
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 25 January 2022
Sec. Applied Genetic Epidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.792313
Background and Aims: Coffee consumption has been suggested to increase the risk of migraine. However, causality remains inconclusive. In the present study, we performed a two-sample Mendelian randomization (MR) analysis to investigate the causal relationship between coffee consumption and migraine.
Methods: We obtained nine single-nucleotide polymorphisms (SNPs) associated with coffee consumption at genome-wide significance (p < 5 × 10−8) from a large genome-wide association study (GWAS) based on the UK Biobank study (included 375,833 individuals). Summary-level data for any migraine (AM) and its subtypes (migraine with aura (MA) and migraine without aura (MO)) were obtained from the largest available GWAS of migraine conducted by the International Headache Genetics Consortium (IHGC) (included 59,674 cases and 316,078 controls). MR estimates were pooled using fixed-effect inverse-variance weighted (IVW) as the main method. Sensitivity analyses were further performed using weighted median, MR-Egger, and MR-PRESSO to assess the robustness of our findings.
Results: Genetically-predicted 50% increase of coffee consumption was not causally associated with the risk of AM (odds ratio (OR), 0.97; 95% confidence interval (CI), 0.83–1.14; p = 0.71), MA (OR, 0.81; 95%CI, 0.58, 1.12; p = 0.19), or MO (OR, 0.97; 95%CI, 0.72, 1.30; p = 0.83) in the fixed-effect IVW methods. Sensitivity analyses returned similar results. No directional pleiotropy was found.
Conclusion: This MR study does not support a causal relationship between genetically predicted coffee consumption and the risk of migraine. Coffee consumption is likely not a trigger nor a prevention strategy for migraine headaches.
Migraine is a disease with unilateral, pulsating, activity-aggravated headache lasting for 4–72 h accompanied by nausea, phonophobia, photophobia, or both (Goadsby et al., 2017). In 2016, ∼1.04 billion people worldwide suffered from migraines (Burch et al., 2019). Moreover, migraine remains the sixth largest cause of years lived with disability in the world (Collaborators, 2017).
Coffee consumption has been associated with migraine for many years (Bigal et al., 2002; Takeshima et al., 2004; Hagen et al., 2009; Tai et al., 2018) and was suggested as one of the dietary triggers for the disorder (Mollaoğlu, 2013; Zaeem et al., 2016; Tai et al., 2018). On the other hand, accumulating evidence demonstrated that caffeine, as an analgesic adjuvant, can reduce pain sensation during migraine attacks (Derry et al., 2014; Baratloo et al., 2017). Since available data on coffee consumption and migraine risk come from observational studies that may be influenced by biases such as residual confounding, misclassification, and reverse causation (Smith and Ebrahim, 2003), causality in the association remains inconclusive. A question arises: is coffee consumption a trigger for migraine, or is the association entirely due to the fact that migraineurs are more likely to drink coffee to relieve the pain? Given that coffee consumption is one of the most common modifiable exposures, recognition of the causal link may advance the development of the preventive strategy for migraine.
Mendelian randomization (MR) study is an approach to investigate the causal relationship between exposures and outcomes by using germline genetic variants as instrumental variables (IVs) (Lawlor et al., 2008). The method is considered as a “nature” RCT and diminishes confounding and reverse causation in observation research (Lawlor et al., 2008). Therefore, the single-nucleotide polymorphisms (SNPs) influencing coffee consumption can be used to analyze the association. In the present study, we aimed to use the two-sample MR analysis to determine the causal effect of coffee consumption on any migraines (AM), migraines with aura (MA), and migraines without aura (MO).
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) guide (Skrivankova et al., 2021). A diagram of this two-sample MR analysis is displayed in Figure 1. Specifically, the analysis is based on the following key assumptions: (1) the IVs (SNPs) should be robustly associated (p < 5 × 10−8) with the exposure (coffee consumption); (2) the IVs should be independent of any potential confounders; and (3) the IVs should not directly affect the outcome (migraine) except through their effect on the exposure.
