
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet., 01 July 2022
Sec. Statistical Genetics and Methodology
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.899923
Background: Allergic rhinitis (AR) is an especially common disorder associated with both environmental and genetic factors, and a lot of researchers have attempted to find polymorphisms which predisposed to the disease. We conducted a meta-analysis of the most frequently researched polymorphisms to find those genes which may be susceptible to AR and then may be of value in diagnosis.
Methods: Pubmed and China National Knowledge Infrastructure (CNKI) databases were searched to screen out eligible studies focusing on the correlation between polymorphisms and AR susceptibility, and then polymorphisms cited in at least 3 studies were selected.
Results: The 142 papers originally selected cited 78 genes. Twelve genes (coinciding with 23 polymorphisms) were reported in more than three papers. Twenty-three polymorphisms were involved in the meta-analysis. Among the 23 polymorphisms, only 4 were found to be related to the risk of AR: IL-13 rs20541, CTLA-4 rs11571302, IL-4R RS1801275 and ACE (I/D). The remaining 19 of the 23 polymorphisms were not associated with AR.
Conclusion: We found polymorphisms that could be used for AR diagnosing and those that were unrelated to AR. This may be the first step in detecting polymorphic combinations susceptible to AR (IL-13 RS20541, CTLA-4 RS11571302, IL-4R RS1801275 and ACE (I/D). In addition, our results may improve AR diagnosis and contribute to the intensive study of AR.
Allergic rhinitis (AR) is a chronic inflammatory disorder of the nasal mucosa mediated by allergic hypersensitivity responses to environmental allergens (Greiner et al., 2011). As reported, 10%–20% of the world’s population suffers from AR (Brożek et al., 2010), which has become a global health problem. However, the underlying cause for AR is poorly understood and prevention of the disease is impossible. AR is a complex heterogeneous disease involving genetic and environment factors (Barnes, 2000). Studies of twins have shown that genetic factors play a significant role in the pathogenesis of allergic disease (Khan et al., 2018), and individuals with a family history of allergic disease are more likely to develop allergic symptoms (Sih and Mion, 2010). Single nucleotide polymorphisms (SNPs) are considered to be an extremely crucial genetic factor for allergic diseases (Suzuki et al., 2016; Ashley et al., 2017) which can upregulate or downregulate the susceptibility of AR (Black et al., 2009). Tang et al. (2020) elucidated that IL13 rs20541 SNP may contribute to the susceptibility to AR and increase the risk of AR in Asian population. Besides, Qian et al. (2010) demonstrated that the A allele of rs11466651 in TLR10 will reduce the susceptibility of AR, which mean that the polymorphism protects against AR. Furthermore, the association of SNPs with AR also exists controversial. For example, some researchers have found that IL-4 rs2243250 allele T could increase or decrease the risk of AR (Lu et al., 2011; Movahedi et al., 2013; Shirkani et al., 2019). In contrast, others believed that allele T is not related to an increased prevalence of AR (Valatabar et al., 2020).
To solve the limitations of individual researches, a comprehensive evaluation of the relationship between SNPs and vulnerability to AR is required in synthesized studies. The purpose of this study was, first, to provide a detailed review of the SNPs researched in AR, and second, to evaluate the correlation between the most frequently reported SNPs and the susceptibility to AR by meta-analysis, so as to further understand the molecular mechanism of this disease and provide underlying therapeutic targets or approaches for the reuse of existing drugs.
We performed a exhaustive literature search on PubMed and CNKI, with the search strategies being (“allergic rhinitis” or “AR”) and (“mutation” or “polymorphism”). The detailed search strategy is supplied in Supplementary Table S1. There were no restrictions on language or date of publication. The literature search was finally updated on 11 February 2022. Eligibility for this publication was independently evaluated by two researchers (FX and LW). For purpose of reviewing the literature as widely and efficiently as possible, we also reviewed references of relevant studies to supplement the valuable literature. In the event of uncertainty, the full text was acquired and any disagreement was resolved by the reviewer’s agreement.
In order to gain the credibility of our results and restrict the amount of meta-analyses completed, only the genes and polymorphisms reported in at least three diverse studies were considered in the analysis.
For meta-analysis, studies must conform to the following inclusion standard: 1) original case-control studies of AR; 2) An association between AR and at least one polymorphism was reported and sufficient data were available to calculate odds ratio (OR) and corresponding 95% CI; 3) Genotype frequency did not deviate from Hardy-Weinberg equilibrium (HWE) significantly; 4) Based on the symptoms and allergen tests or confirmed histologically, people were diagnosed as AR. Duplicate researches and researches with overlapping data were excluded. We also excluded retrospective studies, systematic reviews, and meta-analyses.
Exclusion criteria were as follows: 1) not a case-control study; 2) irrelevant to SNPs and AR risk; 3) Lack of detailed data.
One investigator (FX) extracted the data from every eligible study, which was checked by the second investigator (LW). Divergences were settled by consultation with the third researcher (YP). For each study, the following data were collected: the title, the authors, the journal, the year of publication, the country of study team, the sample capacity, and the method of diagnose.
After data extraction, meta-analyses for polymorphisms in at least three publications were performed. The RevMan 5.3 software was employed to complete all statistical analyses. The degree of association between AR and interested polymorphism was evaluated. A random-effect OR fixed-effect model was applied to completed meta-analysis through merging OR (95% CI) values of the involved publications. Before merging the publications, heterogeneity needs to be found correctly and effectively, that is, heterogeneity test. I2 test is the heterogeneity evaluation index proposed by Higgins et al. (2003). When I2 = 0, it indicates that no heterogeneity is observed, and the higher I2 value, the greater heterogeneity. The selection of model lies with the heterogeneity of inter-studies. When there was no evidence of heterogeneity, the fixed-effect model was selected (I2 < 50%, p > 0.05) (Li et al., 2018; Li et al., 2019; Li et al., 2020). If not, a random-effect model was used. A value of OR greater than 1 with a CI lower limit greater than 1 and p < 0.05 is believed to be statistically significant, or less than 1 with the upper CI boundary less than 1.
The publication bias was judged intuitively by drawing a funnel plot. The funnel plot symmetric, no publication bias was found; otherwise, publication bias existed. Besides, publication bias was quantitatively estimated by the Egger’s Test. For every polymorphism involved in this research, articles search was conducted to identify prior meta-analyses. And our results were compared with the prior results to identify potential records.
After screening (Figure 1), a total of 23 polymorphisms that had been reported in at least 3 literatures were identified. 142 papers (Supplementary Table S1) reported 78 diverse genes, and 12 of those were studied in at least three publications. 55 polymorphisms were identified totally in the present study (Supplementary Table S2). Then, a systematic review were completed for each of the 23 screening polymorphisms, which were studied in more than three publications (Table 1). The number of publications including every polymorphism, the degree of heterogeneity, the amount of patients and controls, the OR [95% CI] and the application of a random-effect or fixed-effect model are showed in Table 1.
