- 1Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, Chapel Hill, NC, United States
- 2Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Department of Social Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 4Department of Medical Ethics and Health Policy, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 5Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
An emerging role for DNA sequencing is to identify people at risk for an inherited cancer syndrome in order to prevent or ameliorate the manifestation of symptoms. Two cancer syndromes, Hereditary Breast and Ovarian Cancer and Lynch Syndrome meet the “Tier 1” evidence threshold established by the Centers for Disease Control and Prevention (CDC) for routine testing of patients with a personal or family history of cancer. Advancements in genomic medicine have accelerated public health pilot programs for these highly medically actionable conditions. In this brief report, we provide descriptive statistics from a survey of 746 US respondents from a Qualtrics panel about the public’s awareness of genetic testing, interest in learning about their cancer risk, and likelihood of participating in a population genetic screening (PGS) test. Approximately of half the respondents were aware of genetic testing for inherited cancer risk (n = 377/745, 50.6%) and would choose to learn about their cancer risk (n-309/635, 48.7%). Characteristics of those interested in learning about their cancer risk differed by educational attainment, age, income, insurance status, having a primary care doctor, being aware of genetic testing, and likelihood of sharing information with family (p < 0.05). A sizeable majority of the respondents who were interested in about learning their cancer risk also said that they were likely to participate in a PGS test that involved a clinical appointment and blood draw, but no out-of-pocket cost (n = 255/309, 82.5%). Reasons for not wanting to participate included not finding test results interesting or important, concerns about costs, and feeling afraid to know the results. Overall, our results suggest that engaging and educating the general population about the benefits of learning about an inherited cancer predisposition may be an important strategy to address recruitment barriers to PGS.
Introduction
DNA-based screening of healthy individuals has enormous, yet untapped potential to improve cancer-related health outcomes through early detection and cancer prevention before symptoms manifest. Multidisciplinary research supporting the clinical utility and validity of DNA-based population screening for certain medically actionable conditions is increasing. (Adams et al., 2016; Hunter et al., 2016; Milko et al., 2019; Zhang et al., 2019; Hendricks- Sturrup et al., 2020; Roman et al., 2020). A point of consensus for DNA-based screening is that the benefit to harm ratio can be maximized by screening for pathogenic genomic variants in well-understood causative genes for conditions with effective, evidence-based clinical interventions. (Jarvik et al., 2014; Berg et al., 2016; Green et al., 2019; Hendricks- Sturrup et al., 2020; Murray et al., 2020; Bean et al., 2021; Murray et al., 2021).
The US Centers for Disease Control and Prevention (CDC) has defined two hereditary cancer syndromes as “Tier 1” based on their clinical actionability: Lynch syndrome (LS) and Hereditary Breast and Ovarian Cancer (HBOC). Evidence indicates that population screening could significantly reduce morbidity and mortality for millions of Americans each year. (Green et al., 2019; Bean et al., 2021; Murray et al., 2021). Cost-effectiveness analyses demonstrate that screening in the general population yields good value for money and even potential cost savings for health care systems, especially when cascade screening (i.e., family testing) is considered. (Manchanda et al., 2018; Zhang et al., 2019).
The National Academies of Sciences, Engineering and Medicine’s Genomics and Public Health Action Collaborative has endorsed “an accelerated implementation science agenda” (Khoury et al., 2018) for the Tier 1 conditions, including LS, HBOC, and familial hypercholesterolemia (FH), to understand the potential impact of population genomic screening in healthy adults. Increasingly, these three conditions and eleven associated genes are being adopted for Population Genetic Screening (PGS) pilot programs to investigate clinical and implementation outcomes in various health care settings around the country. (Brown-Johnson et al., 2021; Christensen et al., 2021; East et al., 2021).
Though the capacity for clinical PGS programs to transform personalized public health in the United States is widely acknowledged (Evans et al., 2013; Green et al., 2020), current public interest in participating in PGS is an essential yet understudied aspect of equitable implementation. Several studies examining the public’s interest in population genetic screening have shown that awareness about genetic screening for certain types of cancer is associated with being non-Hispanic White (Hay et al., 2018; Rubinsak et al., 2019), willingness to pay for testing, having a family history of cancer, and higher educational attainment. (Shen et al., 2022). Other population characteristics such as access to a primary care provider, rurality, income, insurance, sexual orientation, and gender have been shown to be related to genetic services use; however little is understood about their association with interested in and likelihood to participate in PGS. To this end, we conducted a survey to more comprehensively understand the characteristics of those who are interested in participating in PGS for learning about cancer risk. The results of this study may inform strategies to increase awareness and participation in PGS programs among diverse populations.
Methods
Population
In December 2020, the UNC Lineberger Cancer Prevention and Control Program recruited an online convenience sample of US adults through the Qualtrics Online Panel platform (n = 746, Qualtrics, Seattle WA). Participants were eligible if they were over the age of 18 and resided in the US. Qualtrics panel members are recruited from multiple sources including but not limited to, targeted email lists, gaming sites, customer loyalty web portals, and social media. Panel members were sent an email invitation or were prompted on the survey platform to respond to the online survey for a specific compensation amount. Because panel members can be reimbursed in different ways (e.g., gather points, donate funds, etc.), incentive amount for this study is estimated to be $2.50 per person. Interested respondents clicked on the survey link provided. The University of North Carolina Institutional Review Board approved this study (#20–2338).
Measures
Primary outcomes of interest were adapted from existing surveys and included:
(1) awareness about genetic tests (i.e., Genetic tests that analyze your DNA for potential cancer risks are currently available. Have you heard or read about these genetic tests? Yes/no) (HINTS, 2022);
(2) receipt of genetic testing (among those aware of genetic testing: Have you ever had a genetic test to determine if you have an increased risk of developing cancer? Yes/no) (HINTS, 2022);
(3) interest in learning genetic risk (among those who have not received genetic testing: How interested would you be in learning whether you have a genetic risk factor for cancer that can be prevented or treated? Likert 1–5) (Peterson et al., 2022);
(4) likelihood of getting a PGS test (among those who were interested in learning genetic risk: To learn whether you have a genetic risk factor for cancer, you would need to make an appointment at a local clinic, get your blood drawn, and set up an online account to access your test results. Assuming there is no cost to you, how likely would you be to get this test? Likert 1–5); and
(4a) reasons why you selected “unlikely/very unlikely” or “neither likely or unlikely” or “likely/very likely” (open-ended response). (See Supplementary Figure S1).
