
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 31 March 2022
Sec. Toxicogenomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.871820
This article is part of the Research TopicLong-Term Toxicity and Epigenetic Effects of Environmental ExposuresView all 8 articles
 Jia Guo1,2
Jia Guo1,2 Kylie W. Riley1,3
Kylie W. Riley1,3 Teresa Durham1,3
Teresa Durham1,3 Amy E. Margolis1,4
Amy E. Margolis1,4 Shuang Wang1,2
Shuang Wang1,2 Frederica Perera1,3
Frederica Perera1,3 Julie B. Herbstman1,3*
Julie B. Herbstman1,3*Introduction: Prenatal environmental exposures have been associated with children’s cognitive, behavioral, and mental health problems, and alterations in DNA methylation have been hypothesized as an underlying biological mechanism. However, when testing this hypothesis, it is often difficult to overcome the problem of multiple comparisons in statistical testing when evaluating a large number of developmental outcomes and DNA methylation sites as potential mediators. The objective of this study is to implement a ‘meet-in-the-middle’ approach with a sequential roadmap to address this concern.
Methods: In the Columbia Center for Children’s Environmental Health birth cohort study, we implemented a 5-step sequential process for identifying CpG sites that mediate associations between prenatal environmental exposures and cognitive, behavioral, and mental health problems as measured by the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) and the Child Behavior Checklist (CBCL). These steps include 1) the identification of biological pathways that are relevant to each outcome of interest; 2) selection of a set of genes and CpGs on genes that are significantly associated with the outcomes; 3) identification of exposures that are significantly associated with selected CpGs; 4) examination of exposure-outcome relationships among those where significant CpGs were identified; and 5) mediation analysis of the selected exposures and corresponding outcomes. In this study, we considered a spectrum of environmental exposure classes including environmental phenols, pesticides, phthalates, flame retardants and air pollutants.
Results: Among all considered exposures and outcomes, we found one CpG site (cg27510182) on gene (DAB1) that potentially mediates the effect of exposure to PAH on CBCL social problems at children aged 7.
Conclusion: This ‘meet-in-the-middle’ approach attenuates concerns regarding multiple comparisons by focusing on genes and pathways that are biologically relevant for the hypothesis.
Recent studies have discovered a number of associations between prenatal environmental exposures and children’s cognitive, behavioral, and mental health problems. For example, prenatal exposure to polybrominated diphenyl ether (PBDE) was found to be associated with decreased verbal performance and full-scale IQ (Herbstman et al., 2010), decreased cognitive capacity (Eskenazi et al., 2013; Chen et al., 2014), decreased language capacity (Eskenazi et al., 2013; Ding et al., 2015), decreased visual memory (Cowell et al., 2018), and decreased efficiency of the brain’s reading circuit at age 5 (Zhang et al., 2017); prenatal exposure to chlorpyrifos (CPF) has been associated with reduced full-scale IQ at ages three and 7 (Rauh et al., 2006; Rauh et al., 2011); prenatal polycyclic aromatic hydrocarbon (PAH) exposure has been linked with lower full-scale IQ, perceptual reasoning and working memory scores at age 7 (Vishnevetsky et al., 2015), and also linked with children’s lower mental development index measured by Child Behavior Checklist (CBCL) at age 3 (Perera et al., 2006), and attention-deficit/hyperactivity disorder (ADHD) behavior problems at age 9 (Perera et al., 2018), as well as alterations in the development of self-regulation capacity and social problems at age 11 (Margolis et al., 2016) and problems with inhibitory control in childhood that mediate problems with academic skills in adolescence (Margolis et al., 2021). Moreover, prenatal Bisphenol A (BPA) concentration was found to be associated with CBCL anxious/depressed and aggressive behavior (Perera et al., 2012), and CBCL internalizing and externalizing problems (Roen et al., 2015).
Environmental exposures have also been associated with epigenetic alterations including DNA methylation. Specifically, alterations in DNA methylation have been associated with exposure to BPA (Wolstenholme et al., 2011), PAH exposure and increased PAH–DNA adducts (Perera et al., 2011; Herbstman et al., 2012), exposure to phthalates (Kang and Lee, 2005), as well as exposure to high level of nitrogen dioxide (NO2) and fine particulate matter (PM2.5) (Prunicki et al., 2018). We recently developed and validated a pipeline method that predicts dichotomous high/low level of exposures such as NO2, PM2.5 and PAH using DNA methylation patterns in umbilical cord blood (Wang et al., 2021), which further demonstrated associations between DNA methylation and environmental exposures.
