
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 01 March 2022
Sec. Livestock Genomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.764965
This article is part of the Research TopicGenetic Engineering in Farm AnimalsView all 7 articles
Myostatin (MSTN) is a protein that negatively regulates growth of skeletal muscle, and inactivation of MSTN improves the mass of skeletal muscle. Our previous work found that MSTN+/− pigs have higher muscle depth and lower fat depth compared to wild type without any developmental problems. Therefore, MSTN-edited pigs are most likely to appear as heterozygotes in the potential future market, but the characteristics of organs in digestive and reproductive system of pigs with MSTN gene editing remains unclear. Here, we investigated the histological of the organs in the digestive system and reproductive system in MSTN gene heterozygotes at adult stages. The length of intestine was further compared between adult heterozygous and wild type pigs. We found no significant differences in histomorphology of organs, including heart, duodenum, jejunum, ileum, cecum, colon, testis, epididymis, ovaries, oviducts and uterus, between individuals from two genotypes. Moreover, there was no significant difference in the average length of intestine in adult pigs. Our data provide a reference for further clarifying the applications of MSTN gene edited pigs.
In 1997, McPherron first identified MSTN (also known as growth differentiation factor-8) as a member of the TGF-β (transforming growth factor-β) superfamily (McPherron et al., 1997). MSTN is highly expressed in skeletal muscle, but lowly expressed in adipose tissue and cardiac muscle (McPherron et al., 1997; Sharma et al., 1999). MSTN strongly affects skeletal muscle development (McPherron and Lee, 1997; Zhao et al., 2005; Guo et al., 2009; Ahad et al., 2017). The MSTN mutation lead to a double muscling phenotype in cattle (Grobet et al., 1997; McPherron and Lee, 1997), mice (Bünger et al., 2004), sheep (Clop et al., 2006) and other animals (Schuelke et al., 2004; Mosher et al., 2007).
Since pigs are farmed as a major source of animal protein for humans, generation of pigs with MSTN loss-of-function mutations has become a research priority in order to obtain breeding stock with better meat quality and higher economic value. Previous studies have reported the successful production of healthy MSTN gene-inactivated Chinese Meishan pigs (Qian et al., 2015) and Erhualian pigs (Wang et al., 2017). In addition to phenotypic changes to muscle and fat tissues, organs weights were generally significantly reduced among MSTN−/− animals compared with the organs in their wild type (WT) counterparts (Bünger et al., 2004; Fiems, 2012; Luo et al., 2019). Bünger (2004) found that in MSTN knockout mice, the significant increase in muscle weight and decrease in body fat content were accompanied by significant reductions in the weights of heart, liver, kidney and digestive tract (Bünger et al., 2004). Luo (2019) examined the organ weights of MSTN−/−, MSTN+/− and WT piglets and found that the visceral weight of MSTN−/− homozygous piglets was significantly lower than that of MSTN+/− heterozygotes and WT piglets (Luo et al., 2019). These studies show that animals with double site knockout of MSTN have lighter internal organs than WT individuals. The development of organs has a direct impact on an individual’s health, and the weights of body tissues and organs directly affect the slaughter rate, an important index of pig slaughtering performance, which in turn affects economic benefits. However, the mechanism of how MSTN regulates visceral development remains unclear. These findings all raise concerns about the health and welfare of MSTN edited animals.
