- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Multi-Modal Monitoring Technology for Severe Cerebrovascular Disease of Human Engineering Research Center, Xiangya Hospital, Central South University, Changsha, China
Background: The relationship between methylenetetrahydrofolate reductase (MTHFR) gene C677T and A1298C polymorphism with the risk of intracerebral hemorrhage (ICH) has remained to be controversial in recent years. This meta-analysis is aimed to confirm the association of these.
Methods: Systematically searching the related studies from the PubMed, Embase, Cochrane Library, China national knowledge internet database from 1 January 1990 to 1 June 2022. The odd ratio (ORs) and 95% confidence interval (CIs) of gene-disease correlation in various gene models were calculated by fixed or random effect model of meta-analysis. We included 20 case-control studies in this meta-analysis with a total of 1,989 ICH patients and 4,032 health controls originated from Asian, Caucasian, and African populations.
Results: The statistical analysis demonstrated the association of MTHFR C677T gene polymorphism with ICH in allele model [ORT VS. C = 1.20 (95%CI: 1.06–1.36)]; homozygote model [OR TT VS. CC = 1.50 (95%CI: 1.20–1.88)]; dominant model [OR CT+ TT VS. CC = 1.23 (95%CI: 1.03–1.48)] and recessive model [ORTT VS. CT+CC = 1.37 (95%CI: 1.17–1.60)]. Besides, we also found the relationship of MTHFR C677T gene polymorphism with Asian in four comparison model (ORT VS. C = 1.19.95%CI:1.09–1.37, ORTT VS. CC = 1.46.95%CI: 1.15–1.85, OR CT+ TT VS. CC = 1.25.95%CI: 1.01–1.54, ORTT VS. CT+CC = 1.34.95%CI: 1.54–1.17) and Caucasian in four comparison model (ORT VS. C = 1.90.95%CI: 1.22–2.97, ORTT VS. CC = 2.67.95%CI: 1.42–5.00, OR CT+ TT VS. CC = 1.56.95%CI: 1.05–2.32, ORTT VS. CT+CC = 2.25.95%CI: 1.46–4.00). But no statistically significant correlation between A1298C polymorphism and the occurrence of ICH was detected in four studies.
Conclusion: MTHFR C677T gene polymorphism increases the risk of ICH in Asian and Caucasian populations but has no impact on the incidence in African communities. More importantly, the risk of ICH increases in TT genotype individuals in comparison to CT and CC genotype individuals in Asian and Caucasian populations.
Introduction
ICH is a common acute cerebrovascular disease with much higher mortality rate than that of ischemic stroke (van Asch et al., 2010; Krishnamurthi et al., 2013; Krishnamurthi et al., 2020), accounted for 10–30% of strokes with incidence about 24/100,000 people per year (van Asch et al., 2010; Krishnamurthi et al., 2020) and prevalence about 300/100,000 people per year (Krishnamurthi et al., 2020). ICH is a serious threat to physical and mental health which brings huge economic burden to healthcare systems across human societies (Feigin et al., 2009; Krishnamurthi et al., 2013). Generally accepted risk factors for ICH include hypertension, use of anticoagulants or antiplatelet agents, cerebral arteriovenous malformations, cerebral amyloidosis, smoking, excessive drinking, and other environmental and generic factors (An et al., 2017). Commensurate with its implication in health and economy, more and more studies on the occurrence mechanism of ICH have shown that genes play a very important role in the pathogenesis of ICH (Chen et al., 2018; Wahab et al., 2019). However, the effect of gene polymorphism on ICH remains controversial.
MTHFR is an important enzyme in the regulation of plasma homocysteine level. Under normal physiological conditions, MTHFR catalyzes the reduction of 5, 10-methylene tetrahydrofolic acid to 5-methyl tetrahydrofolic acid and the resulting 5-methylenetetrahydrofolate is a source of methyl for the conversion of homocysteine to methionine (Goyette et al., 1994; Ogino and Wi lson, 2003). The encoded gene of MTHFR is located at 1p36.3. MTHFR C677T gene polymorphism causes valine to be replaced by alanine, leading to the decrease of MTHFR activity and the increase of plasma homocysteine concentration (Munshi et al., 2008; Zhu et al., 2015; Chen et al., 2018). High plasma homocysteine concentration in humans contributes to accelerated atherosclerosis, as well as excessive inflammation, long-term endothelial pressure, and increased plaque rupture, all of which increase susceptibility to ICH (Lai and Kan, 2015; Vacek et al., 2015; Zhou et al., 2018). In addition, previous studies have indicated that hyperhomocysteinemia is a risk factor for coronary artery disease, peripheral vascular disease, venous thrombosis and other vascular diseases (Veeranna et al., 2011; Vacek et al., 2015; Zhu et al., 2015; Zhou et al., 2018). In addition to C677T, A1298C is another common gene polymorphism in MTHFR with locus at rs1801131. The main pathological change of exon seven is the replacement of glutamate by alanine, which leads to the decrease of MTHFR activity in human body. In comparison to the C677T gene polymorphism, the MTHFR activity resulting from A1298C polymorphism is relatively higher (Viel et al., 1997; Weisberg et al., 1998).
Previously, some meta-analyses explored the relationship between MTHFR C677T or A1298C gene polymorphism and ischemic stroke, hemorrhagic stroke or coronary artery disease (Lv et al., 2013; Luo et al., 2018; Wang et al., 2021). However, there are only few meta-analyses to explore the relationship between MTHFR C677T and A1298C gene polymorphisms and ICH. Moreover, with the continuous emergence of emerging studies, there are some disputes between MTHFR C677T gene polymorphism and ICH. Recent studies in Morocco (Abidi et al., 2018), India (Somarajan et al., 2011; Sagar et al., 2018) and other countries reported contradictive results that the genetic MTHFR C677T polymorphism is not related to ICH. Interestingly, a Zambian study showed no C677TTT genotype in their population (Atadzhanov et al., 2013). Related to C677T polymorphism, significant association between the A1298C polymorphism of MTFHR and the risk of ischemic stroke in Asian population (Kang et al., 2014; Zhang et al., 2014; Kumar et al., 2020) has also been reported although no correlation studies on the susceptibility of A1298C polymorphism to ICH has been published. Therefore, we included emerging studies to update the relationship between MTHFR C677T gene polymorphism and intracerebral hemorrhage, and analyzed the impact of MTHFR C677T gene polymorphism on intracerebral hemorrhage among different populations and different regions. Besides, we also explore the relationship between MTHFR A1298C gene polymorphism with intracerebral hemorrhage.
Methods
Database search
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines. We systematically searched the studies published in PubMed, Embase, Cochrane Library, China national knowledge internet (CNKI) and other databases from 1 January 1990 to 1 June 2022 regarding the correlation between MTHFR C677T and A1298C polymorphism and ICH. The retrieval strategies: “methylene tetrahydrofolate reductase” or “methyl tetrahydrofolate reductase” or “MTHFR” and “polymorphism” or “mutation” or “genotype” or “A1298C” or “C677T” and “cerebral hemorrhage” or “intracerebral hemorrhage “or “hemorrhagic stroke” or “ICH” or “cerebrovascular disease” were executed for the search. The research population is limited to “human”. And the publication language is only allowed to include Chinese and English. Moreover, manual searches of the reference lists of retrieved study, review articles, and previous meta-analyses were performed to collect more relevant studies that were omitted during electronic database retrieval. Our review of literature inclusion consists of three main steps, first on the title of the study, then on the abstract, and finally on the complete text. If there is a dispute, discuss it in depth. As all data in this study are from published studies, no additional ethical approval is required.
