- 1School of Medicine, Guizhou University, Guiyang, China
- 2School of Life and Health Sciences, Hangzhou Institute for Advanced Study University of Chinese Academy of Sciences, Hangzhou, China
- 3Department of Pediatrics, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 4State Key Laboratory of Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai, China
- 5NHC Key Laboratory of Pulmonary Immune-related Diseases, Guizhou Provincial People’s Hospital, Guiyang, China
- 6State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Autism spectrum disorder (ASD) is a heritable neurodevelopmental disorder with the underlying etiology yet incompletely understood and no cure treatment. Patients of fragile X syndrome (FXS) also manifest symptoms, e.g. deficits in social behaviors, that are core traits with ASD. Several studies demonstrated that a mutual defect in retinoic acid (RA) signaling was observed in FXS and ASD. However, it is still unknown whether RA replenishment could pose a positive effect on autistic-like behaviors in FXS. Herein, we found that RA signaling was indeed down-regulated when the expression of FMR1 was impaired in SH-SY5Y cells. Furthermore, RA supplementation rescued the atypical social novelty behavior, but failed to alleviate the defects in sociability behavior or hyperactivity, in Fmr1 knock-out (KO) mouse model. The repetitive behavior and motor coordination appeared to be normal. The RNA sequencing results of the prefrontal cortex in Fmr1 KO mice indicated that deregulated expression of Foxp2, Tnfsf10, Lepr and other neuronal genes was restored to normal after RA treatment. Gene ontology terms of metabolic processes, extracellular matrix organization and behavioral pathways were enriched. Our findings provided a potential therapeutic intervention for social novelty defects in FXS.
Introduction
Individuals with autism spectrum disorder (ASD) show early-onset social dysfunction and abnormally restricted, repetitive behaviors (Lord et al., 2018). ASD affects approximately one in 44 children, and the incidence is 4-fold higher in males than females (Maenner et al., 2021). The causes of autism are complex, including environmental, genetic and metabolic factors (Peça et al., 2011; Krakowiak et al., 2012; Modabbernia et al., 2017). Fragile X syndrome (FXS) is a X-linked hereditary intellectual disability associated with ASD. FXS mainly results from the abnormal CGG amplification (>200 repeats) of the fragile X mental retardation 1 (Fmr1) gene that leads to loss of the expression of fragile X intellectually retarded protein (FMRP) (Saldarriaga et al., 2014). FMRP is an RNA-binding protein that regulates the synaptic development and plasticity (Richter and Zhao, 2021). FXS is the most common genetic cause of ASD, accounting for about 2–6% of the cases (Hogan et al., 2017), and approximately 30% of FXS patients are also diagnosed with ASD (Hagerman and Harris, 2008). Shared symptoms between FXS and ASD, such as repetitive behaviors and social deficits (Kazdoba et al., 2014), indicate an overlap of molecular mechanisms in these diseases (Salcedo-Arellano et al., 2021).
All-trans retinoic acid (RA) is a naturally occurring metabolite from retinol (vitamin A) (Kumar and Duester, 2011). As a critical signaling molecule, RA is involved in synaptic plasticity, neuronal differentiation and brain maturation (Aoto et al., 2008; Chen et al., 2014). Disruption of RA signaling is closely related to the abnormal patterns of the central nervous system, especially the synaptic plasticity homeostasis (Chen et al., 2014). Study in Fmr1 knock-out (KO) mice, a disease model of FXS, indicated an interaction between FMRP and retinoic acid receptor alpha (RARα), an essential component in RA signaling (Park et al., 2021). Furthermore, RA-mediated synaptic strength regulation was abolished in Fmr1 KO hippocampal neurons and FXS patient-derived induced pluripotent stem (iPS) cells, thus leading to abnormal synaptic function (Zhang et al., 2018; Zhong et al., 2018). These studies suggested that FXS might result from impaired synaptic plasticity homeostasis caused by dysregulation of RA signaling. Most recently, we have discovered that RA synthesis and RA signaling were down-regulated in the mouse ASD model induced by excessive UBE3A expression, and the ASD-like behaviors caused by repression in RA signaling were successfully ameliorated by oral supplementation of RA in mice (Xu et al., 2018). It was fascinating to ask whether RA replenishment would have beneficial effects on core traits of ASD in Fmr1 KO mice.
In this study, we have thus first examined whether RA signaling was indeed down-regulated when the expression of FMR1 was disrupted. Subsequently, we went on to directly test the effect of RA supplementation on the social deficits manifested by the Fmr1 KO mice, and investigated the potential molecular mechanism by analyzing RNA-seq data. Our findings provide RA replenishment as a potential therapeutic intervention for the social novelty deficit in FXS.
Materials and Methods
Animals
Fmr1(-/y) (Fmr1 KO) mice (aged 2–3 months, FVB background) were gifted from Prof. Chen Zhang of Capital Medical University, Beijing. These mice were then backcrossed for ten generations to our C57BL/6J strain. This Fmr1 strain was maintained in C57 background. To obtain hemizygous males and WT males, heterozygous females and wildtype males were intercrossed. Mice were housed in a specific-pathogen-free (SPF) facility with 12-h light/dark cycle and ad libitum access to food and water. Per cage was housed three to five mice by genotype. All animal experiments were performed strictly in accordance with the instructions of the Institutional Animal Care and Use Committee (IACUC) at the Center for Excellence in Molecular Cell Science, CAS.
Plasmid Construction
The pGL4-RARE-TK-EGFP-CLPEST plasmid was modified based on pCBG99-Control (Promega) plasmid. The DNA fragment between the two polyA signals, including SV40 promoter, Puromycin and polyA signals, were amplified by PCR from pGL4.22-RARE-TK-luciferase (Xu et al., 2018) plasmid and inserted into pCBG99-Control between XmaⅠ and BamHⅠ. The CL1-PEST sequence from pGL4.22-RARE-TK-luciferase was amplified together with EGFP from pEGFP-N1 plasmid before inserted between XmaⅠ and NheⅠ. The retinoic acid response element (RARE) together with thymidine kinase (TK) promoter were amplified from pGL4.22 and inserted between KpnⅠ and NheⅠ.
The pGL4-RARE-TK-EGFP-CLPEST plasmid contains three copies of DR5 (direct repeat with 5 bp of spacing) variant of RARE in different directions (one in forward direction and the other two in reverse direction according to the sequence) and a EGFP reporter gene with CL1-PEST sequence, which could promote the degradation of EGFP and hence result in rapid turnover of the reporter.
Cell Line and Transfection
SH-SY5Y(ATCC) cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Corning) supplemented with 10% fetal bovine serum (FBS, Gibco) and 50 μg/ml penicillin/streptomycin (Life Technologies). Cells were maintained at 37°C in a saturated humidity atmosphere containing 5% CO2.
SH-SY5Y cells were transfected with indicated plasmids and siRNAs using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The siRNA sequences were listed below (Khalil et al., 2008):
si-FMR1-1-F: GGGUGAGUUUUAUGUGAUA
si-FMR1-1-R: UAUCACAUAAAACUCACCC
si-FMR1-2-F: GGAUGAUAAAGGGUGAGUU
si-FMR1-2-R: AACUCACCCUUUAUCAUCC.
Flow Cytometry
The cells transfected with siRNAs and plasmids for 48 h were digested and suspended with PBS, before subjected to flow cytometry analysis on Beckman CytoFlex. GFP positive cells were selected and calculated for the proportion. The fluorescence intensity of all live, single cells was also recorded for further analysis.
RA Administration
Mice (4 weeks old) were administered daily with RA (Sigma, USA) dissolved in olive oil (Aladdin, China) via oral gavage for 1 month, at the dosage of 5 mg/kg. The control group received the olive oil only. Body weights of the mice were measured every 2 days.