Given that cost is a known barrier to genetic testing among patients (Steffen et al., 2017), we asked specifically about interest in a screening test that would be at no-cost to patients. Participants were also asked to explain how they arrived at their answer about their likelihood of getting a PGS test in an open field question (question 4a above).
Because we were interested in understanding which subpopulations are aware of, engaged in or interested in engaging in PGS programs, we collected sociodemographic information that has been associated with awareness about or use of genetic services in prior studies. These characteristics included: gender (women/men) (Sanderson et al., 2004; Childers et al., 2018), ethnicity (Hispanic, non-Hispanic) (Childers et al., 2018; Salloum et al., 2018), race (white, black, other) (Salloum et al., 2018; Chapman-Davis et al., 2021), education (less than HS/HS/GED, some college/technical school, AD, BS, graduate/professional degree) (Sanderson et al., 2004; Armstrong et al., 2005; Childers et al., 2018), age (<25, 25–49, 50–74, 75+) (Sanderson et al., 2004; Orlando et al., 2019), income ($0–34,999, $35,000–99,999, $100K+) (Armstrong et al., 2005), insurance (Medicare/Medical Assistance/any kind of government-assistance plan for those with low incomes or a disability, Employer-based, any Medicaid, and other) (The National Academies Collection: Reports funded by National Institutes of Health, 2018), sexual orientation (straight, gay or lesbian, bisexual, prefer to self-describe) (Nathan et al., 2019), and rurality (urban, suburban, rural). (Salloum et al., 2018). We also included several additional variables: having a primary care doctor (yes/no), (Armstrong et al., 2005), perceived comparative cancer risk (5 point Likert scale) (Chopra and Kelly, 2017), and intentions to share results with family (8 point Likert scale) (Chopra and Kelly, 2017). These variables were included as conversations with primary care providers, (Armstrong et al., 2005), having higher perceived cancer risk (often due to family or personal cancer history) (Chopra and Kelly, 2017), and intentions to share results with family members have been associated with increased genetic services use (Chopra and Kelly, 2017).
Analysis
We calculated descriptive statistics to examine whether there were differences between the characteristics of respondents according to our primary outcomes. We used chi-square tests to examine differences between those who were 1) aware of genetic tests versus not aware, 2) previously tested versus not tested, 3) interested (very interested, interested) in PGS versus not interested (neutral, uninterested, very uninterested), and 4) likely to participate in PGS versus not likely (neutral, unlikely, very unlikely).
We used thematic analysis to understand respondents free text responses to their likelihood of participating in PGS (question 4a above). We report the top five themes among those who were likely, unlikely, and neutral about getting a PGS test (MCR and LVM). Independently coders reviewed open-ended responses and identified emergent themes for each response category (unlikely: unlikely/very unlikely; neutral: neither likely or unlikely; likely: likely/very likely) and applied them to 10% of responses in each category. Coders compared themes and their application, modified the list of themes, and then independently applied the codes to 20% of responses (n = 60). We repeated this process until we achieved 100% agreement (in one round), and then we independently coded the remaining responses. We calculated the level of agreement between coders (87.6%). Conflicts were then reconciled through discussion. We reported the major themes and exemplar quotes.
Results
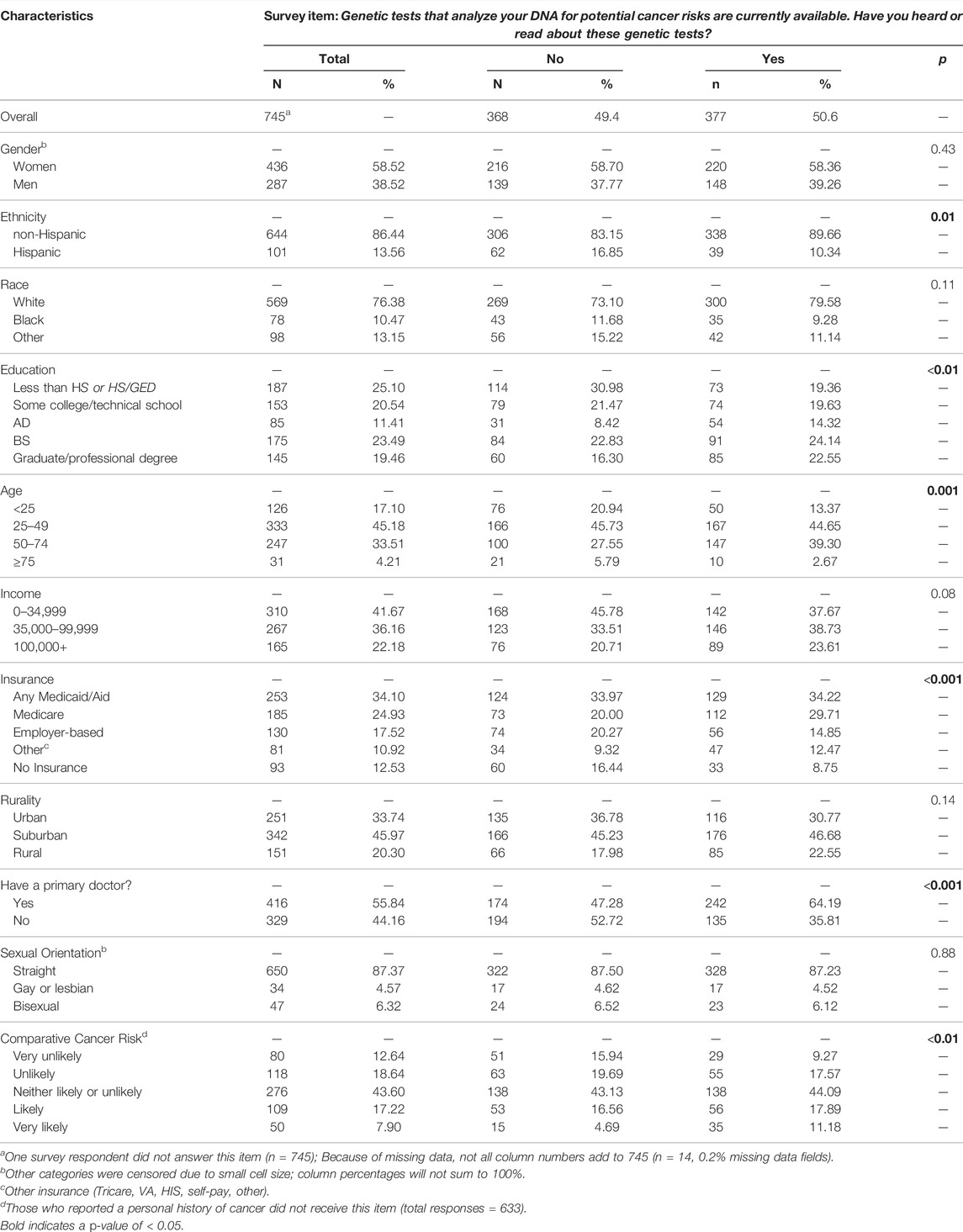
Of the overall sample of respondents, 377 (50.6%) were aware of genetic tests. Among those who were aware of genetic tests, a higher proportion were non-Hispanic, in the middle age categories, as well as had higher educational attainment, Medicare or other insurance, a primary care doctor and higher perceived comparative cancer risk. No significant differences between gender, race, income, rurality, and sexual orientation were identified (Table 1).
