
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 04 October 2022
Sec. Epigenomics and Epigenetics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.934277
This article is part of the Research TopicInsights in Epigenomics and Epigenetics: 2022View all 7 articles
 Congrong Wang1
Congrong Wang1 Rossella Alfano1
Rossella Alfano1 Brigitte Reimann1
Brigitte Reimann1 Janneke Hogervorst1
Janneke Hogervorst1 Mariona Bustamante2,3,4,5
Mariona Bustamante2,3,4,5 Immaculata De Vivo6,7
Immaculata De Vivo6,7 Michelle Plusquin1
Michelle Plusquin1 Tim S. Nawrot1,8
Tim S. Nawrot1,8 Dries S. Martens1*
Dries S. Martens1*Telomere length at birth determines later life telomere length and potentially predicts ageing-related diseases. However, the genetic and epigenetic settings of telomere length in newborns have not been analyzed. In addition, no study yet has reported how the interplay between genetic variants and genome-wide cytosine methylation explains the variation in early-life telomere length. In this study based on 281 mother-newborn pairs from the ENVIRONAGE birth cohort, telomere length and whole-genome DNA methylation were assessed in cord blood and 26 candidate single nucleotide polymorphism related to ageing or telomere length were genotyped. We identified three genetic variants associated with cord blood telomere length and 57 cis methylation quantitative trait loci (cis-mQTLs) of which 22 mQTLs confirmed previous findings and 35 were newly identified. Five SNPs were found to have significant indirect effects on cord blood telomere length via the mediating CpGs. The association between rs911874 (SOD2) and newborn telomere length was modified by nearby DNA methylation indicated by a significant statistical interaction. Our results suggest that DNA methylation in cis might have a mediation or modification effect on the genetic difference in newborn telomere length. This novel approach warrants future follow-up studies that are needed to further confirm and extend these findings.
Telomeres are ribonucleoprotein complexes that cap the end of eukaryotic chromosomes and function to protect genomic stability. In normal somatic cells, telomere length declines with cell divisions until it reaches a critical length, which triggers cellular senescence and finally leads to subsequent programmed cell death. Therefore, telomere length determines the replicative capacity and the lifespan of a cell (O'Sullivan and Karlseder, 2010; Allsopp et al., 1992; Codd et al., 2013). At the level of an individual, somatic cell telomeres are the longest at birth and normal telomere attrition occurs throughout the life course (Frenck et al., 1998). At birth and among same-aged individuals, considerable variability exists in telomere length (Slagboom et al., 1994), which is partly due to heritability and partly to potential accelerated telomere shortening induced by environmental and cellular stressors (Aubert and Lansdorp, 2008). Telomere length tracks over different life stages (Bijnens et al., 2017; Martens et al., 2021), and shorter telomere lengths in adulthood is associated with age-related diseases (Zhao et al., 2013; Blackburn et al., 2015) and mortality (Wang et al., 2018).
The genetic determinants of adult telomere length have been widely investigated. Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) related to telomere length (Levy et al., 2010; Prescott et al., 2011; Mangino et al., 2012; Codd et al., 2013; Liu et al., 2014; Mitchell et al., 2014; Mangino et al., 2015; Codd et al., 2021) and lifespan (Lunetta et al., 2007; TenNapel et al., 2014). Previous twin studies have indicated that heritable factors could account for up to 78% of the variation in telomere length (Slagboom et al., 1994; Hannon et al., 2018). Besides genetic variants related to telomere length, epigenetic signatures (DNA cytosine methylation) of telomere length (Buxton et al., 2014; Lee et al., 2019) and telomere attrition (Wang et al., 2021a) have been described. However, little is known on the potential interplay between genetic and epigenetic regulations of telomere length. Gene-specific allelic variations are closely associated with differences in DNA methylation in proximal regions of this allelic variation, as shown by published DNA methylation quantitative trait (mQTL) analyses (Gaunt et al., 2016; Volkov et al., 2016; Ciuculete et al., 2017). Given the role of the epigenome in regulating gene expression (Moore et al., 2013), the potential mediation effect of DNA methylation has been studied on the genetic liability to a specific phenotype (Howe et al., 2019).
In this study, within a subset of the ongoing prospective birth cohort, ENVIRonmental influence ON early AGEing (ENVIRONAGE), we aimed to investigate the combined genetic-epigenetic regulation of newborn telomere length, assessing biological ageing-related SNPs and DNA methylation. The following hypotheses were tested: 1) genetic variants in adult biological ageing-related genes are associated with newborn telomere length, 2) these genetic variants affect the DNA methylation within the same or nearby genes, which mediate the genetic association with newborn telomere length, and 3) the association between a genetic variant and newborn telomere length is modified by DNA methylation levels in nearby regions.
This study took place within the context of the ongoing prospective ENVIRONAGE birth cohort (Janssen et al., 2017) initiated in 2010. This cohort study has been approved by the ethical committee of Hasselt University and East-Limburg Hospital (Genk, Belgium), and is carried out according to the Declaration of Helsinki. Between February 2010 and May 2015, 885 mothers were recruited upon arrival in the hospital for delivery, and were considered eligible if they were able to fill out a questionnaire in Dutch. Written informed consent was obtained from the participating mothers and questionnaires were filled out by the mothers to collect information on lifestyles and socioeconomic status. Information on newborns’ sex, birth weight and gestational age, and maternal age and parity was collected from medical records in the hospital. The date of conception was estimated based on the first day of the mother’s last menstrual period in combination with the first ultrasonographic examination. Maternal body mass index (BMI) was determined as the ratio of the maternal weight to the squared maternal height measured at the first antenatal visit (weeks 7-9 of pregnancy). The ethnicity of a newborn was categorized as European if at least two grandparents were Europeans and classified as non-European otherwise. The educational level of the mothers was coded as “0” if they did not obtain any diploma, as “1” if they obtained at highest a high school diploma, as “2” if the highest diploma obtained was from a 3-year college and as “3” if they obtained a 4-year college or university degree. Maternal smoking status was classified into “never smoker”, “former smoker” (quit smoking before pregnancy), or “smoker” (continued smoking during pregnancy). Mothers were regarded to have had pregnancy complications if they had any of the following conditions: gestational diabetes, hypertension, hyper- or hypothyroidism, infectious disease, preeclampsia, vaginal bleeding, phenylketonuria and allergy or asthma during pregnancy. The ENVIRONAGE birth cohort is representative of all births in Flanders with regard to maternal age and education, parity, newborn’s sex, ethnicity, and birth weight (Janssen et al., 2017). Among the recruited mother-newborn pairs, 855 had telomere length measurement at birth, of which 365 were also profiled on genome-wide DNA methylation. After excluding the newborns without SNP genotyping and one mother missing information on smoking status, 281 mother-newborn pairs were included in the current study (Supplementary Figure S1).