In our previous study, MSTN+/− Large White (LW) pigs underwent exon 3 editing were prepared. The lean meat production of the MSTN+/− LW pigs were significantly higher than that of the WT (75.50%: 69.44%, p < 0.0001). Conversely, the fat meat rates of MSTN+/− pigs were significantly lower than that of the WT pigs (5.11%: 10.36%, p = 0.0022). The majority of amino acids content in the MSTN-edited LW pork were significantly higher than that in the WT pork; and the levels of polyunsaturated fatty acids were enriched in the MSTN-edited LW lean meat (Fan et al., 2021). Pigs with a naturally occurring single copy MSTN mutation have higher muscle depth and lower fat depth compared to the WT (Matika et al., 2019). Therefore, the heterozygous of MSTN gene-edited pigs have excellent production performance and are likely to be used in market applications in the future. However, there has been no systematic study on the digestive tract organs and reproductive organs of MSTN+/− pigs. The structure and function of intestine can affect nutrient absorption from feed. And the function of reproductive system organs determines the reproductive efficiency of the breeding herd. These are important factors to the economic benefit of pig production. Therefore, we examined the histological features of major organs in digestive and reproductive system in adults, and also contrasted the intestine length of MSTN+/− LW pigs and their WT half-sibs. This work will provide an important reference for MSTN gene edited pigs that can be used in future pig breeding.
The pigs had ad libitum access to a commercial pig diet and water throughout the experimental period. All animal studies were approved by the Animal Welfare and Research Ethics Committee at the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences. Here, we used newborn, 5-month-old and 8-month-old MSTN+/− and WT pigs to study the viscera development characteristics.
Paraffin-embedded sections (4 μm thick) of various organs of MSTN+/−, and WT piglets, 5-month-old pigs, and 8-month-old pigs were made using a histotome. The slices were dewaxed, hydrated with gradient alcohol, stained with hematoxylin solution for 15 min, counterstained with 0.5% eosin solution for 5 min, dehydrated with gradient alcohol, cleared, and sealed (Feldman and Wolfe, 2014). After staining, images were acquired using a LEICA DMi8 microscope. Then, we assessed which cell types were in the images and the morphological characteristics of the cells to detect whether there were any changes in the tissue structure between the two genotypes.
In total, 17 MSTN+/− and 18 WT LW pigs were slaughtered at 8 months old, and the length of intestinal length was measured as follows:
Small intestinal length (m): Length from the pylorus to the ileocecal junction.
Large intestine length (m): The length from the ileocecal junction to the anus.
Data were analyzed using SPSS 19 software (SPSS, Inc., Chicago, IL, United States). All data were expressed as Mean ± SEM. The independent sample t-test (Student’s t-test) was used to calculate the significance, and p < 0.05 was used as the criterion for significance.
The formalin-fixed paraffin-embedded heart tissue of MSTN+/− and WT piglets were stained by Hematoxylin and Eosin (H&E) for histomorphological comparison. Results showed that the myocardial fibers of newborn WT and MSTN pigs were both short and cylindrical, branched and interconnected in a network, and there were intercalated discs at the junction between adjacent myocardial fibers (Figures 1A,B). Furthermore, we compared the sizes and weights of heart of 17 MSTN+/− and 18 WT 8-month-old pigs. The appearance and sizes of heart did not markedly differ between the MSTN+/− and WT adult pigs (Figure 1C). There was also, no significant difference of average heart weight between MSTN+/−(406.5 ± 15 g) and WT (452.3 ± 32.4 g).
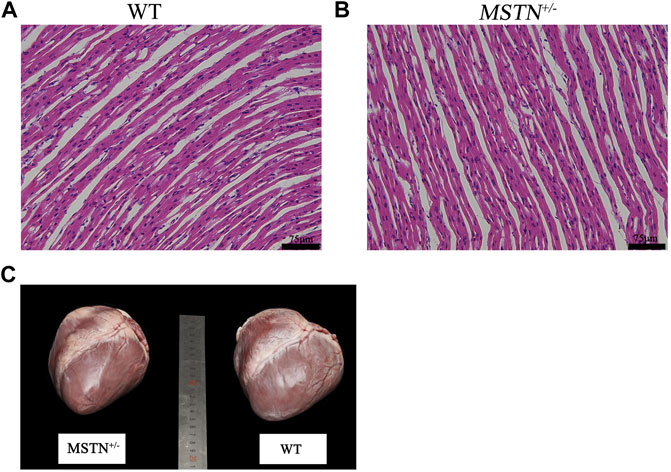
FIGURE 1. Morphological analysis of heart in MSTN+/− and WT pigs. (A) HE staining results of heart tissue in WT piglets. (B) HE staining results of heart tissue in MSTN+/− piglets. (C) Morphology of heart in 8-month-old MSTN+/− and WT pigs.
