- 1Department of Genetics and Plant Breeding, Chaudhary Charan Singh University, Meerut, India
- 2Division of Genetics and Plant Breeding, Faculty of Agriculture, Sher-e-Kashmir University of Agricultural Sciences and Technology, Wadura, India
- 3Department of Plant Science and Landscape Architecture, University of Maryland, College Park, MD, United States
Wheat is one of the most important cereal crops in the world. The production and productivity of wheat is adversely affected by several diseases including leaf rust, which can cause yield losses, sometimes approaching >50%. In the present mini-review, we provide updated information on (i) all Lr genes including those derived from alien sources and 14 other novel resistance genes; (ii) a list of QTLs identified using interval mapping and MTAs identified using GWAS (particular those reported recently i.e., after 2018) and their association with known Lr genes; (iii) introgression/pyramiding of individual Lr genes in commercial/prominent cultivars from 18 different countries including India. Challenges and future perspectives of breeding for leaf rust resistance are also provided at the end of this mini-review. We believe that the information in this review will prove useful for wheat geneticists/breeders, not only in the development of leaf rust-resistant wheat cultivars, but also in the study of molecular mechanism of leaf rust resistance in wheat.
Introduction
Leaf rust caused by the fungal pathogen Puccinia triticina Eriks. & E. Henn is an important disease in wheat, which causes significant yield losses, sometimes approaching up to >50% (Riaz and Wong 2017). The study of the genetic basis of this disease and breeding for leaf rust resistance in wheat has been an important area of research (Dyck 1993; Kolmer and Liu, 2002; Oelke and Kolmer 2005; Datta et al., 2008; Rosa et al., 2016; Aoun et al., 2017). Each individual Lr gene apparently shows resistance against a specific race of P. triticina (Pt), which must carry the corresponding avirulence (Avr) gene, such that a specific Lr gene in the host and the corresponding specific Avr gene in the pathogen always follow a “gene-for-gene” relationship (Flor 1946). The pathogen Pt keeps on developing new virulent races through mutations or recombination involving Avr genes; new strains may also migrate from other geographical areas, and may carry one or more new Avr genes for which the corresponding R gene may be absent in the host (Samborski 1985; Bolton et al., 2008). Therefore, the host resistance breaks down and is short-lived. It is thus obvious that a majority of race specific Lr genes individually do not provide durable resistance (Johnson 1984).
Lr genes provide either seedling resistance (SR), also described as all stage resistance (ASR), or adult plant resistance (APR genes), the latter expressed only at the adult plant stage, particularly after booting. It is also known that ASR genes provide resistance, which breaks down within a few years, while APR provides long-term durable resistance (Ellis et al., 2014). Some of the APR genes like Lr34 and Lr67 have also been cloned and were found to be complex loci including Lr34/Sr57/Yr18/Pm38 and Lr67/Sr55/Yr46/Pm46 (Lagudah et al., 2006; Moore et al., 2015). These gene complexes confer durable resistance not only against leaf rust, but also against stripe rust, stem rust, powdery mildew, and barley yellow dwarf virus (Singh and Rajaram, 1993). The use of APR genes along with 4–5 Lr genes is a strategy that provides durable resistance.
A number of reviews on leaf rust resistance in wheat have already been published (Kolmer 1996; Kolmer 2013; McCallum et al., 2016; Pinto da Silva et al., 2018; Dinh et al., 2020; Figlan et al., 2020; Ghimire et al., 2020; Prasad et al., 2020). Information about QTLs for leaf rust resistance has also been recently reviewed (Pinto da Silva et al., 2018). However, considerable literature has appeared during the last 3–4 years, where many more QTLs and as many as 600 new MTAs have been added thus warranting a fresh look on the subject, hence this minireview.
According to some recent reports, currently more than 80 Lr genes and 14 other genes for leaf rust resistance are known in wheat (McIntosh et al., 2017, 2020). The above 14 genes have not been assigned a new number in Lr series, perhaps because these genes have not been subjected to test of allelism with the known Lr genes to ascertain their novelty. Since literature on Lr genes keep on appearing on a regular basis, any review published soon becomes out of date thus creating the need for a fresh review. The present mini-review caters to this need and provides an updated information on all Lr genes and other genes including genes derived from alien species. The mini-review includes information about chromosomal location of all these genes (including 14 other resistance genes, which could not be assigned to any of the known Lr genes; modified names were used for these 14 genes based on the cultivar in which they were identified). We also provide information about the wild relatives of wheat as a source of Lr genes and the molecular markers associated with most of these genes (wherever known). Information about cloning and characterisation of Lr genes has also been included, wherever available. The wheat varieties carrying different Lr genes developed in 18 different countries are also listed.
Lr Genes/Novel Lr Genes Catalogued so far
More than 80 Lr genes (∼50% derived from alien species) are already known to be distributed on all the 21 wheat chromosomes, with majority of genes located on the short arms of individual chromosomes (Table 1; Supplementary Tables S1, S2). Most of the Lr genes are located on the B sub-genome, relative to those located on either A sub-genome or D sub-genome. Maximum number of ten Lr genes (including two novel genes LrZH22 and LrG6) are located on chromosome 2B. At least two of these genes, namely Lr18 and LrZH22, are known to be temperature sensitive; Lr18 exhibits resistance at 15–18°C, ineffective at >18°C (Carpenter et al., 2017). The other gene LrZH22 confers resistance at higher temperatures (22–25°C; Wang et al., 2016). Lr genes conferring APR include the following: Lr34, Lr46, Lr67, Lr68, Lr74, Lr75, Lr77 and Lr78. Information on QTLs/MTAs was also included in an earlier review (Pinto da Silva et al., 2018) and has been compiled by us also in this mini-review (Supplementary Tables S1, S2). A set of 14 novel resistance genes (including three genes from alien species) are known, which differ from other available Lr genes, since they show seedling reaction pattern, which was different from reaction patterns known for different Lr genes studied so far. These 14 genes along with associated markers are also listed in Table 1. These genes were mapped on 10 out of the 21 wheat chromosomes with maximum number of these genes available on B sub-genome (8) followed by sub-genome D (4) and sub-genome A (2).
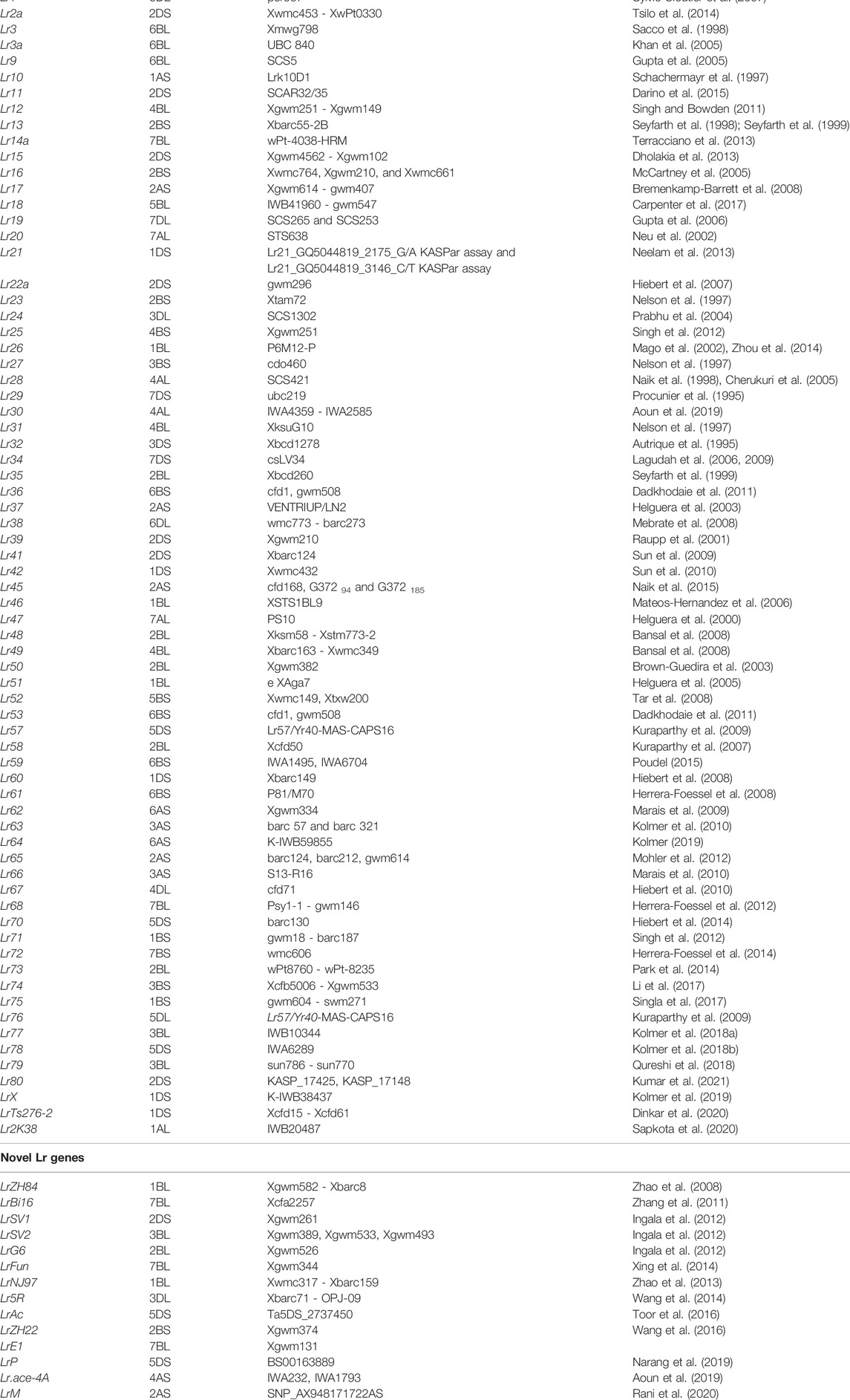
TABLE 1. Details of leaf rust (Lr) resistant genes including novel Lr genes identified in bread wheat.