Cord blood was collected immediately after delivery in BD Vacutainer® plastic whole blood tubes with spray-coated K2EDTA (BD, Franklin Lakes, NJ, USA). Samples were centrifuged at 3,200rpm for 15 min. Plasma was removed and the remainder of the samples with the buffy coats was stored at -80 °C until further analysis.
Fetal placental biopsies (1–2 cm3) were taken at the fetal side directly underneath the chorioamniotic membrane, at approximately 4 cm from the umbilical cord. Contamination by chorioamniotic membrane was avoided by visual examination and dissection. Histological examination confirmed that the fetal placental biopsies mainly contained cytotrophoblasts and syncytiotrophoblast differentiated from trophoblasts.
Cord blood and placental DNA were extracted using the QIAamp DNA mini kit (Qiagen, Inc., Venlo, Netherlands). DNA purity and concentration were verified using a Nanodrop 1,000 spectrophotometer (Isogen, Life Science, Belgium). DNA was considered pure when the A260/280 was greater than 1.80 and A260/230 was greater than 2.0. DNA integrity was assessed with agarose gel electrophoresis.
Sixteen SNPs reported to be related to telomere length in previous GWAS (Prescott et al., 2011; Mangino et al., 2012; Codd et al., 2013; Liu et al., 2014; Mitchell et al., 2014; Mangino et al., 2015) and 18 SNPs shown to be associated with lifespan in a GWAS (Lunetta et al., 2007) or in a candidate gene study (TenNapel et al., 2014) were selected a priori for genotyping in placental DNA. SNP genotyping was conducted using the Biotrove OpenArray SNP Genotyping Platform at the Dana Farber/Harvard Cancer Center Genotyping and Genetics for Population Sciences Facility. After excluding SNPs with a SNP call rate lower than 95% and samples with a sample call rate lower than 90%, genotype imputation was performed with R package statgenGWAS (version 1.0.7.1) (van Rossum et al., 2020) based on the Beagle software (Browning and Browning, 2016). The genotyping data were further filtered to remove SNPs with a minor allele frequency (MAF) < 0.01 and a Hardy-Weinberg equilibrium chi-squared test p-value < 0.05. After preprocessing, 26 candidate SNPs related to telomere length or ageing traits were included in this study. The selection of SNPs and the original publications are shown in Supplementary Table S1. The pairwise linkage-disequilibrium (LD) was evaluated by plotting the allelic correlation r2 matrix in a heatmap, using R package LDheatmap (version 1.0–4). SNP genotype frequency data can be found in the European Variation Archive with accession numbers PRJEB53351 (project) and ERZ11081188 (analyses).
Cord blood DNA samples were bisulphite-converted, amplified and hybridized to the Illumina HumanMethylationEPIC Bead-Chip array (Illumina, San Diego, CA, USA), at the service lab GenomeScan (Leiden, Netherlands), for 377 cord blood samples from ENVIRONAGE. Array measurements were scanned using an Illumina iScan and the data quality was assessed using the R script MethylAid. DNA methylation data were preprocessed using the minfi package (version 1.38.0) in R (Aryee et al., 2014). Briefly, a probe was excluded when the probe call rate was lower than 95% based on a detection p-value larger than 10e-16 (Lehne et al., 2015). One sample was excluded because of the sample call rate lower than 99%, and four other samples were excluded due to discordant sex prediction using shinyMethyll (version 1.28.0) (Fortin et al., 2014a). Methylation data were normalized using functional normalization (Fortin et al., 2014b). For each CpG locus, the methylation level was expressed as M value calculated using the signal intensity from methylated probes and unmethylated probes (Du et al., 2010). Preprocessing resulted in a DNA methylation dataset with 857,898 CpGs. Missingness was imputed by K-nearest neighbor (KNN) imputation (K = 10) and technical confounding effects (batch and position) were removed from the M-value using an empirical Bayes method (Johnson et al., 2007). Subsequently, we trimmed the data per CpG for outliers defined as lower than three inter-quarter-ranges (IQR) below the first quartile, or higher than three IQRs above the third quartile. CpG probes were filtered to exclude CpGs on X and Y chromosomes, those known to be common SNPs and those having cross-reactivity with multiple genomic locations (Pidsley et al., 2016). 787,264 CpGs remained available for the statistical analyses.
Based on the DNA methylation data, blood cell proportions (nucleated red blood cells, granulocytes, monocytes, natural killer cells, B cells, CD4+ T cells, and CD8+ T cells) in the cord blood samples were estimated using Bakulski algorithm (Bakulski et al., 2016).
Average relative telomere length was measured in cord blood samples in triplicate using a previously described quantitative, real-time polymerase chain reaction (qPCR) protocol (Martens et al., 2016). Detailed specifications of the assay are provided in the supplementary Method. Telomere length was measured as the ratio of telomere copy number to single-copy gene number (T/S) relative to the average T/S ratio of the entire sample set. The inter-assay intra-class correlation coefficient (ICC) (TELOMERE RESEARCH NETWORK, 2020) was 0.936 (95% CI: 0.808–0.969) and the intra-assay ICC was 0.952 (95% CI: 0.947–0.956). A coefficient of variation (CV) of 6.4% was achieved within triplicates of the T/S ratios.
The statistical analyses workflow is shown in Supplementary Figure S2. In each step, the models were adjusted for a set of a priori selected covariates: newborn’s sex, gestational age, ethnicity and birth weight, maternal pregnancy complications, pre-pregnancy BMI, parity, education level and smoking status and paternal age, as well as cell type heterogeneity estimated from the DNA methylation data.
In the first step, cord blood telomere length was regressed on each SNP in a multiple regression model. Dominant coding was applied, where the genotype at each SNP was classified into either of the two categories: major homozygote and heterozygote/minor homozygote. To ensure robustness of the results, we additionally applied an additive coding based on the number of minor alleles, which is a commonly used SNP coding method in the literature (Tam et al., 2019). Statistical significance of a model term was defined as having a nominal p-value lower than 0.05.