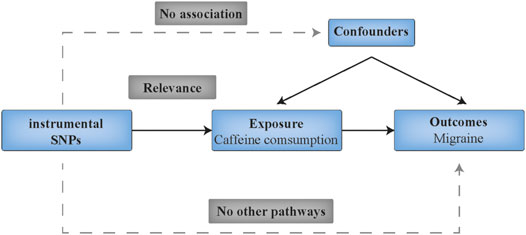
FIGURE 1. Schematic diagram showing the assumptions of Mendelian randomization analysis. SNPs indicates single nucleotide polymorphisms.
The current MR analyses were performed using published GWAS datasets (Supplementary Table S1). No specific ethical approval and written informed consent of participants is required.
Genetic IVs for coffee consumption were obtained from the largest available genome-wide association study (GWAS) meta-analysis using the UK Biobank Resource (Zhong et al., 2019). The UK Biobank study included over 500,000 participants aged 37–73 years from 22 centers across England, Wales, and Scotland in 2006–2010. In this study, 375,833 samples were included after the exclusion of outliers based on heterozygosity and missingness, individuals with sex discrepancy between self-reported and inferred sex, and closely related individuals (kinship coefficient of >0.0442). In addition, the analysis was restricted to those who self-reported as “British” and shared similar ancestral backgrounds (Zhong et al., 2019). Self-reported coffee consumption was retrieved from all participants based on the question “How many cups of coffee do you drink each day (include decaffeinated coffee)?” (Zhong et al., 2019). The authors identified 15 SNPs with genome-wide significance (p < 5 × 10−8) (Zhong et al., 2019). To select valid instrumental SNPs, we first pruned SNPs with horizontal pleiotropic effects to meet the second key assumption–the IVs should be independent of any potential confounders. By searching the PhenoScanner database (Kamat et al., 2019), 5 SNPs were removed for being associated with potential confounders (p < 5 × 10−8), such as body mass index and alcohol consumption (Supplementary Table S2). Second, we excluded rs4719497 since it was in linkage disequilibrium (r2 < 0.01; region size, 10000 kb) with other SNPs (rs4410790 and rs12699844). Finally, nine coffee-associated SNPs were used as IVs for the MR analyses (Supplementary Table S3). F-statistics were calculated to assess the strength of each SNP using the formula
Summary statistics for the associations of the coffee-related SNPs with migraine were derived from the hitherto largest GWAS meta-analysis of migraine conducted by the International Headache Genetics Consortium (IHGC), which comprised data from 22 studies with a total of 59,674 cases and 316,078 controls of European ancestry (Gormley et al., 2016). Migraine was defined based on previously published diagnostic criteria from the International Headache Society. (2013). Two prevalent sub-forms of migraine were also included in the present study: MA (included 6,332 cases and 144,883 controls) and MO (included 8,348 cases and 139,622 controls) (Supplementary Table S1).
After harmonizing the SNPs across the data sources via the effect alleles, we calculated the effect estimate for each instrumental SNP on migraine with the Wald estimator, and assessed the possible measurement errors using the Delta method (Lawlor et al., 2008). The fixed-effects inverse variance-weighted (IVW) method was used as standard analysis to derive the final effect estimates. Heterogeneity among estimates of SNPs was measured by Cochran Q-derived p, I2, and the funnel plot (Sterne et al., 2011; Greco M et al., 2015). Sensitivity analyses included the multiplicative random-effects IVW(Bowden et al., 2017), the weighted median (Bowden et al., 2016), the MR-Egger regression method (Burgess and Thompson, 2017), and the MR-pleiotropy residual sum and outlier (MR-PRESSO) method (Verbanck et al., 2018). Where heterogeneity existed (I2 > 25% or Cochran Q-derived p < 0.05) (Greco M et al., 2015), the multiplicative random-effects IVW method was adopted to avoid the bias of weak SNP-exposure associations (Bowden et al., 2017). The weighted median method can provide valid estimates even when up to 50% of the information in the analysis comes from invalid IVs(Bowden et al., 2016). The MR-Egger method provides more conservative causal estimates in the presence of pleiotropic variants and is less likely to generate inflated test statistics (Burgess and Thompson, 2017). The MR-PRESSO method was used to detect the presence of outliers that could bias the results (Verbanck et al., 2018). We applied the intercept test from MR-Egger to assess horizontal pleiotropy (Bowden et al., 2015). In addition, we employed leave-one-out analyses to determine whether a single SNP drove the causal relationship. With this approach, we excluded one SNP in turn and then re-evaluated the causal effect. Scatter plots depicting the associations were also provided.