QTLs/MTAs Linked to Lr Genes
In recent years, a number of newer approaches (based on DNA markers) led to the discovery of a large number of QTLs/QRLs and marker-trait associations (MTAs) for resistance against plant diseases including leaf rust. Qualitative resistance provided by Lr genes is generally compromised within a short period of time (Goyeau et al., 2006; Goyeau and Lannou, 2011), but quantitative disease resistance (QDR) provides effective and durable resistance involving major reduction in the level of disease (Mundt et al., 2002; Parlevliet, 2002; Stuthman et al., 2007). The QDR generally depends upon the presence of few major QTLs/genes and a fairly large number of minor QTLs (Ballini et al., 2008; Clair, 2010). Only a solitary example, where QDR for leaf rust resistance has been utilized is the French wheat cultivar Apache, which carried sustained resistance against leaf rust for a fairly long time (Papaïx et al., 2011). The availability of large number of QTLs/MTAs in wheat, as demonstrated in several studies, suggests that QDR against leaf rust is common in this crop, but has not been fully exploited.
A large number of QTLs, mostly associated with Lr genes were listed in some earlier reviews. For instance, in one report, 250 QTLs (reported till 2017) were listed, which were reported in 70 different studies (Pinto da silva et al., 2018). In second study, 35 meta-QTLs (MQTLs) were listed, which were identified using QTLs reported in several studies (Soriano and Royo, 2015). During the last 4 years (after 2017), additional 103 QTLs were reported in 18 studies; 29 of these QTLs were shown to be associated with Lr genes and Lr/Yr genes (Supplementary Table S1).
In addition to QTLs, ∼200 MTAs based on GWAS involving seven association panels (AM) were also reported earlier (Pinto da silva et al., 2018). As mentioned earlier, after publication of this review, ∼600 MTAs were reported in eight genome-wide association studies (GWAS); 42 of these MTAs were found to be linked to Lr genes (Supplementary Table S2). The maximum number of QTLs and Lr genes for leaf rust resistance are present in the B sub-genome. The PVE of the individual QTLs ranged from 4.63% to 75.3%; 29 of these QTLs had a PVE >20% suggesting their utility in MAS for breeding (Supplementary Table S1).
Wild Relatives as a Source of Lr Genes
At least 50% of Lr genes are derived from wild relatives (alien resources). One of the important alien sources from Fertile Crescent region is Sharon goatgrass (Aegilops sharonensis), which is a very valuable source of unique genes/QTLs for resistance to several wheat diseases including leaf rust (for reviews see Ghimire et al., 2020; Figlan et al., 2020). Following other important wild relatives of wheat have also been identified as sources of Lr genes/QTLs: (i) Tausch’s goatgrass (Ae. tauschii) (Lr21, Lr22a, and Lr39), (ii) wheatgrass (Thinopyrum ponticum) (Lr24), (iii) Ae. geniculate (Lr57), (iv) Ae. ventricosa (Lr37/Yr17), (v) Ae. umbellulata (Lr9), (vi) Thinopyrum elongatum Zhuk. (Lr 19), (vii) Agropyron elongatum (Lr24), (viii) Secale cereale L. (Lr26), (ix) Ae. peregrina (Lr59), (x) Ae. kotschyi (Lr54), (xi) Ae. sharonensis (Lr56), (xii) Ae. triuncialis (Lr58), and (xiii) Ae. neglecta (Lr62); however this list is not exclusive (McIntosh, 1975; Autrique et al., 1995; McIntosh et al., 1995; Marais and Botes, 2003; Kumar et al., 2022).
MAS for Pre-Breeding
There are ∼700 cultivars/varieties from 18 different countries (including India), each cultivar carrying one to six resistance genes for leaf rust including both ASR and APR genes (the details of varieties and their country of origin, are available in Supplementary Tables S2, S4). Two different approaches (including conventional breeding and marker assisted breeding, including pre-breeding) are available for developing resistant cultivars (Figure 1). Since markers associated with each of a number of Lr genes and QTLs including MTAs are available, MAS has become routine for supplementing conventional breeding (Supplementary Table S5). These markers are particularly useful for pyramiding of resistance genes, since introgression of additional resistance genes in the presence of existing resistance genes using phenotypic selection is rather difficult. There are at least a dozen examples (seven from India involving PBW343 and HD2329), where associated markers have been used to supplement conventional breeding including pre-breeding. A number of wheat varieties belonging to hard red winter or soft red winter wheats from United States were also developed using MAS (USDA website; https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1124692/1/Doc188-online-Sandra-Brammer.pdf. Using MAS, up to 10 Lr genes could be pyramided into the same wheat cultivar.
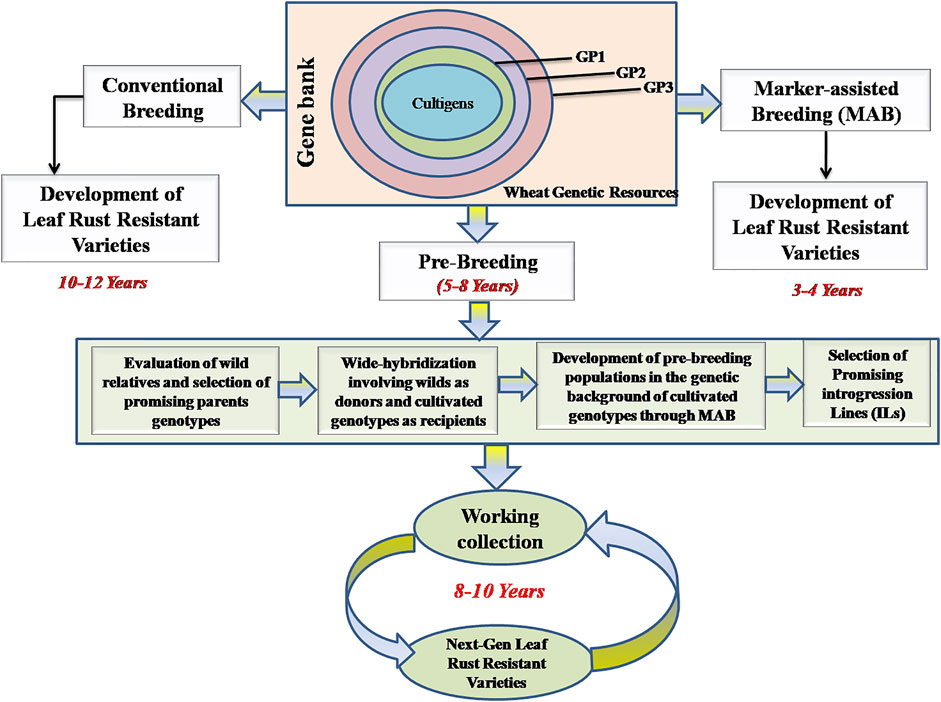
FIGURE 1. Various pre—breeding steps involved in use of wild relatives in the development of leaf rust resistant wheat varieties. The panels show how wheat genetic resources including wild relatives belonging to primary (GP1), secondary (GP2) and tertiary (GP3) gene pools conserved in different gene banks can be used in pre-breeding programs. The panels also shows the advantages of use of marker—assisted breeding (only 3–4 years in developing new cultivars) over conventional breeding (taking 10–12 years in cultivar development).
Conclusion and Future Perspectives
The present mini-review is yet another effort to provide a summary of updated published literature on resistance against leaf rust in wheat, including known R genes (∼80 Lr genes and 14 novel genes) (Supplementary Table S1), known QTLs/MTAs (Supplementary Tables S1, S2) and details of varieties containing one or more of these important leaf rust genes/QTLs/MTAs (Supplementary Tables S3, S4). Some details about the use of MAS for introgression of Lr genes into wheat varieties are also included (Supplementary Table S5).
One of the major challenges for wheat breeders is the regular development of new cultivars or improvement of old cultivars using new resistance genes, since new virulence pathotypes and races keep on appearing (Figlan et al., 2020; Ghimire et al., 2020). Therefore, continuous rigorous efforts are needed to locate sources for novel genes/QTLs to overcome new emerging races of pathogen and gain long-term resistance in the field. There are several other areas, which need attention and will certainly be the subject for future research. These will be briefly discussed as the future perspectives.
Although most R genes encode NLR proteins (with NBS-LRR domain), there are several other mechanisms involved as shown in a recent review, where work done during last 25 years involving >300 cloned R genes is reviewed. At least 60% of these R genes were shown to encode NLR proteins, the remaining 40% encoding RLKs/RLPs (Kourelis and van der Hoorn, 2018). Based on the study of these cloned R genes and the corresponding Avr genes of the pathogens, nine different mechanisms for the function of R genes have also been identified and summarised (Kourelis and van der Hoorn, 2018). However, the resistance mechanism of reported Lr genes is not clear and therefore can be a subject for future research.