Each of the selected 26 SNPs was analyzed against CpG sites available in cis (within ±0.5 Mb from the SNP), by fitting multiple regression models of the M-value of each CpG on the dominant-coded or additive-coded SNPs. The R package limma (version 3.48.1) was used for model fitting and inference. Statistical significance was determined under multiple testing control by Bonferroni correction at a level of 0.05. The identified significant SNP-CpG pairs were compared with a published methylation quantitative trait loci (mQTL) database (www.mqtldb.org), specifying the timepoint as “newborns” and the distance defining cis-mQTLs as 500 kb Gaunt et al., 2016.
The CpGs involved in the significant mQTL were assessed for their association with cord blood telomere length. Using the CpGs that were identified both in mQTL and in association with cord blood telomere length, a path model was constructed for the mediating effect of the CpGs on the SNP-telomere length association, based on the following assumptions: 1) genotypes determined at the very beginning of life precede both the change in DNA methylation and the shortening of telomere length, and 2) genotypes are less likely to be changed than DNA methylation and telomere length shortening, which are regulated through more complex pathways. Therefore, it was assumed that the genetic variants at a certain SNP altered the methylation level at certain CpGs, which subsequently led to a change in newborn telomere length. Mediation analyses were performed with the R package mediation (version 4.5.0). Tingley et al., (2014). Dominant coding was used for SNPs. The direct effect (DE), the indirect effect (IE) and the total effect (TE) were estimated, and the proportion of mediated was estimated in case that the DE and ID were in the same direction. Statistical inference was performed using bootstrap percentiles with 1,000 bootstrap samples and nominal significance level of 0.05 was adopted for each effect.
The cis-interaction between CpG and SNP was assessed by including a statistical interaction term (SNP*CpG) in the model of cord blood telomere length. The model is stated as:
where
Characteristics of the study population, comprising 281 mother-newborn pairs, are summarized in Table 1. The numbers of newborn girls and boys were balanced, and the majority (93.6%) of the newborns were of European origin. The gestational age ranged between 29 and 41 weeks, and around 6.0% were born preterm (before week 37). The newborns had an average birth weight of 3.38 ± 0.48 kg and around 2.8% were of low birth weight (lower than 2.5 kg). Mothers had a mean age of 30.11 ± 4.17 years and a pre-pregnancy body-mass index (BMI) of 24.03 ± 4.21 kg/m2. Among all the mothers, 65.8% had received a higher education. Thirty-three mothers (11.7%) reported having smoked during pregnancy, whereas most mothers (73.0%) never smoked. More than half of the newborns were from the first pregnancy of the mother (51.6%). In total 48 mothers (17.1%) experienced one or more pregnancy complications.
The model estimates under dominant coding and additive coding, respectively, are shown in Table 2. Without correction for multiple testing, the results with both coding methods showed weak associations. Three SNPs, rs9419958 (OBFC1), rs9420907 (OBFC1) and rs17653722 (KRT80), had estimates significantly different from zero in the dominant coding model. Rs9419958 (OBFC1) was confirmed in the additive coding model, while the other two SNPs, rs9420907 (OBFC1) and rs17653722 (KRT80), were only borderline-significant under additive coding.
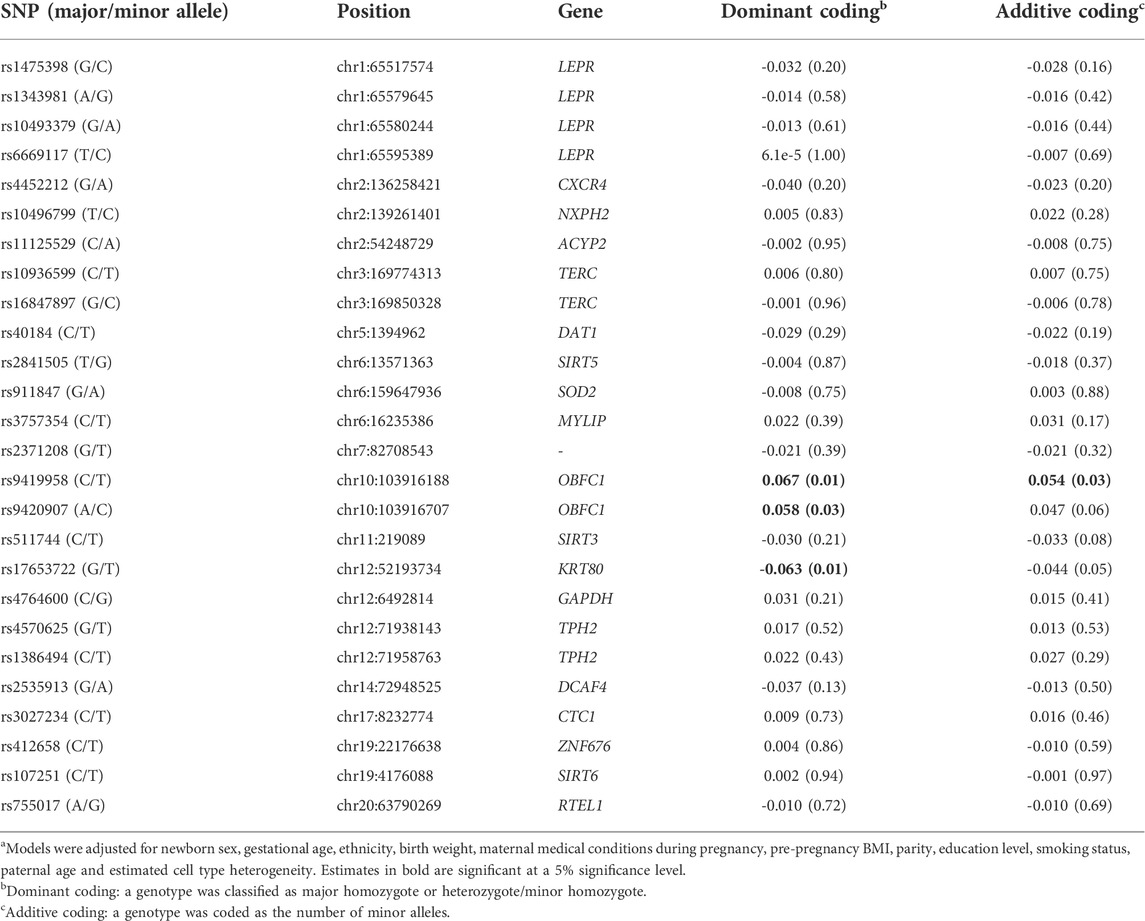
TABLE 2. Association (nominal p-value) between SNPs and newborn telomere lengtha.
