
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 22 July 2022
Sec. RNA
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.933009
This article is part of the Research TopicMachine Learning-Based Methods for RNA Data Analysis - Volume IIIView all 11 articles
Lung cancer is one of the leading causes of cancer-related deaths. Thus, it is important to find its biomarkers. Furthermore, there is an increasing number of studies reporting that long noncoding RNAs (lncRNAs) demonstrate dense linkages with multiple human complex diseases. Inferring new lncRNA-disease associations help to identify potential biomarkers for lung cancer and further understand its pathogenesis, design new drugs, and formulate individualized therapeutic options for lung cancer patients. This study developed a computational method (LDA-RLSURW) by integrating Laplacian regularized least squares and unbalanced bi-random walk to discover possible lncRNA biomarkers for lung cancer. First, the lncRNA and disease similarities were computed. Second, unbalanced bi-random walk was, respectively, applied to the lncRNA and disease networks to score associations between diseases and lncRNAs. Third, Laplacian regularized least squares were further used to compute the association probability between each lncRNA-disease pair based on the computed random walk scores. LDA-RLSURW was compared using 10 classical LDA prediction methods, and the best AUC value of 0.9027 on the lncRNADisease database was obtained. We found the top 30 lncRNAs associated with lung cancers and inferred that lncRNAs TUG1, PTENP1, and UCA1 may be biomarkers of lung neoplasms, non-small–cell lung cancer, and LUAD, respectively.
Cancers are posing threat for the health of humans (Yang et al., 2013; Liu et al., 2021). Lung cancer is the most common cancer worldwide and one of the leading causes of cancer-relevant deaths, and it has been so for many years. Thus, in 2008, the global statistical analysis demonstrated that approximately 1.6 million new lung cancer cases were diagnosed, and 1.4 million deaths were confirmed globally. In 2012, there were 1.8 million of new lung cancer diagnoses and 1.6 million deaths (de Groot et al., 2018; Howlader et al., 2020). In 2018, the number of new lung cancer cases exceeded 2 million and the number of deaths exceeded 1.7 million (Yuan et al., 2019). In the United States, approximately 234,000 cases of lung cancer were diagnosed the same year. This year, lung cancer diagnosis account for 14 and 13% of new cases in men and women, respectively. Estimation of mortality is 83,550 and 70,500 deaths in men and women, respectively. Lung carcinoma is one of cancers with the lowest survival rate. It is usually not diagnosed until an advanced stage (de Groot et al., 2018; Howlader et al., 2020).
Despite the fast development of lung cancer therapy, high morbidity and mortality rates still pose a severe challenge for cancer researchers. The majority of patients with advanced-stage lung cancer have been ultimately poorly diagnosed. Thus, designing efficient therapy strategies is extremely important for lung cancer patients. However, existing techniques applied to diagnosis and therapies of lung cancer remain suboptimal. Thus, better strategies supplementing or replacing the existing techniques are urgent. Genome-wide association studies have found numerous genetic variants relevant to various cancers, one-third of which are densely linked to noncoding regions. The noncoding RNAs can be used as biomarkers of lung cancers. Therefore, accurate biomarker identification is urgently required to effectively diagnose lung cancer and boost the survival rate while decreasing its mortality and morbidity (Huang et al., 2017; Roointan et al., 2019; Yang et al., 2020).
