- 1Department of Nephrology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Pediatrics, Ministry of Education Key Laboratory of Child Development and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, National Clinical Research Center for Child Health and Disorders, Pediatric Research Institute, Chongqing, China
- 3Key Laboratory of Molecular Biology for Infectious Diseases, Department of Infectious Diseases, Ministry of Education, Centre for Lipid Research, The Second Affiliated Hospital, Institute for Viral Hepatitis, Chongqing Medical University, Chongqing, China
Background: PAX2 is a nuclear transcription factor gene that is highly conserved among species. Variants within PAX2 could result in optic nerve colobomas and kidney hypoplasia. However, little clinical and genetic information is currently available about PAX2 variants in Chinese children.
Objective: This study aims to further understand the clinical manifestations and genetic characteristics of PAX2 variants in Chinese population.
Methods: In this single-center retrospective study, we analyzed the clinical data of 10 children identified as carriers of PAX2 variants by gene sequencing. All the variants found in this study were analyzed using in silico prediction and American College of Medical Genetics and Genomics (ACMG) standards and guidelines.
Results: The mean age for developing the first symptom in 10 unrelated children was 7.2 years old. Proteinuria and bilateral kidney dysplasia were found in every patient. Two children underwent kidney histological examination; one child showed high-intensity C1q deposition in the kidney, and the other child showed focal segmental glomerular sclerosis (FSGS). Three children had PAX2-related ocular abnormalities, including nystagmus, retinal exudation, amblyopia, microphthalmia, microcornea, and total blindness. In addition, one patient had the comorbidity of oculocutaneous albinism (OCA). Eight different PAX2 variants were found in ten patients, three of which were reported for the first time.
Conclusion: We reported some patients with unique manifestations and comorbidities, and we reported three variants that have not been previously identified. The PAX2 gene is prone to spontaneous variants, and the outcome of patients is unfavorable. Because of the lack of specific therapy, genetic testing should be recommended for individuals with obvious evidence of kidney dysplasia and eye abnormalities, and kidney protective treatment should be initiated early.
Introduction
PAX2 is a member of the PAX family of transcription factors, which localizes to human chromosome band 10q24, spans 84.2 kb, and contains 12 coding exons (Rossanti et al., 2020). It is usually expressed in the urogenital system, eye, ear, and central nervous system. Animal model findings show that PAX2 plays a vital role in cellular regeneration and organ development (Bower et al., 2012; Naiman et al., 2017; Zhang et al., 2018). PAX2 variants are inherited in an autosomal dominant fashion, and these variants were initially characterized through the presence of kidney dysplasia and optic nerve abnormalities.
Patients with a homozygous variant of PAX2 die soon after birth due to the lack of organogenesis; in contrast, patients with a heterozygous variant of PAX2 may present with renal coloboma syndrome (RCS) and FSGS (Grimley and Dressler, 2018; Deng et al., 2019). Abnormal kidney structure or function (92% of individuals) and ophthalmological abnormalities (77% of individuals) have been reported (Zhang et al., 2018). Reported kidney findings with the PAX2 variants include kidney hypoplasia, multicystic dysplastic kidney, horseshoe kidney, oligomeganephronia, and vesicoureteral reflux (VUR). Many patients eventually develop kidney failure (KF) and need kidney replacement therapy. However, visual impairment is known to have a wide variation that can be asymmetric, ranging from normal to complete blindness (Deng et al., 2019). In addition to kidney and ocular anomalies, some rare clinical features have been reported in patients with PAX2 variants, such as hearing loss, skeletal deformities, growth retardation, microcephaly, heart defects, ovarian teratoma, and gout (Bower et al., 2012; Deng et al., 2019).
Although there have been several reports on PAX2 variants in Chinese children, the reports are similar to other previous works in the literature. In this article, we will present the cases of 10 patients with PAX2 variants with widely different clinical manifestations and some novel variants. Their kidney histological results are entirely different, and some patients have unique manifestations and comorbidities such as C1q kidney deposition and OCA. Our study further explores the clinical manifestations and genetic characteristics of PAX2 variants in the Chinese population.
Materials and Methods
Subjects
We enrolled 10 patients with a PAX2 (NM_003,990.4) variant by gene sequencing in our single-center study from May 2017 to April 2021. In our cohort, six patients were admitted to the hospital with proteinuria, three manifested abnormal kidney function, and one presented with kidney ultrasound abnormalities. Before the genetic tests, informed consent was obtained from all families. In addition to these ten patients, we also found one patient with recurrent painless gross hematuria, who had left renal vein entrapment (LRVE) and a PAX2 variant (c.725G > A). However, his kidney structure and function were normal, and the pathogenicity of his variant was uncertain. His symptoms were totally relieved after conservative medical treatment. This result suggested that the LRVE, instead of the PAX2 variant, was the leading cause of his gross hematuria. Because he did not have a confirmed genetic diagnosis, we finally excluded this patient.
