
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet., 16 May 2022
Sec. Genetics of Common and Rare Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.858668
Cardinal features of CDK13-related disorders are characterized by intellectual disability, developmental delay, dysmorphic facial features, structural heart defect and structural brain abnormality. A 9-year-old boy presented with intellectual disability, development delay, characteristic craniofacial features, brain malformation, cryptorchidism, autism spectrum disorder, and recently, recurrent hemophagocytic lymphohistiocytosis (HLH) in a half year period. Further investigation revealed the diagnosis of CDK13-related disorder. Finally, we found the underlying cause of HLH is acute lymphoblastic leukemia. Probably leukemia was a coincidental finding in this boy with CDK13-related disorder, but the case herein suggests that individuals with CDK13-related disorder also face risk of developing cancers. Further detailed information could enable us to clarify this presentation because of only limited investigation in affected cases.
The protein encoded by cyclin-dependent kinase 13 (CDK13) gene is a member of the cyclin-dependent serine/threonine protein kinase family. Members of this family are well known for their essential roles as master switches in cell cycle control (Even et al., 2016). CDK12 and CDK13 both are heterodimers containing a Cyclin K subunit, and they are structurally similar kinases. Many studies on CDK12 have clearly shown that it is a tumor suppressor, notably for ovarian and prostate cancers (Greenleaf, 2019; Fan et al., 2020). In contrast, the exact function of CDK13 has not yet been determined. Recent studies have linked heterozygous variants in CDK13 to a syndromic form of intellectual disability and other abnormalities with variable phenotypes (Sifrim et al., 2016; Hamilton et al., 2018; van den Akker et al., 2018). Cardinal features of CDK13-related disorders are characterized by intellectual disability, developmental delay, dysmorphic facial features, feeding difficulties, structural heart defect and structural brain abnormality. Additional features are widely described with multisystem involvement (Hamilton and Suri, 2019). However, no malignant diseases have been reported in those with CDK13-related disorder. Herein, we report a rare case of a boy with CDK13-related disorder, who presented with recurrent hemophagocytic lymphohistiocytosis (HLH) initially with progression to leukemia in a half year period.
This boy was the first child of non-consanguineous Chinese parents and born at term with normal physical examination apart from mild hypotonia. During the first years of life, he presented with obviously delayed developmental milestones: he gained head control at 8 months, and walked alone without support at 3.5 years of age. Feeding difficulties were not reported but he had a chronic constipation. He had a history of febrile convulsions in infancy. He was diagnosed with cerebral hypoplasia at 8 months, cryptorchidism at 1 year, and autism spectrum disorder (ASD) at 5 years of age. His family history was unremarkable.
At the age of 9 years, this boy presented with recurrent fever of unknown origin and experienced a prolonged hospitalization in the local hospital from April to May 2019. Other symptoms/signs included anorexia, low mood, obvious hypotonia, increased sleep and reduced performance over usual. Subjective symptoms of discomfort were limited due to his communication deficits. His laboratory evaluation revealed an excessive inflammation and multiorgan involvement with clinically mild respiratory infections but without evidence of malignancies as summarized in Table 1 (first, 02/04 to 08/05, 2019). He received intravenous immunoglobulin (IVIG) and anti-infective therapy with third generation cephalosporins but also higher classes of antibiotics for several weeks, but his symptoms did not improve until receiving a short course of corticosteroid. He was discharged in a stable condition with a clinical diagnosis of a suspicion of HLH.
However unfortunately, he suffered a relapse within 4 months. At the second admission in the local hospital, his laboratory evaluation strongly resembled previous records, as shown in Table 1 (second, 06/09 to 30/09, 2019). Treatment with corticosteroid was initiated and his condition improved significantly in the short term. Considering his congenital anomalies, trio-based whole exome sequencing (WES) was performed using peripheral blood samples from this patient and his parents.