Long noncoding RNAs (lncRNAs) are a type of noncoding RNAs that has over 200 nucleotides and post-transcriptional modifications including splicing, capping, and polyadenylation. lncRNAs can be used as a guide for protein-DNA interactions, protein-RNA interactions, and protein–protein interactions (Peng et al., 2020a). With the fast advancement of cancer genomics, many lncRNAs have been demonstrated to be aberrantly expressed in diverse cancers and play key action in the development of tumors through modulation of cancer-related signaling pathways. lncRNAs can regulate survival, metastasis, angiogenesis, and proliferation of tumor cells. Therefore, lncRNAs can be used as potential biomarkers and therapeutic targets in cancers by interacting with proteins (Chandra Gupta and Nandan Tripathi, 2017). For example, Peng et al. and her groups (Peng et al., 2021a; Zhou L. Q. et al., 2021; Peng et al., 2021b; Zhou L. et al., 2021; Tian et al., 2021; Peng et al., 2022) designed a series of state-of-the-art lncRNA-protein interaction prediction methods and significantly improved biomarker identification for various diseases. In addition, lncRNA SNHG14, BCRT1, DSCAM-AS1, MaTAR24, and HOTAIR have been validated to densely link to breast cancer (Niknafs et al., 2016; Dong et al., 2018; Chang et al., 2020; Liang et al., 2020; Yang et al., 2022; Xue et al., 2016). HOTAIR has been reported to be highly expressed in non-small–cell lung cancer (NSCLC) and affect NSCLC tumorigenesis and metastasis. In addition, many biomarkers (for example, CA125, NSE, CEA, VEGF, and EGFR (Khanmohammadi et al., 2020) have been validated to associate with lung cancer.
More importantly, many machine learning methods, especially deep-learning methods, have been applied to identify lncRNA biomarkers of various diseases through lncRNA-disease association prediction. Thus, Fan et al. (2022) designed an LDA prediction method (GCRFLDA) using the graph convolutional matrix completion. Ma Y (Ma, 2022) exploited a deep multi-network embedding-based LDA inference framework. Wu et al. (2021) integrated graph auto-encoder and random forest for LDA prediction. Sheng et al. (2021) developed an attentional multi-level representation encoding method to find new LDAs combining convolutional and variance autoencoders. Zhao et al. (2022) proposed a heterogeneous graph attention network-based LDA identification model. These methods significantly improved the LDA prediction.
With the development of single cell RNA sequencing technologies (Peng et al., 2020b), we can obtain numerous RNA data. These data can improve the analyses of RNA data, for example, SARS-CoV-2 (Xu et al., 2020; Li et al., 2021). By finding new lncRNA biomarkers, we can design corresponding therapeutic strategies for lung cancer based on drug repositioning (Peng et al., 2015; Liu et al., 2020; Meng et al., 2022; Shen et al., 2022).
Although experimental methods found a few biomarkers for lung cancer, they are time-consuming and waste of resources. Therefore, computational techniques have been exploited to infer potential biomarkers for lung cancer. However, the majority of computational approaches need to improve the inference performance. In this study, to analyze the diagnostic, prognostic, and therapeutical potential of lncRNAs in lung cancer patients, we exploit a computational model combining Laplacian regularized least square and unbalanced bi-random walk, LDA-RLSURW, to predict possible lncRNA biomarkers for lung cancer.
First, the lncRNA-disease association dataset was collected. The dataset can be obtained from the lncRNADisease database at http://www.cuilab.cn/lncrnadisease (Chen et al., 2012). We obtained 82 lncRNAs, 157 diseases, and 701 associations after excluding lncRNAs without record in the lncRNADisease database and diseases with inappropriate names or without MeSH tree numbers.
This study developed an lncRNA-disease association prediction method LDA-RLSURW. First, LDA-RLSURW computed disease semantic similarity and lncRNA functional similarity. Second, LDA-RLSURW calculated the initial association probability of each lncRNA-disease pair using unbalanced bi-random walk based on disease similarity matrix and lncRNA similarity, respectively. In conclusion, the computed initial lncRNA-disease association probabilities were further updated Laplacian regularized least squares. The flowchart of LDA-RLSURW is presented in Figure 1.
