- 1Key Laboratory for Molecular Genetic Mechanisms and Intervention Research on High Altitude Disease of Tibet Autonomous Region, Xianyang, China
- 2Key Laboratory of High Altitude Environment and Genes Related to Diseases of Tibet Autonomous Region, School of Medicine, Xizang Minzu University, Xianyang, China
- 3School of Public Health, Sun Yat-sen University, Shenzhen, China
Background: The incidence of gallstones in the Tibetan population is increasing rapidly. Previous studies indicated that genetic variation located in the cholesterol metabolism pathway may be associated with the incidence of gallstones.
Methods: By recruiting 132 Tibetan gallstone patients and 52 normal Tibetan controls, we performed next-generation sequencing for 508 genes in the cholesterol metabolism pathway. Additionally, by integrating the sequence data of 41 normal Tibetan subjects in the public database, we finally obtained 93 normal Tibetan controls. Single nucleotide polymorphisms (SNPs) calling were performed by using the GATK pipeline. The quality control criteria for SNPs were: missing rate <0.05; minor allele frequency (MAF) > 0.01; and p value >0.001 in the Hardy-Weinberg Equilibrium (HWE) test. To eliminate the influence of population heterogeneity, Principal Component Analysis (PCA) was carried out by using the smartpca software. Association analyses were performed by Plink software. Multiple tests were adjusted by the false discovery rate (FDR) method.
Results: A total of 2,401 SNPs were obtained by analyzing 508 genes, and 2,011 SNPs left after quality control. After adjusting the eigen vectors, we found that 10 SNPs (SNV05997, rs80145081, rs80005560, rs79074685, rs748546375, rs201880593, rs142559357, rs750769471, rs869789 and rs4072341) were significantly associated with gallstone. Subsequently, by comparing the case group with our control group and the public database control group separately, we further found that the SNP rs869789 was consistently significantly associated with gallstone (p = 9.04 × 10–3 in cases vs. our controls and 5.73 × 10–3 in cases vs. public controls, respectively).
Conclusion: By systematically analyzed SNPs in the cholesterol metabolism pathway, we identified one polymorphic locus rs869789 significantly associated with the pathogenesis of gallstone in the Tibetan population. This study will provide clue for further mechanism study of gallstone in the Tibetan population.
Introduction
Gallstone disease (GD) is the most common gallbladder disease (Pang et al., 2020), affecting 10–20% adult worldwide. The incidence of gallstone increased sharply during the past decades. Complications of GD include cholecystitis, pancreatitis, and so on. And, it can also significantly increase the risk of cardiovascular disease, finally leading to serious public health problems (Hu F. et al., 2021). In addition to bile acids, other components of bile including cholesterol, fatty acids, phospholipids and bilirubin (Grigor’eva and Romanova, 2020). Cholesterol stones account for more than 80% of gallstones (Hu F. et al., 2021). Most gallstones are caused by the excessive and rapid mobilization of cholesterol into the bile through the liver. The high secretion of free cholesterol in the bile leads to the oversaturation of cholesterol, resulting in the accelerated crystallization of cholesterol in the gallbladder bile, the excessive secretion of bile mucin, and the weakening of gallbladder power, and finally the formation of stone core (Di Ciaula et al., 2019; Grigor’eva and Romanova, 2020; Sun et al., 2021). In general, the formation of GD is due to the imbalance of cholesterol, bile acid, lecithin, and other components in the bile.
GD is a complex disease, and its main pathogenic factors include living environment, eating habits and genetic background (Di Ciaula et al., 2018). Studies have shown that the risk of gallstones in East Asian countries including China is lower than other areas (Lammert et al., 2016), however, the incidence rate of gallstones in Qinghai-Tibet Plateau is high. Tibet is located in the southwest frontier of China, with an average altitude of more than 4,000 m and a thin oxygen content. Hypoxia inducible factor 1α (HIF1α) gene expression is up-regulated in hypoxia. HIF1α leads to bile concentration by inhibiting the activity of aquaporin 8 (AQP8) in liver, which is one of the reasons for promoting the occurrence of cholesterol stones (Asai et al., 2017). GD is a digestive system disease, which is largely affected by eating habits. Tibetan people consume less vegetables and fruits in their daily diet, but more beef and mutton. They drink butter tea and sweet tea, but less drinking water. Their lifestyle is quite different from that of people in other regions of China.
With the development of sequencing technology, the genetic characteristics of many complex diseases have been gradually revealed. Approximately 25% of the risk of cholelithiasis can be explained by genetics (Katsika et al., 2005). Mutations in some lithogenic genes may be the cause of gallstone formation. Common lithogenic genes include liver cholesterol transporter ATP binding cassette subfamily G member 5 (ABCG5) (Di Ciaula et al., 2018), ABCG8 (Lammert et al., 2016), ATP binding cassette subfamily B member 4 (ABCB4), and UDP glucuronosyltransferase family 1 member A1 (UGT1A1) (Sun et al., 2021). In addition, other genes related to GD include CYP7A1, GCKR, SULT21, TM4SF4, TTC39B, etc. (Rebholz et al., 2018).
However, previous studies mainly focused on one or two genes, or several specific exons. Few studies systematically investigated the relationship of genes in the cholesterol metabolism pathway and gallbladder diseases. Here, by targeting all genes in cholesterol metabolism pathway, we explored the relationship of polymorphic loci in cholesterol metabolism pathway and gallstone in the Tibetan populations.
Materials and Methods
Study Subjects
A total of 184 subjects were selected from the Tibetan population. Among them, 132 were gallstone patients and 52 were health controls. Gallstone was diagnosed with the Doppler ultrasound of fasting examination by professional doctors. In addition, whole exon sequencing data of 41 healthy Tibetans were downloaded from the CNCB (China National Center for Bioinformation) database (8 samples were downloaded from accession Number PRJCA000600; 33 of 38 samples were downloaded from accession Number PRJNA382306, 5 Sherpa were excluded). This study was approved by the ethics committee of Xizang Minzu University, and all subjects signed informed consent.
DNA Extraction
3 ml peripheral venous blood were collected from each subject, and was placed in an anticoagulant tube containing ethylenediaminetetraacetic acid (EDTA). DNA was extracted using a blood genomic DNA extraction kit. The concentration, purity, and quality of extracted DNA were examined, and only qualified samples were stored in −20°C refrigerator.
Genotyping and Quality Control
Genes located in the cholesterol metabolism pathway were sequenced in all samples by the next-generation sequencing method. Single nucleotide polymorphisms (SNPs) calling were implemented by using the standard GATK pipeline (Genome Analysis ToolKit, v4.0.4.0). The criteria for SNPs quality control were: 1) missing rate <0.05; 2) minor allele frequency (MAF) > 0.01; 3) p value >0.001 in Hardy-Weinberg equilibrium (HWE) test.