Genomic DNA was extracted from each sample, to an average size of 180 bp with a Bioruptor sonicator (Diagenode). Paired-end sequencing libraries then were prepared using a DNA sample prep reagent set 1 (NEBNext). Library preparation included end repair, adapter ligation and PCR enrichment, and was carried out as recommended by Illumina protocols. All sequencing was performed on the Nova seq (6,000 platform (Illumina) (MyGenostics Inc. Beijing, China). Variants were further annotated by ANNOVAR (Wang et al., 2010) and associated with multiple databases, such as, 1000genomes, ESP6500, dbSNP, ExAC, Inhouse (MyGenostics), ClinVar, and the Human Gene Mutation Database (HGMD). Variants with a minor allele frequency of less than 5% in population databases or were presented in variant databases were included in the analysis. Filtered candidate variants were confirmed by Sanger sequencing. The American College of Medical Genetics and Genomics (ACMG) Standard guidelines for the interpretation of sequence variants were followed in this study. A heterozygous missense variant in CDK13 gene located on 7p14.1, NM_003,718, exon 4, c.2141G > T (p.G714V) was identified (full report of the variant found by WES in the trio was summarized in Supplementary Table S1), and Sanger sequencing showed the variant was de novo since his unaffected parents did not have the variant (Figure 1). Residue Gly714, lying within the nucleotide binding domain of CDK13 (Figure 2), is highly conserved, and this variant is predicted to be pathogenic by SIFT, PolyPhen-2, MutationTaster, GERP++ and REVEL. This variant is not found in all population databases, and has not been described in variant databases and therefore is novel (Figure 2A), and moreover, alternative variants in the same position are classified as pathogenic by ClinVar. This novel sidechain is putative to cause changes in the CDK13 structure (Figures 2B,C). In summary, c.2141G > T (p.G714 V) is considered to be likely pathogenic according to ACMG (PS2+PM2+PP3). Therefore, the diagnosis of CDK13-related disorder is made.
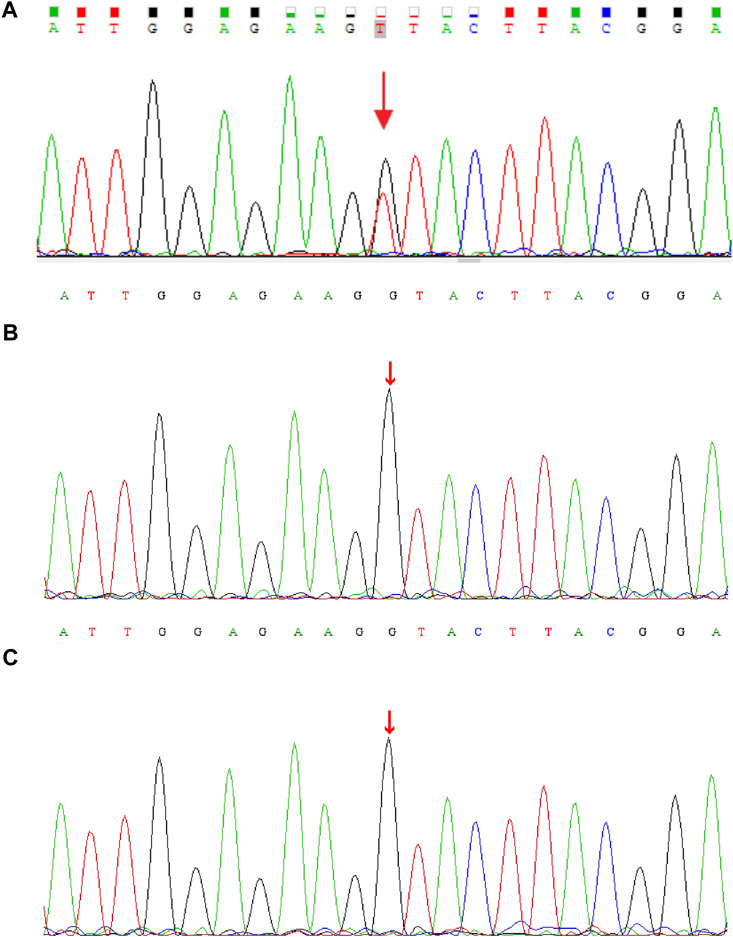
FIGURE 1. DNA electrophoregram with the (C)2141G > T [p.G714 V] in CDK13 gene of (A) proband (B) father, and (C) mother.
