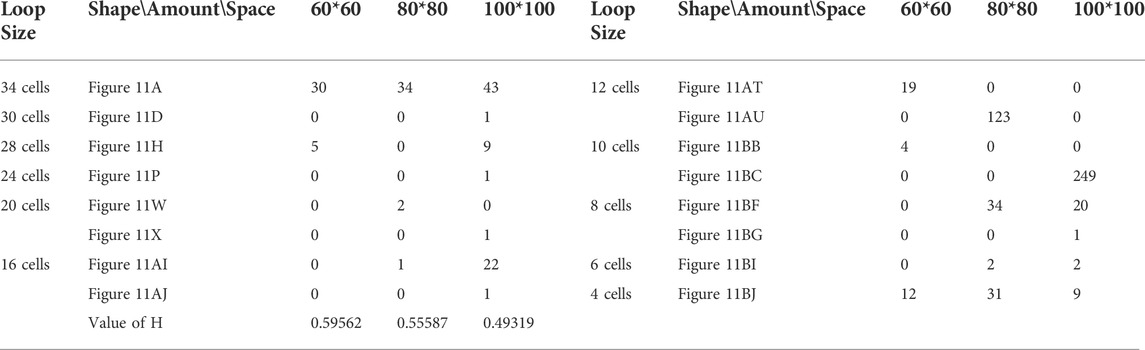
TABLE 11. Statistical numbers of the loops with various structures for the greedy selection mechanism on different cellular spaces starting from the initial configuration in Figure 7D.
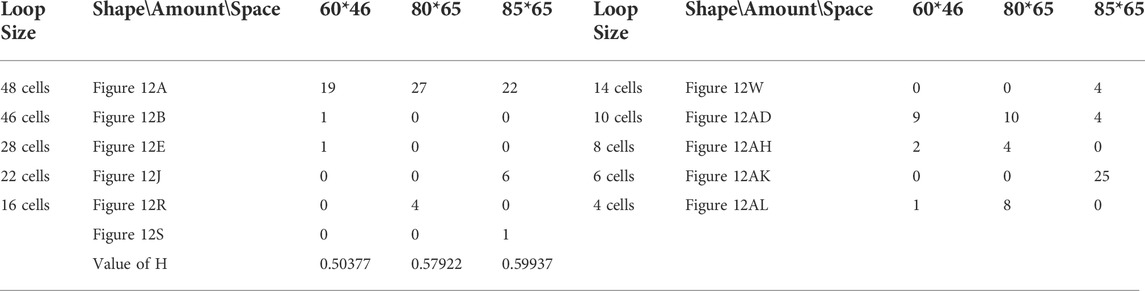
TABLE 14. Statistical numbers of the loops with various structures for the greedy selection mechanism on different cellular spaces starting from the initial configuration in Figure 7E.
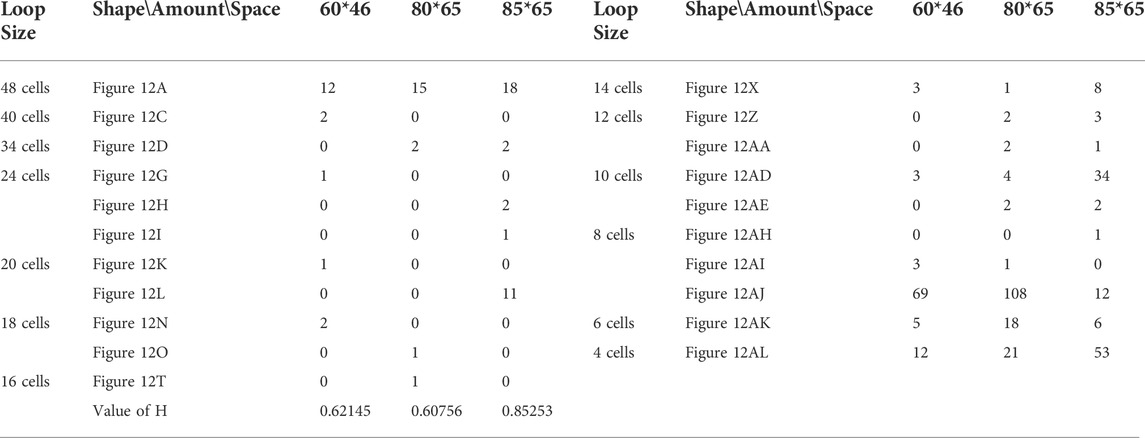
TABLE 15. Statistical numbers of the loops with various structures for the adding mechanism on different cellular spaces starting from the initial configuration in Figure 7E.