Clinical Assessment
We retrospectively reviewed the clinical findings of 10 patients, including the onset age, kidney findings, extrarenal manifestations, and kidney histological examination findings. The definitions of the main clinical manifestations, including kidney and ocular symptoms, and the methods of evaluating kidney function are shown in Supplementary Table S1 (Ketteler et al., 2018). The indications for gene sequencing were as follows: 1) highly suspected inherited metabolic diseases; 2) significant urinary system malformation; 3) nephropathy with poor response to corticosteroid therapy; and 4) kidney tubule disease. The indications for kidney histological examination were as follows: 1) recurrent proteinuria and/or glomerular hematuria without a definite cause; 2) worsened kidney function without a definite cause; 3) diagnosed with glomerulopathies, such as Henoch-Schonlein nephritis, IgA nephropathy, lupus nephritis, and anti-neutrophilic cytoplasmic antibody nephritis; and 4) nephropathy with poor response to corticosteroid therapy.
Gene Sequencing and Genetic Analyses
Peripheral blood samples were collected from 10 patients and their parents, and genomic DNA was extracted according to standard procedures. The standard Illumina libraries were prepared by using a DNA Sample Prep Reagent Set (MyGenostics, Beijing, China). The amplified DNA was captured using the capture kit of urinary system genes. The enrichment libraries were sequenced on Illumina HiSeq 2000, Illumina Nova6000, and MGI DNBSEQ-T7. The raw data were saved in FASTQ format after sequencing. Both Illumina sequencing adapters and low-quality reads (<80 bp) were filtered by cut adaptor software. BWA was used to map the clean reads to the UCSC hg19 human reference genome.
All variants were identified by GATK or Sentieon and then annotated by ANNOVAR software, as well as the following multiple databases: 1,000 Genomes Project, ESP6500, ExAC, and ExAC-EAS. In downstream analysis, we used five steps to select the potential pathogenic mutations: 1) The mutation reads had to be more than 5, and the mutation ratio had to be no less than 30%. 2) The mutations for which the frequencies were more than 5% in the 1000g, ESP6500 ExAC, and ExAC-EAS database were removed. 3) If the mutations existed in InNormal database (MyGenostics), then they were dropped. 4) The synonymous mutations were removed. 5) After (1), (2), and (3), if the mutations were synonymous and they were reported in HGMD, then they were retained. Then, we performed pathogenicity analysis of the variants according to the ACMG standards and guidelines, and variants were classified as pathogenic, likely pathogenic, uncertain significance, likely benign, or benign (Richards et al., 2015). The ACMG guidelines and the matching degree between the patient’s phenotype and the gene-related disease were taken into consideration when identifying the pathogenic variant. In silico prediction of the variants was calculated from the output of the programs Sorting Intolerant from Tolerant (SIFT), Polymorphism Phenotyping v2 (PolyPhen_2), MutationTaster, GERP++, and REVEL. In addition, genomic DNA from all patients’ parents was obtained for Sanger sequencing, which was used to confirm the variants identified by next-generation sequencing (NGS).
Results
General Information
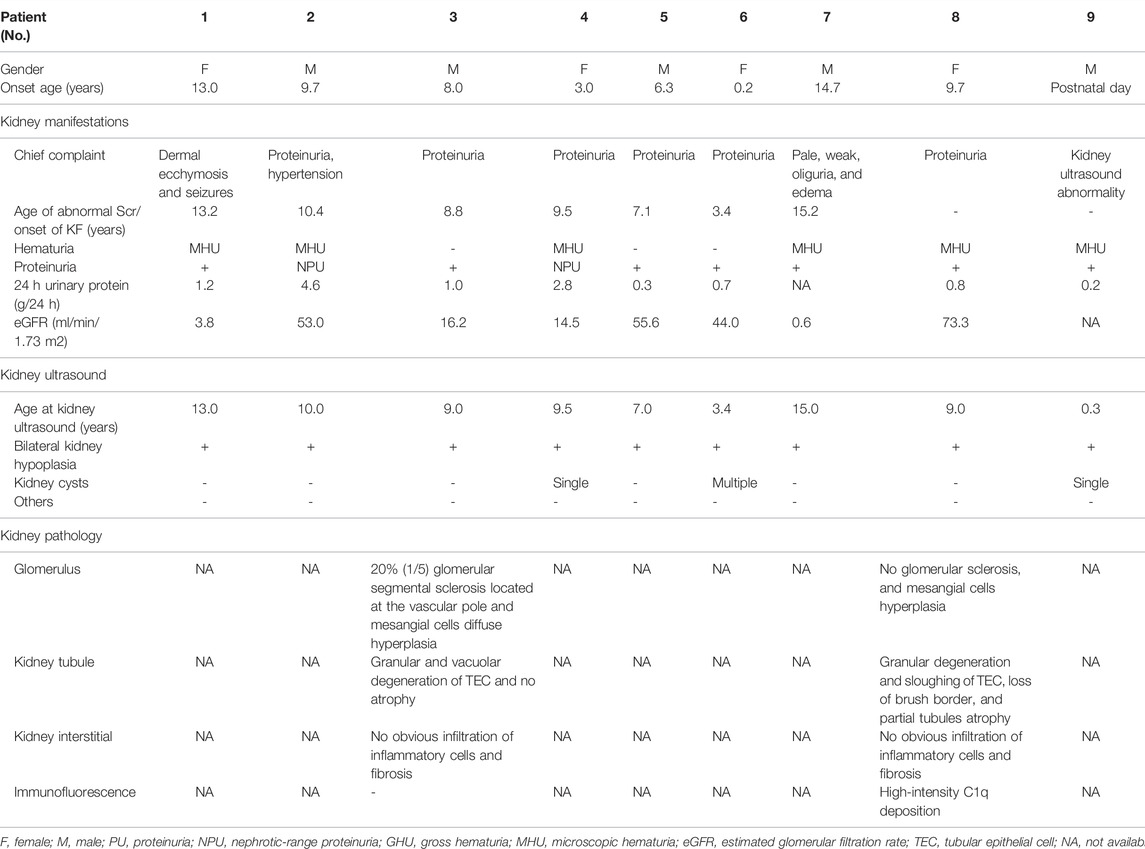
A total of 10 patients were enrolled in this study, including six boys and four girls, and their cases were sporadic. The ages of the patients on admission ranged from 0.3 to 15.2 years, with an average age of 8.8 years, while the age at the development of the first symptom was 7.2 (ranging from postnatal day to 14.7) years old. Six patients (patients 2, 3, 4, 5, 6, and 8) were identified with proteinuria as the first symptom, three patients (patients 1, 7, and 10) were admitted to the hospital due to KF, and kidney hypoplasia was detected in patient 9 after birth. Every patient in our cohort had confirmed congenital kidney hypoplasia and decreased estimated glomerular filtration rate (eGFR).
