- 1Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran
- 2Department of Prosthetic Dentistry, Sechenov First Moscow State Medical University, Moscow, Russia
- 3Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, Kurdistan Region, Erbil, Iraq
- 4Center of Research and Strategic Studies, Lebanese French University, Kurdistan Region, Erbil, Iraq
- 5Department of Biology, College of Education, Salahaddin University-Erbil, Kurdistan Region, Erbil, Iraq
- 6Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Institute of Human Genetics, Jena University Hospital, Jena, Germany
- 8Department of Immunology, School of Medicine, Zanjan University of Medical Sciecnes, Zanjan, Iran
- 9Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 10Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 11Critical Care Quality Improvement Research Center, Loghman Hakin Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
These days, mesenchymal stem cells (MSCs), because of immunomodulatory and pro-angiogenic abilities, are known as inevitable factors in regenerative medicine and cell therapy in different diseases such as ocular disorder. Moreover, researchers have indicated that exosome possess an essential potential in the therapeutic application of ocular disease. MSC-derived exosome (MSC-DE) have been identified as efficient as MSCs for treatment of eye injuries due to their small size and rapid diffusion all over the eye. MSC-DEs easily transfer their ingredients such as miRNAs, proteins, and cytokines to the inner layer in the eye and increase the reconstruction of the injured area. Furthermore, MSC-DEs deliver their immunomodulatory cargos in inflamed sites and inhibit immune cell migration, resulting in improvement of autoimmune uveitis. Interestingly, therapeutic effects were shown only in animal models that received MSC-DE. In this review, we summarized the therapeutic potential of MSCs and MSC-DE in cell therapy and regenerative medicine of ocular diseases.
Introduction
Eye disorders that cause visual impairment include a variety of disorder with severe impacts on health (Flaxman et al., 2017). Treatment of these disorders is complicated in many cases. Recent investigations have shown the beneficial effects of mesenchymal stem cells (MSCs) in treatment of eye disorders. These cells can exert mmunosuppressive, anti-inflammatory, and trophic effects through production of several biological factors (Joe and Gregory-Evans, 2010). These cells produce a wide array of microvesicles, particularly exosomes that carry these biomolecules. The therapeutic effects of MSCs in eye disorders is mainly mediated through secretion of these microvesicle. In this review, we summarized the therapeutic potential of MSCs and these microvesicles in cell therapy and regenerative medicine of ocular diseases.
Role of Exosomes in Immune-Mediated Ocular Disorder
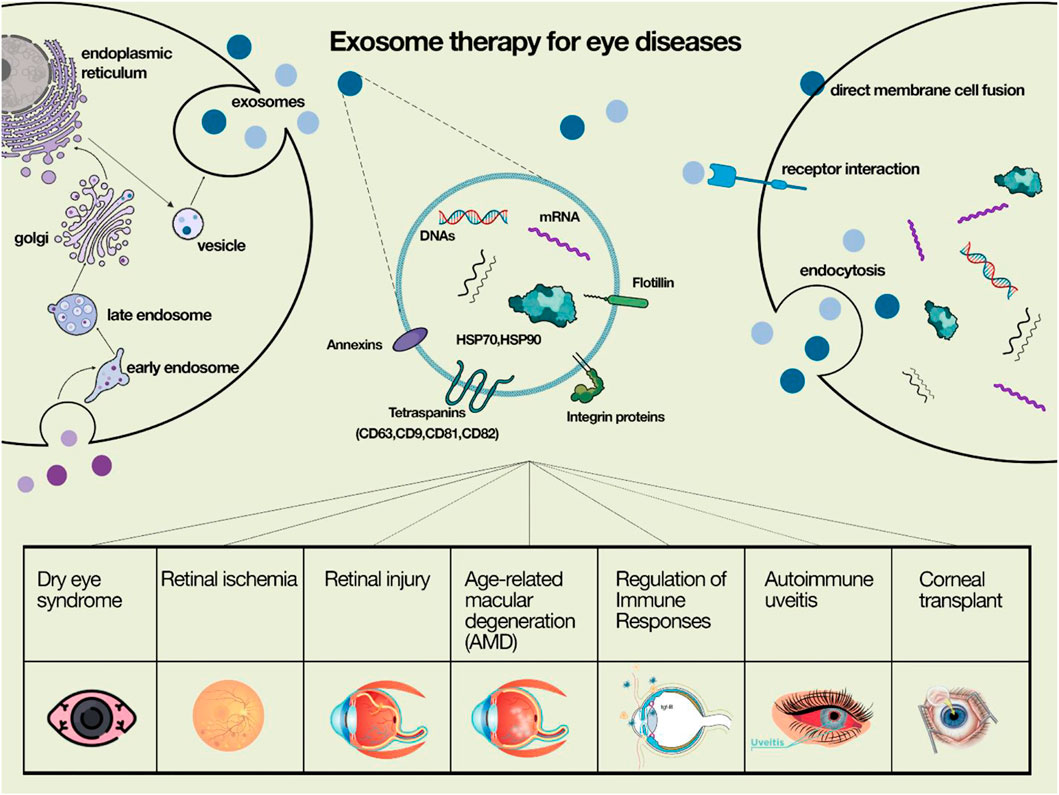
Exosomes have been introduced as a novel agent in cell-free therapy in eye diseases (Figure 1) In the comong sections, we describe the impact of these agents in each eye disorder.
Dry Eye or Sjögren’s Syndrome
Sjögren’s syndrome (SS) is an autoimmune disorder which is characterized by infiltration of lymphocyte in salivary glands (SGs) and lacrimal glands (LGs). This disorder is manifested by oral and ocular dryness (Shiboski et al., 2012; López-Miguel et al., 2016). This situation is known as one of the severe dry eye diseases (Bose et al., 2016). Several different pathways such as interferon (IFN) signatures, and NF-kB signalling are responsible for the pathogenesis of SS (Mavragani, 2017; Sandhya et al., 2017). Salivary gland epithelial cells (SGECs) in SS play active roles in the autoimmune and inflammatory responses (Manoussakis and Kapsogeorgou, 2010; Generali et al., 2017). Mainly, lymphocyte T CD4+ and B cells invade to epithelial cells (Mavragani et al., 2014; Goules et al., 2017). One study showed that SGECs release some exosomes containing Sm RNPs and Ro/SS-A, which can activate lymphocytes B. This mechanism might signify a mechansim for presentation of intracellular autoantigens to the immune system which might have either an immunogenic or tolerogenic consequence (Kapsogeorgou et al., 2005). In EBV, EBV-miRBART13–3p is transferred from B cells to SGECs by the exosome. EBV-miRBART13–3p targeted aquaporin 5 (AQP5), which can interfere with the secretion of SGs. LG is responsible for the production of tear; thus this disorder is mainly due to immune cells invasion (Li et al., 2019a). One study showed that MSC therapy efficiently alleviated autoimmune dacryoadenitis in vivo animal models (Li et al., 2016). MSC-derived exosomes (MSC-DEs) can modulate the immunosuppressive effects and play essential roles in treating eye disorders (Ren, 2019). Recently, it has been shown that injection of MSC-DEs efficiently alleviated inflammation in lacrimal glands compared to those treated with saline. Probably, MSC-DEs provide a promising cell-free therapy for eye disease.
Corneal Transplant Rejection
The most crucial cause of corneal graft failure is transplant rejection is ascribed to the detection of donor MHC antigens by recipient T cells (Marino et al., 2016; Mahabadi et al., 2018). Exosomes released by donor cells are responsible for rejection and tolerance under certain situations (Gonzalez-Nolasco et al., 2018). It has been revealed that Treg CD4+CD25− derived EXs and MSCs-DEs prolong survival of kidney transplants through their specific components (Wen et al., 2016; Aiello et al., 2017). These data suggest that exosomes can serve as a cell-free therapy to increase immune tolerance in corneal transplantation. Due to the absence of enough donated corneas, other alternatives such as collagen gels, synthetic polymers, tissue-engineered scaffolds has also been introduced (Gain et al., 2016; Williams et al., 2018). Epithelial cell-derived exosomes increase the proliferation of fibroblast and alleviate wound healing in corneal injuries (Han et al., 2017). It has been shown that limbal keratocytes-derived exosomes activate Akt signalling and increase wound healing in limbal epithelial cells (Leszczynska et al., 2018; Ghafouri-Fard et al., 2021a). Another study has revealed that corneal mesenchymal stem cell-derived exosomes could increase wound healing in corneal injuries (Samaeekia et al., 2018). Recently, it has been shown that exosomes have an essential role in corneal injury and regenerative medicine of eye disease (Basu and Ludlow, 2016).
Autoimmune Uveitis
Autoimmune uveitis is described as inflammation of the uvea tissue and adjacent tissues (Papotto et al., 2014). This autoimmune disease is mostly due to the unsuitable function of T-cells (Krishna et al., 2017). Th17 lymphocytes and other innate immune cells such as natural killer (NK) cells and dendritic cells (DCs) mediate autoimmune uveitis by secretion of various cytokines (Caspi, 2010; Chong et al., 2015; Patel and Kuchroo, 2015; Pepple and Lin, 2018). Retinal pigment epithelium (RPE) cells may be destroyed in inflammatory actions in posterior uveitis (Sevgi et al., 2017). RPE cells could release exosomes as an immunosuppressive agent and suppress Th17 and Th22 cells (Shi et al., 2015). According to the immunoregulatory effect of RPE-derived exosomes, they may be used as a therapeutic agent to treat uveitis in the future. It has been shown that bone marrow MSCs-DEs could alleviate autoimmune uveoretinitis (Shigemoto-Kuroda et al., 2017). It has been proven that cord blood MSCs derived EXs (CB-MSC-DEs) can not inhibit the function of T cell lymphocytes, but they suppress the migration of immune cells (Bai et al., 2017a). The mentioned result suggested that MSC-DEs have therapeutic application in the treatment of autoimmune uveitis. Further investigation is necessary to reveal the anti-inflammatory and immunomodulatory mechanisms of these processes.