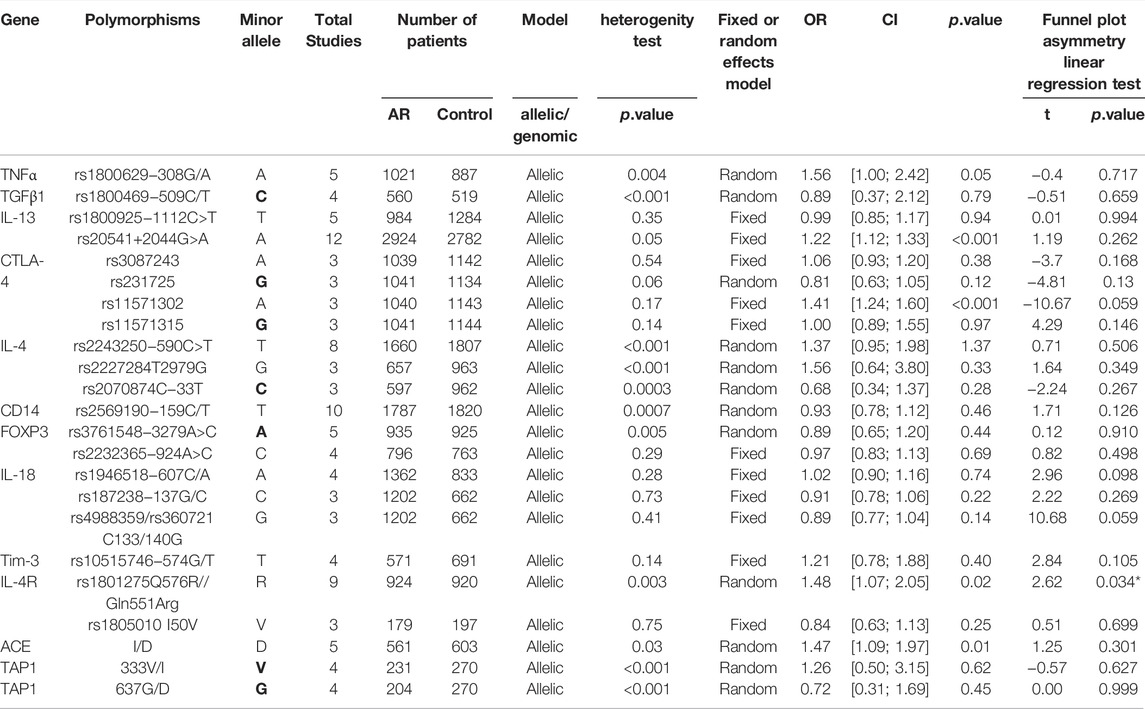
TABLE 1. The primary results of the meta-analyses in exploring the relationship between gene polymorphisms and AR.
Five studies evaluated six tumor necrosis factor (TNF) variants, of which only TNF RS1800629 was considered in our work. Five literatures were included in this meta-analysis (30/59/102/103/104). We didn’t observe significant associations (OR 1.56; 95% CI 1.00-2.42) applying a random-effect model (Figure 2A), and publication bias wasn’t observed as well (t = −0.4; p = 0.717) (Supplementary Figure S1A).
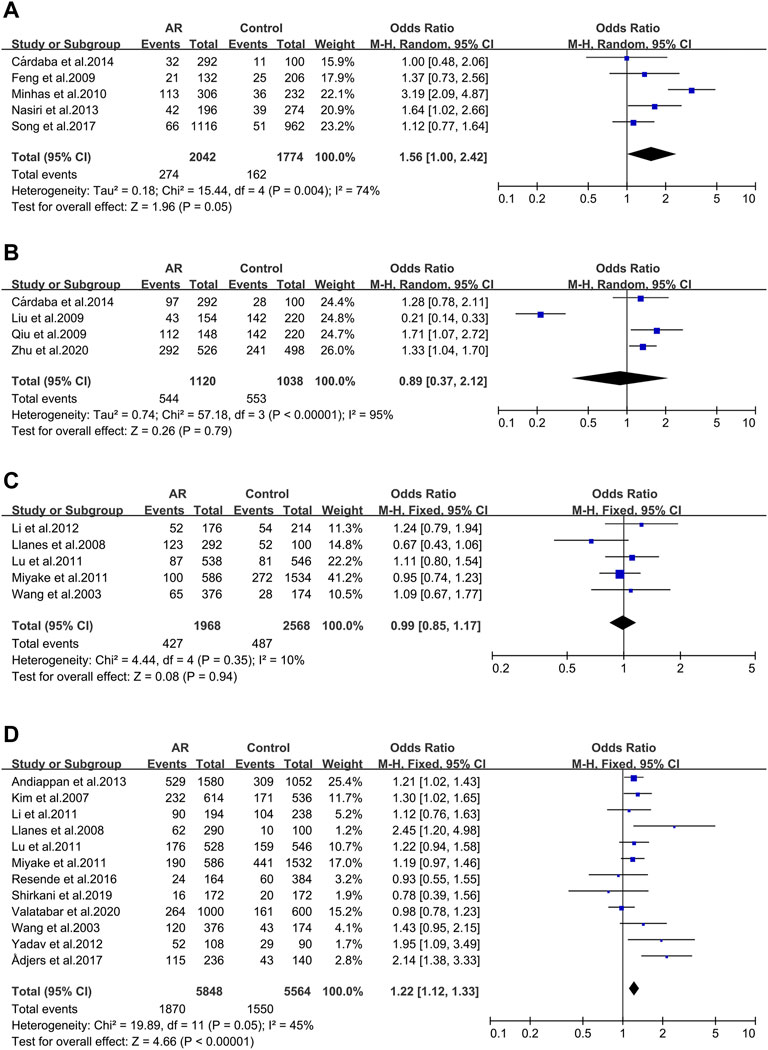
FIGURE 2. Forest plots for TNFα, TGFβ1 and IL-13 polymorphisms. (A) TNFα(rs1800629): A allele; (B) TGFβ1 (rs1800469): allele G; (C) IL-13 (rs1800925): allele T; (D) IL-13 (rs20541): allele A.
Four studies of the Transforming growth factor β1 (TGFβ1) rs1800469 polymorphism were included (32/65/104/141). We didn’t observe significant associations (OR 0.89; 95% CI 0.37–2.12) applying a fixed-effect model (Figure 2B) and publication bias wasn’t observed as well (t = −0.51; p = 0.659) (Supplementary Figure S1B).
Thirteen full-text articles on Interleukin-13(IL-13) SNPs of interest were selected. Two genetic variants of IL-13 were analyzed: rs1800925 (Figure 2C), rs20541 (Figure 2D).
IL-13 _ rs1800925 (-1112C > T/1055). IL-13 _ rs1800925 (-1112C > T/1055) was considered in five studies (41/100/107/117/118) and meta-analysis was completed on them. We didn’t observe significant associations (OR 0.99; 95% CI 0.85–1.17) in a fixed-effect model (Figure 2C), and publication bias wasn’t seen as well (t = 0.01; p = 0.994) (Supplementary Figure S1C).
IL-13_rs20541 (130/ + 2044G > A). Twelve studies dealing with the IL-13_rs20541 A allele were considered (42/100/105/107/108/110/111/113/116/117/118/119) for the meta-analysis. An association was observed (OR 1.22; 95% CI 1.12–1.33) applying a fixed-effect model (Figure 2D) without publication bias (t = 1.19; p = 0.262) (Supplementary Figure S1D).