When pigs were 5 months old, we carried out histomorphological examination of the duodenum, jejunum, ileum, cecum, and colon. The structure of duodenum, jejunum and ileum of pigs was intact, and the small intestinal villi were slender and orderly arranged, the morphology and structure of epithelial cells were intact and clear, the cup cells were evenly distributed and in large number, the villi height was normal, the morphology and structure of crypt were clear, and the depth of crypt was normal (Figures 2A–C). The colonic structure was intact. The epithelial cells of the mucosa are arranged neatly and clearly defined, and the boundary between the epithelial layer and lamina propria is clear. The columnar cells are tall and the nuclei are elliptic, and the goblet cells are goblet shaped near the base of the cells (Figure 3A). The structure of the cecum is complete and closely arranged as that of WT pigs. The villi are orderly, uniform in length and very dense (Figure 3B). Furthermore, we compared the intestinal length of 17 MSTN+/− and 18 WT 8-month-old pigs. No any significant difference between genotypes in total intestinal length (combined length of small and large intestines, Table 1). According to the results, there were no significant morphological and length differences between MSTN+/− and WT in these tissues.
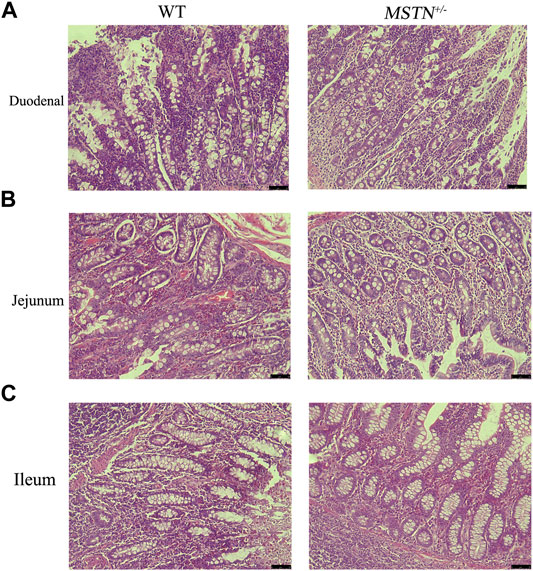
FIGURE 2. Histological analysis of duodenum, jejunum, and ileum in 5-month-old MSTN+/− and WT pigs. (A–C) Representative pictures of HE staining in duodenum (A), jejunum (B), and ileum tissue of WT (left) and MSTN+/− (right) pigs.
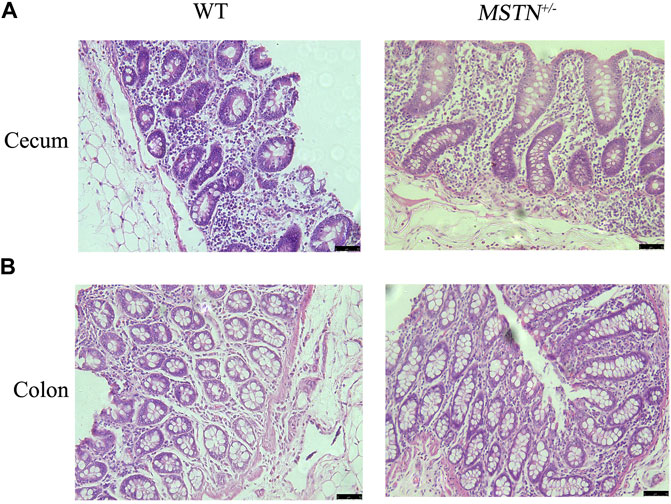
FIGURE 3. Histological analysis of cecum and colon in 5-month-old MSTN+/− and WT pigs. (A,B) Histology of cecum (A) and colon (B) from WT (left) and MSTN+/− (right) pigs was investigated by HE staining.