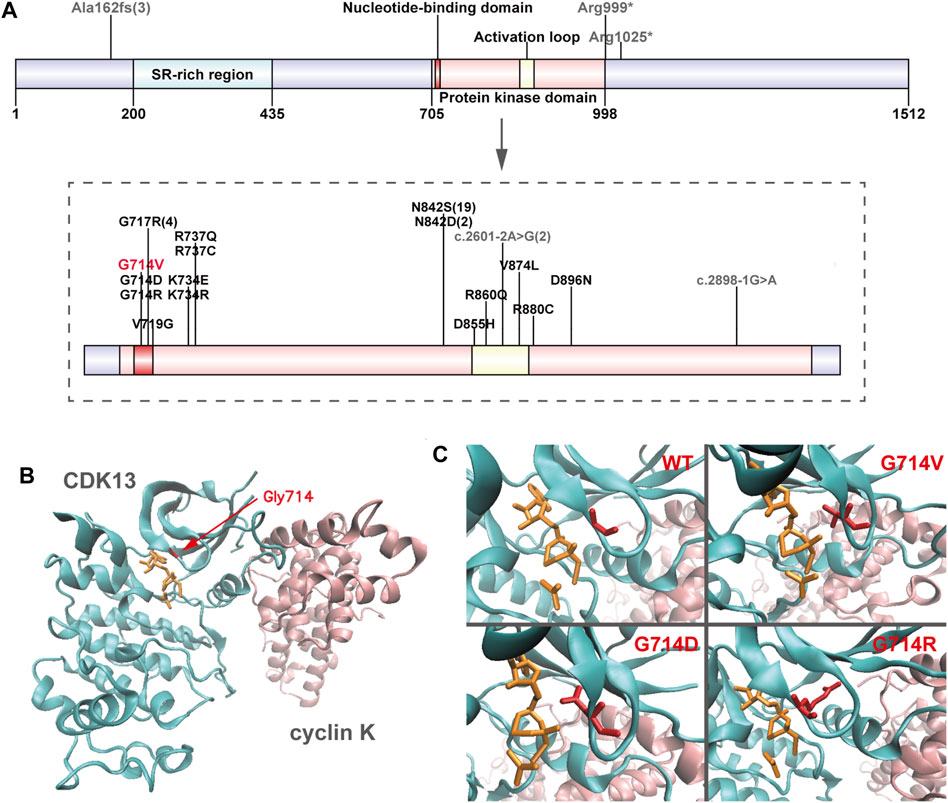
FIGURE 2. Overview of CDK13 variants (A) Location of pathogenic variants in the CDK13 protein of all presently reported individuals. Missense variants are annotated in black (presently reported variant in red), and others in grey. Numbers in brackets refer to the corresponding variants presented in more than one individual. Up to half (21/46) of the variants perturb the wild-type asparagine residue at amino acid position 842. (B) Crystal structure of one heterodimer (chains A-B) of CDK13-cyclin K (PDB id: 5efq); CDK13 and cyclin K are shown in new-cartoon format, colored cyan and pink, respectively, with sidechains of selected residues (red) and ligands (orange) shown in licorice format. (C) The exact positions of CDK13 residue 714 in wild-type (WT) CDK13, Gly714Val (G714 V) variant, Gly714Asp (G714D) variant, and Gly714Arg (G714R) variant, showing in red licorice format.
At the end of October 2019, he suffered a second relapse in the course of tapering corticosteroid, and finally, he was referred to our hospital by the local hospital. Upon hospital admission, the boy was noted to have atypical facial anomalies (Figure 3A). His physical examination revealed obvious developmental delay: his height was 122.0 cm [standard deviation (SD), - 3], his weight was 25.4 kg (SD, -2), and his head circumference was 49.8 cm (SD, -2). The remainder of his physical examination was unremarkable showing in Table 1 (23/10 to 23/11, 2019). After reviewing his medical history, a diagnosis of a high suspicion of HLH was made by the diagnostic criteria used in the HLH-2004 trial (Bergsten et al., 2017). After two chemotherapy cycles of corticosteroid, cyclosporin A and etoposide (VP16), his condition returned back to stable at the middle of November 2019, and bone marrow morphology was revaluated, showing acute lymphoblastic leukemia (ALL) with a proportion of leukemic blasts of 48% (reference intervals (RI): <5%) (Figure 3B). Continued chemotherapy and hematopoietic stem cell transplantation (HSCT) were recommended, but his parents refused. This boy was asked to abandon continued treatment and be taken home without further evaluation. One month later, death occurred at home owing to disease progression.