While previous research supports the hypotheses that prenatal exposures affect cord blood DNA methylation and also subsequent child health outcomes, it does not imply that DNA methylation is necessarily on the causal pathway between exposure and outcome. Few studies have explored the relationship among the three—environmental exposures, DNA methylation and children’s cognitive, behavioral, and mental health outcomes—simultaneously considering a spectrum of environmental exposures and a spectrum of developmental outcomes. Prior studies have focused on a single exposure or a single outcome. For example, a recent study showed that DNA methylation partly mediates the association between Bisphenol F (BPF) exposure and lower cognition in boys (Engdahl et al., 2021); another study showed that DNA methylation mediated the association between early-life lead (Pb) exposure and infant neurodevelopmental outcomes such as psychomotor development index and rating scale of emotional regulation (Rygiel et al., 2021). Similarly, another study considered a single outcome, body mass index (BMI), and a spectrum of exposures, with the goal of examining whether DNA methylation mediates the relationship between an array of environmental exposures and BMI (Cadiou et al., 2020). All of these studies were limited because they only consider a single exposure or a single outcome, because when considering many exposures or many outcomes and a large number of DNA methylation sites, the problem of multiple comparisons becomes the main limiting factor.
One study examined the effects of multiple exposures on a health outcome, BMI, via DNA methylation, by developing a “Meet-in-the-Middle” approach that attenuates the multiple comparisons problem by reducing DNA methylation dimensions a priori, identifying relevant genes and pathways (Cadiou et al., 2020).
In the present study, we aimed to examine the associations between a spectrum of environmental exposures and a range of children’s cognitive, behavioral, and mental health problems that might potentially be mediated through DNA methylation. We employ a similar dimension reduction approach as in (Cadiou et al., 2020) and only consider relevant biological pathways that potentially connect environmental exposures and children’s cognitive, behavioral, and mental health problems based on a priori knowledge obtained from the KEGG database (Kanehisa et al., 2000). We applied this approach in a longitudinal birth cohort from the Columbia Center for Children’s Environmental Health (CCCEH), seeking to identify methylation sites and corresponding genes that mediate the effect of environmental exposures on children’s cognitive, behavioral, and mental health problems.
The prospective cohort study was conducted by the Columbia Center for Children’s Environmental Health, with a complete description of the study design in (Whyatt et al., 2002; Perera et al., 2002). Study subjects included 727 pregnant Dominican and African-American women recruited through local prenatal care clinics between 1998 and 2006. All women delivered at New York Presbyterian Hospital, Harlem Hospital, or their satellite clinics and were between the ages of 18–35; non-active cigarette smokers; free of diabetes, hypertension, or known HIV, having initiated prenatal care by the 20th week of pregnancy. Participants were of low-income status, with 90% of women on Medicaid.
We explored a spectrum of prenatal environmental exposures including BPA, CPF, the sum of phthalate DEHP metabolites, PBDEs, PAH, PAH-DNA adducts in maternal and cord blood, PM2.5, and NO2. For PAH and PAH adducts, we used the raw measurement, log-transformed values, and binary indicators dichotomized at the limit of detection. For PM2.5 and NO2, we used the average daily measurements within each trimester (at first, second, and third trimester separately) and average measurements across the entire pregnancy. For other exposures, we used continuous exposure measures. Detailed descriptions of exposures are reported elsewhere (Perera et al., 2016; Rauh et al., 2011; Herbstman et al., 2010; Perera et al., 2018; Factor-Litvak et al., 2014).
We considered a range of children’s cognitive, behavioral, and mental health outcomes. Cognition was measured with the Full-Scale Intelligence Quotient (FSIQ) of the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV). The WISC-IV subtest scores were used to derive the FSIQ. Behavioral and mental health problems were measured with the Child Behavior Checklist (CBCL) and one DSM-IV oriented scale (Attention Deficit/Hyperactivity problems). Herein we analyzed WISC-IV FSIQ at age five and age 7, CBCL internalizing and externalizing composite scores at age seven and age 9, the empirically based social and attention problems syndrome scales at age seven and age 9, and the DSM-IV oriented ADHD scale at age seven and age 9. These outcomes were chosen because they have been found to be potentially associated with some environmental exposures in the literatures (Herbstman et al., 2010; Rauh et al., 2011; Perera et al., 2006; Perera et al., 2018; Margolis et al., 2016; Roen et al., 2015). Note that we considered these outcomes at different ages as separate outcomes because questionnaires that measure these outcomes at different ages might have different questions.
DNA methylation in 432 cord blood samples was measured using the 450K array (485,577 CpG sites) and the EPIC array (866,895 CpG sites). A full description of the preprocessing and data analysis steps was included in (Wang et al., 2021). Briefly, we conducted standard quality control steps separately for 450K arrays and EPIC arrays, including requiring 95% CpG coverage per sample and 70% sample coverage per CpG, as well as removing CpGs on sex chromosomes. We also corrected for type I/II probe bias separately for two arrays using the “wateRmelon” R-package (Pidsley et al., 2013) and then combined samples with the 450K/EPIC arrays, where we kept the overlapping CpG sites that were covered by both arrays, resulting in 379,639 CpG sites. We did not perform the calibration between 450K arrays and EPIC arrays, because the BMIQ calibration method (Horvath 2013) would result in a shift between the distribution of 450K array data and the distribution of EPIC array data, while the arrays are aligned better before the calibration (Supplementary Figure S1). We used logit2 transformation to obtain M-values from methylation β-values, and adjusted for cell composition to obtain the M-value residuals by regressing the M-values on cell proportions, which were estimated from cord blood DNA methylation measures using the R-package “minfi” (Aryee et al., 2014). The M-value residuals were used in the following analyses.