Statistical Analysis
SPSS v23.0 statistical software was used for data analysis and processing. Genetic association analysis was performed using the Plink software (Purcell et al., 2007). Principal Component Analysis (PCA) was performed by using Smartpca software. After adjusting different numbers of eigen vectors, Q-Q (quantile-quantile) plot and inflation factor were used to evaluate the impact of population heterogeneity on the association results. Association analyses were implemented by using logistic regression under the additive assumption. Manhattan plot was depicted by using R software. Expression Quantitative Trait Locus (eQTL) analyses were implemented in GTEx database. Multiple tests were adjusted by the False Discovery Rate (FDR) method and FDR-P < 0.2 was used as the criteria of significance.
Results
Sample Population
A total of 225 Tibetans were included in this study. Of which, 132 were gallstone patients and 93 were healthy controls. No abnormal deviation was found between the case group and the control group in the PCA analyses (Figure 1A). Further, by combing our subject with the subjects of the International 1000 Genomes Project (Auton et al., 2015), we found that our subjects only aggregated with Han Chinese in Beijing (CHB) population, which indicated that no obvious population heterogeneity were found in our study (Figure 1B).
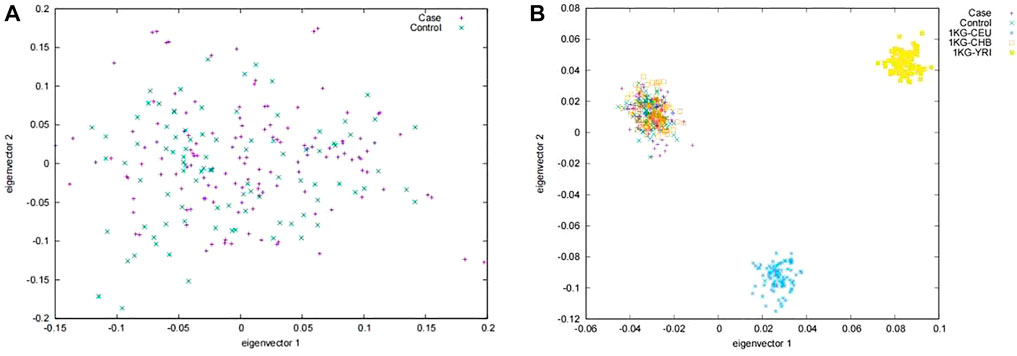
FIGURE 1. Principal component analysis in (A) the Chinese Tibetan population and (B) the reference sample in the international 1,000 genomes project. Case, Patients with gallstones; Control, Healthy control population; CEU, Utah Residents (CEPH) with Northern and Western European Ancestry; CHB, Han Chinese in Beijing, China; YRI, Yoruba in Ibadan, Nigeria.
Sequencing Region Information
Target sequencing includes 1,042 loci regions of 508 genes in the cholesterol metabolism pathway. The basic information and location of these regions were summarized in Supplementary Table S1. By using the GATK analysis pipeline, a total of 2,401 SNPs were obtained. And, after quality control, 2,011 high-quality SNPs were finally obtained for subsequent analysis.
The Overall Association Analysis
Although the previous PCA analyses did not find obvious population heterogeneity (Figure 1), we still adjusted the top eigen vectors in the following association analyses. By depicting Q-Q plots and calculating the inflation factors, we found that the association result after adjusting top two eigen vectors have the smallest inflation factor (Figure 2). Thus, in the following association analyses, top two eigen vectors were adjusted. Then, by evaluating the association of all 2,011 SNPs with gallstone, we found that 10 SNPs were significantly associated with Gallstone (FDR-P < 0.2, Figure 3A and Table 1). All 2,011 loci are listed in Supplementary Table S2.
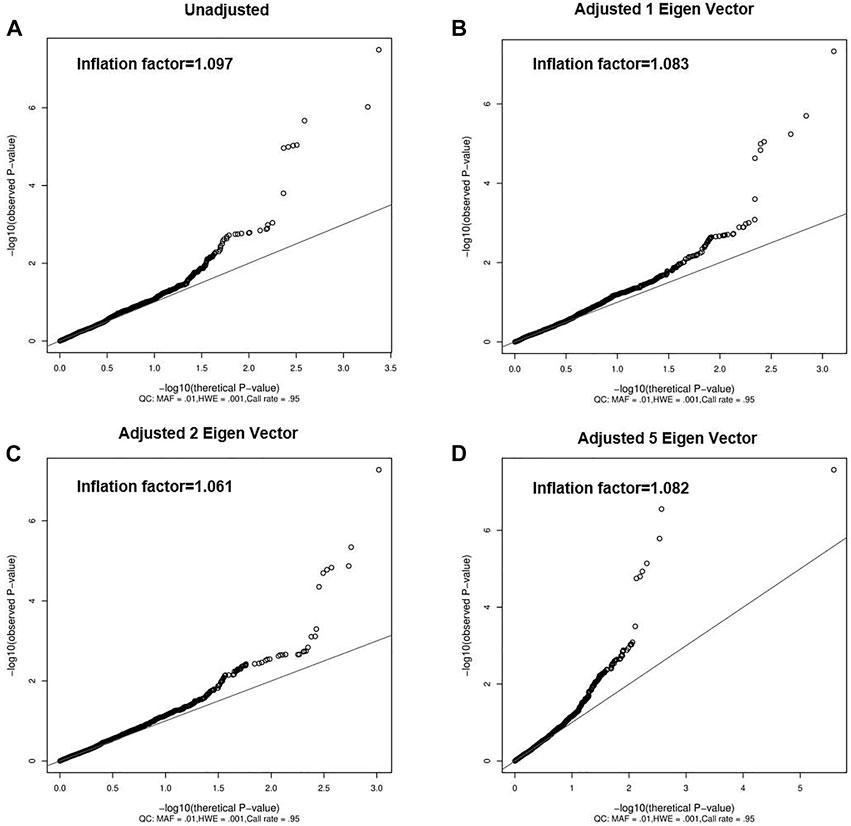
FIGURE 2. Quantile-Quantile Plot of gallstones. (A) does not correct the eigen vector, (B) correct for the first eigen vector, (C) correct for the top 2 eigen vectors and (D) correct for the top 5 eigen vectors. Solid lines indicate the null-hypothesis.