Semantic similarity between diseases can be computed using the directed acyclic graph (DAGs) based on their MeSH descriptors (Fan et al., 2020). Given a disease
where
In Eq. 1, we assume that terms at one identical layer from
Considering this situation, we compute another semantic contribution value for disease
where
where
where
Thus, the contribution of all diseases in
In summary, the semantic similarity between diseases A and B can be computed by Eq. 6:
We calculate the lncRNA similarity using the approach provided by Fan et al. (2020). Assuming that DG(u)/DG(v) denotes diseases associated with lncRNA
Third, the similarity between DG(u) to DG(v) and one between DG(v) to DG(u) can be calculated by Eqs. 9 and 10:
In conclusion, the similarity between two lncRNAs
where
In this section, inspired by Shen et al. (2022), we consider that the lncRNA similarity network and the disease network and design an unbalance bi-random walk model to score lncRNA-disease pairs. The two networks exhibit different topological structures. Therefore, we use different optimal walking step sizes when randomly walking on these two networks. That is, we propose an unbalanced bi-random walk algorithm. First, we compute lncRNA-disease association scores by randomly walking with the maximal iteration number of
In Eq. 12, at each step, the lncRNA similarity is fused with the random walk step by multiplying
Second, we compute lncRNA-disease association scores by randomly walking with the maximal iteration number of
In Eq. 13, at each step, disease similarity is fused with the random walk step by multiplying
In the last section, we compute the association probability for each lncRNA and disease using unbalanced bi-random walk method. However, for the algorithm, the jump condition is determined by known LDA data and the two similarity matrices. For a node
where
Second, to optimize the above minimum problems, the loss functions in the lncRNA and disease spaces are defined based on Laplacian matrices
where
To comprehensively detect the effect of unbalanced bi-random walk on the inference performance, we replace
At the
In conclusion, the LDA-RLSURW calculates the association score for each lncRNA-disease pair by combining association scores from the lncRNA and disease networks using Eq. 24:
The semantic contribution weight
To assess the performance of our proposed LDA-RLSURW method, we compare it with other 10 classical LDA prediction methods, that is, LNCSIM1, LNCSIM2, ILNCSIM, and IDSSIM (Fan W. et al., 2020). LNCSIM1 and LNCSIM2 measured the disease similarity separately using DAGs and the information content and computed association score for each lncRNA-disease pair by Laplacian regularized least squares. IDSSIM designed novel lncRNA functional similarity and disease semantic similarity computation approaches and computed the lncRNA-disease association scores using the computed similarity matrices and weighed K nearest known neighbor method. Table 1 shows the AUC values of LDA prediction methods on the lncRNADisease dataset. From Table 1, we can see that LDA-RLSURW computes the best AUC, which demonstrates the powerful LDA prediction performance of LDA-RLSURW.
In this section, we conduct case studies to find potential lncRNA biomarkers for lung neoplasms, NSCLC, and adenocarcinoma of lung after confirming the performance of the proposed LDA-RLSURW method.
Lung neoplasms are one of the leading causes of death associated with malignant tumors in China (Khanmohammadi et al., 2020). Thus, Wang et al. (2020) investigated 14,528 lung cancer patients suffering from multiple primary malignant neoplasms (MPMN) and found 364 MPMN cases. In this section, we inferred the top 30 lncRNA biomarkers associated with lung neoplasms. The results are shown in Table 2 and Figure 2. From Table 2 and Figure 2, we can find that 15 lncRNAs are known to be associated with lung neoplasms in the lncRNADisease database, 3 lncRNAs (MINA, PVT1, and XIST) are unknown to be associated with lung neoplasms in the lncRNADisease database, which can be validated by the MNDR database (Cui et al., 2018). In addition, 12 lncRNAs are predicted to link to lung neoplasms and may be possible biomarkers of lung neoplasms.
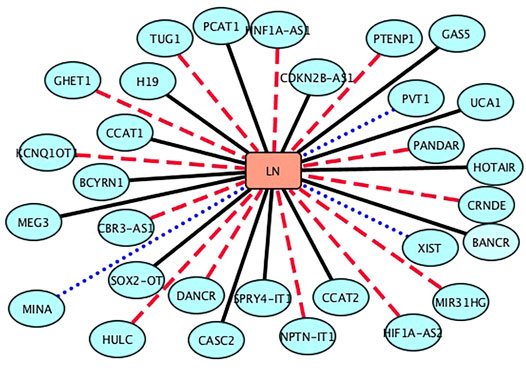
FIGURE 2. Associations between the inferred top 30 lncRNAs and lung neoplasms (LN). Black solid lines represent known LDAs in the lncRNADisease database. Blue-dot lines represent LDAs that can be observed in the MNDR database. Red-dash lines represent LDAs predicted to be potential lncRNA biomarkers of LN.