Clinical Manifestations and Follow-Up
Proteinuria is the most common kidney manifestation in children with PAX2 variants. Ten patients had varying degrees of proteinuria in our study; three of them (30.0%) presented nephrotic-range proteinuria. Six patients (60.0%) were found to have hematuria. In general, the patients with the PAX2 variants had various clinical manifestations except for proteinuria, but the other nephrotic manifestations, such as edema and hypoproteinemia, were rare. Bilateral kidney dysplasia without ureterectasia was found in all patients by kidney ultrasound examination. Kidney cysts were detected in three patients (30.0%); one patient had multiple cysts, and the other two had a single cyst. The kidney ultrasound results of patients in our single-center study showed that the more severe the kidney dysplasia was, the worse kidney function of the patient. The relevant information is summarized in Table 1.
Only two patients underwent histological examinations, but the results were completely different (Figure 1). The kidney function of patient 3 deteriorated rapidly after onset, and histological examination indicated FSGS. In contrast, the kidney function of patient 8 was stable. The histological examination of patient 8 showed diffuse high-intensity C1q deposition in the mesangial area on immunofluorescence and a mild proliferation of mesangial cells and the stroma under light and electron microscopy.
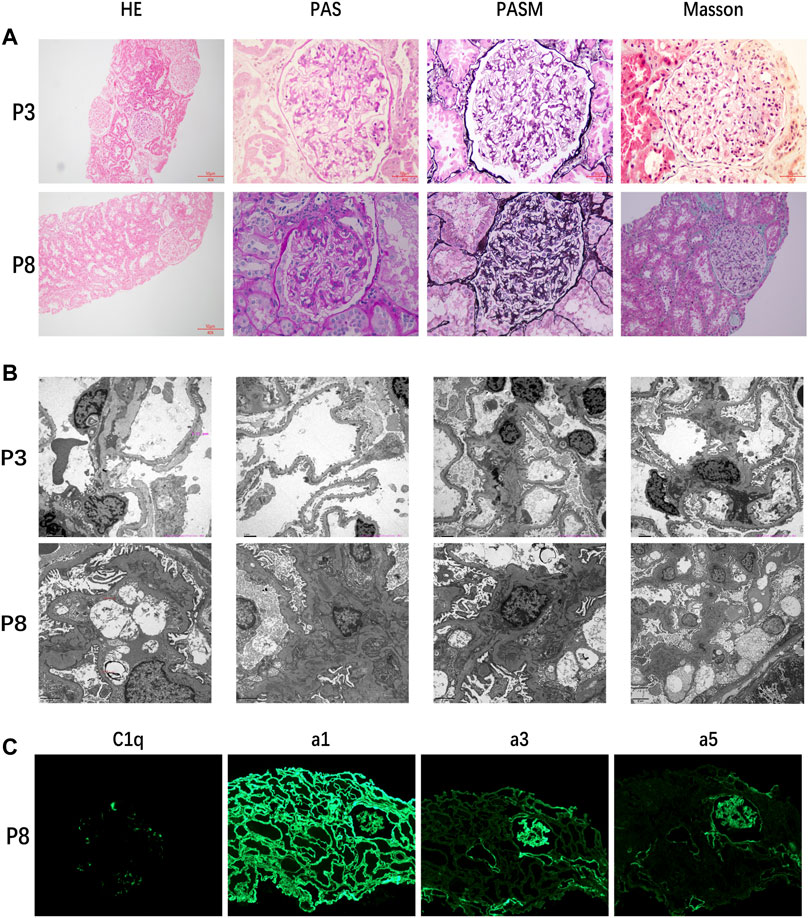
FIGURE 1. (A) Kidney histological examination of patient 3 showed FSGS and that of patient 8 showed mild proliferation of mesangial cells and stroma. (B) Electron microscopic results of patient 3 still supported FSGS and those of patient 8 showed high-density electron-dense deposition. (C) Results of immunofluorescence of patient 8 indicated diffuse high-intensity C1q deposition.