FIGURE 3. Facial features of the patient with CDK13-related disorder and bone marrow smears. (A) Anterior and profile facial photographs show his facial anomalies with mild exophthalmos, blepharoptosis, broad nasal bridge, big front teeth with wide space, low set and posteriorly rotated ears. He presented with drowsiness, emotional apathy after HLH onset (Left). (B) Bone marrow smears showing morphologic blasts cells with a proportion of 48% at diagnosis of acute lymphoblastic leukemia (ALL).
Review of relevant literature revealed 45 cases with CDK13-related disorder to date (Hamilton and Suri, 2019; Yakubov et al., 2019), and genotype-phenotype correlations are not significant, despite of the potential generality in genotypes that 37 of all 45 affected individuals carry missense variants within the protein kinase domain (Figure 2A; Table 2) (Sifrim et al., 2016; Bostwick et al., 2017; Deciphering Developmental Disorders Study, 2017; Hamilton et al., 2018; Uehara et al., 2018; van den Akker et al., 2018; Hamilton and Suri, 2019; Yakubov et al., 2019). In 45 reported individuals with CDK13-related disorder, all variants have risen de novo, which hints they may not have the ability to reproduce. The median age of the reported cases is 8.3 years (age range, 0.5–54 years) with only three adults. This older adult appears to offer some assurance that his underlying genetic defect did not affect his long-term survival (van den Akker et al., 2018). In contrast, the case herein shows the prognosis concerning quality of life and longevity is not optimistic.
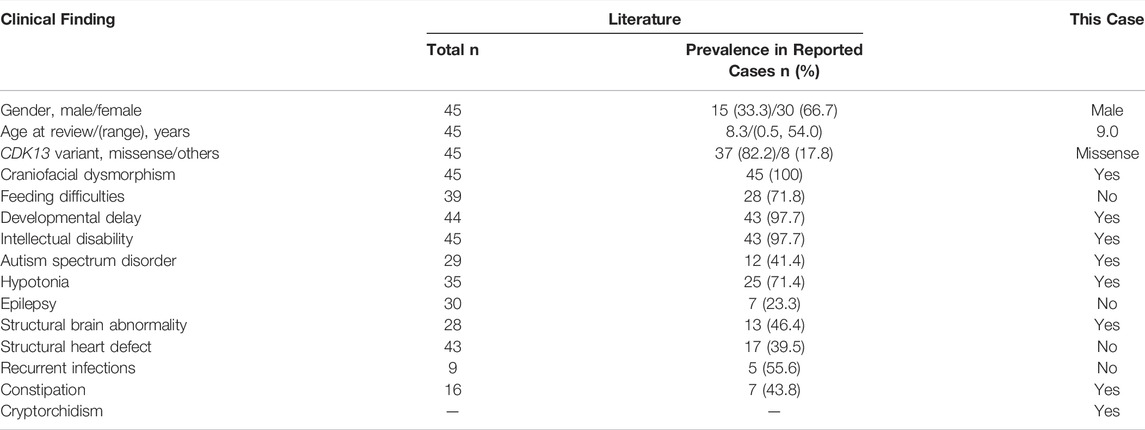
TABLE 2. Summary of major clinical findings of 45 already published individuals with CDK13-related disorder.
Notably, five of nine reported cases present with immunological complications marked specifically by recurrent ear, upper respiratory tract, or gastrointestinal infections (Table 2), one with recurrent mouth ulcers and one with low levels of IgM and IgA (Hamilton et al., 2018). The current case does not present with remarkable infections during the first years of life, but at 9 years of age, he appears to present with recurrent infections in a half year period and more severe phenotypes—excessive immune activation—HLH. Indeed, it is possible that isolated low level of IgM is a secondary condition related to HLH or leukemia. Patients with immune disorders are at an increased risk of HLH and malignancies compared with a normal population (Shapiro, 2011; Bode et al., 2015), but to our knowledge, no malignant diseases have been reported in those with CDK13-related disorder.