Eligibility criteria
Inclusion criteria: 1) a case-control study investigating the correlation between MTHFR C677T or A1298C gene polymorphism and ICH; 2) ICH was clinically confirmed by clinical and computed tomography or magnetic resonance imaging scans; 3) Genotype frequencies are available to estimate the odds ratio of the 95% confidence interval. Exclusion criteria were: 1) Duplicate publications with overlapping topics in the same study; 2) No available data were reported; 3) low-quality studies with a quality score (Newcastle-Ottawa Scale) below 4.
Data extraction
According to the above inclusion and exclusion criteria, two investigators independently extracted data from the included literature. The extracted information includes the first author’s name, published journal, published year, country, race, an average age of population, number of case group and control group, genotyping method, A1298C and C677T genotype distribution and allele frequency. The allele frequency distribution was calculated by Hardy Weinberg equilibrium (HWE) (Wang and Shete, 2017) in studies which did not provide allele frequency information.
Quality assessment
The quality of the included study was evaluated by Newcastle-Ottawa Scale (Stang, 2010) and was assessed from three aspects: population selection, comparability between groups, and measurement of exposure factors. The score ranged from 0 to 9, a score of 0–3 is considered as in poor quality, and a score of seven or above is considered as in high quality. Investigators strictly control the quality of articles included.
Statistical analysis
We assessed the association between the allelic model (T versus C), homozygous model (TT versus CC), heterozygous model (CT versus CC), recessive model (TT versus TC + CC), and dominant model (TC + TT versus CC) alleles of MTHFR C677T and A1298C and the hazard of ICH. Using a fixed effect model (Mantel-Haenszel method) or random effect model (DerSimonian and Laird’s method) to calculate the odds ratio (ORs) of 95%CI (Mantel and Haenszel, 1959; DerSimonian, 1996). Heterogeneity between studies was compared using Cochran’s Q statistics and I2 measures (Higgins and Thompson, 2002; Higgins et al., 2003). I2 was used to assess the degree of heterogeneity between the included studies, in which 0–25% indicated no heterogeneity, a higher value indicated increased heterogeneity, 25–50% was considered as low, 50–75% medium, and 75–100% high. If the heterogeneity is high, we will conduct subgroup analysis and meta regression to analyze the source of heterogeneity.
In order to verify the reliability of the results of the meta-analysis, sensitivity analysis was performed by case-by-case exclusion (Tobias, 1999). Furthermore, we conducted HWE in the control group and observed changes in sensitivity by excluding studies that did not conform to HWE. In terms of publication bias, the Begg funnel graph was used for evaluation and the Egger graph for verification (Egger et al., 1997). All statistical analyses were performed with STATA 14.0, and all p values were bilateral. When p < 0.05, it was considered statistically significant.
Results
Literature search
146 studies were preliminarily searched in PubMed, Embase, Cochrane Library and CNKI databases. After screening, we excluded 90 studies that were not strongly relevant to this study, 14 studies that did not have abstracts in English, and 12 meta-analyses and reviews. Then thirty studies were left. Furthermore, we conducted an in-depth review of the full text of the 30 studies, excluding five of them with insufficient information or low quality. Finally, 25 studies were included for this meta-analysis (as showed in Figure 1).
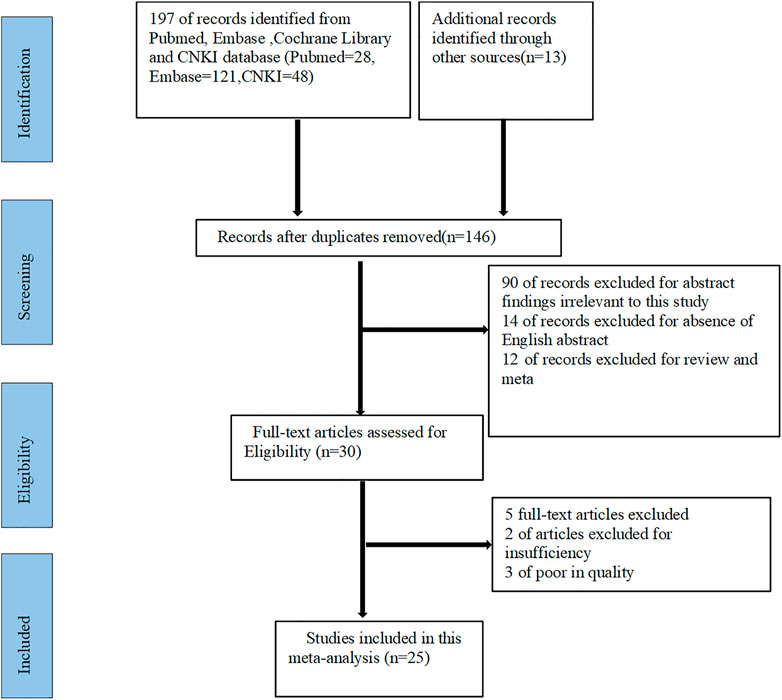
FIGURE 1. Flow diagram for the selection of studies in this meta-analysis. CNKI: China national knowledge internet.
Study characteristics
The basic features of the 25 case-control studies (Nakata et al., 1998; Zheng et al., 2000; Yingdong et al., 2002; Li et al., 2003; Zhang et al., 2004; Yan et al., 2004; Fang and Wu, 2004; Ye et al., 2004; Żur-Wyrozumska et al., 2017; Fang et al., 2005; Dikmen et al., 2006; Sazci et al., 2006; Hu et al., 2007; Shen et al., 2007; Zhang et al., 2008; Ruigrok et al., 2010; Hultdin et al., 2011; Somarajan et al., 2011; Atadzhanov et al., 2013; Rui-Rui et al., 2013; Das et al., 2015; Shao et al., 2016; Hu et al., 2016; Abidi et al., 2018; Jiang et al., 2018) included are shown in Table 1. The incorporated studies ranged from 1998 to 2018, involving mainly Asian populations and some European and African communities. Three different races were involved in these 25 selected studies: two (Atadzhanov et al., 2013; Abidi et al., 2018) African, three (Dikmen et al., 2006; Sazci et al., 2006; Hultdin et al., 2011) Caucasian and the remaining 17 (Nakata et al., 1998; Zheng et al., 2000; Yingdong et al., 2002; Zhang et al., 2004; Yan et al., 2004; Fang and Wu, 2004; Ye et al., 2004; Żur-Wyrozumska et al., 2017; Hu et al., 2007; Zhang et al., 2008; Rui-Rui et al., 2013; Shao et al., 2016; Hu et al., 2016; Jiang et al., 2018) Asian. The control population in three of the 25 studies (Li et al., 2003; Shen et al., 2007; Das et al., 2015) did not meet Hardy-Weinberg equilibrium and be excluded. One study due to high heterogeneity (Fang et al., 2005) and one study of rebleeding after secondary subarachnoid hemorrhage (Ruigrok et al., 2010) were excluded (Table 2). All the 20 included studies had moderately high-quality scores. In four of the 20 studies (Dikmen et al., 2006; Sazci et al., 2006; Hultdin et al., 2011; Abidi et al., 2018), A1298C and C677T gene polymorphism and ICH were simultaneously studied, and a total of 3,280 patients with ICH and 9,324 controls were included in this meta-analysis.