The 26 SNPs were not completely independent, as suggested by Supplementary Figure S3, in which three clusters of SNPs were detected by their pairwise linkage disequilibrium. Highly correlated SNPs (r2 > 0.5), such as rs1475398 (LEPR), rs10493379 (LEPR) and rs1343981 (LEPR), rs9419958 (OBFC1) and rs9420907 (OBFC1), behaved consistently in their association with cord blood telomere length in terms of the effect size, direction and effect significance.
The number of CpGs tested on each SNP is summarized in Supplementary Table S2. Among the 26 SNPs, rs755017 (RTEL1) had no CpGs within the 0.5 Mb neighborhood. In total, the association was tested on 12,194 SNP-CpG pairs. There were 57 cis-mQTL identified from both the dominant models and the additive models under Bonferroni correction (Supplementary Table S3). Matching our results with the external mQTL database mQTLdb (http://www.mqtldb.org/), (Gaunt et al., 2016) 22 out of the 57 SNP-CpG pairs were confirmed with the same direction and comparable effect size. The top association identified in the current study was between rs4764600 (GAPDH) and cg07142400 (NCAPD2; MRPL51), which confirmed the findings in mQTLdb, where one more copy of the minor allele G at rs4764600 was associated with more than 0.50 increase in the M-value at cg07142400 (NCAPD2; MRPL51). Most of the SNPs were associated with multiple CpGs that were not located in the same gene. SNP rs3027234 (CTC1) had the largest number of associated CpGs and the involved twelve CpGs mapped to seven genes. The SNP with the second largest number of associated CpGs was rs4764600 (GAPDH), with the nine CpGs distributed over five genes. On the other hand, five CpGs were associated with more than one SNP in other genes and all the other CpGs were only associated with one SNP.
Among the 48 CpGs involved in the 57 mQTLs, five CpGs were found to be associated with cord blood telomere length at a nominal significance level (Supplementary Table S4). These five SNP-CpG pairs were used to construct mediation models as shown in Figure 1, where all SNPs’ indirect effect was mediated by only one CpG. All five indirect effects were significant at the 5% significance level, and none of the direct effects or the total effects was significant (Figure 1 and Supplementary Table S5). For rs2535913 (DCAF4), the direct and indirect effects were in the same direction and the proportion mediated was 68% (p-value = 0.15, Figure 1). For all the other SNPs, the direct effects were in the opposite direction of the corresponding indirect effects.
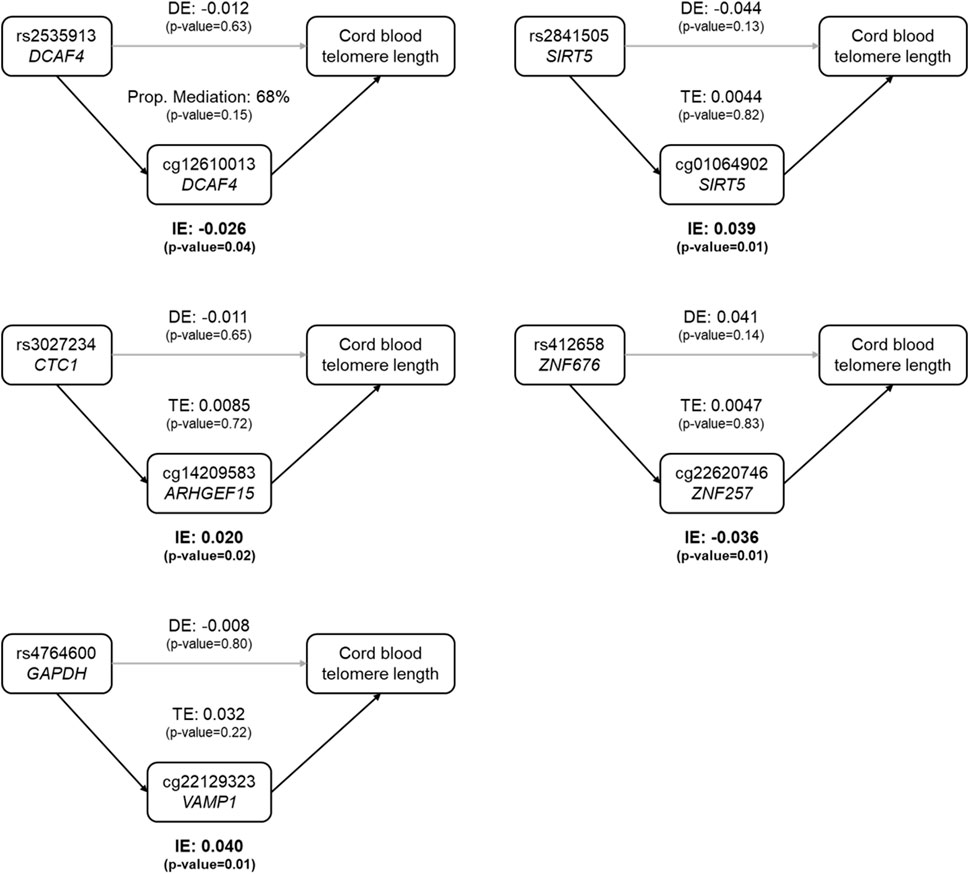
FIGURE 1. Estimated proportion of the association between SNP genotypes and cord blood telomere length mediated by the DNA methylation level at a CpG. In each sub-figure, the estimate of the indirect effect (IE) via the CpG, and the estimate of the direct effect (DE) of the SNP on cord blood telomere length are displayed. For the first SNP-CpG pair (rs2535913 and cg12610013, top left), where IE and DE were in the same direction, the proportion of mediation (IE/DE + IE) was shown. In the other 4 mediation relationships, IE and DE had opposite directions, and therefore only the total effect (TE) was shown. Grey arrows stood for insignificant associations, while black arrows denoted the statistically significant ones (at a nominal significance level of 0.05). Dominant coding of SNPs was used and the mediation model was adjusted for newborn’s sex, gestational age, ethnicity and birth weight, maternal pregnancy complications, pre-pregnancy BMI, parity, education level and smoking status, paternal age, and estimated cell type heterogeneity.
