
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 22 June 2022
Sec. Genetics of Common and Rare Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.868159
This article is part of the Research Topic Advancing Genomics for Rare Disease Diagnosis and Therapy Development Vol II View all 42 articles
 Hongyan Yu1†
Hongyan Yu1† Fucheng Liu2†
Fucheng Liu2† Kaining Chen1
Kaining Chen1 Yufen Xu1
Yufen Xu1 Yishuai Wang1
Yishuai Wang1 Lanyan Fu1
Lanyan Fu1 Huazhong Zhou1
Huazhong Zhou1 Lei Pi1
Lei Pi1 Di Che1
Di Che1 Hehong Li3*
Hehong Li3* Xiaoqiong Gu1,4*
Xiaoqiong Gu1,4*Background: Kawasaki disease (KD) is an acute, self-limited vasculitis disorder of unknown etiology in children. Immunologic abnormalities were detected during the acute phase of KD, which reflected that the effect cells of the activated immune system markedly increased cytokine production. High-dose intravenous immunoglobulin (IVIG) therapy is effective in resolving inflammation from KD and reducing occurrence of coronary artery abnormalities. However, 10%–20% of KD patients have no response to IVIG therapy, who were defined as IVIG resistance. Furthermore, these patients have persistent inflammation and increased risk of developing coronary artery aneurysm (CAA). EIF2AK4 is a stress sensor gene and can be activated by pathogen infection. In addition, the polymorphisms of EIF2AK4 were associated with various blood vessel disorders. However, it remains unclear whether the EIF2AK4 gene polymorphisms were related to IVIG therapy outcome in KD patients.
Methods: EIF2AK4/rs4594236 polymorphism was genotyped in 795 IVIG response KD patients and 234 IVIG resistant KD patients through TaqMan, a real-time polymerase chain reaction. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to assess the strength of association between EIF2AK4/rs4594236 polymorphism and IVIG therapeutic effects.
Results: Our results showed that the EIF2AK4/rs4594236 AG/GG genotype was significantly associated with increased risk to IVIG resistance compared to the AA genotype (AG vs. AA: adjusted ORs = 1.71, 95% CIs = 1.17–2.51, and p = 0.0061; GG vs. AA: adjusted ORs = 2.09, 95% CIs = 1.36–3.23, and p = 0.0009; AG/GG vs. AA: adjusted ORs = 1.82, 95% CIs = 1.27–2.63, and p = 0.0013; and GG vs. AA/AG: adjusted ORs = 1.45, 95% CI = 1.04–2.02, and p = 0.0306). Furthermore, the stratified analysis of age and gender in the KD cohort indicated that male patients carrying the rs4594236 AG/GG genotype tends to be more resistant to IVIG therapy than female patients.
Conclusion: These results suggested that EIF2AK4/rs4594236 polymorphism might be associated with increased risk of IVIG resistance in southern Chinese KD patients.
Kawasaki disease (KD) is an acute, self-limited vasculitis disease in children aged from 6 months to 5 years (Burns and Glodé, 2004). Immunologic abnormalities were detected during the acute phase of KD, which reflected that the effect cells of the activated immune system markedly increased cytokine production (Burns and Glodé, 2004). The etiology of KD is unknown, while several epidemiological and clinical reports have suggested that KD might be triggered by infectious agents or viruses (Sharma et al., 2021). This was evidenced by the fact that proinflammatory cytokines (IL-6, IL-10, TNFα, and IFNγ) were increased significantly in the acute stage of KD (Wang et al., 2013). Since lots of cytokines and activated immune cells attacked medium-sized arteries, especially coronary arteries, 20%–25% of untreated patients will develop coronary artery aneurysm (CAA) (Gersony, 2009), which has made KD the leading cause of acquired heart disease among children in developed countries (Kato et al., 1975).
Intravenous immunoglobulin (IVIG) contains pooled immunoglobulin G (IgG) from the plasma of over thousand blood donors and is widely used in people with weakened immune systems or other diseases to fight off infections (Lünemann et al., 2015). High-dose IVIG therapy is effective in resolving inflammation from KD and reducing occurrence of CAA. However, 10%–20% of KD patients will develop IVIG resistance, defined by recrudescent or persistent fever for over 36 h after the end of the IVIG infusion primary therapy. In addition, these patients have persistent inflammation and increased risk of developing CAA (McCrindle et al., 2017). Therefore, uncovering the mechanism of IVIG resistance in KD is urgently needed. While the mechanism of IVIG action is complicated and how it works on KD is still confused and unknown, at present, several studies have shown that genetic polymorphisms, especially some immune functional genes, are associated with IVIG resistance, such as inositol 1.4.5-trisposhate 3-kinase C (ITPKC), Fcγ IgG receptor 2A (FCGR2A), CD40, and interferon-gamma (IFN-γ) (Onouchi et al., 2008; Khor et al., 2011; Lee et al., 2012; Onouchi et al., 2013; Huang et al., 2016). These studies suggested that genetic factors might be involved in IVIG resistance.
Eukaryotic translation initiation factor 2-alpha kinase 4 (EIF2AK4, also known as GCN2) is a member of the kinase family that phosphorylates the alpha subunit of eukaryotic translation initiation factor-2 (eIF2a)(Wang et al., 2019). EIF2AK4 phosphorylates eIF2a on the serine 51 site and reduces GDP/GTP exchange activity subsequently. In addition, this resulted in mRNA translation changes and subsequently modulated cellular physiological activities (McGaha et al., 2012).