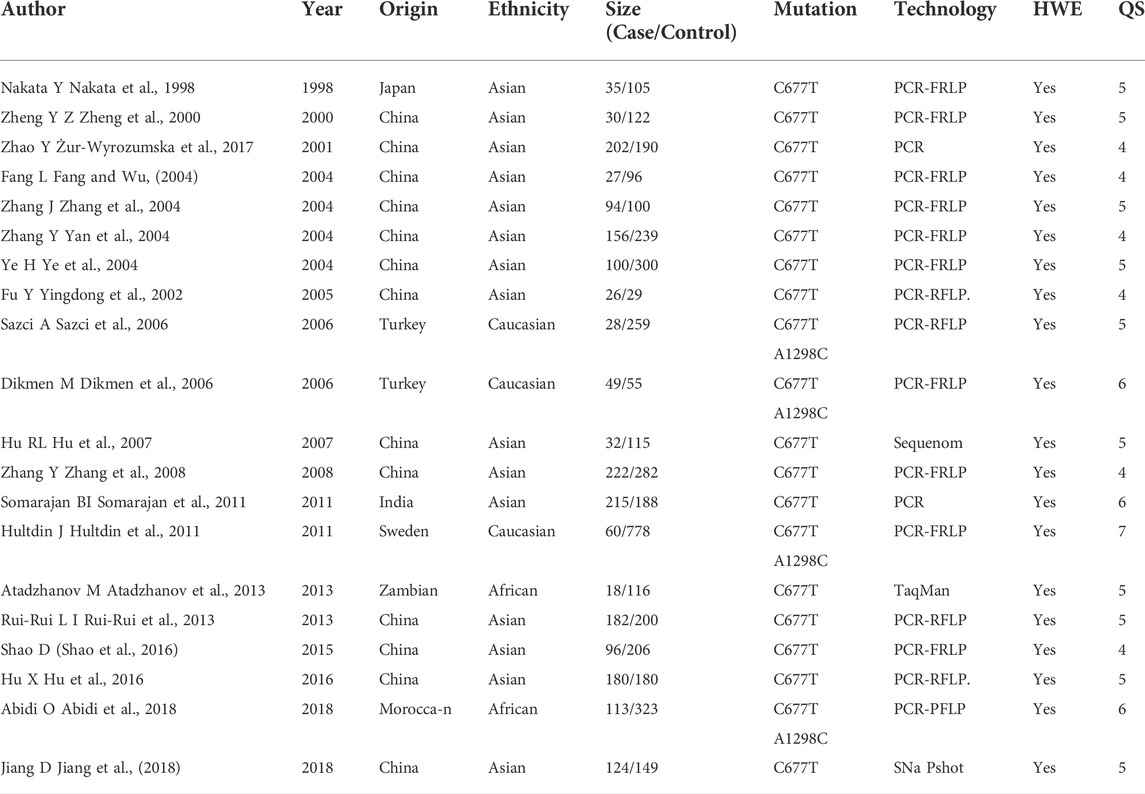
TABLE 1. The characteristics of all studies in the meta-analysis of the association between MTHFR gene C677T and A1298C polymorphisms with the risk of intracerebral hemorrhage. PCR-RFLP = Polymerase Chain Reaction-Restriction Fragment Length Polymorphism. SNP = Single Nucleotide Polymorphism. HWE = Hardy Weinberg Equilibrium. QS = Quality score.

TABLE 2. Information for some strong correlation research not included in the data collection process.
The association of methylenetetrahydrofolate reductase C677T gene polymorphism with the risk of intracerebral hemorrhage
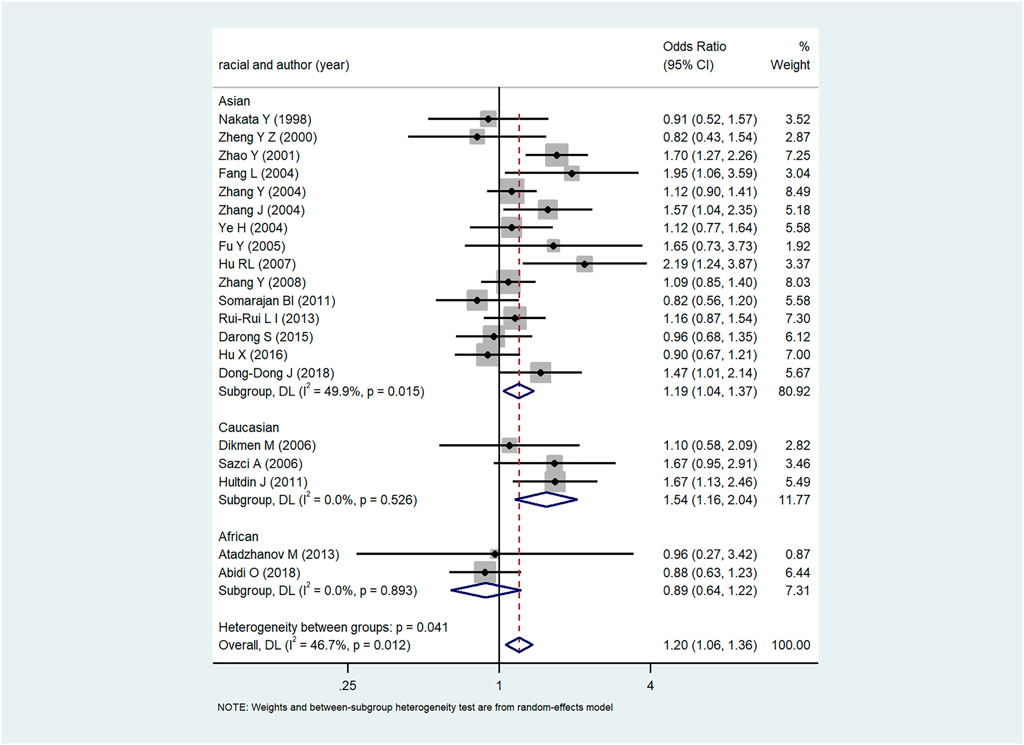
The forest plot of the relationship between MTHFR C667T gene polymorphism and ICH in various gene models were demonstrated in Figure 2 and Supplementary Figure S1 with the OR and p-values of each gene model shown in Table 3. In each gene model, a significant association was observed between MTHFR C677T gene polymorphism and the risk of ICH. The results of subgroup analysis by ethnicity varied with the risk of ICH in the study populations. MTHFR C677T gene polymorphism in the dominant model [OR CT+ TT VS. CC = 1.23 (95%CI: 1.03–1.48)], allele model [OR = 1.20 (95%CI: 1.06–1.36)], homozygous model [OR = 1.50 (95%CI: 1.20–1.88)] and recessive model [OR = 1.37 (95%CI: 1.17–1.60] all revealed significant association with the risk of ICH. In addition, four comparison model (ORT VS. C = 1.19.95%CI:1.09–1.37, ORTT VS. CC = 1.46.95%CI: 1.15–1.85, OR CT+ TT VS. CC = 1.25.95%CI: 1.01–1.54, ORTT VS. CT+CC = 1.34.95%CI: 1.54–1.17) also significantly related to Asian population. Likewise, in Caucasian population, allele model [(OR = 1.90 (95%CI: 1.22–2.97)] homozygous model [OR = 2.67 (95%CI: 1.42–5.00)], recessive model [OR = 2.25 (95%CI: 1.46–4.00)] and dominant model [OR CT+ TT VS. CC = 1.56.95%CI: 1.05–2.32)] showed significant association with the risk of ICH, while the association in heterozygous model [OR = 1.39 (95%CI:0.91–2.13)], were not evidenced. In African population, no substantial correlation between MTHFR C667T gene polymorphism and ICH was observed in any one of the five models with heterozygote model [OR = 0.86 (95%CI: 0.56–1.32)], dominant model [OR = 0.85 (95%CI: 0.57–1.28), allele model [OR = 0.89 (95%CI: 0.64–1.22)], homozygous model [OR = 0.82 (95%CI: 0.39–1.73)] and recessive model [OR = 0.89 (95%CI: 0.43–1.81)].