Behavioral Analysis
Male mice at 8 weeks of age were subjected to behavioral tests. Mice were tested at a room with lighting maintained at 230 Lux. Before the experiments began, mice were transferred to the testing room and acclimated for at least 1 h. After each test, wipe the instrument with 75% ethanol to remove any residual odors which may affect subsequent tests. All the behavioral experiments except for self-grooming test and rotarod test were tracked by EthoVision XT (Noldus) tracking system. All data acquisition and analysis were carried out by an individual blinded to the genotype and treatment.
(1) Self-grooming test
The Self-grooming test was performed as previously described (Wang et al., 2020). Mice were placed individually to a clean cage covered with beddings (∼0.5 cm). Prior to the test, animals were allowed to habituate to the novel environment for 10 min. Then the time spent in grooming behaviors was recorded for 10 min. All instances of face-wiping, head and ears scratching/rubbing, and full-body grooming were counted as grooming behavior.
(2) Three-chamber social test
The Three-chamber social test was executed according to previously reported with minor modifications (Rein et al., 2020). In brief, a transparent acrylic box (60 cm × 40 cm × 20 cm) was equally divided into three chambers with removable doors in each partition. Two days prior to the test, the stranger mice (sex and age were matched with test mice) were habituated to the wire cages for 1 h per day. The test mouse was introduced to the central chamber to explore the apparatus freely for 10 min for habituation prior to the experiment.
In the sociability test phase, a stranger mouse (stranger Ⅰ) and an inanimate object were placed into the right and left cages, respectively. The test mouse was allowed to explore all three chambers freely for 10 min and the amount of time spent in each chamber was recorded. Then the test mouse was asked to spend an extra 5 min in the stranger I chamber to get more familiar with stranger I before the next phase.
In the social novelty test phase, the inanimate object was replaced with a novel mouse (stranger II). Similarly, the test animal was allowed to freely explore all three sections of the apparatus for 10 min and the amount of time spent in each chamber was recorded. The sociability preference index = (time spent in stranger I chamber-time spent in object chamber)/(total time in the two chambers); social novelty preference index = (time spent in stranger II chamber-time spent in stranger I chamber)/(total time in the two chambers).
(3) Open-field test
Locomotor activity was evaluated in an acrylic box (40 cm × 40 cm × 40 cm, Med Associates) and videotaped by an overhead camera. The mouse was initially placed in the center of the device and allowed to explore the arena freely for 10 min. The central zone is defined as a 20 cm × 20 cm area in the center of the bottom. The distance travelled and average speed were measured by EthoVision XT (Noldus) tracking system.
(4) Rotarod test
To assess motor coordination and balance, mice were placed on a rotarod apparatus (Columbus Instruments) that accelerates from 4 to 40 rpm for 5 min. The latency to fall was automatically recorded by the infrared detection system. Each mouse was tested for three trials, with 1–2 h between trials in the same day.
RNA Sequencing
Total RNA samples were extracted from the PFC tissues with Trizol reagent (Tiangen, China) according to the manufacturer’s instruction. PFC tissues from 2 mice of the same genotype and treatment were pooled together as one sample. A total of three samples from six mice in each group were used for high-throughput sequencing. Differential expression was determined using DESeq2. The differentially expressed genes (DEGs) were determined by using 1.5-fold change, with p value <0.05 as threshold. GO enrichment analysis of the identified DEGs was performed with ‘clusterProfiler ’package in R. Volcano plots, heatmap and dot plot were drawn in RStudio with the ‘ggplot2’ packages. The generated RNA-seq data have been deposited in the Gene Expression Omnibus (The GEO accession number is: GSE201672).
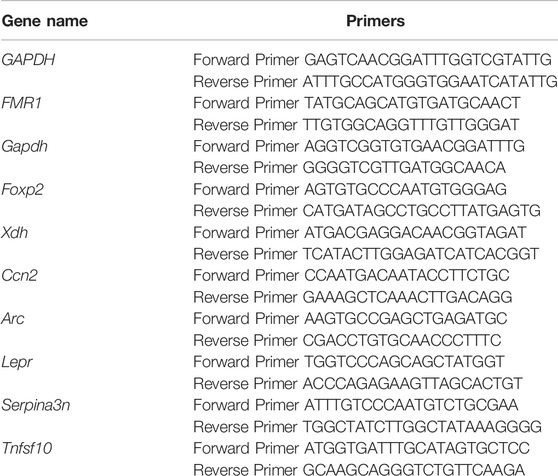
Quantitative Real-Time PCR
Total RNA was converted to complementary DNA (cDNA) by using the HiScript® III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) amplifications of various genes were performed using ChamQ universal SYBR qPCR Master Mix (Vazyme, China) in a Roche LightCycler® 384 (Roche, Switzerland). The relative expression level of each transcript was normalized to Gapdh using the 2ΔΔCt method. Sequences for the primers used in this study were listed below. All data were obtained from three independent experiments.
Western Blot
Protein lysates from tissues were extracted using RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate and 1% NP-40 pH 7.6), supplemented with protease inhibitor cocktail and quantified with a BCA kit (Beyotime, China). The protein lysates were denatured at 100°C for 10 min in 1× SDS loading buffer and then separated by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, United States) and blocked in 10% fat-free milk for 1 h at room temperature. Then the membranes were immunoblotted with the primary antibodies overnight at 4°C: anti-FMRP (1:1000, Abcam, ab17722); anti-GAPDH (1:3000, Proteintech, 60004-1-Ig). The corresponding HRP-conjugated secondary antibodies were used at room temperature for 1 h to detect the primary antibody and finally visualized with ECL Western Blotting Reagent (Tanon, Shanghai, China) using Tanon 5200 Imaging System.
Statistics
Data were analyzed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, United States). Statistical tests were conducted as stated in the figure legends. Values are presented as means ± SEM.
Results
To investigate whether RA signaling was down-regulated when the expression of FMR1 was decreased, we first knocked down FMR1 gene expression by siRNA in SH-SY5Y cells, a commonly used cell line in RA research (Cheung et al., 2009). The mRNA level (Figure 1A) and protein level (Figure 1B) of FMR1 were markedly decreased in cells transfected with siRNAs targeting FMR1. We then co-transfected SH-SY5Y cells with siRNA and a GFP reporter, the expression of which was driven by RA-response element (RARE), to examine the RA signaling (Figure 1C). The proportion of GFP positive cells (Figure 1D) and the mean value of GFP fluorescence intensity (Figure 1E) were decreased in cells with FMR1 siRNAs. Overall, these results suggested that RA signaling was indeed down-regulated in cells with decreased level of FMR1.