EIF2AK4 mutation was found in patients classified as having idiopathic and heritable pulmonary arterial hypertension (Hadinnapola et al., 2017). Histopathology of EIF2AK4 mutation carriers in pulmonary veno-occlusive disease (PVOD) patients was distinctive from noncarriers regarding arterial remodeling, with significantly more severe intimal fibrosis and less severe medial hypertrophy (Nossent et al., 2018). Furthermore, under nutrient-deprived conditions, EIF2AK4 could promote angiogensis of endothelial cells by increasing VEGF expression (Longchamp et al., 2018). These literature studies showed that EIF2AK4 could modulate vascular remodeling and angiogensis, which are closely associated with coronary arterial lesions (CALs) of KD (Takahashi et al., 2013). What’s more, EIF2AK4 was also found to regulate cytokine production and macrophage function in several infectious diseases. Eif2ak4 knockout mice challenged with lipopolysaccharide (LPS) showed reduced inflammatory response, including decreased IL-6 and IL-12 expression, as compared to wild-type mice (Liu et al., 2014). Interestingly, IL-6 and IL-10 were at a high level in the IVIG resistant group compared to the IVIG response group after IVIG treatment (Wang et al., 2013). In inflammatory kidney disease, IFN-γ–activated EIF2AK4 could suppress proinflammatory cytokine production in glomeruli and reduce macrophage recruitment to the kidneys (Chaudhary et al., 2015), while Ravindran et al. (2016) showed that EIF2AK4 also controlled intestinal inflammation through inhibiting inflammasome activation and IL-1β production. In conclusion, these studies indicate that EIF2AK4 may be associated with KD.
Our team has worked on the area of the etiology and therapy effect of KD for many years (Che et al., 2018; Lin et al., 2021; Wang et al., 2021). We found that the single-nucleotide polymorphism (SNP) of immune and/or cardiovascular-related genes were usually related to IVIG therapy outcome of KD, such as IL-1β (Fu et al., 2019), PLA2G7 (Gu et al., 2020), P2RY12 (Wang et al., 2020), and MRP4 (Wang et al., 2021). However, evidence regarding the polymorphisms of EIF2AK4 and IVIG resistance of KD is very scarce. Based on this background, we performed this epidemiology study to investigate whether EIF2AK4 is related to IVIG resistance of KD by examining the association between EIF2AK4 polymorphism (rs4594236) and the risk of IVIG resistance of KD.
A total of 1,029 KD patients from the Guangzhou Women and Children’s Medical Center between January 2014 and December 2019 were enrolled in this study. All individuals with KD were diagnosed by pediatricians based on the criteria of the American Heart Association (Newburger et al., 2004; McCrindle et al., 2017). IVIG resistance was defined as persistent or recrudescent fever (temperature ≥38.0°C, measured axilla or orally) for over 36 h, but for a period of less than 7 days, after completion of the first IVIG infusion (2 g/kg).
Peripheral blood was collected from KD patients after treatment completion. Genomic DNA was extracted with a TIANamp Blood DNA Kit (DP318, TIANGEN Biotech, Beijing) following the guidance of the manufacturer’s instructions (Wu et al., 2020). Specific fluorescent allele probes for rs4594236 were purchased from ABI (Thermo Fisher Scientific, United States). PCR was performed in 384-well plates with an ABI-Q6 Sequence Detection System machine (Thermo Fisher Scientific).
The genotyping of the SNP was conducted using a TaqMan SNP genotyping assay (Lin et al., 2021). Laboratory technicians were blind to the sample information, including the identities of the replicate aliquots. 10% of the samples from both groups were arbitrarily chosen to repeat the genotyping results. A concordance rate of 100% was obtained.
Statistical analysis of this study was performed by using SAS software (version 9.4; SAS Institute, Cary, NC). Pearson’s chi square test was used to evaluate the significant differences between IVIG response and IVIG resistant cases in the distribution of demographic variables and genotype frequency. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression analysis for measuring the association between the EIF2AK4/rs4594236 polymorphism and the risk of IVIG treatment resistance in KD patients. Furthermore, stratification analysis was performed, classified by age and gender. We also performed the eQTL analysis using the GTEx Portal web site (https://www.gtexportal.org/home/) to predict potential associations between the SNP and gene-expression levels (Consortium, 2013). A p-value of less than 0.05 was regarded as statistically significant.
The characteristic distribution of 795 IVIG therapy response KD patients and 234 IVIG therapy resistant KD patients is shown in Table 1. The average age of the IVIG response group was 25.14 ± 20.33 months (rang 1–131 months), and it was 26.08 ± 21.80 months (rang 2–132 months) for the IVIG resistant group. 67.17% of the KD patients who were responsive to IVIG therapy were men, and the male ratio was 72.65% in KD patients who were resistant to IVIG therapy. The proportion of women was 32.83% and 27.35%, respectively, while there were no significant difference in age (p = 0.3750) and gender (p = 0.1096) between the IVIG response group and the resistant group.
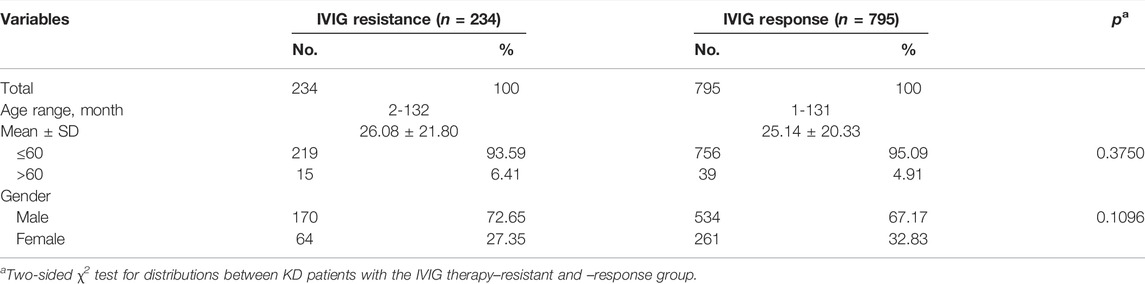
TABLE 1. Characteristic distribution in the IVIG therapy resistant group and the response group of KD patients.
