- Department of Cardiothoracic Surgery, Taihe Hospital, Hubei University of Medicine, Shiyan, China
Esophageal cancer (ESCA) is one of the common malignant tumors. The roles and signaling mechanisms of spindle apparatus coiled-coil protein 1 (SPDL1) in ESCA progression have not been reported previously. Therefore, the expression levels and potential clinical roles of SPDL1 were investigated using data from multiple databases and tissue samples of 53 ESCA patients who underwent 18F-FDG positron emission tomography (PET)/computed tomography (CT) before therapy. The signaling mechanisms of SPDL1 involved in ESCA progression were investigated via bioinformatics analysis. The effects of SPDL1 on the growth and migration of ESCA cells were investigated using CCK-8, Edu, and transwell assays. SPDL1 was upregulated in ESCA tissues. Increased SPDL1 expression was associated with age, grade, drinking history, cancer stage, lymph node metastasis, TP53 mutation, and poor prognosis in patients with ESCA. SPDL1 overexpression was significantly correlated with SUVmax, SUVmean, and TLG of PET/CT. SPDL1 silencing inhibited cell proliferation, migration, and invasion. SPDL1 was significantly enriched in cell cycle, spliceosome, DNA replication, and other processes. The hub genes of a constructed protein–protein interaction network included CDK1, BUB1, CCNB1, BUB1B, CCNA2, CDC20, MAD2L1, AURKB, NDC80, and PLK1, which were related to SPDL1 expression. The findings of this study suggest that SPDL1 may serve as a biomarker of ESCA prognosis.
1 Introduction
Esophageal cancer (ESCA) is one of the most common cancers worldwide, including China, with the ninth-highest incidence and sixth-highest mortality worldwide (Chen et al., 2016; Bray et al., 2018). At present, due to the lack of target molecules for early diagnosis, drug treatment, and evaluation of prognosis of ESCA, the five-year survival rate of cancer patients remains low (Zarean et al., 2018; Jingu et al., 2019; Lin et al., 2019). Therefore, new biomarkers are sought after to improve the diagnosis rate and to find new targeted therapies to improve the overall survival (OS) of ESCA patients.
Under physiological conditions, the mitotic spindle can regulate the microtubule cytoskeleton. However, under pathological conditions, abnormal regulation of microtubule cytoskeleton leads to genomic instability, which is associated with cancer progression (Qin et al., 2017). For example, Polo-like kinase 1 (PLK1) is a key kinase that regulates mitosis. The PLK1-specific inhibitor BI2536 can activate spindle assembly checkpoint (SAC) in non-small cell lung cancer cells. Excessive activation of SAC results in cell death (Choi et al., 2015). Mitotic arrest defective protein 2 (MAD2) is a key gene that regulates mitosis. MAD2 expression level is correlated with age, lymph node metastasis, and survival time of small-cell lung cancer patients (Wu et al., 2018). Spindle apparatus coiled-coil protein 1 (SPDL1), also known as CCDC99, located at 5q35.1, encodes a protein containing a helical domain, which plays an important role in mitotic spindle formation and chromosome segregation (Barisic et al., 2010; Kodama et al., 2019). The myocardium-associated transcription factor B (MRTFB) can suppress the invasion and migration of colorectal cancer (CRC) cells. Disrupting SPDL1 expression in CRC cells significantly increases the invasion and migration of CRC cells. In the intestinal tract of mice, the knockdown of MRTFB inhibits SPDL1 expression. Decreased SPDL1 expression is associated with a low survival rate of CRC. Regulation of melanoma cell adhesion molecule and SPDL1 promotes the development of xenografts in nude mice (Kodama et al., 2019). This indicates that SPDL1 functions as a tumor suppressor gene in CRC development. However, the role of SPDL1 in ESCA development has not been reported. Therefore, we evaluated SPDL1 expression in ESCA tissues via UALCAN, The Cancer Genome Atlas (TCGA), and TIMER database analyses, and used clinical tissues in the present study. We aimed to determine the relationship between SPDL1 expression and clinical values in patients with ESCA and determine the signaling mechanisms associated with SPDL1 that are involved in the progression of ESCA.
2 Materials and Methods
2.1 UALCAN Database
The correlation between SPDL1 expression in ESCA tissues and the clinicopathological features (race, sex, age, drinking history, body weight, histological subtype, smoking history, cancer stage, tumor grade, and TP53 mutation status) of ESCA patients were investigated using the UALCAN (http://ualcan.path.uab.edu) database (Chandrashekar et al., 2017).
2.2 The Cancer Genome Atlas and Genotype-Tissue Expression Databases
The gene expression data of pan-cancer cases were downloaded from the TCGA (https://portal.gdc.cancer.gov/) and GTEx (https://gtexportal.org/home/datasets) databases and were analyzed to determine SPDL1 expression levels. In total, 11,093 tissue profiles of cancer patients were downloaded from the TCGA database, 4,124 tissue profiles were downloaded from the GTEx database, and 15,776 tissue profiles were downloaded from the XENA-TCGA- GTEx database. The data from TCGA and GTEx databases were corrected, normalized, processed for fold-changes, and merged. In total, 171 cases of TCGA ESCA HTSeq-FPKM, including 11 cases of normal esophagus tissues and 160 cases of ESCA tissues, were obtained, and 8 esophagus tissues and 8 ESCA tissues were derived from the same patients. SPDL1 expression in ESCA tissues was analyzed for TCGA-derived data. The clinical data of 183 patients with ESCA obtained from the TCGA database were analyzed to determine the prognosis of ESCA patients after collating the clinical data.
2.3 Clinical ESCA Tissue Samples
Cancer tissues and adjacent tissues were collected from 53 ESCA patients, and SPDL1 expression in cancer tissues was determined via immunohistochemistry (IHC). This study was approved by the ethics committee of Taihe Hospital. The clinical data of patients, including diagnosis age, gender, T stage, and positron emission tomography (PET)/computed tomography (CT) index, were collected and applied to analyze the SPDL1 expression level in clinical roles.
2.4 Immunohistochemistry
ESCA paraffin sections were deparaffinized. The sections were incubated with 3% H2O2 at room temperature for 5 min, after which antigen retrieval was performed. The samples were blocked with 10% goat serum and incubated with 1:100 SPDL1 (Proteintech, China) antibody working solution at 4°C overnight. Subsequently, an appropriate amount of biotin-labeled secondary antibody working solution was added following incubation at 37°C. After dolichos biflorus agglutinin staining, counterstaining, dehydration, transparency, mounting and photographing, and other processes, the SPDL1 expression level in ESCA tissues was determined.