The OR estimates of AM, MA, and MO were scaled per 50% increase in coffee consumption (0.5 more cups of coffee). Two-sided p values of < 0.0167 (= 0.05/3 outcomes) were set as the thresholds for significance. Statistical power was calculated with an online tool (https://shiny.cnsgenomics.com/mRnd/) (Brion et al., 2013). All MR analyses were conducted using R software (version 4.1.0) with R packages including TwoSampleMR (Hemani et al., 2018), MendelianRandomization (Yavorska and Burgess, 2017), and MR-PRESSO(Verbanck et al., 2018).
All IVs were estimated to account for 0.5% of the observed variance of coffee consumption. None of these IVs had an F-statistic lower than the threshold of 10, suggesting no weak instrument bias in the present study (Supplementary Table S3). Our MR analyses had over 80% statistical power to detect an odds ratio (OR) of 1.17 (or 0.83) for the coffee consumption-AM relationship, 1.49 (or 0.51) for the coffee consumption-MA relationship, and 1.43 (or 0.57) for the coffee consumption-MO relationship.
In the standard IVW method, genetically-predicted 50% increase of coffee consumption was not associated with the risk of AM (OR, 0.97; 95% confidence interval (CI), 0.83–1.14; p = 0.71), MA (OR, 0.81; 95%CI, 0.58, 1.12; p = 0.19), or MO (OR, 0.97; 95%CI, 0.72, 1.30; p = 0.83) (Figure 2). No outliers were detected with the MR-PRESSO test (Figure 2). However, there was some evidence of heterogeneity in the IVW analyses as demonstrated by Cochran Q-derived p, I2 (Supplementary Table S4), and funnel plots (Supplementary Figure S1); thereby we applied the multiplicative random-effects IVW method, which yielded similar results (Figure 2). The scatter plots for AM, MA, and MO are displayed in Supplementary Figure S2. Forest plots of the effect of each single SNP on the outcomes are provided in Supplementary Figure S3. However, we may not have reached sufficient statistical power to detect such weak associations.
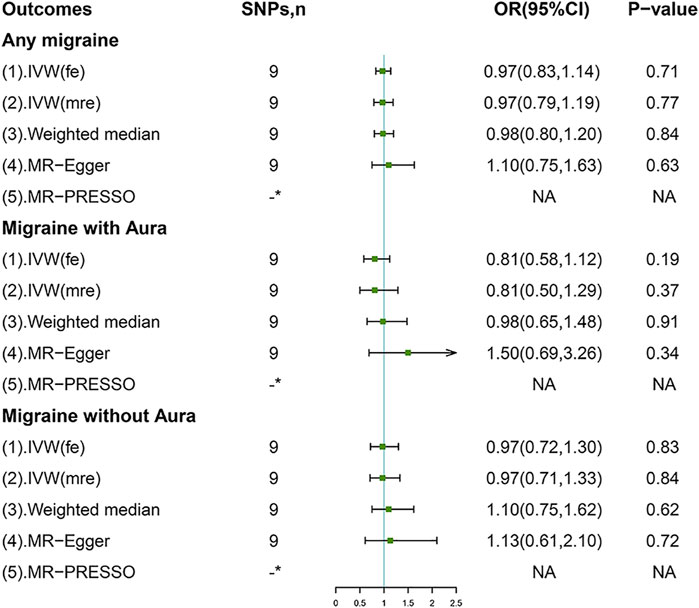
FIGURE 2. Associations of genetically predicted coffee consumption with migraine and its subtypes. IVW (fe), fixed-effects inverse-variance weighted; IVW (mre), multiplicative random-effects inverse-variance weighted; MR-Egger, Mendelian randomization-Egger; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier method; NA, not available. *No outliers detected.
Sensitivity analyses including weighted median and MR-Egger methods returned consistent results (Figure 2). Leave-one-out analyses indicated the non-causal associations were not biased by any single genetic variant (Supplementary Figure S4). Importantly, no evidence of directional pleiotropy was found as measured by the MR-Egger intercept and the MR-PRESSO global test (p for intercept > 0.264; p for global test > 0.075; Supplementary Table S4).