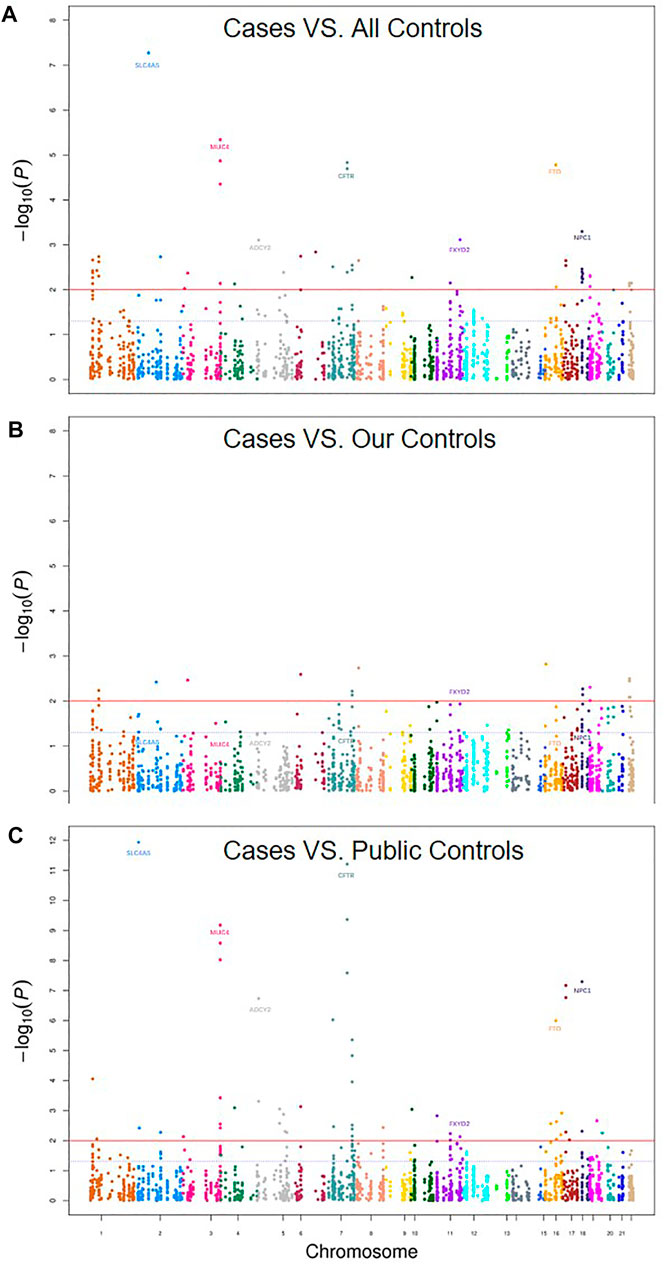
FIGURE 3. Manhattan Plot of gallstones for (A) Cases vs. all controls (our 52 controls +41 public controls), (B) Cases. our 52 controls and (C) Cases vs. 41 public controls. The plot shows −log10 p value for each SNP against the chromosomal location. The x-axis represents chromosomal position and the y-axis shows −log10 p values. The blue dashed line indicates the p value of 0.05 while the solid red line indicates the p value of 0.01.
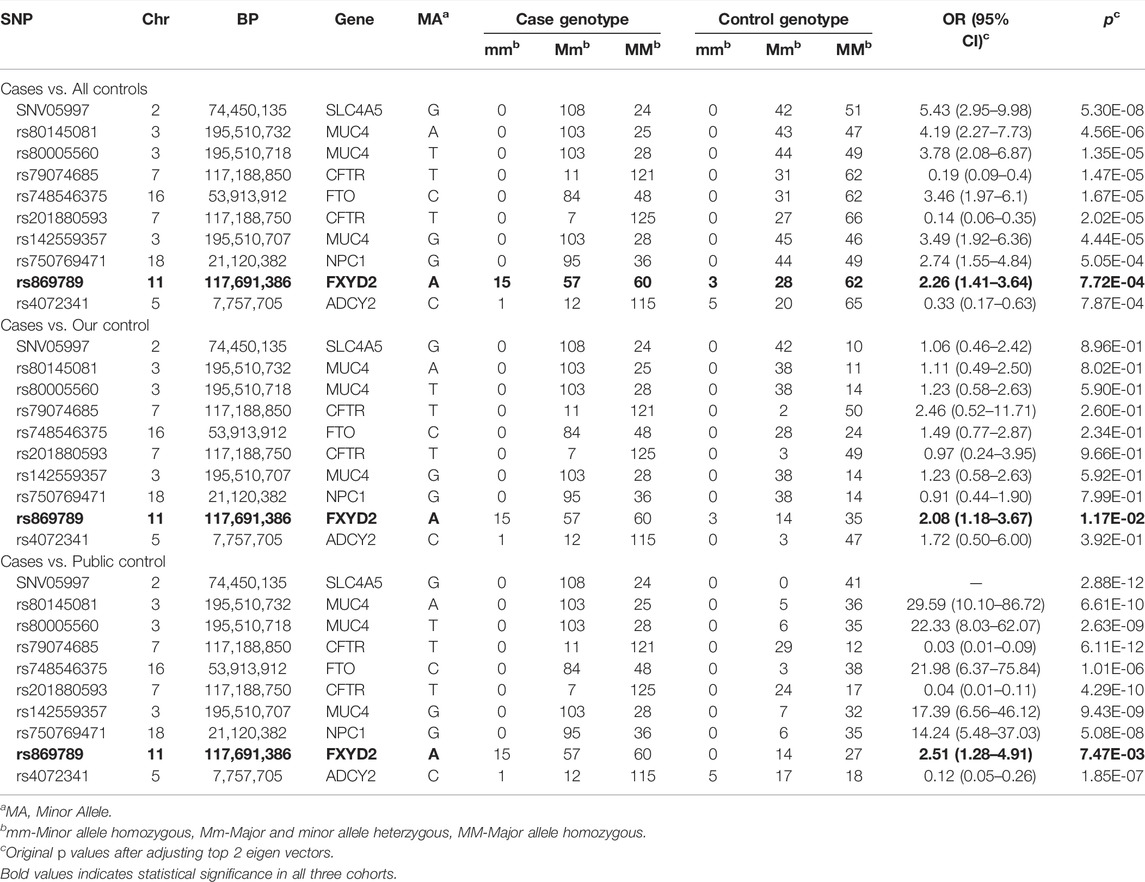
TABLE 1. Overall and subgroup analyses for SNPs which were significantly associated with gallstone (FDR-P < 0.2).