Reproductive organs, including testis, epididymis, ovaries, oviducts and uterus, were collected from pigs aged 8 months. And these tissues were processed for H&E staining. The MSTN+/− pigs showed normal histology of testis and epididymides, and sperm were present in testis and epididymides (Figure 4). Different follicular stages and atretic follicles existed in ovarian sections (Figure 5A). Compared with the WT group, histological analysis under light microscopy showed no morphologic differences in the ovaries, oviducts, and uterus histology of the MSTN group (Figure 5).
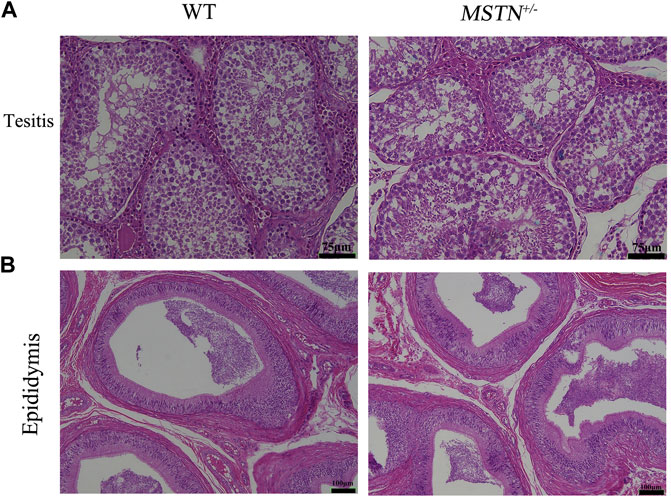
FIGURE 4. Histological of testis and epididymitis in 8-month-old MSTN+/− and WT pigs. (A,B) HE staining of testicular (A) and epididymal (B) tissue in WT (left) and MSTN+/− (right) pigs.
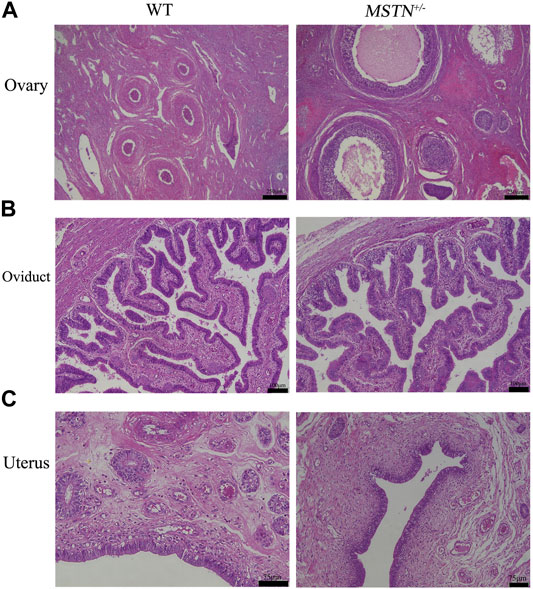
FIGURE 5. Histological analysis of ovary, oviduct and uterus in 8-month-old MSTN+/− and WT sow. (A–C) Ovarian (A), tubal (B), and uterine (C) histology image from WT (left) and MSTN+/− (right) sow.