The genotype frequency distribution of EIF2AK4/rs4594236 polymorphism in the KD IVIG resistant group and response group is described in Table 2. To explore the association between EIF2AK4/rs4594236 polymorphism and the risk to IVIG therapy resistance, we performed χ2 test analysis. We found that EIF2AK4/rs4594236 polymorphism was significantly associated with increased IVIG therapy resistance risk in KD patients (AG vs. AA: adjusted OR = 1.71, 95% confidence interval (CI) = 1.17–2.51, and p = 0.0061; GG vs. AA: adjusted OR = 2.09, 95% confidence interval (CI) = 1.36–3.23, and p = 0.0009; AG/GG vs. AA: adjusted OR = 1.82, 95% CI = 1.27–2.63, and p = 0.0013; GG vs. AG/AA: adjusted OR = 1.45, 95% confidence interval (CI) = 1.04–2.02, and p = 0.0306). The results indicated that patients with a GG/AG genotype had a higher risk of suffering IVIG therapy resistance than patients with an AA genotype, suggesting the resistive effect of this SNP against IVIG therapy.
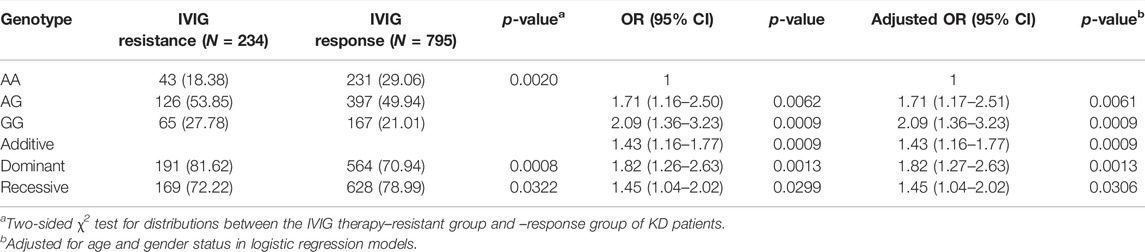
TABLE 2. Genotype distribution frequency of EIF2AK4/rs4594236 polymorphism in the IVIG therapy–resistant group and –response group of KD patients.
It is well known that EIF2AK4 is involved in vascular remodeling (Nossent et al., 2018), which is the critical step for CAL formation. Therefore, the association between EIF2AK4/rs4594236 polymorphism and CAL formation was explored. Patients with KD were then divided into the CAL group and the NCAL group depending on whether they had CAL or not, and EIF2AK4/rs4594236 genotyping was performed on the two groups (Table 3). As shown in Table 3, EIF2AK4/rs4594236 was not associated with CAL formation. We then analyzed the relation between EIF2AK4/rs4594236 polymorphism and CAA formation (the serious lesions of the coronary artery) of KD. However, there was no significant association observed between EIF2AK4/rs4594236 and CAA (Table 4).
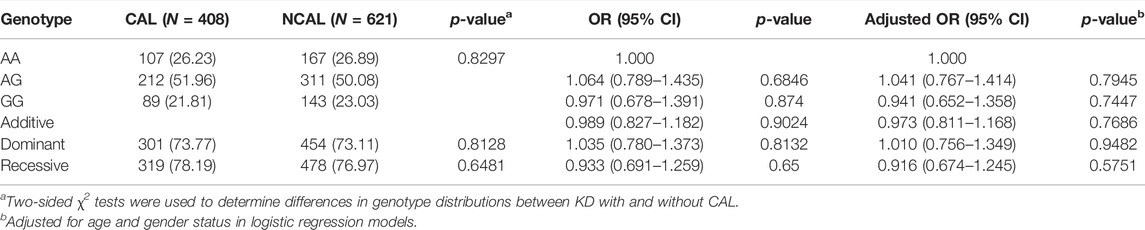
TABLE 3. Genotype distribution frequency of EIF2AK4/rs4594236 polymorphism in the NCAL group and CAL group of KD patients.
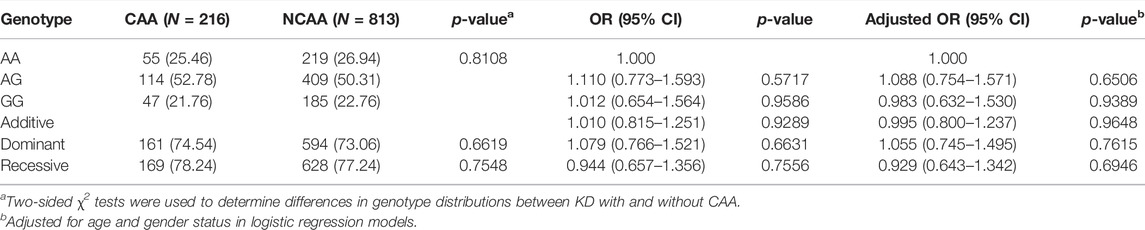
TABLE 4. Genotype and allele frequencies of EIF2AK4 in KD Patients with (CAA) or without CAA (NCAA).
We further explored the association between EIF2AK4/rs4594236 polymorphism and the risk effect of IVIG resistance on certain subgroups classified by age and gender (Table 5). Compared with the rs4594236 AA genotype, the risk effect of the rs4594236 GG/AG genotype was more prominent in male patients of all ages (adjust OR = 1.91, 95% CI = 1.23–2.95, and p = 0.0039).
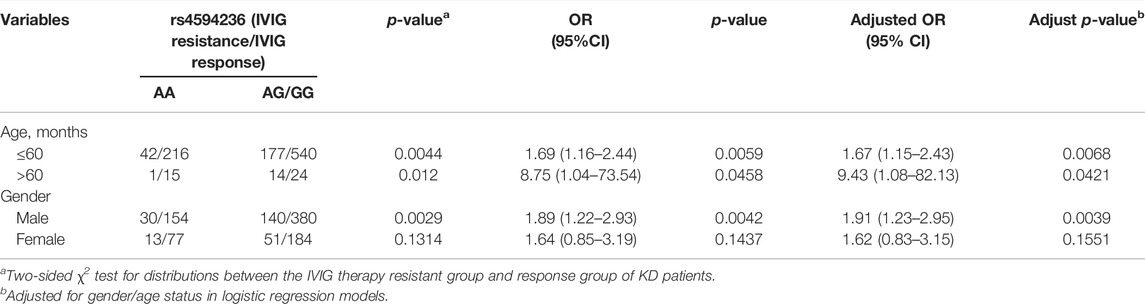
TABLE 5. Stratification analysis of EIF2AK4/rs4594236 polymorphism in the IVIG therapy resistant group and response group of KD patients.