A SNP-CpG interaction effect was identified between rs911874 (SOD2) and cg24223887 (gene unknown) under Bonferroni correction (β = -0.330, p-value = 6.2e-5), indicating that the higher the methylation level at cg24223887 (gene unknown), the weaker the association between the number of A allele at rs911847 (SOD2) and cord blood telomere length. Within the same model, the SNP rs911874 (SOD2) main effect was significant under the Bonferroni correction for SNP main effects, which has not been identified in the candidate SNP association analysis without SNP-CpG interactions (Table 2). The number of A allele was associated with a 1.186 T/S ratio (p-value = 6.4e-5) increase in cord blood telomere length. The CpG main effect of cg24223887 was nominally significant (0.263, p-value = 8.2e-04).
Telomere length is a biomarker of ageing and early-life telomere length relates to later life telomere length and may underlie ageing-related diseases. How the interplay between genetic and epigenetic factors is related to newborn telomere length remains to be understood. In the present study, we showed that DNA methylation on specific CpGs in the near genomic distance to biological age-related genetic variants might mediate the genetic regulation of newborn telomere length. In addition, the genetic difference in newborn telomere length might be altered by the DNA methylation level in a nearby region.
In the candidate gene association analysis of newborn telomere length, we studied 26 telomere length- or lifespan-related candidate SNPs that were previously described in adults. Although these candidate SNPs were identified in previous studies mostly by GWAS under stringent control of type I error, we did not observe clear associations with newborn telomere length under stringent control of type I error. While limited statistical power due to sample size could be one explanation, a previous candidate gene study of mother-newborn pairs was similarly unable to reproduce the GWAS findings of cord blood telomere length for three of the SNPs used in the current study (rs11125529 (ACYP2), rs10936599 (TERC) and rs755017 (RTEL1)) (Weng et al., 2016). This might be explained by the fact that these variants are involved in telomere length maintenance against risk factors that arise from external stimuli in later life, rather than in the programming of telomere length at birth. In the current study, SNPs in OBFC1 (rs9419958 and rs9420907) and in KRT80 (rs17653722) were found nominally significantly associated with cord blood telomere length, where the associations for the two SNPs in OBFC1 gene were robust across coding methods and were of the same effect direction as the published GWAS’s (Prescott et al., 2011; Mangino et al., 2012; Codd et al., 2013; Codd et al., 2021). The OBFC1 gene encodes the OB Fold-containing Protein 1, which is involved in telomere elongation (Wan et al., 2009). KRT80-encoded keratin 80 is involved in cell and tissue differentiation especially in epithelia (Langbein et al., 2010). Conditional on the SNP-CpG interaction, rs911847 (SOD2) was found strongly associated with cord blood telomere length, while in the discovery study it was associated with age at death and a skeletal marker of biological age (Lunetta et al., 2007). SOD2 encodes a manganese ion-binding mitochondrial protein that converts superoxide byproducts of oxidative phosphorylation to hydrogen peroxide and oxygen. Deficiency of SOD2 has been associated with premature epidermal thinning in mice, which is also an age-associated phenotype in human beings (Weyemi et al., 2012).
We assessed cis-mQTL for the candidate SNPs and all available nearby CpGs. Only cis-acting mQTL and interactions were investigated since the trans-interplay explains much less variation and tends to be polygenic (Gaunt et al., 2016). Part of the identified mQTLs in the current study were in line with an mQTL database published for a large-scale study of newborns (Gaunt et al., 2016) where the effect directions were also consistent. Only four SNPs, rs4452212 (CXCR4), rs11125529 (ACYP2), rs511744 (SIRT3) and rs17653722 (KRT80), were significantly associated with one CpG each, and all the other SNPs were associated with multiple CpGs. While rs3027234 (CTC1) was the mQTL with the largest number of CpGs, the SNP with the largest number of CpGs in the 500 kb neighborhood was rs40184 (DAT1). This might indicate that the presence of mQTL is not randomly distributed across the genome, but is enriched within a certain set of hub SNPs.
We found that DNA methylation at specific CpGs mediated the genetic association with newborn telomere length. All indirect effects were nominally significant, which is logical because the CpGs used to construct the mediation model were involved in mQTLs and associated with cord blood telomere length at the same time (meeting the minimal assumptions to be a mediator). None of the SNPs in the mediation model was associated with cord blood telomere length in the candidate gene association analysis, and neither were they when conditioned on the mediation by a CpG. Therefore, no SNP had a significant direct effect. Among the five SNP-CpG pairs used in the mediation analysis, rs2841505 (SIRT5)-cg01064902 (SIRT5) and rs412658 (ZNF676)-cg22620746 (ZNF257) confirmed the findings reported in mQTLdb. However, these SNP-CpG pairs have not been reported yet as being involved in a causal relationship with any diseases or phenotypes.
In addition to mediation, we also investigated effect modification by DNA methylation on the association between SNPs and cord blood telomere length. The variance explained by the model increased by 36% after adding the interaction between rs911847 (SOD2) and cg24223887 (gene unknown) (results not shown). Published epigenome-wide studies have reported DNA methylation modulating the genetic impact on cardiovascular disease-related traits (Veenstra et al., 2018; Wang et al., 2021b) and type II diabetes (Vohra et al., 2020), whereas for newborn telomere length, the current study is to our knowledge the first to identify SNP-CpG interactions.
In addition to the role of DNA methylation in the interplay with SNP genotypes that explained newborn telomere length, we previously have investigated DNA methylation through constructing a DNA methylation-based explanatory model for newborn telomere length (Wang et al., 2021a). However, we did not identify the same CpGs within an earlier reported DNAmTL prediction model (Lu et al., 2019), which was constructed using data from adult populations. Both telomere length and DNA methylation have highly dynamic patterns at birth as well as over life. Therefore, the epigenetic mechanisms of telomere length may be different throughout the stages of life.
Understanding genetic, epigenetic and environmental factors which can explain the variation of telomere length in early life is of interest as telomere length at birth is linked with telomere length in childhood and early adulthood (Martens et al., 2021). Moreover, telomere length at birth is a predictive factor for life expectancy (Heidinger et al., 2012) and has been reported to be associated with blood pressure in childhood (Martens et al., 2022). Our findings can potentially suggest clinical utility in prediction and intervention. The genotype at the SNPs under study might indicate different liability to telomere length shortening. While genotypes cannot be modified and can only be used for prognostic use, it is possible to alter the level of DNA methylation. For instance, the identified CpGs could possibly serve as targets in epigenetic therapies (Berdasco and Esteller, 2019). However, some identified CpGs were not mapped to any gene or with a known function, which requires further investigation for their biological or clinical relevance. Moreover, the current study is only exploratory and the findings still need validation in external populations in larger scale studies.