More importantly, we predict that lncRNA taurine-upregulated gene 1 (TUG1) may be associated with lung neoplasms. TUG1 is one of lncRNAs that were first identified to associate with human disease. It is linked to diverse physiological processes, for example, gene regulation involved in translation, post-translation, transcription, and post-transcription. In this section, we infer that TUG1 may be the biomarker of lung neoplasms (Guo et al., 2020).
The NSCLC is a subtype of lung cancer. It is one of the leading causes of cancer death in the United States and accounts for 85% of lung cancers among all its subtypes. Although we have achieved important advancements in the NSCLC treatment, our understanding about the biology and mechanisms of NSCLC progression and early detection is still superficial. In this section, we aim to infer new lncRNA biomarkers for NSCLC after confirming the performance of LDA-RLSURW. The predicted top 30 lncRNAs associated with NSCLC are presented in Table 3 and Figure 3. From Table 3 and Figure 3, we can find that 18 lncRNAs associated with NSCLC are known in the lncRNADisease database, 10 lncRNAs associated with NSCLC have been validated in the MNDR database, and 2 lncRNAs (MINA and PTENP1) associated with NSCLC are unknown and require validation. The lncRNA PTENP1 has exerted the tumor-suppressive function through modulating PTEN expression in multiple malignancies. We predict that the PTENP1 may be a potential biomarker of NSCLC (Herbst et al., 2018; Arbour and Riely, 2019; Fan et al., 2020; Leighl et al., 2019).
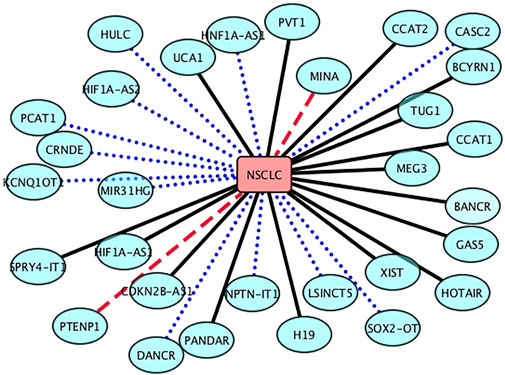
FIGURE 3. Associations between the inferred top 30 lncRNAs and NSCLC. Black solid lines represent known LDAs in the lncRNADisease database. Blue-dot lines represent LDAs that can be observed in the MNDR database. Red-dash lines represent LDAs predicted to be potential lncRNA biomarkers of LN.
The NSCLC is divided into three main subtypes: lung squamous cell carcinoma, large-cell lung cancer, and lung adenocarcinoma (LUAD), among which lung squamous cell carcinoma and LUAD are the most prevalent. In this section, we predict possible lncRNAs associated with LUAD. The results are shown in Table 4 and Figure 4. From Table 4 and Figure 4, we can find that 6 lncRNAs are known to associate with LUAD, 2 lncRNAs are not known to associate with LUAD in the lncRNADisease database, although they are known in the MNDR database, and 22 lncRNAs have not been confirmed to associate with LUAD.
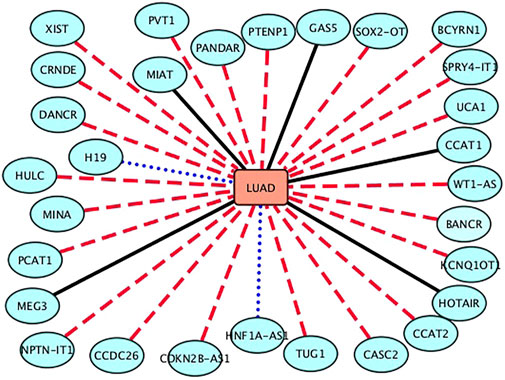
FIGURE 4. Associations between the inferred top 30 lncRNAs and LUAD. Black solid lines represent known LDAs in the lncRNADisease database. Blue-dot lines represent LDAs that can be observed in the MNDR database. Red-dash lines represent LDAs predicted to be potential lncRNA biomarkers of adenocarcinoma of lung.