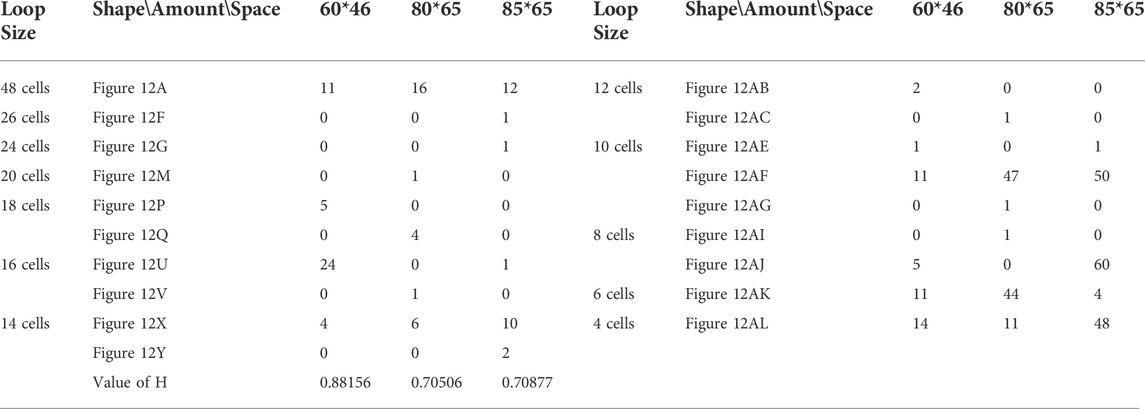
TABLE 16. Statistical numbers of the loops with various structures for the changing mechanism on different cellular spaces starting from the initial configuration in Figure 7E.
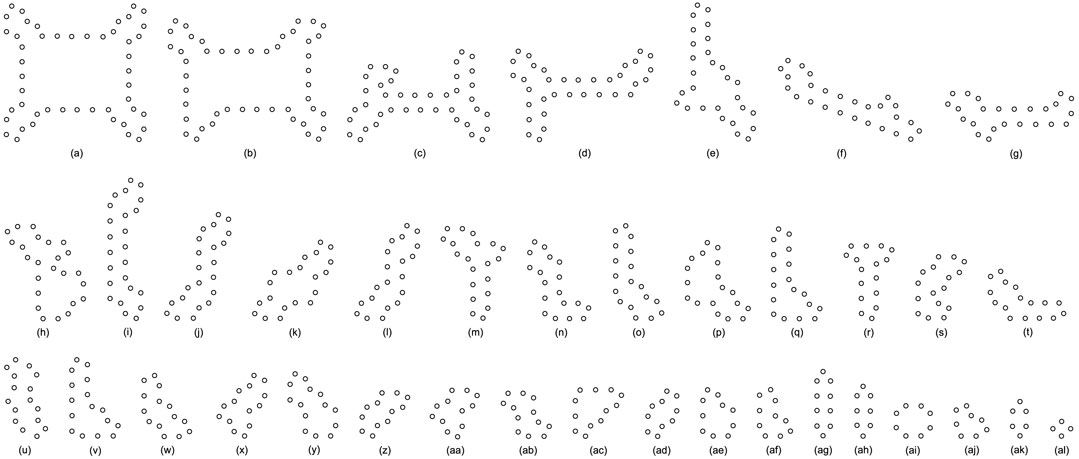
FIGURE 12. All final loop structures starting from the configuration in Figure 7E by the greedy selection, adding, and changing mechanisms.