Age-Related Macular Degeneration
One of the reasons for blindness in older adults is AMD, a multifactorial disease (Wong et al., 2014). AMD is divided into 1) early non-exudative and 2) late exudative forms (Pennington and DeAngelis, 2016). It has been proven that the complement system and inflammatory elements have an essential role in the pathogenesis of AMD (Bora et al., 2015; Perez and Caspi, 2015). Point mutations in complement factor H, the regulator of the complement pathway, or deficiency of protein C3 complement enhance the risk of developing AMD (Liszewski et al., 2017; Toomey et al., 2018; Park et al., 2019). It has been shown that RPE cell-derived exosomes are responsible for the formation of innate immunity in AMD. In exudative AMD, especially in the choroidal neovascular membranes, macrophages are the main cells that attract inflammatory cells (Nussenblatt et al., 2013). Vascular endothelial growth factor (VEGF) and macrophages polarization have been determined as essential mechanisms that trigger pathologic neovascularization in AMD (Sene et al., 2015). It has been proven that MSC-DEs could regulate macrophage polarization, decrease secretion of VEGF, control abnormal neovascularization and, alleviate pathogenesis of AMD (Pakravan et al., 2017; Li et al., 2019b).
Table 1 summarizes the role of exosome in eye disease.
Regulation of Immune Responses in Eye Diseases
MSCs release a wide range of extracellular vesicles, e.g., microvesicles and exosomes (Yeo et al., 2013). MSC-DEs play an essential role in physiological and pathological processes such as cell-cell communication, modulation of inflammation, and immune response (Théry et al., 2009; Zöller, 2009). Amniotic fluid MSC-DEs are enriched by TGF-β as one of the most immunosuppressive factors. TGF-β inhibits cell cycle of T cells and decreases adaptive immunity and T cell-mediated inflammation (Di Nicola et al., 2002; Volarevic et al., 2017). Also, TGF-β could suppress the function of B cells and decrease the production of antibodies (Balbi et al., 2017). Interestingly, amniotic fluid MSC-DEs can not inhibit the function of CD4 + CD25 + FoxP3+ T regulatory cells, proving the importance of immunosuppressive and therapeutic application of amniotic fluid MSC-DEs in inflammation. Recently, an ophthalmic solution has been introduced that contains IL-1 receptor antagonist (IL-1Ra) and amniotic fluid MSC-DEs. Due to its immunomodulatory effect, this product has a therapeutic application in treating dry eye syndrome (DES) and corneal injuries. Corneal injuries are generally induced by inflammation and engagement of immune cells (Dana et al., 2000). IL-1Ra attaches to IL-1 receptor and inhibits activation of IL-1R and subsequently prevents secretion of various pro-inflammatory cytokines, chemokines, and invasion of immune cells in injured corneas (Balbi et al., 2017). In DES inflammation, DCs and Th17 cells participate in the pathogenesis of the disease. (De Paiva et al., 2009; Théry et al., 2009). Amniotic fluid MSC-DEs cargo immunomodulatory factor, increase the number of regulatory DCs, decrease maturation of DC and attenuate the immune response (Gayton, 2009; Yi and Song, 2012; Nakamura et al., 2015; Merino-González et al., 2016).
Exosome Therapy in Retinal Injury
It has been indicated that ischemia, infection and other injuries can damage retinal cells and result in visual dysfunction (Yoles and Schwartz, 1998). Recently, it has been shown that exosome and MSCs have a therapeutic role in treating retinal disorders. MSC-DE and MSCs therapy can modulate the immune response and cytokine levels in laser-induced retinal injury (LIRI). Also, MSC-DEs decrease inflammation and CD68 + macrophages and diminish apoptosis of retinal cells (Yu et al., 2016). MSC-DEs modulate secretion of different chemokines such as TNF-α and MCP-1 in retinal injuries and decrease monocytes migration (Yu et al., 2016). Another study showed that bone marrow MSCs derived exosomes (BM-MSC-DE) have therapeutic potential in the regeneration of retinal ganglion cells (RGCs) in optic nerve crush (Mead and Tomarev, 2017). BM-MSC-DE increases survival and preserves the function of RGCs in animal models. Furthermore, due to the diffusion of BM-MSC-DE into the inner retina, they have shown more therapeutic effects than BM-MSCs therapy that remain in the vitreous after injection (Yu et al., 2016; Mead and Tomarev, 2017).
MSCDEs increase neuroprotection of retinal cells after heat-induced injuries in animal models (59). MSC-DEs also decrease MCP-1 in retinal cells, consequently suppressing migration of macrophages and microglial cells toward the injured area and diminish degeneration. In vitro, retinal pigment epithelium (RPE) injured by laser produces a large amount of VEGF. Conversely, UC-MSC-DE down-regulates VEGF expression in laser damage RPE cells (Huang et al., 2018). In addition, it has been demonstrated that retinal astrocyte-derived exosomes can suppress neovascularization (Hajrasouliha et al., 2013).
Retinal ischemia occurs by an obstruction in blood vessels or detachment of the retina resulting in severe damage such as blindness. It has been indicated that MSCs therapy can inhibit degeneration of the retina in this process (Dreixler et al., 2014). Moisseiev et al. have demonstrated that BM-MSC-DE could simulate the therapeutic effect of MSCs in the treatment of retinal ischemia (Moisseiev et al., 2017). Furthermore, it has been shown that exosomes therapy inhibit microglia/astrocyte activation and subsequently enhance the secretion of pro-inflammatory cytokines such as IL-1β and IL-6 and afterwards decrease retinal ganglion cells (van der Merwe et al., 2019). Also, it has been indicated that intravitreal injection of exosomes could decrease dysfunction of retinal ganglion cells (van der Merwe et al., 2019). In the animal model, BM-MSC-DEs decrease secretion of pro-inflammatory cytokines and, increase autophagy (Ma et al., 2020). Although the exact mechanism of exosome therapy in the regeneration of the retina is not completely clear, the role of the exosome component on the neuroprotective and anti-inflammatory process has been proved.
Exosome Therapy in Corneal Injuries
Mechanical/chemical damage, immune responses and genetic disorders can induce corneal injuries with inflammation and neovascularization. Therefore, delayed or inappropriate medical intervention may lead to blindness. Recently, MSC therapy has been demonstrated to possess an influential role in reducing inflammation and modulating anti-angiogenesis in corneal injuries. Likewise, the anti-inflammation, anti-angiogenesis, and wound repair potential of MSC-DEs in different tissue models have been illustrated. Thus, these therapeutic strategies may effectively promote corneal wound repair by affecting inflammation, angiogenesis, and tissue regeneration. Furthermore, during the culture of corneal stromal cells with adipose MSC-DEs, corneal stromal cells manifested increased proliferation and inhibited apoptosis with grated deposition of collagen (Shen et al., 2018b).
Furthermore, in the stromal wound caused in an animal model, corneal stromal stem cell-derived exosomes alleviated inflammation by suppressing neutrophil infiltration (Hertsenberg et al., 2017). Also, a corneal epithelial wound was treated by corneal stromal stem cell-derived exosome (Samaeekia et al., 2018). Thus, mentioned studies propose the importance of MSC-DE in ocular and corneal disorders.
MSCs Therapy in Corneal Regeneration
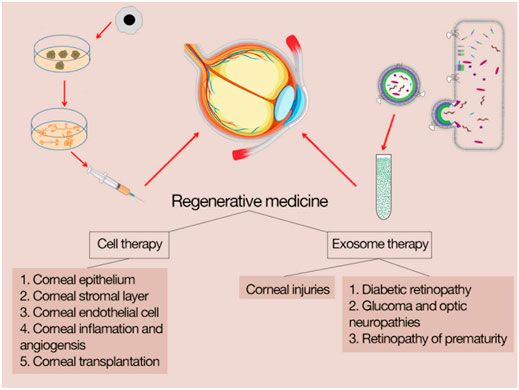
MSCtherapy in the regeneration of corneal tissue is related to two mechanisms; 1) direct cell replacement (Karp and Leng Teo, 2009) and 2) paracrine effect of regulating immune response, modulating inflammation and adjusting wound repair (Phinney and Prockop, 2007; Burrello et al., 2016). Figure 2 shows the outlines of cell therapy and exosome therapy in corneal diseases.
MSCs Therapy in Cornea Epithelium
CE includes several layers of stratified squamous that are located in the most outer part of the cornea. Physical trauma, infection, and limbal stem cell deficiency can break the structure of CE and cause continuous defects, such as neovascularization, opacities, and immune response that result in corneal blindness (Dua et al., 2005). It has been demonstrated that MSCs can transdifferentiate into epithelial cells and other neuroectoderm-derived cells, for example, astrocytes and neurons (Phinney and Prockop, 2007). Mesenchymal-epithelial-transition (MET) and epithelial-mesenchymal transition (EMT) have a critical role in organogenesis and regenerative medicine in animal models (Kim et al., 2017; Shao et al., 2018; Sivagurunathan et al., 2018). Coculture of BM-MSC with limbal stem cells resulted in polygonal epithelial-like cells with overexpression of CK3 and CK12 (corneal epithelium-specific cytokeratin 3) (Gu et al., 2009; Jiang et al., 2010).
On the other hand, AD-MSCs cultured in the supernatant of CE cell culture indicated epithelial-like cells with overexpression of CK3 and CK12 (Nieto-Miguel et al., 2013). Nevertheless, transplantation of BM- MSCs on injured corneas did not affect expression of corneal epithelium-specific cytokeratin (Ma et al., 2006). Conversely, the combination of BM-MSCs with fibrin gel to injured corneal shows an excellent therapeutic effect and manifested upregulation of CK3 (Gu et al., 2009). Recently, the application of small molecules has revealed much concentration to manipulate target gene reprogramming and change the cellular fate in the MET process (Teng et al., 2015; Ghosh et al., 2018). Epithelial progenitors derived from adipose MSC induce overexpression of N-cadherin, E-cadherin, and feature of MET progression. The mixture of these progenitors with fibrin gel can improve transparency and increase the healing of corneal injuries. MSCs can be accessible for treating corneal epithelial injuries and regenerative medicine in ocular disorders. Also, transplantation of limbal epithelial cells combined with amniotic membrane or fibrin gel is another procedure for severe limbal stem cell deficiency (Basu et al., 2012; Rama et al., 2017).
Furthermore, it has been shown that ex vivo expansion of limbal derived epithelial stem cells possesses a theoretical advantage for treating an ocular surface disorder (Teng et al., 2015; Ghosh et al., 2018). The efficacy of MSC therapy has been compared with cultivated limbal epithelial cells and the result indicated both could reconstruct corneal epithelial disease (Calonge et al., 2019). The mentioned result has manifested the application of MSC in the regeneration of corneal epithelial disorder. However, further evaluation is necessary to explain other aspects of the therapeutic procedure.