Urothelial carcinoma associated 1 (UCA1) is an oncogenic lncRNA. It is highly expressed in many cancers. UCA1 can bind to tumor-suppressive microRNAs, activate a few pivotal signaling pathways, and alter epigenetic and transcriptional regulation. More importantly, its high expression is linked to poor clinicopathological characteristics. In this section, we predict that UCA1 may associate with LUAD and require validation (Yao et al., 2019).
LNCSIM1 and LNCSIM2 obtained better performance improvements based on cross-validation and case analyses. However, LNCSIM1 cannot effectively distinguish the semantic contributions of various disease terms from the identical layer. LNCSIM2 computed the IC values only through integrating DAG information. ILNCSIM is an edge-based prediction model. It combined the concept of information content and the hierarchical structure of DAGs to compute disease semantic similarity.
The RWRlncD conducted random walk with restart on the lncRNA similarity network. However, the RWRlncD cannot be used to predict associated information for diseases without any associated lncRNAs. The IRWRLDA improved random walk-based method through setting an initial probability vector to reduce the disadvantages of random walk with restart. The SIMCLDA used an inductive matrix completion model to complement missing LDA information. The LRLSLDA utilized Laplacian regularized least square model to predict LDAs. The LLCLPLDA first applied a locality-constraint linear coding model to project the local-constraint characteristics of lncRNAs and diseases, and then propagated LDAs by the initial LDA. The LDA-LNSUBRW used linear neighborhood similarity measurement and unbalanced bi-random walk algorithm to find possible LDAs.
The LDA-RLSURW obtains better performance for lncRNA-disease association prediction. It has three advantages: First, it utilizes the biological features to compute the lncRNA and disease similarity. Second, it uses unbalanced bi-random walk to compute the lncRNA-disease association probability. In conclusion, it further computes the lncRNA-disease association probability combining Laplacian regularized least squares.
Lung cancer is one of the most threatening cancer forms worldwide. In this study, we designed a computational method, LDA-RLSURW, to find possible lncRNA biomarkers for lung cancer. LDA-RLSURW effectively combines unbalanced bi-random walk and Laplacian regularized least square. We predict that TUG1, PTENP1, and UCA1 may be the biomarkers of lung neoplasms, NSCLC and LUAD, respectively.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conceptualization: ZG, YH, FK, and XL; methodology: ZG, YH, FK, and XL; project administration: XL; software: XL; writing original draft: ZG; writing review and editing: ZG and XL.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arbour, K. C., and Riely, G. J. (2019). Systemic Therapy for Locally Advanced and Metastatic Non-small Cell Lung Cancer. Jama 322 (8), 764–774. doi:10.1001/jama.2019.11058
Chandra Gupta, S., and Nandan Tripathi, Y. (2017). Potential of Long Non-coding RNAs in Cancer Patients: From Biomarkers to Therapeutic Targets. Int. J. Cancer 140 (9), 1955–1967. doi:10.1002/ijc.30546
Chang, K. C., Diermeier, S. D., Yu, A. T., Brine, L. D., Russo, S., Bhatia, S., et al. (2020). Matar25 Lncrna Regulates the Tensin1 Gene to Impact Breast Cancer Progression. Nat. Commun. 11, 1–19. doi:10.1038/s41467-020-20207-y