Among those aware of genetic tests, 110 respondents (29.2%) had received a genetic test for an inherited cancer risk in the past. Compared to the 267 respondents who had not previously received a genetic test, the tested group had a higher proportion of men, higher educational attainment, higher income, had insurance coverage, lived in urban areas, were in the middle age categories, and identified as not straight (Table 2).
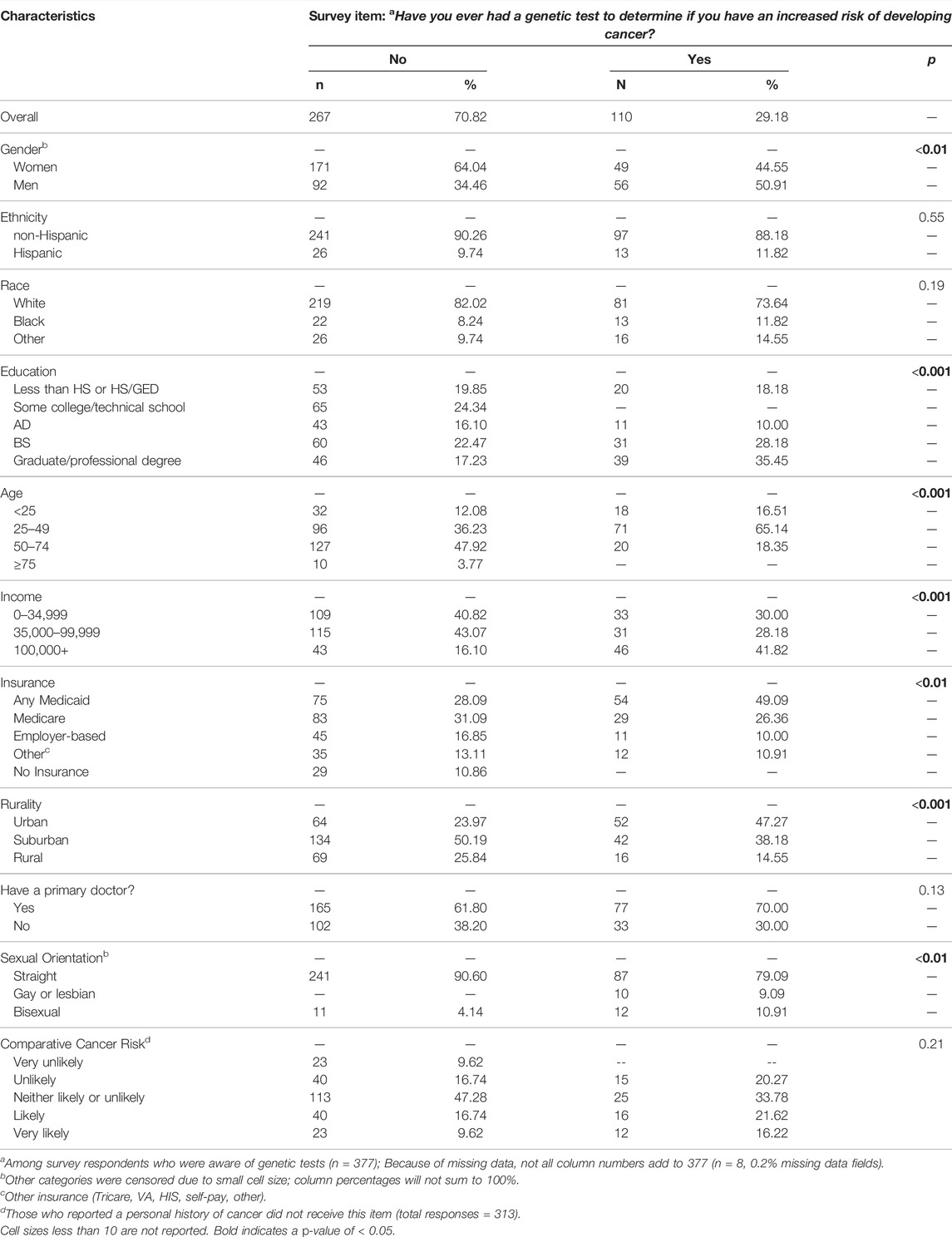
TABLE 2. Descriptive characteristics of those how have and have not received a genetic test for cancer risk.