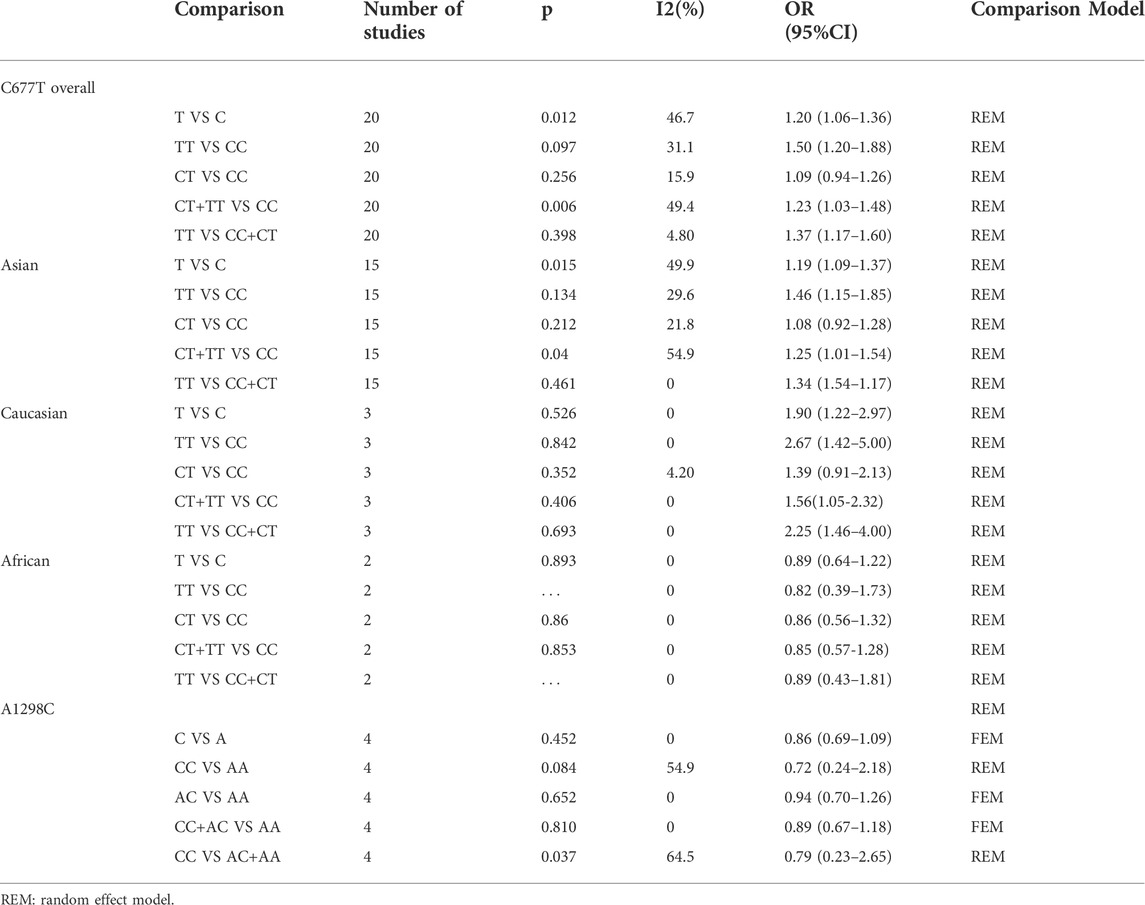
TABLE 3. Results of association between C677T and A1298C with intracerebral hemorrhage in this meta-analysis.
Publication bias
In this paper, Begg and Egger funnel plots were applied to assess the publication bias of the 20 included studies. No significant asymmetry was observed in any gene model by either of our two researchers (Figure 3 and Supplementary Figure S2). The p-values of Begg and Egger’s test in all the five gene models were all over 0.05 with the dominant model (P Begg = 0.098, P Egger = 0.149), homozygous model (P Begg = 0.294, P Egger = 0.183), heterozygous model (P Begg = 0.098, P Egger = 0.200), allelic model (P Begg = 0.381, P Egger = 0.398), recessive model (P Begg = 0.162, P Egger = 0.264), which confirmed the absence of obvious publication bias in the 20 included studies.
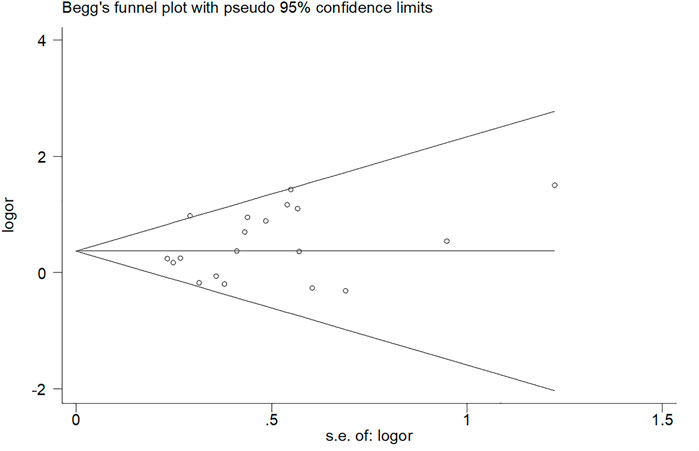
FIGURE 3. Begg’s funnel plot of the relationship between MTHFR gene (C677T) polymorphism and intracerebral hemorrhage risk.
Sensitivity analyses
A sensitivity analysis of the 20 included studies was performed to assess the impact of each study on ORs inclusion by sequentially omitting individual inclusion studies. No significant change in OR value was observed in the process of removing the study one by one. Therefore, overall sensitivity analysis reinforced that the results of this meta-analysis are statistically reliable. The correlation of MTHFR gene C677T polymorphism with the risk of ICH in this meta-analysis was validated by the following meta regression analysis of the selected 21 studies, i.e., published year (p = 0.086), the quality score (p = 0.795), race [Caucasian populations (p = 0.100), African populations (p = 0.243), the Asian population (p = 0.931)] as a covariate meta regression analysis, with all the p-values above 0.05 which indicated no statistically significant. And the subgroup analysis did not find obvious source of heterogeneity.
The association of methylenetetrahydrofolate reductase A1298C gene polymorphism with the risk of intracerebral hemorrhage
At present, there are still few studies on the relationship between MTHFR A1298C gene polymorphism and the risk of ICH only in Caucasian and African populations. Therefore, only four related studies (Dikmen et al., 2006; Sazci et al., 2006; Hultdin et al., 2011; Abidi et al., 2018) were included in the current paper. The meta-analysis results of the four studies were shown in Table 3 and Supplementary Figure S3 with corresponding OR and 95%CI for the five gene models as follows—heterozygous model [OR = 0.94 (95%CI: 0.7–1.26)], dominant model [OR = 0.87 (95%CI: 0.67–1.18), homozygous model [OR = 0.72 (95%CI: 0.24–2.18), recessive model [OR = 0.64 (95%CI: 0.34–1.2)] and allele model [OR = 0.86 (95%CI: 0.69–1.09)]. Hence, no significant correlation between MTHFR A1298C allele polymorphism and ICH was detected in any one of the five gene models in Caucasian and African communities. Furthermore, no obvious publication bias was found in Begg funnel chart (Supplementary Figure S4). Considering the absence of adequate studies, sensitivity analysis, subgroup analysis or meta-regression was not conducted.