2.5 Genes Co-Expressed With SPDL1
R (version: 3.6.1) was used to filter genes co-expressed with SPDL1 in 160 ESCA tissues. Genes associated with p < 0.001 and Pearson’s coefficient (r > 0.4 or < −0.4) were considered to be strongly co-expressed with SPDL1 (Guo et al., 2020). Pearson’s coefficient indicated the association between the two genes to demonstrate the roles of SPDL1.
2.6 Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, and Gene Set Enrichment Analysis
The biological functions and signaling pathways of genes co-expressed with SPDL1 were investigated using GO and KEGG. The 160 ESCA cases were assigned to high-SPDL1 and low-SPDL1 expression groups according to the median value of SDPL1 expression. The effect of SPDL1 expression level on each gene was investigated using GSEA, and each analysis was performed 1,000 times (Guo et al., 2021; Zhang et al., 2021).
2.7 Protein–Protein Interaction Network in the Search Tool for the Retrieval of Interacting Genes
The STRING (https://string-db.org/) database was applied to investigate the PPI of multiple genes. Genes co-expressed with SPDL1 were evaluated using the STRING database to establish the PPI network using a combined score >0.7. The top 10 highly connected co-expressed genes were identified using CytoHubba plug-in in Cytoscape 3.6.1 and defined as hub genes. The TCGA and GTEx databases were analyzed using the Gene Expression Profiling Interactive Analysis (GEPIA) database to determine the expression levels of hub genes.
2.8 Cell Culture and Transfection
EC109 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) (HyClone, China), and cells showing optimal growth were plated in 6-well plates and cultured for 24 h. SPDL1 small interfering RNA (siRNA) or microRNA (miRNA) mimics/inhibitors were transfected using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA). SPDL1 mRNA and protein expression levels in SPDL1 siRNA-transfected (si-SPDL1) and negative control siRNA-transfected (si-NC) groups were determined via quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blotting (WB), respectively. SPDL1 siRNA was purchased from GenePharma (Shanghai, China), and the sense sequence is GGGAGAAGUUUAUCGAUUATT, and the antisense sequence is UAAUCGAUAAACUUCUCCCTT that interfere with SPDL1 expression. Total RNA and proteins were extracted according to standard procedures, and PCR and WB were performed (Liu et al., 2020). PCR primers were purchased from Genecopoeia (HQP105394) in China, and anti-SPDL1 antibodies were purchased from Proteintech (China).
2.9 Cell Proliferation
Transfected EC109 cells were digested using trypsin and resuspended in 96-well plates. Each group was dispensed in three duplicate wells. Subsequently, 10 µL/well of CCK-8 was added daily, following incubation for 1 h, and the optical density at 450 nm was determined for each well. Transfected EC109 cells (5 × 103 cells/well) were seeded in the 96-well plate. The plates were incubated after addition of pre-warmed Edu working solution (Genecopoeia, China) and an equal volume of medium. EC109 cells were fixed in 4% neutral paraformaldehyde for 15–30 min. Subsequently, the fixative was removed and cells were washed, followed by Edu detection, DNA counterstaining, imaging, and analysis. The experiment was repeated thrice.
2.10 Cell Migration and Invasion
Transwell chambers were coated with 1:10 diluted Matrigel, and the transwell chambers were placed in a 24-well plate. Transfected EC109 cells were suspended in serum-free DMEM medium and seeded in transwell chambers. DMEM (500 μL) and 10% fetal bovine serum were added to the bottom of the chamber following incubation at 37°C. After 24 h, the chamber was removed, fixed with 4% neutral paraformaldehyde, stained with crystal violet, and the cells were photographed and counted. The experiment was repeated thrice.
2.11 Statistical Analysis
SPDL1 expression in cancer tissues and the effects of SPDL1 on proliferation, migration, and invasion of ESCA cells were evaluated using the t-test. The values of SPDL1 expression level in ESCA patient diagnosis and prognosis were analyzed via the receiver operating characteristic (ROC) curve and survival analysis. The chi-square test was applied to investigate the relationship between SPDL1 expression in clinical tissues and clinicopathological characteristics. Values of p < 0.05 were considered significant.
3 Results
3.1 SPDL1 Was Abnormally Expressed in Pan-Cancer Tissues
SPDL1 was abnormally expressed in pan-cancer tissues based on data obtained from the TCGA and XENA-TCGA-GTEx databases (Figure 1). The pan-cancer data from the TCGA database were sorted which showed that SPDL1 was abnormally expressed in several matched cancer tissues (Supplementary Figure S1). SPDL1 was upregulated in bladder cancer, breast cancer, cholangiocarcinoma, ESCA, squamous cell cancer in the head and neck region, kidney renal clear cell carcinoma, and other tissues, but was downregulated in kidney chromophobe tissues (Supplementary Figure S1).

FIGURE 1. Pan-cancer data from multiple databases showed that SPDL1 was abnormally expressed in pan-cancer tissues. (A) TPM-type data from the TCGA database. (B) TPM-type data from the TCGA + GTEx databases. (C) FPKM-type data from the TCGA database. SPDL1, spindle apparatus coiled-coil protein 1; TCGA, The Cancer Genome Atlas; GTEx, Genotype-Tissue Expression; TPM, transcripts per million; FPKM, fragments per kilobase per million; NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3.2 SPDL1 Was Upregulated in ESCA
In the TCGA and XENA-TCGA-GTEx databases, SPDL1 expression was increased in both unpaired ESCA patients (Figure 1) and in 8 pairs of ESCA patients (Supplementary Figure S1). In 53 ESCA cases, the SPDL1 protein level was increased in most paired tissues (Figure 2). Among them, SPDL1 expression level was increased in 44 ESCA tissues and significantly decreased in 9 cancer patients.

FIGURE 2. SPDL1 was overexpressed in clinical ESCA tissues obtained in our hospital. ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
3.3 Relationship of SPDL1 Expression With Clinical Features of ESCA
The SPDL1 expression level was evaluated with respect to the clinical features of ESCA patients available on the UALCAN database (Figure 3). SDPL1 expression level was associated with lymph node metastasis (N0-vs-N1; N0-vs-N2; N0-vs-N3), TP53 mutation (TP53-mutant-vs-TP53-non-mutant), age ((age (41–60yrs)-vs-age (61–80yrs)) and (age (41–60yrs)-vs-age (81–100yrs)), grade (grade2-vs-grade3), drinking history (0 Ddys/week-vs-7 Ddays/week, 1 day/week-vs-4days/week, 4 ay/week-vs-5 days/week, 4 day/week-vs-7 days/week, and 5 day/week-vs-7 days/week), and cancer stage (stage1-vs-stage2, stage1-vs-stage3, and stage1-vs-stage4) of ESCA patients which was significant.