Of the 635 respondents who had not received genetic test, 309 (48.7%) would be interested in learning whether they had a genetic risk factor for cancer. Among those interested in learning about their risk, a higher proportion were aware of genetic tests, had higher income, had a primary care doctor, had insurance coverage, were in older age categories, had higher educational attainment, and would be more likely to share information with family (Table 3).
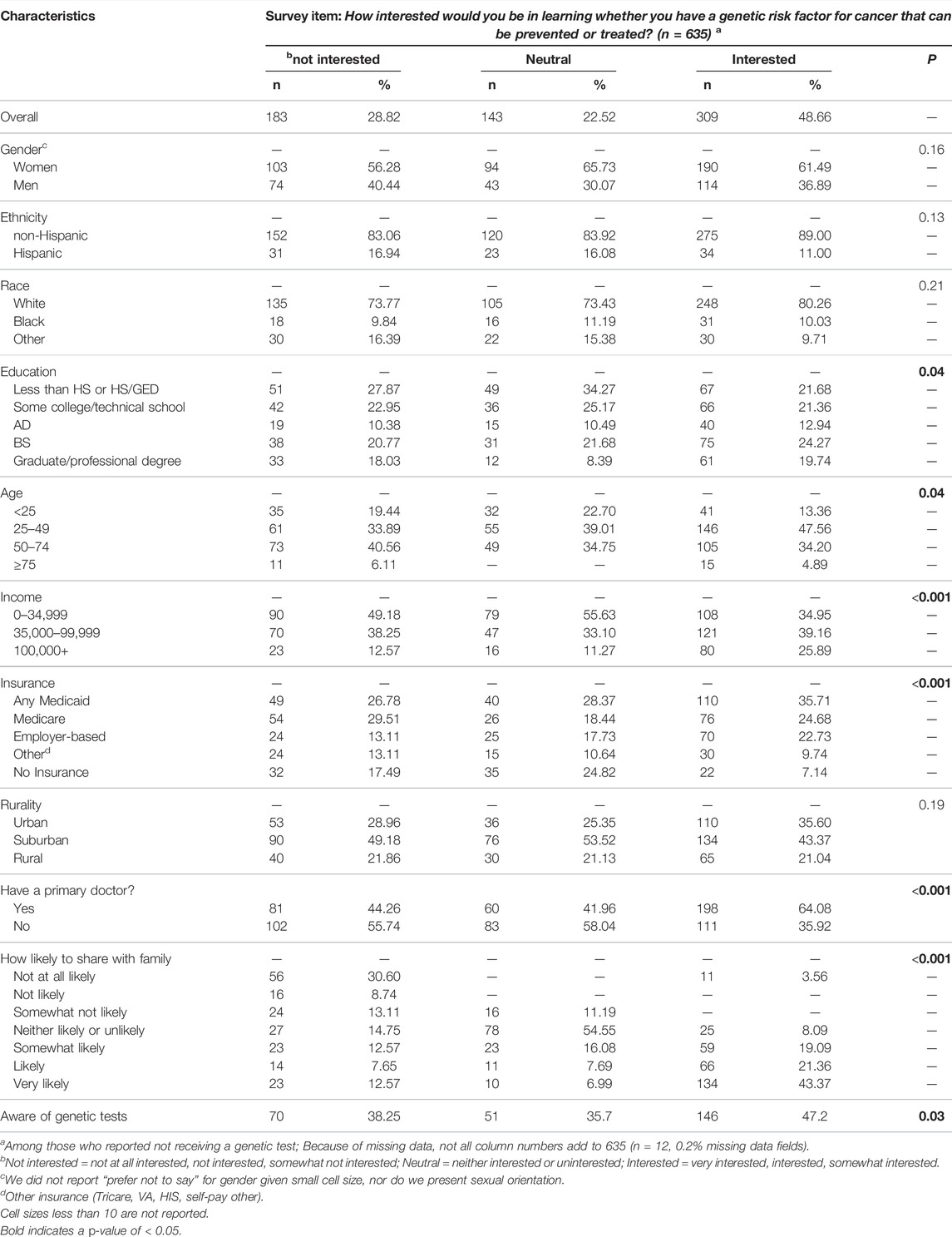
TABLE 3. Descriptive characteristics among those with different levels of interest in learning about genetic cancer risks.
Finally, of 309 respondents interested in learning whether they had a genetic risk factor for cancer, a substantial majority, 255 respondents (82.5%), would be likely or highly likely to get a PGS test (Table 4). Within this group, a higher proportion were male, in middle age categories, and were more likely to share information with family. Major themes emerged for being likely to get a PGS test and they included 1) believing the test could inform their health and/or plan for the future (n = 51 of 255), 2) wanting to know their risk of cancer (n = 46 of 255), 3) finding the test “important” (n = 45 of 255), 4) finding the test easy/available/free (n = 35 of 255), 5) having a family history of cancer (n = 21 of 255), and 6) having a personal history of cancer or other risk factors for cancer (n = 11 of 255). Only 14 respondents (4.5%) were unlikely to get a PGS test, and the top reasons were 1) not being interested or finding the test important (n = 5), 2) concerns about costs (n = 2), and 3) not wanting to or being scared to know (n = 2). The 40 respondents who were neutral (12.9%) were not sure if they wanted the test yet (n = 8), concerned about logistics (n = 4), felt the test was not interesting or important for them (n = 4), among other less common reasons (See Supplementary Table S1).