Among 341 cord samples with DNA methylation data and with at least one of the outcomes considered, we randomly selected 240 samples (70%) as a discovery set and other 101 samples (30%) as a validation set. We first applied our method on the discovery set and then used the validation set to validate results.
There are five steps in our approach to investigate whether associations between prenatal environmental exposures and children’s cognitive, behavioral, and mental health problems are mediated by methylation. Before the analysis, extreme outliers (values falling outside of 4 standard deviations from the mean) are removed, where less than two samples are removed for each outcome and exposure. The steps are described in detail below and shown in Figure 1. In brief, for each outcome of interest, Step 1 selects biological pathways that are relevant to each outcome of interest based on the KEGG database, which in turn helps identify relevant genes and CpGs on these genes. Step 2 selects a set of CpGs that are significantly associated with the outcome. Step 3 identifies exposures that are significantly associated with selected CpGs from Step 2. Step 4 examines the associations between selected exposures from Step 3 and the corresponding outcome. Step 5 conducts a mediation analysis with selected CpGs and selected exposures, for each outcome of interest.
Step 1: For each neurodevelopmental outcome, we defined a set of keywords (Table 1) and then identified biological pathways related to these keywords in the KEGG database. We searched for biologically relevant pathways based on key words among all available pathways using the R-package “KEGGREST” (Tenenbaum 2016). If a keyword appears at least once in the pathway’s “Name”, “Description”, “Disease”, or titles of “Reference”, we then say the keyword is related to this pathway. A pathway is included for an outcome if this pathway is related to at least one of the predefined keywords. Examples of identified pathways related to the keyword “depression” can be found in the Supplementary material SA. We tried a large set of keywords (Supplementary material SB), and in Table 1 we only showed the keywords that are related to at least one biological pathway. For each outcome, we then extracted genes in the identified pathways and CpGs on these genes.
Step 2: For each outcome, we tested its associations with each of the preselected CpGs in Step 1 using regression models, adjusting for children’s sex (male vs. female), ethnicity (Dominican vs. African American), and children’s age of testing when outcomes are measured (Eqs 1, 2, where
Step 3: For each outcome, we tested associations between each of the environmental exposures and each of the significant CpGs identified in Step 2, adjusting for children’s sex and ethnicity (Eq. 3, where
Step 4: For each outcome, we tested associations with environmental exposures selected in Step 3, adjusting for children’s sex, ethnicity, and children’s age of testing (Eqs 4, 5). We used FDR to adjust for multiple comparisons (number of tests = number of outcomes ∗ number of exposures).
Step 5: For each exposure-outcome pair with a significant association identified in Step 4, we conducted mediation analysis using the R-package ‘MMA’, where mediators are CpGs that are associated with both the outcome (Step 2) and the exposure (Step 3). The ‘MMA’ package uses a bootstrap sampling method to estimate indirect and direct effects of the exposure on the outcome. The indirect effect measures the extent to which the exposure influences the outcome through CpGs, while the direct effect constitutes the extent to which the exposure directly influences the outcome without CpGs. We reported the percentage of the mediation effect in the total effect, calculated as in Eq. 6:
Table 2 shows the numbers of pathways, genes and CpGs that are selected from KEGG database using keywords for different outcomes (from Step 1). Table 2 also shows the number of CpGs (from Step 2) that are significantly associated with each outcome in the CCCEH dataset after FDR adjustment for multiple testing. CBCL internalizing problems at age nine and CBCL social problems at age seven were found to be associated with two preselected CpGs after FDR adjustment for multiple comparisons, while no CpGs were found to be significantly associated with other outcomes. Full results of Step 2 were showed in Supplementary Figure S2; Supplementary Material SC.