MSCs Therapy in Corneal Stromal Layer
CSL, the thickest corneal layer, consists of different components such as collagen fibrils, specialized extracellular matrix (ECM), etc. Corneal stromal keratocytes (CSKs) along with keratan sulfate proteoglycans, stromal crystallins, collagenous lamellae and ECM proteins have a critical role in the transparency and integrity of CSL (West-Mays and Dwivedi, 2006). Usually, mechanical damage and disease induce the death of CSK, resulting in decreased proteoglycan synthesis and disruption of collagen fibrils. In these situations, surviving CSKs around the damaged site are activated and start treating stromal wound healing. Unfortunately, some CSKs transform into highly contractile myofibroblasts and form corneal opacities and scars. These opacities can interfere with the transmission of light, resulting in blindness. Several studies have demonstrated that the differentiation of BM-MSCs and UCB-MSCs into keratocyte-like cells can reconstruct corneal stromal clarity (Liu et al., 2010; Liu et al., 2012; Park et al., 2012). Lumican knockout (Lum−/−) animal model with deformation of collagen fibrils show stromal opacities (Kao and Liu, 2002). However, injection of UCB-MSCs can recover disturbed collagen and restore corneal transparency with the formation of CSK-like cells and inhibition of the inflammatory reaction (Liu et al., 2010). Corneal stromal-derived stem cells and MSCs showed similar features and have been shown to have the same potential for regeneration of stromal tissue, decrease scarring and inhibition of inflammation (Du et al., 2009; Kureshi et al., 2014; Funderburgh et al., 2016; Hertsenberg et al., 2017). It has been shown that dental MSCs possess an essential role in regenerative medicine. Among the identified dental stem cells, periodontal ligament and dental pulp derived stem cells due to similar origin with CSKs and neural crest, have the same developmental pathway (Gronthos et al., 2000; Seo et al., 2004; Huang et al., 2009; Yam et al., 2015). Therefore, MSC therapy could be a beneficial procedure for the treatment of severe keratoconus eyes. However, further study will be necessary to explain the functionality of MSCs and delineate their translational potential for therapeutic application in treating stromal diseases.
MSCs Therapy in Corneal Endothelial Cells Regeneration
The CEC is the most inner layer of the corneal that consists of a hexagonal cells monolayer (Bourne, 2003). Mature CECs have an active ATPase pump that transport ions from CSL to aqueous humour and regulate hydration level and oedema in CSL, resulting in normal vision (Bonanno, 2012). Due to the overexpression of negative cell cycle regulators and mitogenic inhibitors, CECs have been introduced as non-mitotic cells (Joyce, 2012). Therefore, mechanical damage, ageing, and disease can lead to stromal oedema, CEC dysfunction, and disturbance in normal vision. Recently, cell therapy and corneal transplantation have been introduced as alternative therapies (Kinoshita et al., 2018). MSCs can be used as beneficial therapeutic tools for CEC production in vitro and treating CEC diseases such as Fuchs’ endothelial dystrophy (Joyce et al., 2012). Recent studies have shown differentiation and generation of endothelial-like cells through UC-MSC (Yamashita et al., 2018). Further studies are necessary to indicate the application of MSC in CSL regeneration.
MSCs Therapy in Corneal Inflammation and Angiogenesis
It has been shown that MSCs have anti-inflammatory potential and angiogenic ability which are important in treatment of corneal diseases. BM-MSCs could alleviate injuries and regulate angiogenesis in the animal models (Ma et al., 2006; Ye et al., 2006; Jiang et al., 2010). MSCs therapy in corneal disease inhibits infiltration of immune cells such as CD68+ macrophages and decreases pro-inflammatory cytokines, such as IL-2, IL-17, VEGF and matrix metallopeptidase 2 (MMP2). Cell therapy in corneal enhances anti-inflammatory cytokines, for example, IL-6, IL-10, thrombospondin-1 (Ma et al., 2006; Ye et al., 2006; Jiang et al., 2010).
Mentioned anti-inflammatory and pro-inflammatory cytokines modified the corneal environment, increasing wound healing in injured sites (Yao et al., 2012). In addition, MSCs exert an angiogenesis effect by secretion of innumerable pro-angiogenic and anti-angiogenic factors. However, MSCs increase the expression of TSP-1 that enhances endothelial cell apoptosis, suppresses angiogenesis and decreases MMP2 (Oh et al., 2008; Kaur et al., 2010; Bazzazi et al., 2018; Ahani-Nahayati et al., 2021a). Thus, it has been demonstrated that corneal stromal stem cells (CSSCs) have the effective potential for the regulation of inflammation and wound healing (Pinnamaneni and Funderburgh, 2012).
Importance of MSCs Therapy in Corneal Transplantation
Several studies have demonstrated the roles of MSC in the survival of corneal after transplantation (Oh et al., 2008; Casiraghi et al., 2012; Oh et al., 2012). MSCs have different immunomodulatory features that inhibit the expansion and activation of antigen-presenting cells (APCs), NK cells and other cytotoxic cells (De Miguel et al., 2012). Likewise, MSCs trigger the secretion of IL-10 from immature APCs and increase T-cell inhibitors that modulate the immune response (Beyth et al., 2005; Oh et al., 2012). Decreased mature APC population increase immune tolerance during MSCs therapy (Casiraghi et al., 2012; Oh et al., 2012). It has been illustrated that MSCs therapy suppresses expansion, cytokine secretion, and maturation of T/B-cells and modulates T regulatory cells generation (Crop et al., 2009; Casiraghi et al., 2014; Coulson-Thomas et al., 2014; Ghafouri-Fard et al., 2020). Finally, MSCs have a membrane covered by anti-inflammatory molecules, glycocalyx and versican, which regulate the host inflammatory immune response (Coulson-Thomas et al., 2014; Kao and Coulson-Thomas, 2016).
Exosome Therapy in Other Ocular Disorders
Diabetic Retinopathy
Diabetic retinopathy (DR) is one of the most critical complications of diabetes caused by high glucous levels, resulting in microangiopathy and injuries in the back of the eye (retina). DR is classified into three pathological stages; 1) non-proliferative, 2) pre-proliferative, 3) proliferative. Vascular leakage in diabetic persons induces macular oedema, a subtype of non-proliferative of DR (Stitt et al., 2016; Wong et al., 2016). The pre-proliferative stage is associated with the occlusion of blood vessels and the manifestation of the non-perfusion region. Neovascularization is one of the most important criteria in proliferative DR (Stitt et al., 2016; Wong et al., 2016). It has been demonstrated that hypoxia plays an essential role in the proliferative DR stage (Wong et al., 2016). Retinal ischemia-reperfusion injury (IRI) is associated with the complications of DR (Stitt et al., 2016; Wong et al., 2016). IRI increases the production of reactive oxygen species (ROS) that causes necrosis, apoptosis resulting in neurons and RGCs death (Liu et al., 2016; Yamashita et al., 2018). MSC-DE could improve complications of DR (Zhang et al., 2019b). Exosomes containing angiogenic active cargo have been indicated to treat diabetic microangiopathy and retinal ischemia in animal models (Moisseiev et al., 2017). A study has shown that a high level of glucose induces DR phenotype in retinal photoreceptors. In addition, a high level of glucose enhances VEGF and decreases anti-angiogenic miRNA in photoreceptors and exosomes (Maisto et al., 2020). Recently, researchers have shown that AD-MSC containing miR-192 or miR-222 has therapeutic application in treating DR and can inhibit inflammatory response and angiogenesis (Safwat et al., 2018). Likewise, injection of UC-MSC-DE with overexpression of miR-126 in a diabetic animal model has been shown to suppress HMGB1 signalling pathway and decrease inflammation caused by diabetes (60).
Exosome Therapy in Glaucoma and Optic Neuropathies
Glaucoma is an ocular disorder characterized by degeneration of the optic nerve, resulting in a visual disturbance. Glaucoma is usually because of insufficient blood supply and/or increased intraocular pressure (IOP). Glaucoma is classified into two groups 1) open-angle glaucoma (primary) 2) closed-angle glaucoma (secondary) (Davis et al., 2016). Patients with open-angle glaucoma possess normal or elevated IOP without and identified reasons (Razeghinejad et al., 2011; Weinreb et al., 2014; Davis et al., 2016).
Conversely, secondary glaucoma has several reasons, such as increased IOP (Razeghinejad et al., 2011; Weinreb et al., 2014; Davis et al., 2016). In glaucoma, increased IOP damages RGCs resulting in a visual interruption (Almasieh et al., 2012). Therefore, the essential method in treating glaucoma is decreasing intraocular pressure that diminishes the impairment of RGCs. Drain aqueous humour by trabecular meshwork (TM) has been defined as clinical treatment when intraocular pressure increases (Harrell et al., 2019). It has been demonstrated that treating TM by pigmented ciliary epithelium (NPCE) derived exosomes down-regulates Wnt signalling and expression of COL3A1.
Furthermore, these studies showed that mentioned exosomes could modulate the visual water system (Yang et al., 2020). Moreover, MSC-Des have therapeutic application in glaucoma and traumatic optic neuropathies. MSC-DEs have been shown to increase the survival of RGCs, regeneration of axons and exert neuroprotective effect in an animal model (Mead and Tomarev, 2017; Mead et al., 2018).
Exosome Therapy in Retinopathy of Prematurity
ROP is one of the reasons that cause blindness in premature infants. ROP is characterized by retinal vasculature abnormalities that involve the retina (Fulton et al., 2009; Kong et al., 2012; Hansen et al., 2017). This disease is divided into two oxygen-dependent phases; 1) inhibition of vascular growth 2) vascular proliferation (Hellström et al., 2013). In phase 1, hyperoxia (increased oxygen pressure) suppresses the secretion of VEGF and is the main inducer of disease (Hellström et al., 2013). In phase 2, the baby has grown, and metabolism increases, resulting in a hypoxic state and enhancing VEGF expression (Hellström et al., 2013). It has been shown that MSC-DEs can modulate hyperoxia-induced retinopathy, suggesting a new safe non-cellular therapy for ROP (Moisseiev et al., 2017). Also, researchers have demonstrated the influential role of microglia derived-exosome in treating retina-related diseases (Ebneter et al., 2017; Xu et al., 2019; Ahani-Nahayati et al., 2021b). Injection of microglia derived exosome into vitreous has induced down-regulation of VEGF, TGF-β and suppresses apoptosis of photoreceptor, indicating the therapeutic application of exosome in ROP (Xu et al., 2019).