Therefore, the aforementioned experiments show that the adding mechanism and the changing mechanism can bring higher diversity than the greedy selection mechanism. Moreover, for those loops with the same number of cells, the adding mechanism and the changing mechanism can obtain more variable loops with different phenotypes. Phenotype change is a sufficient factor for achieving such a functional evolution Kampis and Gulyás (2008). In the process of self-replicating, once a minimal loop is created, the loop will quickly replicate itself, because the minimal loop can track its body much faster. As a result, the minimal loops will become the vast majority of the population after reaching saturation, thereby reducing the diversity. Such a tendency is similar to the basic orientation of the evolution paths in Sayama (2004).
Moreover, in order to further test the diversity that the active mechanisms can bring, we conducted experiments on the initial configuration in 7(d) with 60*60 cellular space using three mechanisms. From Figure 13, we can see that the greedy mechanism mostly can obtain the highest value on the total quantity of loops, but significantly lower than the active mechanisms in terms of species and diversity, which may imply that the greedy mechanism tends to produce smaller loops. Generally speaking, smaller loops can replicate themselves rapidly and be more likely to survive.
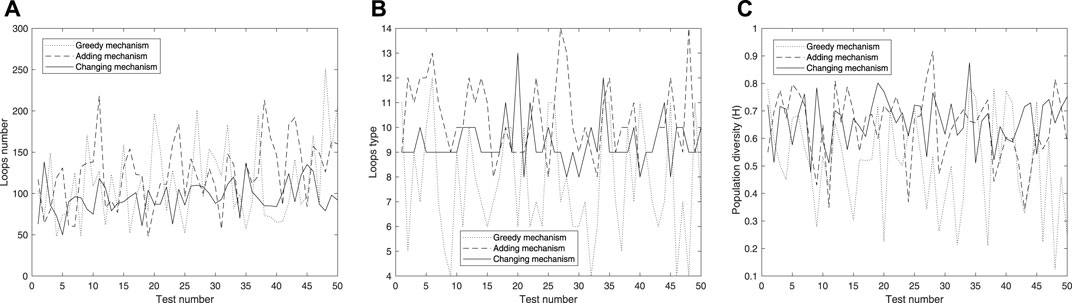
FIGURE 13. Further results on the initial configuration in Figure 7D with 60*60 cellular space using the three mechanisms.
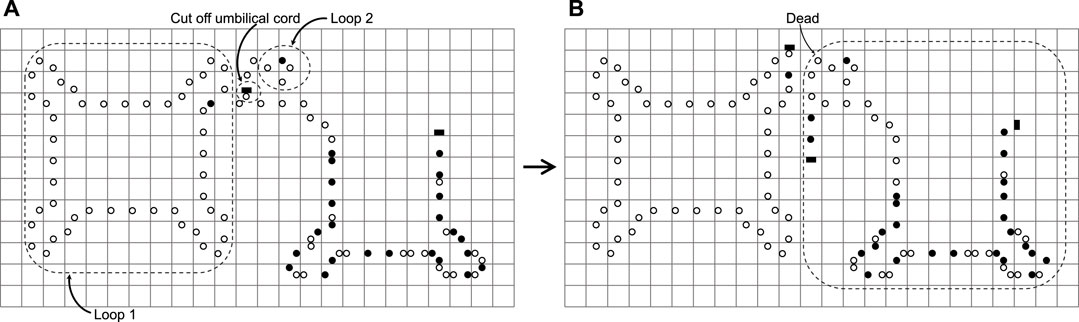
However, mistakes may occur in the process of self-replication and the details are shown in Figure 14. There are several conditions for the error to occur (see also Huang et al. (2013)): 1) Loop 1 is on the inner side of the arm of the loop 2 in Figure 14A; 2) The arm of loop 1 contains no construction code, which means the head of the arm is in the state ◦■; 3)The construction arm of loop 2 has been scanned by a validation signal, which means the state about the part of the arm turns state • to state ◦. Especially, there is a parallel arm that is made up of state ◦ shown in Figure 14B. However, this error seldom happens. Under these conditions, loop 2 may have an erroneous cognition that it thinks of the arm of loop 2 as its own; thereby it will cut off the umbilical cord at the arm head. Fortunately, loop 1 is unaffected by this error and goes on self-replicating. Loop 2, however, is not so lucky, and dies. What is worse, the dead loop 2 and the discarded arm of loop 1 waste many spaces. Nevertheless, enhancing the function of a validation signal may seem reasonable to avoid erroneous cognition. On the plus side, an erroneous cognition may possibly be regarded as some non-trivial co-action between loops Sayama (1999). Moreover, an erroneous cognition may create an offspring the size of which is bigger than the mother loop Salzberg (2003).