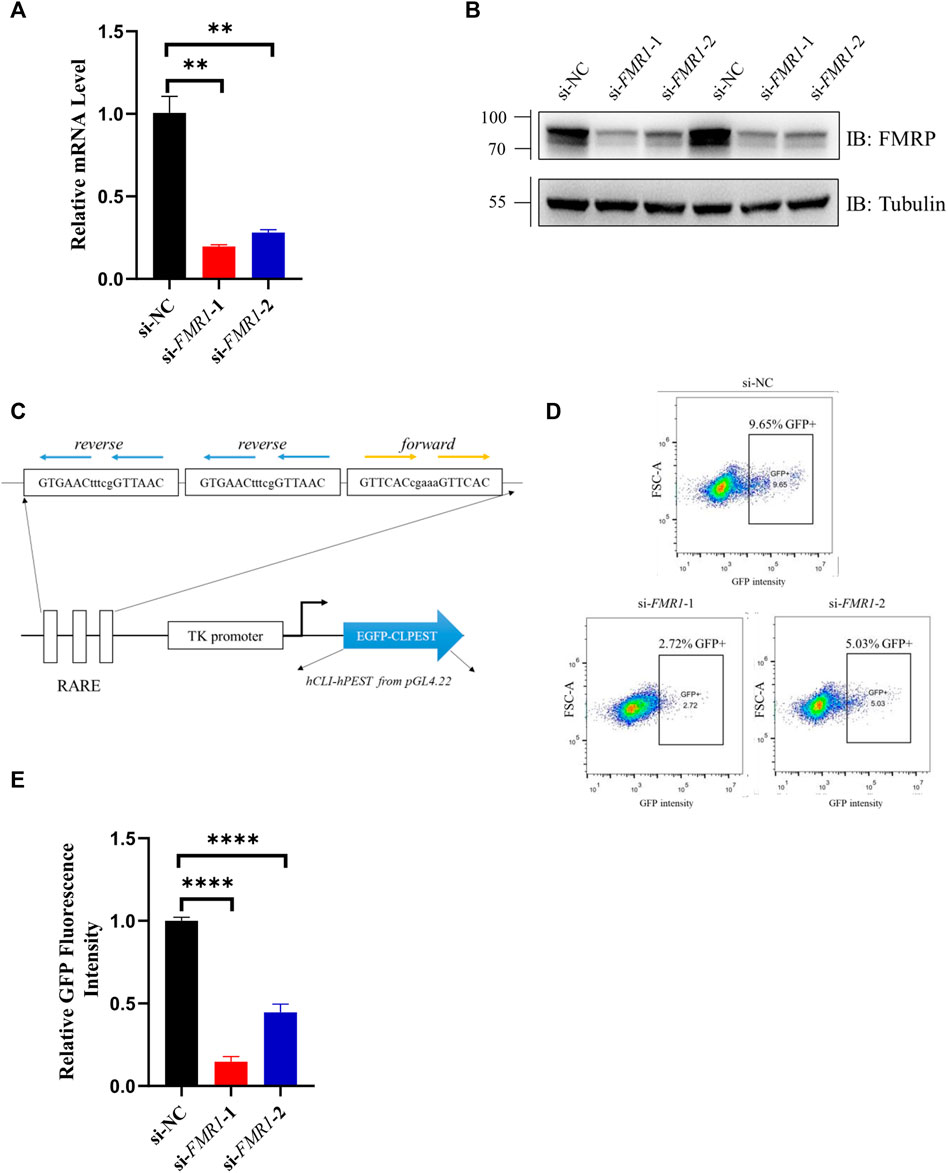
FIGURE 1. RA-induced gene expression was down-regulated in FMR1 knockdown cells. (A,B) Analysis with (A) RT-qPCR and (B) Western blotting for the effects of siRNAs on the expression level of FMR1 in SH-SY5Y cells. (C) Schematic diagram of 3×RARE-EGFP reporter construct. The expression of EGFP was regulated by three copies of RA-response element (RARE). CL1PEST sequence was attached to the C terminal of EGFP to promote its turnover. (D) Flow cytometry analysis of SH-SY5Y cells transfected with RARE-EGFP reporter and indicated siRNAs. The proportion of GFP positive (GFP+) cells were noted (E). Quantification of normalized GFP fluorescence intensity from flow cytometry analysis. n = 3 biological replicates. Data are presented as means ± SEM. **p < 0.01, ****p < 0.0001. One-way ANOVA with Dunnett post hoc test.
We used male Fmr1 KO mice (Fmr1(-/y)) and their wild-type (WT) littermates to explore the effect of RA exerting on the behaviors. Both WT and KO mice were treated with RA by oral gavage at 5 mg/kg/day or olive oil as control from 4 weeks of age for 1 month as previously described (Pasqualetti et al., 2001; Xu et al., 2018), followed by behavior tests at postnatal day 58 (Figure 2A). During the intragastric administration, no significant weight differences were observed among the four groups of mice (WT + Oil, WT + RA, KO + Oil, KO + RA) (Figure 2B). The shared symptoms between FXS and ASD are impaired social skills and repetitive, stereotyped behaviors (Kazdoba et al., 2014), which were tested by the Three-chamber social task and Self-grooming task. Compared with WT mice, Fmr1 KO mice spent comparable time with an object or a live mouse regardless of RA administration (Figures 2C,D), manifesting impaired sociability (Figure 2E). WT mice spent longer time with a novel mouse (stranger II) than with a familiar mouse (stranger I), while Fmr1 KO mice, if not treated with RA, spent similar time in each chamber (Figures 2F,G), showing defects in social novelty behavior (Figure 2H). The supplementation of RA, however, significantly increased the time that KO mice spent with a novel stranger, thus restoring the defective social novelty behavior (Figures 2F,H). We found that RA supplementation could rescue the deficits in social novelty, yet not in sociability, of Fmr1 KO mice, without significantly changing the behaviors of the WT mice.
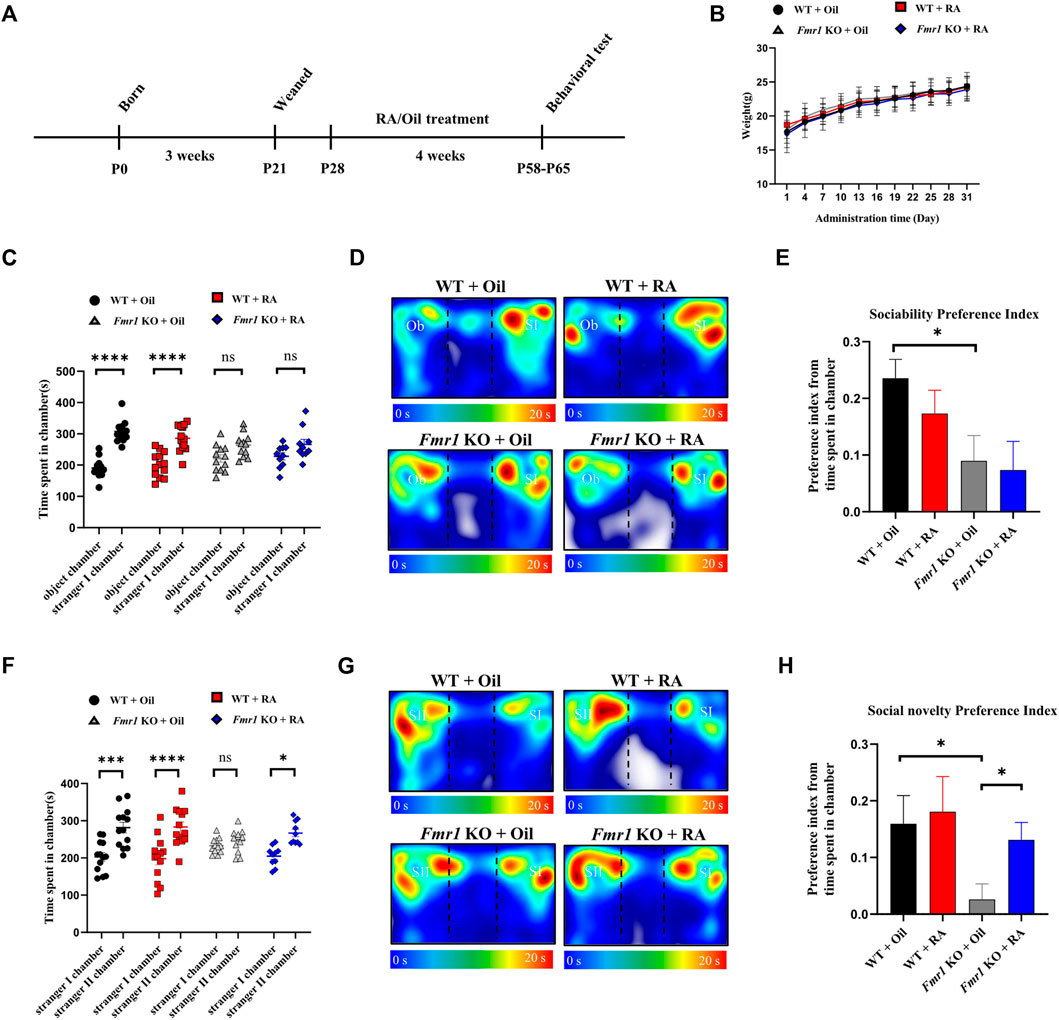
FIGURE 2. RA administration restores social novelty behavior in Fmr1 KO mice. (A) Schematic diagram of experimental design. WT and Fmr1 KO male mice were orally administered with Oil or RA (5 mg/kg/d) for 4 weeks, before subjected to behavioral tests at postnatal day P58-P65. (B) Body weights of mice recorded every 4 days for 1 month after Oil or RA administration. WT + Oil (n = 13), WT + RA (n = 13), Fmr1 KO + Oil (n = 13), Fmr1 KO + RA (n = 12). (C–E) Time spent in each chamber (C), representative heat map (D) and the preference index (E) for the sociability test performed with 4 groups of mice. The Ob and SI indicate the object and stranger Ⅰ, respectively. WT + Oil (n = 13), WT + RA (n = 13), Fmr1 KO + Oil (n = 12), Fmr1 KO + RA (n = 10). (F–H) Time spent in each chamber (F), representative heat map (G) and the preference index (H) for the social novelty test performed with 4 groups of mice. The SI and SII indicate the stranger Ⅰ and stranger Ⅱ, respectively. WT + Oil (n = 13), WT + RA (n = 13), Fmr1 KO + Oil (n = 12), Fmr1 KO + RA (n = 10). Data are presented as means ± SEM. *p < 0.05, ***p < 0.001, ****p < 0.0001, ns, not significant. (B,C,F) Two-way ANOVA with Bonferroni post hoc test; (E,H) Unpaired two-tailed t test with Welch’s correction.