Uveal Melanoma
UM is intraocular cancer that involves the choroid, ciliary body, or iris (Chattopadhyay et al., 2016; Lande et al., 2020). Nearly more than fifty percent of patients with UM possess organ metastases. Metastasis in UM is a common phenomenon and usually involve the liver (Carvajal et al., 2017). Isolation of exosomes from local blood circulation in patients with liver involvement indicated UM origin of exosomes with enrichment of Melan-A and melanoma-related microRNAs (Eldh et al., 2014). It has been manifested that isolated exosomes from patients with UM are a specific diagnostic marker (Ragusa et al., 2015). However, exosomes have not been thoroughly examined in UM; for this reason, more studies are necessary to show their importance in eye malignancies.
Importance of Exosomes in Angiogenesis
In the last decade, it has been shown that the exosome has an essential role in angiogenesis. For example, several studies demonstrated that the injection of hematopoietic stem cells CD34 + stem cell-derived exosomes (HSC-DE) into the limb ischemia animal model increases the level of miRNA-126–3p and angiogenesis (Mathiyalagan et al., 2017; Mathiyalagan and Sahoo, 2017; Sahoo et al., 2017; Ghafouri-Fard et al., 2021b). Furthermore, pericardial effusion-derived exosomes can enhance angiogenesis in patients with heart failure (Vicencio et al., 2015; Beltrami et al., 2017). Moreover, it has been manifested that MSC-derived exosomes can decrease VEGF and inhibit angiogenesis (Lee et al., 2013). Retinal ischemia is the main trigger of the proliferative stage in DR, which increase neovascularization. Drug prescription and surgery can not alleviate this issue. However, today there is a therapeutic approach that exosome has treating potential in neovascularization (Stępień et al., 2012).
Conclusion and Future Perspectives
Although cell therapy with MSCs have a critical role in treating disease, recent studies demonstrated that cell-free therapy with MSC-DE has more advantages than MSCs. MSCs-therapy has several side effects such as allogeneic reaction, unexpected differentiation and impede of microvascularization, whereas exosome therapy is safe for these problems. Exosome therapy has crucial therapeutic applications as MSCs without mentioned side effects. Moreover, exosomes can transfer their component through biological barriers and deliver their cargo to target organs because of their small size (Yu et al., 2016; Niazi et al., 2021).
Nevertheless, further evaluations are necessary to solve some present challenges. For example, freezing and thawing, investigating the effects of lyophilization, optimum dose and injection intervals to keep the long-lasting effects of exosome in eye disease are essential issues that need more examination. In addition, the possibility of toxicity in higher dosage levels injection should be determined. Moreover, exosomes are extremely heterogeneous depending on the source of MSC and the origin of MSC is associated with the therapeutic effect of exosomes in eye disease.
Author Contributions
VN, SG-F, and MH wrote the draft and revised it. MT designed and supervised the study. SR, BH, HH, and AY collected the data and designed the figures and tables. All the authors read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboul Naga, S. H., Dithmer, M., Chitadze, G., Kabelitz, D., Lucius, R., Roider, J., et al. (2015). Intracellular Pathways Following Uptake of Bevacizumab in RPE Cells. Exp. Eye Res. 131, 29–41. doi:10.1016/j.exer.2014.12.010
Ahani-Nahayati, M., Niazi, V., Moradi, A., Pourjabbar, B., Roozafzoon, R., Baradaran-Rafii, A., et al. (2021). Cell-based Therapy for Ocular Disorders: A Promising Frontier. Curr. Stem Cel Res. Ther. 17 (2), 147–165. doi:10.2174/1574888x16666210622124555
Ahani-Nahayati, M., Niazi, V., Moradi, A., Pourjabbar, B., Roozafzoon, R., Baradaran-Rafii, A., et al. (2021). Umbilical Cord Mesenchymal Stem/stromal Cells Potential to Treat Organ Disorders; an Emerging Strategy. Curr. Stem Cel Res. Ther. 17 (2), 126–146. doi:10.2174/1574888x16666210907164046
Aiello, S., Rocchetta, F., Longaretti, L., Faravelli, S., Todeschini, M., Cassis, L., et al. (2017). Extracellular Vesicles Derived from T Regulatory Cells Suppress T Cell Proliferation and Prolong Allograft Survival. Sci. Rep. 7 (1), 11518–11519. doi:10.1038/s41598-017-08617-3
Almasieh, M., Wilson, A. M., Morquette, B., Cueva Vargas, J. L., and Di Polo, A. (2012). The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 31 (2), 152–181. doi:10.1016/j.preteyeres.2011.11.002
Atienzar‐Aroca, S., Serrano‐Heras, G., Freire Valls, A., Ruiz de Almodovar, C., Muriach, M., Barcia, J. M., et al. (2018). Role of Retinal Pigment Epithelium‐derived Exosomes and Autophagy in New Blood Vessel Formation. J. Cell. Mol. Med. 22 (11), 5244–5256.
Bai, L., Shao, H., Wang, H., Zhang, Z., Su, C., Dong, L., et al. (2017). Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Scientific Rep. 7 (1), 1–11. doi:10.1038/s41598-017-04559-y
Bai, L., Shao, H., Wang, H., Zhang, Z., Su, C., Dong, L., et al. (2017). Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 7 (1), 4323. doi:10.1038/s41598-017-04559-y
Balbi, C., Piccoli, M., Barile, L., Papait, A., Armirotti, A., Principi, E., et al. (2017). First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential. Stem Cell translational Med. 6 (5), 1340–1355. doi:10.1002/sctm.16-0297
Basu, J., and Ludlow, J. W. (2016). Exosomes for Repair, Regeneration and Rejuvenation. Expert Opin. Biol. Ther. 16 (4), 489–506. doi:10.1517/14712598.2016.1131976
Basu, S., Ali, H., and Sangwan, V. S. (2012). Clinical Outcomes of Repeat Autologous Cultivated Limbal Epithelial Transplantation for Ocular Surface burns. Am. J. Ophthalmol. 153 (4), 643–650. doi:10.1016/j.ajo.2011.09.016
Bazzazi, H., Zhang, Y., Jafarnejad, M., Isenberg, J. S., Annex, B. H., and Popel, A. S. (2018). Computer Simulation of TSP1 Inhibition of VEGF-Akt-eNOS: An Angiogenesis Triple Threat. Front. Physiol. 9, 644. doi:10.3389/fphys.2018.00644
Beltrami, C., Besnier, M., Shantikumar, S., Shearn, A. I. U., Rajakaruna, C., Laftah, A., et al. (2017). Human Pericardial Fluid Contains Exosomes Enriched with Cardiovascular-Expressed microRNAs and Promotes Therapeutic Angiogenesis. Mol. Ther. 25 (3), 679–693. doi:10.1016/j.ymthe.2016.12.022
Beyth, S., Borovsky, Z., Mevorach, D., Liebergall, M., Gazit, Z., Aslan, H., et al. (2005). Human Mesenchymal Stem Cells Alter Antigen-Presenting Cell Maturation and Induce T-Cell Unresponsiveness. Blood 105 (5), 2214–2219. doi:10.1182/blood-2004-07-2921
Biasutto, L., Chiechi, A., Couch, R., Liotta, L. A., and Espina, V. (2013). Retinal Pigment Epithelium (RPE) Exosomes Contain Signaling Phosphoproteins Affected by Oxidative Stress. Exp. Cel. Res. 319 (13), 2113–2123. doi:10.1016/j.yexcr.2013.05.005
Bonanno, J. A. (2012). Molecular Mechanisms Underlying the Corneal Endothelial Pump. Exp. Eye Res. 95 (1), 2–7. doi:10.1016/j.exer.2011.06.004
Bora, N. S., Matta, B., Lyzogubov, V. V., and Bora, P. S. (2015). Relationship between the Complement System, Risk Factors and Prediction Models in Age-Related Macular Degeneration. Mol. Immunol. 63 (2), 176–183. doi:10.1016/j.molimm.2014.07.012
Bose, T., Diedrichs-Möhring, M., and Wildner, G. (2016). Dry Eye Disease and Uveitis: a Closer Look at Immune Mechanisms in Animal Models of Two Ocular Autoimmune Diseases. Autoimmun. Rev. 15 (12), 1181–1192. doi:10.1016/j.autrev.2016.09.001
Bourne, W. M. (2003). Biology of the Corneal Endothelium in Health and Disease. Eye 17 (8), 912–918. doi:10.1038/sj.eye.6700559
Burrello, J., Monticone, S., Gai, C., Gomez, Y., Kholia, S., and Camussi, G. (2016). Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cel Dev. Biol. 4, 83. doi:10.3389/fcell.2016.00083
Calonge, M., Pérez, I., Galindo, S., Nieto-Miguel, T., López-Paniagua, M., Fernández, I., et al. (2019). A Proof-Of-Concept Clinical Trial Using Mesenchymal Stem Cells for the Treatment of Corneal Epithelial Stem Cell Deficiency. Translational Res. 206, 18–40. doi:10.1016/j.trsl.2018.11.003
Carvajal, R. D., Schwartz, G. K., Tezel, T., Marr, B., Francis, J. H., and Nathan, P. D. (2017). Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br. J. Ophthalmol. 101 (1), 38–44. doi:10.1136/bjophthalmol-2016-309034
Casiraghi, F., Azzollini, N., Todeschini, M., Cavinato, R. A., Cassis, P., Solini, S., et al. (2012). Localization of Mesenchymal Stromal Cells Dictates Their Immune or Proinflammatory Effects in Kidney Transplantation. Am. J. Transplant. 12 (9), 2373–2383. doi:10.1111/j.1600-6143.2012.04115.x
Casiraghi, F., Remuzzi, G., and Perico, N. (2014). Mesenchymal Stromal Cells to Promote Kidney Transplantation Tolerance. Curr. Opin. Organ Transplant. 19 (1), 47–53. doi:10.1097/mot.0000000000000035