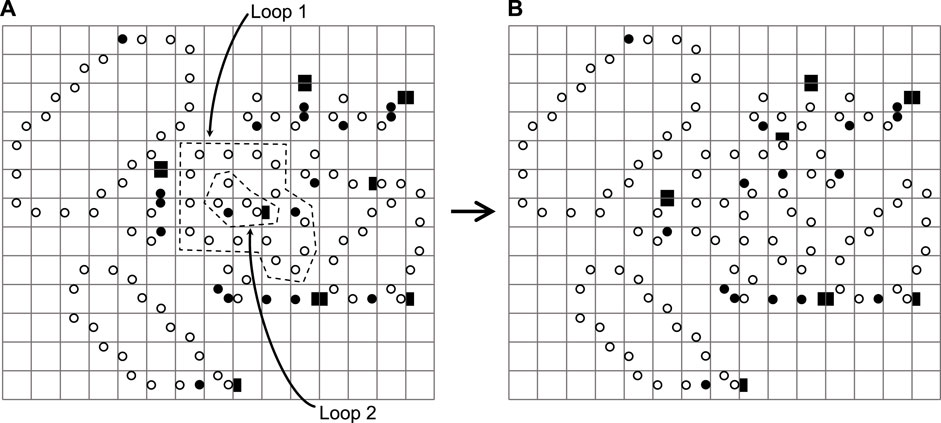
Furthermore, from Figure 15, we can see that Loop 2 takes up the space thanks to the faster replication capability during the process of generating Loop 1, and Loop 1 exactly forms a closed loop that wraps around Loop 2. This situation is similar to the phagocytosis of immune cell Stossel (1974). Luckily, Loop 1 and Loop 2 are alive. Thus, if there are enough spaces, the loops can self-replicate.
5 Discussion
Many studies have considered the self-replication on various cellular automata to simulate the process of biological self-replication, including the reversible cellular automata (Morita and Imai, 1996b), polymorphic cellular automata (Sekanina and Komenda, 2011), and graph automata (Tomita et al., 2002). Moreover, self-replication on cellular automata has been applied to several fields, such as worm propagation in smartphones (Peng et al., 2013), artificial chemistry (Hutton, 2007), and image processing (Sahin et al., 2015). In this article, we provided a different approach to enhance the diversity of artificial self-replicating structures, instead of abandoning partial structural information or destroying the whole loop. In order to obtain these effects better, on the basis of existing ordinary self-replication, we change a greedy selection mechanism to two active mechanisms when dealing with collision, which add an orientation and change the construction signal under the dead head. Experiments showed that active adaptations using our schemes can actually improve the possibility of survival and replication of any self-replicating structure in a wide variety of environments than the passive one. In particular, the changing mechanism involves abandoning one building-block from the original structure of a mother loop when every collision happens, even though the mechanism changes the construction signal. Also, the adding mechanism does not seem to lose the block of information coming from the parent, while some constructional information is left for the offspring to complete the replication. This may result in the shrinkage of both shape and size of the offspring.