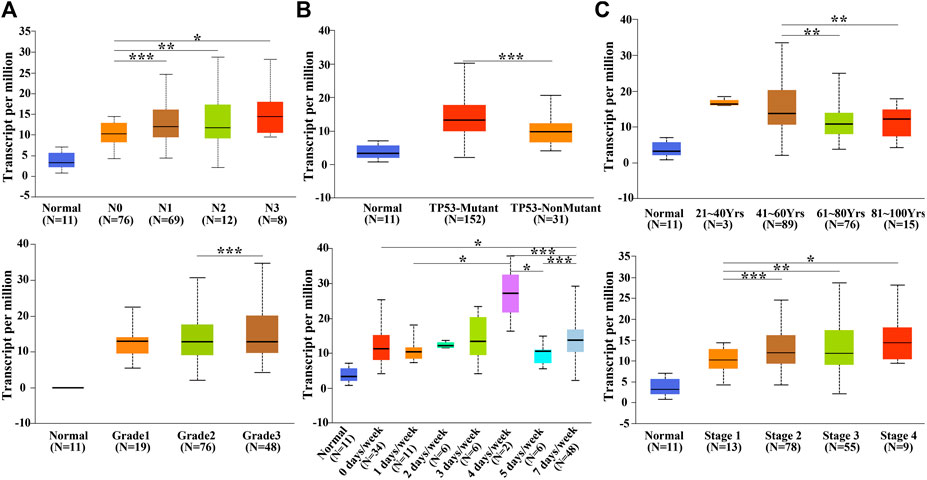
FIGURE 3. SPDL1 expression level was related to the clinicopathological features of patients with ESCA in the UALCAN database. (A) Lymph node metastasis; (B) TP53 mutation; (C) age; (D) grade; (E) drinking history; and (F) clinical stage. Normal esophageal tissues; ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
The demographic data of 53 ESCA patients are shown in Figure 4. The relationship between SPDL1 expression level and clinicopathological data (age, gender, grade, T stage, lymph node metastasis, nerve invasion, and vascular invasion) of ESCA patients was investigated. The results were not significant (Table 1). SPDL1 expression level was significantly correlated with maximum standardized uptake value (SUVmax), SUVmean, and total legion glycolysis (TLG), but was not correlated with metabolic tumor volume, tumor diameter, and tumor invasion depth of PET/CT (Table 2).
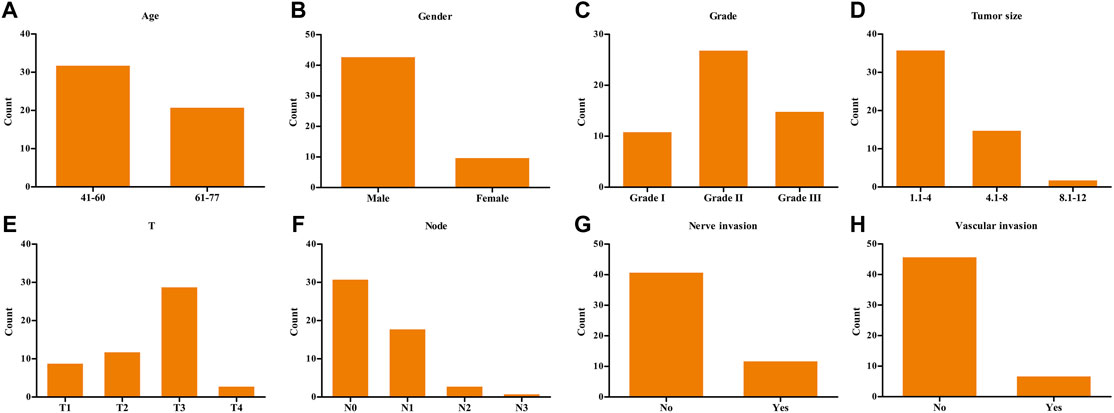
FIGURE 4. Demographic data of 53 clinical ESCA patients. (A) Age; (B) gender; (C) grade; (D) tumor size; (E) T stage; (F) lymph nodes; (G) nerve invasion; and (H) vascular invasion. ESCA, esophageal cancer.
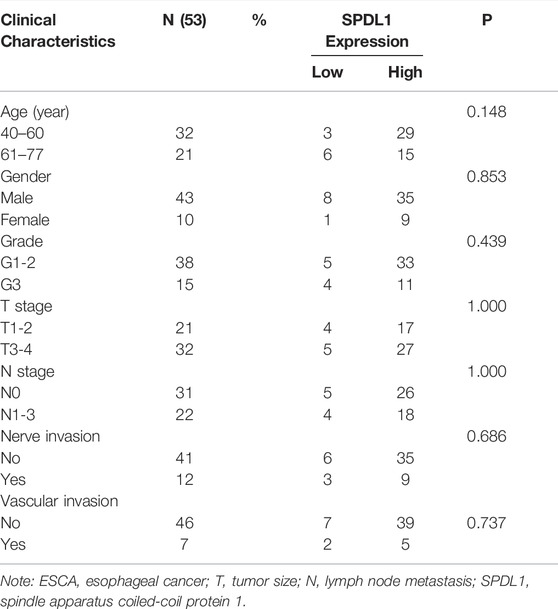
TABLE 1. Relationship between SPDL1 expression level and the clinicopathological data of 53 ESCA patients.
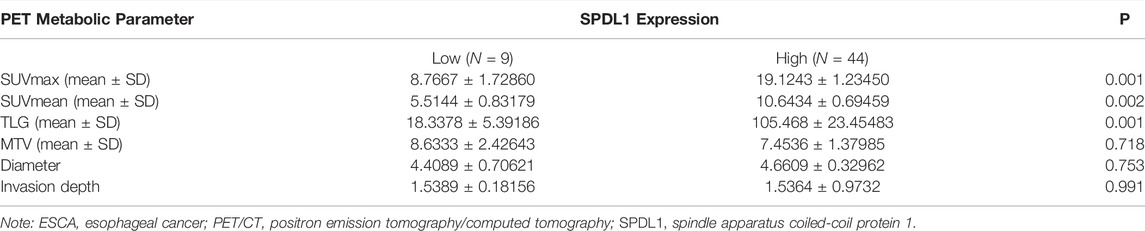
TABLE 2. SPDL1 overexpression was associated with 18F-FDG PET/CT metabolic parameters of ESCA patients.