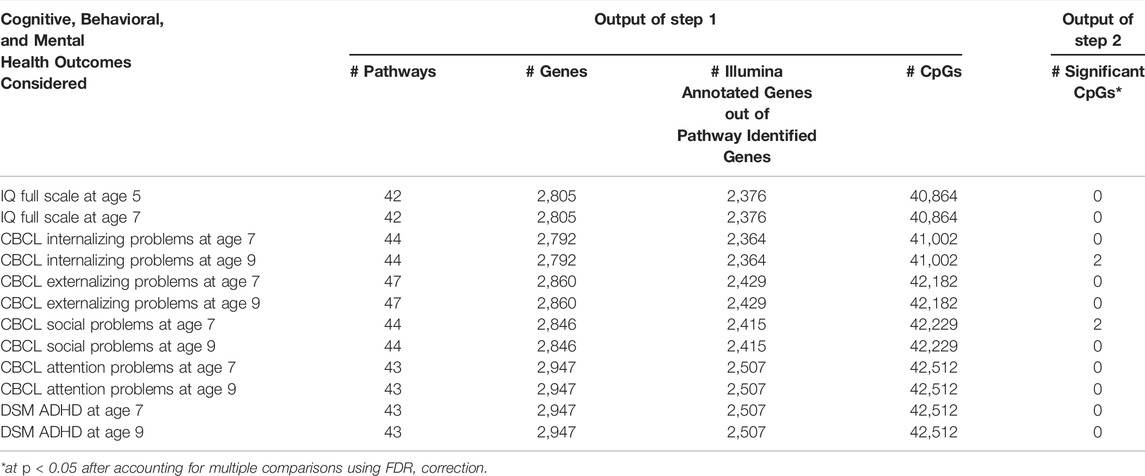
TABLE 2. Numbers of selected pathways/genes/CpGs based on keywords and numbers of CpGs that are significantly associated with outcomes (Output of Step 1 and Step 2).
For each outcome identified in Step 2 (Table 2) with at least one associated CpG site, Step 3 examines the relationship between each environmental exposure and the subset of CpGs identified in Step 2. Table 3 lists the exposures that are significantly associated with at least one CpG after FDR adjustment for multiple comparisons, representing the output of Step 3. For the two CpGs (cg27510182 and cg24713878) that were associated with CBCL social problems at age 7, only CpG cg27510182 is significantly associated with log-transformed PAH after accounting for multiple comparisons using FDR. No exposures were found to be significantly associated with these two CpGs that are associated with CBCL internalizing problems at age 9. Full results of Step 3 were included in Supplementary Material SD.
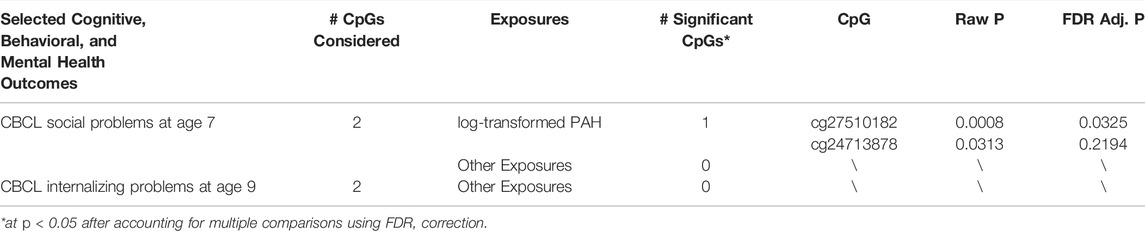
TABLE 3. Selected exposures for each outcome and the number of CpGs that are significantly associated with the exposures (Output of Step 3).
For the exposure and outcomes that show significant findings in Step 3, Step 4 evaluates the exposure-outcome relationships. The only association needs to be tested is the association between CBCL social problems at age seven and log-transformed PAH. Table 4 shows that the log-transformed PAH is positively and significantly associated with CBCL social problems at age 7. The direction is expected because higher scores of CBCL indicates more problems.

TABLE 4. Association tests between outcome and selected exposures which were significantly associated with some outcome-related CpGs (Output from Step 4).
For the significant exposure-outcome relationship of Step 4, Step 5 evaluates the percentage of mediation effects in the total effect, when those potential CpGs are found to be mediators (Table 5). For the effect of log-transformed PAH on CBCL social problems at age 7, cg27510182 has a mediation effect about 46.7% of the total effect. In Table 6, we summarized our overall finding in the discovery dataset with 240 samples with cg27510182, including regression coefficients and raw p-values for each step that involve this CpG from CpG-outcome, exposure-CpG and exposure-outcome relationships. The corresponding results in the validation dataset with 101 samples are included in Supplementary Table S1, where the associations were not significant, possibly due to the small sample size, but the directions of CpG-outcome and exposure-CpG relationships were replicated as those in the discovery dataset.
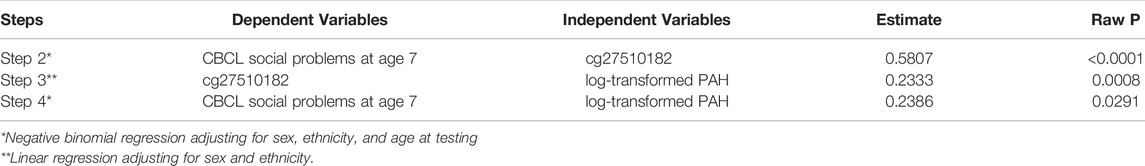
TABLE 6. Overall findings of CpG cg27510182 from each step in the discovery dataset with 240 samples.
We further summarized the identified CpG cg27510182 and the corresponding gene together with their related keywords and pathways from the KEGG database in Table 7.