To assess the putative functional relevance of rs4594236 polymorphism affecting EIF2AK4 mRNA expression, we used the data released from Genotype-Tissue Expression (GTEx) website. It was found that individuals carrying the rs4594236 G allele displayed significantly higher EIF2AK4 mRNA levels in the artery of the aorta and tibia, the atrial appendage, and the left ventricle of the heart than those with the rs4594236 A allele (Figure 1). Furthermore, we evaluated the impact of the rs4594236 polymorphism on the mRNA level of the neighboring genes in the above-mentioned tissues and found that signal recognition particle 14 (SRP14) (or divergent transcript (SRP14-AS1)) mRNA levels in tissues with the rs4594236 G allele were significantly lower than those with the rs4594236 A allele (Figure 2).
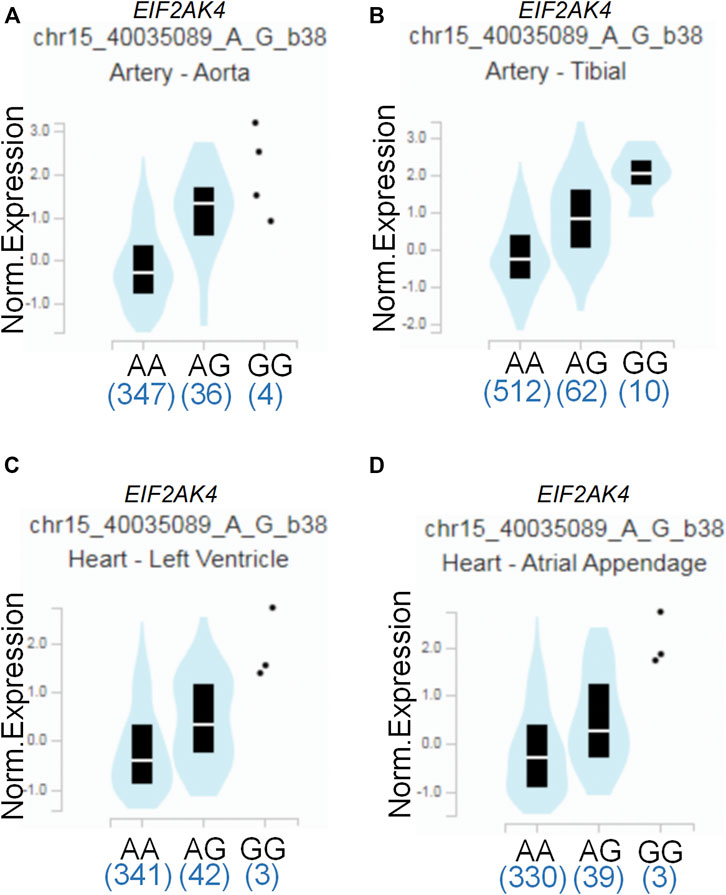
FIGURE 1. Functional implication of the EIF2AK4 gene rs4594236 polymorphism in human tissues. (A) The genotype of rs4594236 and expression of the EIF2AK4 gene in the artery of the aorta were searched on the public database GTEx Portal. p = 2.6 × 10−20. (B) The genotype of rs4594236 and expression of the EIF2AK4 gene in the artery of the tibia were searched on the public database GTEx Portal. p = 2.4 × 10−18. (C) The genotype of rs4594236 and expression of the EIF2AK4 gene in the left ventricle of the heart were searched on the public database GTEx Portal. p = 3.4 × 10−8. (D) The genotype of rs4594236 and expression of the EIF2AK4 gene in the atrial appendage of the heart were searched on the public database GTEx Portal. p = 2.1 × 10−8.
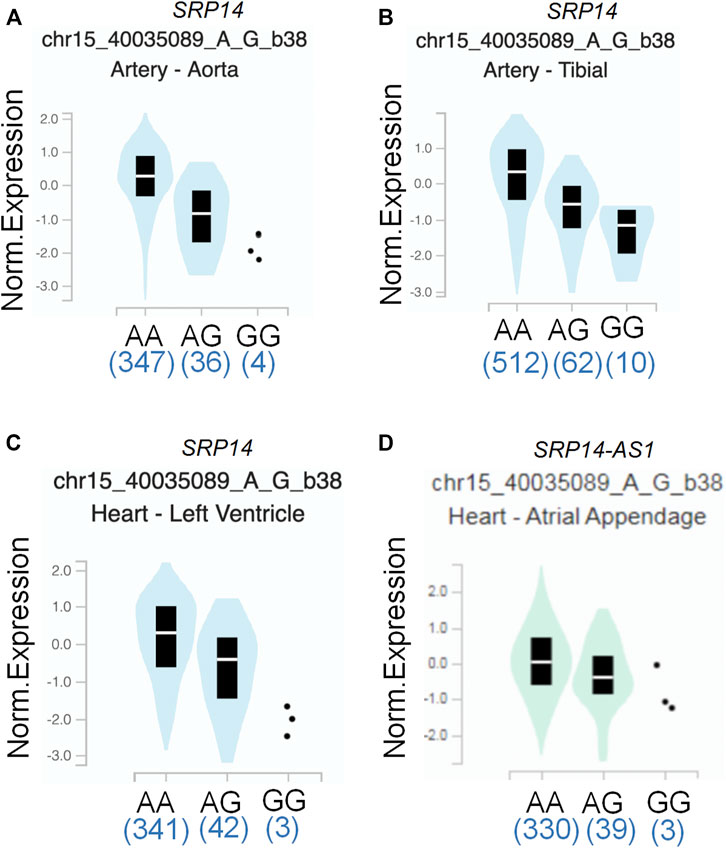
FIGURE 2. Functional prediction of rs4594236 on a neighboring gene. (A) Correlation between rs4594236 and SRP14 gene expression in the artery of the aorta. p = 6.9 × 10−10. (B) Correlation between rs4594236 and SRP14 gene expression in the artery of the tibia. p = 2.3 × 10−9. (C) Correlation between rs4594236 and SRP14 gene expression in the left ventricle of the heart. p = 3.4 × 10−8. (D) Correlation between rs4594236 and SRP14-AS1 gene expression in the atrial appendage of the heart. p = 9.8 × 10−9.