Although the adding and changing mechanisms enable more active self-adaptation than the greedy selection mechanism, they still look somewhat passive in the sense that the adaptation can only be activated when collision occurs. In living organisms, mutation on genes will occur in a probabilistic manner. As with self-adaptation, self-recovery or self-healing is also an interesting feature of organisms. In the future work, we will consider how to endow self-replicating loops with a self-repairing ability (Tempesti et al., 1998), use random inputs (Griffith et al., 2005) to generate interesting patterns, and genetic algorithms to automatically discover rules (Lohn and Reggia, 1997).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
WX: methodology and writing—original draft; CW: conceptualization and writing—original draft; QP: software and formal analysis; JL: conceptualization and supervision; YX: methodology and validation; and SK: formal analysis.
Acknowledgments
The authors are grateful to the reviewers for their careful reading and valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.958069/full#supplementary-material
References
Abou-Jaoudé, W., Traynard, P., Monteiro, P. T., Saez-Rodriguez, J., Helikar, T., Thieffry, D., et al. (2016). Logical modeling and dynamical analysis of cellular networks. Front. Genet. 7, 94. doi:10.3389/fgene.2016.00094
Agrawal, A. A. (2001). Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326. doi:10.1126/science.1060701
Aminetzach, Y. T., Macpherson, J. M., and Petrov, D. A. (2005). Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science 309, 764–767. doi:10.1126/science.1112699
Anzalone, A. V., Randolph, P. B., Davis, J. R., Sousa, A. A., Koblan, L. W., Levy, J. M., et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157. doi:10.1038/s41586-019-1711-4
Bargmann, C. I., Hung, M.-C., and Weinberg, R. A. (1986). Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 45, 649–657. doi:10.1016/0092-8674(86)90779-8
Baris, Y., Taylor, M. R. G., Aria, V., and Yeeles, J. T. P. (2022). Fast and efficient dna replication with purified human proteins. Nature 606, 204–210. doi:10.1038/s41586-022-04759-1
Becerra-Rodríguez, C., Marsit, S., and Galeote, V. (2021). Diversity of oligopeptide transport in yeast and its impact on adaptation to winemaking conditions. Front. Genet. 11. doi:10.3389/fgene.2020.00602
Bilotta, E., and Pantano, P. (2006). Structural and functional growth in self-reproducing cellular automata. Complexity 11, 12–29. doi:10.1002/cplx.20131
Bookstein, R., Shew, J.-Y., Chen, P.-L., Scully, P., and Lee, W.-H. (1990). Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science 247, 712–715. doi:10.1126/science.2300823
Buckley, W. R., and Mukherjee, A. (2005). “Constructibility of signal-crossing solutions in von neumann 29-state cellular automata,” in International conference on computational science (Atlanta, GA: Springer), 395–403.
Buisson, R., Langenbucher, A., Bowen, D., Kwan, E. E., Benes, C. H., Zou, L., et al. (2019). Passenger hotspot mutations in cancer driven by apobec3a and mesoscale genomic features. Science 364, eaaw2872. doi:10.1126/science.aaw2872
Byl, J. (1989). Self-reproduction in small cellular automata. Phys. D. Nonlinear Phenom. 34, 295–299. doi:10.1016/0167-2789(89)90242-x
Cea, V., Cipolla, L., and Sabbioneda, S. (2015). Replication of structured dna and its implication in epigenetic stability. Front. Genet. 6, 209. doi:10.3389/fgene.2015.00209
Domingo, E., and Holland, J. J. (1997). RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51, 151–178. doi:10.1146/annurev.micro.51.1.151
Duan, Y., Lu, Z., Zhou, Z., Sun, X., and Wu, J. (2019a). Data privacy protection for edge computing of smart city in a dikw architecture. Eng. Appl. Artif. Intell. 81, 323–335. doi:10.1016/j.engappai.2019.03.002
Duan, Y., Sun, X., Che, H., Cao, C., Li, Z., Yang, X., et al. (2019b). Modeling data, information and knowledge for security protection of hybrid iot and edge resources. IEEE Access 7, 99161–99176. doi:10.1109/access.2019.2931365
Duan, Y. (2019). “Towards a periodic table of conceptualization and formalization on state, style, structure, pattern, framework, architecture, service and so on,” in 2019 20th IEEE/ACIS International Conference on Software Engineering, Artificial Intelligence, Networking and Parallel/Distributed Computing (SNPD), Toyama, Japan, 08-11 July 2019, 133–138.