The present study reveals no causal effect of the genetic increase in coffee consumption on any type of migraine, and the results are consistent across sensitivity analyses. To our knowledge, this is the first MR study to explore the causal association between coffee consumption and the risk of migraine.
Previous observational studies have reported the association between regular coffee consumption and migraine. A prospective cross-sectional study demonstrated that coffee consumption is significantly associated with migraine prevalence (OR, 1.73; 95% CI, 1.12–2.68; p = 0.014) (Tai et al., 2018). In the Head-Hunt study, after adjusting for confounders such as age, gender, smoking, and level of education, coffee consumption is accompanied by an increased migraine incidence (OR, 1.13; 95% CI, 1.07-1.20; p < 0.05) (Hagen et al., 2009). Similarly, findings from a randomized case-control study revealed a positive correlation between coffee consumption and chronic migraine (OR, 2.9; 95% CI, 1.5–5.3; p < 0.0001) (Bigal et al., 2002). These findings suggested that coffee may act as a trigger for migraine. The prevalence of coffee as a migraine trigger ranges from 6.3 to 14.5% (Zaeem et al., 2016). On the other hand, coffee was suggested as an analgesic adjuvant for migraine. A double-blind RCT study showed that pain relief after caffeine monotherapy is faster than after placebo treatment (Diener et al., 2005). Mechanically, caffeine can inhibit NO synthase production and produce cerebral vasoconstriction, thereby reducing pain sensation during migraine attacks (Nowaczewska et al., 2020). Therefore, it is also possible that migraineurs drink coffee to relieve the pain, leading to the wrong conclusion in traditional studies.
In this study, we aimed to determine whether coffee consumption, as one of the most common modifiable exposures, is associated with higher migraine risk. However, the analyses did not suggest a causal association of coffee consumption with migraine risk as anticipated. This can be explained from the following perspectives. First, caffeine has been suggested as an analgesic adjuvant for migraine; migraineurs tend to drink coffee to relieve the pain, leading to a cause-effect inversion bias in traditional studies. Second, although some confounders in observational studies were adjusted, unmeasured risk factors cannot be completely ruled out. Third, it is well-known that migraines are usually preceded by some premonitory symptoms. Drinking coffee might simply be a consequence of premonitory symptoms that herald a headache. Food craving and other symptoms like yawning and sleepiness in the premonitory phase may be responsible for drinking coffee, leading to a false connection between coffee consumption and migraine (Nowaczewska et al., 2020). Our results were in agreement with a prospective cross-sectional study in which no subjects reported coffee as a trigger for migraine (Yadav et al., 2010).
The Mendelian randomization design is one of the major strengths of this study. By using randomly allocated genetic variants as IVs, our study largely mitigated confounding or reverse causation bias, thus providing compelling evidence. In addition, since the analyses were restricted to individuals of European ancestries, the bias introduced by population structure was unlikely to affect our results. Other strengths included the stability of the causal estimates across different sensitivity analyses, the large sample size derived from several GWAS datasets, and the strong estimated effects of each IVs (all F-statistic >10).
There are several limitations in our study. First, we are unable to explore the potential non-linear association between coffee consumption and migraine since this study was based on summary-level data. Second, since the population in this study was restricted to Europe, we are not sure if the same conclusion can be reached in non-European populations. Third, the statistical power for the present study may be insufficient since only 0.5% of the observed variance in coffee consumption was explained by IVs. Therefore, we should be cautious with interpreting the negative results; the null association might be due to a lack of power. Fourth, data on self-reported coffee consumption might be imprecise and is likely to introduce measurement bias. In addition, coffee consumption may not be very highly heritable; it is commonly not a life-long exposure, which reduced the clinical relevance of this MR study. Finally, potential directional pleiotropy that may bias estimation of causal inference cannot be completely ruled out, despite the lack of evidence from MR-Egger regression and MR-PRESSO methods.
This MR study provides no evidence of a causal association between increased coffee consumption and the risk of migraine in European populations. Coffee consumption is likely not a trigger nor a prevention strategy for migraine headaches.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HC and HZ designed the study, conducted the MR analyses, and wrote the manuscript; LZ contributed to data acquisition and revision of the manuscript.