The most common product of R genes, the NLRs have recently been shown to function through an assembly of a high-resolution structure called ‘resistosome’ which was first resolved in Arabidopsis and is responsible for providing resistance (Wang et al., 2019). Two additional examples of the high-resolution structures of interaction between NLRs and the effector molecules, through formation of resistosome also became available, thus suggesting that formation of the resistosome may be of wide occurrence (Ma et al., 2020; Martin et al., 2020). These three recent studies improved our understanding of the action of NLR at the molecular level. However, no Lr gene has been subjected to such studies involving formation of a resistosome. Therefore this is also an important area of future research.
Another important challenge in breeding for leaf rust resistance is the limited number of Lr genes that have been cloned (Lr1, Lr10, Lr21, Lr22a, Lr34, Lr67) and therefore cloning more genes is needed to understand the variation between such a large number of Lr genes and the mechanism used for their operation for providing resistance (Dinh et al., 2020; Prasad et al., 2020). According to some optimistic views, it will be possible in the next 15 years to clone most of the ∼460 known wheat resistance genes and their corresponding effectors, making it possible to design suitable strategies for resistance breeding in wheat (Wulff and Krattinger 2022). We, however feel that cloning of so many genes in a short period of 15 years may not be immediately possible. Therefore, closely linked markers may be used to identify which of the Lr genes encode NLR proteins and which other proteins may be encoded by other Lr genes. Bioinformatics may be used for this purpose and the results of this exercise may then be verified using suitable experiments.
Genomics of the pathogen is another important area, since genomes of a number of races of the pathogen have already been sequenced (Kiran et al., 2016; Wu et al., 2020; Fellers et al., 2021). This should facilitate use of bioinformatics for identification of effectors, using knowledge about conserved domains that have been discovered to be present in effector molecules. The genome sequences of different races of Pt have been worked out and many more genomes from the pathogen will also allow us to know the pangenome of Pt, which includes core genome, dispensible genome and unique genome. This knowledge will also allow to identify effectors and in planning suitable strategies for wheat breeding involving resistance against leaf rust.
It may also be necessary to study the effect of environment on expression of many resistance genes in the host since expression of genes has been found to vary with changing temperature (Figlan et al., 2020). This will involve study of the mode of action of resistance genes in the host, their interactions with other host genes, interactions with Avr gene while providing stable and durable resistance across environments. The recent advances in genomics tools and techniques including whole genome sequencing, genome annotation and high-throughput genomics tools like pathogenomics, gene cloning, genome editing are expected to offer deeper insights into host-pathogen interactions. This should eventually help in achieving durable rust resistance (Dinh et al., 2020). Molecular biology tools including HIGS/VIGS have also become very important for understanding and analyzing different facets of host and pathogen biology that includes secretome analysis, transcriptional profiling, putative virulence gene identification, structural gene annotation, and alternative transcript splicing. Another important area of future research is identification of vir genes, and effectors, which together make the subject of effectoromics and effector based breeding. This will allow the use of knowledge about effectors to screen the germplasm for resistance.
Epigenomics is another area, which has started attracting the attention of wheat geneticists. This will allow us to understand the role of DNA methylation, histone modifications, noncoding RNAs (e.g., miRNAs, lncRNAs) and chromatin states, thus further resolving the mechanism of resistance at the molecular level (Saripalli et al., 2020a; Saripalli et al., 2020b; Jain et al., 2020; Prasad et al., 2020).
Author Contributions
PG, HB, and PS conceived and outlined the review. KK, IJ, and GS collected the literature and wrote the first draft of the review. PG, HB, PS, and RM edited and finalized the manuscript with the help of KK, IJ, and GS.
Funding
Department of Biotechnology (DBT), Govt. of India provided funds in the form of a research project (Award number: BT/PR21024/AGIII/103/925/2016). No grant has been received for publishing this review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks are due to the Department of Biotechnology (DBT), Govt of India for providing funds in the form of a research project (Award number: BT/PR21024/AGIII/103/925/2016) and fellowships to KK and IJ for undertaking the research work related to this review, to National Agricultural Science Fund (NASF)-Indian Council of Agricultural Research (ICAR) for providing fellowship to GS and to Indian National Science Academy (INSA) for the award of position of INSA Honorary Scientist to HB.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.816057/full#supplementary-material
References
Aoun, M., Kolmer, J. A., Rouse, M. N., Chao, S., Bulbula, W. D., and Elias, E. M. (2017). Inheritance and Bulked Segregant Analysis of Leaf Rust and Stem Rust Resistance in Durum Wheat Genotypes. Phytopathol 107, 1496–1506. doi:10.1094/phyto-12-16-0444-r
Aoun, M., Kolmer, J. A., Rouse, M. N., Elias, E. M., Breiland, M., Bulbula, W. D., et al. (2019). Mapping of Novel Leaf Rust and Stem Rust Resistance Genes in the Portuguese Durum Wheat Landrace. G 9, 2535–2547. doi:10.1534/g3.119.400292
Autrique, E., Tanksley, S. D., Sorrells, M. E., and Singh, R. P. (1995). Molecular Markers for Four Leaf Rust Resistance Genes Introgressed into Wheat from Wild Relatives. Genome 38 (1), 75–83. doi:10.1139/g95-009
Ballini, E., Morel, J. B., Droc, G., Price, A., Courtois, B., Notteghem, J. L., et al. (2008). A Genome-wide Meta-Analysis of rice Blast Resistance Genes and Quantitative Trait Loci Provides New Insights into Partial and Complete Resistance. Mol. Plant Microbe Interact 21, 859–868. doi:10.1094/mpmi-21-7-0859
Bansal, U. K., Hayden, M. J., Venkata, B. P., Khanna, R., Saini, R. G., and Bariana, H. S. (2008). Genetic Mapping of Adult Plant Leaf Rust Resistance Genes Lr48 and Lr49 in Common Wheat. Theor. Appl. Genet. 117 (3), 307–312. doi:10.1007/s00122-008-0775-6
Bhardwaj, S. C., Prashar, M., Jain, S. K., Kumar, S., and Datta, D. (2010). Adult Plant Resistance in Some Indian Wheat Genotypes and Postulation of Leaf Rust Resistance Genes. Indian Phytopathol 63, 174–180.
Bhardwaj, S. C., Singh, G. P., Gangwar, O. P., Prasad, P., and Kumar, S. (2019). Status of Wheat Rust Research and Progress in Rust Management-Indian Context. J. Agron. 9, 892. doi:10.3390/agronomy9120892
Bokore, F. E., Knox, R. E., Cuthbert, R. D., Pozniak, C. J., McCallum, B. D., N’Diaye, A., et al. (2020). Mapping Quantitative Trait Loci Associated with Leaf Rust Resistance in Five spring Wheat Populations Using Single Nucleotide Polymorphism Markers. Plos One 15, e0230855. doi:10.1371/journal.pone.0230855
Bolton, M. D., Kolmer, J. A., and Garvin, D. F. (2008). Wheat Leaf Rust Caused by Pucciniatriticina. Mol. Plant Pathol. 9, 563–575. doi:10.1111/j.1364-3703.2008.00487.x
Bremenkamp‐Barrett, B., Faris, J. D., and Fellers, J. P. (2008). Molecular Mapping of the Leaf Rust Resistance Gene Lr17a in Wheat. Crop Sci. 48 (3), 1124–1128.
Brown-Guedira, G. L., Singh, S., and Fritz, A. 2. (2003). Performance and Mapping of Leaf Rust Resistance Transferred to Wheat from Triticum Timopheevii Subsp. Armeniacum. Phytopathology 93 (7), 784–789. doi:10.1094/phyto.2003.93.7.784
Carpenter, N. R., Griffey, C. A., Malla, S., Chao, S., and Brown-Guedira, G. L. (2017). Mapping Lr18: a Leaf Rust Resistant Gene Widely Deployed in Soft Red winter Wheat. J. Plant Dis. Biomark 1, 4–10.
Charpe, A., Koul, S., Gupta, S. K., Singh, A., Pallavi, S. K., and andPrabhu, K. V. (2012). Marker Assisted Gene Pyramiding of Leaf Rust Resistance Genes Lr24, Lr28 and Lr9 in a Bread Wheat Cultivar HD2329. J. Wheat Res. 4, 20–28.
Cherukuri, D. P., Gupta, S. K., Charpe, A., Koul, S., Prabhu, K. V., Singh, R. B., et al. (2005). Molecular Mapping of Aegilops Speltoides Derived Leaf Rust Resistance Gene Lr28 in Wheat. Euphytica 143 (1), 19–26. doi:10.1007/s10681-005-1680-6
Chhuneja, P., Vikal, Y., Kaur, S., Singh, R., Juneja, N. S., Bains, S., et al. (2011). Marker-assisted Pyramiding of Leaf Rust Resistance Genes Lr24 and Lr28 in Wheat (Triticumaestivum). Ind. J. Agrisci 81, 214–218.
Clair, St. D. A. (2010). Quantitative Disease Resistance and Quantitative Resistance Loci in Breeding. Ann. Rev. Phytopathol 48, 247–268. doi:10.1146/annurev-phyto-080508-081904
Cloutier, S., McCallum, B. D., Loutre, C., Banks, T. W., Wicker, T., Feuillet, C., et al. (2007). Leaf Rust Resistance Gene Lr1, Isolated from Bread Wheat (Triticum aestivum L.) Is a Member of the Large Psr567 Gene Family. Plant Mol. Biol. 65 (1), 93–106. doi:10.1007/s11103-007-9201-8
Cuddy, W., Park, R., Bariana, H., Bansal, U., Singh, D., Roake, J., et al. (2016). Expected Responses of Australian Wheat, Triticale and Barley Varieties to the Cereal Rust Diseases and Genotypic Data for Oat Varieties. Cereal Rust Rep. 14, 1–8.