Currently, ALL combination therapy typically results in cure rates of more than 90 percent in the developed world. However, in the developing world, patients with ALL continue to have relatively poor outcomes in part due to abandonment of treatment (Allemani et al., 2015; Cai et al., 2019), The reasons are multifaceted regarding this case forgoing needed health care. First of all, the underlying CDK13-related disorder is not diagnosed in time at his first onset. His ASD also poses difficulties in assessing response to treatment because of his deficits in social communication and the symptom of apathy more obvious after malignancy-associated HLH (M-HLH) onset. Moreover, he has already experienced a prolonged hospitalization and clinical deterioration, but also has received incorrect and irregular treatment for early-stage M-HLH, which may potentially lead to a poorer outcome. Unfortunately, due to his abandonment for continued treatment, we do not further evaluate additional prognostic indicators or determine whether he may benefit from more intensive therapy.
Overall prognosis is quite poor for any M-HLH despite of the favorable cure rates for childhood ALL. Despite clinical advances in recent decades, misdiagnosis of M-HLH remains a significant concern. Notably, HLH is often more immediately life-threatening than the malignancy itself (Lehmberg et al., 2015). Frequently in clinical practice, a diagnosis of HLH is made in the patient who partly meets the criteria. Prompt initiation of immunochemotherapy is essential for survival, although in many cases, corticosteroid monotherapy is sufficient (Jordan et al., 2011). However, this, in return, increases the risk of misdiagnosis because the underlying causes may be obscured.
It is possible that the leukemia was a coincidental finding in this boy with a CDK13 gene variant. Besides, ALL could also be a secondary consequence of the extensive clinical deterioration of the patient and the previous incorrect treatment. However, only limited information is known about CDK13-related disorders, and further studies are necessary to determine whether those with CDK13-related disorders may share long-term survival with the absence of significant life-limiting sequelae or confer increased susceptibility to cancer development, and whether the cases with such specific conditions may benefit from gene therapy or HSCT.
Interestingly, several recent studies have described the connection of CDK13 influencing cancer development, such as, ovarian cancer, prostate cancer, gastric cancers, hepatocellular carcinoma, etc. (Dong et al., 2018; Greenleaf, 2019; Qi et al., 2021; Tadesse et al., 2021; Wu et al., 2021). However, the role of CDK13 in transcription remains poorly understood. CDK13 is a complicated multi-tasking protein of central importance to proper gene expression and to genome stability, but it also probably plays yet undiscovered roles in other processes. Many studies have shown that CDK12 and CDK13 influence a large set of common processes, but they also influence a smaller set of distinct processes (Greenleaf, 2019). The data of a recent study fundamentally characterized both CDK12 and CDK13 as critical regulators of RNA processing (Fan et al., 2020). These findings have important implications in the context of tumorigenesis. We know that the loss of CDK12 activity will lead to aberrant CTD (C-terminal repeat domain of RNA polymerase II) phosphorylation which can lead to ovarian and prostate cancers with unusual genome instabilities. In contrast to CDK12, few functional analyses of genetic aberrations in CDK13 have been undertaken, and thus, their precise impact on cellular metabolism remains speculative (Hamilton and Suri, 2019). It is predicted that impaired function of CDK13 protein would result in reduced phosphorylation of the CTD of RNA polymerase II (Greifenberg et al., 2016). Expression studies in the lymphoblastoid cells derived from patient with CDK13-related disorder suggested that the mutant transcript was subject to nonsense-mediated decay, hence would not produce a stable protein product (Hamilton et al., 2018; Hamilton and Suri, 2019). Therefore, a major goal of future work will be to determine what the in vivo activities and functions of CDK13 actually are, and further detailed information may enable us to discern how its loss can lead to cancers.
However, there are certain limitations in the present study, such as without experimental verification, thus further studies are needed to elucidate the underlying mechanisms of the phenotypes described in individuals with CDK13-related disorder.