Caspi, R. R. (2010). A Look at Autoimmunity and Inflammation in the Eye. J. Clin. Invest. 120 (9), 3073–3083. doi:10.1172/jci42440
Chattopadhyay, C., Kim, D. W., Gombos, D. S., Oba, J., Qin, Y., Williams, M. D., et al. (2016). Uveal Melanoma: from Diagnosis to Treatment and the Science in between. Cancer 122 (15), 2299–2312. doi:10.1002/cncr.29727
Chong, W. P., van Panhuys, N., Chen, J., Silver, P. B., Jittayasothorn, Y., Mattapallil, M. J., et al. (2015). NK-DC Crosstalk Controls the Autopathogenic Th17 Response through an Innate IFN-γ-IL-27 axis. J. Exp. Med. 212 (10), 1739–1752. doi:10.1084/jem.20141678
Coulson-Thomas, V. J., Gesteira, T. F., Hascall, V., and Kao, W. (2014). Umbilical Cord Mesenchymal Stem Cells Suppress Host Rejection. J. Biol. Chem. 289 (34), 23465–23481. doi:10.1074/jbc.m114.557447
Crop, M., Baan, C., Weimar, W., and Hoogduijn, M. (2009). Potential of Mesenchymal Stem Cells as Immune Therapy in Solid-Organ Transplantation. Transpl. Int. 22 (4), 365–376. doi:10.1111/j.1432-2277.2008.00786.x
Dana, M. R., Qian, Y., and Hamrah, P. (2000). Twenty-five-Year Panorama of Corneal Immunology. Cornea 19 (5), 625–643. doi:10.1097/00003226-200009000-00008
Davis, B. M., Crawley, L., Pahlitzsch, M., Javaid, F., and Cordeiro, M. F. (2016). Glaucoma: the Retina and beyond. Acta Neuropathol. 132 (6), 807–826. doi:10.1007/s00401-016-1609-2
De Miguel, M. P., Fuentes-Julian, S., Blazquez-Martinez, A., Pascual, C. Y., Aller, M. A., Arias, J., et al. (2012). Immunosuppressive Properties of Mesenchymal Stem Cells: Advances and Applications. Cmm 12 (5), 574–591. doi:10.2174/156652412800619950
De Paiva, C. S., Chotikavanich, S., Pangelinan, S. B., Pitcher, , Fang, B., Zheng, X., et al. (2009). IL-17 Disrupts Corneal Barrier Following Desiccating Stress. Mucosal Immunol. 2 (3), 243–253. doi:10.1038/mi.2009.5
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., et al. (2002). Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 99 (10), 3838–3843. doi:10.1182/blood.v99.10.3838
Dreixler, J. C., Poston, J. N., Balyasnikova, I., Shaikh, A. R., Tupper, K. Y., Conway, S., et al. (2014). Delayed Administration of Bone Marrow Mesenchymal Stem Cell Conditioned Medium Significantly Improves Outcome after Retinal Ischemia in Rats. Invest. Ophthalmol. Vis. Sci. 55 (6), 3785–3796. doi:10.1167/iovs.13-11683
Du, Y., Carlson, E. C., Funderburgh, M. L., Birk, D. E., Pearlman, E., Guo, N., et al. (2009). Stem Cell Therapy Restores Transparency to Defective Murine Corneas. Stem cells 27 (7), 1635–1642. doi:10.1002/stem.91
Dua, H. S., Shanmuganathan, V., Powell-Richards, A., Tighe, P., and Joseph, A. (2005). Limbal Epithelial Crypts: a Novel Anatomical Structure and a Putative Limbal Stem Cell Niche. Br. J. Ophthalmol. 89 (5), 529–532. doi:10.1136/bjo.2004.049742
Ebneter, A., Kokona, D., Schneider, N., and Zinkernagel, M. S. (2017). Microglia Activation and Recruitment of Circulating Macrophages during Ischemic Experimental branch Retinal Vein Occlusion. Invest. Ophthalmol. Vis. Sci. 58 (2), 944–953. doi:10.1167/iovs.16-20474
Elbay, A., Ercan, Ç., Akbaş, F., Bulut, H., and Ozdemir, H. (2019). Three New Circulating microRNAs May Be Associated with Wet Age-Related Macular Degeneration. Scand. J. Clin. Lab. Invest. 79 (6), 388–394. doi:10.1080/00365513.2019.1637931
Eldh, M., Olofsson Bagge, R., Lässer, C., Svanvik, J., Sjöstrand, M., Mattsson, J., et al. (2014). MicroRNA in Exosomes Isolated Directly from the Liver Circulation in Patients with Metastatic Uveal Melanoma. BMC cancer 14 (1), 962–1010. doi:10.1186/1471-2407-14-962
Flaxman, S. R., Bourne, R. R. A., Resnikoff, S., Ackland, P., Braithwaite, T., Cicinelli, M. V., et al. (2017). Global Causes of Blindness and Distance Vision Impairment 1990-2020: a Systematic Review and Meta-Analysis. Lancet Glob. Health 5 (12), e1221–e1234. doi:10.1016/s2214-109x(17)30393-5
Fulton, A. B., Hansen, R. M., Moskowitz, A., and Akula, J. D. (2009). The Neurovascular Retina in Retinopathy of Prematurity. Prog. Retin. Eye Res. 28 (6), 452–482. doi:10.1016/j.preteyeres.2009.06.003
Funderburgh, J. L., Funderburgh, M. L., and Du, Y. (2016). Stem Cells in the Limbal Stroma. Ocul. Surf. 14 (2), 113–120. doi:10.1016/j.jtos.2015.12.006
Gain, P., Jullienne, R., He, Z., Aldossary, M., Acquart, S., Cognasse, F., et al. (2016). Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 134 (2), 167–173. doi:10.1001/jamaophthalmol.2015.4776
Gallo, A., Jang, S.-I., Ong, H. L., Perez, P., Tandon, M., Ambudkar, I., et al. (2016). Targeting the Ca 2+ Sensor STIM1 by Exosomal Transfer of Ebv-miR-BART13-3p Is Associated with Sjögren's Syndrome. EBioMedicine 10, 216–226. doi:10.1016/j.ebiom.2016.06.041
Gayton, J. (2009). Etiology, Prevalence, and Treatment of Dry Eye Disease. Opth 3, 405. doi:10.2147/opth.s5555
Generali, E., Costanzo, A., Mainetti, C., and Selmi, C. (2017). Cutaneous and Mucosal Manifestations of Sjögren's Syndrome. Clinic Rev. Allerg Immunol. 53 (3), 357–370. doi:10.1007/s12016-017-8639-y
Ghafouri-Fard, S., Niazi, V., Hussen, B. M., Omrani, M. D., Taheri, M., and Basiri, A. (2021). The Emerging Role of Exosomes in the Treatment of Human Disorders with a Special Focus on Mesenchymal Stem Cells-Derived Exosomes. Front. Cel. Dev. Biol. 9. doi:10.3389/fcell.2021.653296
Ghafouri-Fard, S., Glassy, M. C., Abak, A., Hussen, B. M., Niazi, V., and Taheri, M. (2021). The Interaction between miRNAs/lncRNAs and Notch Pathway in Human Disorders. Biomed. Pharmacother. 138, 111496. doi:10.1016/j.biopha.2021.111496
Ghafouri-Fard, S., Niazi, V., and Taheri, M. (2020). Role of miRNAs in Conveying Message of Stem Cells via Extracellular Vesicles. Exp. Mol. Pathol. 117, 104569. doi:10.1016/j.yexmp.2020.104569
Ghosh, A., Degyatoreva, N., Kukielski, C., Story, S., Bhaduri, S., Maiti, K., et al. (2018). Targeting miRNA by Tunable Small Molecule Binders: Peptidic Aminosugar Mediated Interference in miR-21 Biogenesis Reverts Epithelial to Mesenchymal Transition. Med. Chem. Commun. 9 (7), 1147–1154. doi:10.1039/c8md00092a
Gonzalez-Nolasco, B., Wang, M., Prunevieille, A., and Benichou, G. (2018). Emerging Role of Exosomes in Allorecognition and Allograft Rejection. Curr. Opin. Organ Transplant. 23 (1), 22–27. doi:10.1097/mot.0000000000000489
Goules, A. V., Kapsogeorgou, E. K., and Tzioufas, A. G. (2017). Insight into Pathogenesis of Sjögren's Syndrome: Dissection on Autoimmune Infiltrates and Epithelial Cells. Clin. Immunol. 182, 30–40. doi:10.1016/j.clim.2017.03.007
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., and Shi, S. (2000). Postnatal Human Dental Pulp Stem Cells (DPSCs) In Vitro and Invivo. Proc. Natl. Acad. Sci. 97 (25), 13625–13630. doi:10.1073/pnas.240309797
Gu, S., Xing, C., Han, J., Tso, M. O., and Hong, J. (2009). Differentiation of Rabbit Bone Marrow Mesenchymal Stem Cells into Corneal Epithelial Cells In Vivo and Ex Vivo. Mol. Vis. 15, 99–107.
Hajrasouliha, A. R., Jiang, G., Lu, Q., Lu, H., Kaplan, H. J., Zhang, H.-G., et al. (2013). Exosomes from Retinal Astrocytes Contain Antiangiogenic Components that Inhibit Laser-Induced Choroidal Neovascularization. J. Biol. Chem. 288 (39), 28058–28067. doi:10.1074/jbc.m113.470765
Han, K. Y., Tran, J. A., Chang, J. H., Azar, D. T., and Zieske, J. D. (2017). Potential Role of Corneal Epithelial Cell-Derived Exosomes in Corneal Wound Healing and Neovascularization. Sci. Rep. 7 (1), 40548–40614. doi:10.1038/srep40548
Hansen, R. M., Moskowitz, A., Akula, J. D., and Fulton, A. B. (2017). The Neural Retina in Retinopathy of Prematurity. Prog. Retin. Eye Res. 56, 32–57. doi:10.1016/j.preteyeres.2016.09.004
Harrell, C. R., Fellabaum, C., Arsenijevic, A., Markovic, B. S., Djonov, V., and Volarevic, V. (2019). Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cell Int 2019, 7869130. doi:10.1155/2019/7869130
Hellström, A., Smith, L. E., and Dammann, O. (2013). Retinopathy of Prematurity. The lancet 382 (9902), 1445–1457.
Hertsenberg, A. J., Shojaati, G., Funderburgh, M. L., Mann, M. M., Du, Y., and Funderburgh, J. L. (2017). Corneal Stromal Stem Cells Reduce Corneal Scarring by Mediating Neutrophil Infiltration after Wounding. PloS one 12 (3), e0171712. doi:10.1371/journal.pone.0171712
Huang, C., Fisher, K. P., Hammer, S. S., Navitskaya, S., Blanchard, G. J., and Busik, J. V. (2018). Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 67 (8), 1639–1649. doi:10.2337/db17-1587
Huang, G. T.-J., Gronthos, S., and Shi, S. (2009). Mesenchymal Stem Cells Derived from Dental Tissuesvs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent Res. 88 (9), 792–806. doi:10.1177/0022034509340867
H. Y. Kim, T. R Jackson, and L. A Davidson (Editors) (2017). “On the Role of Mechanics in Driving Mesenchymal-To-Epithelial Transitions,” Seminars in Cell & Developmental Biology (Elsevier).