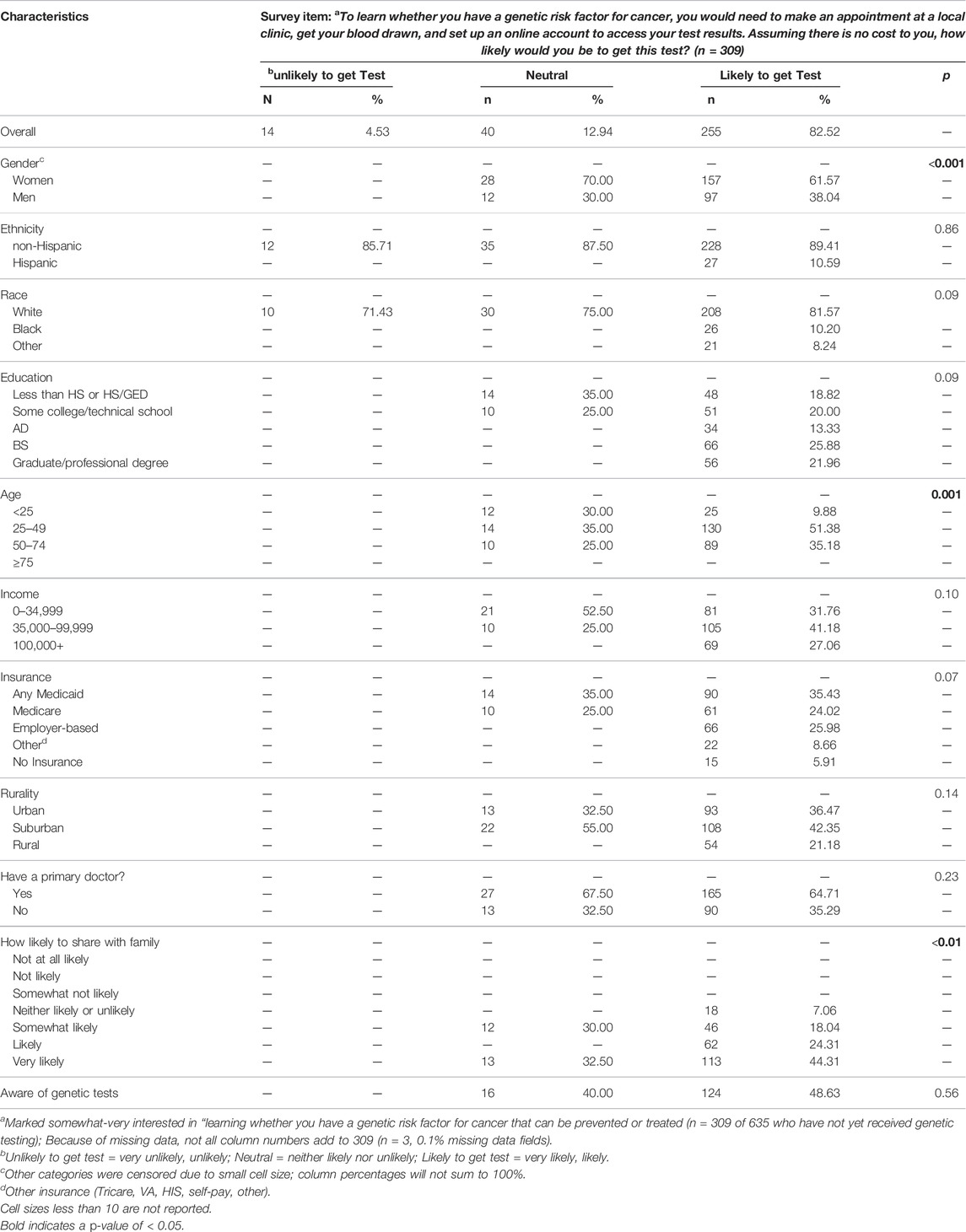
TABLE 4. Descriptive characteristics among those with different likelihoods of getting a population genetic screening test for cancer risk.
Discussion
Despite increasing availability of direct-to-consumer (DTC) testing and PGS programs, we found that awareness of genetic testing for cancer predisposition in the general population remains at around 50%. This aligns with prior research from 2017 from the National Cancer Institute (NCI) Health Information National Trends Survey in which 57% of respondents reported being aware of genetic tests used for health reasons. (Roberts et al., 2019). This percentage is higher than a decade ago, at which time awareness about DTC genetic testing was 38.1% (Apathy et al., 2018). Our results were also consistent with prior reports of the association between awareness of genetic testing and education level, (Sanderson et al., 2004; Armstrong et al., 2005; Childers et al., 2018), demonstrating a persistent need to reach individuals with lower educational attainment to prevent widening disparities in access to precision health care.
Though racial disparities in genetic testing utilization are well established in the literature, (Salloum et al., 2018; Chapman-Davis et al., 2021), we did not find statistically significant disparities in our data, likely because only individuals who reported being aware of genetic tests were asked about their genetic testing history. This aligns with data from the NCI using a similar measure about awareness of DTC genetic testing in which differences by race were not observed. (Agurs -Collins et al., 2015). We did find that people of Hispanic ethnicity were significantly less likely to be aware of testing, indicating that efforts to increase accessibility to precision health care should also include native Spanish speakers.
Among those aware of genetic testing who have not already had genetic testing, almost half would be interested in learning whether they had a genetic risk factor for cancer that can be prevented or treated, which is lower than what has been reported elsewhere in public samples. (Donovan and Tucker, 2000; Alvord et al., 2020). For example, in a 2020 study of public perception of predictive cancer genetic testing in Oregon, 87% of participants reported an interest in cancer genetic testing and receiving genetic information about themselves; however, it is important to note that 85% of individuals in this study had a personal or family diagnosis of cancer. As better understanding of family cancer risks is a known motivator for testing reported by participants in that study, this study also highlights an urgent need for more data from participants with no prior personal or family history of cancer. (HINTS, 2019). In our data we found that respondents who were likely to share their results with family were also more likely to be interested in learning whether they had a genetic risk factor for cancer. Deeper understanding about the reasons for overall disinterest in testing (e.g. uninformed about benefits vs mistrust of health system) will be important for developing strategies to engage the broader population in genetic screening. We also found socioeconomic factors (educational attainment, insurance, income), age, and having a primary care provider differed, such that larger proportions of those who are traditionally underserved and those without a primary care provider reported being uninterested in learning about their genetic risk for cancer. This aligns with prior work that has demonstrated potential disparities in genetic services use among these populations. (Shen et al., 2022; Sanderson et al., 2004; Childers et al., 2018; Armstrong et al., 2005; Orlando et al., 2019; The National Academies Collection: Reports funded by National Institutes of Health, 2018).