Edwards, G., Garcia, J., Tajalli, H., Popescu, D., Medvidovic, N., Sukhatme, G., et al. (2009). “Architecture-driven self-adaptation and self-management in robotics systems,” in 2009 ICSE Workshop on Software Engineering for Adaptive and Self-Managing Systems, Vancouver, BC, Canada, 18-19 May 2009, 142–151.
Gemble, S., Wardenaar, R., Keuper, K., Srivastava, N., Nano, M., Macé, A.-S., et al. (2022). Genetic instability from a single s phase after whole-genome duplication. Nature 604, 146–151. doi:10.1038/s41586-022-04578-4
Gindin, Y., Meltzer, P. S., and Bilke, S. (2014). Replicon: A software to accurately predict dna replication timing in metazoan cells. Front. Genet. 5, 378. doi:10.3389/fgene.2014.00378
Griffith, S., Goldwater, D., and Jacobson, J. M. (2005). Robotics: Self-replication from random parts. Nature 437, 636. doi:10.1038/437636a
Huang, X., Lee, J., Sun, T.-H., and Peper, F. (2013). Self-adaptive self-reproductions in cellular automata. Phys. D. Nonlinear Phenom. 263, 11–20. doi:10.1016/j.physd.2013.07.012
Hutton, T. J. (2007). Evolvable self-reproducing cells in a two-dimensional artificial chemistry. Artif. Life 13, 11–30. doi:10.1162/artl.2007.13.1.11
Ibáñez, J., Anabitarte, D., Azpeitia, I., Barrera, O., Barrutieta, A., Blanco, H., et al. (1995). “Self-inspection based reproduction in cellular automata,” in European conference on artificial life (Berlin: Springer), 564–576.
Imai, K., Hori, T., and Morita, K. (2002). Self-reproduction in three-dimensional reversible cellular space. Artif. Life 8, 155–174. doi:10.1162/106454602320184220
Kampis, G., and Gulyás, L. (2008). Full body: The importance of the phenotype in evolution. Artif. Life 14, 375–386. doi:10.1162/artl.2008.14.3.14310
Langton, C. G. (1984). Self-reproduction in cellular automata. Phys. D. Nonlinear Phenom. 10, 135–144. doi:10.1016/0167-2789(84)90256-2
Lawson, A. R. J., Abascal, F., Coorens, T. H. H., Hooks, Y., O’Neill, L., Latimer, C., et al. (2020). Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 370, 75–82. doi:10.1126/science.aba8347
Lei, Y., and Duan, Y. (2021). Trusted service provider discovery based on data, information, knowledge, and wisdom. Int. J. Soft. Eng. Knowl. Eng. 31, 3–19. doi:10.1142/s0218194021400015
Li, Y., Duan, Y., Maamar, Z., Che, H., Spulber, N.-B., Fuentes, S., et al. (2021). Swarm differential privacy for purpose-driven data-information-knowledge-wisdom architecture. Mob. Inf. Syst. 6671628, 1–15. doi:10.1155/2021/6671628
Lohn, J. D., and Reggia, J. A. (1997). Automatic discovery of self-replicating structures in cellular automata. IEEE Trans. Evol. Comput. 1, 165–178. doi:10.1109/4235.661547
Marchal, P. (1998). John von Neumann: The founding father of artificial life. Artif. Life 4, 229–235. doi:10.1162/106454698568567
Merkle, R. (1992). Self replicating systems and molecular manufacturing. Br. Interplanet. Soc. J. 45, 407–413.