We acknowledge the limitations of the current study. Firstly, the placenta is a heterogeneous organ containing both fetal and maternal tissues. It is, however, unlikely that the SNPs were genotyped in maternal DNA, since standard sampling strategies and histological examinations was applied to minimize maternal tissue contamination, and studies have shown a largely differential molecular pattern between placental biopsies from different locations (Wyatt et al., 2005; Sood et al., 2006). Secondly, given the statistical power due to a small sample size and limited variation within a healthy population, we were not able to reproduce most of the associations between SNPs and telomere length that were identified in previous large-scale studies, which were mostly adult population-based. A haplotype-based approach that summarizes blocks of 10–100 kb might increase the power (Daly et al., 2001). However, since the SNPs included in the current study were selected a priori and were not from genome-wide genotyping, they only suggested SNP blocks of limited size (2–4 kb). Thirdly, the SNP selection was only based on genome-wide association studies, while it could be more precise and sensible to select targets based on genes that are well studied and known to be relevant for telomere length, such as telomerase or shelterin genes as suggested in the Telomerase Database (Podlevsky et al., 2008). In future investigations, such an improved target selection might further reveal important insights in the methylation mediating role of the genetic regulation of telomere biology. Fourthly, although indirect effects have been found via the DNA methylation at a few CpGs, the mediation effects should be interpreted with caution. The interplay between DNA methylation and telomere attrition is intertwined, because not only epigenetic modification but also telomere length can regulate gene expression (Kim and Shay, 2018), through which they might cause changes in each other. Further causal inference might be achieved by a Mendelian randomization study using genetic variants that are strongly associated with newborn telomere length as the instrumental variables (Davies et al., 2018). Finally, we acknowledge that the current study is hypothesis generating and that our identified markers should be further validated in larger follow-up studies and via using a targeted DNA methylation approach.
To conclude, our study for the first time modelled newborn telomere length in a context of multiple molecular layers (genetic variants and DNA methylation). We partly confirmed telomere length- or ageing-related genetic variants and mQTLs in newborns identified in previous studies. Our results suggest that DNA methylation might alter the effect of genetic variants on newborn telomere length, and identified DNA methylation loci that might be crucial targets in future investigations of telomere biology. Potential mediating effects by DNA methylation might be present, but knowledge of biological pathways is required for a sound causal inference. Through the current study, we have shown the potential importance of investigating the interplay between different molecular levels in regulating telomere length. This novel approach should be evaluated in future large-scale studies.
The genetic variants frequency data is publicly available in the European Variation Archive with accession numbers PRJEB53351 (project) and ERZ11081188 (analyses). Other data are available from the corresponding author, DSM, upon reasonable request.
The studies involving human participants were reviewed and approved by the Ethical Committee of Hasselt University and East-Limburg Hospital in Genk (Belgium). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
TN coordinates the ENVIRONAGE birth cohort. TN, DM and CW designed the current study. JH and IDV coordinated the genotyping assay. DM performed DNA extractions and telomere length measurements. RA performed DNA methylation data pre-preprocessing. CW conducted all the statistical analyses. RA, BR, MP and MB advised on the analysis. CW, DM and TN prepared the first draft of the manuscript. All authors contributed in critical revision of the manuscript and approved the final manuscript.
The ENVIRONAGE birth cohort is supported by grants from the European Research Council (Grant No. ERC-2012-StG310898), the Flemish Scientific Fund (FWO, Grant No. G073315N) and Kom Op Tegen Kanker (KOTK). DSM (FWO grant 12X9620N) is postdoctoral fellows of the Flanders Research Foundation. JH was partly funded by a postdoctoral research fellow grant from the Research Foundation–Flanders (FWO) (no. 12J9516N). This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 874583 (ATHLETE).
We acknowledge the cohort participants, midwives, the staff of the maternity ward and the clinical laboratory of East-Limburg Hospital in Genk.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.934277/full#supplementary-material
Allsopp, R. C., Vaziri, H., Patterson, C., Goldstein, S., Younglai, E. V., Futcher, A. B., et al. (1992). Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 89 (21), 10114–10118. doi:10.1073/pnas.89.21.10114
Aryee, M. J., Jaffe, A. E., Corrada-Bravo, H., Ladd-Acosta, C., Feinberg, A. P., Hansen, K. D., et al. (2014). Minfi: A flexible and comprehensive bioconductor package for the analysis of infinium DNA methylation microarrays. Bioinformatics 30 (10), 1363–1369. doi:10.1093/bioinformatics/btu049
Aubert, G., and Lansdorp, P. M. (2008). Telomeres and aging. Physiol. Rev. 88 (2), 557–579. doi:10.1152/physrev.00026.2007
Bakulski, K. M., Feinberg, J. I., Andrews, S. V., Yang, J., Brown, S., Stephanie, L. M., et al. (2016). DNA methylation of cord blood cell types: Applications for mixed cell birth studies. Epigenetics 11 (5), 354–362. doi:10.1080/15592294.2016.1161875
Berdasco, M., and Esteller, M. (2019). Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 20 (2), 109–127. doi:10.1038/s41576-018-0074-2
Bijnens, E. M., Zeegers, M. P., Derom, C., Martens, D. S., Gielen, M., Hageman, G. J., et al. (2017). Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 15 (1), 205. doi:10.1186/s12916-017-0964-8
Blackburn, E. H., Epel, E. S., and Lin, J. (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350 (6265), 1193–1198. doi:10.1126/science.aab3389
Browning, B. L., and Browning, S. R. (2016). Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 98 (1), 116–126. doi:10.1016/j.ajhg.2015.11.020
Buxton, J. L., Suderman, M., Pappas, J. J., Borghol, N., McArdle, W., Blakemore, A. I. F., et al. (2014). Human leukocyte telomere length is associated with DNA methylation levels in multiple subtelomeric and imprinted loci. Sci. Rep. 4 (1), 4954. doi:10.1038/srep04954
Ciuculete, D. M., Boström, A. E., Voisin, S., Philipps, H., Titova, O. E., Bandstein, M., et al. (2017). A methylome-wide mQTL analysis reveals associations of methylation sites with GAD1 and HDAC3 SNPs and a general psychiatric risk score. Transl. Psychiatry 7 (1), e1002. doi:10.1038/tp.2016.275