IVIG has been the optimal and effective treatment for KD to reduce the prevalence of coronary-artery abnormalities and systemic inflammation until now (Newburger et al., 1986). Although the molecular and cellular basis of IVIG function is complicated and remains unknown, some evidence indicated that genetic factors played an important role in IVIG treatment activities. Taking the fact that several genes were associated with the susceptibility of KD and the rates of IVIG resistant patients differ among different ethnic groups (Kashef et al., 2005; Uehara et al., 2008; Tremoulet et al., 2011), some hot genetic factors, especially immune functional genes, were examined to be associated with IVIG resistance, such as FcγR2C and FcγR3B (Makowsky et al., 2013). Furthermore, Weng et al. (2010) had found that patients with IL-1β (−511 TT) and IL-1β (−31 CC) genotypes had increased risk of IVIG resistance and were associated with initial IVIG treatment failure based on a study of 156 KD patients (136 with and 20 without response to IVIG treatment) among Taiwanese children .
Herein, we demonstrated a potentially contributing role of EIF2AK4/rs4594236 polymorphism in IVIG resistance in KD and for the first time reported that EIF2AK4/rs4594236 polymorphism could predispose to IVIG resistance in southern Chinese KD children.
EIF2AK4 is a high molecular weight protein kinase activated by uncharged tRNA (Wek et al., 1990; Nakamura et al., 2018; Schmidt et al., 2019). Activated EIF2AK4 can phosphorylate eIF2a to upregulate ATF4 translation, which in turn increases amino acid biosynthetic and activated transport pathways (Harding et al., 2000; Harding et al., 2003). The physiological functions of EIF2AK4 currently remain poorly understood, but its function in human diseases has recently been emphasized.
Several studies demonstrated that EIF2AK4 was associated with vascular remodeling (Lu et al., 2014; Nossent et al., 2018; Chen et al., 2021). One possible mechanism was that EIF2AK4 dysfunction enhanced collagen I gene transcription via the ATF3/p38 pathway, which led to increased collagen deposition in the pulmonary artery (Chen et al., 2021). As vascular remodeling is critical for CAL formation of KD, we also investigated the association between EIF2AK4/rs4594236 polymorphism and CAL or CAA formation of KD. However, we found that EIF2AK4/rs4594236 polymorphism was not associated with either CAL or CAA formation. The possible reason may be that the EIF2AK4 expression level was decreased significantly in EIF2AK4 mutation PVOD patients who had undergone vascular modeling. While the data from GTEx showed that individuals carrying the rs4594236 G allele displayed significantly higher EIF2AK4 mRNA levels in the artery of the aorta and tibia, the atrial appendage, and the left ventricle of the heart than those with the rs4594236 A allele (Figure 1), it is indicated that the patients with an rs4594236 G allele have a higher risk of IVIG therapy resistance and the EIF2AK4 expression level. In other words, the patients with higher EIF2AK4 expression tend to have a higher IVIG resistant incidence rate. Hence, different expression levels of EIF2AK4 stimulated diverse downstream signals to regulate cell physiological functions differently.
On the other hand, McGaha et al. found loss of EIF2AK4-enhanced inflammatory macrophage transcription with a crowd of proinflammatory cytokine expression and production. In addition, the activated regulatory macrophage was attenuated with a decrease in the Arg1 and CCL22 mRNA expression and IL-10 protein level at the same time. Mechanistically, EIF2AK4 altered the myeloid function by activating the CREB-2/ATF4 signal pathway, which was required for maturation and polarization of macrophages in both mice and humans (Halaby et al., 2019). Interestingly, IVIG treatment promotes tumor-associated macrophages from M2 to M1 polarization, and the IVIG effect was dependent on the activation/polarization state of macrophages (Domínguez-Soto et al., 2014). It is possible that the immunomodulatory effect of IVIG observed in other autoimmune diseases such as KD follows a similar pattern. In addition, our results showed the IVIG resistant risk of KD patients may be linked to the upregulated expression levels of the EIF2AK4 gene (Figure 1); thus, we deduced the hypothesis that IVIG treatment promoted macrophage M1 polarization while the immunomodulatory function of the M1 macrophage was inhibited by upregulated EIF2AK4, which caused persistent inflammation. However, more functional experiments need to be carried out to support the possible mechanism.
To date, studies have been conducted regarding the epidemiologic assessment of EIF2AK4 gene SNPs. Deng et al. carried out a genome-wide association study of body mass index (BMI) from a cohort containing 597 northern Chinese patients and reported 281,533 SNPs. They found that two adjacent SNPs (rs4432245 and rs711906) of EIF2AK4 were significantly associated with BMI (Yang et al., 2014). Given the critical role of EIF2AK4 in immunity reactions, it is necessary to investigate the association between EIF2AK4 gene SNPs and IVIG resistance of KD. The current study revealed that the SNP rs4594236 polymorphism in the EIF2AK4 gene was associated with increased risk to IVIG resistance of KD in southern Chinese population. Compared with the rs4594236 AA genotype, the AG/GG genotype increased the IVIG resistant risk significantly, especially in male KD patients. We then explored the potential mechanism for the risk role of rs4594236 polymorphism in IVIG resistance. The results from eQTLs analysis indicated that IVIG resistant risk of KD was associated with upregulated expression levels of the EIF2AK4 gene (Figure 1). We also evaluated the impact of rs4594236 polymorphism on the mRNA level of the neighboring genes. We found that the SRP14 (or SRP14-AS1) mRNA level with the rs4594236 G genotype was significantly lower than that in cells with the rs4594236 A genotype (Figure 2). SRP14 is a universal ribonucleoprotein, and combined with SRP9 as a heterodimer, it can recognize the RNA UGUNR motif to regulate target gene translation (Bovia et al., 1995; Hasler and Strub, 2006). Thus, we hypothesized that SRP14 combined with the specific RNA motif of EIF2AK4 and negatively regulated the EIF2AK4 mRNA translation level to trigger downstream physiological reactions to inhibit IVIG therapy response.