In the CCCEH birth cohort, we have explored the associations between a spectrum of environmental exposures, DNA methylation, and a range of children’s cognitive, behavioral, and mental health problems using a “Meet-in-the-Middle” approach. As previously noted, this approach takes advantage of relevant biological pathways to initially reduce the number of DNA methylation CpG sites tested. Using sequential steps that further reduce the number of comparisons, this methodology can be used to test exposure-DNA methylation-outcome relationships where a priori information supports the biological plausibility of findings. To compare with our method, we also conducted a standard EWAS (epigenome-wide association study) for each considered outcome, without using relevant biological pathways to preselect CpGs. Although we found CpGs that are significantly associated with some outcomes (Supplementary Material SE) in EWAS after FDR adjustment, there was no significant finding from the following steps based on these significant CpGs. This emphasizes the benefit of our method.
Among all the exposure-DNA methylation-outcome relationships we examined, we found one CpG site and one gene that potentially mediates the effect of exposure to PAH on CBCL social problems at age 7. Specifically, we identified the methylation CpG cg27510182 and corresponding gene DAB1 (DAB Adaptor Protein 1), that potentially mediate the effect of exposure to PAH on CBCL social problems. Close investigation of the identified gene and its relevant pathways suggests that they are biologically relevant. The identified gene DAB1 has been reported to be associated with many neurodevelopmental and psychiatric disorders, such as schizophrenia (SCZ) and autism spectrum disorders (ASD) (Wang et al., 2014; Li et al., 2015; Chen et al., 2017; Stessman et al., 2017; Sánchez-Sánchez et al., 2018; Nawa et al., 2020), because DAB1 is involved in the Reelin signaling pathway which plays a critical role in the central nervous system such as regulating neuronal position in the developing brain (Howell et al., 1997; Rice et al., 2001; Trotter et al., 2013). In addition, the identified gene DAB1 is in the KEGG pathway of a group of progressive neurodegenerative diseases “Spinocerebellar ataxia” (hsa05017), which are usually due to the dysfunction of the cerebellum (Paulson 2009; Matilla-Dueñas et al., 2010), and it has been reported that cerebellar damage is associated with an increased risk of the ASD (Becker et al., 2013; D'Mello et al., 2015). Thus, prenatal exposure to PAH may associate with the malfunction of cerebellum and the Reelin signaling pathway via epigenetic processes, which then associate with children’s neurodevelopmental problems, such as the ASD. In conclusion, the Meet-in-the-Middle approach has revealed that DNA methylation may mediate the effect of prenatal exposure of PAH on neurodevelopmental problems, by affecting the potentially relevant portion of the brain and neurological pathways.
There have been many studies focusing on the relationships between environmental exposures and neurodevelopmental outcomes through DNA methylation. Some studies considered one single exposure and one single outcome (Engdahl et al., 2021); some studies considered one single exposure and multiple outcomes (Rygiel et al., 2021); while some studies considered multiple exposures and one single outcome (Cadiou et al., 2020). Our research is distinct from these existing studies in that we simultaneously consider a spectrum of exposures and a spectrum of correlated cognitive, behavioral, and mental health problems outcomes. To overcome the multiple comparison problem in our work with many exposures and many outcomes as well as high dimensional DNA methylation, we identified a subset of genes in relevant biological pathways with neurodevelopmental outcomes through keywords search.
The step of keywords search is also a limitation of our study, as different keywords will identify different subsets of genes, which may lead to different methylation mediation effects. A more rigorous and more specific method to choose the keywords is necessary. Another limitation is that the identified biological pathways and genes are obtained from a single database, KEGG, which may only contain limited knowledge from existing literature. Besides, the relatively small sample size and lack of adjusting other potential confounders are also limitations of our study. In this study, we randomly selected 70% of our samples as the discovery set and the other 30% as the validation set, which further reduces the sample sizes in each set. We acknowledge the randomness in sample selection, which may affect the findings in both discovery set and the validation set. However, we want to emphasize that we our method is promising and can overcome small sample size problems when large prospective birth cohort studies with multiple cognitive, behavioral, and mental health outcomes of children and multiple prenatal environmental exposures, together with epigenetics are relatively hard to find.