Dadkhodaie, N. A., Karaoglou, H., Wellings, C. R., and Park, R. F. (2011). Mapping Genes Lr53 and Yr35 on the Short Arm of Chromosome 6B of Common Wheat with Microsatellite Markers and Studies of Their Association with Lr36. Theor. Appl. Genet. 122 (3), 479–487. doi:10.1007/s00122-010-1462-y
Darino, M. A., Dieguez, M. J., Singh, D., Ingala, L. R., Pergolesi, M. F., Park, R. F., et al. (2015). Detection and Location of Lr11 and Other Leaf Rust Resistance Genes in the Durably Resistant Wheat Cultivar Buck Poncho. Euphytica 206 (1), 135–147. doi:10.1007/s10681-015-1486-0
Datta, D., Nayar, S. K., Bhardwaj, S. C., Prashar, M., and Kumar, S. (2008). Detection and Inheritance of Leaf Rust Resistance in Common Wheat Lines Agra Local and IWP94. Euphytica 159, 343–351. doi:10.1007/s10681-007-9522-3
Dholakia, B. B., Rajwade, A. V., Hosmani, P., Khan, R. R., Chavan, S., Reddy, D. M. R., and Gupta, V. S. (2013). Molecular Mapping of Leaf Rust Resistance Gene Lr15 in Hexaploid Wheat. Mol. Breed. 31 (3), 743–747. doi:10.1007/s11032-012-9813-9
Dinh, H. X., Singh, D., Periyannan, S., Park, R. F., and Pourkheirandish, M. (2020). Molecular Genetics of Leaf Rust Resistance in Wheat and Barley. Theor. Appl. Genet. 133, 2035–2050. doi:10.1007/s00122-020-03570-8
Dinkar, V., Jha, S. K., Mallick, N., Niranjana, M., Agarwal, P., and Sharma, J. B. (2020). Molecular Mapping of a New Recessive Wheat Leaf Rust Resistance Gene Originating from Triticumspelta. Sci. Rep. 10, 1–9. doi:10.1038/s41598-020-78679-3
Draz, I. S., Abou-Elseoud, M. S., Kamara, A. E., Alaa-Eldein, O. A., and El-Bebany, A. F. (2015). Screening of Wheat Genotypes for Leaf Rust Resistance along with Grain Yield. Ann. Agric Sci 60, 29–39. doi:10.1016/j.aoas.2015.01.001
Dyck, P. L. (1993). The Inheritance of Leaf Rust Resistance in the Wheat Cultivar Pasqua. Canad J. Plant Sci. 73, 903–906. doi:10.4141/cjps93-118
El-Orabey, W. M., and Nagaty, H. H. (2013). Detection of the Leaf Rust Resistance Gene Lr9 in Some Egyptian Wheat Varieties. Minufiya J. Agric. Res. 38, 895–907.
Ellis, J. G., Lagudah, E. S., Spielmeyer, W., and Dodds, P. N. (2014). The Past, Present and Future of Breeding Rust Resistant Wheat. Front. Plant Sci. 5, 641. doi:10.3389/fpls.2014.00641
Fellers, J. P., Sakthikumar, S., He, F., McRell, K., Bakkeren, G., Cuomo, C. A., et al. (2021). Whole-genome Sequencing of Multiple Isolates of Puccinia Triticina Reveals Asexual Lineages Evolving by Recurrent Mutations. G 11 (9), jkab219. doi:10.1093/g3journal/jkab219
Figlan, S., Ntushelo, K., Mwadzingeni, L., Terefe, T., Tsilo, T. J., and Shimelis, H. (2020). Breeding Wheat for Durable Leaf Rust Resistance in Southern Africa: Variability, Distribution, Current Control Strategies, Challenges and Future Prospects. Front. Plant Sci. 11, 549. doi:10.3389/fpls.2020.00549
Gautam, T., Dhillon, G. S., Saripalli, G., Singh, V. P., Prasad, P., Kaur, S., et al. (2020). Marker-assisted Pyramiding of Genes/QTL for Grain Quality and Rust Resistance in Wheat (Triticumaestivum L.). Mol. Breed. 40, 49. doi:10.1007/s11032-020-01125-9
Genievskaya, Y., Abugalieva, S., Rsaliyev, A., Yskakova, G., Turuspekov, Y., Griffiths, S., et al. (2020a). QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva× Paragon Mapping Population of Bread Wheat. J. Agron. 10 (9), 1285. doi:10.3390/agronomy10091285
Genievskaya, Y., Fedorenko, Y., Sarbayev, A., Amalova, A., Abugalieva, S., et al. (2019). Identification of OTLs for Resistance to Leaf and Stem Rusts in Bread Wheat (Triticumaestivum L.) Using a Mapping Population of ‘PamyatiAzieva X Paragon’Vavilov. J. Genet. Breed. 23, 887–895. doi:10.18699/vj19.563
Genievskaya, Y., Turuspekov, Y., Rsaliyev, A., and Abugalieva, S. (2020b). Genome-wide Association Mapping for Resistance to Leaf, Stem, and Yellow Rusts of Common Wheat under Field Conditions of South Kazakhstan. Peer J. 8, 9820. doi:10.7717/peerj.9820
Ghimire, B., Sapkota, S., Bahri, B. A., Martinez-Espinoza, A. D., Buck, J. W., and Mergoum, M. (2020). Fusarium Head Blight and Rust Diseases in Soft Red winter Wheat in the Southeast United States: State of the Art, Challenges and Future Perspective for Breeding. Front. Plant Sci. 11, 1080. doi:10.3389/fpls.2020.01080
Goyeau, H., and Lannou, C. (2011). Specific Resistance to Leaf Rust Expressed at the Seedling Stage in Cultivars Grown in France from 1983 to 2007. Euphytica 178, 45–62. doi:10.1007/s10681-010-0261-5
Goyeau, H., Park, R., Schaeffer, B., and andLannou, C. (2006). Distribution of Pathotypes with Regard to Host Cultivars in French Wheat Leaf Rust Populations. Phytopathology 96, 264–273. doi:10.1094/phyto-96-0264
Gul’tyaeva, E. I., Kanyuka, I. A., Alpat’eva, N. V., Baranova, O. A., Dmitriev, A. P., and Pavlyushin, V. A. (2009). Molecular Approaches in Identifying Leaf Rust Resistance Genes in Russian Wheat Varieties. Russ. Agricsci 35, 316–319.
Gupta, A., Singh, C., Kumar, V., Tyagi, B. S., Tiwari, V., Chatrath, R., et al. (2018). Wheat Varieties Notified in india since 1965. Karnal: ICAR-Indian Institute of Wheat and Barley Research, 101.