Discussion
In this meta-analysis, we included a total of 20 case-control studies, all 20 of which examined the correlation of the polymorphism of the MTHFR C677T gene with the risk of ICH (including 1,989 cases of ICH patients and 4,032 controls), while only four of the 20 studies interrogated the relationship of MTHFR A1298C gene polymorphism with the risk of ICH (with a total of 250 cases of ICH and 1,415 controls included). The results of the meta-analysis revealed significant association between MTHFR C677T gene polymorphism and risk of ICH under all the four genetic models in Asian populations. Funnel figure, subgroup analysis and sensitivity analysis all confirmed the reliability of the results. A subgroup analysis and a meta regression was performed to analyze the source of the heterogeneity, but no covariate was found to attribute to the source of heterogeneity.
Subgroup analysis based on ethnic classification uncovered a strong association between MTHFR C677T gene polymorphism and the risk of ICH in Asian, and Caucasians populations, while no association was found in Africans. What’s more, compared with genotype CC or, CT, TT genotype substantially increases the susceptibility to ICH with OR TT VS. CC = 1.50 (95%CI: 1.20–1.88) and ORTT VS. CT+CC = 1.37 (95%CI: 1.17–1.60). Considering the influence of MTHFR C677T gene polymorphism on homocysteine level, individuals with TT genotype have a higher risk of ICH which may be related to the significantly increased level of homocysteine. The revealed correlation provides a logic scientific basis for further in-depth study of gene polymorphism and molecular epidemiology of ICH.
The T-allele of methylene tetrahydrofolate reductase C677T can increase homocysteine levels in human body to a mild to moderate level (Cronin et al., 2005; Holmes et al., 2011). Artificially induced hyperhomocysteinemia mice were significantly more vulnerable to vascular inflammation, atherosclerosis, and hypercoagulability (Hofmann et al., 2001; Zhou et al., 2008; Veeranna et al., 2011; Vacek et al., 2015; Zhou et al., 2018). Likewise, MTHFR gene polymorphism also increases the risk of ICH by affecting the blood clotting function via hyperhomocysteinemia which accelerates the activation of coagulation factors V,X, and XII and rises the risk of arterial thrombosis and atherosclerotic cerebrovascular diseases of large and small arteries (Diaz-Arrastia, 2000; Kelly et al., 2002; Dikmen et al., 2006). Thinking on, investigations on reduction the level of homocysteine via intentionally ingestion of additional folic acid to mitigate the risk of ICH and coronary heart disease have been implemented. However, the clinical efficacy of folic acid on alleviation of ICH risk remains contentious (Lewis et al., 2005; Aléssio et al., 2011). Considering the common occurrence of T allele of C677T in Asian and Caucasian populations, the importance of correlation investigations for the prediction, prevention and treatment of ICH in these populations cannot be overlooked.
The current and previous studies (Gao et al., 2012; Kang et al., 2013; Zhao and Jiang, 2013; Kang et al., 2014) on the association between MTHFR gene polymorphism and the risk of ICH were mainly concentrated on C677T polymorphism in Asian populations. No research on the influence of A1298C polymorphism on the danger of ICH has been published in Asian populations. Consequently, only four studies were included in this paper involving the association of MTHFR A1298C polymorphism with ICH in Caucasian and African populations. Considering the potential correlation of A1298C gene polymorphism with the susceptibility to ICH, further studies are warranted, especially for the Asian populations with high frequency of occurrence.
Compared with the previous meta-analysis (Gao S, et al., 2012), which only focused on MTHFR C677T, this systematic review and meta-analysis analyzed the relationship between MTHFR A1298C gene polymorphism and intracerebral hemorrhage. We also included emerging studies to update the relationship between MTHFR C677T gene polymorphism and intracerebral hemorrhage, and analyzed the impact of MTHFR C677T gene polymorphism on intracerebral hemorrhage among different populations and different regions. In our study, we found that MTHFR C677T gene polymorphism increases the risk of ICH in Asian and Caucasian populations but has no impact on the incidence in African communities. More importantly, the risk of ICH increases in TT genotype individuals in comparison to CT and CC genotype individuals in Asian populations. Therefore, our study not only revealed the important role of MTHFR A1298C in the prevention and treatment of ICH, but also indicated that the populational specific strategies for ICH prevention via MTHFR C677T and A1298C should be considered in Asians and Caucasians instead of Africans firstly.
Due to availability of data, there are several deficiencies in this research. First of all, only a small number of studies were included, mainly on A1298C genotype, which would affect the representativeness of the results of the meta-analysis in this part. Although we searched a large number of databases, only four relevant literatures were found. Secondly, all the included studies were case-control studies, which were basically retrospective, and there was probable bias caused by partial design when comparing with prospective studies. Moreover, some gene models showed moderate to low degree of heterogeneity, but meta-regression and subgroup analysis based on publication year, ethnicity, and quality score could not designate the source of heterogeneity, which may be related to study design, genetic and environmental interaction, and the number of included patients. Finally, we have not registered this search protocol in an online database such as PROSPERO.
In summary, this meta-analysis confirmed that MTHFR C677T gene polymorphism is related to the risk of ICH, mainly in Asian populations, and that TT genotype individuals have a higher risk of ICH than CC and CT genotypes. No substantial correlation of MTHFR A1298C polymorphisms with the risk of ICH were found. The high incidence of ICH in Asian population may be related to the polymorphism of MTHFR C677T gene, which may be a predictor for the susceptibility of ICH in Asian. The detection of MTHFR C677T gene polymorphism in clinical will help to predict, prevent and reduce the pathological cases of cerebral hemorrhage in Asian population. Further studies are needed to clarify the prevention of ICH in Asian population targeting MTHFR C677T gene polymorphism.
Main messages
1) MTHFR is an important enzyme in the regulation of plasma homocysteine level. High plasma homocysteine concentration in humans contributes to increase susceptibility to ICH.
2) MTHFR C677T gene polymorphism is a biomarker gene of ICH in Asian and Caucasian populations, which has guiding significance for the prevention of ICH.
3) The risk of ICH increases in C677TTT genotype individuals in comparison to CT and CC genotype individuals in Asian and Caucasian populations.
Current research questions
1) Are C677T and A1298C gene polymorphisms of MTHFR associated with the risk of ICH?
2) Is there population difference in the effect of MTHFR gene polymorphism on the risk of ICH?
3) Are the different alleles of C677T and A1298C responsible for the difference in the risk of ICH in different populations? Can these allele differences become biomarkers for the prevention and treatment of ICH?
4) What is the potential mechanism of MTHFR C677T and A1298C gene polymorphisms on ICH?
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LZ and X-LZ designed the research and determined the structure of the paper. X-LZ, T-XY, LD, LC, and YL selected the references and contributed to the writing. X-LZ and T-XY collected the data. X-LZ, T-XY, and LZ helped to analyze the results of this meta-analysis. LZ contributed to the revision and finalization of the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Science & Technology Fundamental Resources Investigation Program of China to LZ (No.2018FY100900), The Hunan Provincial Natural Science Foundation of China Grant to YZ (No.2021JJ30923), The Provincial Science and Technology Innovation Leading Talents Project to LZ (No.2021RC4014), National Clinical Research Center for Geriatric Disorders (XiangYa Hospital).