In summary, the methodology outlined in this analysis provides a roadmap for analyses that reserve hypothesis testing to relationships along the exposure-DNA methylation-outcome pathway that have enhanced biological plausibility and thus increased the potential to provide meaningful results. In this study, we detected one CpG cg27510182 on the gene DAB1 that partially mediates the association between prenatal exposure to PAH and CBCL social problems at ages 7. The results are consistent with most recent literature, where PAH could produce a long-lasting effect on self-regulatory capacities and PAH-DNA adduct had a positive association with the CBCL social competence problem (Margolis et al., 2016). PAH exposure during pregnancy has been reported to be positively correlated with CBCL social scores and also positively correlated with the Autism Behavior Checklist (ABC) total scores, which suggest that PAH could be one of the risk factors of ASD-related behaviors for children (Liu et al., 2019). Future epidemiologic studies that can replicate/confirm these associations and mechanistic studies that can evaluate these pathways will enhance our understanding of how prenatal exposure of PAH may lead to neurodevelopmental problems in children.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Columbia University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conceptualization and methodology, JG, KR, TD, AM, SW, FP and JH; Formal Analysis JG and SW.; Validation, JG, SW, FP and JH; Writing and Editing, JG, KR, TD, AM, SW, FP and JH; Funding Acquisition, JH and FP All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.871820/full#supplementary-material
Aryee, M. J., Jaffe, A. E., Corrada-Bravo, H., Ladd-Acosta, C., Feinberg, A. P., Hansen, K. D., et al. (2014). Minfi: a Flexible and Comprehensive Bioconductor Package for the Analysis of Infinium DNA Methylation Microarrays. Bioinformatics 30 (10), 1363–1369. doi:10.1093/bioinformatics/btu049
Becker, E. B. E., and Stoodley, C. J. (2013). Autism Spectrum Disorder and the Cerebellum. Int. Rev. Neurobiol. 113, 1–34. doi:10.1016/b978-0-12-418700-9.00001-0
Cadiou, S., Bustamante, M., Agier, L., Andrusaityte, S., Basagaña, X., Carracedo, A., et al. (2020). Using Methylome Data to Inform Exposome-Health Association Studies: An Application to the Identification of Environmental Drivers of Child Body Mass index. Environ. Int. 138, 105622. doi:10.1016/j.envint.2020.105622
Chen, A., Yolton, K., Rauch, S. A., Webster, G. M., Hornung, R., Sjödin, A., et al. (2014). Prenatal Polybrominated Diphenyl Ether Exposures and Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ. Health Perspect. 122 (8), 856–862. doi:10.1289/ehp.1307562
Chen, N., Bao, Y., Xue, Y., Sun, Y., Hu, D., Meng, S., et al. (2017). Meta-analyses of RELN Variants in Neuropsychiatric Disorders. Behav. Brain Res. 332, 110–119. doi:10.1016/j.bbr.2017.05.028
Cowell, W. J., Margolis, A., Rauh, V. A., Sjödin, A., Jones, R., Wang, Y., et al. (2018). Associations between Prenatal and Childhood PBDE Exposure and Early Adolescent Visual, Verbal and Working Memory. Environ. Int. 118, 9–16. doi:10.1016/j.envint.2018.05.004
D'Mello, A. M., and Stoodley, C. J. (2015). Cerebro-cerebellar Circuits in Autism Spectrum Disorder. Front. Neurosci. 9, 408. doi:10.3389/fnins.2015.00408
Ding, G., Yu, J., Cui, C., Chen, L., Gao, Y., Wang, C., et al. (2015). Association between Prenatal Exposure to Polybrominated Diphenyl Ethers and Young Children's Neurodevelopment in China. Environ. Res. 142, 104–111. doi:10.1016/j.envres.2015.06.008
Engdahl, E., Svensson, K., Lin, P.-I. D., Alavian-Ghavanini, A., Lindh, C., Rüegg, J., et al. (2021). DNA Methylation at GRIN2B Partially Mediates the Association between Prenatal Bisphenol F Exposure and Cognitive Functions in 7-Year-Old Children in the SELMA Study. Environ. Int. 156, 106617. doi:10.1016/j.envint.2021.106617
Eskenazi, B., Chevrier, J., Rauch, S. A., Kogut, K., Harley, K. G., Johnson, C., et al. (2013). In Uteroand Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ. Health Perspect. 121 (2), 257–262. doi:10.1289/ehp.1205597
Factor-Litvak, P., Insel, B., Calafat, A. M., Liu, X., Perera, F., Rauh, V. A., et al. (2014). Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PloS one 9 (12), e114003. doi:10.1371/journal.pone.0114003
Herbstman, J. B., Sjödin, A., Kurzon, M., Lederman, S. A., Jones, R. S., Rauh, V., et al. (2010). Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect. 118 (5), 712–719. doi:10.1289/ehp.0901340
Herbstman, J. B., Tang, D., Zhu, D., Qu, L., Sjödin, A., Li, Z., et al. (2012). Prenatal Exposure to Polycyclic Aromatic Hydrocarbons, Benzo[a]pyrene-DNA Adducts, and Genomic DNA Methylation in Cord Blood. Environ. Health Perspect. 120 (5), 733–738. doi:10.1289/ehp.1104056
Horvath, S. (2013). DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 14 (10), R115–R120. doi:10.1186/gb-2013-14-10-r115
Howell, B. W., Hawkes, R., Soriano, P., and Cooper, J. A. (1997). Neuronal Position in the Developing Brain Is Regulated by Mouse Disabled-1. Nature 389 (6652), 733–737. doi:10.1038/39607
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28 (1), 27–30. doi:10.1093/nar/28.1.27
Kang, S. C., and Lee, B. M. (2005). DNA Methylation of Estrogen Receptor Alpha Gene by Phthalates. J. Toxicol. Environ. Health A. 68 (23-24), 1995–2003. doi:10.1080/15287390491008913
Li, W., Guo, X., and Xiao, S. (2015). Evaluating the Relationship between Reelin Gene Variants (Rs7341475 and Rs262355) and Schizophrenia: a Meta-Analysis. Neurosci. Lett. 609, 42–47. doi:10.1016/j.neulet.2015.10.014
Liu, X. Y., Wang, B. L., Yi, M. J., and Zhang, F. H. (2019). Association of Exposure to Polycyclic Aromatic Hydrocarbons during Pregnancy with Autism Spectrum Disorder-Related Behaviors in Toddlers: a Birth Cohort Study. Zhongguo Dang Dai Er Ke Za Zhi 21 (4), 332–336.