Association Analyses in Subset Datasets
Considering that our controls including two parts: our own 52 subjects and 41 subjects from the public database. We further did subgroup analyses by using our own 52 subjects only and by using 41 public subjects only. We found that the SNP rs869789, which located in the 3′ UTR region of FXYD2, were consistently significantly associated gallstone in the subgroup analyses (Figures 3B,C; Table 1). The frequency of A allele in cases was 0.330, which were significantly higher than that in our control (0.192, OR = 2.06, p = 9.04 × 10–3) and in public controls (0.171, OR = 2.39, p = 5.73 × 10–3). By implementing eQTL analyses in GTEx, we found that A allele of rs869789 was significantly correlated with a higher expression of FXYD2 in colon-sigmoid tissue, thyroid tissue, colon-transverse tissue, esophagus-gastroesophageal junction tissue and nerve-tibial tissue (Supplementary Figure S1).
Discussion
The disease composition of people in the high altitude environment is different from that in other areas, and the incidence of gallbladder diseases is increasing year by year. Cholesterol gallstones are the most common type of gallstones, and the change of cholesterol homeostasis may be one of the causes of gallstones. Vegetarians can reduce the risk of gallstones by reducing cholesterol levels in the body (Chang et al., 2019). Interestingly, another prospective cohort study pointed out that vegetarians had a significantly higher risk of symptomatic gallstone disease than non-vegetarians after adjusting for risk factors, including body mass index (McConnell et al., 2017). We speculated that this might be related to the difference in gene expression in the cholesterol metabolism pathway. There are many genes in the cholesterol metabolism pathway, which directly or indirectly affect the metabolism level of cholesterol in the body, which may have some unknown correlation with the occurrence of GD. In this study, through the detection of cholesterol metabolism pathway genes in Tibetan people living in Tibet, it was found that ten SNPs loci were associated with gallstone, of which SNP rs869789 was most significantly associated with gallstone in Tibetan people. We analyzed the information of 10 SNPs screened. These loci are located in seven different genes, including SLC4A5, MUC4, FTO, NPC1 and FXYD2 genes that may increase the risk of gallstone in the Tibetan population, while CFTR and ADCY2 genes that reduce the risk of gallstone in the Tibetan population.
The Solute carrier family 4 Member 5 (SLC4A5) gene encodes Na+-HCO3− cotransporter, which plays a role in regulating sodium and bicarbonate transport and affects intracellular, extracellular, interstitial and ultimately plasma Ph (Felder et al., 2016). The sodium bicarbonate cotransporter SLC4A5 plays an important role in the recovery of cerebrospinal fluid pH during hypercapnia-induced acidosis, which can protect the brain from acid damage (Christensen et al., 2018). In gallbladder diseases, some elements in bile (such as Ca2+, Fe3+, Cu2+) are very significant in the development of GD (Khan et al., 2017). The higher density and pH value of bile and the higher concentration of transition elements may be important factors for the formation of different types of GD. In a population survey, it was found that people with hypertension have a higher risk of gallstones than the general population, which may be related to the mutation of SLC4A5 gene loci (Song et al., 2020). A number of studies have pointed out that there is a genetic association between SLC4A5 gene polymorphism and hypertension (Parker, 2018; Barbuskaite et al., 2020). A healthy diet can reduce the risk of gallstone disease while preventing high blood pressure (Wirth et al., 2018). Our results showed that SNV05997 in SLC4A5 gene is a risk factor for GD in Tibetan people, and it is speculated that the dietary structure of Tibetan people may induce the mutation of SLC4A5 gene, and changing diet may play a certain role in the prevention of gallstone.
The microflora of the gastrointestinal tract and biliary tract are involved in the formation of bile, and are related to various complications of gallstone (such as acute and chronic cholecystitis, cholangitis, pancreatitis, etc.), and also related to dysbacteriosis. Studies have shown that oral pathogenic bacteria affect gallbladder movement and the expression of mucin genes (MUC1, MUC3, and MUC4) through the immune regulation mechanism, regulate cholesterol metabolism and promote the formation of gallstone (Grigor’eva and Romanova, 2020). Hu et al. (Hu et al., 2021a) analyzed the gallbladder tissue and bile samples of patients with gallstones and found that the Gram-positive microflora and MUC4 protein in the bile of patients with gallstones were positively correlated with the calcification of cholesterol stones, and there was a synergistic effect. MUC family genes are a contributing factor to the formation of cholesterol crystal nuclei. Cholesterol stones and gallbladder infections are associated with increased expression of mucin genes MUC3 and MUC5B (Yoo et al., 2016). In the study of mouse models, it was found that the mRNA expression levels of MUC2, MUC5AC, MUC5B and MUC6 genes in cholesterol gallstone mice were significantly increased (Kim et al., 2012). The increase of MUC1 mucin in the gallbladder epithelium can promote the absorption of cholesterol in mice and inhibit the movement of the gallbladder, thereby promoting the formation of gallstones (Wang et al., 2006; Chuang et al., 2011) found MUC1 and MUC2 gene loci significantly associated with gallstones in Chinese males. We found that MUC4 gene rs80145081, rs80005560 and rs142559357 are risk factors for GD in the Tibetan population of China, which is an effective supplement to the susceptibility site of MUC family gene in GD, and provides clues for exploring the mechanism of MUC family gene co-action to increase susceptibility to gallstones in Chinese population.
The human alpha-ketoglutarate dependent dioxygenase (FTO) genes is involved in DNA repair and fatty acid metabolism (Franczak et al., 2018). In mice with fused-toe (Ft) mutation, there is a gene named fat mass and obesity associated (FTO) gene, which is the orthologs gene of human, causing obesity (Chen and Du, 2019). In humans, the overexpression of FTO gene is manifested in the increase of body mass index (BMI) and basal metabolic rate (BMR) (Shaikh et al., 2021; Stender et al., 2013) found that people with elevated BMI are more likely to suffer from symptomatic gallstone disease by comparing gallstone people with healthy people. Obese people usually show an increase in fasting gallbladder volume and a decrease in postprandial gallbladder emptying, which lead to gallbladder stasis and promote the formation of gallstones (Di Ciaula et al., 2012). FTO gene is highly expressed in the hypothalamus, which is a key region regulating feeding and energy consumption (Chen and Du, 2019). It is speculated that the FTO gene may have the function of regulating the feeding center. Studies have shown that the increase of abdominal fat mass leads to decreased gallbladder motility and cholestasis. Therefore, factors affecting adipocyte secretion or metabolism may also affect the formation of gallstones (Stender et al., 2013). Gallstones are closely related to obesity. The emptying time of the gallbladder in obese people is similar to that of normal people, while the refilling time of the gallbladder is significantly shortened, showing enhanced enterohepatic circulation dynamics, resulting in a significant increase in blood cholesterol levels and a higher risk of cholesterol stone formation (Kubica and Balbus, 2021). Population studies have shown that FTO gene has SNP loci regulating fat metabolism in human body, leading to the occurrence of obesity (Mehrdad et al., 2020; Shaikh et al., 2021). We speculated that obese patients are more prone to GD, which is related to the mutation site of FTO gene to a certain extent. In this study, we found that rs748546375 was related to the increased risk of GD in the Tibetan population.