After screening, four of the nine main allelic mutants of Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) were analyzed: rs3087243 (Figure 3A), rs231725 (Figure 3B), rs11571302 (Figure 3C) and rs11571315 (Figure 3D), and only rs11571302 was significantly associated with AR.
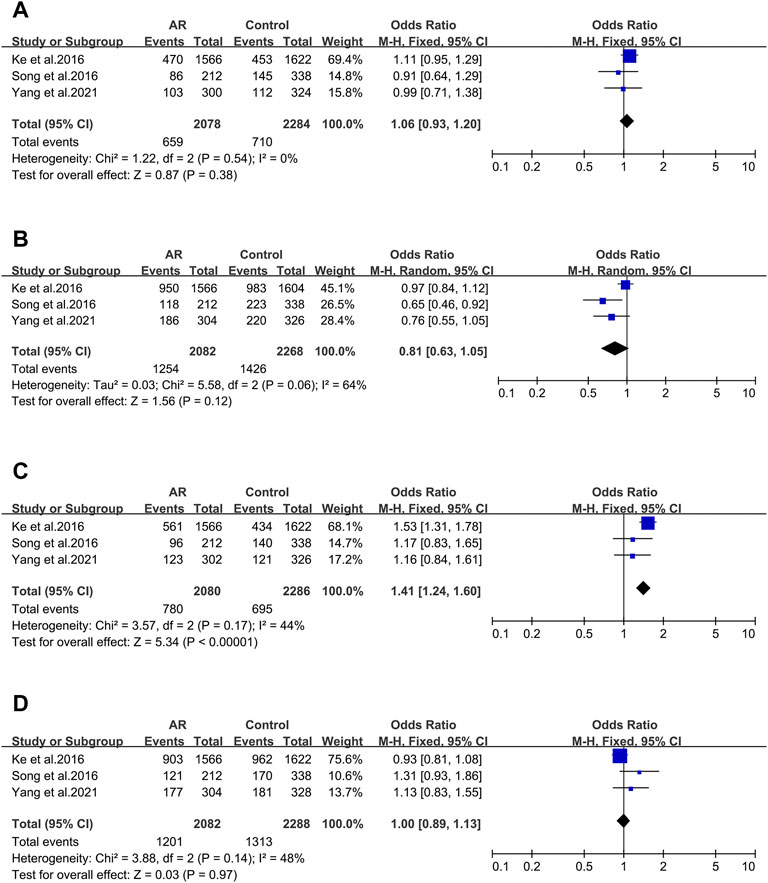
FIGURE 3. Forest plots for CTLA-4 polymorphism. (A) CTLA-4 (rs3087243); (B) CTLA-4 (rs231725); (D) CTLA-4 (rs11571302); (D) CTLA-4 (rs11571315).
CTLA-4 _ rs3087243. Three studies were included (12/94/106). We didn’t observe significant associations (OR 1.06; 95% CI 0.93–1.20) applying a fixed-effect model (Figure 3A). And asymmetry wasn’t seen in the funnel plot test for bias as well (t = −3.7; p = 0.168) (Supplementary Figure S1E).
CTLA-4 _ rs231725. Three studies (12/94/106) were involved in the meta-analysis. We didn’t find significant associations (OR 0.81; 95% CI 0.63–1.05) applying a random-effect model (Figure 3B), and publication bias wasn’t seen as well (t = -4.81; p = 0.13) (Supplementary Figure S2F).
CTLA-4 _ rs11571302. CTLA-4 variant (rs11571302) was considered in three studies (12/94/106). When we considered the A allele as the risk allele, a correlation was found (OR 1.41; 95% CI 1.24–1.60) in a fixed-effect model (Figure 3C) with no publication bias (t = −10.67; p = 0.059) (Supplementary Figure S2G).
Three studies were considered (12/94/106). We didn’t observe significant associations (OR 1.00; 95% CI 0.89–1.55) in a fixed-effect model (Figure 3D), and publication bias wasn’t seen as well (t = 4.29; p = 0.146) (Supplementary Figure S2H).
In the 8 studies of AR and Interleukin-4(IL-4) polymorphisms five IL-4 variants were reported. Only three of them were considered.
IL-4 _ rs2243250 (−590C > T/589). IL-4 _ rs2243250 was considered in eight studies (107/108/109/110/112/113/114/115). When we considered the T allele as the risk allele, associations were not identified (OR 1.37; 95% CI 0.95–1.98) in a random-effect model (Figure 4A), and publication bias wasn’t seen as well (t = 0.71; p = 0.506) (Supplementary Figure S2A).
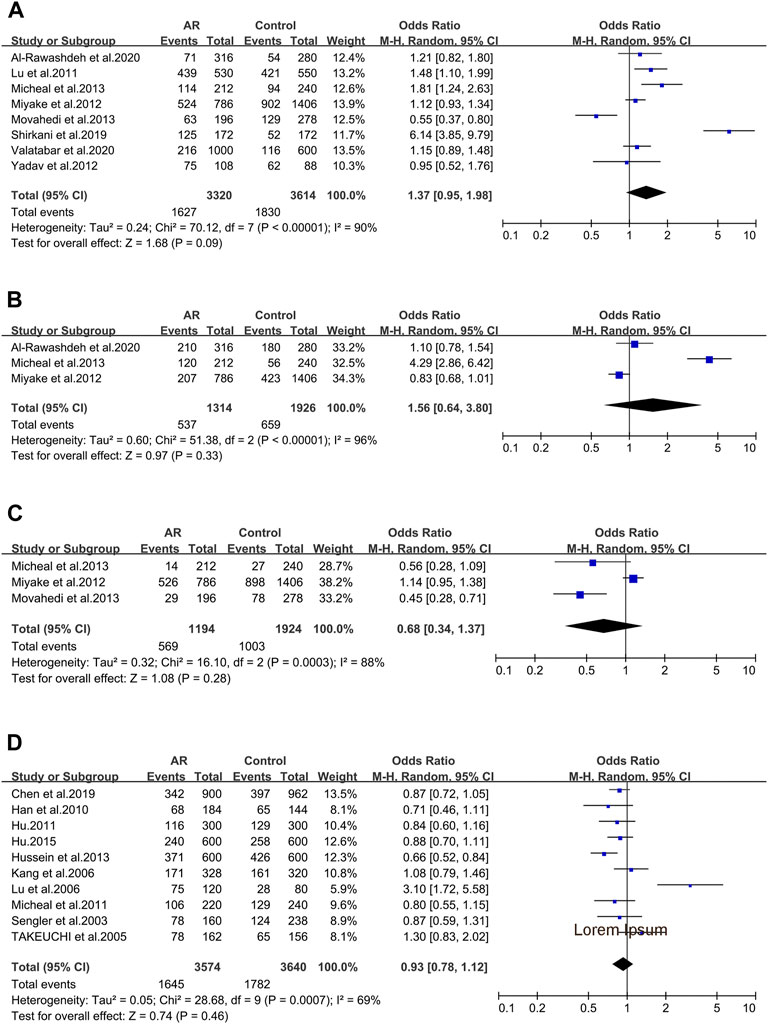
FIGURE 4. Forest plots for IL-4 and CD14 polymorphisms. (A) IL-4 (rs2243250): T allele; (B) IL-4 (rs2227284): allele G; (C) IL-4 (rs2070874): allele T; (D) CD14 (rs2569190): allele T.