Gupta, S. K., Charpe, A., Koul, S., Prabhu, K. V., and Haq, Q. M. R. (2005). Development and Validation of Molecular Markers Linked to an Aegilops Umbellulata–Derived Leaf-Rust-Resistance Gene, Lr9, for Marker-Assisted Selection in Bread Wheat. Genome 48 (5), 823–830. doi:10.1139/g05-051
Gupta, S. K., Charpe, A., Prabhu, K. V., and Haque, Q. M. R. (2006). Identification and Validation of Molecular Markers Linked to the Leaf Rust Resistance Gene Lr19 in Wheat. Theor. Appl. Genet. 113 (6), 1027–1036. doi:10.1007/s00122-006-0362-7
Helguera, M., Khan, I. A., and Dubcovsky, J. (2000). Development of PCR Markers for the Wheat Leaf Rust Resistance Gene Lr47. Theor. Appl. Genet. 100 (7), 1137–1143. doi:10.1007/s001220051397
Helguera, M., Khan, I. A., Kolmer, J., Lijavetzky, D., Zhong‐Qi, L., and Dubcovsky, J. (2003). PCR Assays for the Lr37‐Yr17‐Sr38 Cluster of Rust Resistance Genes and Their Use to Develop Isogenic Hard Red spring Wheat Lines. Crop Sci. 43 (5), 1839–1847. doi:10.2135/cropsci2003.1839
Helguera, M., Vanzetti, L., Soria, M., Khan, I. A., Kolmer, J., and Dubcovsky, J. (2005). PCR Markers for Triticum Speltoides Leaf Rust Resistance Gene Lr51 and Their Use to Develop Isogenic Hard Red spring Wheat Lines. Crop Sci. 45 (2), 728–734. doi:10.2135/cropsci2005.0728
Herrera-Foessel, S. A., Huerta-Espino, J., Calvo-Salazar, V., Lan, C. X., and Singh, R. P. (2014). Lr72 Confers Resistance to Leaf Rust in Durum Wheat Cultivar Atil C2000. Plant Dis. 98 (5), 631–635. doi:10.1094/pdis-07-13-0741-re
Herrera-Foessel, S. A., Singh, R. P., Huerta-Espino, J., Rosewarne, G. M., Periyannan, S. K., Viccars, L., et al. (2012). Lr68: a New Gene Conferring Slow Rusting Resistance to Leaf Rust in Wheat. Theor. Appl. Genet. 124 (8), 1475–1486. doi:10.1007/s00122-012-1802-1
Herrera-Foessel, S. A., Singh, R. P., Huerta-Espino, J., William, H. M., Djurle, A., and Yuen, J. (2008). Molecular Mapping of a Leaf Rust Resistance Gene on the Short Arm of Chromosome 6B of Durum Wheat. Plant Dis. 92 (12), 1650–1654. doi:10.1094/pdis-92-12-1650
Hiebert, C. W., McCallum, B. D., and Thomas, J. B. (2014). Lr70, a New Gene for Leaf Rust Resistance Mapped in Common Wheat Accession KU3198. Theor. Appl. Genet. 127 (9), 2005–2009. doi:10.1007/s00122-014-2356-1
Hiebert, C. W., Thomas, J. B., McCallum, B. D., Humphreys, D. G., DePauw, R. M., Hayden, M. J., et al. (2010). An Introgression on Wheat Chromosome 4DL in RL6077 (Thatcher* 6/PI 250413) Confers Adult Plant Resistance to Stripe Rust and Leaf Rust (Lr67). Theor. Appl. Genet. 121 (6), 1083–1091. doi:10.1007/s00122-010-1373-y
Hiebert, C. W., Thomas, J. B., McCallum, B. D., and Somers, D. J. (2008). Genetic Mapping of the Wheat Leaf Rust Resistance Gene Lr60 (LrW2). Crop Sci. 48 (3), 1020–1026. doi:10.2135/cropsci2007.08.0480
Hiebert, C. W., Thomas, J. B., Somers, D. J., McCallum, B. D., and Fox, S. L. (2007). Microsatellite Mapping of Adult-Plant Leaf Rust Resistance Gene Lr22a in Wheat. Theor. Appl. Genet. 115 (6), 877–884. doi:10.1007/s00122-007-0604-3
Ingala, L., López, M., Darino, M., Pergolesi, M. F., Diéguez, M. J., and Sacco, F. (2012). Genetic Analysis of Leaf Rust Resistance Genes and Associated Markers in the Durable Resistant Wheat Cultivar Sinvalocho MA. Theor. Appl. Genet. 124, 1305–1314. doi:10.1007/s00122-012-1788-8
Jain, N., Sinha, N., Krishna, H., Singh, P. K., Gautam, T., Prasad, P., et al. (2020). A Study of miRNAs and lncRNAs during Lr28-Mediated Resistance against Leaf Rust in Wheat (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 112, 101552. doi:10.1016/j.pmpp.2020.101552
Johnson, R. (1984). A Critical Analysis of Durable Resistance. Ann. Rev. Phtopathol 22, 309–330. doi:10.1146/annurev.py.22.090184.001521
Joukhadar, R., Hollaway, G., Shi, F., Kant, S., Forrest, K., Wong, D., et al. (2020). Genome-wide Association Reveals a Complex Architecture for Rust Resistance in 2300 Worldwide Bread Wheat Accessions Screened under Various Australian Conditions. Theor. Appl. Genet 133, 2695–2712. doi:10.1007/s00122-020-03626-9
Kandiah, P., Chhetri, M., Hayden, M., Ayliffe, M., Bariana, H., and Bansal, U. (2020). Mapping of Adult Plant Leaf Rust Resistance in Aus27506 and Validation of Underlying Loci by In-Planta Fungal Biomass Accumulation. Agronomy 10, 943. doi:10.3390/agronomy10070943
Khan, H., Bhardwaj, S. C., Gangwar, O. P., Prasad, P., Kashyap, P. L., Savadi, S., et al. (2017). Identifying Some Additional Rust Resistance Genes in Indian Wheat Varieties Using Robust Markers. Cereal Res. Commun. 45, 633–646. doi:10.1556/0806.45.2017.041
Khan, R. R., Bariana, H. S., Dholakia, B. B., Naik, S. V., Lagu, M. D., Rathjen, A. J., et al. (2005). Molecular Mapping of Stem and Leaf Rust Resistance in Wheat. Theor. Appl. Genet. 111 (5), 846–850. doi:10.1007/s00122-005-0005-4
Kiran, K., Rawal, H. C., Dubey, H., Jaswal, R., Devanna, B. N., Gupta, D. K., et al. (2016). Draft Genome of the Wheat Rust Pathogen (Puccinia Triticina) Unravels Genome-wide Structural Variations during Evolution. Genome Biol. Evol. 8 (9), 2702–2721. doi:10.1093/gbe/evw197
Kolmer, J. A., Anderson, J. A., and Flor, J. M. (2010). Chromosome Location, Linkage with Simple Sequence Repeat Markers, and Leaf Rust Resistance Conditioned by Gene Lr63 in Wheat. Crop Sci. 50 (6), 2392–2395. doi:10.2135/cropsci2010.01.0005
Kolmer, J. A., Bernardo, A., Bai, G., Hayden, M. J., and Anderson, J. A. (2019). Thatcher Wheat Line RL6149 Carries Lr64 and a Second Leaf Rust Resistance Gene on Chromosome 1DS. Theor. Appl. Genet. 132 (10), 2809–2814. doi:10.1007/s00122-019-03389-y
Kolmer, J. A., Bernardo, A., Bai, G., Hayden, M. J., and Chao, S. (2018b). Adult Plant Leaf Rust Resistance Derived from Toropi Wheat Is Conditioned by Lr78 and Three Minor QTL. Phytopathology 108 (2), 246–253. doi:10.1094/phyto-07-17-0254-r
Kolmer, J. A. (1996). Genetics of Resistance to Wheat Leaf Rust. Ann. Rev. Phytopathol 34, 435–455. doi:10.1146/annurev.phyto.34.1.435
Kolmer, J. A., and Liu, J. Q. (2002). Inheritance of Leaf Rust Resistance in the Wheat Cultivars AC Majestic, AC Splendor, and AC Karma. Canad J. Plant Pathol. 24, 327–331. doi:10.1080/07060660209507017
Kolmer, J. A., Su, Z., Bernardo, A., Bai, G., and Chao, S. (2018a). Mapping and Characterization of the New Adult Plant Leaf Rust Resistance Gene Lr77 Derived from Santa Fe winter Wheat. Theor. Appl. Genet. 131 (7), 1553–1560. doi:10.1007/s00122-018-3097-3
Kolmer, J. A. (2013). Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 4, 70–84. doi:10.3390/f4010070
Kolmer, (2019). Virulence of Puccinia Triticina, the Wheat Leaf Rust Fungus in the United States in 2017. Plant Dis. 103, 2113–2120. doi:10.1094/pdis-09-18-1638-sr
Kourelis, J., and van der Hoorn, R. A. (2018). Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. The Plant Cell 30 (2), 285–299. doi:10.1105/tpc.17.00579
Kthiri, D., Loladze, A., N’Diaye, A., Nilsen, K. T., Walkowiak, S., Dreisigacker, S., et al. (2019). Mapping of Genetic Loci Conferring Resistance to Leaf Rust from Three Globally Resistant Durum Wheat Sources. Front. Plant Sci. 10, 1247. doi:10.3389/fpls.2019.01247
Kumar, A., Choudhary, A., Kaur, H., and Mehta, S. (2022). A Walk towards Wild Grasses to Unlock the Clandestine of Gene Pools for Wheat Improvement: A Review. Plant Stress 3. doi:10.1016/j.stress.2021.100048
Kumar, D., Kumar, A., Chhokar, V., Gangwar, O. P., Bhardwaj, S. C., Sivasamy, M., et al. (2020). Genome-wide Association Studies in Diverse spring Wheat Panel for Stripe, Stem, and Leaf Rust Resistance. Front. Plant Sci. 11, 748. doi:10.3389/fpls.2020.00748
Kumar, S., Bhardwaj, S. C., Gangwar, O. P., Sharma, A., Qureshi, N., Kumaran, V. V., et al. (2021). Lr80: A New and Widely Effective Source of Leaf Rust Resistance of Wheat for Enhancing Diversity of Resistance Among Modern Cultivars. Theor. Appl. Genet. 134, 849–858. doi:10.1007/s00122-020-03735-5
Kuraparthy, V., Sood, S., Chhuneja, P., Dhaliwal, H. S., Kaur, S., Bowden, R. L., et al. (2007). A Cryptic Wheat–Aegilops Triuncialis Translocation with Leaf Rust Resistance Gene Lr58. Crop Sci. 47 (5), 1995–2003. doi:10.2135/cropsci2007.01.0038
Kuraparthy, V., Sood, S., See, D. R., and Gill, B. S. (2009). Development of a PCR Assay and Marker‐assisted Transfer of Leaf Rust and Stripe Rust Resistance Genes Lr57 and Yr40 into Hard Red winter Wheats. Crop Sci. 49 (1), 120–126. doi:10.2135/cropsci2008.03.0143
Lagudah, E. S., Krattinger, S. G., Herrera-Foessel, S., Singh, R. P., Huerta-Espino, J., Spielmeyer, W., et al. (2009). Gene-specific Markers for the Wheat Gene Lr34/Yr18/Pm38 Which Confers Resistance to Multiple Fungal Pathogens. Theor. Appl. Genet. 119 (5), 889–898. doi:10.1007/s00122-009-1097-z
Lagudah, E. S., McFadden, H., Singh, R. P., Huerta-Espino, J., Bariana, H. S., and Spielmeyer, W. (2006). Molecular Genetic Characterization of the Lr34/Yr18 Slow Rusting Resistance Gene Region in Wheat. Theor. Appl. Genet. 114 (1), 21–30. doi:10.1007/s00122-006-0406-z
Li, C., Wang, Z., Li, C., Bowden, R., Bai, G., Li, C., et al. (2017). Mapping of Quantitative Trait Loci for Leaf Rust Resistance in the Wheat Population Ning7840× Clark. Plant Dis. 101 (12), 1974–1979. doi:10.1094/pdis-12-16-1743-re
Li, J., Shi, L., Wang, X., Zhang, N., Wei, X., Zhang, L., et al. (2018). Leaf Rust Resistance of 35 Wheat Cultivars (Lines). J. Plant Pathol. Microbiol. 9, 429.