Codd, V., Nelson, C. P., Albrecht, E., Mangino, M., Deelen, J., Buxton, J. L., et al. (2013). Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 45 (4), 422–427. doi:10.1038/ng.2528
Codd, V., Wang, Q., Allara, E., Musicha, C., Kaptoge, S., Stoma, S., et al. (2021). Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 53 (10), 1425–1433. doi:10.1038/s41588-021-00944-6
Daly, M. J., Rioux, J. D., Schaffner, S. F., Hudson, T. J., and Lander, E. S. (2001). High-resolution haplotype structure in the human genome. Nat. Genet. 29 (2), 229–232. doi:10.1038/ng1001-229
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ Clin. Res. ed) 362, k601. doi:10.1136/bmj.k601
Du, P., Zhang, X., Huang, C-C., Jafari, N., Kibbe, W. A., Hou, L., et al. (2010). Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinforma. 11 (1), 587. doi:10.1186/1471-2105-11-587
Fortin, J-P., Fertig, E., and Hansen, K. (2014). shinyMethyl: interactive quality control of Illumina 450k DNA methylation arrays in R. F1000Res. 3, 175. doi:10.12688/f1000research.4680.2
Fortin, J. P., Labbe, A., Lemire, M., Zanke, B. W., Hudson, T. J., Fertig, E. J., et al. (2014). Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol. 15 (12), 503. doi:10.1186/s13059-014-0503-2
Frenck, R. W., Blackburn, E. H., and Shannon, K. M. (1998). The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. U. S. A. 95 (10), 5607–5610. doi:10.1073/pnas.95.10.5607
Gaunt, T. R., Shihab, H. A., Hemani, G., Min, J. L., Woodward, G., Lyttleton, O., et al. (2016). Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 17 (1), 61. doi:10.1186/s13059-016-0926-z
Hannon, E., Knox, O., Sugden, K., Burrage, J., Wong, C. C. Y., Belsky, D. W., et al. (2018). Characterizing genetic and environmental influences on variable DNA methylation using monozygotic and dizygotic twins. PLoS Genet. 14 (8), e1007544. doi:10.1371/journal.pgen.1007544
Heidinger, B. J., Blount, J. D., Boner, W., Griffiths, K., Metcalfe, N. B., and Monaghan, P. (2012). Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. U. S. A. 109 (5), 1743–1748. doi:10.1073/pnas.1113306109
Howe, L. J., Richardson, T. G., Arathimos, R., Alvizi, L., Passos-Bueno, M. R., Stanier, P., et al. (2019). Evidence for DNA methylation mediating genetic liability to non-syndromic cleft lip/palate. Epigenomics 11 (2), 133–145. doi:10.2217/epi-2018-0091
Janssen, B. G., Madhloum, N., Gyselaers, W., Bijnens, E., Clemente, D. B., Cox, B., et al. (2017). Cohort profile: The ENVIRonmental influence on early AGEing (ENVIRONAGE): A birth cohort study. Int. J. Epidemiol. 46 (5), 1386–1387. doi:10.1093/ije/dyw269
Johnson, W. E., Li, C., and Rabinovic, A. (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostat. Oxf. Engl. 8 (1), 118–127. doi:10.1093/biostatistics/kxj037
Kim, W., and Shay, J. W. (2018). Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation. 99, 1–9. doi:10.1016/j.diff.2017.11.005
Langbein, L., Eckhart, L., Rogers, M. A., Praetzel-Wunder, S., and Schweizer, J. (2010). Against the rules: Human keratin K80: Two functional alternative splice variants, K80 and K80.1, with special cellular localization in a wide range of epithelia. J. Biol. Chem. 285 (47), 36909–36921. doi:10.1074/jbc.M110.161745
Lee, Y., Sun, D., Ori, A. P. S., Lu, A. T., Seeboth, A., Harris, S. E., et al. (2019). Epigenome-wide association study of leukocyte telomere length. Aging 11 (16), 5876–5894. doi:10.18632/aging.102230
Lehne, B., Drong, A. W., Loh, M., Zhang, W., Scott, W. R., Tan, S-T., et al. (2015). A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 16 (1), 37. doi:10.1186/s13059-015-0600-x
Levy, D., Neuhausen, S. L., Hunt, S. C., Kimura, M., Hwang, S. J., Chen, W., et al. (2010). Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc. Natl. Acad. Sci. U. S. A. 107 (20), 9293–9298. doi:10.1073/pnas.0911494107
Liu, Y., Cao, L., Li, Z., Zhou, D., Liu, W., Shen, Q., et al. (2014). A genome-wide association study identifies a locus on TERT for mean telomere length in Han Chinese. PLoS One 9 (1), e85043. doi:10.1371/journal.pone.0085043
Lu, A. T., Seeboth, A., Tsai, P. C., Sun, D., Quach, A., Reiner, A. P., et al. (2019). DNA methylation-based estimator of telomere length. Aging 11 (16), 5895–5923. doi:10.18632/aging.102173
Lunetta, K. L., D'Agostino, R. B., Sr, , Karasik, D., Benjamin, E. J., Guo, C. Y., et al. (2007). Genetic correlates of longevity and selected age-related phenotypes: A genome-wide association study in the framingham study. BMC Med. Genet. 8 (1), S13. doi:10.1186/1471-2350-8-S1-S13
Mangino, M., Christiansen, L., Stone, R., Hunt, S. C., Horvath, K., Eisenberg, D. T. A., et al. (2015). DCAF4, a novel gene associated with leucocyte telomere length. J. Med. Genet. 52 (3), 157–162. doi:10.1136/jmedgenet-2014-102681<
Mangino, M., Hwang, S. J., Spector, T. D., Hunt, S. C., Kimura, M., Fitzpatrick, A. L., et al. (2012). Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 21 (24), 5385–5394. doi:10.1093/hmg/dds382
Martens, D. S., Plusquin, M., Gyselaers, W., De Vivo, I., and Nawrot, T. S. (2016). Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 14 (1), 148. doi:10.1186/s12916-016-0689-0
Martens, D. S., Sleurs, H., Dockx, Y., Rasking, L., Plusquin, M., and Nawrot, T. S. (2022). Association of newborn telomere length with blood pressure in childhood. JAMA Netw. Open 5 (8), e2225521–e. doi:10.1001/jamanetworkopen.2022.25521
Martens, D. S., Van Der Stukken, C., Derom, C., Thiery, E., Bijnens, E. M., and Nawrot, T. S. (2021). Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. EBioMedicine 63, 103164. doi:10.1016/j.ebiom.2020.103164
Mitchell, C., Hobcraft, J., McLanahan, S. S., Siegel, S. R., Berg, A., Brooks-Gunn, J., et al. (2014). Social disadvantage, genetic sensitivity, and children's telomere length. Proc. Natl. Acad. Sci. U. S. A. 111 (16), 5944–5949. doi:10.1073/pnas.1404293111
Moore, L. D., Le, T., and Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology 38 (1), 23–38. doi:10.1038/npp.2012.112
O'Sullivan, R. J., and Karlseder, J. (2010). Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11 (3), 171–181. doi:10.1038/nrm2848
Pidsley, R., Zotenko, E., Peters, T. J., Lawrence, M. G., Risbridger, G. P., Molloy, P., et al. (2016). Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 17 (1), 208. doi:10.1186/s13059-016-1066-1
Podlevsky, J. D., Bley, C. J., Omana, R. V., Qi, X., and Chen, J. J. (2008). The telomerase database. Nucleic Acids Res. 36, D339–D343. doi:10.1093/nar/gkm700
Prescott, J., Kraft, P., Chasman, D. I., Savage, S. A., Mirabello, L., Berndt, S. I., et al. (2011). Genome-wide association study of relative telomere length. PLOS ONE 6 (5), e19635. doi:10.1371/journal.pone.0019635
Slagboom, P. E., Droog, S., and Boomsma, D. I. (1994). Genetic determination of telomere size in humans: A twin study of three age groups. Am. J. Hum. Genet. 55 (5), 876–882.