Acknowledgments
We thank all researchers who have contributed to the study of the association between MTHFR gene polymorphism and intracerebral hemorrhage included in this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.829672/full#supplementary-material
Supplementary Figure S1 | Forest plot of the association between MTHFR C677T gene polymorphism and the risk of ICH in Asians, Caucasians, and Africans. (A) Allele model (T VS C) in Asians, Caucasians, and Africans. T and C are the case group and T1 and C1 are the control group. (B) Recessive gene model (TT VS CC+CT) in Asians, Caucasians, and Africans. where R=CC+CT, R1=CT1+CC1, R and CC are the case group, and R1 and CC1 are the control group. (C) Dominant gene model (TT+CT VS CC) in Asians, Caucasians, and Africans, in which TT and CC are case groups and TT1 and CC1 are control groups. (D) Heterozygote gene model (CT VS CC) in Asians, Caucasians, and Africans, in which CT and CC are case groups and CT1 and CC1 are control groups.
Supplementary Figure S2 | Sensitivity analysis of MTHFR C677T polymorphism and risk of ICH in a dominant gene model.
Supplementary Figure S3 | Forest plot of the relationship between MTHFR A1298C gene polymorphism and the risk of ICH. (A) Allele model (C VS A), where C and A are case groups and C1 and A1 are control groups.(B) Homozygous model (CC VS AA), in which CC and AA are case groups and CC1 and AA1 are control groups. (C) Dominant model (CC+AC VS AA), where F=CC+AC, F1=CC1+AC1.F and AA are case groups, and F1 and AA1 are control groups. (D) Recessive model (CCVS AA+AC), where B=AA+AC, B1=AA1+AC1.B, AA is the case group, and B1, AA1 are the control group. (E) Heterozygous model (AC VS AA), in which AC and AA are case groups and AC1 and AA1 are control groups.
Supplementary Figure S4 | The Begg funnel plot of the relationship between MTHFR A1298C gene polymorphism and the risk of ICH.
Supplementary Table S1 | Differences between other meta-analyses similar to this study.
Supplementary Table S2 | Information for some strong correlation research not included in the data collection process.
Abbreviations
MTHFR, methylenetetrahydrofolate reductase; ICH, intracerebral hemorrhage; OR, odd ratio; CI, confidence interval; CNKI, China national knowledge internet; HWE, hardy weinberg equilibrium; REM, random effect model; FEM, fixed effect model.
References
Abidi, O., Haissam, M., Nahili, H., and El Azhari, A. (2018). Methylenetetrahydrofolate reductase gene polymorphisms (C677T and A1298C) and hemorrhagic stroke in Moroccan patients. J. Stroke Cerebrovasc. Dis. 27 (7), 1837–1843. doi:10.1016/j.jstrokecerebrovasdis.2018.02.029
Aléssio, A. C., Santos, C. X., Debbas, V., Oliveira, L. C., Haddad, R., Annichino-Bizzacchi, J. M., et al. (2011). Evaluation of mild hyperhomocysteinemia during the development of atherosclerosis in apolipoprotein E-deficient and normal mice. Exp. Mol. Pathol. 90 (1), 45–50. doi:10.1016/j.yexmp.2010.07.008
An, S. J., Kim, T. J., and Yoon, B. W. (2017). Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke 19 (1), 3–10. doi:10.5853/jos.2016.00864
Atadzhanov, M., Mwaba, M. H., Mukomena, P. N., Lakhi, S., Rayaprolu, S., Ross, O. A., et al. (2013). Association of the APOE, MTHFR and ACE genes polymorphisms and stroke in Zambian patients. Neurol. Int. 5 (4), e20. doi:10.4081/ni.2013.e20
Chen, Y. C., Chang, K. H., and Chen, C. M. (2018). Genetic polymorphisms associated with spontaneous intracerebral hemorrhage. Int. J. Mol. Sci. 19 (12), 3879. doi:10.3390/ijms19123879
Cronin, S., Furie, K. L., and Kelly, P. J. (2005). Dose-related association of MTHFR 677T allele with risk of ischemic stroke: Evidence from a cumulative meta-analysis. Stroke 36 (7), 1581–1587. doi:10.1161/01.STR.0000169946.31639.af
Das, S., Roy, S., Kaul, S., and Jyothy, A. (2015). MTHFR gene (C677t) polymorphism in ischemic stroke, its subtypes and hemorrhagic stroke in a south Indian population[J]. Acta Medica Int. 2 (2), 28–33. doi:10.5530/ami.2015.2.7
DerSimonian, R. (1996). Meta-analysis in the design and monitoring of clinical trials. Stat. Med. 15 (12), 1237–1248. doi:10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N
Diaz-Arrastia, R. (2000). Homocysteine and neurologic disease. Arch. Neurol. 57 (10), 1422–1427. doi:10.1001/archneur.57.10.1422
Dikmen, M., Ozbabalik, D., Gunes, H. V., Degirmenci, I., Bal, C., Ozdemir, G., et al. (2006). Acute stroke in relation to homocysteine and methylenetetrahydrofolate reductase gene polymorphisms. Acta Neurol. Scand. 113 (5), 307–314. doi:10.1111/j.1600-0404.2005.00556.x
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Fang, L., Wu, Y. Q., and Wang, T. W. (2004). Correlation of polymorphism of gene methylenetetrahydrofolate reductase and cystathionine beta-synthase with heredity of cerebral infarction and cerebral hemorrhage in northern Chinese Han people. Chin. J. Clin. Rehabil. 8, 4654–4656.
Fang, X., Namba, H., Akamine, S., and Sugiyama, K. (2005). Methylenetetrahydrofolate reductase gene polymorphisms in patients with cerebral hemorrhage. Neurol. Res. 27 (1), 73–76. doi:10.1179/016164105X18313
Feigin, V. L., Lawes, C. M., Bennett, D. A., Barker-Collo, S. L., and Parag, V. (2009). Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet. Neurol. 8 (4), 355–369. doi:10.1016/S1474-4422(09)70025-0
Gao, S., Li, H., Xiao, H., Yao, G., Shi, Y., Wang, Y., et al. (2012). Association of MTHFR 677T variant allele with risk of intracerebral haemorrhage: A meta-analysis. J. Neurol. Sci. 323 (1-2), 40–45. doi:10.1016/j.jns.2012.07.038
Goyette, P., Sumner, J. S., Milos, R., Duncan, A. M., Rosenblatt, D. S., Matthews, R. G., et al. (1994). Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nat. Genet. 7 (2), 195–200. doi:10.1038/ng0694-195
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hofmann, M. A., Lalla, E., Lu, Y., Gleason, M. R., Wolf, B. M., Tanji, N., et al. (2001). Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J. Clin. Investig. 107 (6), 675–683. doi:10.1172/JCI10588
Holmes, M. V., Newcombe, P., Hubacek, J. A., Sofat, R., Ricketts, S. L., Cooper, J., et al. (2011). Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 378 (9791), 584–594. doi:10.1016/S0140-6736(11)60872-6
Hu, R. L., Zhao, S. G., Niu, G. M., Zhang, C. Y., Hu, R. L., Wang, Z. G., et al. (2007). The association between gene polymorphisms of N5, 10-methylene tetrahydrofolate reductase(MTHFR) and Mongol nation patients with primarily hypertension disease and hypertension complicating cerebrovascular disease. Stroke Nerv. Dis. 14, 13–15. Available at: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZZYS200701003.htm.