Li, Z., Yuan, C., Herrera-Foessel, S. A., Randhawa, M. S., Huerta-Espino, J., Liu, D., et al. (2020). Four Consistent Loci Confer Adult Plant Resistance to Leaf Rust in the Durum Wheat Lines Heller# 1 and Dunkler. Phytopathology 110, 892–899. doi:10.1094/phyto-09-19-0348-r
Ma, S., Lapin, D., Liu, L., Sun, Y., Song, W., Zhang, X., et al. (2020). Direct Pathogen-Induced Assembly of an NLR Immune Receptor Complex to Form a Holoenzyme. Science 370 (6521), eabe3069. doi:10.1126/science.abe3069
Mago, R., Spielmeyer, W., Lawrence, G., Lagudah, E., Ellis, J., and Pryor, A. (2002). Identification and Mapping of Molecular Markers Linked to Rust Resistance Genes Located on Chromosome 1RS of rye Using Wheat-rye Translocation Lines. Theor. Appl. Genet. 104 (8), 1317–1324. doi:10.1007/s00122-002-0879-3
Malaker, P. K., and Reza, M. M. (2011). Resistance to Rusts in Bangladeshi Wheat. Czech J. Genet. Plant Breed. 47, S155–S159. doi:10.17221/3271-cjgpb
Mallick, N., Sharma, J. B., Tomar, R. S., Sivasamy, M., and Prabhu, K. V. (2015). Marker Assisted Back-Cross Breeding to Combine Multiple Rust Resistance in Wheat. Plant Breed 134, 172–177. doi:10.1111/pbr.12242
Marais, G. F., and Botes, W. C. (2003). “Recurrent Mass Selection as a Means to Pyramid Major Genes for Pest Resistance in Spring Wheat,” in Proceedings of the 10th International Wheat Genetics Symposium, September, 1–6.
Marais, F., Marais, A., McCallum, B., and Pretorius, Z. (2009). Transfer of Leaf Rust and Stripe Rust Resistance Genes Lr62 and Yr42 from Aegilops Neglecta Req. Ex Bertol. To Common Wheat. Crop Sci. 49 (3), 871–879. doi:10.2135/cropsci2008.06.0317
Marais, G. F., Bekker, T. A., Eksteen, A., McCallum, B., Fetch, T., and Marais, A. S. (2010). Attempts to Remove Gametocidal Genes Co-transferred to Common Wheat with Rust Resistance from Aegilops Speltoides. Euphytica 171 (1), 71–85. doi:10.1007/s10681-009-9996-2
Martin, R., Qi, T., Zhang, H., Liu, F., King, M., Toth, C., and Staskawicz, B. J. (2020). Structure of the Activated ROQ1 Resistosome Directly Recognizing the Pathogen Effector XopQ. Science 370 (6521), eabd9993. doi:10.1126/science.abd9993
Mateos-Hernandez, M., Singh, R. P., Hulbert, S. H., Bowden, R. L., Huerta-Espino, J., Gill, B. S., et al. (2006). Targeted Mapping of ESTs Linked to the Adult Plant Resistance Gene Lr46 in Wheat Using Synteny with rice. Funct. Integr. Genomic 6 (2), 122–131. doi:10.1007/s10142-005-0017-9
McCallum, B. D., Hiebert, C. W., Cloutier, S., Bakkeren, G., Rosa, S. B., Humphreys, D. G., et al. (2016). A Review of Wheat Leaf Rust Research and the Development of Resistant Cultivars in Canada. Canad J. Plant Pathol. 38, 1–8. doi:10.1080/07060661.2016.1145598
McCartney, C. A., Somers, D. J., McCallum, B. D., Thomas, J., Humphreys, D. G., Menzies, J. G., et al. (2005). Microsatellite Tagging of the Leaf Rust Resistance Gene Lr16 on Wheat Chromosome 2BSc. Mol. Breed 15 (4), 329–337. doi:10.1007/s11032-004-5948-7
McIntosh, R. A., Dubcovsky, J., Rogers, W. J., Morris, C., and Xia, X. C. (2017). Catalog of Gene Symbols for Wheat: 2017. Aberdeen, USA: supplement.
McIntosh, R. A. (1975). Cytogenetical Studies in Wheat VIII. Telocentric Mapping and Linkage Studies Involving Sr22 and Other Genes in Chromosome 7AL. Aust. J. Biol. Sci. 28 (6), 531–8.
McIntosh, R. A., Friebe, B., Jiang, J., and Gill, B. S. (1995). Cytogenetical Studies in Wheat XVI. Chromosome Location of a New Gene for Resistance to Leaf Rust in a Japanese Wheat-Rye Translocation Line. Euphytica 83 (2), 141–7.
McIntosh, R. A., Dubcovsky, J., Rogers, W. J., Xia, X. C., and Raupp, W. J. (2020). Catalogue of Gene Symbols for Wheat: 2020 Supplement. Ann. Wheat Newslett 66.
Mebrate, S. A., Oerke, E. C., Dehne, H., and Pillen, K. (2008). Mapping of the Leaf Rust Resistance Gene Lr38 on Wheat Chromosome Arm 6DL Using SSR Markers. Euphytica 162 (3), 457–466. doi:10.1007/s10681-007-9615-z
Mohler, V., Singh, D., Singrün, C., and Park, R. F. (2012). Characterization and Mapping of Lr65 in Spelt Wheat ‘Altgold Rotkorn. Plant breed 131 (2), 252–257. doi:10.1111/j.1439-0523.2011.01934.x
Moore, J. W., Herrera-Foessel, S., Lan, C., Schnippenkoetter, W., Ayliffe, M., Huerta-Espino, J., et al. (2015). A Recently Evolved Hexose Transporter Variant Confers Resistance to Multiple Pathogens in Wheat. Nat. Genet. 47, 1494–1498. doi:10.1038/ng.3439
Moullet, O., Fossati, D., Mascher, F., Guadangnolo, R., and andSchori, A. (2009). “Use of Marker-Assisted Selection (MAS) for Pyramiding Two Leaf Rust Resistance Genes, (Lr9,Lr24 and Lr22a) in Wheat,” in Int. Conf. Conventional Mol. Breed. Field Veg. Crops (Novi Sad, Serbia: Springer).
Muhammad, S., Ahmad, A., Awan, F. S., Khan, A. I., Qasim, M., Rehman, A., et al. (2018). Genome Wide Association Analysis for Leaf Rust Resistance in spring Wheat (Triticumaestivum L.) Germplasm. Int. J. Agric. Biol. 20, 2387–2394.
Mundt, C. C., Cowger, C., and Garrett, K. A. (2002). Relevance of Integrated Disease Management to Resistance Durability. Euphytica 124, 245–252. doi:10.1023/a:1015642819151
Naik, B. K., Sharma, J. B., Sivasamy, M., Prabhu, K. V., Tomar, R. S., and Tomar, S. M. S. (2015). Molecular Mapping and Validation of the Microsatellite Markers Linked to the Secale Cereale-Derived Leaf Rust Resistance Gene Lr45 in Wheat. Mol. Breed 35 (2), 1–10. doi:10.1007/s11032-015-0234-4
Naik, S., Gill, K. S., Prakasa Rao, V. S., Gupta, V. S., Tamhankar, S. A., Pujar, S., et al. (1998). Identification of a STS Marker Linked to the Aegilops Speltoides-Derived Leaf Rust Resistance Gene Lr28 in Wheat. Theor. Appl. Genet. 97 (4), 535–540. doi:10.1007/s001220050928
Narang, D., Kaur, S., Steuernagel, B., Ghosh, S., Dhillon, R., Bansal, M., et al. (2019). Fine Mapping of Aegilopsperegrina Co-segregating Leaf and Stripe Rust Resistance Genes to Distal-Most End of 5DS. Theor. Appl. Genet. 132, 1473–1485. doi:10.1007/s00122-019-03293-5
Neelam, K., Brown-Guedira, G., and Huang, L. (2013). Development and Validation of a Breeder-Friendly KASPar Marker for Wheat Leaf Rust Resistance Locus Lr21. Mol. Breed 31 (1), 233–237. doi:10.1007/s11032-012-9773-0
Nelson, J. C., Singh, R. P., Autrique, J. E., and Sorrells, M. E. (1997). Mapping Genes Conferring and Suppressing Leaf Rust Resistance in Wheat. Crop Sci. 37 (6), 1928–1935. doi:10.2135/cropsci1997.0011183x003700060043x
Neu, C., Stein, N., and Keller, B. (2002). Genetic Mapping of the Lr20 Pm1 Resistance Locus Reveals Suppressed Recombination on Chromosome Arm 7AL in Hexaploid Wheat. Genome 45 (4), 737–744. doi:10.1139/g02-040
Nocente, F., Gazza, L., and Pasquini, M. (2007). Evaluation of Leaf Rust Resistance Genes Lr1, Lr9, Lr24, Lr47 and Their Introgression into Common Wheat Cultivars by Marker-Assisted Selection. Euphytica 155, 329–336. doi:10.1007/s10681-006-9334-x
Oelke, L. M., and Kolmer, J. A. (2005). Genetics of Leaf Rust Resistance in spring Wheat Cultivars Alsen and Norm. Phytopathol 95, 773–778. doi:10.1094/phyto-95-0773
Papaïx, J., Goyeau, H., DuCheyron, P., Monod, H., and Lannou, C. (2011). Influence of Cultivated Landscape Composition on Variety Resistance: an Assessment Based on Wheat Leaf Rust Epidemics. New Phytol. 191, 1095–1107. doi:10.1111/j.1469-8137.2011.03764.x
Park, R. F., Mohler, V., Nazari, K., and Singh, D. (2014). Characterisation and Mapping of Gene Lr73 Conferring Seedling Resistance to Puccinia Triticina in Common Wheat. Theor. Appl. Genet. 127 (9), 2041–2049. doi:10.1007/s00122-014-2359-y
Parlevliet, J. E. (2002). Durability of Resistance against Fungal, Bacterial and Viral Pathogens; Present Situation. Euphytica 124, 147–156. doi:10.1023/a:1015601731446
Pathan, A. K., and Park, R. F. (2006). Evaluation of Seedling and Adult Plant Resistance to Leaf Rust in European Wheat Cultivars. Euphytica 149, 327–342. doi:10.1007/s10681-005-9081-4
Pietrusińska, A., Czembor, J. H., and Czembor, P. C. (2011). Pyramiding Two Genes for Leaf Rust and Powdery Mildew Resistance in Common Wheat. Cereal Res. Commun. 39, 577–588.