This work was supported by the National Natural Science Foundation of China (grant number 81873484); the Youth Program of National Natural Science Foundation of China (grant number 82000316); and the Nature Science Foundation of Zhejiang Province (grant number LZ16H020001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We extend sincere thanks to Dr. Zhong VW, etc. for releasing GWAS summary statistics for coffee consumption, and the International Headache Genetics Consortium for conducting GWAS and sharing summary-level data on migraine. The members of the International Headache Genetics Consortium are listed in the Supplementary Material.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.792313/full#supplementary-material
Baratloo, A., Mirbaha, S., Kasmaei, H. D., Payandemehr, P., Elmaraezy, A., and Negida, A. (2017). Intravenous Caffeine Citrate vs. Magnesium Sulfate for Reducing Pain in Patients with Acute Migraine Headache; a Prospective Quasi-Experimental Study. Korean J. Pain 30, 176–182. doi:10.3344/kjp.2017.30.3.176
Bigal, M. E., Sheftell, F. D., Rapoport, A. M., Tepper, S. J., and Lipton, R. B. (2002). Chronic Daily Headache: Identification of Factors Associated with Induction and Transformation. Headache: J. Head Face Pain 42, 575–581. doi:10.1046/j.1526-4610.2002.02143.x
Bowden, J., Del Greco M, F., Minelli, C., Smith, G. D., Sheehan, N., and Thompson, J. (2017). A Framework for the Investigation of Pleiotropy in Two-Sample Summary Data Mendelian Randomization. Statist. Med. 36, 1783–1802. doi:10.1002/sim.7221
Bowden, J., Smith, G. D., and Burgess, S. (2015). Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 44, 512–525. doi:10.1093/ije/dyv080
Bowden, J., Smith, G. D., Haycock, P. C., and Burgess, S. (2016). Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 40, 304–314. doi:10.1002/gepi.21965
Brion, M.-J. A., Shakhbazov, K., and Visscher, P. M. (2013). Calculating Statistical Power in Mendelian Randomization Studies. Int. J. Epidemiol. 42, 1497–1501. doi:10.1093/ije/dyt179
Burch, R. C., Buse, D. C., and Lipton, R. B. (2019). Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 37, 631–649. doi:10.1016/j.ncl.2019.06.001
Burgess, S., and Thompson, S. G. (2011). Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 40, 755–764. doi:10.1093/ije/dyr036
Burgess, S., and Thompson, S. G. (2017). Interpreting Findings from Mendelian Randomization Using the MR-Egger Method. Eur. J. Epidemiol. 32, 377–389. doi:10.1007/s10654-017-0255-x
Collaborators, G. (2017). Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi:10.1016/S0140-6736(17)32154-2
Derry, C. J., Derry, S., and Moore, R. A. (2014). Caffeine as an Analgesic Adjuvant for Acute Pain in Adults. Cochrane database Syst. Rev. 12, CD009281. doi:10.1002/14651858.CD009281.pub3
Diener, H., Pfaffenrath, V., Pageler, L., Peil, H., and Aicher, B. (2005). The Fixed Combination of Acetylsalicylic Acid, Paracetamol and Caffeine Is More Effective Than Single Substances and Dual Combination for the Treatment of Headache: a Multicentre, Randomized, Double-Blind, Single-Dose, Placebo-Controlled Parallel Group Study. Cephalalgia 25, 776–787. doi:10.1111/j.1468-2982.2005.00948.x
Goadsby, P. J., Holland, P. R., Martins-Oliveira, M., Hoffmann, J., Schankin, C., and Akerman, S. (2017). Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 97, 553–622. doi:10.1152/physrev.00034.2015
Gormley, P., Anttila, V., Anttila, V., Winsvold, B. S., Palta, P., Esko, T., et al. (2016). Meta-analysis of 375,000 Individuals Identifies 38 Susceptibility Loci for Migraine. Nat. Genet. 48, 856–866. doi:10.1038/ng.3598
Greco M, F. D., Minelli, C., Sheehan, N. A., and Thompson, J. R. (2015). Detecting Pleiotropy in Mendelian Randomisation Studies with Summary Data and a Continuous Outcome. Statist. Med. 34, 2926–2940. doi:10.1002/sim.6522