We then examined the repetitive behavior, the other core symptom of ASD, in Fmr1 KO mice. We found no significant difference in self-grooming time, a manifestation of repetitiveness, either with or without RA treatment (Figure 3A). In addition to the behavioral study of mutual symptoms mentioned above, motor activity and coordination in Fmr1 KO mice were also detected. Consistent with previous studies (Ding et al., 2014; Gantois et al., 2017; Nolan et al., 2017), KO mice showed increased travel distance and average speed in the open field test, indicating hyperactivity, which was not ameliorated by RA (Figures 3B–D). The time spent in central area (Figure 3E) and the ratio of total distance travelled in the central area (Figure 3F) were significantly increased in Fmr1 KO mice, suggesting that Fmr1 KO mice manifested reduced anxiety-like behavior compared with the WT mice, which was consistent with other reports (Yan et al., 2004; Zieba et al., 2019). The motor coordination was not significantly affected by either elimination of Fmr1 expression or RA treatment (Figure 3G). Taken together, these results suggest that RA supplementation can alleviate the defects in social novelty, but not in sociability or hyperactivity, in Fmr1 KO mice.
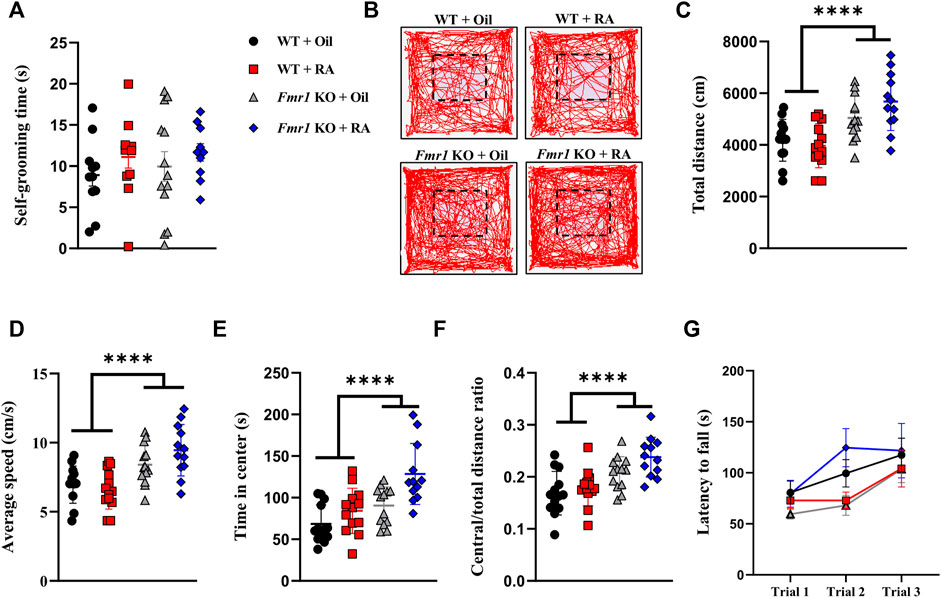
FIGURE 3. RA administration does not affect repetitive behavior, locomotion, or motor coordination in Fmr1 KO mice. (A) Time spent in self-grooming for repetitive behavioral test performed with 4 groups of mice. WT + Oil (n = 11), WT + RA (n = 12), Fmr1 KO + Oil (n = 13), Fmr1 KO + RA (n = 10). (B–F) Representative activity traces (B), total distance moved (C), average speed (D), time spent in center (E), and ratio of central distance to total distance (F) in the open-field test performed with 4 groups of mice. The dotted line indicates the central area. WT + Oil (n = 13), WT + RA (n = 13), Fmr1 KO + Oil (n = 13), Fmr1 KO + RA (n = 12). (G) Latency to falling in the rotarod test performed with 4 groups of mice. WT + Oil (n = 11), WT + RA (n = 12), Fmr1 KO + Oil (n = 13), Fmr1 KO + RA (n = 11). Data are presented as means ± SEM. ****p < 0.0001. (A) One-way ANOVA with Bonferroni post hoc test; (C–G) Two-way ANOVA with Bonferroni post hoc test.
The behavioral results have revealed the therapeutic potential of RA for rescuing aberrant social novelty behavior. Since RA treatment did not restore the protein level of FMRP in the hippocampus or prefrontal cortex (PFC) of Fmr1 KO mice (Figure 4A), we performed RNA sequencing (RNA-seq) of PFC samples from three groups of mice (WT + Oil, KO + Oil, KO + RA) to acquire further insight into the underlying mechanisms of RA treatment. The PFC region has been shown to be one of the primary brain regions that regulating social behaviors (Amodio and Frith, 2006; Brumback et al., 2018). Differentially expressed genes (DEGs) (∣FoldChange∣> 1.5, p value <0.05) were identified by comparing the sequencing results between WT + Oil and KO + Oil, as well as KO + Oil and KO + RA. As shown in the Venn diagram (Figure 4B), 293 and 263 DEGs were found, respectively, with 57 of them overlapped (Detailed information in Supplementary Table S1). In specific, compared with WT + Oil group, 110 genes were up-regulated and 183 genes were down-regulated in KO + Oil group. While compared with KO + Oil group, there were 149 up-regulated genes and 114 down-regulated genes in KO + RA group (Figure 4C). Our intention was to find out the DEGs in the KO + Oil group, of which the expression levels were restored to the similar level as those in the WT + Oil group after RA supplementation. As shown in Figures 4D, 56 out of 57 overlapped DEGs (except Tagap, T cell activation RhoGTPase activating protein) meet the criteria mentioned above, including some autism-related genes (Simons Foundation Autism Research Initiative, SFARI), such as period circadian clock 1 (Per1), period circadian clock 2 (Per2) and forkhead box P2(Foxp2). Several DEGs associated with neuronal functions were also identified (Figure 4C). The abnormal increase in the expression of activity-regulated cytoskeleton-associated protein (Arc), cellular communication network factor 2 (Ccn2), forkhead box P2 (Foxp2), and xanthine dehydrogenase (Xdh) in Fmr1 KO mice was down-regulated after RA administration, while the expression level of leptin receptor (Lepr), serine peptidase inhibitor clade A member 3N (Serpina3n) and tumor necrosis factor superfamily member 10 (Tnfsf10) was increased to normal as WT. These findings were verified by quantitative Real-time PCR (qRT-PCR) (Figure 4E). In order to probe the functional associations of DEGs caused by RA supplementation, we performed Gene Ontology (GO) enrichment analysis on the DEGs between KO + Oil and KO + RA groups, and identified significant changes in 40 terms of Biological Process (BP) (P, adjust <0.05, complete list in Supplementary Table S2; top 20 pathways in Figure 4F). The most enriched pathways were various metabolic processes, such as glycogen metabolic, glucan metabolic and glutathione metabolic, etc. Besides, the pathways associated with memory, cognition, eating behavior and extracellular matrix organization were also enriched. The alterations of these pathways were previously implicated in FXS (Lumaban and Nelson, 2015; O’Leary and Nolan, 2015; Reinhard et al., 2015; Bostrom et al., 2016; Westmark, 2021). Collectively, these results suggested that RA alleviated defective social novelty behavior in Fmr1 KO mice possibly through restoring anomalous expressed genes and biological processes to normal.