Pinto da Silva, G. B., Zanella, C. M., Martinelli, J. A., Chaves, M. S., Hiebert, C. W., McCallum, B. D., et al. (2018). Quantitative Trait Loci Conferring Leaf Rust Resistance in Hexaploid Wheat. Phytopathol 108, 1344–1354. doi:10.1094/phyto-06-18-0208-rvw
Ponce-Molina, L. J., Huerta-Espino, J., Singh, R. P., Basnet, B. R., Alvarado, G., Randhawa, M. S., et al. (2018). Characterization of Leaf Rust and Stripe Rust Resistance in spring Wheat ‘Chilero. Plant Dis. 102, 421–427. doi:10.1094/pdis-11-16-1545-re
Poudel, R. S. (2015). The Acquisition of Useful Disease Resistance Genes for Hard Red winter Wheat Improvement. Fargo, North Dakota: North Dakota State University. Doctoral dissertation.
Prabhu, K. V., Gupta, S. K., Charpe, A., and Koul, S. (2004). SCAR Marker Tagged to the Alien Leaf Rust Resistance Gene Lr19 Uniquely Marking the Agropyron Elongatum‐derived Gene Lr24 in Wheat: a Revision. Plant Breed 123 (5), 417–420. doi:10.1111/j.1439-0523.2004.00971.x
Prasad, P., Savadi, S., Bhardwaj, S. C., and Gupta, P. K. (2020). The Progress of Leaf Rust Research in Wheat. Fungal Biol. 124 (6), 537–550. doi:10.1016/j.funbio.2020.02.013
Procunier, J. D., Townley-Smith, T. F., Fox, S., Prashar, S., Gray, M., Kim, W. K., et al. (1995). PCR-based RAPD/DGGE Markers Linked to Leaf Rust Resistance Genes Lr29 and Lr25 in Wheat (Triticum aestivum L.). J. Genet. Breed. 49 (1), 87–91.
Qureshi, N., Bariana, H., Kumran, V. V., Muruga, S., Forrest, K. L., Hayden, M. J., et al. (2018). A New Leaf Rust Resistance Gene Lr79 Mapped in Chromosome 3BL from the Durum Wheat Landrace Aus26582. Theor. Appl. Genet. 131 (5), 1091–1098. doi:10.1007/s00122-018-3060-3
Rani, K., Raghu, B. R., Jha, S. K., Agarwal, P., Mallick, N., Niranjana, M., et al. (2020). A Novel Leaf Rust Resistance Gene Introgressed from Aegilopsmarkgrafii Maps on Chromosome Arm 2AS of Wheat. Theor. Appl. Genet. 133, 2685–2694. doi:10.1007/s00122-020-03625-w
Raupp, W. J., Brown-Guedira, G. L., and Gill, B. S. (2001). Cytogenetic and Molecular Mapping of the Leaf Rust Resistance Gene Lr39 in Wheat. Theor. Appl. Genet. 102 (2), 347–352. doi:10.1007/s001220051652
Revathi, P., Tomar, S. M. S., and Singh, N. K. (2010). Marker Assisted Gene Pyramiding of Leaf Rust Resistance Genes Lr24, Lr28 along with Stripe Rust Resistance Gene Yr15 in Wheat (Triticumaestivum L.). Ind. J. Genet. 70, 349–354.
Riaz, M., and Wong, Y. (2017). Estimation of Yield Losses Due to Leaf Rust and Late Seeding on Wheat (Triticum aestivum L) Variety Seher-06 in District Faisalabad, Punjab, Pakistan. Adv. Biotech. Micro 4. doi:10.19080/AIBM.2017.04.555657
Roelfs, A. P. (1992). Rust Diseases of Wheat: Concepts and Methods of Disease Management. Mexico: Cimmyt.
Rosa, S. B., McCallum, B., Brûlé-Babel, A., Hiebert, C., Shorter, S., Randhawa, H. S., et al. (2016). Inheritance of Leaf Rust and Stripe Rust Resistance in the Brazilian Wheat Cultivar ‘Toropi. Plant Dis. 100, 1132–1137. doi:10.1094/pdis-10-15-1128-re
Rosa, S. B., Zanella, C. M., Hiebert, C. W., Brûlé-Babel, A. L., Randhawa, H. S., Shorter, S., et al. (2019). Genetic Characterization of Leaf and Stripe Rust Resistance in the Brazilian Wheat Cultivar Toropi. Phytopathol 109, 1760–1768. doi:10.1094/phyto-05-19-0159-r
Sacco, F., Suarez, E. Y., and Naranjo, T. (1998). Mapping of the Leaf Rust Resistance Gene Lr3 on Chromosome 6B of Sinvalocho MA Wheat. Genome 41 (5), 686–690. doi:10.1139/g98-067
Samborski, D. J. (1985). “Wheat Leaf Rust,” in Diseases, Distribution, Epidemiology, and Control (Academic Press), 39–59. doi:10.1016/b978-0-12-148402-6.50010-9
Samsampour, D., Zanjani, B. M., Singh, A., Pallavi, J. K., and Prabhu, K. V. (2009). Marker Assisted Selection to Pyramid Seedling Resistance Gene Lr24 and Adult Plant Resistance Gene Lr48 for Leaf Rust Resistance in Wheat. Ind. J. Genet. 69, 1–9.
Sapkota, S., Hao, Y., Johnson, J., Buck, J., Aoun, M., and andMergoum, M. (2019). Genome-wide Association Study of a Worldwide Collection of Wheat Genotypes Reveals Novel Quantitative Trait Loci for Leaf Rust Resistance. Plant Genome 12, 190033. doi:10.3835/plantgenome2019.05.0033
Sapkota, S., Mergoum, M., Kumar, A., Fiedler, J. D., Johnson, J., Bland, D., et al. (2020). A Novel Adult Plant Leaf Rust Resistance Gene Lr2K38 Mapped on Wheat Chromosome 1AL. Plant Genome 13 (3), e20061. doi:10.1002/tpg2.20061
Saripalli, G., Sharma, C., Gautam, T., Singh, K., and Jain, N. (2020a). Complex Relationship between DNA Methylation and Gene Expression Due to Lr28 in Wheat-Leaf Rust Pathosystem. Mol. Bio Rep. 47, 1339–1360. doi:10.1007/s11033-019-05236-1
Saripalli, G., Singh, K., Gautam, T., Santosh, K., Saurabh, R., Prasad, P., et al. (2020b). Genome-wide Analysis of H3K4me3 and H3K27me3 Modifications Due to Lr28 for Leaf Rust Resistance in Bread Wheat (Triticum aestivum). Plant Mol. Biol. 104, 113–136. doi:10.1007/s11103-020-01029-4
Schachermayr, G., Feuillet, C., and Keller, B. (1997). Molecular Markers for the Detection of the Wheat Leaf Rust Resistance Gene Lr10 in Diverse Genetic Backgrounds. Mol. Breed. 3 (1), 65–74. doi:10.1023/a:1009619905909
Seyfarth, R., Feuille, C., and Keller, B. (1998). “Development and Characterization of Molecular Markers for the Adult Plant Leaf Rust Resistance Genes Lr13 and Lr35 in Wheat,” in Proc 9th Int Wheat Genet Symp. Editors A. E. Slinkard (Saskatoon, Canada: University Extension Press), Vol. 3, 154.