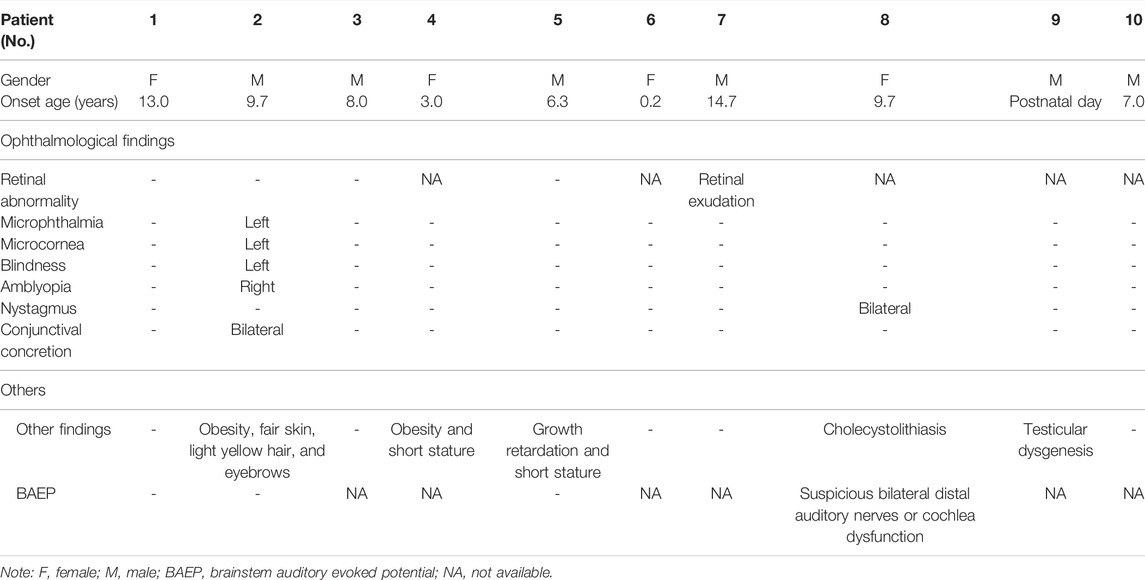
Ocular manifestations are the most common extrarenal manifestations in children with PAX2 variants. In our cohort, only patients 2 and 8 had subjective ocular abnormalities. Five patients (patients 1, 2, 3, 5, and 7) underwent ophthalmic fundus examination. Patient 2 presented with severe ocular lesions since birth, including microphthalmia, microcornea, congenital amblyopia of the right eye, and congenital blindness of the left eye. After hospitalization, bilateral conjunctival concretions were also discovered in patient 2. Retinal exudation without macular coloboma was detected in patient 7. Furthermore, patient 8 had binocular nystagmus, and the results of the brainstem auditory evoked potential (BAEP) were abnormal, showing suspicious bilateral distal auditory nerves or cochlear dysfunction. In addition to ocular and auditory lesions, malnutrition, obesity, short stature, growth retardation, cholecystolithiasis, and testicular dysgenesis could be observed in our group. Among them, cholecystolithiasis and testicular dysgenesis were rare extrarenal phenotypes that have not been reported before. The relevant information is summarized in Table 2.
It is worth mentioning that we revealed suspicious OCA in patient 2, such as fair skin, light yellow hair, and eyebrows. The gene test of this patient revealed compound heterozygous variants in the OCA2 gene (c.1441G>A, c.727C>T), originating from his father and his mother, respectively. The variants have both been reported as pathological variants of OCA (Lee et al., 1994; Wei et al., 2010; Hwang et al., 2014; Bullich et al., 2018; Ishiwa et al., 2019). This meant that patient 2 could also be diagnosed with OCA, except the PAX2 variant. The ocular symptoms of OCA are mainly reduced pigmentation and congenital nystagmus, which were inconspicuous in patient 2. Therefore, we supposed that the PAX2 variant was the leading cause of the ocular lesions of patient 2.
In our single-center study, the longest follow-up time of 10 children was 4.4 years, the shortest was 0.67 years, and the average was 2.3 years. Among them, five patients (patients 1, 3, 4, 7, and 10) had developed KF, and the kidney function of three patients deteriorated drastically within a few months but that of the other two patients deteriorated over several years. However, almost all five patients were in adolescence at the onset of KF, and their onset ages ranged from 8.8 to 15.2 years. The kidney function of the remaining five patients was relatively stable. To date, four patients (patients 1, 4, 7, and 10) are undergoing maintenance hemodialysis. The other six patients are receiving oral drug treatment to protect kidney function. None of the patients had new ocular abnormalities.