Jangamreddy, J. R., Haagdorens, M. K. C., Mirazul Islam, M., Lewis, P., Samanta, A., Fagerholm, P., et al. (2018). Short Peptide Analogs as Alternatives to Collagen in Pro-regenerative Corneal Implants. Acta Biomater. 69, 120–130. doi:10.1016/j.actbio.2018.01.011
Jiang, T. S., Cai, L., Ji, W. Y., Hui, Y. N., Wang, Y. S., Hu, D., et al. (2010). Reconstruction of the Corneal Epithelium with Induced Marrow Mesenchymal Stem Cells in Rats. Mol. Vis. 16, 1304–1316.
Joe, A. W., and Gregory-Evans, K. (2010). Mesenchymal Stem Cells and Potential Applications in Treating Ocular Disease. Curr. Eye Res. 35 (11), 941–952. doi:10.3109/02713683.2010.516466
Joyce, N. C., Harris, D. L., Markov, V., Zhang, Z., and Saitta, B. (2012). Potential of Human Umbilical Cord Blood Mesenchymal Stem Cells to Heal Damaged Corneal Endothelium. Mol. Vis. 18, 547–564.
Joyce, N. C. (2012). Proliferative Capacity of Corneal Endothelial Cells. Exp. Eye Res. 95 (1), 16–23. doi:10.1016/j.exer.2011.08.014
Kamalden, T. A., Macgregor-Das, A. M., Kannan, S. M., Dunkerly-Eyring, B., Khaliddin, N., Xu, Z., et al. (2017). Exosomal microRNA-15a Transfer from the Pancreas Augments Diabetic Complications by Inducing Oxidative Stress. Antioxid. Redox Signaling 27 (13), 913–930. doi:10.1089/ars.2016.6844
Kang, G.-Y., Bang, J. Y., Choi, A. J., Yoon, J., Lee, W.-C., Choi, S., et al. (2014). Exosomal Proteins in the Aqueous Humor as Novel Biomarkers in Patients with Neovascular Age-Related Macular Degeneration. J. Proteome Res. 13 (2), 581–595. doi:10.1021/pr400751k
Kao, W. W., and Coulson-Thomas, V. J. (2016). Cell Therapy of Corneal Diseases. Cornea 35 Suppl 1 (Suppl. 1), S9. doi:10.1097/ICO.0000000000001010
Kao, W. W., and Liu, C. Y. (2002). Roles of Lumican and Keratocan on Corneal Transparency. Glycoconj J. 19 (4), 275–285. doi:10.1023/A:1025396316169
Kapsogeorgou, E. K., Abu-Helu, R. F., Moutsopoulos, H. M., and Manoussakis, M. N. (2005). Salivary Gland Epithelial Cell Exosomes: a Source of Autoantigenic Ribonucleoproteins. Arthritis Rheum. 52 (5), 1517–1521. doi:10.1002/art.21005
Karp, J. M., and Leng Teo, G. S. (2009). Mesenchymal Stem Cell Homing: the Devil Is in the Details. Cell stem cell 4 (3), 206–216. doi:10.1016/j.stem.2009.02.001
Katome, T., Namekata, K., Mitamura, Y., Semba, K., Egawa, M., Naito, T., et al. (2015). Expression of Intraocular Peroxisome Proliferator-Activated Receptor Gamma in Patients with Proliferative Diabetic Retinopathy. J. Diabetes its Complications 29 (2), 275–281. doi:10.1016/j.jdiacomp.2014.10.010
Kaur, S., Martin-Manso, G., Pendrak, M. L., Garfield, S. H., Isenberg, J. S., and Roberts, D. D. (2010). Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting its Association with CD47. J. Biol. Chem. 285 (50), 38923–38932. doi:10.1074/jbc.m110.172304
Kinoshita, S., Koizumi, N., Ueno, M., Okumura, N., Imai, K., Tanaka, H., et al. (2018). Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 378 (11), 995–1003. doi:10.1056/nejmoa1712770
Kong, L., Fry, M., Al-Samarraie, M., Gilbert, C., and Steinkuller, P. G. (2012). An Update on Progress and the Changing Epidemiology of Causes of Childhood Blindness Worldwide. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 16 (6), 501–507. doi:10.1016/j.jaapos.2012.09.004
Krishna, U., Ajanaku, D., Denniston, A. K., and Gkika, T. (2017). Uveitis: a Sight-Threatening Disease Which Can Impact All Systems. Postgrad. Med. J. 93 (1106), 766–773. doi:10.1136/postgradmedj-2017-134891
Kureshi, A. K., Funderburgh, J. L., and Daniels, J. T. (2014). Human Corneal Stromal Stem Cells Exhibit Survival Capacity Following Isolation from Stored Organ-Culture Corneas. Invest. Ophthalmol. Vis. Sci. 55 (11), 7583–7588. doi:10.1167/iovs.14-14448
Lande, K., Gupta, J., Ranjan, R., Kiran, M., Torres Solis, L. F., Solís Herrera, A., et al. (2020). Exosomes: Insights from Retinoblastoma and Other Eye Cancers. Ijms 21 (19), 7055. doi:10.3390/ijms21197055
Lee, J.-K., Park, S.-R., Jung, B.-K., Jeon, Y.-K., Lee, Y.-S., Kim, M.-K., et al. (2013). Exosomes Derived from Mesenchymal Stem Cells Suppress Angiogenesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PloS one 8 (12), e84256. doi:10.1371/journal.pone.0084256
Leszczynska, A., Kulkarni, M., Ljubimov, A. V., and Saghizadeh, M. (2018). Exosomes from normal and Diabetic Human Corneolimbal Keratocytes Differentially Regulate Migration, Proliferation and Marker Expression of Limbal Epithelial Cells. Sci. Rep. 8 (1), 15173–15213. doi:10.1038/s41598-018-33169-5
Li, J., Xue, H., Li, T., Chu, X., Xin, D., Xiong, Y., et al. (2019). Exosomes Derived from Mesenchymal Stem Cells Attenuate the Progression of Atherosclerosis in ApoE−/- Mice via miR-Let7 Mediated Infiltration and Polarization of M2 Macrophage. Biochem. biophysical Res. Commun. 510 (4), 565–572. doi:10.1016/j.bbrc.2019.02.005
Li, N., Zhao, L., Wei, Y., Ea, V. L., Nian, H., and Wei, R. (2019). Recent Advances of Exosomes in Immune-Mediated Eye Diseases. Stem Cel Res Ther 10 (1), 278–310. doi:10.1186/s13287-019-1372-0
Li, X., Lu, X., Sun, D., Wang, X., Yang, L., Zhao, S., et al. (2016). Adipose-derived Mesenchymal Stem Cells Reduce Lymphocytic Infiltration in a Rabbit Model of Induced Autoimmune Dacryoadenitis. Invest. Ophthalmol. Vis. Sci. 57 (13), 5161–5170. doi:10.1167/iovs.15-17824
Liszewski, M. K., Java, A., Schramm, E. C., and Atkinson, J. P. (2017). Complement Dysregulation and Disease: Insights from Contemporary Genetics. Annu. Rev. Pathol. Mech. Dis. 12, 25–52. doi:10.1146/annurev-pathol-012615-044145
Liu, H., Zhang, J., Liu, C.-Y., Hayashi, Y., and Kao, W. W.-Y. (2012). Bone Marrow Mesenchymal Stem Cells Can Differentiate and Assume Corneal Keratocyte Phenotype. J. Cel. Mol. Med. 16 (5), 1114–1124. doi:10.1111/j.1582-4934.2011.01418.x
Liu, H., Zhang, J., Liu, C.-Y., Wang, I.-J., Sieber, M., Chang, J., et al. (2010). Cell Therapy of Congenital Corneal Diseases with Umbilical Mesenchymal Stem Cells: Lumican Null Mice. PloS one 5 (5), e10707. doi:10.1371/journal.pone.0010707
Liu, L., Sun, Q., Wang, R., Chen, Z., Wu, J., Xia, F., et al. (2016). Methane Attenuates Retinal Ischemia/reperfusion Injury via Anti-oxidative and Anti-apoptotic Pathways. Brain Res. 1646, 327–333. doi:10.1016/j.brainres.2016.05.037
López-Miguel, A., Tesón, M., Martín-Montañez, V., Enríquez-de-Salamanca, A., Stern, M. E., González-García, M. J., et al. (2016). Clinical and Molecular Inflammatory Response in Sjögren Syndrome–Associated Dry Eye Patients under Desiccating Stress. Am. J. Ophthalmol. 161, 133–141.e2.