Among respondents who had not had any previous genetic testing, about half were interested in learning about a genetic risk for cancer predisposition. Furthermore, a large majority said they would be likely to participate in a PGS test in a clinical setting that required making an appointment, getting a blood draw, and creating an online account for a patient portal. All respondents provided contextual information about how and why they responded to this question and, interestingly, only one respondent mentioned mistrust or concerns about genetic discrimination, data privacy or security, which have been commonly reported in the literature. (Hann et al., 2017). This may be due to our small sample size of individuals who reported being very unlikely or unlikely to participate in the PGS (n = 14) and also that these concerns are reflected in the high proportion of respondents who were not interested in learning about a genetic risk for cancer predisposition. Overall, we found it telling that most survey respondents who were interested in learning their risk for developing an inherited cancer syndrome would also hypothetically be willing to commit the time and effort participate in a clinical offering that included a blood draw and a patient portal. Of note, cost was still a concern of respondents despite explicitly noting that the hypothetical clinical screening test would be at no-cost to patients. A deeper awareness about participants’ downstream financial concerns is warranted and may help us better understand barriers related to follow-up medical costs or costs associated with taking time off to get a blood draw.
While these descriptive findings provide foundational data on public awareness, use and interest in genetic testing and screening, they should be interpreted within the context of several limitations. First, because of small sample size, we are unable to examine multivariable associations between key variables and certain outcomes (receipt of a genetic test, interest in learning genetic risk and likelihood of getting a PGS test). Further, while Qualtrics survey samples can provide a diverse sample, (Miller et al., 2020), respondents may not be representative of the general population. We used convenience sampling from a geographically diverse area in the US to rapidly gather data about the public’s opinions and to generate hypotheses about potentially important factors related to stakeholder engagement around PGS. However, this sampling approach has several drawbacks including lack of generalizability, as well as selection, sampling, and positivity biases. Further, we are unable to determine the denominator required to calculate a response rate given the ways in which participants were invited to take the survey. Future work should include larger nationally representative samples to better understand the association between key sociodemographic variables and key outcomes which will be essential for ensuring equity in the implementation of population genetic screening program. In addition, we asked about individuals’ intentions to learn about genetic risk and likelihood of participating in a PGS test. Intentions are associated with health behaviors, as are other factors, some of which are known, such as perceived control (data not collected) and others which are less certain. (Ajzen, 1991). Thus, it will be important for clinical PGS programs to collect data on actual uptake of PGS to understand how the public engages with PGS in real world settings. This work should be expanded to include other clinical contexts, such as familial hypercholesterolemia, which is also a CDC Tier One condition for which population genetic screening has the potential to improve precision public health. (CDC, 2021). Because we test multiple comparisons, our chances of having a type I error are higher; results should be interpreted in this light. Finally, the population genetic screening program described in the survey item mentioned that the test would be free, clinic-based, and require a blood draw, limiting the generalizability of our findings to PGS programs with different characteristics. Future studies to compare different PGS models will further inform the implementation of PGS models moving forward.
Our findings identified two key challenges for the implementation of population genetic screening: increasing awareness of the potential benefits of genetic testing and interest in learning one’s genetic risk for cancer. This may be especially important for subpopulations with lower socioeconomic status and those without a primary source of care. Future work to better understand and develop strategies to overcome these challenges will be essential as PGS programs are increasingly implemented into clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This human subjects research study was reviewed and determined to be exempt by The University of North Carolina Institutional Review Board.
Author Contributions
MR, KF, GH, SP, KS, KW, and LM contributed to the design of the study. MR conducted the data analysis and MR, KF, GH, SP, KS, KW, and LM all contributed to the interpretation of the study results. MR and LM wrote the manuscript and all authors MR, KF, GH, SP, KS, KW, and LM contributed revisions and approved this manuscript for publication.
Funding
The University of North Carolina’s University Cancer Research Fund provided funding for data collection.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Lauren Passero for her assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.886640/full#supplementary-material
Supplementary Figure S1 | Survey flow and primary measures of interest.
Supplementary Table S1 | Open-ended responses about reasons for respondents’ perceived likelihood of getting a population genetic screening test.
References
Adams, M. C., Evans, J. P., Henderson, G. E., and Berg, J. S. (2016). The Promise and Peril of Genomic Screening in the General Population. Genet. Med. 18 (6), 593–599. doi:10.1038/gim.2015.136
Agurs-Collins, T., Ferrer, R., Ottenbacher, A., Waters, E. A., O’Connell, M. E., and Hamilton, J. G. (2015). Public Awareness of Direct-To-Consumer Genetic Tests: Findings from the 2013 U.S. Health Information National Trends Survey. J. Canc Educ. 30 (4), 799–807. doi:10.1007/s13187-014-0784-x
Ajzen, I. (1991). The Theory of Planned Behavior. Organ. Behav. Hum. Decis. Process. 50 (2), 179–211. doi:10.1016/0749-5978(91)90020-T
Alvord, T. W., Marriott, L. K., Nguyen, P. T., Shafer, A., Brown, K., Stoller, W., et al. (2020). Public Perception of Predictive Cancer Genetic Testing and Research in Oregon. Jrnl Gene Coun 29 (2), 259–281. doi:10.1002/jgc4.1262
Apathy, N. C., Menser, T., Keeran, L. M., Ford, E. W., Harle, C. A., and Huerta, T. R. (2018). Trends and Gaps in Awareness of Direct-To-Consumer Genetic Tests from 2007 to 2014. Am. J. Prev. Med. 54 (6), 806–813. doi:10.1016/j.amepre.2018.02.013
Armstrong, K., Micco, E., Carney, A., Stopfer, J., and Putt, M. (2005). Racial Differences in the Use of BRCA1/2 Testing Among Women with a Family History of Breast or Ovarian Cancer. JAMA 293 (14), 1729–1736. doi:10.1001/jama.293.14.1729
Bean, L. J. H., Scheuner, M. T., Murray, M. F., Biesecker, L. G., Green, R. C., Monaghan, K. G., et al. (2021). DNA-based Screening and Personal Health: a Points to Consider Statement for Individuals and Health-Care Providers from the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 23 (6), 979–988. doi:10.1038/s41436-020-01083-9
Berg, J. S., Foreman, A. K. M., O'Daniel, J. M., Booker, J. K., Boshe, L., Carey, T., et al. (2016). A Semiquantitative Metric for Evaluating Clinical Actionability of Incidental or Secondary Findings from Genome-Scale Sequencing. Genet. Med. 18 (5), 467–475. doi:10.1038/gim.2015.104
Brown-Johnson, C. G., Safaeinili, N., Baratta, J., Palaniappan, L., Mahoney, M., Rosas, L. G., et al. (2021). Implementation Outcomes of Humanwide: Integrated Precision Health in Team-Based Family Practice Primary Care. BMC Fam. Pract. 22 (1), 28. doi:10.1186/s12875-021-01373-4
CDC (2021). Tier 1 Genomics Applications and Their Importance to Public Health | CDC. Published March 16. 2021Available at: https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm (Accessed February 14, 2022).