IL-4 _ rs2227284 (T2979G). Three studies was also considered (112/114/115) and meta-analysis was completed on these studies. We didn’t find significant associations (OR 1.56; 95% CI 0.64–3.80) in a random-effect model (Figure 4B), and publication bias wasn’t seen as well (t = 1.64; p = 0.349) (Supplementary Figure S2B).
IL-4 _ rs2070874(C-33T). Three studies (109/112/114) were included in this meta-analysis. We didn’t observe significant associations (OR 0.68; 95% CI 0.34–1.37) applying a random-effect model (Figure 4C), and publication bias wasn’t detected as well (t = −2.24; p = 0.267) (Supplementary Figure S2C).
When considering the 10 studies of CD14 variants and AR, solely the rs2569190 polymorphism conformed to the inclusion standard. The ten publications were pooled (5/7/47/63/91/120/121/122/123/124), and we didn’t find significant associations (OR 0.93 95% IC0.78–1.12) applying a random-effect model (Figure 4D), as well as the publication bias (t = 1.71; p = 0.126) (Supplementary Figure S2D).
Two of the four primary genetic variants of forkhead box P3(FOXP3) were analyzed: rs3761548 (Figure 5A) and rs2232365 (Figure 5B), and none was significantly associated with AR.
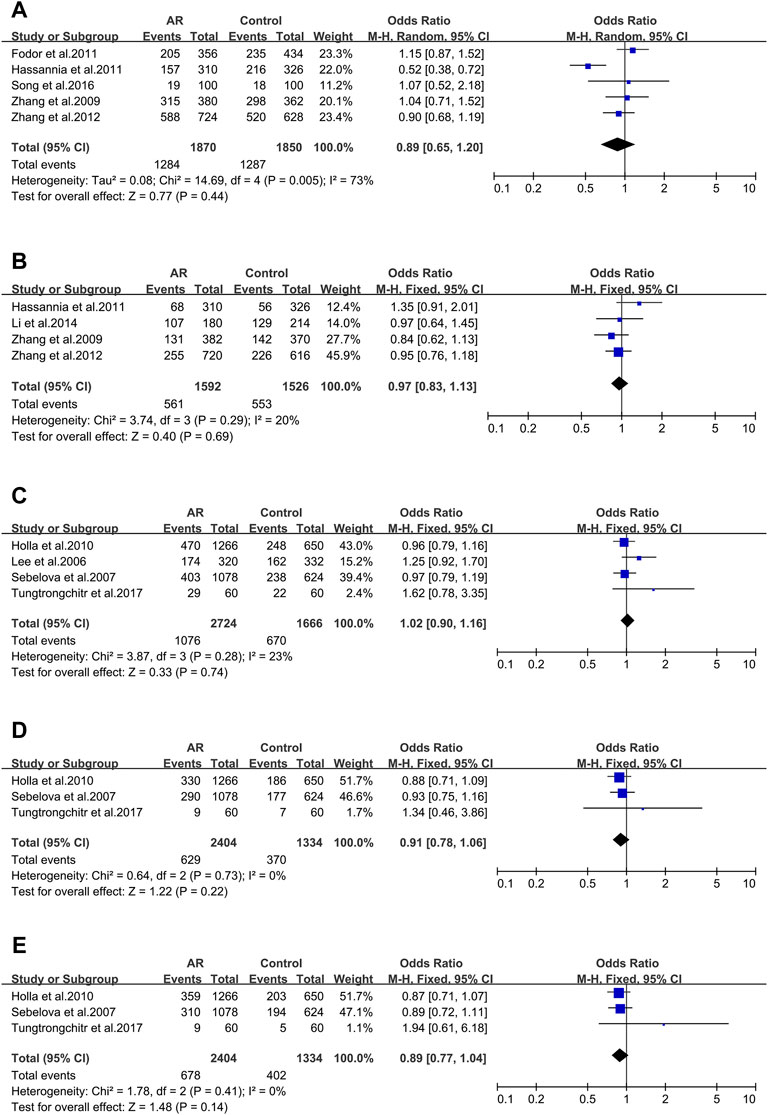
FIGURE 5. Forest plots for FOXP3 and IL-18 polymorphisms. (A) FOXP3 (rs3761548): C allele; (B) FOXP3 (rs2232365): C allele; (C) IL-18 rs1946518): allele A; (D) IL-18 (rs187238): allele C; (E) IL-18 (rs4988359): allele G.
FOXP3_ rs3761548 (−3279A > C). Five studies (50/84/125/126/127) were considered for meta-analysis. Association wasn’t identified (OR 0.89; 95% CI 0.65–1.20) employing the random-effect model (Figure 5A) and publication bias wasn’t found (t = 0.12; p = 0.910) (Supplementary Figure S2E).
FOXP3_ rs2232365 (−924A > C). Four studies (125/126/127/128) were involved (Supplementary Figure S26A). We didn’t find significant associations (OR 0.97; 95% CI 0.83–1.13) in a fixed-effect model (Figure 5B), and publication bias wasn’t found as well (t = 0.82; p = 0.498) (Supplementary Figure S2F).
Seven Interleukin-18(IL-18) variants were reported. Only three of them were considered.
IL-18_ rs1946518 (−607C/A). meta-analysis was completed with data of four publications (73/129/130/131). We didn’t find significant associations (OR 1.02; 95% CI 0.90–1.16) applying a fixed-effect model (Figure 5C), and publication bias wasn’t detected in funnel plot (t = 2.96; p = 0.098) (Supplementary Figure S3A).
IL-18 _ rs187238 (−137G/C). Three relevant studies were considered in this meta-analysis (129/130/131). We didn’t observe significant associations (OR 1.10; 95% CI 0.94–1.28) in a fixed-effect model (Figure 5D), and publication bias wasn’t seen (t = 2.96; p = 0.098) (Supplementary Figure S3B).
IL-18 _rs360721(C133/140G). Three studies (129/130/131) were involved in the meta-analysis. We didn’t observe significant associations (OR 0.89; 95% CI 0.77–1.04) applying the fixed-effect model (Figure 5E). No potential publication bias was identified (t = 10.68; p = 0.059) (Supplementary Figure S3C).
After screening, only one of the main allelic mutants of T cells immunoglobulin domain and mucin domain protein-3(Tim-3) were analyzed.
Tim-3_ rs10515746 (−574G/T). Four studies (6/44/49/132) were involved in this meta-analysis. We didn’t find significant associations (OR 1.21; 95% CI 0.78–1.88) in a fixed-effect model (Figure 6A), and publication bias wasn’t identified as well (t = 2.84; p = 0.105) (Supplementary Figure S3D).