3.4 SPDL1 Upregulation Was an Adverse Effect on the Poor Prognosis of ESCA Patients
SPDL1 is valuable in the diagnosis and prognosis of ESCA (Figure 5). ROC analysis showed that SPDL1 has the ESCA diagnostic value based on TCGA data (Figure 5A), and the area under the curve of SPDL1 was 0.938. SPDL1 upregulation represented an adverse effect on the prognosis of ESCA patients based on survival analysis using TCGA database and clinical samples (Figures 5B,C). SPDL1 expression was significantly abnormal in the disease-specific survival (DSS) and progression-free interval (PFI) events, and was relatively higher in case of deaths (Figures 5D,E).
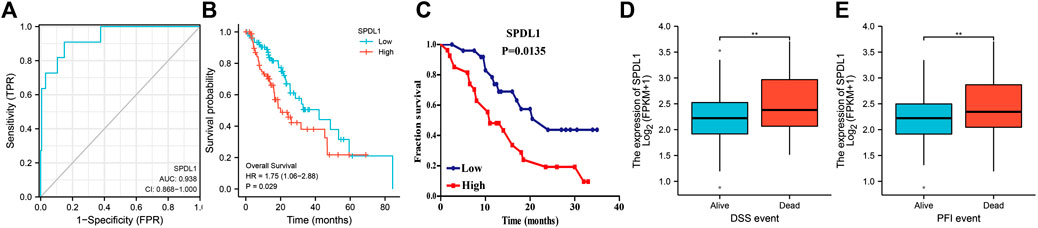
FIGURE 5. SPDL1 expression levels in diagnosing ESCA and evaluating the prognosis of patients. (A) Diagnostic value; (B, C) OS; (D) DSS; (E) and PFI. OS, overall survival; DSS, disease-specific survival; PFI, progression-free interval; ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
3.5 Biological Functions and Signaling Mechanisms of SPDL1
In total, 393 genes were found to be co-expressed with SDPL1 (Supplementary Table S1). Figure 6 shows the top 9 co-expressed genes of SDPL1. The co-expressed genes mainly participated in chromosome segregation, positive regulation of cell cycle process, DNA replication, cell cycle G2/M phase transition, and meiotic cell cycle based on GO annotation (Figures 7A–C and Supplementary Table S2). KEGG analysis showed that the co-expressed genes mainly participated in DNA replication, homologous recombination (HR), p53 signaling pathway, RNA degradation, and other signaling pathways (Figure 7D and Table 3). GSEA showed that DNA replication, HR, and p53 signaling pathway were highly enriched in the high-SPDL1 expression group (Figure 8 and Table 4).
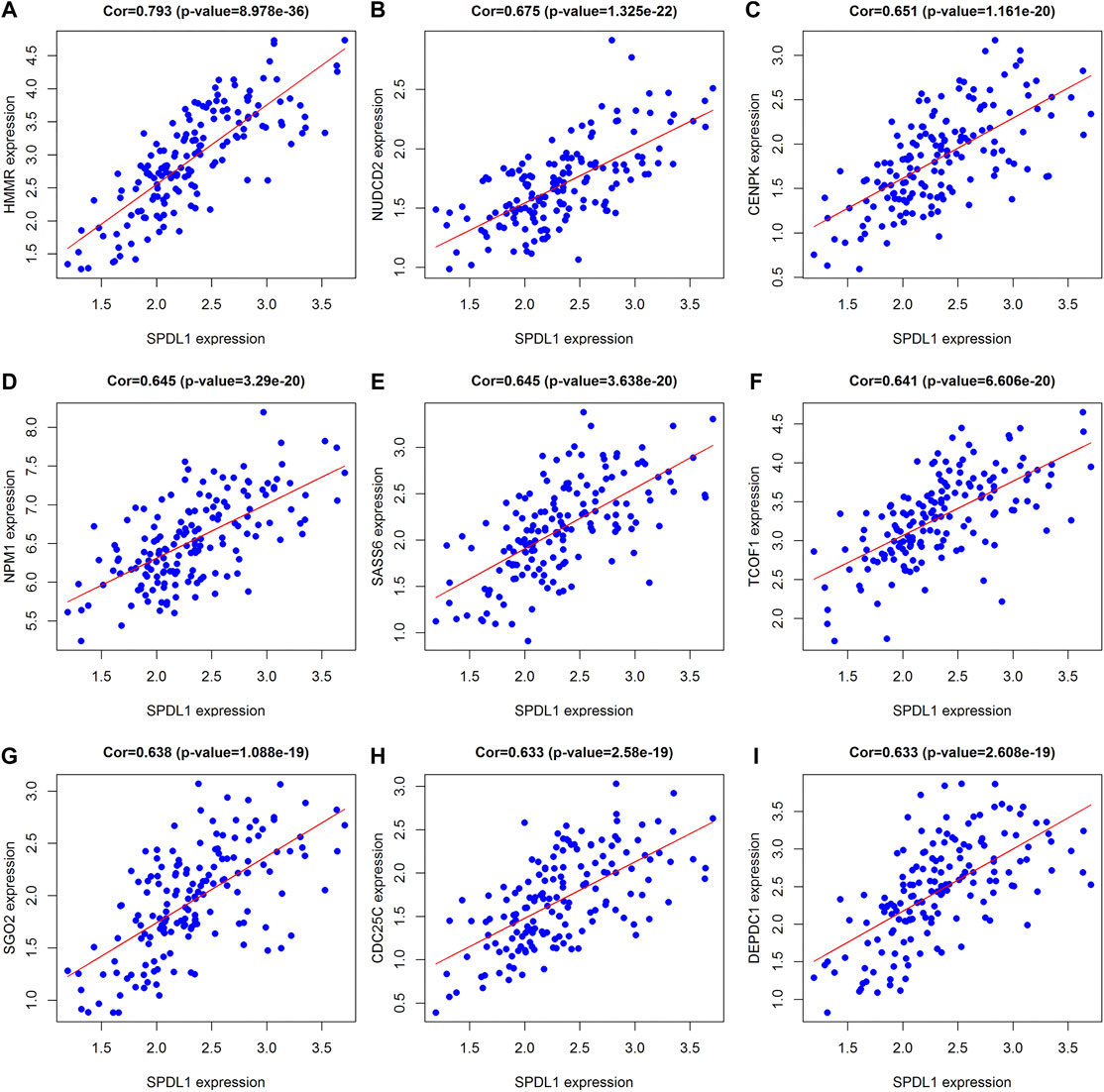
FIGURE 6. Genes co-expressed with SPDL1 ranked are shown using correlation coefficient. (A) HMMR; (B) NUDCD2; (C) CENPK; (D) NPM1; (E) SASS6; (F) TCOF1; (G) SGO2; (H) CDC25C; and (I) DEPDC1. ESCA, esophageal cancer tissue; SPDL1, spindle apparatus coiled-coil protein 1.