The major strength of this study is its novelty; as we know, this is the first study that focuses on the EIF2AK4 function in IVIG therapy resistance of KD at present. However, our study still has some limitations. First, the enrolled patients of this study were mainly from the southern Chinese population; the study needs multicenter subjects from other geographic populations to support and evaluate the applicability of the findings to other ethnicities. Second, only one functional SNP in the EIF2AK4 gene was included in this study; more potentially functional SNPs of EIF2AK4 need to be investigated in the future. Last but not least, the exact biological mechanism of EIF2AK4 in IVIG resistance of KD is worthy of further investigation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Review Committee of the Guangzhou Women and Children’s Medical Center (2014073009). All studies had obtained the approval of the local hospital institutional review board, and all the participants were informed of the details of this study and signed the informed consent form.
HY, HL, and XG conceived and designed the study. HY, FL, KC, and YX analyzed and interpreted the data. YW, LF, and HZ provided assistance in performing the study. LP, DC, and HL revised the manuscript. HY, FL, and XG wrote the paper with feedback from all authors. All the authors read and approved the final manuscript.
This study was funded by the Guangdong Natural Science Fund, China (Grant numbers: 2019A1515012061 and 2021A1515011207), the Guangzhou Science and Technology Program Project, China (Grant numbers: 201904010486 and 202102010197), the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center Fund, China (Grant numbers: GCP-2019-003, GCP-2019-006, and YIP-2019-050), and the Postdoctoral Research Initiation Fund from Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center (Grant number 3001162).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the Clinical Biological Resource Bank of the Guangzhou Women and Children’s Medical Center for providing all the clinical samples and greatly appreciate all the patients who donated the samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.868159/full#supplementary-material
Bovia, F., Fornallaz, M., Leffers, H., and Strub, K. (1995). The SRP9/14 Subunit of the Signal Recognition Particle (SRP) Is Present in More Than 20-fold Excess over SRP in Primate Cells and Exists Primarily Free but Also in Complex with Small Cytoplasmic Alu RNAs. MBoC 6 (4), 471–484. doi:10.1091/mbc.6.4.471
Burns, J. C., and Glodé, M. P. (2004). Kawasaki Syndrome. Lancet 364 (9433), 533–544. doi:10.1016/s0140-6736(04)16814-1
Chaudhary, K., Shinde, R., Liu, H., Gnana-Prakasam, J. P., Veeranan-Karmegam, R., Huang, L., et al. (2015). Amino Acid Metabolism Inhibits Antibody-Driven Kidney Injury by Inducing Autophagy. J. I. 194 (12), 5713–5724. doi:10.4049/jimmunol.1500277
Che, D., Pi, L., Fang, Z., Xu, Y., Cai, M., Fu, L., et al. (2018). ABCC4 Variants Modify Susceptibility to Kawasaki Disease in a Southern Chinese Population. Dis. Markers 2018, 8638096. doi:10.1155/2018/8638096
Chen, Z., Zhang, J., Wei, D., Chen, J., and Yang, J. (2021). GCN2 Regulates ATF3-P38 MAPK Signaling Transduction in Pulmonary Veno-Occlusive Disease. J. Cardiovasc Pharmacol. Ther. 26 (6), 677–689. doi:10.1177/10742484211015535
Consortium, G. T. (2013). The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 45 (6), 580–585. doi:10.1038/ng.2653
Domínguez-Soto, A., de las Casas-Engel, M., Bragado, R., Medina-Echeverz, J., Aragoneses-Fenoll, L., Martín-Gayo, E., et al. (2014). Intravenous Immunoglobulin Promotes Antitumor Responses by Modulating Macrophage Polarization. J. I. 193 (10), 5181–5189. doi:10.4049/jimmunol.1303375
Fu, L. Y., Qiu, X., Deng, Q. L., Huang, P., Pi, L., Xu, Y., et al. (2019). The IL-1B Gene Polymorphisms Rs16944 and Rs1143627 Contribute to an Increased Risk of Coronary Artery Lesions in Southern Chinese Children with Kawasaki Disease. J. Immunol. Res. 2019, 4730507. doi:10.1155/2019/4730507
Gersony, W. M. (2009). The Adult after Kawasaki Disease. J. Am. Coll. Cardiol. 54 (21), 1921–1923. doi:10.1016/j.jacc.2009.06.057
Gu, X., Lin, W., Xu, Y., Che, D., Tan, Y., Lu, Z., et al. (2020). The Rs1051931 G>A Polymorphism in the PLA2G7 Gene Confers Resistance to Immunoglobulin Therapy in Kawasaki Disease in a Southern Chinese Population. Front. Pediatr. 8, 338. doi:10.3389/fped.2020.00338
Hadinnapola, C., Bleda, M., Haimel, M., Screaton, N., Swift, A., Dorfmüller, P., et al. (2017). Phenotypic Characterization of EIF2AK4 Mutation Carriers in a Large Cohort of Patients Diagnosed Clinically with Pulmonary Arterial Hypertension. Circulation 136 (21), 2022–2033. doi:10.1161/CIRCULATIONAHA.117.028351