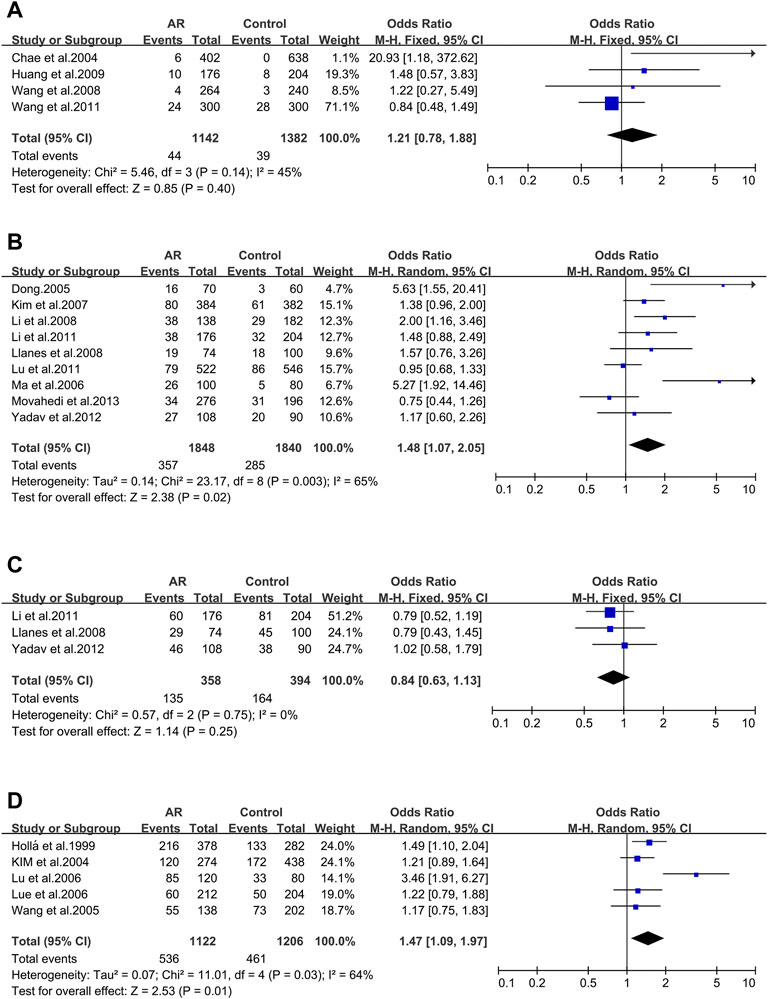
FIGURE 6. Forest plots for Tim-3, IL-4R and ACE polymorphisms. (A) Tim-3 (rs10515746): T allele; (B) IL-4R (rs1801275): allele R; (C) IL-4R (rs1805010): allele V; (D) ACE (I/D): allele D.
AFTER our data screening, both of the primary allelic mutants of IL-4R were analyzed.
IL-4R_ rs1801275 (Q576R/Gln551Arg). Nine studies (35/36/43/107/109/113/116/117/142) were considered in this meta-analysis. We observed significant associations (OR 1.48; 95% CI 1.07–2.05) applying a random-effect model (Figure 6B), and a publication bias across studies was detected (t = 2.62; p = 0.034) (Supplementary Figure S4A).
IL-4R_ rs1805010 (Ile50Val). Three studies (113/117/142) were included for this variant. No association was identified (OR 0.84; 95% CI 0.63–1.13) (Figure 6C) with no publication bias (t = 0.51; p = 0.699) (Supplementary Figure S4B).
ACE_ I/D. When all the five studies were pooled together, we observed a significant association in a random-effect model (OR 1.47; 95% CI 1.09–1.97) (Figure 6D). And publication bias wasn’t identified (t = 1.25; p = 0.301) (Supplementary Figure S4C).
TAP1_333V/I. Four studies dealing with ACE and AR were identified. Based on these four remaining studies (21/56/137/138), we didn’t find significant associations (OR 1.26; 95% CI 0.50–3.15) in a random-effect model (Figure 7A) and no potential publication bias was detected as well (t = −0.57; p = 0.627) (Supplementary Figure S4D).
TAP1_637G/D. Of the four selected studies for TAP1_637G/D (21/56/137/138), meta-analysis was completed with information of these publications, and we didn’t find significant associations (OR 0.72; 95% CI 0.31–1.69) employing a random-effect model (Figure 7B), as well as the publication bias (t = 0.00; p = 0.999) (Supplementary Figure S4E).
The aim of this research was to offer a detailed review of polymorphisms about AR and to conduct a meta-analysis of those most frequently studied polymorphisms. Finally, 23 polymorphisms (in 12 genes) were meta-analyzed. Four polymorphisms were identified to be significantly related to the risk of AR. Thus, we were allowed to identify those polymorphisms that had the potential to constitute a screening test for AR and put forward combinations of SNPs that could prerecognize AR: IL-13 rs20541, CTLA-4 rs11571302, IL-4R rs1801275 and ACE (I/D).
To be as detailed as possible, we conducted extensive PubMed and CNKI search queries. The amount of 142 papers (involving 78 genes) were found to be related. To increase efficiency, only the genes that had been researched in three or more studies was considered in our study. Of the 142 publications cited in the Results section, 38 was augmented manually for the reason that the keyword “AR” was missing. The manual augment of references was not unexpected, because we were allowed to find relevant data quickly and accurately by our strategy.
To the best of our knowledge, our study is the first to have emphasized the potential association between AR and the CTLA-4 rs11571302 polymorphisms through meta-analysis (Figure 2C). The CTLA-4 gene plays a negative regulatory role in antigen-presenting cells (APC) activation of T cells (Walunas et al., 1996). Inducible CTLA-4 under-expression with Th2/Treg imbalance is associated to AR (Botturi et al., 2011). In our meta-analyses, CTLA-4 rs11571302 was related to AR (with OR of 1.41 [95% CI 1.24–1.60]). The frequencies of the rs11571302 A allele was obviously associated with the risk of AR. In contrast to the result for CTLA-4 rs11571302, the literature data on CTLA-4 rs3087243, CTLA-4 rs231725, and CTLA-4 rs11571315 show that they have no noticeable association with the AR risk.
Inflammation and immunization are known to be involved in the pathogenesis of AR. The Type 2 cytokines (IL-13, IL-4 and IL-18) are the initiating factors of airway inflammatory response (Wan et al., 2015; Che et al., 2018). Several studies have unveiled that levels of IL-13, IL4 and IL18 in peripheral secretion appear to be abnormally high in patients with AR (Shamji et al., 2021). Our meta-analysis emphasized an association between the IL-13 rs20541 polymorphism and AR risk. IL-13 rs20541 imparts susceptibility to the development of AR. However, we observed no link between IL-13 rs1800925 and AR. These results were similar to the previous meta-analyses (Chen et al., 2018; Tang et al., 2020).
No associations with AR were detected here for IL-4 rs2243250, IL-4 rs2227284 and IL-4 rs2070874. In contrast, one previous study reported that the IL-4 rs2243250 genotype is associated with AR susceptibility (Jiang and Yan, 2021). We excluded one study not published online any more which was taken into account in the previous meta-analysis. Obviously, the selection of control participants affects the final result. Considering the instability of the results, further studies are required to get a more comprehensive result to ensure or refute the underlying association between AR and the IL-4 rs2243250 polymorphism. Associations were not also found for IL-18 rs1946518, IL-18 rs187238 and IL-18 rs360721, which confirmed with the earlier meta-analysis (Tang et al., 2020). Further researches are deemed unnecessary.