Margolis, A. E., Herbstman, J. B., Davis, K. S., Thomas, V. K., Tang, D., Wang, Y., et al. (2016). Longitudinal Effects of Prenatal Exposure to Air Pollutants on Self-Regulatory Capacities and Social Competence. J. Child. Psychol. Psychiatr. 57 (7), 851–860. doi:10.1111/jcpp.12548
Margolis, A. E., Ramphal, B., Pagliaccio, D., Banker, S., Selmanovic, E., Thomas, L. V., et al. (2021). Prenatal Exposure to Air Pollution Is Associated with Childhood Inhibitory Control and Adolescent Academic Achievement. Environ. Res. 202, 111570. doi:10.1016/j.envres.2021.111570
Matilla-Dueñas, A., Sánchez, I., Corral-Juan, M., Dávalos, A., Alvarez, R., and Latorre, P. (2010). Cellular and Molecular Pathways Triggering Neurodegeneration in the Spinocerebellar Ataxias. The Cerebellum 9 (2), 148–166.
Nawa, Y., Kimura, H., Mori, D., Kato, H., Toyama, M., Furuta, S., et al. (2020). Rare Single-Nucleotide DAB1 Variants and Their Contribution to Schizophrenia and Autism Spectrum Disorder Susceptibility. Hum. Genome 7 (1), 37–39. doi:10.1038/s41439-020-00125-7
Paulson, H. L. (2009). The Spinocerebellar Ataxias. J. neuro-ophthalmology: official J. North Am. Neuro-Ophthalmology Soc. 29 (3), 227–237. doi:10.1097/wno0b013e3181b416de
Perera, F., and Herbstman, J. (2011). Prenatal Environmental Exposures, Epigenetics, and Disease. Reprod. Toxicol. 31 (3), 363–373. doi:10.1016/j.reprotox.2010.12.055
Perera, F., Nolte, E. L. R., Wang, Y., Margolis, A. E., Calafat, A. M., Wang, S., et al. (2016). Bisphenol A Exposure and Symptoms of Anxiety and Depression Among Inner City Children at 10-12 Years of Age. Environ. Res. 151, 195–202. doi:10.1016/j.envres.2016.07.028
Perera, F. P., Illman, S. M., Kinney, P. L., Whyatt, R. M., Kelvin, E. A., Shepard, P., et al. (2002). The challenge of Preventing Environmentally Related Disease in Young Children: Community-Based Research in New York City. Environ. Health Perspect. 110 (2), 197–204. doi:10.1289/ehp.02110197
Perera, F. P., Rauh, V., Whyatt, R. M., Tsai, W.-Y., Tang, D., Diaz, D., et al. (2006). Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbonson Neurodevelopment in the First 3 Years of Life Among Inner-City Children. Environ. Health Perspect. 114 (8), 1287–1292. doi:10.1289/ehp.9084
Perera, F. P., Wheelock, K., Wang, Y., Tang, D., Margolis, A. E., Badia, G., et al. (2018). Combined Effects of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Material Hardship on Child ADHD Behavior Problems. Environ. Res. 160, 506–513. doi:10.1016/j.envres.2017.09.002
Perera, F., Vishnevetsky, J., Herbstman, J. B., Calafat, A. M., Xiong, W., Rauh, V., et al. (2012). Prenatal Bisphenol a Exposure and Child Behavior in an Inner-City Cohort. Environ. Health Perspect. 120 (8), 1190–1194. doi:10.1289/ehp.1104492
Pidsley, R., Y Wong, C. C., Volta, M., Lunnon, K., Mill, J., and Schalkwyk, L. C. (2013). A Data-Driven Approach to Preprocessing Illumina 450K Methylation Array Data. BMC genomics 14 (1), 293. doi:10.1186/1471-2164-14-293
Prunicki, M., Stell, L., Dinakarpandian, D., de Planell-Saguer, M., Lucas, R. W., Hammond, S. K., et al. (2018). Exposure to NO2, CO, and PM2.5 Is Linked to Regional DNA Methylation Differences in Asthma. Clin. Epigenetics 10 (1), 2–11. doi:10.1186/s13148-017-0433-4
Rauh, V. A., Garfinkel, R., Perera, F. P., Andrews, H. F., Hoepner, L., Barr, D. B., et al. (2006). Impact of Prenatal Chlorpyrifos Exposure on Neurodevelopment in the First 3 Years of Life Among Inner-City Children. Pediatrics 118 (6), e1845–e1859. doi:10.1542/peds.2006-0338