Pathogenic Variants in the PAX2 Gene
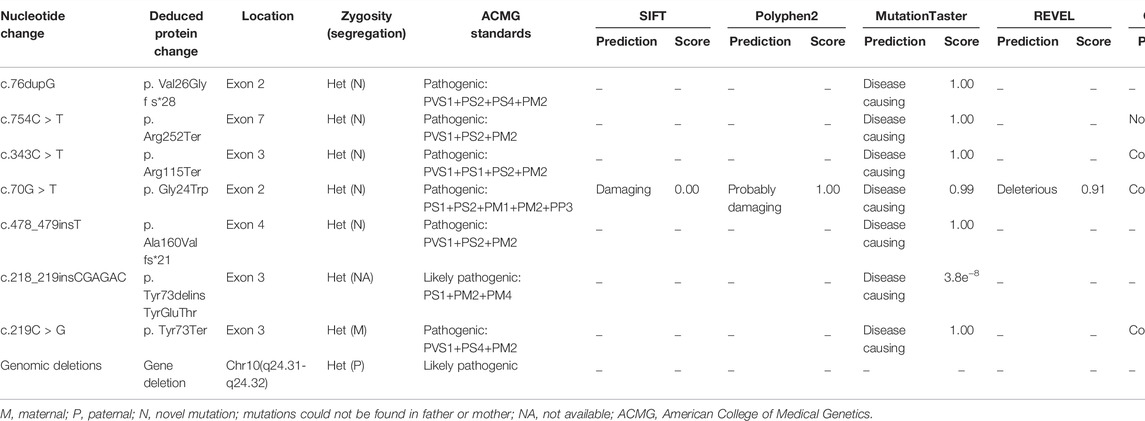
Eight different variants, most of which occurred in exons 2–4, were found in the 10 patients. Among all the variants, variants c.754C > T and c.478_479insT and the large genomic deletions of patient 4 were not previously reported in the Human Gene Variant Database (HGMD) or ClinVar (Figure 2). According to ACMG standards, two novel variants (c.754C>T and c.478_479insT) were identified as pathogenic variants, and the remaining variant (genomic deletions) was considered to be likely pathogenic. The details and bioinformatic analyses of all the PAX2 variants are summarized in Table 3. We validated two novel variants, and the secondary protein structure of those variants was changed, which is shown in Supplementary Figure S1.
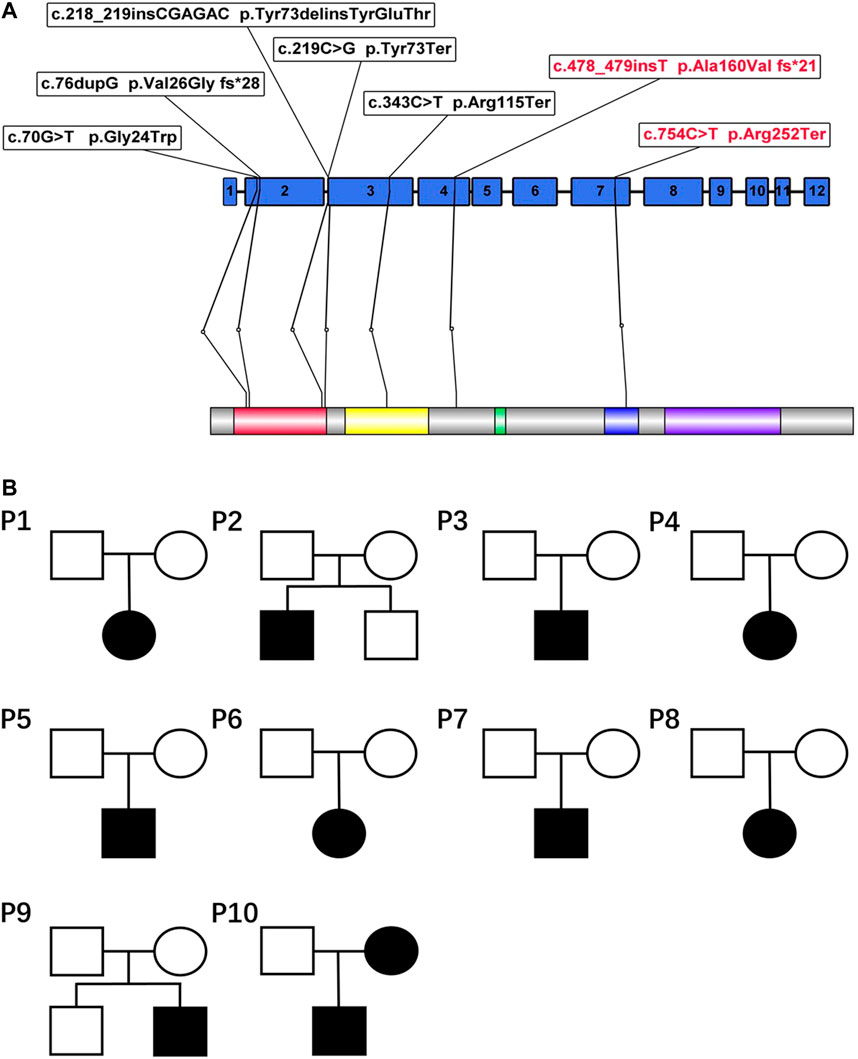
FIGURE 2. (A) PAX2 domain structure and localization of seven variants in this article. The variants marked with red in the figure refer to the novel variants reported for the first time. PAX2 is characterized by an N-terminal paired domain consisting of the N terminus (red) and C terminus (yellow). The relative locations of the other domains are also indicated, including the octapeptide motif (green), the homeodomain(blue), and a transactivation domain (violet). (B) Pedigrees of 10 families. The genetic variants of patient 10 originated from the mother, and his mother had clinical manifestations. The variant of patient 4 originated from her father, whose manifestations were not obvious.