Seyfarth, R., Feuillet, C., Schachermayr, G., Winzeler, M., and Keller, B. (1999). Development of a Molecular Marker for the Adult Plant Leaf Rust Resistance Gene Lr35 in Wheat. Theor. Appl. Genet. 99 (3), 554–560. doi:10.1007/s001220051268
Singh, A., Pallavi, J. K., Gupta, P., and Prabhu, K. V. (2012). Identification of Microsatellite Markers Linked to Leaf Rust Resistance Gene Lr25 in Wheat. J. Appl. Genet. 53 (1), 19–25. doi:10.1007/s13353-011-0070-0
Singh, M., Mallick, N., Chand, S., Kumari, P., Sharma, J. B., Sivasamy, M., et al. (2017). Marker-assisted Pyramiding of Thinopyrum-Derived Leaf Rust Resistance Genes Lr19 and Lr24 in Bread Wheat Variety HD2733. J. Genet. 96, 951–957. doi:10.1007/s12041-017-0859-7
Singh, R. P., and Rajaram, S. (2002). Breeding for Disease Resistance in Wheat. Rome, Italy: FAO plant production and protection series, 30.
Singh, R. P., and Rajaram, S. (1993). Genetics of Adult Plant Resistance to Stripe Rust in Ten spring Bread Wheats. Euphytica 72, 1–7. doi:10.1007/bf00023766
Singh, S., and Bowden, R. L. (2011). Molecular Mapping of Adult-Plant Race-specific Leaf Rust Resistance Gene Lr12 in Bread Wheat. Mol. Breed. 28 (2), 137–142. doi:10.1007/s11032-010-9467-4
Singla, J., Lüthi, L., Wicker, T., Bansal, U., Krattinger, S. G., and Keller, B. (2017). Characterization of Lr75: a Partial, Broad-Spectrum Leaf Rust Resistance Gene in Wheat. Theor. Appl. Genet. 130 (1), 1–12. doi:10.1007/s00122-016-2784-1
Sivasamy, M. (2014). Deployment of Rust Resistance Genes in Wheat Varities Increased Wheat Production in India in National Seminar on Challenges and Innovative Approaches in Crop Improvement. Madurai, Tamilnadu, India: Agricultural college and research institute.
Slikova, S., Gregova, E., Bartos, P., and andKraic, J. (2003). Marker-assisted Selection for Leaf Rust Resistance in Wheat by Transfer of Gene Lr19. Plant Protect Sci. 39, 13–17.
Soriano, J. M., and Royo, C. (2015). Dissecting the Genetic Architecture of Leaf Rust Resistance in Wheat by QTL Meta-Analysis. Phytopathol 105, 1585–1593. doi:10.1094/phyto-05-15-0130-r
Stuthman, D. D., Leonard, K. J., and Miller‐Garvin, J. (2007). Breeding Crops for Durable Resistance to Disease. AdvAgron 95, 319–367. doi:10.1016/s0065-2113(07)95004-x
Sun, X., Bai, G., Carver, B. F., and Bowden, R. (2010). Molecular Mapping of Wheat Leaf Rust Resistance Gene Lr42. Crop Sci. 50 (1), 59–66. doi:10.2135/cropsci2009.01.0049
Sun, X., Bai, G., and Carver, B. F. (2009). Molecular Markers for Wheat Leaf Rust Resistance Gene Lr41. Mol. Breed 23 (2), 311–321. doi:10.1007/s11032-008-9237-8
Tar, M., Purnhauser, L., and Csősz, M. (2008). Identification and Localization of Molecular Markers Linked to the Lr52 Leaf Rust Resistance Gene of Wheat. Cereal Res. Commun. 36 (3), 409–415. doi:10.1556/crc.36.2008.3.5
Terracciano, I., Maccaferri, M., Bassi, F., Mantovani, P., Sanguineti, M. C., Salvi, S., et al. (2013). Development of COS-SNP and HRM Markers for High-Throughput and Reliable Haplotype-Based Detection of Lr14a in Durum Wheat (Triticum Durum Desf.). Theor. Appl. Genet. 126 (4), 1077–1101. doi:10.1007/s00122-012-2038-9
Tomar, S. M. S., Singh, S. K., and Sivasamy, M. (2014). Wheat Rusts in India: Resistance Breeding and Gene Deployment-A Review. Ind. J. Genet. Plant Breed. 74, 129–156. doi:10.5958/0975-6906.2014.00150.3
Toor, P. I., Kaur, S., Bansal, M., Yadav, B., and andChhuneja, P. (2016). Mapping of Stripe Rust Resistance Gene in an Aegilops Caudate Introgression Line in Wheat and its Genetic Association with Leaf Rust Resistance. J. Genet. 95, 933–938. doi:10.1007/s12041-016-0718-y
Tsilo, T. J., Kolmer, J. A., and Anderson, J. A. (2014). Molecular Mapping and Improvement of Leaf Rust Resistance in Wheat Breeding Lines. Phytopathol 104 (8), 865–870. doi:10.1094/phyto-10-13-0276-r
Wang, C., Yin, G., Xia, X., He, Z., Zhang, P., Yao, Z., et al. (2016). Molecular Mapping of a New Temperature-Sensitive Gene LrZH22 for Leaf Rust Resistance in Chinese Wheat Cultivar Zhoumai 22. Mol. Breed. 36, 18. doi:10.1007/s11032-016-0437-3
Wang, J., Hu, M., Wang, J., Qi, J., Han, Z., Wang, G., and Chai, J. (2019). Reconstitution and Structure of a Plant NLR Resistosome Conferring Immunity. Science 364 (6435), eaav5870. doi:10.1126/science.aav5870
Wang, J., Shi, L., Li, X., and Liu, D. (2014). Genetic Analysis and Molecular Mapping of Leaf Rust Resistance Genes in the Wheat Line 5R618. Czech J. Genet. Plant Breed. 50, 262–267. doi:10.17221/164/2014-cjgpb
Wu, J. Q., Dong, C., Song, L., and Park, R. F. (2020). Long-Read–Based De Novo Genome Assembly and Comparative Genomics of the Wheat Leaf Rust Pathogen Puccinia Triticina Identifies Candidates for Three Avirulence Genes. Front. Genet. 11, 521. doi:10.3389/fgene.2020.00521
Wulff, B. B., and Krattinger, S. G. (2022). The Long Road to Engineering Durable Disease Resistance in Wheat. Curr. Opin. Biotechnol. 73, 270–275. doi:10.1016/j.copbio.2021.09.002
Xing, L., Wang, C., Xia, X., He, Z., Chen, W., Liu, T., et al. (2014). Molecular Mapping of Leaf Rust Resistance Gene LrFun in Romanian Wheat Line Fundulea 900. Mol. Breed. 33, 931–937. doi:10.1007/s11032-013-0007-x
Yuan, C., Singh, R. P., Liu, D., Randhawa, M. S., Huerta-Espino, J., and Lan, C. (2020). Genome-wide Mapping of Adult Plant Resistance to Leaf Rust and Stripe Rust in CIMMYT Wheat Line Arableu# 1. Plant Dis. 104, 1455–1464. doi:10.1094/pdis-10-19-2198-re
Zhang, H., Xia, X., He, Z., Li, X., Li, Z., and Liu, D. (2011). Molecular Mapping of Leaf Rust Resistance Gene LrBi16 in Chinese Wheat Cultivar Bimai 16. Mol. Breed. 28, 527–534. doi:10.1007/s11032-010-9501-6
Zhang, P., Yin, G., Zhou, Y., Qi, A., Gao, F., Xia, X., et al. (2017). QTL Mapping of Adult-Plant Resistance to Leaf Rust in the Wheat Cross Zhou 8425B/Chinese Spring Using High-Density SNP Markers. Front. Plant Sci. 8, 793. doi:10.3389/fpls.2017.00793
Zhao, X. L., Zheng, T. C., Xia, X. C., He, Z. H., Liu, D. Q., Yang, W. X., et al. (2013). Molecular Mapping of Leaf Rust Resistance Gene LrNJ97 in Chinese Wheat Line Neijiang 977671. Theor. Appl. Genet. 126, 2141–2147. doi:10.1007/s00122-013-2124-7
Zhao, X. L., Zheng, T. C., Xia, X. C., He, Z. H., Liu, D. Q., Yang, W. X., et al. (2008). Molecular Mapping of Leaf Rust Resistance Gene LrZH84 in Chinese Wheat Line Zhou 8425B. Theor. Appl. Genet. 117, 1069–1075. doi:10.1007/s00122-008-0845-9
Keywords: bread wheat, leaf rust, genes, QTLs, markers, molecular breeding
Citation: Kumar K, Jan I, Saripalli G, Sharma PK, Mir RR, Balyan HS and Gupta PK (2022) An Update on Resistance Genes and Their Use in the Development of Leaf Rust Resistant Cultivars in Wheat. Front. Genet. 13:816057. doi: 10.3389/fgene.2022.816057
Received: 16 November 2021; Accepted: 28 February 2022;
Published: 31 March 2022.
Edited by:
Karthikeyan Adhimoolam, Jeju National University, South KoreaReviewed by:
Suraj Sapkota, Crop Genetics and Breeding Research (USDA-ARS), United StatesPrashant Vikram, International Center for Biosaline Agriculture (ICBA), United Arab Emirates
Copyright © 2022 Kumar, Jan, Saripalli, Sharma, Mir, Balyan and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: P. K. Gupta, cGtndXB0YTM2QGdtYWlsLmNvbQ==, cGtndXB0YUBjY3N1bml2ZXJzaXR5LmFjLmlu
†These authors have contributed equally to this work and share first authorship
 Kuldeep Kumar
Kuldeep Kumar Irfat Jan
Irfat Jan Gautam Saripalli
Gautam Saripalli P. K. Sharma
P. K. Sharma Reyazul Rouf Mir
Reyazul Rouf Mir H. S. Balyan
H. S. Balyan P. K. Gupta
P. K. Gupta