Many laboratories have encountered problems at different stages in the process of MSTN deletion or editing. For example, MSTN-knockout Large White/Landrace×Duroc piglets died within 24 h after birth, and weighed less at birth than WT piglets. The single surviving piglet was sacrificed at 29 days old because of weakness in its hindlimbs (Rao et al., 2016). Similarly, eight of the MSTN−/− Landrace newborns generated with CRISPR/Cas9 editing died within a week (Wang et al., 2015). Pigs with naturally occurring MSTN mutations were reported in 2019 (Matika et al., 2019). However, piglets that were homozygous for this mutant allele suffered from lameness syndrome and did not survive when growth with the live weight above 40 kg (Matika et al., 2019). MSTN homozygous knockout in LW pigs leads to lameness, in agreement with other studies. Subsequently, pigs used for this study carrying single, edited copies of MSTN were propagated to expand the population for up to 8 years, which was sufficient to show that these MSTN+/− swine exhibited no developmental defects related to the mutant allele. MSTN+/− heterozygotes had a significant decrease in fat content that was accompanied by an increase in lean meat content (Fan et al., 2021). Therefore, MSTN-edited pigs are most likely to appear as heterozygotes in the potential future market.
Several internal organs have a reduced size in MSTN−/− homozygous mutant animals compared with WT (Bünger et al., 2004; Fiems, 2012; Luo et al., 2019). The Belgian Blue double-muscled (BBDM) calves (11 base-pare deletion of the MSTN (Kambadur et al., 1997)) had a reduction of 18% for the digestive tract, and 14% for the heart (Fiems, 2012). Another study reported that mice carrying homozygous mutations in murine MSTN had significantly lower weights (12–20%) for organs including liver, kidney, heart and digestive tract compared to homozygous WT mice (Bünger et al., 2004). Similarly, neonatal MSTN−/− piglets had decreased heart, liver, lungs, kidney, and stomach weights relative to body weight by 21.4, 21.3, 29.8, 16.7, and 20.0%, respectively (Luo et al., 2019).
Cardiac performance, reserve, and capability of the smaller heart are reduced in MSTN−/− animals compared to WT (Amory et al., 1993). The lower cardiac performance of MSTN−/− animals leading more quickly to exhaustion after severe exercise (Monin et al., 1974), which may even terminate in sudden death (Holmes et al., 1973). Our results showed that there were no histological abnormalities of cardiac muscle structures, and no significant difference in weight between individuals from two genotypes adults. MSTN+/− LW pigs did not suffer from reduced heart function, so there is no need to take extra precautions for animals.
It is well known that the small intestine consists of duodenum, jejunum, and ileum, is responsible for digestion and absorption of nutrients, water, and electrolytes, while the large intestine dividing into cecum and colon, is responsible to absorb water. Notably, the digestive disorders are related to intestinal dysfunction (Johnson, 1985; Soybel, 2005; Iji, 2008). Besides, the length of the digestive tract is positively correlated with nutrient absorption, while the decreases in the digestive tract length may limit the basic metabolism and nutrient availability of the body (Ortigues and Doreau, 1995; Greig et al., 2019). As reported by several authors, a consequence of the smaller digestive tract is reduced feed intake capacity (Clinquart et al., 1995; Fiems et al., 1997). The decrease in feed intake means that MSTN−/− animals do not efficiently utilize low quality feed, and the costs of rearing will increase. According to our results, the histological structure and length of intestinal in MSTN+/− swine were not affected by single copy MSTN mutation. This coincides with our previous finding that no differences were detected in feed conversion ratio values between MSTN+/− and WT LW (Fan et al., 2021). Hence, there were no differences between MSTN+/− and WT LW pigs in the function of digestive tract.