All the variants were heterozygous. Segregation analysis was performed in every patient and almost all first-degree relatives using Sanger sequencing. Most variants could not be detected in their first-degree relatives, suggesting that they may be de novo variants. The variants of two patients (patients 4 and 10) were confirmed to originate from their parents (Figure 2). The variant of patient 10 was derived from his mother. Although she had no obvious clinical symptoms, she also had proteinuria and decreased kidney function. The variant of patient 4 originated from her father, whose routine urine test results were normal.
Discussion
This study presents ten patients with eight different PAX2 variants, and three of these variants were reported for the first time. Every patient in our cohort had confirmed congenital kidney hypoplasia and decreased eGFR, and they all had proteinuria. One patient had a unique histological presentation of high-intensity C1q deposition, and one patient was found to have the comorbidity of OCA, which had not been previously reported. Furthermore, we also reported several novel manifestations of PAX2 variants, including cholecystolithiasis and testicular dysgenesis.
Previous studies have reported that kidney hypoplasia and ocular abnormalities are the most common manifestations of PAX2-associated disease. Except for these symptoms, there may be rare manifestations such as intellectual disability, joint laxity, and sensorineural deafness (Bower et al., 2012; Liu et al., 2018). In our single-center study, kidney hypoplasia was detected in every patient, and several patients had ocular lesions. Among the ten patients, three patients (patients 1, 2, and 9) carried the same PAX2 variant (c.76dupG), and they all had decreased eGFR and kidney hypoplasia. Nevertheless, the results of ophthalmic examinations were not the same. They were not related to each other, neither were they relatives. The variant (c.76dupG) was a recurring mutation that had been reported by many studies, rather than a common mutation derived from a single founder. Compared with the three patients in our study, some patients have been reported to have other presentations, such as microcephaly, mental retardation, soft skin, and VUR (Schimmenti et al., 1997; Ford et al., 2001). This phenomenon also agrees with our earlier observations, which showed that each individual might have a different phenotype even if they carry the same variant within the same family (Iatropoulos et al., 2012). Previous studies found that the PAX2 gene is likely modulated by other genes, amplifying or reducing the genetic effect, which may be explained by haploinsufficiency (Barua et al., 2014). Although many studies have focused on the expression patterns of the mutated gene, several questions remain to be answered, such as incomplete penetrance and parental germline mosaicism. Our study’s de novo rate was 70.0%, higher than that reported (50%) previously (Bower et al., 2012). The small sample size may be the major reason.
Unexpectedly, we found that patient 8 with the PAX2 variant was diagnosed with C1q nephropathy by kidney histological examination, while the gene test showed normal sequences of C1q-related genes. C1q nephropathy is rare, with prevalence ranging from 2.1 to 9.2% in pediatric biopsies (Devasahayam et al., 20152015). As far as we know, this is the first report of C1q nephropathy in a patient with a PAX2 variant. However, the relationship between PAX2 variants and C1q nephropathy is still unclear. It is recognized that PAX2 variants are one of the important causes of FSGS, as reported by many studies (Rachwani Anil et al., 2019; Vivante et al., 2019; Nagano et al., 2020). The systemic activation of complement in primary FSGS has been identified (Huang et al., 2020). In a large study of C1qN, C1q deposition disappeared in some patients throughout the follow-up period, with FSGS developing in repeat biopsy samples (Hisano et al., 2008). As a transcription factor, PAX2 has not been reported to be involved in the pathogenesis of immune diseases. Therefore, we suppose that more studies are needed to clarify the correlation between PAX2 and C1q nephropathy, which could be a research direction in the future.
In our research, five patients (50.0%) developed KF, while the kidney function of the other patients was relatively stable. Although PAX2 variants have been reported every year, it is still unclear what factors affect patients’ disease progression. Unlike most common kidney diseases, the onset age and the duration of progression to KF of this disease vary greatly. In some patients, kidney function deteriorates drastically within a few months, whereas in some patients, kidney function remains stable for decades (Rasmussen et al., 2018; Connaughton et al., 2019; Saida et al., 2020). Compared with the patients with other hereditary kidney diseases, patients with PAX2 variants develop KF mainly in adolescence rather than in infancy or childhood.
There are some limitations to our study. First, our study was retrospective, and most of them had no subjective visual or hearing abnormalities. Therefore, not all the patients had undergone ocular and hearing examinations. Second, only two patients had received a kidney histological examination, and one of them was diagnosed with C1q nephropathy, which is most likely the first report to date. However, the reason for C1q deposition is still unknown and needs to be further explored. Third, except for immediate family members, the other family members’ information was not available in all the patients of our center. However, no suspicious family history was found.
In summary, our study identified three unrecognized variants, two patients with novel presentations, and several novel manifestations of the PAX2 variants. Patients with PAX2 variants developed KF mainly in adolescence rather than in early life, and the clinical manifestations of those patients were highly heterogeneous. Many patients with PAX2 variants developed KF requiring long-term kidney replacement therapy and even transplantation. Thus, individuals with clear evidence of kidney hypoplasia and ocular abnormalities should be suggested for genetic testing. If such individuals have PAX2 variants, they should be examined for PAX2-related organ damage, closely followed up for kidney function, professionally managed to avoid KF development, and indicated for appropriate genetic counseling.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA773192.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Children’s Hospital of Chongqing Medical University. Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MW and QL conceived and designed the work. GZ, HY, and DW performed clinical examinations and interpretation. H-YX collected the clinical data and analyzed the sequencing data. Y-QS, CZ, and QY assisted with data acquisition. H-YX drafted the manuscript, which was revised by YC, QL, and MW.