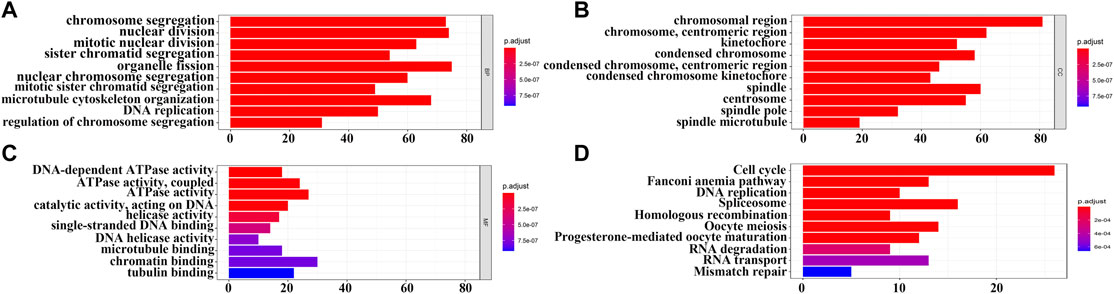
FIGURE 7. Biological functions and signaling mechanisms of genes co-expressed with SPDL1 were determined via GO and KEGG analysis. (A) BP; (B) CC; (C) MF; and (D) KEGG. BP, biological processes; MF, molecular functions; CC, cellular components; KEGG, Kyoto encyclopedia of genes and genomes; ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
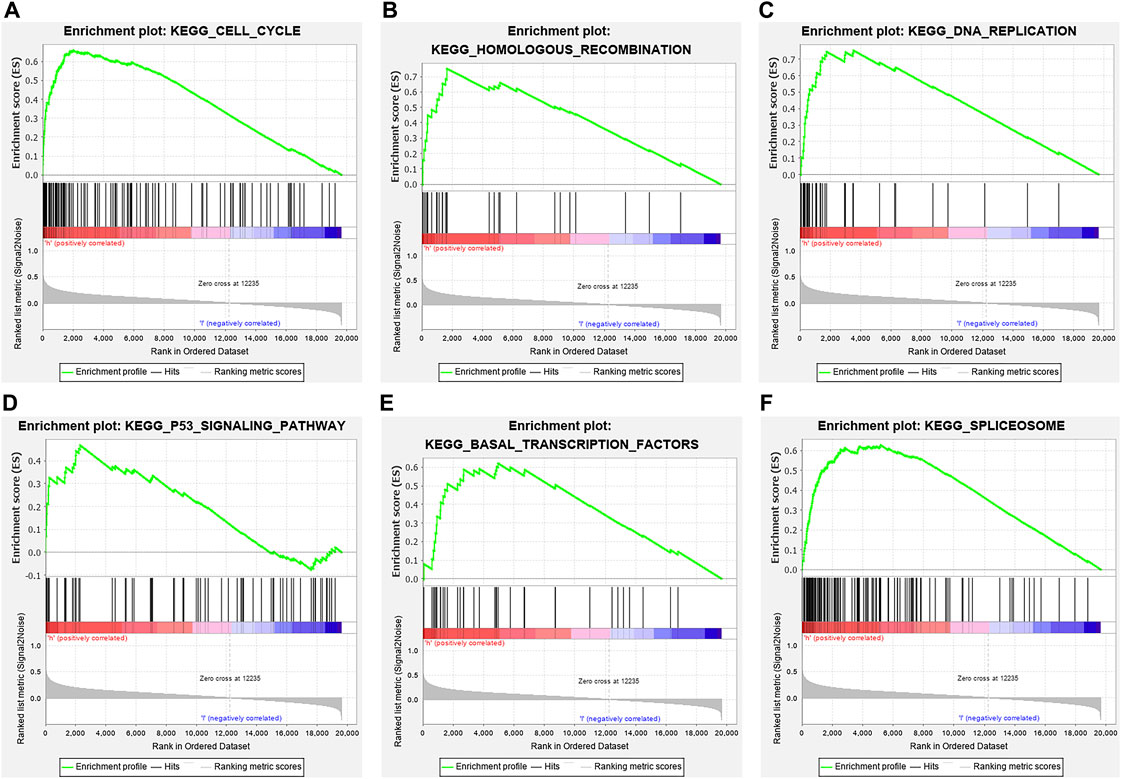
FIGURE 8. ESCA-related signaling pathways enriched in the high-SPDL1 expression group via GSEA. (A) Cell cycle; (B) HR; (C) DNA replication; (D) p53 signaling pathway; (E) basal transcription factor; and (F) spliceosome. GSEA, gene set enrichment analysis; HR, homologous recombination; ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
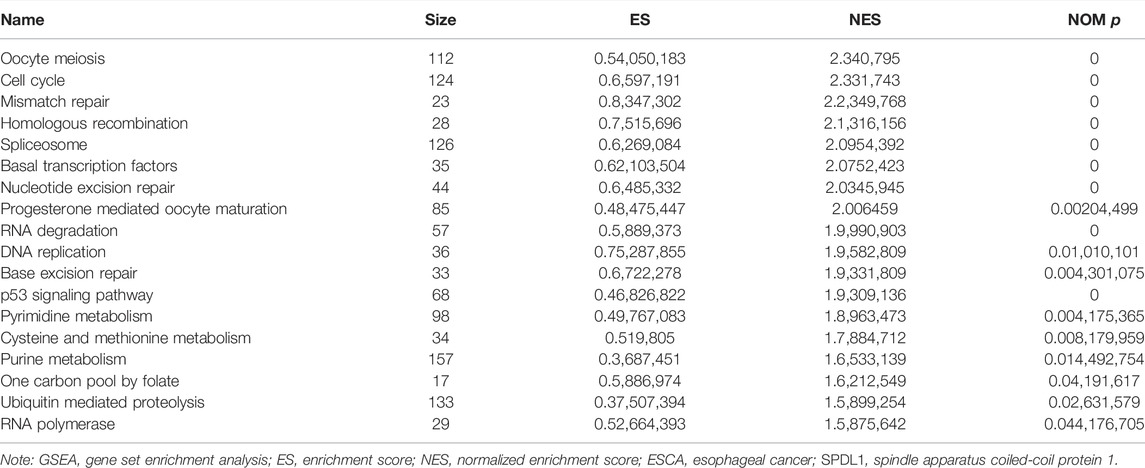
TABLE 4. Mechanisms of upregulated SPDL1 involved in the occurrence and development of ESCA based on GSEA.