Rauh, V., Arunajadai, S., Horton, M., Perera, F., Hoepner, L., Barr, D. B., et al. (2011). Seven-year Neurodevelopmental Scores and Prenatal Exposure to Chlorpyrifos, a Common Agricultural Pesticide. Environ. Health Perspect. 119 (8), 1196–1201. doi:10.1289/ehp.1003160
Rice, D. S., and Curran, T. (2001). Role of the Reelin Signaling Pathway in central Nervous System Development. Annu. Rev. Neurosci. 24 (1), 1005–1039. doi:10.1146/annurev.neuro.24.1.1005
Roen, E. L., Wang, Y., Calafat, A. M., Wang, S., Margolis, A., Herbstman, J., et al. (2015). Bisphenol A Exposure and Behavioral Problems Among Inner City Children at 7-9 Years of Age. Environ. Res. 142, 739–745. doi:10.1016/j.envres.2015.01.014
Rygiel, C. A., Dolinoy, D. C., Bakulski, K. M., Aung, M. T., Perng, W., Jones, T. R., et al. (2021). DNA Methylation at Birth Potentially Mediates the Association between Prenatal lead (Pb) Exposure and Infant Neurodevelopmental Outcomes. Environ. Epigenet 7 (1), dvab005. doi:10.1093/eep/dvab005
Sánchez-Sánchez, S. M., Magdalon, J., Griesi‐Oliveira, K., Yamamoto, G. L., Santacruz‐Perez, C., Fogo, M., et al. (2018). Rare RELN Variants Affect Reelin–DAB1 Signal Transduction in Autism Spectrum Disorder. Hum. Mutat. 39 (10), 1372–1383.
Stessman, H. A. F., Xiong, B., Coe, B. P., Wang, T., Hoekzema, K., Fenckova, M., et al. (2017). Targeted Sequencing Identifies 91 Neurodevelopmental-Disorder Risk Genes with Autism and Developmental-Disability Biases. Nat. Genet. 49 (4), 515–526. doi:10.1038/ng.3792
Trotter, J., Lee, G. H., Kazdoba, T. M., Crowell, B., Domogauer, J., Mahoney, H. M., et al. (2013). Dab1 Is Required for Synaptic Plasticity and Associative Learning. J. Neurosci. 33 (39), 15652–15668. doi:10.1523/jneurosci.2010-13.2013
Vishnevetsky, J., Tang, D., Chang, H.-W., Roen, E. L., Wang, Y., Rauh, V., et al. (2015). Combined Effects of Prenatal Polycyclic Aromatic Hydrocarbons and Material Hardship on Child IQ. Neurotoxicology and teratology 49, 74–80. doi:10.1016/j.ntt.2015.04.002
Wang, Y., Perera, F., Guo, J., Riley, K. W., Durham, T., Ross, Z., et al. (2021). A Methodological Pipeline to Generate an Epigenetic Marker of Prenatal Exposure to Air Pollution Indicators. Epigenetics 2021, 1–9. doi:10.1080/15592294.2021.1872926
Wang, Z., Hong, Y., Zou, L., Zhong, R., Zhu, B., Shen, N., et al. (2014). Reelin Gene Variants and Risk of Autism Spectrum Disorders: An Integrated Meta-Analysis. Am. J. Med. Genet. 165 (2), 192–200. doi:10.1002/ajmg.b.32222
Whyatt, R. M., Camann, D. E., Kinney, P. L., Reyes, A., Ramirez, J., Dietrich, J., et al. (2002). Residential Pesticide Use during Pregnancy Among a Cohort of Urban Minority Women. Environ. Health Perspect. 110 (5), 507–514. doi:10.1289/ehp.02110507
Wolstenholme, J. T., Rissman, E. F., and Connelly, J. J. (2011). The Role of Bisphenol A in Shaping the Brain, Epigenome and Behavior. Horm. Behav. 59 (3), 296–305. doi:10.1016/j.yhbeh.2010.10.001
Keywords: prenatal exposure, DNA methylation, children, PM2. 5, CBCL social problems, autism, PAH
Citation: Guo J, Riley KW, Durham T, Margolis AE, Wang S, Perera F and Herbstman JB (2022) Association Studies of Environmental Exposures, DNA Methylation and Children’s Cognitive, Behavioral, and Mental Health Problems. Front. Genet. 13:871820. doi: 10.3389/fgene.2022.871820
Received: 08 February 2022; Accepted: 14 March 2022;
Published: 31 March 2022.
Edited by:
Kai Wang, University of Michigan, United StatesReviewed by:
Joseph Kochmanski, Michigan State University, United StatesCopyright © 2022 Guo, Riley, Durham, Margolis, Wang, Perera and Herbstman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie B. Herbstman, amgyNjc4QGN1bWMuY29sdW1iaWEuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.