Hu, X., Tao, C., Xie, Z., Li, Y., Zheng, J., Fang, Y., et al. (2016). The MTHFR C677T polymorphism and risk of intracerebral hemorrhage in a chinese han population. Med. Sci. Monit. 22, 127–33. doi:10.12659/msm.896315
Hultdin, J., Van Guelpen, B., Winkvist, A., Hallmans, G., Weinehall, L., Stegmayr, B., et al. (2011). Prospective study of first stroke in relation to plasma homocysteine and MTHFR 677C>T and 1298A>C genotypes and haplotypes - evidence for an association with hemorrhagic stroke. Clin. Chem. Lab. Med. 49 (9), 1555–1562. doi:10.1515/CCLM.2011.234
Jiang, D., Sheng, W., and Luo, M. (2018). Relationship between methylenetetrahydrofolate reductase gene C677T position polymorphism and intracerebral hemorrhage in southern Chinese Han population[J]. J. Clin. Neurology, 10–14. Available at: https://doc.paperpass.com/journal/20180155lcsjbxzz.html.
Kang, S., Wu, Y., Liu, L., Zhao, X., and Zhang, D. (2014). Association of the A1298C polymorphism in MTHFR gene with ischemic stroke. J. Clin. Neurosci. 21 (2), 198–202. doi:10.1016/j.jocn.2013.04.017
Kang, S., Zhao, X., Liu, L., Wu, W., and Zhang, D. (2013). Association of the C677T polymorphism in the MTHFR gene with hemorrhagic stroke: A meta-analysis. Genet. Test. Mol. Biomarkers 17 (5), 412–417. doi:10.1089/gtmb.2012.0295
Kelly, P. J., Barron, M., and Furie, K. L. (2002). Hyperhomocysteinemia, MTHFR 677C-->T polymorphism, and stroke. author reply. 33 (6), 1452–1453. MTHFR 677C-->T polymorphism, and stroke. doi:10.1161/01.str.0000018602.09015.c1
Krishnamurthi, R. V., Feigin, V. L., Forouzanfar, M. H., Mensah, G. A., Connor, M., Bennett, D. A., et al. (2013). Global burden of diseases, injuries, risk factors study 2010 (GBD 2010); GBD stroke experts group. global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. Glob. Health 1 (5), e259–81. doi:10.1016/S2214-109X(13)70089-5
Krishnamurthi, R. V., Ikeda, T., Global, Feigin V. L., Regional, , and Burden, Country-Specific (2020). Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: A systematic analysis of the global burden of disease study 2017. Neuroepidemiology 54 (2), 171–179. doi:10.1159/000506396
Kumar, A., Sharma, R., Misra, S., Nath, M., and Kumar, P. (2020). Relationship between methylenetetrahydrofolate reductase (MTHFR) gene (A1298C) polymorphism with the risk of stroke: A systematic review and meta-analysis. Neurol. Res. 42, 913–922. doi:10.1080/01616412.2020.1798107
Lai, W. K., and Kan, M. Y. (2015). Homocysteine-induced endothelial dysfunction. Ann. Nutr. Metab. 67 (1), 1–12. doi:10.1159/000437098
Lewis, S. J., Ebrahim, S., and Davey Smith, G. (2005). Davey smith G. Meta-Analysis of MTHFR 677C->T polymorphism and coronary heart disease: Does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 331 (7524), 1053. doi:10.1136/bmj.38611.658947.55
Li, Z., Sun, L., Zhang, H., Liao, Y., Wang, D., Zhao, B., et al. (2003). Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: A multicenter case-control study in China. Stroke 34 (9), 2085–2090. doi:10.1161/01.STR.0000086753.00555.0D
Luo, Z., Lu, Z., Muhammad, I., Chen, Y., Chen, Q., Zhang, J., et al. (2018). Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: A systematic review and updated meta-analysis. Lipids Health Dis. 17 (1), 191. doi:10.1186/s12944-018-0837-yPMID: 30115070; PMCID: PMC6097444
Lv, Q., Lu, J., Wu, W., Sun, H., and Zhang, J. (2013). Association of the methylenetetrahydrofolate reductase gene A1298C polymorphism with stroke risk based on a meta-analysis. Genet. Mol. Res. 12 (4), 6882–6894. doi:10.4238/2013.December.19.7PMID: 24391036
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22 (4), 719–748. doi:10.1093/jnci/22.4.719
Munshi, A., Sultana, S., Kaul, S., Reddy, B. P., Alladi, S., and Jyothy, A. (2008). Angiotensin-converting enzyme insertion/deletion polymorphism and the risk of ischemic stroke in a South Indian population. J. Neurol. Sci. 272 (1-2), 132–135. doi:10.1016/j.jns.2008.05.017
Nakata, Y., Katsuya, T., Takami, S., Sato, N., Fu, Y., Ishikawa, K., et al. (1998). Methylenetetrahydrofolate reductase gene polymorphism: Relation to blood pressure and cerebrovascular disease. Am. J. Hypertens. 11 (8 Pt 1), 1019–1023. doi:10.1016/s0895-7061(98)00046-6
Ogino, S., and Wilson, R. B. (2003). Genotype and haplotype distributions of MTHFR677C>T and 1298A>C single nucleotide polymorphisms: A meta-analysis. J. Hum. Genet. 48 (1), 1–7. doi:10.1007/s100380300000
Ou, W., Liu, X., Shen, Y., Li, J., He, L., Yuan, Y., et al. (2014). Association of CVD candidate gene polymorphisms with ischemic stroke and cerebral hemorrhage in Chinese individuals. PLoS One 9 (8), e105516. PMID: 25144711; PMCID: PMC4140791. doi:10.1371/journal.pone.0105516
Rui-Rui, L. I., Xiao, P., and Cardiology, D. O. (2013). Correlation between MTHFR gene polymorphism and cerebral hemorrhage in essential hypertensive patients[J]. Chin. J. Geriatric Heart Brain Vessel Dis. 15 (9), 941–944.
Ruigrok, Y. M., Slooter, A. J., Rinkel, G. J., Wijmenga, C., and Rosendaal, F. R. (2010). Genes influencing coagulation and the risk of aneurysmal subarachnoid hemorrhage, and subsequent complications of secondary cerebral ischemia and rebleeding. Acta Neurochir. 152 (2), 257–262. doi:10.1007/s00701-009-0505-0
Sagar, R., Kumar, A., Yadav, A. K., Raj, R., Misra, S., Shukla, G., et al. (2018). Association between methylenetetrahydrofolate reductase (MTHFR) gene polymorphism and risk of intracerebral hemorrhage (ICH) in north Indian population: A case control study. Int. J. Stroke 13, 2. Supplement 1 (100-101). doi:10.1177/1747493018789544
Sazci, A., Ergul, E., Tuncer, N., Akpinar, G., and Kara, I. (2006). Methylenetetrahydrofolate reductase gene polymorphisms are associated with ischemic and hemorrhagic stroke: Dual effect of MTHFR polymorphisms C677T and A1298C. Brain Res. Bull. 71 (1-3), 45–50. doi:10.1016/j.brainresbull.2006.07.014
Shao, D., Meng, Z., Hua, W. U., and Neurology, D. O. (2016). Association of MTHFR C677T polymorphism with ischemic and hemorrhagic stroke risks[J]. Anhui Med. J. doi:10.3969/j.issn.1000–0399.2016.04.004
Shen, C. D., Zhang, W. L., Sun, K., Wang, Y. B., Zhen, Y. S., Hui, R. T., et al. (2007). Interaction of genetic risk factors confers higher risk for thrombotic stroke in male Chinese: A multicenter case-control study. Ann. Hum. Genet. 71 (Pt 5), 620–629. doi:10.1111/j.1469-1809.2007.00364.x
Somarajan, B. I., Kalita, J., Mittal, B., and Misra, U. K. (2011). Evaluation of MTHFR C677T polymorphism in ischemic and hemorrhagic stroke patients. A case-control study in a Northern Indian population. J. Neurol. Sci. 304 (1-2), 67–70. doi:10.1016/j.jns.2011.02.010
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Tobias, A. (1999). Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 8, 15–17. Available at: https://www.researchgate.net/publication/312625232_Assessing_the_influence_of_a_single_study_in_the_meta-analysis_estimate.