3.6 Construction of Protein–Protein Interaction Network
A PPI network was constructed using the STRING database (Supplementary Figure S2A). The hub genes in the PPI network were found to be cyclin-dependent kinase 1 (CDK1), mitotic checkpoint serine/threonine-protein kinase BUB1 (BUB1), G2/mitotic-specific cyclin-B1 (CCNB1), BUB1B, cyclin A2 (CCNA2), cell division cycle 20 (CDC20), mitotic arrest deficient 2 like 1 (MAD2L1), Aurora kinase B (AURKB), kinetochore protein NDC80 homolog (NDC80), and PLK1 (Supplementary Figure S2). Correlation analysis showed that SPDL1 levels and CDK1, BUB1, CCNB1, BUB1B, CCNA2, CDC20, MAD2L1, AURKB, NDC80, and PLK1 levels were significantly correlated (Table 5).
3.7 SDPL1 Silencing Inhibited ESCA Cell Proliferation and Migration
The SDPL1 mRNA expression level was significantly decreased in the si-SPDL1 group based on qRT-PCR analysis (Figure 9A). The sequence of SPDL1 which showed the best interference was selected to verify the protein expression of SPDL1 in si-SPDL1 and si-NC groups via WB. SPDL1 protein level was significantly decreased in the si-SPDL1 group (Figure 9B). CCK-8 and Edu analyses showed that cell proliferation was significantly decreased after SPDL1 silencing. Cells in the si-SPDL1 group showed inhibited cell proliferation (Figures 9C,D). Transwell analyses showed that cells in the si-SPDL1 group showed significantly decreased cell migration and invasion abilities (Figures 9E,F).

FIGURE 9. SPDL1 silencing inhibits ESCA cell growth and migration. (A,B) Establishment of ESCA cell model; (C,D) cell proliferation determined via Cell Counting Kit-8 and Edu methods and (E,F) cell migration and invasion determined using Transwell assays. ESCA, esophageal cancer; SPDL1, spindle apparatus coiled-coil protein 1.
4 Discussion
The five-year survival time of patients with ESCA remains low due to the lack of target molecules for early diagnosis, drug treatment, and evaluation of prognosis of ESCA. Consequently, new biomarkers are sought after to improve the overall survival of ESCA patients. SPDL1 has been found to play a role in cancer progression; however, its role in ESCA remains unknown. Under physiological conditions, mitosis plays an important role in maintaining normal cell growth and development. Abnormal mitosis is closely associated with cell proliferation and apoptosis (Kalimutho et al., 2018; Matsunuma et al., 2018; Liu et al., 2019). For example, in dihydropyrimidinase-like 3 (DPYSL3)-positive breast cancer CLOW cells, cell proliferation decreases and the expression of marker genes in epithelial-mesenchymal transformation increases in the DPYSL3 knockdown group. The low proliferation of DPYSL3-negative CLOW cells is associated with the accumulation of multinucleated cells, suggesting that it is associated with mitotic defects and increased vimentin levels and vimentin phosphorylation (Matsunuma et al., 2018). SPDL1 plays an important role in mitotic spindle formation and chromosome segregation (Barisic et al., 2010; Kodama et al., 2019). Tian et al. reported that abnormal spindle-like microcephaly-associated protein, BUB1B, and SPDL1 are highly expressed in pancreatic ductal adenocarcinoma and are associated with poor OS and disease-free survival (Tian and Wang, 2020). Our results revealed that SPDL1 expression was increased in ESCA tissues based on TCGA database and clinical samples, and was associated with lymph node metastasis, TP53 mutation, age, grade, drinking history, and cancer stage of ESCA patients based on bioinformatics analysis. SPDL1 was found to have an ESCA diagnostic value, and SPDL1 upregulation had an adverse effect on the prognosis of ESCA patients. In 53 ESCA patients, SPDL1 expression was significantly correlated with SUVmax, SUVmean, and TLG of PET/CT, and was not related to the age, gender, grade, T stage, lymph node metastasis, nerve invasion, and vascular invasion. These results indicate that SPDL1 may serve as a diagnostic and prognostic marker of ESCA.
SPDL1 is a target molecule downstream of MRTFB. MRTFB inhibits the invasion and migration of CRC cells. However, disruption of SPDL1 expression in CRC cells significantly increases invasion and migration. Disruption of MRTFB expression suppresses SPDL1 expression in the intestinal tract of mice. SPDL1 expression has been found to be significantly correlated to the survival rate of mice. Disruption of SPDL1 expression promotes the development of xenografts in nude mice (Kodama et al., 2019). In our cell model, interference with SPDL1 expression inhibited ESCA cell growth and migration of cells transfected with SPDL1 siRNA. In addition, cell cycle, HR, DNA replication, and p53 signaling pathway, which were associated with SPDL1 expression, represent the most common mechanisms of cancer progression (Zou et al., 2016; Fu et al., 2018; Lv et al., 2018; Mahajan et al., 2019). For example, Fu et al. reported that ARC15 and ARC17 induce apoptosis in colon cancer cells by increasing PUMA expression and activating mitochondria, which is related to cell cycle arrest induced by increased p21 expression, inhibition of proteasome activity and MDM2 expression, and p53 activation and accumulation (Fu et al., 2018). Cell proliferation has been found to increase with abnormal checkpoint expression when cancer cells show HR deficiency. Tumor suppressor proteins BRCA2 and p53 checkpoint regulators may counteract abnormal cell proliferation. RAD52 attenuates the inhibition of HR in BRCA2-deficient cells via p53 (Mahajan et al., 2019). In our study, SPDL1 was significantly associated with mitotic nuclear division, DNA replication, cell cycle, HR, and p53 signaling pathway based on GO and KEGG analyses, and SPDL1 silencing inhibited ESCA cell growth and migration. These results indicated that SPDL1 may participate in the progression of ESCA via regulation of the cell cycle, HR, DNA replication, and p53 signaling pathway. The mechanisms associated with the role of SPDL1 in ESCA progression should be investigated in future studies.