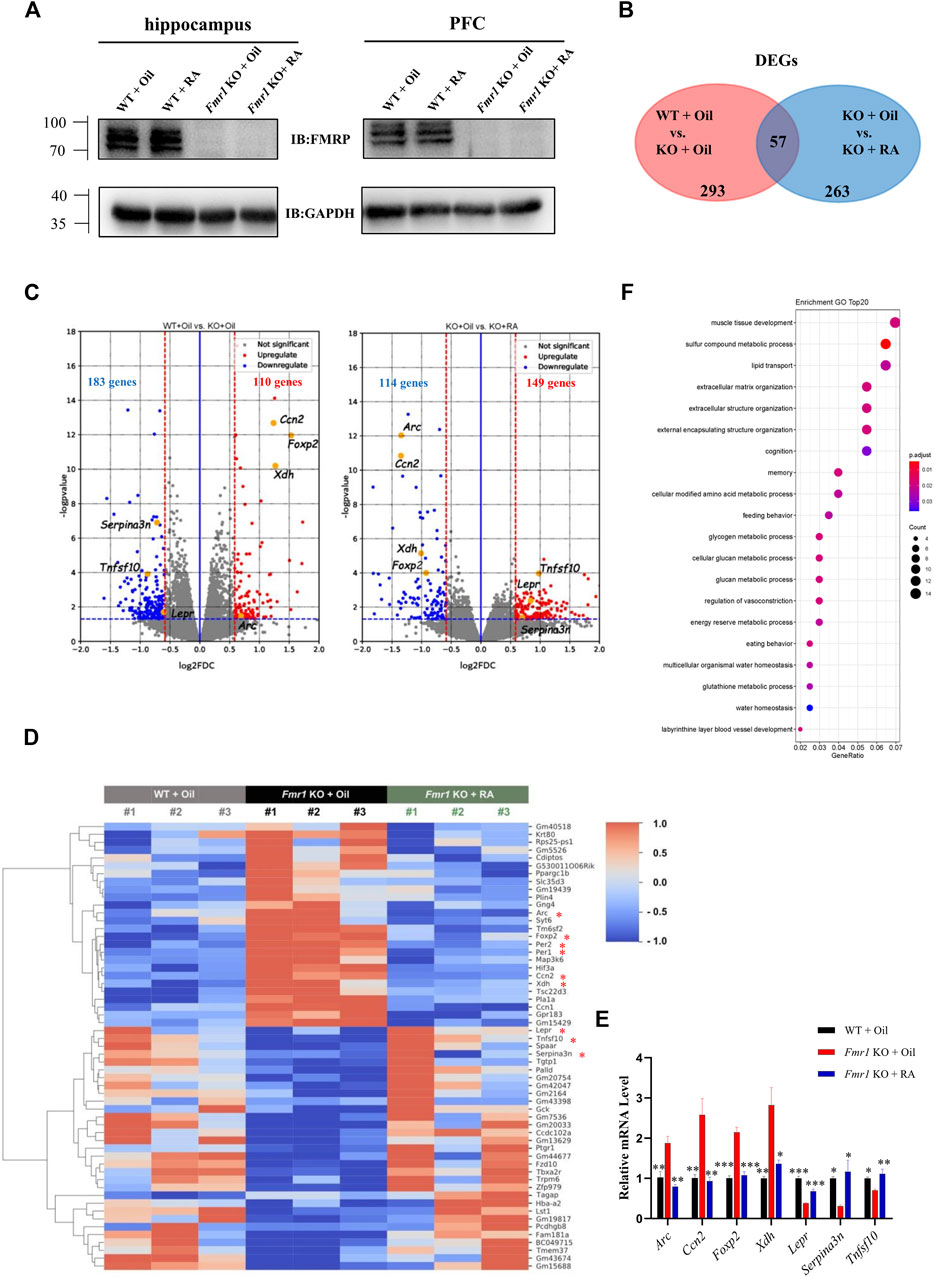
FIGURE 4. RA administration restores mRNA expression in the PFC of Fmr1 KO mice. (A) Representative western blots of FMRP proteins in the hippocampus and prefrontal cortex from 4 groups of mice. (n = 3 per group). (B) Venn diagram for overlap analysis (57 genes) between differentially expressed genes (DEGs) in WT + Oil vs KO + Oil groups (293 genes) and DEGs in KO + Oil vs KO + RA groups (263 genes). (C) Volcano plots for differentially expressed genes (DEGs). Left: WT + Oil and KO + Oil groups; Right: KO + Oil and KO + RA groups. Blue dots represent down-regulated genes while red dots represent upregulated genes. The blue dashed line indicates p = 0.05. The red dashed lines indicate ∣FoldChange∣> 1.5 (∣log2(FoldChange)∣> 0.5849). (n = 3 pooled from six mice per group). (D) Heatmap represents the changes in expression of overlapped genes (57 genes). Blue stripes indicate low expression level; red stripes indicate high expression level. Genes mentioned in the main texts were marked with an asterisk. (E) Quantitative real-time PCR analysis of indicated genes mRNA expression in the prefrontal cortex of mice from WT + Oil, KO + Oil and KO + RA groups. (n = 3 samples pooled from six mice. (F) Top 20 biological process (BP) pathways in the Gene Ontology (GO) enrichment analysis. Data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. (E) One-way ANOVA with Bonferroni post hoc test.
Discussion
Fmr1 is a strong candidate gene associated with ASD, and its deficiency was implicated in autism development (Niu et al., 2017). Several potential treatments have been proved to be able to alleviate abnormalities in Fmr1 KO mice through different pathways. Application of dopamine rescued the impaired social novelty behaviors by reduction of striatal tyrosine hydroxylase protein (Chao et al., 2020). Weekly treatment with purinergic antagonist suramin restored the social behaviors by regulating purinergic signaling (Naviaux et al., 2015). Metformin, a widely-used anti-diabetic drug, was found to rescue the social novelty deficit, repetitive behaviors, and abnormal incidence of seizures in Fmr1 KO mice through normalizing ERK signaling (Gantois et al., 2017). Recently, increasing amount of evidence has suggested an association between impaired RA signaling and ASD (Pavăl et al., 2017; Chen et al., 2018; Zhou and Li, 2018; Hao et al., 2019). Furthermore, the perturbation of synaptic plasticity homeostasis mediated by RA was observed in Fmr1 KO mice and FXS patient-derived induced pluripotent stem (iPS) cells (Soden and Chen, 2010; Zhang et al., 2018). This prompted us to investigate the role that RA plays in FXS behavioral traits.
Our work demonstrated that Fmr1 KO mice displayed atypical social behaviors and hyperactivity, yet no defect in repetitive behavior or motor coordination was noted. RA replenishment rescued social novelty behavior, probably due to the normalization of anomalous gene expression and defective pathways. Since the synaptic plasticity homeostasis mediated by RA was abolished in Fmr1 KO neuron (Soden and Chen, 2010; Sarti et al., 2013), the treatment with RA could not increase the mEPSC amplitude as in WT neurons. This suggested that the improvement in social novelty behavior induced by RA might not result from the restoration in synaptic strength, but from a transcriptional regulation of neuronal genes. Therefore, we performed RNA-seq and identified several genes associated with behavioral traits.