Vacek, T. P., Rehman, S., Neamtu, D., Yu, S., Givimani, S., Tyagi, S. C., et al. (2015). Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 11, 173–183. doi:10.2147/VHRM.S68415
van Asch, C. J., Luitse, M. J., Rinkel, G. J., van der Tweel, I., Algra, A., Klijn, C. J., et al. (2010). Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet. Neurol. 9 (2), 167–176. doi:10.1016/S1474-4422(09)70340-0
Veeranna, V., Zalawadiya, S. K., Nirai, A., Pradhan, J., Ference, B., Burack, R. C., et al. (2011). Homocysteine and reclassification of cardiovascular disease risk. J. Am. Coll. Cardiol. 58, 1025–1033. doi:10.1016/j.jacc.2011.05.028
Viel, A., Dall'Agnese, L., Simone, F., Canzonieri, V., Capozzi, E., Visentin, M. C., et al. (1997). Loss of heterozygosity at the 5, 10-methylenetetrahydrofolate reductase locus in human ovarian carcinomas. Br. J. Cancer 75 (8), 1105–1110. doi:10.1038/bjc.1997.191
Wahab, K. W., Tiwari, H. K., Ovbiagele, B., Sarfo, F., Akinyemi, R., Traylor, M., et al. (2019). Genetic risk of Spontaneous intracerebral hemorrhage: Systematic review and future directions. J. Neurol. Sci. 407, 116526. doi:10.1016/j.jns.2019.116526
Wang, F., Xu, Z., Jiao, H., Wang, A., and Jing, Y. (2021). Associations between MTHFR gene polymorphisms and the risk of intracranial hemorrhage: Evidence from a meta-analysis, e01840. Brain Behav. 11 (1), e01840. Epub 2020 Nov 27. PMID: 33247557; PMCID: PMC7821613. doi:10.1002/brb3.1840
Wang, J., and Shete, S. (2017). Testing departure from hardy-weinberg proportions. Methods Mol. Biol. 1666, 83–115. doi:10.1007/978-1-4939-7274-6_6
Weisberg, I., Tran, P., Christensen, B., Sibani, S., and Rozen, R. (1998). A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 64 (3), 169–172. doi:10.1006/mgme.1998.2714
Yan, Z., Ruping, x., Dafang, C., and Yu, F. (2004). Association of methylenetetrahydrofolate reductase polymorphism with cerebral hemorrhage, a case-control study[J]. Beijing Med. J. 26 (4), 219–221.
Ye, H., Yan, J. T., Shao, J. M., Zhang, F., Hong, M. L., Wang, D. W., et al. (2004). A case-control study on the relationship between stroke and plasma homocysteine level and the mutation of MTHFR gene. Zhonghua Liu Xing Bing Xue Za Zhi 25 (11), 958–961. Chinese. doi:10.1016/j.csr.2003.12.006
Yingdong, Z., Zhigang, Z., and Yang, L. (2002). Association of plasma homocysteine level and N5, N10-methylenetetrahydrofolate reductase gene polymorphism with cerebral infarction. Chin. Med. Sci. J. 17 (4), 231–235. Available at: https://www.researchgate.net/publication/10626752_Association_of_plasma_homocysteine_level_and_N5N10-methylenetetrahydrofolate_reductase_gene_polymorphism_with_cerebral_infarction.
Zhang, J., Lu, L., Shi, H., Jiang, Q. Q., Weng, Q., and Jia, W. P. (2004). The relationship between MTHFR gene polymorphism and cerebral hemorrhage[J]. J. Clin. Neurology 17 (4), 267–269.
Zhang, M. J., Hu, Z. C., Yin, Y. W., Li, B. H., Liu, Y., Liao, S. Q., et al. (2014). A meta-analysis of the relationship between MTHFR gene A1298C polymorphism and the risk of adult stroke. Cerebrovasc. Dis. 38 (6), 425–432. doi:10.1159/000369122
Zhang, Y., Xie, R. P., Shen, Y., and Fan, D. S. (2008). Interaction between methylenetetrahydrofolate reductase C677T gene polymorphism and sleep duration on risk of stroke pathogenesis. Beijing Da Xue Xue Bao Yi Xue Ban. 40 (3), 262–269.
Zhao, X., and Jiang, H. (2013). Quantitative assessment of the association between MTHFR C677T polymorphism and hemorrhagic stroke risk. Mol. Biol. Rep. 40 (1), 573–578. doi:10.1007/s11033-012-2094-x
Zheng, Y. Z., Tong, J., Do, X. P., Pu, X. Q., and Zhou, B. T. (2000). Prevalence of methylenetetrahydrofolate reductase C677T and its association with arterial and venous thrombosis in the Chinese population. Br. J. Haematol. 109 (4), 870–874. doi:10.1046/j.1365-2141.2000.02112.x
Zhou, J., Werstuck, G. H., Lhoták, S., Shi, Y. Y., Tedesco, V., Trigatti, B., et al. (2008). Hyperhomocysteinemia induced by methionine supplementation does not independently cause atherosclerosis in C57BL/6J mice. FASEB J. 22 (7), 2569–2578. doi:10.1096/fj.07-105353
Zhou, Z., Liang, Y., Qu, H., Zhao, M., Guo, F., Zhao, C., et al. (2018). Plasma homocysteine concentrations and risk of intracerebral hemorrhage: A systematic review and meta-analysis. Sci. Rep. 8 (1), 2568. doi:10.1038/s41598-018-21019-3
Zhu, X. Y., Hou, R. Y., Pan, X. D., Wang, Y. C., Zhang, Z. S., Guo, R. Y., et al. (2015). Association between the methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and ischemic stroke in the Chinese population: A meta-analysis. Int. J. Neurosci. 125 (12), 885–894. doi:10.3109/00207454.2014.984295
Keywords: methylenetetrahydrofolate reductase, C677T, A1298C, gene polymorphism, intracerebral hemorrhage, meta-analysis
Citation: Zou X-L, Yao T-X, Deng L, Chen L, Li Y and Zhang L (2022) A systematic review and meta-analysis expounding the relationship between methylene tetrahydrofolate reductase gene polymorphism and the risk of intracerebral hemorrhage among populations. Front. Genet. 13:829672. doi: 10.3389/fgene.2022.829672
Received: 08 December 2021; Accepted: 04 July 2022;
Published: 03 August 2022.
Edited by:
Timo Siepmann, University Hospital Carl Gustav Carus, GermanyReviewed by:
Shiek S. S. J. Ahmed, Chettinad Hospital and Research Institute, IndiaZhiyuan Yu, Sichuan University, China
Copyright © 2022 Zou, Yao, Deng, Chen, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Zhang, emx6ZHpsemRAY3N1LmVkdS5jbg==
 Xue-Lun Zou
Xue-Lun Zou Tian-Xing Yao
Tian-Xing Yao Lu Deng1
Lu Deng1 Le Zhang
Le Zhang