At present, many studies have shown that the hub genes CDC20, CDK1, BUB1, CCNB1, and BUB1B identified in our PPI network are involved in cancer progression (Zhang et al., 2018; Piao et al., 2019; Li et al., 2020; Zhang et al., 2020; Gao et al., 2021). CCNB1 expression is relatively higher in pancreatic cancer tissues. CCNB1 expression in shCCNB1-transfected cells is relatively lower, the ratio of proliferating and S phase cells in shCCNB1-transfected cells is decreased, and the ratio of apoptosis, senescence, and G0/G1 phase cells is increased. CCNB1 silencing inhibits cell proliferation and promotes cell senescence by activating the p53 signaling pathway (Zhang et al., 2018). Overexpression of CDC20 is associated with poor prognosis in patients with osteosarcoma. Downregulation of CDC20 inhibits the proliferation of osteosarcoma cells and induces apoptosis and cell cycle arrest. CDC20 overexpression promotes cell growth and inhibits cell apoptosis (Gao et al., 2021). Therefore, SPDL1 may be of high value in cancer progression.
In conclusion, SPDL1 was overexpressed in ESCA tissues. Increased SPDL1 expression was associated with diagnosis and poor prognosis of ESCA patients. SPDL1 overexpression was associated with 18F-FDG PET/CT metabolic parameters, where ESCA patients that showed high PET/CT SUVmax, SUVmean, and TLG had a poor prognosis. However, we found that the two were not significant when the relationship between PET/CT metabolic parameters (SUVmax, SUVmean, and TLG) and the prognosis of ESCA patients were analyzed. SPDL1 was upregulated in ESCA, and increased SPDL1 expression was associated with poor prognosis, and cancer progression. SPDL1 may regulate ESCA progression via the cell cycle, HR, DNA replication, and p53 signaling pathway.
Data Availability Statement
The data analyzed in this study could be obtained in TCGA (https://portal.gdc.cancer.gov/projects/) and GTEx (https://gtexportal.org/home/datasets) databases, and relevant research materials could be obtained from the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Taihe Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JZ and J-LG contributed to conception of the research topic, prepared the research plan, and directed the implementation of the entire study. H-SL, QG, and HY drafted the manuscript and processed data. MZ and L-QX assisted in collection and analysis of data, and completed the basic experiment. Q-XZ and HL edited the manuscript for English. All authors have read and approved the final manuscript.
Funding
This work was supported by the Health Commission of Hubei Province scientific research project (Number: WJ2019Q014), Hubei Chen Xiaoping science and Technology Development Fund (Number: CXPJJH12000002-2020040), and Research start-up fund of Taihe hospital (Number: 2021LC+JC013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.798020/full#supplementary-material
Abbreviations
ESCA, esophageal cancer; TIMER, Tumor Immune Estimation Resource; TCGA, The Cancer Genome Atlas; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, Gene Set Enrichment Analysis; PPI, protein–protein interaction; OS, overall survival; BP, biological processes; MF, molecular functions; CC, cellular components; NES, Nominal enrichment scores; SPDL1, spindle apparatus coiled-coil protein 1.
References
Barisic, M., Sohm, B., Mikolcevic, P., Wandke, C., Rauch, V., Ringer, T., et al. (2010). Spindly/CCDC99 Is Required for Efficient Chromosome Congression and Mitotic Checkpoint Regulation. MBoC 21 (12), 1968–1981. doi:10.1091/mbc.e09-04-0356
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clinicians 68, 394–424. doi:10.3322/caac.21492
Chandrashekar, D. S., Bashel, B., Balasubramanya, S. A. H., Creighton, C. J., Ponce-Rodriguez, I., Chakravarthi, B. V. S. K., et al. (2017). UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 19 (8), 649–658. doi:10.1016/j.neo.2017.05.002
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA: A Cancer J. Clinicians 66 (2), 115–132. doi:10.3322/caac.21338
Choi, M., Kim, W., Cheon, M. G., Lee, C.-W., and Kim, J.-E. (2015). Polo-like Kinase 1 Inhibitor BI2536 Causes Mitotic Catastrophe Following Activation of the Spindle Assembly Checkpoint in Non-small Cell Lung Cancer Cells. Cancer Lett. 357 (2), 591–601. doi:10.1016/j.canlet.2014.12.023
Fu, J., Soroka, D. N., Zhu, Y., and Sang, S. (2018). Induction of Apoptosis and Cell-Cycle Arrest in Human Colon-Cancer Cells by Whole-Grain Alkylresorcinols via Activation of the P53 Pathway. J. Agric. Food Chem. 66 (45), 11935–11942. doi:10.1021/acs.jafc.8b04442
Gao, Y., Guo, C., Fu, S., Cheng, Y., and Song, C. (2021). Downregulation of CDC20 Suppressed Cell Proliferation, Induced Apoptosis, Triggered Cell Cycle Arrest in Osteosarcoma Cells, and Enhanced Chemosensitivity to Cisplatin. neo 68, 382–390. doi:10.4149/neo_2020_200614N629
Guo, Q., Ke, X.-X., Liu, Z., Gao, W.-L., Fang, S.-X., Chen, C., et al. (2020). Evaluation of the Prognostic Value of STEAP1 in Lung Adenocarcinoma and Insights into its Potential Molecular Pathways via Bioinformatic Analysis. Front. Genet. 11, 242. doi:10.3389/fgene.2020.00242
Guo, Q., Li, D., Luo, X., Yuan, Y., Li, T., Liu, H., et al. (2021). The Regulatory Network and Potential Role of LINC00973-miRNA-mRNA ceRNA in the Progression of Non-small-cell Lung Cancer. Front. Immunol. 12, 684807. doi:10.3389/fimmu.2021.684807