Miles, L. A., Bowman, R. L., Merlinsky, T. R., Csete, I. S., Ooi, A. T., Durruthy-Durruthy, R., et al. (2020). Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587, 477–482. doi:10.1038/s41586-020-2864-x
Monroe, J. G., Srikant, T., Carbonell-Bejerano, P., Becker, C., Lensink, M., Exposito-Alonso, M., et al. (2022). Mutation bias reflects natural selection in arabidopsis thaliana. Nature 602, 101–105. doi:10.1038/s41586-021-04269-6
Moore, L., Cagan, A., Coorens, T. H. H., Neville, M. D. C., Sanghvi, R., Sanders, M. A., et al. (2021). The mutational landscape of human somatic and germline cells. Nature 597, 381–386. doi:10.1038/s41586-021-03822-7
Morita, K., and Imai, K. (1996a). “Logical universality and self-reproduction in reversible cellular automata,” in International conference on evolvable systems (Berlin: Springer), 152–166.
Morita, K., and Imai, K. (1996b). Self-reproduction in a reversible cellular space. Theor. Comput. Sci. 168, 337–366. doi:10.1016/s0304-3975(96)00083-7
Nehaniv, C. L. (2002). “Self-reproduction in asynchronous cellular automata,” in Proceedings 2002 NASA/DoD conference on evolvable hardware (Alexandria, VA: IEEE), 201–209.
Peng, S., Wang, G., and Yu, S. (2013). Modeling the dynamics of worm propagation using two-dimensional cellular automata in smartphones. J. Comput. Syst. Sci. 79, 586–595. doi:10.1016/j.jcss.2012.11.007
Peper, F., Isokawa, T., Kouda, N., and Matsui, N. (2002). Self-timed cellular automata and their computational ability. Future Gener. Comput. Syst. 18, 893–904. doi:10.1016/s0167-739x(02)00069-9
Pesavento, U. (1995). An implementation of von Neumann’s self-reproducing machine. Artif. Life 2, 337–354. doi:10.1162/artl.1995.2.4.337
Poduri, A., Evrony, G. D., Cai, X., and Walsh, C. A. (2013). Somatic mutation, genomic variation, and neurological disease. Sci. (New York, N.Y.) 341, 1237758. doi:10.1126/science.1237758
Reggia, J. A., Chou, H.-H., Armentrout, S. L., and Peng, Y. (1993). Minimizing complexity in cellular automata models of self-replication. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1, 337–344.
Sahin, U., Uguz, S., Akın, H., and Siap, I. (2015). Three-state von Neumann cellular automata and pattern generation. Appl. Math. Model. 39, 2003–2024. doi:10.1016/j.apm.2014.10.025
Salzberg, C. (2003). Emergent evolutionary dynamics of self-reproducing cellular automata (Amsterdam, The Netherlands: Universiteit van Amsterdam). Master’s thesis.
Sasani, T. A., Ashbrook, D. G., Beichman, A. C., Lu, L., Palmer, A. A., Williams, R. W., et al. (2022). A natural mutator allele shapes mutation spectrum variation in mice. Nature 605, 497–502. doi:10.1038/s41586-022-04701-5
Sayama, H. (2004). Self-protection and diversity in self-replicating cellular automata. Artif. Life 10, 83–98. doi:10.1162/106454604322875922
Sayama, H. (1999). A new structurally dissolvable self-reproducing loop evolving in a simple cellular automata space. Artif. Life 5, 343–365. doi:10.1162/106454699568818
Scott, E. (2013). This i believe: We need to understand evolution, adaptation, and phenotype. Front. Genet. 3, 303. doi:10.3389/fgene.2012.00303
Sekanina, L., and Komenda, T. (2011). Global control in polymorphic cellular automata. J. Cell. Autom. 6, 301–321. doi:10.1093/imrn/rnq280
Sha, Y., Wang, S., Bocci, F., Zhou, P., and Nie, Q. (2020). Inference of intercellular communications and multilayer gene-regulations of epithelial–mesenchymal transition from single-cell transcriptomic data. Front. Genet. 11, 604585. doi:10.3389/fgene.2020.604585
Shenhav, L., and Zeevi, D. (2020). Resource conservation manifests in the genetic code. Science 370, 683–687. doi:10.1126/science.aaz9642
Song, Z., Duan, Y., Wan, S., Sun, X., Zou, Q., Gao, H., et al. (2018). Processing optimization of typed resources with synchronized storage and computation adaptation in fog computing. Wirel. Commun. Mob. Comput. 3794175, 1–13. doi:10.1155/2018/3794175
Stossel, T. P. (1974). Phagocytosis (first of three parts). N. Engl. J. Med. 290, 717–723. doi:10.1056/NEJM197403282901306
Suzuki, K., and Ikegami, T. (2003). “Interaction based evolution of self-replicating loop structures,” in European conference on artificial life (Dortmund, Germany: Springer), 89–96.