Hagen, K., Thoresen, K., Stovner, L. J., and Zwart, J.-A. (2009). High Dietary Caffeine Consumption Is Associated with a Modest Increase in Headache Prevalence: Results from the Head-HUNT Study. J. Headache Pain 10, 153–159. doi:10.1007/s10194-009-0114-6
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. Elife 7, e34408. doi:10.7554/eLife.34408
International Headache Society (2013). The International Classification of Headache Disorders, 3rd Edition (Beta Version). Cephalalgia 33, 629–808. doi:10.1177/0333102413485658
Kamat, M. A., Blackshaw, J. A., Young, R., Surendran, P., Burgess, S., Danesh, J., et al. (2019). PhenoScanner V2: an Expanded Tool for Searching Human Genotype-Phenotype Associations. Bioinformatics (Oxford, England) 35, 4851–4853. doi:10.1093/bioinformatics/btz469
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N., and Davey Smith, G. (2008). Mendelian Randomization: Using Genes as Instruments for Making Causal Inferences in Epidemiology. Statist. Med. 27, 1133–1163. doi:10.1002/sim.3034
Mollaoğlu, M. (2013). Trigger Factors in Migraine Patients. J. Health Psychol. 18, 984–994. doi:10.1177/1359105312446773
Nowaczewska, M., Wiciński, M., and Kaźmierczak, W. (2020). The Ambiguous Role of Caffeine in Migraine Headache: From Trigger to Treatment. Nutrients 12, 2259. doi:10.3390/nu12082259
Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Yarmolinsky, J., Davies, N. M., Swanson, S. A., et al. (2021). Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 326, 1614–1621. doi:10.1001/jama.2021.18236
Smith, G. D., and Ebrahim, S. (2003). 'Mendelian Randomization': Can Genetic Epidemiology Contribute to Understanding Environmental Determinants of Disease? Int. J. Epidemiol. 32, 1–22. doi:10.1093/ije/dyg070
Sterne, J. A. C., Sutton, A. J., Ioannidis, J. P. A., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. Bmj 343, d4002. doi:10.1136/bmj.d4002
Tai, M.-L. S., Yap, J. F., and Goh, C. B. (2018). Dietary Trigger Factors of Migraine and Tension-type Headache in a South East Asian Country. Jpr 11, 1255–1261. doi:10.2147/JPR.S158151
Takeshima, T., Ishizaki, K., Fukuhara, Y., Ijiri, T., Kusumi, M., Wakutani, Y., et al. (2004). Population-based Door-To-Door Survey of Migraine in Japan: the Daisen Study. Headache 44, 8–19. doi:10.1111/j.1526-4610.2004.04004.x
Verbanck, M., Chen, C.-Y., Neale, B., and Do, R. (2018). Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 50, 693–698. doi:10.1038/s41588-018-0099-7
Yadav, R. K., Kalita, J., and Misra, U. K. (2010). A Study of Triggers of Migraine in India. Pain Med. 11, 44–47. doi:10.1111/j.1526-4637.2009.00725.x
Yavorska, O. O., and Burgess, S. (2017). MendelianRandomization: an R Package for Performing Mendelian Randomization Analyses Using Summarized Data. Int. J. Epidemiol. 46, 1734–1739. doi:10.1093/ije/dyx034
Zaeem, Z., Zhou, L., and Dilli, E. (2016). Headaches: a Review of the Role of Dietary Factors. Curr. Neurol. Neurosci. Rep. 16, 101. doi:10.1007/s11910-016-0702-1
Keywords: Mendelian Randomization, causal association, coffee consumption, any migraine, migraine with aura, migraine without aura
Citation: Chen H, Zhang H and Zheng L (2022) No Causal Association Between Coffee Consumption and Risk of Migraine: A Mendelian Randomization Study. Front. Genet. 13:792313. doi: 10.3389/fgene.2022.792313
Received: 10 October 2021; Accepted: 11 January 2022;
Published: 25 January 2022.
Edited by:
Cherubino Di Lorenzo, Sapienza University of Rome, ItalyReviewed by:
Penelope Alathea Lind, QIMR Berghofer Medical Research Institute, AustraliaCopyright © 2022 Chen, Zhang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangrong Zheng, MTE5MTA2NkB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.