Ma, M., Li, B., Zhang, M., Zhou, L., Yang, F., Ma, F., et al. (2020). Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes on Retinal Detachment. Exp. Eye Res. 191, 107899. doi:10.1016/j.exer.2019.107899
Ma, Y., Xu, Y., Xiao, Z., Yang, W., Zhang, C., Song, E., et al. (2006). Reconstruction of Chemically Burned Rat Corneal Surface by Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem cells 24 (2), 315–321. doi:10.1634/stemcells.2005-0046
Maisto, R., Trotta, M. C., Petrillo, F., Izzo, S., Cuomo, G., Alfano, R., et al. (2020). Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged with High Glucose. Front. Pharmacol. 11, 235. doi:10.3389/fphar.2020.00235
Manoussakis, M. N., and Kapsogeorgou, E. K. (2010). The Role of Intrinsic Epithelial Activation in the Pathogenesis of Sjögren's Syndrome. J. Autoimmun. 35 (3), 219–224. doi:10.1016/j.jaut.2010.06.011
Marino, J., Paster, J., and Benichou, G. (2016). Allorecognition by T Lymphocytes and Allograft Rejection. Front. Immunol. 7, 582. doi:10.3389/fimmu.2016.00582
Mathew, B., Ravindran, S., Liu, X., Torres, L., Chennakesavalu, M., Huang, C.-C., et al. (2019). Mesenchymal Stem Cell-Derived Extracellular Vesicles and Retinal Ischemia-Reperfusion. Biomaterials 197, 146–160. doi:10.1016/j.biomaterials.2019.01.016
Mathiyalagan, P., Liang, Y., Kim, D., Misener, S., Thorne, T., Kamide, C. E., et al. (2017). Angiogenic Mechanisms of Human CD34 + Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 120 (9), 1466–1476. doi:10.1161/circresaha.116.310557
Mathiyalagan, P., and Sahoo, S. (2017). Cardiac Gene Therapy. Springer, 139–152. doi:10.1007/978-1-4939-6588-5_9Exosomes-based Gene Therapy for microRNA Delivery
Mavragani, C. P., Fragoulis, G. E., and Moutsopoulos, H. M. (2014). Sjögren's Syndrome. Autoimmune Dis., 495–510. doi:10.1016/b978-0-12-384929-8.00035-6
Mavragani, C. P. (2017). Mechanisms and New Strategies for Primary Sjögren's Syndrome. Annu. Rev. Med. 68, 331–343. doi:10.1146/annurev-med-043015-123313
Mead, B., Ahmed, Z., and Tomarev, S. (2018). Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Invest. Ophthalmol. Vis. Sci. 59 (13), 5473–5480. doi:10.1167/iovs.18-25310
Mead, B., Hill, L. J., Blanch, R. J., Ward, K., Logan, A., Berry, M., et al. (2016). Mesenchymal Stromal Cell-Mediated Neuroprotection and Functional Preservation of Retinal Ganglion Cells in a Rodent Model of Glaucoma. Cytotherapy 18 (4), 487–496. doi:10.1016/j.jcyt.2015.12.002
Mead, B., and Tomarev, S. (2017). Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells through miRNA-dependent Mechanisms. Stem Cell translational Med. 6 (4), 1273–1285. doi:10.1002/sctm.16-0428
Merino-González, C., Zuñiga, F. A., Escudero, C., Ormazabal, V., Reyes, C., Nova-Lamperti, E., et al. (2016). Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 7, 24. doi:10.3389/fphys.2016.00024
Moisseiev, E., Anderson, J. D., Oltjen, S., Goswami, M., Zawadzki, R. J., Nolta, J. A., et al. (2017). Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 42 (10), 1358–1367. doi:10.1080/02713683.2017.1319491
Nakamura, Y., Miyaki, S., Ishitobi, H., Matsuyama, S., Nakasa, T., Kamei, N., et al. (2015). Mesenchymal-stem-cell-derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett. 589 (11), 1257–1265. doi:10.1016/j.febslet.2015.03.031
Niazi, V., Ghafouri‐Fard, S., Verdi, J., Jeibouei, S., Karami, F., Pourhadi, M., et al. (2021). Hypoxia Preconditioned Mesenchymal Stem Cell‐derived Exosomes Induce Ex Vivo Expansion of Umbilical Cord Blood Hematopoietic Stem Cells CD133+ by Stimulation of Notch Signalling Pathway. Biotechnol. Prog., e3222. doi:10.1002/btpr.3222
Nieto-Miguel, T., Galindo, S., Reinoso, R., Corell, A., Martino, M., Pérez-Simón, J. A., et al. (2013). In VitroSimulation of Corneal Epithelium Microenvironment Induces a Corneal Epithelial-like Cell Phenotype from Human Adipose Tissue Mesenchymal Stem Cells. Curr. Eye Res. 38 (9), 933–944. doi:10.3109/02713683.2013.802809
Nussenblatt, R. B., Liu, B., Wei, L., and Sen, H. N. (2013). The Immunological Basis of Degenerative Diseases of the Eye. Int. Rev. Immunol. 32 (1), 97–112. doi:10.3109/08830185.2012.740536
Oh, J. Y., Kim, M. K., Shin, M. S., Lee, H. J., Ko, J. H., Wee, W. R., et al. (2008). The Anti-inflammatory and Anti-angiogenic Role of Mesenchymal Stem Cells in Corneal Wound Healing Following Chemical Injury. Stem cells 26 (4), 1047–1055. doi:10.1634/stemcells.2007-0737
Oh, J. Y., Lee, R. H., Yu, J. M., Ko, J. H., Lee, H. J., Ko, A. Y., et al. (2012). Intravenous Mesenchymal Stem Cells Prevented Rejection of Allogeneic Corneal Transplants by Aborting the Early Inflammatory Response. Mol. Ther. 20 (11), 2143–2152. doi:10.1038/mt.2012.165
Pakravan, K., Babashah, S., Sadeghizadeh, M., Mowla, S. J., Mossahebi-Mohammadi, M., Ataei, F., et al. (2017). MicroRNA-100 Shuttled by Mesenchymal Stem Cell-Derived Exosomes Suppresses In Vitro Angiogenesis through Modulating the mTOR/HIF-1α/VEGF Signaling axis in Breast Cancer Cells. Cell Oncol. 40 (5), 457–470. doi:10.1007/s13402-017-0335-7
Papotto, P. H., Marengo, E. B., Sardinha, L. R., Goldberg, A. C., and Rizzo, L. V. (2014). Immunotherapeutic Strategies in Autoimmune Uveitis. Autoimmun. Rev. 13 (9), 909–916. doi:10.1016/j.autrev.2014.05.003
Park, D. H., Connor, K. M., and Lambris, J. D. (2019). The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 10, 1007. doi:10.3389/fimmu.2019.01007
Park, S. H., Kim, K. W., Chun, Y. S., and Kim, J. C. (2012). Human Mesenchymal Stem Cells Differentiate into Keratocyte-like Cells in Keratocyte-Conditioned Medium. Exp. Eye Res. 101, 16–26. doi:10.1016/j.exer.2012.05.009
Patel, D. D., and Kuchroo, V. K. (2015). Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 43 (6), 1040–1051. doi:10.1016/j.immuni.2015.12.003
Pennington, K. L., and DeAngelis, M. M. (2016). Epidemiology of Age-Related Macular Degeneration (AMD): Associations with Cardiovascular Disease Phenotypes and Lipid Factors. Eye Vis. (Lond) 3 (1), 34–20. doi:10.1186/s40662-016-0063-5
Pepple, K. L., and Lin, P. (2018). Targeting Interleukin-23 in the Treatment of Noninfectious Uveitis. Ophthalmology 125 (12), 1977–1983. doi:10.1016/j.ophtha.2018.05.014
Perez, V. L., and Caspi, R. R. (2015). Immune Mechanisms in Inflammatory and Degenerative Eye Disease. Trends Immunology 36 (6), 354–363. doi:10.1016/j.it.2015.04.003
Phinney, D. G., and Prockop, D. J. (2007). Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair-Current Views. Stem cells 25 (11), 2896–2902. doi:10.1634/stemcells.2007-0637
Pinnamaneni, N., and Funderburgh, J. L. (2012). Concise Review: Stem Cells in the Corneal Stroma. Stem cells 30 (6), 1059–1063. doi:10.1002/stem.1100
Ragusa, M., Barbagallo, C., Statello, L., Caltabiano, R., Russo, A., Puzzo, L., et al. (2015). miRNA Profiling in Vitreous Humor, Vitreal Exosomes and Serum from Uveal Melanoma Patients: Pathological and Diagnostic Implications. Cancer Biol. Ther. 16 (9), 1387–1396. doi:10.1080/15384047.2015.1046021
Rama, P., Ferrari, G., and Pellegrini, G. (2017). Cultivated Limbal Epithelial Transplantation. Curr. Opin. Ophthalmol. 28 (4), 387–389. doi:10.1097/icu.0000000000000382
Razeghinejad, M. R., Myers, J. S., and Katz, L. J. (2011). Iatrogenic Glaucoma Secondary to Medications. Am. J. Med. 124 (1), 20–25. doi:10.1016/j.amjmed.2010.08.011
Ren, K. (2019). Exosomes in Perspective: a Potential Surrogate for Stem Cell Therapy. Odontology 107 (3), 271–284. doi:10.1007/s10266-018-0395-9
Safwat, A., Sabry, D., Ragiae, A., Amer, E., Mahmoud, R. H., and Shamardan, R. M. (2018). Adipose Mesenchymal Stem Cells-Derived Exosomes Attenuate Retina Degeneration of Streptozotocin-Induced Diabetes in Rabbits. J. Circ. Biomark 7, 1849454418807827. doi:10.1177/1849454418807827
Sahoo, S., Mathiyalagan, P., and Hajjar, R. J. (2017). Pericardial Fluid Exosomes: a New Material to Treat Cardiovascular Disease. Mol. Ther. 25 (3), 568–569. doi:10.1016/j.ymthe.2017.02.002
Samaeekia, R., Rabiee, B., Putra, I., Shen, X., Park, Y. J., Hematti, P., et al. (2018). Effect of Human Corneal Mesenchymal Stromal Cell-Derived Exosomes on Corneal Epithelial Wound Healing. Invest. Ophthalmol. Vis. Sci. 59 (12), 5194–5200. doi:10.1167/iovs.18-24803
Sandhya, P., Kurien, B., Danda, D., and Scofield, R. (2017). Update on Pathogenesis of Sjogren's Syndrome. Crr 13 (1), 5–22. doi:10.2174/1573397112666160714164149
Sene, A., Chin-Yee, D., and Apte, R. S. (2015). Seeing through VEGF: Innate and Adaptive Immunity in Pathological Angiogenesis in the Eye. Trends Molecular Medicine 21 (1), 43–51. doi:10.1016/j.molmed.2014.10.005
Seo, B.-M., Miura, M., Gronthos, S., Mark Bartold, P., Batouli, S., Brahim, J., et al. (2004). Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. The Lancet 364 (9429), 149–155. doi:10.1016/s0140-6736(04)16627-0
Sevgi, D. D., Davoudi, S., Comander, J., and Sobrin, L. (2017). Retinal Pigmentary Changes in Chronic Uveitis Mimicking Retinitis Pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 255, 1801–1810. doi:10.1007/s00417-017-3689-7