Chen, G., Wang, Z., Wang, D., Qiu, C., Liu, M., Chen, X., et al. (2012). LncRNADisease: a Database for Long-Non-Coding RNA-Associated Diseases. Nucleic acids Res. 41 (D1), D983–D986. doi:10.1093/nar/gks1099
Chen, X., Yan, C. C., Luo, C., Ji, W., Zhang, Y., and Dai, Q. (2015). Constructing lncRNA Functional Similarity Network Based on lncRNA-Disease Associations and Disease Semantic Similarity. Sci. Rep. 5 (1), 1–12. doi:10.1038/srep11338
Cui, T., Zhang, L., Huang, Y., Yi, Y., Tan, P., Zhao, Y., et al. (2018). MNDR v2.0: an Updated Resource of ncRNA-Disease Associations in Mammals. Nucleic Acids Res. 46 (D1), D371–D374. doi:10.1093/nar/gkx1025
de Groot, P. M., Wu, C. C., Carter, B. W., and Munden, R. F. (2018). The Epidemiology of Lung Cancer. Transl. Lung Cancer Res. 7 (3), 220–233. doi:10.21037/tlcr.2018.05.06
Dong, H., Wang, W., Chen, R., Zhang, Y., Zou, K., Ye, M., et al. (2018). Exosome-mediated Transfer of lncRNA-SNHG14 P-romotes T-rastuzumab C-hemoresistance in B-reast C-ancer. Int. J. Oncol. 53, 1013–1026. doi:10.3892/ijo.2018.4467
Fan W, W., Shang, J., Li, F., Sun, Y., Yuan, S., and Liu, J. X. (2020). IDSSIM: an lncRNA Functional Similarity Calculation Model Based on an Improved Disease Semantic Similarity Method. BMC Bioinforma. 21 (1), 1–14. doi:10.1186/s12859-020-03699-9
Fan, Y., Chen, M., and Pan, X. (2022). GCRFLDA: Scoring lncRNA-Disease Associations Using Graph Convolution Matrix Completion with Conditional Random Field. Brief. Bioinform 23 (1), bbab361. doi:10.1093/bib/bbab361
Guo, C., Qi, Y., Qu, J., Gai, L., Shi, Y., and Yuan, C. (2020). Pathophysiological Functions of the lncRNA TUG1. Curr. Pharm. Des. 26 (6), 688–700. doi:10.2174/1381612826666191227154009
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The Biology and Management of Non-small Cell Lung Cancer. Nature 553 (7689), 446–454. doi:10.1038/nature25183
Howlader, N., Forjaz, G., Mooradian, M. J., Meza, R., Kong, C. Y., Cronin, K. A., et al. (2020). The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 383 (7), 640–649. doi:10.1056/nejmoa1916623
Huang, L., Li, X., Guo, P., Yao, Y., Liao, B., Zhang, W., et al. (2017). Matrix Completion with Side Information and its Applications in Predicting the Antigenicity of Influenza Viruses. Bioinformatics 33 (20), 3195–3201. doi:10.1093/bioinformatics/btx390
Khanmohammadi, A., Aghaie, A., Vahedi, E., Qazvini, A., Ghanei, M., Afkhami, A., et al. (2020). Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 206, 120251. doi:10.1016/j.talanta.2019.120251
Leighl, N. B., Page, R. D., Raymond, V. M., Daniel, D. B., Divers, S. G., Reckamp, K. L., et al. (2019). Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non-small Cell Lung Cancer. Clin. Cancer Res. 25 (15), 4691–4700. doi:10.1158/1078-0432.ccr-19-0624
Li, T., Huang, T., Guo, C., Wang, A., Shi, X., Mo, X., et al. (2021). Genomic Variation, Origin Tracing, and Vaccine Development of SARS-CoV-2: A Systematic Review. Innovation 2 (2), 100116. doi:10.1016/j.xinn.2021.100116
Liang, Y., Song, X., Li, Y., Chen, B., Zhao, W., Wang, L., et al. (2020). Lncrna Bcrt1 Promotes Breast Cancer Progression by Targeting Mir-1303/ptbp3 axis. Mol. Cancer 19, 85–20. doi:10.1186/s12943-020-01206-5
Liu, C., Wei, D., Xiang, J., Ren, F., Huang, L., Lang, J., et al. (2020). An Improved Anticancer Drug-Response Prediction Based on an Ensemble Method Integrating Matrix Completion and Ridge Regression. Mol. Ther. - Nucleic Acids 21, 676–686. doi:10.1016/j.omtn.2020.07.003