Sood, R., Zehnder, J. L., Druzin, M. L., and Brown, P. O. (2006). Gene expression patterns in human placenta. Proc. Natl. Acad. Sci. U. S. A. 103 (14), 5478–5483. doi:10.1073/pnas.0508035103
Tam, V., Patel, N., Turcotte, M., Bossé, Y., Paré, G., and Meyre, D. (2019). Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 20 (8), 467–484. doi:10.1038/s41576-019-0127-1
TELOMERE RESEARCH NETWORK (2020). Study Design & analysis. Available at: https://trn.tulane.edu/resources/study-design-analysis/.
TenNapel, M. J., Lynch, C. F., Burns, T. L., Wallace, R., Smith, B. J., Button, A., et al. (2014). SIRT6 minor allele genotype is associated with >5-year decrease in lifespan in an aged cohort. PLoS One 9 (12), e115616. doi:10.1371/journal.pone.0115616
Tingley, D., Yamamoto, T., Hirose, K., Keele, L., and Imai, K. (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software 59 (5), 138. doi:10.18637/jss.v059.i05
van Rossum, B-J., Kruijer, W., Fv, E., Boer, M., Malosetti, M., Bustos-Korts, D., et al. (2020). statgenGWAS: Genome wide association studies. Available at: https://CRAN.R-project.org/package=statgenGWAS.
Veenstra, J., Kalsbeek, A., Koster, K., Ryder, N., Bos, A., Huisman, J., et al. (2018). Epigenome wide association study of SNP-CpG interactions on changes in triglyceride levels after pharmaceutical intervention: A GAW20 analysis. BMC Proc. 12 (9), 58. doi:10.1186/s12919-018-0144-7
Vohra, M., Adhikari, P., Souza, S. C. D., Nagri, S. K., Umakanth, S., Satyamoorthy, K., et al. (2020). CpG-SNP site methylation regulates allele-specific expression of MTHFD1 gene in type 2 diabetes. Lab. Invest. 100 (8), 1090–1101. doi:10.1038/s41374-020-0422-7
Volkov, P., Olsson, A. H., Gillberg, L., Jørgensen, S. W., Brøns, C., Eriksson, K-F., et al. (2016). A genome-wide mQTL analysis in human adipose tissue identifies genetic variants associated with DNA methylation, gene expression and metabolic traits. PloS one 11 (6), e0157776. doi:10.1371/journal.pone.0157776
Wan, M., Qin, J., Songyang, Z., and Liu, D. (2009). OB Fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J. Biol. Chem. 284 (39), 26725–26731. doi:10.1074/jbc.M109.021105
Wang, C., Nawrot, T. S., Van Der Stukken, C., Tylus, D., Sleurs, H., Peusens, M., et al. (2021). Different epigenetic signatures of newborn telomere length and telomere attrition rate in early life. Aging 13 (11), 14630–14650. doi:10.18632/aging.203117
Wang, J., Ma, X., Zhang, Q., Chen, Y., Wu, D., Zhao, P., et al. (2021). The interaction analysis of SNP variants and DNA methylation identifies novel methylated pathogenesis genes in congenital heart diseases. Front. Cell Dev. Biol. 9 (803), 665514. doi:10.3389/fcell.2021.665514
Wang, Q., Zhan, Y., Pedersen, N. L., Fang, F., and Hägg, S. (2018). Telomere length and all-cause mortality: A meta-analysis. Ageing Res. Rev. 48, 11–20. doi:10.1016/j.arr.2018.09.002
Weng, Q., Du, J., Yu, F., Huang, T., Chen, M., Lv, H., et al. (2016). The known genetic loci for telomere length may be involved in the modification of telomeres length after birth. Sci. Rep. 6 (1), 38729. doi:10.1038/srep38729
Weyemi, U., Parekh, P. R., Redon, C. E., and Bonner, W. M. (2012). SOD2 deficiency promotes aging phenotypes in mouse skin. Aging 4 (2), 116–118. doi:10.18632/aging.100433
Wyatt, S. M., Kraus, F. T., Roh, C. R., Elchalal, U., Nelson, D. M., and Sadovsky, Y. (2005). The correlation between sampling site and gene expression in the term human placenta. Placenta 26 (5), 372–379. doi:10.1016/j.placenta.2004.07.003
Keywords: newborn, telomere, ageing, genetic variants, DNA methylation, mQTL, mediation and effect modification
Citation: Wang C, Alfano R, Reimann B, Hogervorst J, Bustamante M, De Vivo I, Plusquin M, Nawrot TS and Martens DS (2022) Genetic regulation of newborn telomere length is mediated and modified by DNA methylation. Front. Genet. 13:934277. doi: 10.3389/fgene.2022.934277
Received: 02 May 2022; Accepted: 06 September 2022;
Published: 04 October 2022.
Edited by:
Michael E. Symonds, University of Nottingham, United KingdomReviewed by:
Nancy Monroy-Jaramillo, National Institute of Neurology and Neurosurgery, MexicoCopyright © 2022 Wang, Alfano, Reimann, Hogervorst, Bustamante, De Vivo, Plusquin, Nawrot and Martens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dries S. Martens, ZHJpZXMubWFydGVuc0B1aGFzc2VsdC5iZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.