Takada, Y., Isokawa, T., Peper, F., and Matsui, N. (2007). Asynchronous self-reproducing loops with arbitration capability. Phys. D. Nonlinear Phenom. 227, 26–35. doi:10.1016/j.physd.2006.12.011
Tempesti, G. (1995). “A new self-reproducing cellular automaton capable of construction and computation,” in European conference on artificial life (Berlin: Springer), 555–563.
Tempesti, G., Mange, D., and Stauffer, A. (1998). Self-replicating and self-repairing multicellular automata. Artif. Life 4, 259–282. doi:10.1162/106454698568585
Tomita, K., Kurokawa, H., and Murata, S. (2002). Graph automata: Natural expression of self-reproduction. Phys. D. Nonlinear Phenom. 171, 197–210. doi:10.1016/s0167-2789(02)00601-2
Vogel, F. (1972). Non-randomness of base replacement in point mutation. J. Mol. Evol. 1, 334–367. doi:10.1007/BF01653962
von Neumann, J. (1966). Theory of self-reproducing automata. Champaign, IL: University of Illinois Press.
Wilke, C. O., Wang, J. L., Ofria, C., Lenski, R. E., and Adami, C. (2001). Evolution of digital organisms at high mutation rates leads to survival of the flattest. Nature 412, 331–333. doi:10.1038/35085569
Williams, G. C., and Burt, A. (1997). Adaptation and natural selection. Princeton, NJ: Princeton University Press.
Xia, Y., Zhou, M., Luo, X., Zhu, Q., Li, J., Huang, Y., et al. (2015). Stochastic modeling and quality evaluation of infrastructure-as-a-service clouds. IEEE Trans. Autom. Sci. Eng. 12, 162–170. doi:10.1109/tase.2013.2276477
Xiao, X., Xue, G.-F., Stamatovic, B., and Qiu, W.-R. (2020). Using cellular automata to simulate domain evolution in proteins. Front. Genet. 11, 515. doi:10.3389/fgene.2020.00515
Keywords: self-replication, self-adaption, cellular automaton, gene mutation, biological resources
Citation: Xu W, Wu C, Peng Q, Lee J, Xia Y and Kawasaki S (2022) Enhancing the diversity of self-replicating structures using active self-adapting mechanisms. Front. Genet. 13:958069. doi: 10.3389/fgene.2022.958069
Received: 31 May 2022; Accepted: 27 June 2022;
Published: 26 July 2022.
Edited by:
Yucong Duan, Hainan University, ChinaReviewed by:
Souvik Roy, Indian Institute of Technology Kanpur, IndiaJiangan Xie, Chongqing University of Posts and Telecommunications, China
Teijiro Isokawa, University of Hyogo, Japan
Copyright © 2022 Xu, Wu, Peng, Lee, Xia and Kawasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunrong Wu, Y3J3dUBjcXUuZWR1LmNu
 Wenli Xu
Wenli Xu Chunrong Wu
Chunrong Wu Qinglan Peng1
Qinglan Peng1 Yunni Xia
Yunni Xia