Funding
This study was sponsored by National Key R&D Program of China (Grant Number: 2021YFC2702002), Research Program of Innovative Research Demonstration Base of Children’s Medical Security (Grant No: NCRCCHD-2019-HP-09), and National Clinical Research Center for Child Health and Disorders (Grant No: NCRC-2020-GP-01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank all of the patients and families in this study. In addition, we would like to thank Wei Jiang and Yi Zhou for their help. Meanwhile, we would like to thank our coordinators from MyGenostics Co.,Ltd. in Beijing for sequencing technology support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.799562/full#supplementary-material
References
Barua, M., Stellacci, E., Stella, L., Weins, A., Genovese, G., Muto, V., et al. (2014). Mutations inPAX2Associate with Adult-Onset FSGS. Jasn 25, 1942–1953. doi:10.1681/ASN.2013070686
Bower, M., Salomon, R., Allanson, J., Antignac, C., Benedicenti, F., Benetti, E., et al. (2012). Update of PAX2 Mutations in Renal Coloboma Syndrome and Establishment of a Locus-specific Database. Hum. Mutat. 33, 457–466. doi:10.1002/humu.22020
Bullich, G., Domingo-Gallego, A., Vargas, I., Ruiz, P., Lorente-Grandoso, L., Furlano, M., et al. (2018). A Kidney-Disease Gene Panel Allows a Comprehensive Genetic Diagnosis of Cystic and Glomerular Inherited Kidney Diseases. Kidney Int. 94, 363–371. doi:10.1016/j.kint.2018.02.027
Connaughton, D. M., Kennedy, C., Shril, S., Mann, N., Murray, S. L., Williams, P. A., et al. (2019). Monogenic Causes of Chronic Kidney Disease in Adults. Kidney Int. 95, 914–928. doi:10.1016/j.kint.2018.10.031
Deng, H., Zhang, Y., Xiao, H., Yao, Y., Liu, X., Su, B., et al. (2019). Diverse Phenotypes in Children with PAX2 ‐related Disorder. Mol. Genet. Genomic Med. 7, e701. doi:10.1002/mgg3.701
Devasahayam, J., Erode-Singaravelu, G., Bhat, Z., Oliver, T., Chandran, A., Zeng, X., et al. (20152015). C1q Nephropathy: The Unique Underrecognized Pathological Entity. Anal. Cell Pathol. 2015, 1–5. doi:10.1155/2015/490413
Ford, B., Rupps, R., Lirenman, D., Van Allen, M. I., Farquharson, D., Lyons, C., et al. (2001). Renal-coloboma Syndrome: Prenatal Detection and Clinical Spectrum in a Large Family. Am. J. Med. Genet. 99, 137–141. doi:10.1002/1096-8628(2000)9999:999<00::aid-ajmg1143>3.0.co;2-f
Grimley, E., and Dressler, G. R. (2018). Are Pax Proteins Potential Therapeutic Targets in Kidney Disease and Cancer? Kidney Int. 94, 259–267. doi:10.1016/j.kint.2018.01.025
Hisano, S., Fukuma, Y., Segawa, Y., Niimi, K., Kaku, Y., Hatae, K., et al. (2008). Clinicopathologic Correlation and Outcome of C1q Nephropathy. Cjasn 3, 1637–1643. doi:10.2215/CJN.00830208
Huang, J., Cui, Z., Gu, Q.-h., Zhang, Y.-m., Qu, Z., Wang, X., et al. (2020). Complement Activation Profile of Patients with Primary Focal Segmental Glomerulosclerosis. PLoS One 15, e0234934. doi:10.1371/journal.pone.0234934
Hwang, D.-Y., Dworschak, G. C., Kohl, S., Saisawat, P., Vivante, A., Hilger, A. C., et al. (2014). Mutations in 12 Known Dominant Disease-Causing Genes Clarify many Congenital Anomalies of the Kidney and Urinary Tract. Kidney Int. 85, 1429–1433. doi:10.1038/ki.2013.508
Iatropoulos, P., Daina, E., Mele, C., Maranta, R., Remuzzi, G., and Noris, M. (2012). Discordant Phenotype in Monozygotic Twins with Renal Coloboma Syndrome and a PAX2 Mutation. Pediatr. Nephrol. 27, 1989–1993. doi:10.1007/s00467-012-2205-x
Ishiwa, S., Sato, M., Morisada, N., Nishi, K., Kanamori, T., Okutsu, M., et al. (2019). Association between the Clinical Presentation of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) and Gene Mutations: an Analysis of 66 Patients at a Single Institution. Pediatr. Nephrol. 34, 1457–1464. doi:10.1007/s00467-019-04230-w
Ketteler, M., Block, G. A., Evenepoel, P., Fukagawa, M., Herzog, C. A., McCann, L., et al. (2018). Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann. Intern. Med. 168, 422–430. doi:10.7326/M17-2640