Shao, J. Z., Qi, Y., Du, S. S., Du, W. W., Li, F. Z., and Zhang, F. Y. (2018). In Vitro inhibition of Proliferation, Migration and Epithelial-Mesenchymal Transition of Human Lens Epithelial Cells by Fasudil. Int. J. Ophthalmol. 11 (8), 1253–1257. doi:10.18240/ijo.2018.08.02
Shen, T., Zheng, Q. Q., Shen, J., Li, Q. S., Song, X. H., Luo, H. B., et al. (2018). Effects of Adipose-Derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. (Engl) 131 (06), 704–712. doi:10.4103/0366-6999.226889
Shen, T., Zheng, Q.-Q., Shen, J., Li, Q.-S., Song, X.-H., Luo, H.-B., et al. (2018). Effects of Adipose-Derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. 131 (6), 704–712. doi:10.4103/0366-6999.226889
Shi, Q., Wang, Q., Li, J., Zhou, X., Fan, H., Wang, F., et al. (2015). A2E Suppresses Regulatory Function of RPE Cells in Th1 Cell Differentiation via Production of IL-1β and Inhibition of PGE2. Invest. Ophthalmol. Vis. Sci. 56 (13), 7728–7738. doi:10.1167/iovs.15-17677
Shiboski, S. C., Shiboski, C. H., Criswell, L. A., Baer, A. N., Challacombe, S., Lanfranchi, H., et al. (2012). American College of Rheumatology Classification Criteria for Sjögren's Syndrome: A Data-Driven, Expert Consensus Approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 64 (4), 475–487. doi:10.1002/acr.21591
Shigemoto-Kuroda, T., Oh, J. Y., Kim, D.-k., Jeong, H. J., Park, S. Y., Lee, H. J., et al. (2017). MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cel. Rep. 8 (5), 1214–1225. doi:10.1016/j.stemcr.2017.04.008
Sivagurunathan, S., Raman, R., and Chidambaram, S. (2018). PIWI-like Protein, HIWI2: A Novel Player in Proliferative Diabetic Retinopathy. Exp. Eye Res. 177, 191–196. doi:10.1016/j.exer.2018.08.018
Stępień, E., Kabłak-Ziembicka, A., Czyż, J., Przewłocki, T., and Małecki, M. (2012). Microparticles, Not Only Markers but Also a Therapeutic Target in the Early Stage of Diabetic Retinopathy and Vascular Aging. Expert Opin. Ther. Targets 16 (7), 677–688. doi:10.1517/14728222.2012.691471
Stitt, A. W., Curtis, T. M., Chen, M., Medina, R. J., McKay, G. J., Jenkins, A., et al. (2016). The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 51, 156–186. doi:10.1016/j.preteyeres.2015.08.001
Teng, N. Y., Liu, Y. S., Wu, H. H., Liu, Y. A., Ho, J. H., and Lee, O. K. (2015). Promotion of Mesenchymal-To-Epithelial Transition by Rac1 Inhibition with Small Molecules Accelerates Hepatic Differentiation of Mesenchymal Stromal Cells. Tissue Eng. Part. A. 21 (7-8), 1444–1454. doi:10.1089/ten.TEA.2014.0320
Théry, C., Ostrowski, M., and Segura, E. (2009). Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 9 (8), 581–593. doi:10.1038/nri2567
Toomey, C. B., Johnson, L. V., and Bowes Rickman, C. (2018). Complement Factor H in AMD: Bridging Genetic Associations and Pathobiology. Prog. Retin. Eye Res. 62, 38–57. doi:10.1016/j.preteyeres.2017.09.001
van der Merwe, Y., Faust, A. E., Sakalli, E. T., Westrick, C. C., Hussey, G., Chan, K. C., et al. (2019). Matrix-bound Nanovesicles Prevent Ischemia-Induced Retinal Ganglion Cell Axon Degeneration and Death and Preserve Visual Function. Scientific Rep. 9 (1), 1–15. doi:10.1038/s41598-019-39861-4
Vicencio, J. M., Yellon, D. M., Sivaraman, V., Das, D., Boi-Doku, C., Arjun, S., et al. (2015). Plasma Exosomes Protect the Myocardium from Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 65 (15), 1525–1536. doi:10.1016/j.jacc.2015.02.026
Volarevic, V., Gazdic, M., Simovic Markovic, B., Jovicic, N., Djonov, V., and Arsenijevic, N. (2017). Mesenchymal Stem Cell-Derived Factors: Immuno-Modulatory Effects and Therapeutic Potential. Biofactors 43 (5), 633–644. doi:10.1002/biof.1374
Wang, A. L., Lukas, T. J., Yuan, M., Du, N., Tso, M. O., and Neufeld, A. H. (2009). Autophagy and Exosomes in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PloS one 4 (1), e4160. doi:10.1371/journal.pone.0004160
Weinreb, R. N., Aung, T., and Medeiros, F. A. (2014). The Pathophysiology and Treatment of Glaucoma. Jama 311 (18), 1901–1911. doi:10.1001/jama.2014.3192
Wen, D., Peng, Y., Liu, D., Weizmann, Y., and Mahato, R. I. (2016). Mesenchymal Stem Cell and Derived Exosome as Small RNA Carrier and Immunomodulator to Improve Islet Transplantation. J. Controlled Release 238, 166–175. doi:10.1016/j.jconrel.2016.07.044
West-Mays, J. A., and Dwivedi, D. J. (2006). The Keratocyte: Corneal Stromal Cell with Variable Repair Phenotypes. Int. J. Biochem. Cel Biol. 38 (10), 1625–1631. doi:10.1016/j.biocel.2006.03.010
Williams, R., Lace, R., Kennedy, S., Doherty, K., and Levis, H. (2018). Biomaterials for Regenerative Medicine Approaches for the Anterior Segment of the Eye. Adv. Healthc. Mater. 7 (10), 1701328. doi:10.1002/adhm.201701328
Wong, T. Y., larsen, M., Cheung, C. M. G., Larsen, M., Sharma, S., and Simó, R. (2016). Diabetic Retinopathy. Nat. Rev. Dis. Primers 2, 16012. doi:10.1038/nrdp.2016.12
Wong, W. L., Su, X., Li, X., Cheung, C. M. G., Klein, R., Cheng, C.-Y., et al. (2014). Global Prevalence of Age-Related Macular Degeneration and Disease burden Projection for 2020 and 2040: a Systematic Review and Meta-Analysis. Lancet Glob. Health 2 (2), e106–e116. doi:10.1016/s2214-109x(13)70145-1
Xu, W., Wu, Y., Hu, Z., Sun, L., Dou, G., Zhang, Z., et al. (2019). Exosomes from Microglia Attenuate Photoreceptor Injury and Neovascularization in an Animal Model of Retinopathy of Prematurity. Mol. Ther. - Nucleic Acids 16, 778–790. doi:10.1016/j.omtn.2019.04.029
Yam, G. H., Peh, G. S., Singhal, S., Goh, B. T., and Mehta, J. S. (2015). Dental Stem Cells: a Future Asset of Ocular Cell Therapy. Expert Rev. Mol. Med. 17, e20. doi:10.1017/erm.2015.16
Yamashita, K., Inagaki, E., Hatou, S., Higa, K., Ogawa, A., Miyashita, H., et al. (2018). Corneal Endothelial Regeneration Using Mesenchymal Stem Cells Derived from Human Umbilical Cord. Stem Cell Dev. 27 (16), 1097–1108. doi:10.1089/scd.2017.0297
Yang, J. J., Yang, Y., Zhang, C., Li, J., and Yang, Y. (2020). Epigenetic Silencing of LncRNA ANRIL Enhances Liver Fibrosis and HSC Activation through Activating AMPK Pathway. J. Cel Mol Med 24 (4), 2677–2687. doi:10.1111/jcmm.14987
Yao, L., Li, Z.-r., Su, W.-r., Li, Y.-p., Lin, M.-l., Zhang, W.-x., et al. (2012). Role of Mesenchymal Stem Cells on Cornea Wound Healing Induced by Acute Alkali Burn. PLoS one 7 (2), e30842. doi:10.1371/journal.pone.0030842
Ye, J., Yao, K., and Kim, J. C. (2006). Mesenchymal Stem Cell Transplantation in a Rabbit Corneal Alkali Burn Model: Engraftment and Involvement in Wound Healing. Eye 20 (4), 482–490. doi:10.1038/sj.eye.6701913
Yeo, R. W. Y., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal Stem Cell: an Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 65 (3), 336–341. doi:10.1016/j.addr.2012.07.001
Yi, T., and Song, S. U. (2012). Immunomodulatory Properties of Mesenchymal Stem Cells and Their Therapeutic Applications. Arch. Pharm. Res. 35 (2), 213–221. doi:10.1007/s12272-012-0202-z
Yoles, E., and Schwartz, M. (1998). Degeneration of Spared Axons Following Partial white Matter Lesion: Implications for Optic Nerve Neuropathies. Exp. Neurol. 153 (1), 1–7. doi:10.1006/exnr.1998.6811
Yu, B., Shao, H., Su, C., Jiang, Y., Chen, X., Bai, L., et al. (2016). Exosomes Derived from MSCs Ameliorate Retinal Laser Injury Partially by Inhibition of MCP-1. Sci. Rep. 6 (1), 34562–34612. doi:10.1038/srep34562
Zhang, W., Dong, X., Wang, T., and Kong, Y. (2019). Exosomes Derived from Platelet-Rich Plasma Mediate Hyperglycemia-Induced Retinal Endothelial Injury via Targeting the TLR4 Signaling Pathway. Exp. Eye Res. 189, 107813. doi:10.1016/j.exer.2019.107813
Zhang, W., Wang, Y., and Kong, Y. (2019). Exosomes Derived from Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation via Targeting HMGB1. Invest. Ophthalmol. Vis. Sci. 60 (1), 294–303. doi:10.1167/iovs.18-25617
Keywords: ocular diseases, mesenchymal stem cells, exosomes, regenerative medicine, ncRNAs
Citation: Rad LM, Yumashev AV, Hussen BM, Jamad HH, Ghafouri-Fard S, Taheri M, Rostami S, Niazi V and Hajiesmaeili M (2022) Therapeutic Potential of Microvesicles in Cell Therapy and Regenerative Medicine of Ocular Diseases With an Especial Focus on Mesenchymal Stem Cells-Derived Microvesicles. Front. Genet. 13:847679. doi: 10.3389/fgene.2022.847679
Received: 03 January 2022; Accepted: 28 February 2022;
Published: 29 March 2022.
Edited by:
Shigeo Yoshida, Kurume University, JapanReviewed by:
Sina Naserian, Hôpital Paul Brousse, FranceHaichao Wei, University of Texas Health Science Center at Houston, United States
Copyright © 2022 Rad, Yumashev, Hussen, Jamad, Ghafouri-Fard, Taheri, Rostami, Niazi and Hajiesmaeili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vahid Niazi, dl9uaWF6aUB5YWhvby5jb20=; Mohammadreza Hajiesmaeili, bXJoYWppZXNtYWVpbGlAc2JtdS5hYy5pcg==
 Lina Moallemi Rad1
Lina Moallemi Rad1 Bashdar Mahmud Hussen
Bashdar Mahmud Hussen Hazha Hadayat Jamad
Hazha Hadayat Jamad Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Mohammad Taheri
Mohammad Taheri Vahid Niazi
Vahid Niazi