It has been reported that NPC intracellular cholesterol transporter 1 (NPC1) gene defect can lead to Niemann-Pick C genetic disease, excessive accumulation of cholesterol in vivo and autophagy dysfunction (Ilnytska et al., 2021). Both NPC gene products participate in the same cholesterol transport pathway and perform different but complementary functions, playing a role in transferring cholesterol from the liver to bile and blood (Klein et al., 2006; Hu P. et al., 2021). Also, the mouse animal model showed that NPC1 gene regulates the formation of gallstones by controlling cholesterol in the liver, suggesting that the expression of NPC1 gene may be related to the formation of cholesterol stones (Morales et al., 2010). Yuan et al. (Yuan et al., 2005) Screened 149 lipid related genes expressed in gallbladder, including gallstone candidate susceptibility gene NPC1. Similarly, our results also found that NPC1 gene SNP rs750769471 may be related to gallstone.
The proteins encoded by FXYD domain containing ion transport regulator (FXYD) gene family belong to a family of small-membrane proteins, which play a key role in the regulation of NA-K-ATPase (Zhao et al., 2020). The activity of NA-K-AtPase depends on phosphatidylserine (PS) and cholesterol. PS and cholesterol bind near the FXYD subunit, so the mutation of FXYD site may be associated with cholesterol (Habeck et al., 2017). It has been reported that the high expression of FXYD2 gene is related to poor overall survival, which may cause changes in tumor microenvironment (TME) and lead to malignant tumors (Zhao et al., 2020). Through the detection of gene expression in normal tissues, it was found that FXYD2 gene was highly expressed in gallbladder, with tissue specificity (Fagerberg et al., 2014). Studies have shown that the region of chromosome 11q22-24 is involved in the occurrence of schizophrenia (Choudhury et al., 2007). FXYD6 gene mutation may affect the brain region and lead to schizophrenia. Chaumette et al. (Chaumette et al., 2020) found that there are pathogenic missense variants of FXYD gene family FXYD1, FXYD6 and FXYD6-FXYD2 readthrough in childhood onset schizophrenia. Among them, FXYD6-FXYD2 readthrough is a connecting gene, which produces transcripts by binding exons of FXYD6 and FXYD2 in the same direction on the same chromosome.
In our study, we found that the FXYD gene family SNP rs869789 was significantly associated with gallstones. (Zhang et al., 2010) found no significant relationship between SNP rs869789 in FXYD gene family and the occurrence of schizophrenia in Chinese population through sequencing analysis. However, Tauroursodeoxycholic acid (TUDCA), a clinical drug for the treatment of gallstones, can play a neuroprotective role in different brain diseases, protect neurons from neurodegeneration and prevent neuroinflammation and oxidative stress (Cheng et al., 2019; Lu et al., 2018). In the process of atherosclerosis, the expression of FXYD3 gene was significantly down-regulated, while the expression of FXYD6 was upregulated (Liu et al., 2007; Dong et al., 2021). Through cell experiments, (Dong et al., 2021) found that lncRNA up-regulated the expression of FXYD6, increased the uptake of cholesterol by macrophages, induced the production of inflammatory molecules, and promoted atherosclerosis. FXYD family genes may lead to cholesterol aggregation, and gene mutations may lead to excessive cholesterol aggregation and gallstone. The specific mechanism of rs869789 and gallstone should be further studied in cell experiments.
In addition, we found that the SNP locus rs79074685 and rs201880593 of the cystic fibrosis transmembrane conductance regulator (CFTR) gene located on chromosome 7 could reduce the risk of GD disease in the Tibetan population at high altitudes. CTFR is involved in gastric acid secretion, chemokine signal transduction, bile secretion, and apoptosis (Wu et al., 2020). The absence of CFTR affects the apical junction complex of the bile duct epithelium. The mislocalization of CFTR in mouse bile duct cells makes mice present a cholestatic phenotype (Fiorotto et al., 2016). CFTR located in the bile secretion pathway is involved in the regulation of pH of rat ameloblasts, and then in the development of tooth germ (Yang et al., 2020). CFTR may be involved in the formation of calcification, leading to the formation of GD. Some SNP mutations in the CFTR gene may have a certain impact on this process. Cystic fibrosis (CF) is an autosomal recessive genetic disease, which is caused by CFTR gene mutation leading to CFTR protein misfolding, defective transport, and impaired function (Kumar et al., 2021). CFTR gene mutation leads to high oxaluric acid urine, acidic urine, and low urine volume, making CF patients more susceptible to urinary calculi (Wright et al., 2021). We speculate that mutations in the CFTR gene can cause changes in various urine indicators, and there may also be mutations that have a certain effect on the biochemistry of bile.
Cholesterol stones may cause obesity and dyslipidemia, and adipocyte dysfunction may lead to gallstone (Han et al., 2019). Our study showed that the alteration of rs4072341 in adenylyl cyclase 2 (ADCY2) gene also reduced the occurrence of gallstones. In addition, (Daily et al., 2019) found that ADCY2 rs326149 affects subcutaneous fat, which is positively correlated with the amount of subcutaneous fat, resulting in a high level of serum high-density lipoprotein cholesterol level. Previous studies have shown that ADCY2 gene is expressed in the brain and encodes a membrane-binding enzyme for the synthesis of cyclic adenosine monophosphate (cAMP). Hypoxic environment causes changes in reactive oxygen species (ROS) levels and also activates cAMP (Wang et al., 2021). cAMP is involved in the regulation of preadipocyte differentiation (Yadav and Jang, 2021). cAMP signaling is associated with adipogenic differentiation of bone marrow mesenchymal stem cells (Chen et al., 2021). Therefore, we speculate that ADCY2 gene may be involved in adipocyte differentiation and thus affect the formation of gallstones.
As a case-control study, we tried our best to match the information of the two groups of subjects. However, in the process of sample recruitment, due to the difference in population distribution in high-altitude areas, the two groups were not completely equal. Therefore, through the application of public databases, we have improved relevant data while saving biological information resources, making this experimental analysis more reliable. Meanwhile, this study was conducted in a high-altitude area in Tibet, China. People living in the same environment had roughly the same diet structure and living habits, so it was a targeted study on the genetic mechanism of GD without the interference of environmental factors.
Conclusion
In summary, this study explored the genetic mechanism of gallstones in high altitude populations based on restricted environment and diet, which is the first systematic sequencing analysis of genes in the cholesterol metabolism signal pathway in the Tibetan gallstone population. We found that ten independent SNPs were located in different positions, especially FXYD2 gene rs869789 was significant in all groups. These gene loci are associated with gallstones and are worthy of in-depth follow-up research, guiding the health of people living in the plateau.