All biological functions of IL-4 are mediated by effector IL-4R, including mediating Th1/Th2 imbalance, promoting B cell proliferation and IgE synthesis, which play a crucial role in allergic rhinitis (Moniuszko et al., 2013). Our result exhibited that IL-4R rs1801275 was associated with the risk of AR (OR 1.48; 95% CI 1.07–2.05), and the R allele increase the susceptibility to AR. But the correlation needs to be validated in more studies, because there is potential publication bias in Egger’s test. In contrast, we detected no association between AR and IL-4R rs1805010 polymorphism.
ACE I/D polymorphism may influence the strength of immunological response and has been reported to be associated with atopic disorders, inflammatory diseases (Eryüksel et al., 2009). Our result revealed that ACE D allele augments the risk of developing AR, concordant with previous researches (Huang et al., 2016; Li et al., 2017).
TNF-α functions as a pro-inflammatory cytokine involving in T cell activation and cytokine release, recruitment of neutrophils, macrophages and monocytes, and increased histamine release from airway mast cells. Here, we ensured the known result of association previously shown by another meta-analysis (Zhou, 2019). We found no correlation between AR and TNF-α (−308G/A).
TGF-β is known to be involved in AR, which can induce initial CD4 + T cells to differentiate into CD4 + CD25 + Treg cells and stabilize the immune function of the body (Gu et al., 2017). Elevated levels of TGF-β1 can alleviate the inflammatory of nasal mucosa in AR (Peng et al., 2022). To date, the results focused on the association between TGF-β1 rs1800469 polymorphism and AR risk were inconsistent. One paper have demonstrated that TGF-β1 polymorphism has a protective effect for AR (Liu et al., 2009). Conversely, the results from other researches shown that the TGF-β1 SNP could increase the susceptibility of developing AR or was not associated with AR (Qiu et al., 2009; Cárdaba et al., 2014; Zhu et al., 2020). Our present work didn’t find a relation of AR and TGF-β1 rs1800469 polymorphism. Additional well-designed case-control researches in large cohorts are needed to confirm the underlying link between AR and TGF-β1 rs1800469 polymorphism.
Studies have shown that CD14/-159 may influence the regulation of CD14 gene expression and thus modulate the influence of CD14-mediated events on innate and adaptive immune responses (LeVan et al., 2001). CD14 specifically binds to LPS to support the conversion of Th0 to Th1, and cytokines produced by Th1 and Th2 control the synthesis of IgE (Baldini et al., 1999). Compared with normal people, the serum CD14 in AR patients is significantly higher, and the risk of AR is significantly associated with the homozygous carriers of the T allele (TT genotype) (Zare Marzouni et al., 2019). Interestingly, it was reported that TT genotype carriers have a significant increase in serum levels of sCD14 and a concomitant decrease in total serum IgE (Qiu et al., 2009). However, TT homozygotes expression was higher in AR patients, but not associated with IgE expression among the Chinese population (Han et al., 2010). In this meta-analysis, CD14 (−159C/T) T allele was not associated with AR susceptibility, which was in line with previous studies (Xu and Wang, 2014; Chen et al., 2018).
FOXP3, as a specific transcription factor of Treg cells, is involved in the development and functional maintenance of Treg cells (Hori et al., 2003). Compared with healthy people, the expression of FOXP3 in peripheral blood of AR patients is significantly decreased, which leads to the dysfunction of Treg cells and is an important factor leading to the onset of AR (Huang et al., 2014). Our result was similar to the earlier meta-analysis (Zhang et al., 2017; Tang et al., 2020). There was no link between AR and FOXP3 rs3761548 or FOXP3 rs2232365.
Tim-3 has a negative regulatory effect on Th1 cell and causes Th1/Th2 imbalance, which is required for the development of AR (Pan et al., 2010). It was reported that increased level of Tim-3 in the peripheral blood of AR patients (Shi and Liu, 2018). However, the meta-analysis result of Tim-3 rs10515746 indicated that it was not significantly related to the AR risk.
Inhaled antigen is presented to CD4 + T cells in AR, and TAP1 gene encodes molecules involved in endogenous antigen processing (Kim et al., 2007). TAP gene polymorphism may affect antigenic peptide selection and transport processes and change immune response regulation in AR. However, we did not identify the potential link between AR and TAP1 333V/I or TAP1 637G/D.
In the end, this study has several limitations. Firstly, for greater efficiency, it only considered the polymorphisms cited in more than three various studies, which may miss some significant associations to AR. Thus, to confirm the underlying correlations between the remaining polymorphisms and the susceptibility of AR, more studies will be necessary. Second, the susceptibility to AR is not only related to heredity, but also to race, age, sex and so on. However, due to the small number of studies and small samples in the present analysis, stratifications for race, age and sex were not performed. Besides, the susceptibility to AR may be affected by multiple gene loci, which coordinate with each other to offset the genetic susceptibility to developing AR. These may be the reasons why only 4 SNPs were observed to have an association with the risk of AR in this study.
As mentioned above, four of the polymorphisms could be analyzed simultaneously as they were associated with AR. For the few polymorphisms not involved in this study, that is, those showing a positive correlation based on two studies, further research is needed. This work allow researchers begin to find the underlying markers for developing a diagnostic method for AR patients. The panel of these polymorphisms exhibits the potential in forming a genetic screening test about AR and may thus lessen the time used in diagnosing AR.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
FX, ZZ, and LW were suitable for the study design, literature searches, statistical analysis, and manuscript preparation. The study was supervised by QZ and YY.