Specifically, the mRNA level of Ccn2, the connective tissue growth factor that negatively regulates myelination (Ercan et al., 2017), was restored to normal after RA administration. Foxp2, a transcription suppressor related to the social defects in ASD patients (Chien et al., 2017), was found irregularly increased in Fmr1 KO mice. Its excessive expression could result in transcription inhibition of mesenchymal-epithelial transition factor (MET) and lead to abnormal neuronal differentiation and growth (Mukamel et al., 2011). The mRNA level of Lepr, whose insufficient level could cause impaired social interaction (Meyer et al., 2014), was decreased when the Fmr1 gene was knocked out. Tnfsf10, also known as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), encodes a membrane-bound cytokine that induces cellular apoptosis (Park et al., 2015). Research implied a contribution from defective programed cell death to the excessive synaptic connections in Fmr1 mutants and behavioral phenotype of children with FXS (Gatto and Broadie, 2011; Cheng et al., 2013), which was also in accordance with our finding that Tnfsf10 mRNA level was significantly decreased in Fmr1 KO mice. RA supplementation restored the expression of these genes (Figure 4E) and normalized neuronal function, which might ameliorate social behaviors in the end.
The biological process pathways enriched in the GO analysis were also found related to the FXS. For instance, extracellular structure plays a pivotal role in neurite outgrowth, neural connectivity, and synaptic plasticity (Cope and Gould, 2019; Peteri et al., 2021). Alterations in connected tissue and extracellular matrix (ECM) have been implied in the pathophysiological development of FXS (Ramírez-Cheyne et al., 2019). According to our GO analysis result, three GO terms concerning extracellular structure were enriched within TOP six terms. This suggested that RA treatment might significantly improve the neural connectivity in the altered ECM from the PFC in Fmr1 KO mice.
The enrichment in glycogen and glucan metabolic process pathways induced by RA (Figure 4F) brought our attention to glycogen synthase kinase 3 (GSK3), the inhibition of which was proved to improve the impaired behaviors of ASD and FXS (Franklin et al., 2014; McCamphill et al., 2020; Rizk et al., 2021). Some reports demonstrated that inhibition of GSK3 could enhance retinoic acid receptor activity (Si et al., 2011). These researches indicated a potential link between enhanced RA signaling and restored symptoms in FXS. It is worth mentioning that knockout of Fmr1 or supplementation of RA did not significantly change the mRNA level of Gsk3a or Gsk3b (data not shown). A crosstalk with RA signaling and GSK3 activity might exist.
The impairment of cognitive abilities and infant diet, was previously implicated in the individuals with FXS (Bostrom et al., 2016; Westmark, 2021). The corresponding GO terms of them, cognition and eating behaviors, were also enriched after RA administration (Figure 4F).
RA has been used for the treatment of several diseases, like acute myelocytic leukemia (Stahl and Tallman, 2019) and skin disorders (Szymański et al., 2020), which suggests the safety of RA and its potential to be used for other diseases. The challenge here is that RA has poor solubility in aqueous solutions, so it is rather difficult to reach an effective concentration in tissues, like brain (Ferreira et al., 2020). In order to increase the stability of RA in human body and the selectivity against RARs, synthetic retinoids have been developed for clinical trials of neurological diseases, for example, Alzheimer’s disease (Wołoszynowska-Fraser et al., 2020). These studies shed light on the possibility of RA treatment in FXS and ASD patients in the future.
Although many questions remained to be addressed, our findings that RA supplementation improved social novelty behavior in Fmr1 KO mice provided a potential therapeutic intervention for FXS, which may further be used in other disease models with defective RA signaling.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC) of Center for Excellence in Molecular Cell Science, CAS. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
RH, ZH and XZ designed and supervised the whole study. LY performed the experiments. ZX and MZ analyzed the RNA-seq data. LY, ZH, JF, PM, YN and RH analyzed the other data and wrote the manuscript. All authors read, and approved the final version of the manuscript.
Funding
This work was supported by National Science and Technology Innovation 2030 Major Project of China (2021ZD0203900); Ministry of Science and Technology of China (2021ZD0203900, 2019YFA0802103); Department of Science and Technology of Zhejiang Province (2021C03104); Guangzhou Science Innovation and Development Program (201803010092); Shenzhen-Hong Kong Institute of Brain Science (NYKFKT2019006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Prof. Chen Zhang of Capital Medical University, Beijing, for generous sharing of the Fmr1(-/y) mice (FVB background). We acknowledge the excellent support from Baojin Wu and his team at the Core Facility of Animal, Wei Bian and her team in the Cell Biology Core Facility, and Ming Chen and his team from the Molecular Biology Facility in SIBCB. We thank Xiangpeng Sheng for his assistance in manuscript preparation. We also thank Lin Ye, Mire Yili and all other members of our laboratory for assistance and support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.928393/full#supplementary-material
References
Amodio, D. M., and Frith, C. D. (2006). Meeting of Minds: the Medial Frontal Cortex and Social Cognition. Nat. Rev. Neurosci. 7, 268–277. doi:10.1038/nrn1884
Aoto, J., Nam, C. I., Poon, M. M., Ting, P., and Chen, L. (2008). Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic Plasticity. Neuron 60, 308–320. doi:10.1016/j.neuron.2008.08.012
Bostrom, C., Yau, S.-y., Majaess, N., Vetrici, M., Gil-Mohapel, J., and Christie, B. R. (2016). Hippocampal Dysfunction and Cognitive Impairment in Fragile-X Syndrome. Neurosci. Biobehav. Rev. 68, 563–574. doi:10.1016/j.neubiorev.2016.06.033
Brumback, A. C., Ellwood, I. T., Kjaerby, C., Iafrati, J., Robinson, S., Lee, A. T., et al. (2018). Identifying Specific Prefrontal Neurons that Contribute to Autism-Associated Abnormalities in Physiology and Social Behavior. Mol. Psychiatry 23, 2078–2089. doi:10.1038/mp.2017.213
Chao, O. Y., Pathak, S. S., Zhang, H., Dunaway, N., Li, J.-S., Mattern, C., et al. (2020). Altered Dopaminergic Pathways and Therapeutic Effects of Intranasal Dopamine in Two Distinct Mouse Models of Autism. Mol. Brain 13, 111. doi:10.1186/s13041-020-00649-7