Data Availability Statement
The data presented in the study are deposited in the Genome Sequence Archive (Genomics, Proteomics and Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA002309) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xizang Minzu University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LK designed the experiments, obtained the funds and revised the manuscript. HTC and HC accomplished the data analyses and prepared the manuscript. LM and ZYZ collected data and specimens. LL, YZ, YL, and ZPZ performed the experiment. All authors read and approved the final manuscript.
Funding
This research was funded by the Science and Technology Department Project of Tibet Autonomous Region (Grant number: Z2014A09G2-3), the National Natural Science Foundation of China (Grant numbers: U20A20395 and 81860329), and the “Tibetan-Qin Himalaya Project—Peak Scholar” of Xizang Minzu University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.902553/full#supplementary-material
References
Asai, Y., Yamada, T., Tsukita, S., Takahashi, K., Maekawa, M., Honma, M., et al. (2017). Activation of the Hypoxia Inducible Factor 1α Subunit Pathway in Steatotic Liver Contributes to Formation of Cholesterol Gallstones. Gastroenterology 152 (6), 1521–1535. doi:10.1053/j.gastro.2017.01.001
Auton, A., Brooks, L., Durbin, R., Garrison, E., Kang, H., Korbel, J., et al. (2015). A Global Reference for Human Genetic Variation. Nature 562 (7571), 68–74. doi:10.1038/nature15393
Barbuskaite, D., Pedersen, F., Christensen, H., Johnsen, L., Praetorius, J., and Damkier, H. (2020). NBCe2 (Slc4a5) Is Expressed in the Renal Connecting Tubules and Cortical Collecting Ducts and Mediates Base Extrusion. Front. Physiol. 11, 560. doi:10.3389/fphys.2020.00560
Chang, C., Chiu, T., Chang, C., Lin, M., and Lin, C. (2019). Plant-Based Diet, Cholesterol, and Risk of Gallstone Disease: A Prospective Study. Nutrients 11 (2), 335. doi:10.3390/nu11020335
Chaumette, B., Ferrafiat, V., Ambalavanan, A., Goldenberg, A., Dionne-Laporte, A., Spiegelman, D., et al. (2020). Missense Variants in ATP1A3 and FXYD Gene Family Are Associated with Childhood-Onset Schizophrenia. Mol. Psychiatry 25 (4), 821–830. doi:10.1038/s41380-018-0103-8
Chen, J., and Du, B. (2019). Novel Positioning from Obesity to Cancer: FTO, an mA RNA Demethylase, Regulates Tumour Progression. J. Cancer Res. Clin. Oncol. 145 (1), 19–29. doi:10.1007/s00432-018-2796-0
Chen, J., Zhang, H., Wu, X., Wang, F., Wang, Y., Gao, Q., et al. (2021). PTHG2 Reduces Bone Loss in Ovariectomized Mice by Directing Bone Marrow Mesenchymal Stem Cell Fate. Stem Cell Int. 2021, 8546739. doi:10.1155/2021/8546739
Cheng, L., Huang, C., and Chen, Z. (2019). Tauroursodeoxycholic Acid Ameliorates Lipopolysaccharide-Induced Depression like Behavior in Mice via the Inhibition of Neuroinflammation and Oxido-Nitrosative Stress. Pharmacology 103, 93–100. doi:10.1159/000494139
Choudhury, K., McQuillin, A., Puri, V., Pimm, J., Datta, S., Thirumalai, S., et al. (2007). A Genetic Association Study of Chromosome 11q22-24 in Two Different Samples Implicates the FXYD6 Gene, Encoding Phosphohippolin, in Susceptibility to Schizophrenia. Am. J. Hum. Genet. 80 (4), 664–672. doi:10.1086/513475
Christensen, H., Barbuskaite, D., Rojek, A., Malte, H., Christensen, I., Füchtbauer, A., et al. (2018). The Choroid Plexus Sodium-Bicarbonate Cotransporter NBCe2 Regulates Mouse Cerebrospinal Fluid pH. J. Physiol. 596 (19), 4709–4728. doi:10.1113/jp275489
Chuang, S., Juo, S., Hsi, E., Wang, S., Tsai, P., Yu, M., et al. (2011). Multiple Mucin Genes Polymorphisms Are Associated with Gallstone Disease in Chinese Men. Clinica Chim. Acta Int. J. Clin. Chem. 412, 599–603. doi:10.1016/j.cca.2010.12.008
Daily, J., Yang, H., Liu, M., Kim, M., and Park, S. (2019). Subcutaneous Fat Mass Is Associated with Genetic Risk Scores Related to Proinflammatory Cytokine Signaling and Interact with Physical Activity in Middle-Aged Obese Adults. Nutr. Metab. 16, 75. doi:10.1186/s12986-019-0405-0
Di Ciaula, A., Garruti, G., Frühbeck, G., De Angelis, M., de Bari, O., Wang, D., et al. (2019). The Role of Diet in the Pathogenesis of Cholesterol Gallstones. Curr. Med. Chem. 26 (19), 3620–3638. doi:10.2174/0929867324666170530080636
Di Ciaula, A., Wang, D., and Portincasa, P. (2018). An Update on the Pathogenesis of Cholesterol Gallstone Disease. Curr. Opin. Gastroenterol. 34 (2), 71–80. doi:10.1097/mog.0000000000000423
Di Ciaula, A., Wang, D., and Portincasa, P. (2012). Gallbladder and Gastric Motility in Obese Newborns, Pre-adolescents and Adults. J. Gastroenterol. Hepatol. 27 (8), 1298–1305. doi:10.1111/j.1440-1746.2012.07149.x
Dong, X., Lu, Z., Kang, C., Li, X., Haworth, K., Ma, X., et al. (2021). The Long Noncoding RNA RP11-728F11.4 Promotes Atherosclerosis. Arteriosclerosis, Thromb. Vasc. Biol. 41 (3), 1191–1204. doi:10.1161/atvbaha.120.315114
Fagerberg, L., Hallström, B., Oksvold, P., Kampf, C., Djureinovic, D., Odeberg, J., et al. (2014). Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteomics : MCP 13 (2), 397–406. doi:10.1074/mcp.M113.035600