This work is supported by the Xinglin Scholars Scientific Research Promotion Plan of Chengdu University of Traditional Chinese Medicine-Innovation team of traditional Chinese medicine otorhinolaryngology discipline, natural science (No. XKTD2021003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.899923/full#supplementary-material
Ashley, S. E., Tan, H.-T. T., Vuillermin, P., Dharmage, S. C., Tang, M. L. K., Koplin, J., et al. (2017). The Skin Barrier Function Gene SPINK5 Is Associated with Challenge-Proven IgE-Mediated Food Allergy in Infants. Allergy 72 (9), 1356–1364. doi:10.1111/all.13143
Baldini, M., Carla Lohman, I., Halonen, M., Erickson, R. P., Holt, P. G., and Martinez, F. D. (1999). A Polymorphism* in the 5 ′ Flanking Region of the CD14 Gene Is Associated with Circulating Soluble CD14 Levels and with Total Serum Immunoglobulin E. Am. J. Respir. Cell Mol. Biol. 20 (5), 976–983. doi:10.1165/ajrcmb.20.5.3494
Barnes, K. C. (2000). Evidence for Common Genetic Elements in Allergic Disease. J. Allergy Clin. Immunol. 106 (5 Suppl. l), S192–S200. doi:10.1067/mai.2000.110150
Black, S., Teixeira, A. S., Loh, A. X. W., Vinall, L., Holloway, J. W., Hardy, R., et al. (2009). Contribution of Functional Variation in theIL13gene to Allergy, Hay Fever and Asthma in the NSHD Longitudinal 1946 Birth Cohort. Allergy 64 (8), 1172–1178. doi:10.1111/j.1398-9995.2009.01988.x
Botturi, K., Lacoeuille, Y., Cavaillès, A., Vervloet, D., and Magnan, A. (2011). Differences in Allergen-Induced T Cell Activation between Allergic Asthma and Rhinitis: Role of CD28, ICOS and CTLA-4. Respir. Res. 12 (1), 25. doi:10.1186/1465-9921-12-25
Brożek, J. L., Bousquet, J., Baena-Cagnani, C. E., Bonini, S., Canonica, G. W., Casale, T. B., et al. (2010). Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 Revision. J. Allergy Clin. Immunol. 126 (3), 466–476. doi:10.1016/j.jaci.2010.06.047
Cárdaba, B., Calzada, D., Baos, S., Aguerri, M., Quiralte, J., and Lahoz, C. (2014). Polymorphisms of Tumor Necrosis Factor-α, Transforming Growth Factor-β, and Interleukin-10 in Asthma Associated with Olive Pollen Sensitization. J. Immunol. Res. 2014, 276345. doi:10.1155/2014/276345
Che, N., Han, B., Liu, S. X., Ge, R. M., and Yu, S. Q. (2018). Experimental Study on Dendritic Cells Modified by Dust Mite Allergen Gene in the Treatment of Allergic Rhinitis in Mouse. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 53 (9), 675–679. doi:10.3760/cma.j.issn.1673-0860.2018.09.007
Chen, M.-L., Zhao, H., Huang, Q.-P., and Xie, Z.-F. (2018). Single Nucleotide Polymorphisms of IL-13 and CD14 Genes in Allergic Rhinitis: a Meta-Analysis. Eur. Arch. Otorhinolaryngol. 275 (6), 1491–1500. doi:10.1007/s00405-018-4975-7
Eryüksel, E., Ceyhan, B. B., Bircan, R., Avşar, M., and Çirakoğlu, B. (2009). Angiotensin Converting Enzyme Gene Polymorphism in Turkish Asthmatic Patients. J. Asthma 46 (4), 335–338. doi:10.1080/02770900802660972
Greiner, A. N., Hellings, P. W., Rotiroti, G., and Scadding, G. K. (2011). Allergic Rhinitis. Lancet 378 (9809), 2112–2122. doi:10.1016/S0140-6736(11)60130-X
Gu, Z. W., Wang, Y. X., and Cao, Z. W. (2017). Neutralization of Interleukin-17 Suppresses Allergic Rhinitis Symptoms by Downregulating Th2 and Th17 Responses and Upregulating the Treg Response. Oncotarget 8 (14), 22361–22369. doi:10.18632/oncotarget.15652
Han, D., She, W., and Zhang, L. (2010). Association of the CD14 Gene Polymorphism C-159T with Allergic Rhinitis. Am. J. Rhinol. Allergy 24 (1), e1–e3. doi:10.2500/ajra.2010.24.3411
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. Bmj 327, 557–560. doi:10.1136/bmj.327.7414.557
Hori, S., Nomura, T., and Sakaguchi, S. (2003). Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 299 (5609), 1057–1061. doi:10.1126/science.1079490
Huang, R.-F., Dong, P., Zhang, T.-Z., Ying, X.-J., and Hu, H. (2016). Angiotensin-converting Enzyme Insertion/deletion Polymorphism and Susceptibility to Allergic Rhinitis in Chinese Populations: a Systematic Review and Meta-Analysis. Eur. Arch. Otorhinolaryngol. 273 (2), 277–283. doi:10.1007/s00405-014-3350-6
Huang, X., Chen, Y., Zhang, F., Yang, Q., and Zhang, G. (2014). Peripheral Th17/Treg Cell-Mediated Immunity Imbalance in Allergic Rhinitis Patients. Braz. J. Otorhinolaryngology 80 (2), 152–155. English, Portuguese. doi:10.5935/1808-8694.20140031
Jiang, F., and Yan, A. (2021). IL-4 Rs2243250 Polymorphism Associated with Susceptibility to Allergic Rhinitis: a Meta-Analysis. Biosci. Rep. 41 (4), BSR20210522. doi:10.1042/BSR20210522
Khan, S. J., Dharmage, S. C., Matheson, M. C., and Gurrin, L. C. (2018). Is the Atopic March Related to Confounding by Genetics and Early‐life Environment? A Systematic Review of Sibship and Twin Data. Allergy 73 (1), 17–28. doi:10.1111/all.13228
Kim, K. R., Cho, S. H., Choi, S. J., Jeong, J. H., Lee, S. H., Park, C. W., et al. (2007). TAP1 and TAP2 Gene Polymorphisms in Korean Patients with Allergic Rhinitis. J. Korean Med. Sci. 22 (5), 825–831. doi:10.3346/jkms.2007.22.5.825
LeVan, T. D., Bloom, J. W., Bailey, T. J., Karp, C. L., Halonen, M., Martinez, F. D., et al. (2001). A Common Single Nucleotide Polymorphism in the CD14 Promoter Decreases the Affinity of Sp Protein Binding and Enhances Transcriptional Activity. J. Immunol. 167 (10), 5838–5844. doi:10.4049/jimmunol.167.10.5838
Li, H.-M., Zhang, R.-J., Gao, H., Jia, C.-Y., Zhang, J.-X., Dong, F.-L., et al. (2018). New Vertebral Fractures after Osteoporotic Vertebral Compression Fracture between Balloon Kyphoplasty and Nonsurgical Treatment PRISMA. Med. Baltim. 97 (40), e12666. doi:10.1097/MD.0000000000012666
Li, H.-M., Zhang, R.-J., and Shen, C.-L. (2020). Accuracy of Pedicle Screw Placement and Clinical Outcomes of Robot-Assisted Technique Versus Conventional Freehand Technique in Spine Surgery From Nine Randomized Controlled Trials. Spine (Phila Pa) 45 (2), E111–E119. doi:10.1097/BRS.0000000000003193
Li, H.-M., Zhang, R.-J., and Shen, C.-L. (2019). Radiographic and Clinical Outcomes of Oblique Lateral Interbody Fusion Versus Minimally Invasive Transforaminal Lumbar Interbody Fusion for Degenerative Lumbar Disease. World Neurosurg. 122, e627–e638. doi:10.1016/j.wneu.2018.10.115
Li, P., Cao, L., and Han, X. (2017). Angiotensin-converting Enzyme (ACE) I/D Polymorphism Is a Risk Factor of Allergic Rhinitis. Cell Mol. Biol. (Noisy-le-grand) 63 (8), 48–50. doi:10.14715/cmb/2017.63.8.11
Liu, Z. W., Li, J. C., and Qiu, L. Z. (2009). Study on TGFβ1-509C/T Polymorphisms in Patients with Allergic Rhinitis. Mod. Hosp. 9 (9), 16–18. [In Chinese].