Chapman-Davis, E., Zhou, Z. N., Fields, J. C., Frey, M. K., Jordan, B., Sapra, K. J., et al. (2021). Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J. Gen. Intern Med. 36 (1), 35–42. doi:10.1007/s11606-020-06064-x
Childers, K. K., Maggard-Gibbons, M., Macinko, J., and Childers, C. P. (2018). National Distribution of Cancer Genetic Testing in the United States. JAMA Oncol. 4 (6), 876–879. doi:10.1001/jamaoncol.2018.0340
Chopra, I., and Kelly, K. M. (2017). Cancer Risk Information Sharing: The Experience of Individuals Receiving Genetic Counseling forBRCA1/2Mutations. J. Health Commun. 22 (2), 143–152. doi:10.1080/10810730.2016.1258743
Christensen, K. D., Bell, M., Zawatsky, C. L. B., Galbraith, L. N., Green, R. C., Hutchinson, A. M., et al. (2021). Precision Population Medicine in Primary Care: The Sanford Chip Experience. Front. Genet. 12, 626845. doi:10.3389/fgene.2021.626845
Donovan, K. A., and Tucker, D. C. (2000). Knowledge about Genetic Risk for Breast Cancer and Perceptions of Genetic Testing in a Sociodemographically Diverse Sample. J. Behav. Med. 23 (1), 15–36. doi:10.1023/a:1005416203239
East, K. M., Kelley, W. V., Cannon, A., Cochran, M. E., Moss, I. P., May, T., et al. (2021). A State-Based Approach to Genomics for Rare Disease and Population Screening. Genet. Med. 23 (4), 777–781. doi:10.1038/s41436-020-01034-4
Evans, J. P., Berg, J. S., Olshan, A. F., Magnuson, T., and Rimer, B. K. (2013). We Screen Newborns, Don't We?: Realizing the Promise of Public Health Genomics. Genet. Med. 15 (5), 332–334. doi:10.1038/gim.2013.11
Green, E. D., Gunter, C., Biesecker, L. G., Di Francesco, V., Easter, C. L., Feingold, E. A., et al. (2020). Strategic Vision for Improving Human Health at the Forefront of Genomics. Nature 586 (7831), 683–692. doi:10.1038/s41586-020-2817-4
Green, R. F., Ari, M., Kolor, K., Dotson, W. D., Bowen, S., Habarta, N., et al. (2019). Evaluating the Role of Public Health in Implementation of Genomics-Related Recommendations: a Case Study of Hereditary Cancers Using the CDC Science Impact Framework. Genet. Med. 21 (1), 28–37. doi:10.1038/s41436-018-0028-2
Hann, K. E. J., Freeman, M., Freeman, M., Fraser, L., Waller, J., Sanderson, S. C., et al. (2017). Awareness, Knowledge, Perceptions, and Attitudes towards Genetic Testing for Cancer Risk Among Ethnic Minority Groups: a Systematic Review. BMC Public Health 17 (1), 503. doi:10.1186/s12889-017-4375-8
Hay, J. L., Zielaskowski, K., Meyer White, K., Kaphingst, K., Robers, E., Guest, D., et al. (2018). Interest and Uptake ofMC1RTesting for Melanoma Risk in a Diverse Primary Care Population. JAMA Dermatol 154 (6), 684–693. doi:10.1001/jamadermatol.2018.0592
Hendricks-Sturrup, R. M., Linsky, A., Lu, C. Y., and Vassy, J. L. (2020). Genomic Testing Is Best Integrated into Clinical Practice when it Is Actionable. Pers. Med. 17 (1), 5–8. doi:10.2217/pme-2019-0106
HINTS (2022). Survey Instruments | HINTS. Available at: https://hints.cancer.gov/data/survey-instruments.aspx (Accessed January 24, 2022).
HINTS (2019). View HINTS Questions | HINTS. Available at: https://hints.cancer.gov/view-questions-topics/all-hints-questions.aspx (Accessed February 14, 2022).