Lee, S.-T., Nicholls, R. D., Bundey, S., Laxova, R., Musarella, M., and Spritz, R. A. (1994). Mutations of the P Gene in Oculocutaneous Albinism, Ocular Albinism, and Prader-Willi Syndrome Plus Albinism. N. Engl. J. Med. 330, 529–534. doi:10.1056/NEJM199402243300803
Liu, J., Wang, P., Huang, J., and Yu, Z. (2018). Rethinking Genotype-Phenotype Correlations in Papillorenal Syndrome: a Case Report on an Unusual Congenital Camptodactyly and Skeletal Deformity with a Heterogeneous PAX2 Mutation of Hexanucleotide Duplication. Gene 641, 74–77. doi:10.1016/j.gene.2017.10.050
Nagano, C., Yamamura, T., Horinouchi, T., Aoto, Y., Ishiko, S., Sakakibara, N., et al. (2020). Comprehensive Genetic Diagnosis of Japanese Patients with Severe Proteinuria. Sci. Rep. 10, 270. doi:10.1038/s41598-019-57149-5
Naiman, N., Fujioka, K., Fujino, M., Valerius, M. T., Potter, S. S., McMahon, A. P., et al. (2017). Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron-Interstitium Boundary during Kidney Development. Developmental Cel 41, 349–365. e3. doi:10.1016/j.devcel.2017.04.022
Rachwani Anil, R., Rocha-de-Lossada, C., Ayala, C. H., and Contreras, M. E. (2019). A New Mutation in the PAX2 Gene in a Papillorenal Syndrome Patient. Am. J. Ophthalmol. Case Rep. 16, 100563. doi:10.1016/j.ajoc.2019.100563
Rasmussen, M., Sunde, L., Nielsen, M. L., Ramsing, M., Petersen, A., Hjortshøj, T. D., et al. (2018). Targeted Gene Sequencing and Whole-Exome Sequencing in Autopsied Fetuses with Prenatally Diagnosed Kidney Anomalies. Clin. Genet. 93, 860–869. doi:10.1111/cge.13185
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and Guidelines for the Interpretation of Sequence Variants: a Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. doi:10.1038/gim.2015.30
Rossanti, R., Morisada, N., Nozu, K., Kamei, K., Horinouchi, T., Yamamura, T., et al. (2020). Clinical and Genetic Variability of PAX2-Related Disorder in the Japanese Population. J. Hum. Genet. 65, 541–549. doi:10.1038/s10038-020-0741-y
Saida, K., Kamei, K., Morisada, N., Ogura, M., Ogata, K., Matsuoka, K., et al. (2020). A Novel Truncating PAX2 Mutation in a Boy with Renal Coloboma Syndrome with Focal Segmental Glomerulosclerosis Causing Rapid Progression to End-Stage Kidney Disease. CEN Case Rep. 9, 19–23. doi:10.1007/s13730-019-00419-y
Schimmenti, L. A., Cunliffe, H. E., McNoe, L. A., Ward, T. A., French, M. C., Shim, H. H., et al. (1997). Further Delineation of Renal-Coloboma Syndrome in Patients with Extreme Variability of Phenotype and Identical PAX2 Mutations. Am. J. Hum. Genet. 60, 869–878.
Vivante, A., Chacham, O. S., Shril, S., Schreiber, R., Mane, S. M., Pode-Shakked, B., et al. (2019). Dominant PAX2 Mutations May Cause Steroid-Resistant Nephrotic Syndrome and FSGS in Children. Pediatr. Nephrol. 34, 1607–1613. doi:10.1007/s00467-019-04256-0
Wei, A., Wang, Y., Long, Y., Wang, Y., Guo, X., Zhou, Z., et al. (2010). A Comprehensive Analysis Reveals Mutational Spectra and Common Alleles in Chinese Patients with Oculocutaneous Albinism. J. Invest. Dermatol. 130, 716–724. doi:10.1038/jid.2009.339
Keywords: PAX2 gene, novel variant, children, C1q nephropathy, kidney hypoplasia
Citation: Xiong H-Y, Shi Y-Q, Zhong C, Yang Q, Zhang G, Yang H, Wu D, Chen Y, Li Q and Wang M (2022) Detection of De Novo PAX2 Variants and Phenotypes in Chinese Population: A Single-Center Study. Front. Genet. 13:799562. doi: 10.3389/fgene.2022.799562
Received: 21 October 2021; Accepted: 01 March 2022;
Published: 31 March 2022.
Edited by:
Tiffany Cook, Wayne State University, United StatesReviewed by:
Muhammad Ansar, Quaid-i-Azam University, PakistanAndrew Mallett, Townsville University Hospital, Australia
Copyright © 2022 Xiong, Shi, Zhong, Yang, Zhang, Yang, Wu, Chen, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Li, bGlxaXU4MDlAMTI2LmNvbQ==; Mo Wang, d2FuZ21vX2NxbXVAMTYzLmNvbQ==
 Hua-Ying Xiong
Hua-Ying Xiong Yong-Qi Shi
Yong-Qi Shi Cheng Zhong
Cheng Zhong Qin Yang1,2
Qin Yang1,2 Gaofu Zhang
Gaofu Zhang Yaxi Chen
Yaxi Chen Qiu Li
Qiu Li Mo Wang
Mo Wang