Halaby, M. J., Hezaveh, K., Lamorte, S., Ciudad, M. T., Kloetgen, A., MacLeod, B. L., et al. (2019). GCN2 Drives Macrophage and MDSC Function and Immunosuppression in the Tumor Microenvironment. Sci. Immunol. 4 (42), 8189. doi:10.1126/sciimmunol.aax8189
Harding, H. P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., Schapira, M., et al. (2000). Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol. Cell 6 (5), 1099–1108. doi:10.1016/s1097-2765(00)00108-8
Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., Calfon, M., et al. (2003). An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell 11 (3), 619–633. doi:10.1016/s1097-2765(03)00105-9
Hasler, J., and Strub, K. (2006). Alu RNP and Alu RNA Regulate Translation Initiation In Vitro. Nucleic Acids Res. 34 (8), 2374–2385. doi:10.1093/nar/gkl246
Huang, Y.-H., Hsu, Y.-W., Lu, H.-F., Wong, H. S.-C., Yu, H.-R., Kuo, H.-C., et al. (2016). Interferon-gamma Genetic Polymorphism and Expression in Kawasaki Disease. Med. Baltim. 95 (17), e3501. doi:10.1097/md.0000000000003501
Kashef, S., Safari, M., and Amin, R. (2005). Initial Intravenous γ-Globulin Treatment Failure in Iranian Children with Kawasaki Disease. Kaohsiung J. Med. Sci. 21 (9), 401–404. doi:10.1016/s1607-551x(09)70141-x
Kato, H., Koike, S., Yamamoto, M., Ito, Y., and Yano, E. (1975). Coronary Aneurysms in Infants and Young Children with Acute Febrile Mucocutaneous Lymph Node Syndrome. J. Pediatr. 86 (6), 892–898. doi:10.1016/s0022-3476(75)80220-4
Khor, C. C., Davila, S., Davila, S., Breunis, W. B., Lee, Y.-C., Shimizu, C., et al. (2011). Genome-wide Association Study Identifies FCGR2A as a Susceptibility Locus for Kawasaki Disease. Nat. Genet. 43 (12), 1241–1246. doi:10.1038/ng.981
Lee, Y.-C., Kuo, H.-C., Chang, J.-S., Chang, L.-Y., Huang, L.-M., Chen, M.-R., et al. (2012). Two New Susceptibility Loci for Kawasaki Disease Identified through Genome-wide Association Analysis. Nat. Genet. 44 (5), 522–525. doi:10.1038/ng.2227
Lin, K., Zhang, L., Wang, Y., Li, J., Xu, Y., Che, D., et al. (2021). FNDC1 Polymorphism (Rs3003174 C > T) Increased the Incidence of Coronary Artery Aneurysm in Patients with Kawasaki Disease in a Southern Chinese Population. Jir 14, 2633–2640. doi:10.2147/jir.s311956
Liu, H., Huang, L., Bradley, J., Liu, K., Bardhan, K., Ron, D., et al. (2014). GCN2-dependent Metabolic Stress Is Essential for Endotoxemic Cytokine Induction and Pathology. Mol. Cell Biol. 34 (3), 428–438. doi:10.1128/mcb.00946-13
Longchamp, A., Mirabella, T., Arduini, A., MacArthur, M. R., Das, A., Treviño-Villarreal, J. H., et al. (2018). Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 173 (1), 117–129. doi:10.1016/j.cell.2018.03.001
Lu, Z., Xu, X., Fassett, J., Kwak, D., Liu, X., Hu, X., et al. (2014). Loss of the Eukaryotic Initiation Factor 2α Kinase General Control Nonderepressible 2 Protects Mice from Pressure Overload-Induced Congestive Heart Failure without Affecting Ventricular Hypertrophy. Hypertension 63 (1), 128–135. doi:10.1161/hypertensionaha.113.02313
Lünemann, J. D., Nimmerjahn, F., and Dalakas, M. C. (2015). Intravenous Immunoglobulin in Neurology-Mode of Action and Clinical Efficacy. Nat. Rev. Neurol. 11 (2), 80–89. doi:10.1038/nrneurol.2014.253
Makowsky, R., Wiener, H. W., Ptacek, T. S., Silva, M., Shendre, A., Edberg, J. C., et al. (2013). FcγR Gene Copy Number in Kawasaki Disease and Intravenous Immunoglobulin Treatment Response. Pharmacogenet Genomics 23 (9), 455–462. doi:10.1097/fpc.0b013e328363686e
McCrindle, B. W., Rowley, A. H., Newburger, J. W., Burns, J. C., Bolger, A. F., Gewitz, M., et al. (2017). Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 135 (17), e927–e99. doi:10.1161/CIR.0000000000000484
McGaha, T. L., Huang, L., Lemos, H., Metz, R., Mautino, M., Prendergast, G. C., et al. (2012). Amino Acid Catabolism: a Pivotal Regulator of Innate and Adaptive Immunity. Immunol. Rev. 249 (1), 135–157. doi:10.1111/j.1600-065x.2012.01149.x
Nakamura, A., Nambu, T., Ebara, S., Hasegawa, Y., Toyoshima, K., Tsuchiya, Y., et al. (2018). Inhibition of GCN2 Sensitizes ASNS-Low Cancer Cells to Asparaginase by Disrupting the Amino Acid Response. Proc. Natl. Acad. Sci. U. S. A. 115 (33), E7776–E85. doi:10.1073/pnas.1805523115
Newburger, J. W., Takahashi, M., Burns, J. C., Beiser, A. S., Chung, K. J., Duffy, C. E., et al. (1986). The Treatment of Kawasaki Syndrome with Intravenous Gamma Globulin. N. Engl. J. Med. 315 (6), 341–347. doi:10.1056/nejm198608073150601
Newburger, J. W., Takahashi, M., Gerber, M. A., Gewitz, M. H., Tani, L. Y., Burns, J. C., et al. (2004). Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease. Circulation 110 (17), 2747–2771. doi:10.1161/01.cir.0000145143.19711.78