Chen, L., Lau, A. G., and Sarti, F. (2014). Synaptic Retinoic Acid Signaling and Homeostatic Synaptic Plasticity. Neuropharmacology 78, 3–12. doi:10.1016/j.neuropharm.2012.12.004
Chen, S., Fragoza, R., Klei, L., Liu, Y., Wang, J., Roeder, K., et al. (2018). An Interactome Perturbation Framework Prioritizes Damaging Missense Mutations for Developmental Disorders. Nat. Genet. 50, 1032–1040. doi:10.1038/s41588-018-0130-z
Cheng, Y., Corbin, J. G., and Levy, R. J. (2013). Programmed Cell Death Is Impaired in the Developing Brain ofFmr1Mutants. Dev. Neurosci. 35, 347–358. doi:10.1159/000353248
Cheung, Y.-T., Lau, W. K.-W., Yu, M.-S., Lai, C. S.-W., Yeung, S.-C., So, K.-F., et al. (2009). Effects of All-Trans-Retinoic Acid on Human SH-Sy5y Neuroblastoma as In Vitro Model in Neurotoxicity Research. Neurotoxicology 30, 127–135. doi:10.1016/j.neuro.2008.11.001
Chien, Y.-L., Wu, Y.-Y., Chen, H.-I., Tsai, W.-C., Chiu, Y.-N., Liu, S.-K., et al. (2017). The Central Nervous System Patterning Gene Variants Associated with Clinical Symptom Severity of Autism Spectrum Disorders. J. Formos. Med. Assoc. 116, 755–764. doi:10.1016/j.jfma.2016.11.015
Cope, E. C., and Gould, E. (2019). Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell. Stem Cell. 24, 690–705. doi:10.1016/j.stem.2019.03.023
Ding, Q., Sethna, F., and Wang, H. (2014). Behavioral Analysis of Male and Female Fmr1 Knockout Mice on C57BL/6 Background. Behav. Brain Res. 271, 72–78. doi:10.1016/j.bbr.2014.05.046
Ercan, E., Han, J. M., Di Nardo, A., Winden, K., Han, M.-J., Hoyo, L., et al. (2017). Neuronal CTGF/CCN2 Negatively Regulates Myelination in a Mouse Model of Tuberous Sclerosis Complex. J. Exp. Med. 214, 681–697. doi:10.1084/jem.20160446
Ferreira, R., Napoli, J., Enver, T., Bernardino, L., and Ferreira, L. (2020). Advances and Challenges in Retinoid Delivery Systems in Regenerative and Therapeutic Medicine. Nat. Commun. 11, 4265. doi:10.1038/s41467-020-18042-2
Franklin, A. V., King, M. K., Palomo, V., Martinez, A., McMahon, L. L., and Jope, R. S. (2014). Glycogen Synthase Kinase-3 Inhibitors Reverse Deficits in Long-Term Potentiation and Cognition in Fragile X Mice. Biol. Psychiatry 75, 198–206. doi:10.1016/j.biopsych.2013.08.00310.1016/j.biopsych.2013.08.003
Gantois, I., Khoutorsky, A., Popic, J., Aguilar-Valles, A., Freemantle, E., Cao, R., et al. (2017). Metformin Ameliorates Core Deficits in a Mouse Model of Fragile X Syndrome. Nat. Med. 23, 674–677. doi:10.1038/nm.4335
Gatto, C. L., and Broadie, K. (2011). Fragile X Mental Retardation Protein Is Required for Programmed Cell Death and Clearance of Developmentally-Transient Peptidergic Neurons. Dev. Biol. 356, 291–307. doi:10.1016/j.ydbio.2011.05.001
Hao, Z., Wu, Q., Li, Z., Li, Y., Li, Q., Lai, X., et al. (2019). Maternal Exposure to Triclosan Constitutes a yet Unrecognized Risk Factor for Autism Spectrum Disorders. Cell. Res. 29, 866–869. doi:10.1038/s41422-019-0220-1
Harris, S. W., Hessl, D., Goodlin-Jones, B., Ferranti, J., Bacalman, S., Barbato, I., et al. (2008). Autism Profiles of Males with Fragile X Syndrome. Am. J. Ment. Retard. 113, 427–438. doi:10.1352/2008.113:427-438
Hogan, A. L., Caravella, K. E., Ezell, J., Rague, L., Hills, K., and Roberts, J. E. (2017). Autism Spectrum Disorder Symptoms in Infants with Fragile X Syndrome: A Prospective Case Series. J. Autism Dev. Disord. 47, 1628–1644. doi:10.1007/s10803-017-3081-9
Kazdoba, T. M., Leach, P. T., Silverman, J. L., and Crawley, J. N. (2014). Modeling Fragile X Syndrome in the Fmr1 Knockout Mouse. Irdr 3, 118–133. doi:10.5582/irdr.2014.01024
Khalil, A. M., Faghihi, M. A., Modarresi, F., Brothers, S. P., and Wahlestedt, C. (2008). A Novel RNA Transcript with Antiapoptotic Function Is Silenced in Fragile X Syndrome. PLoS One 3, e1486. doi:10.1371/journal.pone.0001486
Krakowiak, P., Walker, C. K., Bremer, A. A., Baker, A. S., Ozonoff, S., Hansen, R. L., et al. (2012). Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics 129, e1121–e1128. doi:10.1542/peds.2011-2583
Kumar, S., and Duester, G. (2011). SnapShot: Retinoic Acid Signaling. Cell. 147, 1422. doi:10.1016/j.cell.2011.11.034
Lord, C., Elsabbagh, M., Baird, G., and Veenstra-Vanderweele, J. (2018). Autism Spectrum Disorder. Lancet 392, 508–520. doi:10.1016/S0140-6736(18)31129-2
Lumaban, J. G., and Nelson, D. L. (2015). The Fragile X Proteins Fmrp and Fxr2p Cooperate to Regulate Glucose Metabolism in Mice. Hum. Mol. Genet. 24, 2175–2184. doi:10.1093/hmg/ddu737
Maenner, M. J., Shaw, K. A., Bakian, A. V., Bilder, D. A., Durkin, M. S., Esler, A., et al. (2021). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 70, 1–16. doi:10.15585/mmwr.ss7011a1
McCamphill, P. K., Stoppel, L. J., Senter, R. K., Lewis, M. C., Heynen, A. J., Stoppel, D. C., et al. (2020). Selective Inhibition of Glycogen Synthase Kinase 3α Corrects Pathophysiology in a Mouse Model of Fragile X Syndrome. Sci. Transl. Med. 12, eaam8572. doi:10.1126/scitranslmed.aam8572
Meyer, L. R., Zhu, V., Miller, A., and Roghair, R. D. (2014). Growth Restriction, Leptin, and the Programming of Adult Behavior in Mice. Behav. Brain Res. 275, 131–135. doi:10.1016/j.bbr.2014.08.054
Modabbernia, A., Velthorst, E., and Reichenberg, A. (2017). Environmental Risk Factors for Autism: an Evidence-Based Review of Systematic Reviews and Meta-Analyses. Mol. Autism 8, 13. doi:10.1186/s13229-017-0121-4
Mukamel, Z., Konopka, G., Wexler, E., Osborn, G. E., Dong, H., Bergman, M. Y., et al. (2011). Regulation of MET by FOXP2, Genes Implicated in Higher Cognitive Dysfunction and Autism Risk. J. Neurosci. 31, 11437–11442. doi:10.1523/JNEUROSCI.0181-11.2011
Naviaux, J. C., Wang, L., Li, K., Bright, A., Alaynick, W. A., Williams, K. R., et al. (2015). Antipurinergic Therapy Corrects the Autism-like Features in the Fragile X (Fmr1 Knockout) Mouse Model. Mol. Autism 6, 1. doi:10.1186/2040-2392-6-1
Niu, M., Han, Y., Dy, A. B. C., Du, J., Jin, H., Qin, J., et al. (2017). Autism Symptoms in Fragile X Syndrome. J. Child. Neurol. 32, 903–909. doi:10.1177/0883073817712875
Nolan, S. O., Reynolds, C. D., Smith, G. D., Holley, A. J., Escobar, B., Chandler, M. A., et al. (2017). Deletion of Fmr1 Results in Sex-specific Changes in Behavior. Brain Behav. 7, e00800. doi:10.1002/brb3.800
O’Leary, O., and Nolan, Y. (2015). Glycogen Synthase Kinase-3 as a Therapeutic Target for Cognitive Dysfunction in Neuropsychiatric Disorders. CNS Drugs 29, 1–15. doi:10.1007/s40263-014-0213-z
Park, E., Lau, A. G., Arendt, K. L., and Chen, L. (2021). FMRP Interacts with RARα in Synaptic Retinoic Acid Signaling and Homeostatic Synaptic Plasticity. Ijms 22, 6579. doi:10.3390/ijms22126579