Jingu, K., Umezawa, R., Yamamoto, T., Takeda, K., Ishikawa, Y., Takahashi, N., et al. (2019). FDG-PET Might Not Contribute to Improving Survival in Patients with Locally Advanced Inoperable Esophageal Cancer. Int. J. Clin. Oncol. 24, 927–933. doi:10.1007/s10147-019-01428-8
Kalimutho, M., Sinha, D., Jeffery, J., Nones, K., Srihari, S., Fernando, W. C., et al. (2018). CEP 55 Is a Determinant of Cell Fate during Perturbed Mitosis in Breast Cancer. EMBO Mol. Med. 10 (9). undefined. doi:10.15252/emmm.201708566
Kodama, T., Marian, T. A., Lee, H., Kodama, M., Li, J., Parmacek, M. S., et al. (2019). MRTFB Suppresses Colorectal Cancer Development through Regulating SPDL1 and MCAM. Proc. Natl. Acad. Sci. U.S.A. 116 (47), 23625–23635. doi:10.1073/pnas.1910413116
Li, L., Huang, K., Zhao, H., Chen, B., Ye, Q., and Yue, J. (2020). CDK1-PLK1/SGOL2/ANLN Pathway Mediating Abnormal Cell Division in Cell Cycle May Be a Critical Process in Hepatocellular Carcinoma. Cell Cycle 19, 1236–1252. doi:10.1080/15384101.2020.1749471
Lin, J.-B., Hung, L.-C., Cheng, C.-Y., Chien, Y.-A., Lee, C.-H., Huang, C.-C., et al. (2019). Prognostic Significance of Lung Radiation Dose in Patients with Esophageal Cancer Treated with Neoadjuvant Chemoradiotherapy. Radiat. Oncol. 14 (1), 85. doi:10.1186/s13014-019-1283-3
Liu, H., Zhang, J., Luo, X., Zeng, M., Xu, L., Zhang, Q., et al. (2020). Overexpression of the Long Noncoding RNA FOXD2-AS1 Promotes Cisplatin Resistance in Esophageal Squamous Cell Carcinoma through the miR-195/Akt/mTOR Axis. Oncol. Res. 28 (1), 65–73. doi:10.3727/096504019x15656904013079
Liu, X., Chen, Y., Li, Y., Petersen, R. B., and Huang, K. (2019). Targeting Mitosis Exit: A Brake for Cancer Cell Proliferation. Biochim. Biophys. Acta (Bba) - Rev. Cancer 1871 (1), 179–191. doi:10.1016/j.bbcan.2018.12.007
Lv, W., Su, B., Li, Y., Geng, C., and Chen, N. (2018). KIAA0101 Inhibition Suppresses Cell Proliferation and Cell Cycle Progression by Promoting the Interaction between P53 and Sp1 in Breast Cancer. Biochem. Biophys. Res. Commun. 503 (1), 600–606. doi:10.1016/j.bbrc.2018.06.046
Mahajan, S., Raina, K., Verma, S., and Rao, B. J. (2019). Human RAD52 Protein Regulates Homologous Recombination and Checkpoint Function in BRCA2 Deficient Cells. Int. J. Biochem. Cell Biol. 107, 128–139. doi:10.1016/j.biocel.2018.12.013
Matsunuma, R., Chan, D. W., Kim, B.-J., Singh, P., Han, A., Saltzman, A. B., et al. (2018). DPYSL3 Modulates Mitosis, Migration, and Epithelial-To-Mesenchymal Transition in Claudin-Low Breast Cancer. Proc. Natl. Acad. Sci. U.S.A. 115 (51), E11978–E11987. doi:10.1073/pnas.1810598115
Piao, J., Zhu, L., Sun, J., Li, N., Dong, B., Yang, Y., et al. (2019). High Expression of CDK1 and BUB1 Predicts Poor Prognosis of Pancreatic Ductal Adenocarcinoma. Gene 701, 15–22. doi:10.1016/j.gene.2019.02.081
Qin, J., Yang, Y., Gao, S., Liu, Y., Yu, F., Zhou, Y., et al. (2017). Deregulated ALG-2/HEBP2 axis Alters Microtubule Dynamics and Mitotic Spindle Behavior to Stimulate Cancer Development. J. Cell Physiol 232, 3067–3076. doi:10.1002/jcp.25754
Tian, X., and Wang, N. (2020). Upregulation of ASPM, BUB1B and SPDL1 in Tumor Tissues Predicts Poor Survival in Patients with Pancreatic Ductal Adenocarcinoma. Oncol. Lett. 19 (4), 3307–3315. doi:10.3892/ol.2020.11414
Wu, Y., Tan, L., Chen, J., Li, H., Ying, H., Jiang, Y., et al. (2018). MAD2 Combined with Mitotic Spindle Apparatus (MSA) and Anticentromere Antibody (ACA) for Diagnosis of Small Cell Lung Cancer (SCLC). Med. Sci. Monit. 24, 7541–7547. doi:10.12659/MSM.909772
Zarean, E., Mahmoudi, M., Azimi, T., and Amini, P. (2018). Determining Overall Survival and Risk Factors in Esophageal Cancer Using Censored Quantile Regression. Asian Pac. J. Cancer Prev. 19 (11), 3081–3086. doi:10.31557/APJCP.2018.19.11.3081
Zhang, H., Zhang, X., Li, X., Meng, W. B., Bai, Z. T., Rui, S. Z., et al. (2018). Effect of CCNB1 Silencing on Cell Cycle, Senescence, and Apoptosis through the P53 Signaling Pathway in Pancreatic Cancer. J. Cell Physiol 234, 619–631. doi:10.1002/jcp.26816
Zhang, X., Wang, F., Wang, Z., Yang, X., Yu, H., Si, S., et al. (20202020). ALKBH5 Promotes the Proliferation of Renal Cell Carcinoma by Regulating AURKB Expression in an m6A-dependent Manner. Ann. Transl Med. 8 (10), 646. doi:10.21037/atm-20-3079
Zhang, Y.-Q., Yuan, Y., Zhang, J., Lin, C.-Y., Guo, J.-L., Liu, H.-S., et al. (2021). Evaluation of the Roles and Regulatory Mechanisms of PD-1 Target Molecules in NSCLC Progression. Ann. Transl Med. 9 (14), 1168. doi:10.21037/atm-21-2963
Keywords: spindle apparatus coiled-coil protein 1, esophageal cancer, prognosis, biomarker, positron emission tomography/computed tomography
Citation: Liu H-S, Guo Q, Yang H, Zeng M, Xu L-Q, Zhang Q-X, Liu H, Guo J-L and Zhang J (2022) SPDL1 Overexpression Is Associated With the 18F-FDG PET/CT Metabolic Parameters, Prognosis, and Progression of Esophageal Cancer. Front. Genet. 13:798020. doi: 10.3389/fgene.2022.798020
Received: 19 October 2021; Accepted: 05 April 2022;
Published: 18 May 2022.
Edited by:
Marcelo R. S. Briones, Federal University of São Paulo, BrazilReviewed by:
Rajni Kumari, Albert Einstein College of Medicine, United StatesZhitong Bing, Institute of Modern Physics (CAS), China
Copyright © 2022 Liu, Guo, Yang, Zeng, Xu, Zhang, Liu, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, emhqdW4xNTlAc2luYS5jb20=; Jia-Long Guo, R0pMOTk4OEAxMjYuY29t
†These authors share first authorship
 Hua-Song Liu
Hua-Song Liu Qiang Guo
Qiang Guo Heng Yang†
Heng Yang†