Nossent, E. J., Antigny, F., Montani, D., Bogaard, H. J., Ghigna, M. R., Lambert, M., et al. (2018). Pulmonary Vascular Remodeling Patterns and Expression of General Control Nonderepressible 2 (GCN2) in Pulmonary Veno-Occlusive Disease. J. Heart Lung Transplant. 37 (5), 647–655. doi:10.1016/j.healun.2017.09.022
Onouchi, Y., Gunji, T., Burns, J. C., Shimizu, C., Newburger, J. W., Yashiro, M., et al. (2008). ITPKC Functional Polymorphism Associated with Kawasaki Disease Susceptibility and Formation of Coronary Artery Aneurysms. Nat. Genet. 40 (1), 35–42. doi:10.1038/ng.2007.59
Onouchi, Y., Suzuki, Y., Suzuki, H., Terai, M., Yasukawa, K., Hamada, H., et al. (2013). ITPKC and CASP3 Polymorphisms and Risks for IVIG Unresponsiveness and Coronary Artery Lesion Formation in Kawasaki Disease. Pharmacogenomics J. 13 (1), 52–59. doi:10.1038/tpj.2011.45
Ravindran, R., Loebbermann, J., Nakaya, H. I., Khan, N., Ma, H., Gama, L., et al. (2016). The Amino Acid Sensor GCN2 Controls Gut Inflammation by Inhibiting Inflammasome Activation. Nature 531 (7595), 523–527. doi:10.1038/nature17186
Schmidt, S., Gay, D., Uthe, F. W., Denk, S., Paauwe, M., Matthes, N., et al. (2019). A MYC-GCN2-eIF2α Negative Feedback Loop Limits Protein Synthesis to Prevent MYC-dependent Apoptosis in Colorectal Cancer. Nat. Cell Biol. 21 (11), 1413–1424. doi:10.1038/s41556-019-0408-0
Sharma, K., Vignesh, P., Srivastava, P., Sharma, J., Chaudhary, H., Mondal, S., et al. (2021). Epigenetics in Kawasaki Disease. Front. Pediatr. 9, 673294. doi:10.3389/fped.2021.673294
Takahashi, K., Oharaseki, T., Yokouchi, Y., Naoe, S., and Saji, T. (2013). Kawasaki Disease: Basic and Pathological Findings. Clin. Exp. Nephrol. 17 (5), 690–693. doi:10.1007/s10157-012-0734-z
Tremoulet, A. H., Devera, G., Best, B. M., Jimenez-Fernandez, S., Sun, X., Jain, S., et al. (2011). Increased Incidence and Severity of Kawasaki Disease Among Filipino-Americans in San Diego County. Pediatr. Infect. Dis. J. 30 (10), 909–911. doi:10.1097/inf.0b013e31821e52c6
Uehara, R., Belay, E. D., Maddox, R. A., Holman, R. C., Nakamura, Y., Yashiro, M., et al. (2008). Analysis of Potential Risk Factors Associated with Nonresponse to Initial Intravenous Immunoglobulin Treatment Among Kawasaki Disease Patients in Japan. Pediatr. Infect. Dis. J. 27 (2), 155–160. doi:10.1097/inf.0b013e31815922b5
Wang, K., Cao, Y., Rong, Y., Ning, Q., Jia, P., Huang, Y., et al. (2019). A Novel SNP in EIF2AK4 Gene Is Associated with Thermal Tolerance Traits in Chinese Cattle. Anim. (Basel) 9 (6), 375. doi:10.3390/ani9060375
Wang, Y., Wang, W., Gong, F., Fu, S., Zhang, Q., Hu, J., et al. (2013). Evaluation of Intravenous Immunoglobulin Resistance and Coronary Artery Lesions in Relation to Th1/Th2 Cytokine Profiles in Patients with Kawasaki Disease. Arthritis & Rheumatism 65 (3), 805–814. doi:10.1002/art.37815
Wang, Y., Xu, Y., Huang, P., Che, D., Wang, Z., Huang, X., et al. (2021). Homozygous of MRP4 Gene Rs1751034 C Allele Is Related to Increased Risk of Intravenous Immunoglobulin Resistance in Kawasaki Disease. Front. Genet. 12, 510350. doi:10.3389/fgene.2021.510350
Wang, Z., Xu, Y., Zhou, H., Wang, Y., Li, W., Lu, Z., et al. (2020). Association between P2RY12 Gene Polymorphisms and IVIG Resistance in Kawasaki Patients. Cardiovasc Ther. 2020, 3568608. doi:10.1155/2020/3568608
Wek, R. C., Ramirez, M., Jackson, B. M., and Hinnebusch, A. G. (1990). Identification of Positive-Acting Domains in GCN2 Protein Kinase Required for Translational Activation of GCN4 Expression. Mol. Cell Biol. 10 (6), 2820–2831. doi:10.1128/mcb.10.6.2820-2831.1990
Weng, K.-P., Hsieh, K.-S., Ho, T.-Y., Huang, S.-H., Lai, C.-R., Chiu, Y.-T., et al. (2010). IL-1B Polymorphism in Association with Initial Intravenous Immunoglobulin Treatment Failure in Taiwanese Children with Kawasaki Disease. Circ. J. 74 (3), 544–551. doi:10.1253/circj.cj-09-0664
Wu, Z., Yu, Y., Fu, L., Mai, H., Huang, L., Che, D., et al. (2020). LncRNA SOX2OT Rs9839776 Polymorphism Reduces Sepsis Susceptibility in Southern Chinese Children. Jir 13, 1095–1101. doi:10.2147/jir.s281760
Keywords: Kawasaki disease, IVIG resistance, polymorphism, EIF2AK4/rs4594236, intravenous immunoglobulin
Citation: Yu H, Liu F, Chen K, Xu Y, Wang Y, Fu L, Zhou H, Pi L, Che D, Li H and Gu X (2022) The EIF2AK4/rs4594236 AG/GG Genotype Is a Hazard Factor of Immunoglobulin Therapy Resistance in Southern Chinese Kawasaki Disease Patients. Front. Genet. 13:868159. doi: 10.3389/fgene.2022.868159
Received: 02 February 2022; Accepted: 16 May 2022;
Published: 22 June 2022.
Edited by:
Tieliu Shi, Hunan University of Arts and Science, ChinaReviewed by:
Fatma Savran Oguz, Istanbul University, TurkeyCopyright © 2022 Yu, Liu, Chen, Xu, Wang, Fu, Zhou, Pi, Che, Li and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hehong Li, aG9ybmNAMTYzLmNvbQ==; Xiaoqiong Gu, Z3V4aWFvcWlvbmdAZ3djbWMub3Jn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.