Park, J.-Y., Jeong, J.-K., Lee, J.-H., Moon, J.-H., Kim, S.-W., Lee, Y.-J., et al. (2015). Induction of Cellular Prion Protein (PrPc) under Hypoxia Inhibits Apoptosis Caused by TRAIL Treatment. Oncotarget 6, 5342–5353. doi:10.18632/oncotarget.3028
Pasqualetti, M., Neun, R., Davenne, M., and Rijli, F. M. (2001). Retinoic Acid Rescues Inner Ear Defects in Hoxa1 Deficient Mice. Nat. Genet. 29, 34–39. doi:10.1038/ng702
Pavăl, D., Rad, F., Rusu, R., Niculae, A.-Ş., Colosi, H. A., Dobrescu, I., et al. (2017). Low Retinal Dehydrogenase 1 (RALDH1) Level in Prepubertal Boys with Autism Spectrum Disorder: A Possible Link to Dopamine Dysfunction? Clin. Psychopharmacol. Neurosci. 15, 229–236. doi:10.9758/cpn.2017.15.3.229
Peça, J., Feliciano, C., Ting, J. T., Wang, W., Wells, M. F., Venkatraman, T. N., et al. (2011). Shank3 Mutant Mice Display Autistic-like Behaviours and Striatal Dysfunction. Nature 472, 437–442. doi:10.1038/nature09965
Peteri, U. K., Pitkonen, J., Toma, I., Nieminen, O., Utami, K. H., Strandin, T. M., et al. (2021). Urokinase Plasminogen Activator Mediates Changes in Human Astrocytes Modeling Fragile X Syndrome. Glia 69, 2947–2962. doi:10.1002/glia.24080
Ramírez-Cheyne, J. A., Duque, G. A., Ayala-Zapata, S., Saldarriaga-Gil, W., Hagerman, P., Hagerman, R., et al. (2019). Fragile X Syndrome and Connective Tissue Dysregulation. Clin. Genet. 95, 262–267. doi:10.1111/cge.13469
Rein, B., Ma, K., and Yan, Z. (2020). A Standardized Social Preference Protocol for Measuring Social Deficits in Mouse Models of Autism. Nat. Protoc. 15, 3464–3477. doi:10.1038/s41596-020-0382-9
Reinhard, S. M., Razak, K., and Ethell, I. M. (2015). A Delicate Balance: Role of MMP-9 in Brain Development and Pathophysiology of Neurodevelopmental Disorders. Front. Cell. Neurosci. 9, 280. doi:10.3389/fncel.2015.00280
Richter, J. D., and Zhao, X. (2021). The Molecular Biology of FMRP: New Insights into Fragile X Syndrome. Nat. Rev. Neurosci. 22, 209–222. doi:10.1038/s41583-021-00432-0
Rizk, M., Saker, Z., Harati, H., Fares, Y., Bahmad, H. F., and Nabha, S. (2021). Deciphering the Roles of Glycogen Synthase Kinase 3 (GSK3) in the Treatment of Autism Spectrum Disorder and Related Syndromes. Mol. Biol. Rep. 48, 2669–2686. doi:10.1007/s11033-021-06237-9
Salcedo-Arellano, M. J., Cabal-Herrera, A. M., Punatar, R. H., Clark, C. J., Romney, C. A., and Hagerman, R. J. (2021). Overlapping Molecular Pathways Leading to Autism Spectrum Disorders, Fragile X Syndrome, and Targeted Treatments. Neurotherapeutics 18, 265–283. doi:10.1007/s13311-020-00968-6
Saldarriaga, W., Tassone, F., González-Teshima, L. Y., Forero-Forero, J. V., Ayala-Zapata, S., and Hagerman, R. (2014). Fragile X Syndrome. Colomb. Med. (Cali) 45, 190–198. doi:10.25100/cm.v45i4.1810
Sarti, F., Zhang, Z., Schroeder, J., and Chen, L. (2013). Rapid Suppression of Inhibitory Synaptic Transmission by Retinoic Acid. J. Neurosci. 33, 11440–11450. doi:10.1523/JNEUROSCI.1710-13.2013
Si, J., Mueller, L., and Collins, S. J. (2011). GSK3 Inhibitors Enhance Retinoic Acid Receptor Activity and Induce the Differentiation of Retinoic Acid-Sensitive Myeloid Leukemia Cells. Leukemia 25, 1914–1918. doi:10.1038/leu.2011.171
Soden, M. E., and Chen, L. (2010). Fragile X Protein FMRP Is Required for Homeostatic Plasticity and Regulation of Synaptic Strength by Retinoic Acid. J. Neurosci. 30, 16910–16921. doi:10.1523/JNEUROSCI.3660-10.2010
Stahl, M., and Tallman, M. S. (2019). Differentiation Syndrome in Acute Promyelocytic Leukaemia. Br. J. Haematol. 187, 157–162. doi:10.1111/bjh.16151
Szymański, Ł., Skopek, R., Palusińska, M., Schenk, T., Stengel, S., Lewicki, S., et al. (2020). Retinoic Acid and its Derivatives in Skin. Cells 9, 2660. doi:10.3390/cells9122660
Wang, Z.-J., Zhong, P., Ma, K., Seo, J.-S., Yang, F., Hu, Z., et al. (2020). Amelioration of Autism-like Social Deficits by Targeting Histone Methyltransferases EHMT1/2 in Shank3-Deficient Mice. Mol. Psychiatry 25, 2517–2533. doi:10.1038/s41380-019-0351-2
Westmark, C. J. (2021). Parental Reports on Early Autism Behaviors in Their Children with Fragile X Syndrome as a Function of Infant Feeding. Nutrients 13, 2888. doi:10.3390/nu13082888
Wołoszynowska-Fraser, M. U., Kouchmeshky, A., and McCaffery, P. (2020). Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 40, 247–272. doi:10.1146/annurev-nutr-122319-034227
Xu, X., Li, C., Gao, X., Xia, K., Guo, H., Li, Y., et al. (2018). Excessive UBE3A Dosage Impairs Retinoic Acid Signaling and Synaptic Plasticity in Autism Spectrum Disorders. Cell. Res. 28, 48–68. doi:10.1038/cr.2017.132
Yan, Q. J., Asafo-Adjei, P. K., Arnold, H. M., Brown, R. E., and Bauchwitz, R. P. (2004). A Phenotypic and Molecular Characterization of the Fmr1-tm1Cgr Fragile X Mouse. Genes. Brain Behav. 3, 337–359. doi:10.1111/j.1601-183X.2004.00087.x
Zhang, Z., Marro, S. G., Zhang, Y., Arendt, K. L., Patzke, C., Zhou, B., et al. (2018). The Fragile X Mutation Impairs Homeostatic Plasticity in Human Neurons by Blocking Synaptic Retinoic Acid Signaling. Sci. Transl. Med. 10, eaar4338. doi:10.1126/scitranslmed.aar4338
Zhong, L. R., Chen, X., Park, E., Südhof, T. C., and Chen, L. (2018). Retinoic Acid Receptor RARα-dependent Synaptic Signaling Mediates Homeostatic Synaptic Plasticity at the Inhibitory Synapses of Mouse Visual Cortex. J. Neurosci. 38, 10454–10466. doi:10.1523/JNEUROSCI.1133-18.2018
Zhou, W., and Li, S. (2018). Decreased Levels of Serum Retinoic Acid in Chinese Children with Autism Spectrum Disorder. Psychiatry Res. 269, 469–473. doi:10.1016/j.psychres.2018.08.091
Keywords: fragile X syndrome, autism spectrum disorder, retinoic acid, social behavior, FMR1
Citation: Yang L, Xia Z, Feng J, Zhang M, Miao P, Nie Y, Zhang X, Hao Z and Hu R (2022) Retinoic Acid Supplementation Rescues the Social Deficits in Fmr1 Knockout Mice. Front. Genet. 13:928393. doi: 10.3389/fgene.2022.928393
Received: 25 April 2022; Accepted: 30 May 2022;
Published: 17 June 2022.
Edited by:
Zhexing Wen, Emory University, United StatesReviewed by:
Yuen Gao, Michigan State University, United StatesYing Zhou, Emory University, United States
Fei Li, Shanghai Jiao Tong University, China
Copyright © 2022 Yang, Xia, Feng, Zhang, Miao, Nie, Zhang, Hao and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyan Zhang, enh5MzU3NjJAMTI2LmNvbQ==; Zijian Hao, aGFvemlqaWFuMjAwOEAxNjMuY29t; Ronggui Hu, Y29yeWh1QHNpYmNiLmFjLmNu
†These authors have contributed equally to this work
 Liqin Yang
Liqin Yang Zhixiong Xia
Zhixiong Xia Jianhua Feng
Jianhua Feng Menghuan Zhang4
Menghuan Zhang4 Pu Miao
Pu Miao Ronggui Hu
Ronggui Hu