In conclusion, we report a case of a boy with CDK13-related disorder presenting with HLH prior to ALL. Immune disorders may represent previously unappreciated diagnostic clues to CDK13-related disorder, and should be highlighted and monitored in long-term follow-up of this newly identified syndrome. Probably leukemia was a coincidental finding in this boy, but the case herein suggests that individuals with CDK13-related disorder also face risk of developing cancers. Further investigation could be more informative to clarify this presentation because of limited information in affected cases.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Tongji Medical College, Huazhong University of Science and Technology. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
DC contributed to the acquisition, analysis of data, and writing the paper. AL and QH contributed to the conception of the work and cared the patient. SW and AZ cared patient and collected the data. All authors have substantively revised the work and approved the final manuscript and submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the patient and his parents for their cooperation and consent to this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.858668/full#supplementary-material
Allemani, C., Weir, H. K., Carreira, H., Harewood, R., Spika, D., Wang, X.-S., et al. (2015). Global Surveillance of Cancer Survival 1995-2009: Analysis of Individual Data for 25 676 887 Patients from 279 Population-Based Registries in 67 Countries (CONCORD-2). Lancet 385, 977–1010. doi:10.1016/S0140-6736(14)62038-9
Bergsten, E., Horne, A., Aricó, M., Astigarraga, I., Egeler, R. M., Filipovich, A. H., et al. (2017). Confirmed Efficacy of Etoposide and Dexamethasone in HLH Treatment: Long-Term Results of the Cooperative HLH-2004 Study. Blood 130, 2728–2738. doi:10.1182/blood-2017-06-788349
Bode, S. F., Ammann, S., Al-Herz, W., Bataneant, M., Dvorak, C. C., Gehring, S., et al. (2015). The Syndrome of Hemophagocytic Lymphohistiocytosis in Primary Immunodeficiencies: Implications for Differential Diagnosis and Pathogenesis. Haematologica 100, 978–988. doi:10.3324/haematol.2014.121608
Bostwick, B. L., McLean, S., McLean, S., Posey, J. E., Streff, H. E., Gripp, K. W., et al. (2017). Phenotypic and Molecular Characterisation of CDK13-Related Congenital Heart Defects, Dysmorphic Facial Features and Intellectual Developmental Disorders. Genome Med. 9, 73. doi:10.1186/s13073-017-0463-8
Cai, J., Yu, J., Zhu, X., Hu, S., Zhu, Y., Jiang, H., et al. (2019). Treatment Abandonment in Childhood Acute Lymphoblastic Leukaemia in China: a Retrospective Cohort Study of the Chinese Children's Cancer Group. Arch. Dis. Child. 104, 522–529. doi:10.1136/archdischild-2018-316181
Deciphering Developmental Disorders Study (2017). Prevalence and Architecture of De Novo Mutations in Developmental Disorders. Nature 542, 433–438. doi:10.1038/nature21062
Dong, X., Chen, G., Cai, Z., Li, Z., Qiu, L., Xu, H., et al. (2018). CDK13 RNA Over-editing Mediated by ADAR1 Associates with Poor Prognosis of Hepatocellular Carcinoma Patients. Cell. Physiol. biochem. 47, 2602–2612. doi:10.1159/000491656
Even, Y., Escande, M.-L., Fayet, C., and Genevière, A.-M. (2016). CDK13, a Kinase Involved in Pre-mRNA Splicing, Is a Component of the Perinucleolar Compartment. PLoS One 11, e0149184. doi:10.1371/journal.pone.0149184
Fan, Z., Devlin, J. R., Hogg, S. J., Doyle, M. A., Harrison, P. F., Todorovski, I., et al. (2020). CDK13 Cooperates with CDK12 to Control Global RNA Polymerase II Processivity. Sci. Adv. 6, eaaz5041. doi:10.1126/sciadv.aaz5041
Greenleaf, A. L. (2019). Human CDK12 and CDK13, Multi-Tasking CTD Kinases for the New Millenium. Transcription 10, 91–110. doi:10.1080/21541264.2018.1535211