Lu, M.-P., Chen, R.-X., Wang, M.-L., Zhu, X.-J., Zhu, L.-P., Yin, M., et al. (2011). Association Study on IL4, IL13 and IL4RA Polymorphisms in Mite-Sensitized Persistent Allergic Rhinitis in a Chinese Population. PLoS One 6 (11), e27363. doi:10.1371/journal.pone.0027363
Moniuszko, M., Kowal, K., Jeznach, M., Rusak, M., Dabrowska, M., and Bodzenta-Lukaszyk, A. (2013). Phenotypic Correlations between Monocytes and CD4+ T Cells in Allergic Patients. Int. Arch. Allergy Immunol. 161 (2), 131–141. doi:10.1159/000343687
Movahedi, M., Amirzargar, A. A., Nasiri, R., Hirbod-Mobarakeh, A., Farhadi, E., Tavakol, M., et al. (2013). Gene Polymorphisms of Interleukin-4 in Allergic Rhinitis and its Association with Clinical Phenotypes. Am. J. Otolaryngology 34 (6), 676–681. doi:10.1016/j.amjoto.2013.05.002
Pan, H.-F., Zhang, N., Li, W.-X., Tao, J.-H., and Ye, D.-Q. (2010). TIM-3 as a New Therapeutic Target in Systemic Lupus Erythematosus. Mol. Biol. Rep. 37 (1), 395–398. doi:10.1007/s11033-009-9833-7
Peng, L. F., Feng, J. R., Wang, M. G., and Gui, X. B. (2022). Mechanism of Buqi Tongqiao Formula on Alleviating the Inflammatory Injury of Nasal Mucosa in Rats with Allergic Rhinitis by Regulating Treg/Th17 Balance. CJTCMP 37 (3), 1648–1651. [In Chinese].
Qian, F. H., Zhang, Q., Zhou, L. F., Jin, G. F., Bai, J. L., and Yin, K. S. (2010). Polymorphisms in the Toll-like Receptor 2 Subfamily and Risk of Asthma: a Case-Control Analysis in a Chinese Population. J. Investig. Allergol. Clin. Immunol. 20 (4), 340–346. doi:10.1089/jir.2009.0014
Qiu, L. Z., Huang, Y. H., Lai, H., Zhang, J. G., Chen, S. Q., et al. (2009). Study on TGFβ1-509C/T Polymorphisms in Patients with Allergic Rhinitis. Anat. Res. 31 (2), 85–87. [In Chinese].
Shamji, M. H., Singh, I., Layhadi, J. A., Ito, C., Karamani, A., Kouser, L., et al. (2021). Passive Prophylactic Administration with a Single Dose of Anti-Fel D 1 Monoclonal Antibodies REGN1908-1909 in Cat Allergen-Induced Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 204 (1), 23–33. doi:10.1164/rccm.202011-4107OC
Shi, J., and Liu, Y. (2018). Study on the Mechanism of Yiqi Tuomin Decoction Regulating Th1/Th2 of Allergic Rhinitis by Tim3. CJTCMP 33 (7), 2781–2785. [In Chinese].
Shirkani, A., Mansouri, A., Farid Hosseini, R., Jabbari Azad, F., Alsadat Mahmoudian, R., Montazer, M., et al. (2019). The Role of Interleukin-4 and 13 Gene Polymorphisms in Allergic Rhinitis: A Case Control Study. Rep. Biochem. Mol. Biol. 8 (2), 111–118.
Sih, T., and Mion, O. (2010). Allergic Rhinitis in the Child and Associated Comorbidities. Pediatr. Allergy Immunol. 21 (1 Pt 2), e107–e113. doi:10.1111/j.1399-3038.2009.00933.x
Suzuki, H., Makino, Y., Nagata, M., Furuta, J., Enomoto, H., Hirota, T., et al. (2016). A Rare Variant in CYP27A1 and its Association with Atopic Dermatitis with High Serum Total IgE. Allergy 71 (10), 1486–1489. doi:10.1111/all.12950
Tang, L., Chen, Y., Xiang, Q., Xiang, J., Tang, Y., and Li, J. (2020). The Association between IL18, FOXP3 and IL13 Genes Polymorphisms and Risk of Allergic Rhinitis: a Meta-Analysis. Inflamm. Res. 69 (9), 911–923. doi:10.1007/s00011-020-01368-4
Valatabar, N., Hosseinpourfeizi, M., Safaralizadeh, R., and Sadeghi-Shabestari, M. (2020). Relationships between IL-13 and IL-4 Genotypes and Aeroallergens with Risk of Allergic Rhinitis in Iranian-Azeri. Pediatr. Allergy, Immunol. Pulmonol. 33 (1), 33–38. doi:10.1089/ped.2019.1099
Walunas, T. L., Bakker, C. Y., and Bluestone, J. A. (1996). CTLA-4 Ligation Blocks CD28-dependent T Cell Activation. J. Exp. Med. 183 (6), 2541–2550. doi:10.1084/jem.183.6.2541
Wan, H., Su, H., Wu, Y., Zhao, Y., and Zhou, M. (2015). Expression and Significance of NLPR3 Inflammasome and its Downstream Factors IL-1/IL-18 in Rat Model of Allergic Rhinitis. Chin. J. Otorhinolaryngol. Head. Neck Surg. 50 (2), 145–150. [In Chinese]. doi:10.3760/cma.j.issn.1673-0860.2015.02.011
Xu, Y., and Wang, J. (2014). Association of CD14 Gene -159C/T Polymorphism with Allergic Rhinitis Risk: a Meta-Analysis. Eur. Arch. Otorhinolaryngol. 271 (6), 1601–1607. doi:10.1007/s00405-013-2793-5
Zare Marzouni, H., Farid-Hosseini, R., Jabari-Azad, F., Tavakkol-Afshari, J., Tehranian, F., Khoshkhui, M., et al. (2019). CD14 as A Serum Immune Biomarker and Genetic Predisposition Factor for Allergic Rhinitis. Iran. J. Otorhinolaryngol. 31 (102), 1–9.
Zhang, G., Zhang, D., Shi, W., Sun, P., and Lin, P. (2017). The Impact of FOXP3 Polymorphism on the Risk of Allergic Rhinitis: A Meta-Analysis. Ann. Hum. Genet. 81 (6), 284–291. doi:10.1111/ahg.12205
Zhou, Y. (2019). Association between TNF-α Gene 308G/A Polymorphisms and the Risk of Allergic Rhinitis : A Meta-Analysis. Wuhan, China: Huazhong University of Science and Technology.
Keywords: allergic rhinitis, polymorphism, meta-analysis, IL-13, CTLA-4, IL-4R, ACE
Citation: Xiang F, Zeng Z, Wang L, Yang YP and Zhang QX (2022) Polymorphisms and AR: A Systematic Review and Meta-Analyses. Front. Genet. 13:899923. doi: 10.3389/fgene.2022.899923
Received: 19 March 2022; Accepted: 08 June 2022;
Published: 01 July 2022.
Edited by:
Hugues Aschard, Institut Pasteur, FranceReviewed by:
Fatma Savran Oguz, Istanbul University, TurkeyCopyright © 2022 Xiang, Zeng, Wang, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Xiu Zhang, emhhbmdxaW54aXVAY2R1dGNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.