Felder, R., Jose, P., Xu, P., and Gildea, J. (2016). The Renal Sodium Bicarbonate Cotransporter NBCe2: Is it a Major Contributor to Sodium and pH Homeostasis? Curr. Hypertens. Rep. 18 (9), 71. doi:10.1007/s11906-016-0679-9
Fiorotto, R., Villani, A., Kourtidis, A., Scirpo, R., Amenduni, M., Geibel, P., et al. (2016). CFTR Controls Biliary Epithelial Inflammation and Permeability by Regulating Src Tyrosine Kinase Activity. Hepatol. (Baltimore, Md 64 (6), 2118–2134. doi:10.1002/hep.28817
Franczak, A., Kolačkov, K., Jawiarczyk-Przybyłowska, A., and Bolanowski, M. (2018). Association between FTO Gene Polymorphisms and HDL Cholesterol Concentration May Cause Higher Risk of Cardiovascular Disease in Patients with Acromegaly. Pituitary 21 (1), 10–15. doi:10.1007/s11102-017-0840-8
Grigor'eva, I., and Romanova, T. (2020). Gallstone Disease and Microbiome. Microorganisms 8 (6), 835. doi:10.3390/microorganisms8060835
Habeck, M., Kapri-Pardes, E., Sharon, M., and Karlish, S. (2017). Specific Phospholipid Binding to Na,K-ATPase at Two Distinct Sites. Proc. Natl. Acad. Sci. United States America 114 (11), 2904–2909. doi:10.1073/pnas.1620799114
Han, T., Lv, Y., Wang, S., Hu, T., Hong, H., and Fu, Z. (2019). PPARγ Overexpression Regulates Cholesterol Metabolism in Human L02 Hepatocytes. J. Pharmacol. Sci. 139 (1), 1–8. doi:10.1016/j.jphs.2018.09.013
Hu, F., Chen, H., Guo, F., Yang, M., Jiang, X., Yu, J., et al. (2021a). Biliary Microbiota and Mucin 4 Impact the Calcification of Cholesterol Gallstones. Hepatobiliary Pancreatic Diseases International : HBPD INT. 20 (1), 61–66. doi:10.1016/j.hbpd.2020.12.002
Hu, P., Li, H., Sun, W., Wang, H., Yu, X., Qing, Y., et al. (2021b). Cholesterol-associated Lysosomal Disorder Triggers Cell Death of Hematological Malignancy: Dynamic Analysis on Cytotoxic Effects of LW-218. Acta Pharmaceutica Sinica. B 11 (10), 3178–3192. doi:10.1016/j.apsb.2021.02.004
Ilnytska, O., Lai, K., Gorshkov, K., Schultz, M., Tran, B., Jeziorek, M., et al. (2021). Enrichment of NPC1-Deficient Cells with the Lipid LBPA Stimulates Autophagy, Improves Lysosomal Function, and Reduces Cholesterol Storage. J. Biol. Chem. 297 (1), 100813. doi:10.1016/j.jbc.2021.100813
Katsika, D., Grjibovski, A., Einarsson, C., Lammert, F., Lichtenstein, P., and Marschall, H. (2005). Genetic and Environmental Influences on Symptomatic Gallstone Disease: a Swedish Study of 43,141 Twin Pairs. Hepatol. (Baltimore, Md 41 (5), 1138–1143. doi:10.1002/hep.20654
Khan, M., Kazi, T., Afridi, H., Sirajuddin, , , Bilal, M., Akhtar, A., et al. (2017). Variation of Calcium, Copper and Iron Levels in Serum, Bile and Stone Samples of Patients Having Different Types of Gallstone: A Comparative Study. Clinica Chim. Acta Int. J. Clin. Chem. 471, 254–262. doi:10.1016/j.cca.2017.06.012
Kim, J., Cho, S., Kang, S., Kim, E., Yi, H., Yun, E., et al. (2012). N-3 Polyunsaturated Fatty Acid Attenuates Cholesterol Gallstones by Suppressing Mucin Production with a High Cholesterol Diet in Mice. J. Gastroenterol. Hepatol. 27 (11), 1745–1751. doi:10.1111/j.1440-1746.2012.07227.x
Klein, A., Amigo, L., Retamal, M., Morales, M., Miquel, J., Rigotti, A., et al. (2006). NPC2 Is Expressed in Human and Murine Liver and Secreted into Bile: Potential Implications for Body Cholesterol Homeostasis. Hepatol. (Baltimore, Md 43 (1), 126–133. doi:10.1002/hep.20985
Kubica, K., and Balbus, J. (2021). A Computer Study of the Risk of Cholesterol Gallstone Associated with Obesity and normal Weight. Scientific Rep. 11 (1), 8868. doi:10.1038/s41598-021-88249-w
Kumar, P., Soni, D., Sen, C., Larsen, M., Mazan-Mamczarz, K., Piao, Y., et al. (2021). SFPQ Rescues F508del-CFTR Expression and Function in Cystic Fibrosis Bronchial Epithelial Cells. Scientific Rep. 11 (1), 16645. doi:10.1038/s41598-021-96141-w
Lammert, F., Gurusamy, K., Ko, C., Miquel, J., Méndez-Sánchez, N., Portincasa, P., et al. (2016). Gallstones. Nat. Rev. Dis. primers 2, 16024. doi:10.1038/nrdp.2016.24
Liu, S., Li, Y., Shi, G., Jiang, M., Chang, J., and Wu, H. (2007). The Effect of Statin on the Aortic Gene Expression Profiling. Int. J. Cardiol. 114 (1), 71–77. doi:10.1016/j.ijcard.2006.01.009
Lu, X., Yang, R., Zhang, J., Wang, P., Gong, Y., Hu, W., et al. (2018). Tauroursodeoxycholic Acid Produces Antidepressant-like Effects in a Chronic Unpredictable Stress Model of Depression via Attenuation of Neuroinflammation, Oxido-Nitrosative Stress, and Endoplasmic Reticulum Stress. Fundam. Clin. Pharmacol. 32 (4), 363–377. doi:10.1111/fcp.12367
McConnell, T., Appleby, P., and Key, T. (2017). Vegetarian Diet as a Risk Factor for Symptomatic Gallstone Disease. Eur. J. Clin. Nutr. 71 (6), 731–735. doi:10.1038/ejcn.2016.252
Mehrdad, M., Doaei, S., Gholamalizadeh, M., Fardaei, M., Fararouei, M., and Eftekhari, M. (2020). Association of FTO Rs9939609 Polymorphism with Serum Leptin, Insulin, Adiponectin, and Lipid Profile in Overweight Adults. Adipocyte 9 (1), 51–56. doi:10.1080/21623945.2020.1722550
Morales, M., Amigo, L., Balboa, E., Acuña, M., Castro, J., Molina, H., et al. (2010). Deficiency of Niemann-Pick C1 Protein Protects against Diet-Induced Gallstone Formation in Mice. Liver Int. : official J. Int. Assoc. Study Liver 30 (6), 887–897. doi:10.1111/j.1478-3231.2010.02230.x