Hunter, J. E., Irving, S. A., Biesecker, L. G., Buchanan, A., Jensen, B., Lee, K., et al. (2016). A Standardized, Evidence-Based Protocol to Assess Clinical Actionability of Genetic Disorders Associated with Genomic Variation. Genet. Med. 18 (12), 1258–1268. doi:10.1038/gim.2016.40
Jarvik, G. P., Amendola, L. M., Berg, J. S., Brothers, K., Clayton, E. W., Chung, W., et al. (2014). Return of Genomic Results to Research Participants: the Floor, the Ceiling, and the Choices in between. Am. J. Hum. Genet. 94 (6), 818–826. doi:10.1016/j.ajhg.2014.04.009
Khoury, M. J., Feero, W. G., Chambers, D. A., Brody, L. C., Aziz, N., Green, R. C., et al. (2018). Correction: A Collaborative Translational Research Framework for Evaluating and Implementing the Appropriate Use of Human Genome Sequencing to Improve Health. PLoS Med. 15 (8), e1002650. doi:10.1371/journal.pmed.1002650
Manchanda, R., Patel, S., Gordeev, V. S., Antoniou, A. C., Smith, S., Lee, A., et al. (2018). Cost-effectiveness of Population-Based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 Mutation Testing in Unselected General Population Women. J. Natl. Cancer Inst. 110 (7), 714–725. doi:10.1093/jnci/djx265
Milko, L. V., O'Daniel, J. M., DeCristo, D. M., Crowley, S. B., Foreman, A. K. M., Wallace, K. E., et al. (2019). An Age-Based Framework for Evaluating Genome-Scale Sequencing Results in Newborn Screening. J. Pediatr. 209, 68–76. doi:10.1016/j.jpeds.2018.12.027
Miller, C. A., Guidry, J. P. D., Dahman, B., and Thomson, M. D. (2020). A Tale of Two Diverse Qualtrics Samples: Information for Online Survey Researchers. Cancer Epidemiol. Biomarkers Prev. 29 (4), 731–735. doi:10.1158/1055-9965.EPI-19-0846
Murray, M. F., Evans, J. P., and Khoury, M. J. (2020). DNA-based Population Screening. JAMA 323 (4), 307–308. doi:10.1001/jama.2019.18640
Murray, M. F., Giovanni, M. A., Doyle, D. L., Harrison, S. M., Lyon, E., Manickam, K., et al. (2021). DNA-based Screening and Population Health: a Points to Consider Statement for Programs and Sponsoring Organizations from the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 23 (6), 989–995. doi:10.1038/s41436-020-01082-w
Nathan, M. L., Ormond, K. E., Dial, C. M., Gamma, A., and Lunn, M. R. (2019). Genetic Counselors' and Genetic Counseling Students' Implicit and Explicit Attitudes toward Homosexuality. J. Genet. Couns. 28 (1), 91–101. doi:10.1007/s10897-018-0295-8
Orlando, L. A., Voils, C., Horowitz, C. R., Myers, R. A., Arwood, M. J., Cicali, E. J., et al. (2019). IGNITE Network: Response of Patients to Genomic Medicine Interventions. Mol. Genet. Genomic Med. 7 (5), e636. doi:10.1002/mgg3.636
Peterson, E. B., Taber, J. M., and Klein, W. M. P. (2022). Information Avoidance, Self-Affirmation, and Intentions to Receive Genomic Sequencing Results Among Members of an African Descent Cohort. Ann. Behav. Med. 56 (2), 205–211. doi:10.1093/abm/kaab042
Roberts, M. C., Turbitt, E., and Klein, W. M. P. (2019). Psychosocial, Attitudinal, and Demographic Correlates of Cancer-Related Germline Genetic Testing in the 2017 Health Information National Trends Survey. J. Community Genet. 10 (4), 453–459. doi:10.1007/s12687-018-00405-4
Roman, T. S., Crowley, S. B., Roche, M. I., Foreman, A. K. M., O’Daniel, J. M., Seifert, B. A., et al. (2020). Genomic Sequencing for Newborn Screening: Results of the NC NEXUS Project. Am. J. Hum. Genet. 107 (4), 596–611. doi:10.1016/j.ajhg.2020.08.001
Rubinsak, L. A., Kleinman, A., Quillin, J., Gordon, S. W., Sullivan, S. A., Sutton, A. L., et al. (2019). Awareness and Acceptability of Population-Based Screening for Pathogenic BRCA Variants: Do Race and Ethnicity Matter? Gynecol. Oncol. 154 (2), 383–387. doi:10.1016/j.ygyno.2019.06.009
Salloum, R. G., George, T. J., Silver, N., Markham, M.-J., Hall, J. M., Guo, Y., et al. (2018). Rural-urban and Racial-Ethnic Differences in Awareness of Direct-To-Consumer Genetic Testing. BMC Public Health 18 (1), 277. doi:10.1186/s12889-018-5190-6
Sanderson, S. C., Wardle, J., Jarvis, M. J., and Humphries, S. E. (2004). Public Interest in Genetic Testing for Susceptibility to Heart Disease and Cancer: a Population-Based Survey in the UK. Prev. Med. 39 (3), 458–464. doi:10.1016/j.ypmed.2004.04.051
Shen, E. C., Sriniva, S., Passero, L. E., Allen, C. G., Dixon, M., Foss, K., et al. (2022). Barriers and Facilitators for Population Genetic Screening in Healthy Populations: A Systematic Review. Published online. doi:10.21203/rs.3.rs-1315303/v1
Steffen, L. E., Du, R., Gammon, A., Mandelblatt, J. S., Kohlmann, W. K., Lee, J.-H., et al. (2017). Genetic Testing in a Population-Based Sample of Breast and Ovarian Cancer Survivors from the REACH Randomized Trial: Cost Barriers and Moderators of Counseling Mode. Cancer Epidemiol. Biomarkers Prev. 26 (12), 1772–1780. doi:10.1158/1055-9965.EPI-17-0389
The National Academies Collection: Reports funded by National Institutes of Health(2018). Exploring the Barriers to Accessing Genomic and Genetic Services. (US): National Academies Press. Available at: https://www.ncbi.nlm.nih.gov/books/NBK538442/(Accessed April 25, 2022).
Keywords: population genetic screening, cancer, public awareness, DNA sequencing, barriers
Citation: Roberts MC, Foss KS, Henderson GE, Powell SN, Saylor KW, Weck KE and Milko LV (2022) Public Interest in Population Genetic Screening for Cancer Risk. Front. Genet. 13:886640. doi: 10.3389/fgene.2022.886640
Received: 28 February 2022; Accepted: 06 June 2022;
Published: 22 July 2022.
Edited by:
Manuel Corpas, Cambridge Precision Medicine, United KingdomReviewed by:
Aideen McInerney-Leo, The University of Queensland, AustraliaMaria Katapodi, University of Basel, Switzerland
Copyright © 2022 Roberts, Foss, Henderson, Powell, Saylor, Weck and Milko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan C. Roberts, bWVnYW4ucm9iZXJ0c0B1bmMuZWR1
 Megan C. Roberts
Megan C. Roberts Kimberly S. Foss2
Kimberly S. Foss2 Sabrina N. Powell
Sabrina N. Powell Katherine W. Saylor
Katherine W. Saylor Laura V. Milko
Laura V. Milko