Spermatogenesis occurs in the testis and completes in the epididymis (de Kretser et al., 1998). Meanwhile, the ovary, oviduct and uterus are critical organs responsible for the oogenesis and embryonic development (Bagnell et al., 2017). The BBDM cows have significant longer calving interval than dairy cows (averaging 435 days compared to 393 days) (Hanzen et al., 1994). That may be caused by underdeveloped maternal reproductive tract and increased birth weight of calves, which make cesarean section an elective operation (Kolkman et al., 2007). A longer calving interval can be affected by the cesarean section, which significantly reduces the subsequent pregnancy rate (Patterson et al., 1981), but this is not the sole cause. Compared with Holstein cows, BBDM cows have significantly poorer semen quality (Hoflack et al., 2006). Nevertheless, BBDM cows have better egg quality than Holstein cows (Leroy et al., 2005). Other studies have shown that compared with the control group, MSTN-immunized mice had reduced litter number, but normal embryo development in both groups (Liang et al., 2007). These results are consistent with those obtained with BBDM (Leroy et al., 2005). This indicates that the completely loss of MSTN function does not affect the function of the uterus, but reduces the quality of sperm and increases litter birth weight. Our results previous work showed that the total number born, the number born alive and litter birth weight of the MSTN+/− piglets did not differ from those of the WT (Fan et al., 2021). Here, results showed that there was no significant difference in the histomorphology of testis, epididymis, ovaries, oviducts and uterus in MSTN+/− LW compared with WT pigs. Additionally, our MSTN+/− LW pigs are now in their eight generation (Fan et al., 2021). Therefore, it can be seen that the characteristics of reproductive organs of MSTN+/− LW pigs are similar to WT pigs.
In summary, MSTN+/− LW pigs had no significant differences in the histomorphology of the heart, duodenum, jejunum, ileum, cecum, or colon, nor in the lengths of the large and small intestine compared to WT pigs. Similarly, no morphologic abnormality was evident in testis, epididymis, ovaries, oviducts and uterus. These results indicated that the characteristics of heart, digestive and reproductive organs of MSTN+/− heterozygotes are similar to that of WT LW pigs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Animal Welfare and Research Ethics Committee at the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS2012-11). The pigs used in this paper were prepared by our team.
KL, ZF, and YP designed and managed the project. YP, ZF, YS, and CC analyzed the data and performed all animal works and collected biological samples. YP wrote the manuscript. ZF, YM, BL, HL, and KL revised the paper. All authors approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (32130102), the National Transgenic Breeding Project (2016ZX08006-001), the China Postdoctoral Foundation project (2018M631648), the Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (2019B030301010), the Key Laboratory of Animal Molecular Design and Precise Breeding of Guangdong Higher Education Institutes (2019KSYS011), and the Key-Area Research and Development Program of Guangdong Province (2019B020211004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahad, W. A., Ahmad, S. M., Beigh, S. A., Bhat, R. A., and Shah, R. A. (2017). Applications of Myostatin (MSTN) Gene in the Livestock Animals and Humans: A Review. Int. J. Curr. Microbiol. Appl. ences 6 (9), 1807–1811. doi:10.20546/ijcmas.2017.609.222
Amory, H., McEntee, K., Linden, A. S., Desmecht, D. J. M., Beduin, J. M. L., D'Orio, V., et al. (1993). Comparison of the Cardiac Pumping Capability and Cardiac Pumping reserve in Double-Muscled and Conventional Calves. Can. J. Physiol. Pharmacol. 71 (12), 946–951. doi:10.1139/y93-143