Liu, H., Qiu, C., Wang, B., Bing, P., Tian, G., Zhang, X., et al. (2021). Evaluating DNA Methylation, Gene Expression, Somatic Mutation, and Their Combinations in Inferring Tumor tissue-of-Origin[J]. Front. Cell Dev. Biol. 9, 886. doi:10.3389/fcell.2021.619330
Ma, Y. (2022). DeepMNE: Deep Multi-Network Embedding for lncRNA-Disease Association Prediction[J]. IEEE J. Biomed. Health Inf. 26 (7), 3539–3549. doi:10.1109/JBHI.2022.3152619
Meng, Y., Lu, C., Jin, M., Xu, J., Zeng, X., and Jang, J. (2022). A Weighted Bilinear Neural Collaborative Filtering Approach for Drug Repositioning[J]. Briefings Bioinforma. 23 (2), bbab581. doi:10.1093/bib/bbab581
Niknafs, Y. S., Han, S., Ma, T., Speers, C., Zhang, C., Wilder-Romans, K., et al. (2016). The Lncrna Landscape of Breast Cancer Reveals a Role for Dscam-As1 in Breast Cancer Progression. Nat. Commun. 7, 1–14. doi:10.1038/ncomms12791
Peng, L., Liao, B., Zhu, W., Li, Z., and Li, K. (2015). Predicting Drug-Target Interactions with Multi-Information Fusion. IEEE J. Biomed. Health Inf. 21 (2), 561–572. doi:10.1109/JBHI.2015.2513200
Peng, L., Tan, J., Tian, X., and Zhou, L. (2022). EnANNDeep: An Ensemble-Based lncRNA–Protein Interaction Prediction Framework with Adaptive K-Nearest Neighbor Classifier and Deep Models[J]. Interdiscip. Sci. Comput. Life Sci. 14, 209–232. doi:10.1007/s12539-021-00483-y
Peng, L. H., Wang, C., Tian, X. F., Zhou, L. Q., and Li, K. Q. (2021b). Finding lncRNA-Protein Interactions Based on Deep Learning with Dual-Net Neural Architecture[J]. IEEE/ACM Trans. Comput. Biol. Bioinforma. 2021, 3116232. doi:10.1109/TCBB.2021.3116232
Peng, L. H., Yuan, R. Y., Shen, L., Gao, P. F., and Zhou, L. Q. (2021a). LPI-EnEDT: an Ensemble Framework with Extra Tree and Decision Tree Classifiers for Imbalanced lncRNA-Protein Interaction Data Classification[J]. BioData Min. 14 (1), 1–22. doi:10.1186/s13040-021-00277-4
Peng, L., Liu, F., Yang, J., Liu, X., Meng, Y., Deng, X., et al. (2020a). Probing lncRNA-Protein Interactions: Data Repositories, Models, and Algorithms. Front. Genet. 10, 1346. doi:10.3389/fgene.2019.01346
Peng, L., Tian, X., Tian, G., Xu, J., Huang, X., Weng, Y., et al. (2020b). Single-cell RNA-Seq Clustering: Datasets, Models, and Algorithms. RNA Biol. 17 (6), 765–783. doi:10.1080/15476286.2020.1728961
Roointan, A., Ahmad Mir, T., Wani, S. I., Mati-ur-Rehman, , , Hussain, K. K., Ahmed, B., et al. (2019). Early Detection of Lung Cancer Biomarkers through Biosensor Technology: A Review. J. Pharm. Biomed. analysis 164, 93–103. doi:10.1016/j.jpba.2018.10.017
Shen, L., Liu, F., Huang, L., Liu, G., Zhou, L., and Peng, L. (2022). VDA-RWLRLS: An Anti-SARS-CoV-2 Drug Prioritizing Framework Combining an Unbalanced Bi-random Walk and Laplacian Regularized Least Squares. Comput. Biol. Med. 140, 105119. doi:10.1016/j.compbiomed.2021.105119
Sheng, N., Cui, H., Zhang, T., and Xuan, P. (2021). Attentional Multi-Level Representation Encoding Based on Convolutional and Variance Autoencoders for lncRNA-Disease Association Prediction. Brief. Bioinform 22 (3), bbaa067. doi:10.1093/bib/bbaa067
Tian, X. F., Shen, L., Wang, Z. W., Zhou, L. Q., and Peng, L. H. (2021). A Novel lncRNA–Protein Interaction Prediction Method Based on Deep Forest with Cascade Forest Structure[J]. Sci. Rep. 11 (1), 1–15. doi:10.1038/s41598-021-98277-1
Wang, H., Hou, J., Zhang, G., et al. (2019). Clinical Characteristics and Prognostic Analysis of Multiple Primary Malignant neoplasms in patients with lung cancer[J]. Cancer Gene Therapy 26 (11), 419–426.