Pang, Y., Lv, J., Kartsonaki, C., Yu, C., Guo, Y., Du, H., et al. (2020). Association of Physical Activity with Risk of Hepatobiliary Diseases in China: a Prospective Cohort Study of 0.5 Million People. Br. J. Sports Med. 55 (18), 1024–1033. doi:10.1136/bjsports-2020-102174
Parker, M. (2018). Mouse Models of SLC4-Linked Disorders of HCO-Transporter Dysfunction. Am. J. Physiol. Cel Physiol. 314 (5), C569–C588. doi:10.1152/ajpcell.00301.2017
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A. R., Bender, D., et al. (2007). PLINK: A Toolset for Whole-Genome Association and Population-Based Linkage AnalysisA Global Reference for Human Genetic Variation. Am. J. Hum. GeneticsNature 81526 (37571), 55968–57574. doi:10.1086/519795
Rebholz, C., Krawczyk, M., and Lammert, F. (2018). Genetics of Gallstone Disease. Eur. J. Clin. Invest. 48 (7), e12935. doi:10.1111/eci.12935
Shaikh, F., Shah, T., Madkhali, N., Gaber, A., Alsanie, W., Ali, S., et al. (2021). Frequency Distribution and Association of Fat-Mass and Obesity (FTO) Gene SNP Rs-9939609 Variant with Diabetes Mellitus Type-II Population of Hyderabad, Sindh, Pakistan. Saudi J. Biol. Sci. 28 (8), 4183–4190. doi:10.1016/j.sjbs.2021.06.001
Song, S., Shi, J., Wang, X., Guo, Y., Hu, P., Zhu, F., et al. (2020). Prevalence and Risk Factors for Gallstone Disease: A Population-Based Cross-Sectional Study. J. Dig. Dis. 21 (4), 237–245. doi:10.1111/1751-2980.12857
Stender, S., Nordestgaard, B., and Tybjaerg-Hansen, A. (2013). Elevated Body Mass index as a Causal Risk Factor for Symptomatic Gallstone Disease: a Mendelian Randomization Study. Hepatol. (Baltimore, Md 58 (6), 2133–2141. doi:10.1002/hep.26563
Sun, D., Niu, Z., Zheng, H., Wu, F., Jiang, L., Han, T., et al. (2021). A Mitochondrial DNA Variant Elevates the Risk of Gallstone Disease by Altering Mitochondrial Function. Cell Mol. Gastroenterol. Hepatol. 11 (4), 1211–1226. e1215. doi:10.1016/j.jcmgh.2020.11.015
Wang, H., Afdhal, N., Gendler, S., and Wang, D. (2006). Evidence that Gallbladder Epithelial Mucin Enhances Cholesterol Cholelithogenesis in MUC1 Transgenic Mice. Gastroenterology 131 (1), 210–222. doi:10.1053/j.gastro.2006.04.011
Wang, Y., Jin, H., Wang, Y., Yao, Y., Yang, C., Meng, J., et al. (2021). Sult2b1 Deficiency Exacerbates Ischemic Stroke by Promoting Pro-inflammatory Macrophage Polarization in Mice. Theranostics 11 (20), 10074–10090. doi:10.7150/thno.61646
Wirth, J., Song, M., Fung, T., Joshi, A., Tabung, F., Chan, A., et al. (2018). Diet-quality Scores and the Risk of Symptomatic Gallstone Disease: a Prospective Cohort Study of Male US Health Professionals. Int. J. Epidemiol. 47 (6), 1938–1946. doi:10.1093/ije/dyy210
Wright, J., Craig, W., Lucas, F., Goldfarb, D., Zuckerman, J., and Taylor, E. (2021). Urinary Stone Disease Prevalence and Associations in Cystic Fibrosis. Urolithiasis 49, 415–423. doi:10.1007/s00240-021-01244-8
Wu, Z., Li, J., Zhang, Y., Hu, L., and Peng, X. (2020). CFTR Regulates the Proliferation, Migration and Invasion of Cervical Cancer Cells by Inhibiting the NF-Κb Signalling Pathway. Cancer Manag. Res. 12, 4685–4697. doi:10.2147/cmar.S252296
Yadav, A., and Jang, B. (2021). Inhibition of Lipid Accumulation and Cyclooxygenase-2 Expression in Differentiating 3T3-L1 Preadipocytes by Pazopanib, a Multikinase Inhibitor. Int. J. Mol. Sci. 22 (9), 4884. doi:10.3390/ijms22094884
Yang, J., Lu, X., Liu, S., and Zhao, S. (2020). The Involvement of Genes Related to Bile Secretion Pathway in Rat Tooth Germ Development. J. Mol. histology 51 (1), 99–107. doi:10.1007/s10735-020-09861-0
Yoo, K., Choi, H., Jun, D., Lee, H., Lee, O., Yoon, B., et al. (2016). MUC Expression in Gallbladder Epithelial Tissues in Cholesterol-Associated Gallbladder Disease. Gut and liver 10 (5), 851–858. doi:10.5009/gnl15600
Yuan, Z., Han, T., Jiang, Z., Fei, J., Zhang, Y., Qin, J., et al. (2005). Expression Profiling Suggests a Regulatory Role of Gallbladder in Lipid Homeostasis. World J. Gastroenterol. 11 (14), 2109–2116. doi:10.3748/wjg.v11.i14.2109
Zhang, J., Che, R., Li, X., Tang, W., Zhao, Q., Tang, R., et al. (2010). No Association between the FXYD6 Gene and Schizophrenia in the Chinese Han Population. J. Psychiatr. Res. 44 (6), 409–412. doi:10.1016/j.jpsychires.2009.10.009
Keywords: cholesterol, single nucleotide polymorphism, FXYD2, Tibetans, gallstones
Citation: Ma L, Chen H, Zhang Z, Liu L, Zhao Y, Li Y, Zhao Z, Chen H and Kang L (2022) Association Study Between Polymorphic Loci in Cholesterol Metabolism Pathway and Gallstone in the Tibetan Population. Front. Genet. 13:902553. doi: 10.3389/fgene.2022.902553
Received: 23 March 2022; Accepted: 13 April 2022;
Published: 16 May 2022.
Edited by:
Chuan-Chao Wang, Xiamen University, ChinaReviewed by:
Weiyu Li, University of California, San Francisco, United StatesShengjie Nie, Kunming Medical University, China
Copyright © 2022 Ma, Chen, Zhang, Liu, Zhao, Li, Zhao, Chen and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Chen, Y2hlbmh0NTZAbWFpbC5zeXN1LmVkdS5jbg==; Longli Kang, bG9uZ2xpX2thbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lifeng Ma1,2†
Lifeng Ma1,2† Haitao Chen
Haitao Chen Longli Kang
Longli Kang