Greifenberg, A. K., Hönig, D., Pilarova, K., Düster, R., Bartholomeeusen, K., Bösken, C. A., et al. (2016). Structural and Functional Analysis of the Cdk13/Cyclin K Complex. Cell Rep. 14, 320–331. doi:10.1016/j.celrep.2015.12.025
Hamilton, M. J., Caswell, R. C., Canham, N., Cole, T., Firth, H. V., Foulds, N., et al. (2018). Heterozygous Mutations Affecting the Protein Kinase Domain of CDK13 Cause a Syndromic Form of Developmental Delay and Intellectual Disability. J. Med. Genet. 55, 28–38. doi:10.1136/jmedgenet-2017-104620
Hamilton, M. J., and Suri, M. (2019). CDK13-related Disorder. Adv. Genet. 103, 163–182. doi:10.1016/bs.adgen.2018.11.001
Jordan, M. B., Allen, C. E., Weitzman, S., Filipovich, A. H., and McClain, K. L. (2011). How I Treat Hemophagocytic Lymphohistiocytosis. Blood 118, 4041–4052. doi:10.1182/blood-2011-03-278127
Lehmberg, K., Sprekels, B., Nichols, K. E., Woessmann, W., Müller, I., Suttorp, M., et al. (2015). Malignancy-associated Haemophagocytic Lymphohistiocytosis in Children and Adolescents. Br. J. Haematol. 170, 539–549. doi:10.1111/bjh.13462
Qi, J.-C., Yang, Z., Lin, T., Ma, L., Wang, Y.-X., Zhang, Y., et al. (2021). CDK13 Upregulation-Induced Formation of the Positive Feedback Loop Among circCDK13, miR-212-5p/miR-449a and E2F5 Contributes to Prostate Carcinogenesis. J. Exp. Clin. Cancer Res. 40, 2. doi:10.1186/s13046-020-01814-5
Shapiro, R. S. (2011). Malignancies in the Setting of Primary Immunodeficiency: Implications for Hematologists/oncologists. Am. J. Hematol. 86, 48–55. doi:10.1002/ajh.21903
Sifrim, A., Hitz, M. P., Hitz, M.-P., Wilsdon, A., Breckpot, J., Turki, S. H. A., et al. (2016). Distinct Genetic Architectures for Syndromic and Nonsyndromic Congenital Heart Defects Identified by Exome Sequencing. Nat. Genet. 48, 1060–1065. doi:10.1038/ng.3627
Tadesse, S., Duckett, D. R., and Monastyrskyi, A. (2021). The Promise and Current Status of CDK12/13 Inhibition for the Treatment of Cancer. Future Med. Chem. 13, 117–141. doi:10.4155/fmc-2020-0240
Uehara, T., Takenouchi, T., Kosaki, R., Kurosawa, K., Mizuno, S., and Kosaki, K. (2018). Redefining the Phenotypic Spectrum of De Novo Heterozygous CDK13 Variants: Three Patients without Cardiac Defects. Eur. J. Med. Genet. 61, 243–247. doi:10.1016/j.ejmg.2017.12.004
van den Akker, W. M. R., Brummelman, I., Martis, L. M., Timmermans, R. N., Pfundt, R., Kleefstra, T., et al. (2018). De Novo variants in CDK13 Associated with Syndromic ID/DD: Molecular and Clinical Delineation of 15 Individuals and a Further Review. Clin. Genet. 93, 1000–1007. doi:10.1111/cge.13225
Wang, K., Li, M., and Hakonarson, H. (2010). ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 38, e164. doi:10.1093/nar/gkq603
Wu, Z., Wang, M., Li, F., Wang, F., Jia, J., Feng, Z., et al. (2021). CDK13-Mediated Cell Cycle Disorder Promotes Tumorigenesis of High HMGA2 Expression Gastric Cancer. Front. Mol. Biosci. 8, 707295. doi:10.3389/fmolb.2021.707295
Keywords: CDK13-related disorder, HLH hemophagocytic lymphohistiocytosis, acute lymphobastic leukemia, craniofacial dysmorphic features, children
Citation: Cui D, Wang S, Zhang A, Liu A and Hu Q (2022) Case Report: Hemophagocytic Lymphohistiocytosis Prior to the Onset of Leukemia in a Boy With CDK13-Related Disorder. Front. Genet. 13:858668. doi: 10.3389/fgene.2022.858668
Received: 20 January 2022; Accepted: 25 April 2022;
Published: 16 May 2022.
Edited by:
Enrique Medina-Acosta, State University of the North Fluminense Darcy Ribeiro, BrazilReviewed by:
Thais Louvain De Souza, Faculdade de Medicina de Campos, BrazilCopyright © 2022 Cui, Wang, Zhang, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiguo Liu, ZHJsaXVhaWd1b0AxNjMuY29t; Qun Hu, cXVuaHUyMDEzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.