Wu, Q. W., Xia, J. F., Ni, J. C., and Zheng, C. H. (2021). GAERF: Predicting lncRNA-Disease Associations by Graph Auto-Encoder and Random Forest. Brief. Bioinform 22 (5), bbaa391. doi:10.1093/bib/bbaa391
Xu, J., Cai, L., Liao, B., Zhu, W., and Yang, J. (2020). CMF-impute: an Accurate Imputation Tool for Single-Cell RNA-Seq Data. Bioinformatics 36 (10), 3139–3147. doi:10.1093/bioinformatics/btaa109
Xue, X., Yang, Y. A., Zhang, A., Fong, K.-W., Kim, J., Song, B., et al. (2016). LncRNA HOTAIR Enhances ER Signaling and Confers Tamoxifen Resistance in Breast Cancer. Oncogene 35 (21), 2746–2755. doi:10.1038/onc.2015.340
Yang, J., Grünewald, S., and Wan, X.-F. (2013). Quartet-Net: A Quartet-Based Method to Reconstruct Phylogenetic Networks. Mol. Biol. Evol. 30 (5), 1206–1217. doi:10.1093/molbev/mst040
Yang, J., Ju, J., Guo, L., Ji, B., Shi, S., Yang, Z., et al. (2022). Prediction of HER2-Positive Breast Cancer Recurrence and Metastasis Risk from Histopathological Images and Clinical Information via Multimodal Deep Learning. Comput. Struct. Biotechnol. J. 20, 333–342. doi:10.1016/j.csbj.2021.12.028
Yang, J., Peng, S., Zhang, B., Houten, S., Schadt, E., Zhu, J., et al. (2020). Human Geroprotector Discovery by Targeting the Converging Subnetworks of Aging and Age-Related Diseases. Geroscience 42 (1), 353–372. doi:10.1007/s11357-019-00106-x
Yao, F., Wang, Q., and Wu, Q. (2019). The Prognostic Value and Mechanisms of lncRNA UCA1 in Human Cancer. Cancer Manag. Res. 11, 7685–7696. doi:10.2147/cmar.s200436
Yuan, M., Huang, L. L., Chen, J. H., Wu, J., and Xu, Q. (2019). The Emerging Treatment Landscape of Targeted Therapy in Non-small-cell Lung Cancer. Signal Transduct. Target Ther. 4 (1), 61–14. doi:10.1038/s41392-019-0099-9
Zhao, X., Zhao, X., and Yin, M. (2022). Heterogeneous Graph Attention Network Based on Meta-Paths for lncRNA-Disease Association Prediction. Brief. Bioinform 23 (1), bbab407. doi:10.1093/bib/bbab407
Zhou, L. Q., Duan, Q., Tian, X. F., Tang, J. X., and Peng, L. H. (2021a). LPI-HyADBS: a Hybrid Framework for lncRNA-Protein Interaction Prediction Integrating Feature Selection and Classification[J]. BMC Bioinforma. 22 (1), 1–31. doi:10.1186/s12859-021-04485-x
Keywords: lung cancer, lncRNA, biomarker, lncRNA-disease association, laplacian regularized least squares, unbalanced bi-random walk
Citation: Guo Z, Hui Y, Kong F and Lin X (2022) Finding Lung-Cancer-Related lncRNAs Based on Laplacian Regularized Least Squares With Unbalanced Bi-Random Walk. Front. Genet. 13:933009. doi: 10.3389/fgene.2022.933009
Received: 30 April 2022; Accepted: 03 June 2022;
Published: 22 July 2022.
Edited by:
Lihong Peng, Hunan University of Technology, ChinaCopyright © 2022 Guo, Hui, Kong and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxi Lin, TDE4MDQ3NjY2MDU5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.