Bagnell, C. A., Ho, T. Y., George, A. F., Wiley, A. A., Miller, D. J., and Bartol, F. F. (2017). Maternal Lactocrine Programming of Porcine Reproductive Tract Development. Mol. Reprod. Dev. 84 (9), 957–968. doi:10.1002/mrd.22815
Bünger, L., Ott, G., Varga, L., Schlote, W., Rehfeldt, C., Renne, U., et al. (2004). Marker-assisted Introgression of the Compact Mutant Myostatin Allele MstnCmpt-dl1Abc into a Mouse Line with Extreme Growth Effects on Body Composition and Muscularity. Genet. Res. 84 (3), 161–173. doi:10.1017/s0016672304007165
Clinquart, A., Van Eenaeme, C., Mayombo, A. P., Gauthier, S., and Istasse, L. (1995). Plasma Hormones and Metabolites in Cattle in Relation to Breed (Belgian Blue vs Holstein) and Conformation (Double-muscled vs Dual-Purpose Type). Vet. Res. Commun. 19 (3), 185–194. doi:10.1007/bf01839297
Clop, A., Marcq, F., Takeda, H., Pirottin, D., Tordoir, X., Bibé, B., et al. (2006). A Mutation Creating a Potential Illegitimate microRNA Target Site in the Myostatin Gene Affects Muscularity in Sheep. Nat. Genet. 38 (7), 813–818. doi:10.1038/ng1810
de Kretser, D. M., Loveland, K. L., Meinhardt, A., Simorangkir, D., and Wreford, N. (1998). Spermatogenesis. Hum. Reprod. 13 (Suppl. 1), 1–8. doi:10.1093/humrep/13.suppl_1.1
Fan, Z., Liu, Z., Xu, K., Wu, T., Ruan, J., Zheng, X., et al. (2021). Long-term, Multidomain Analyses to Identify the Breed and Allelic Effects in MSTN-Edited Pigs to Overcome Lameness and Sustainably Improve Nutritional Meat Production. Sci. China Life Sci. 1, 1–14. doi:10.1007/s11427-020-1927-9
Feldman, A. T., and Wolfe, D. (2014). Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 1180, 31–43. doi:10.1007/978-1-4939-1050-2_3
Fiems, L. O., Cottyn, B. G., Boucqué, C. V., Bogaerts, D. F., Eenaeme, C. V., and Vanacker, J. M. (1997). Effect of Beef Type, Body Weight and Dietary Protein Content on Voluntary Feed Intake, Digestibility, Blood and Urine Metabolites and Nitrogen Retention. J. Anim. Physiol. Anim. Nutr. 77 (1-5), 1–9. doi:10.1111/j.1439-0396.1997.tb00731.x
Fiems, L. O. (2012). Double Muscling in Cattle: Genes, Husbandry, Carcasses and Meat. Animals 2 (3), 472–506. doi:10.3390/ani2030472
Greig, C. J., Oh, P. S., Gross, E. R., and Cowles, R. A. (2019). Retracing Our STEPs: Four Decades of Progress in Intestinal Lengthening Procedures for Short Bowel Syndrome. Am. J. Surg. 217 (4), 772–782. doi:10.1016/j.amjsurg.2018.11.025
Grobet, L., Royo Martin, L. J., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., et al. (1997). A Deletion in the Bovine Myostatin Gene Causes the Double-Muscled Phenotype in Cattle. Nat. Genet. 17 (1), 71–74. doi:10.1038/ng0997-71
Guo, T., Jou, W., Chanturiya, T., Portas, J., Gavrilova, O., and McPherron, A. C. (2009). Myostatin Inhibition in Muscle, but Not Adipose Tissue, Decreases Fat Mass and Improves Insulin Sensitivity. PLoS One 4 (3), e4937. doi:10.1371/journal.pone.0004937
Hanzen, C., Laurent, Y., and Ward, W. R. (1994). Comparison of Reproductive Performance in Belgian Dairy and Beef Cattle. Theriogenology 41 (5), 1099–1114. doi:10.1016/s0093-691x(05)80033-0
Hoflack, G., Opsomer, G., Van Soom, A., Maes, D., de Kruif, A., and Duchateau, L. (2006). Comparison of Sperm Quality of Belgian Blue and Holstein Friesian Bulls. Theriogenology 66 (8), 1834–1846. doi:10.1016/j.theriogenology.2006.05.007
Holmes, J. H. G., Ashmore, C. R., and Robinson, D. W. (1973). Effects of Stress on Cattle with Hereditary Muscular Hypertrophy. J. Anim. Sci. 36 (4), 684–694. doi:10.2527/jas1973.364684x
Iji, P. A. (2008). Intestinal Development and Nutrient Utilisation in the Ostrich: a Brief Review. Aust. J. Exp. Agric. 48 (10), 1280–1283. doi:10.1071/ea08140
Johnson, L. R. (1985). Functional Development of the Stomach. Annu. Rev. Physiol. 47 (1), 199–215. doi:10.1146/annurev.ph.47.030185.001215
Kambadur, R., Sharma, M., Smith, T. P. L., and Bass, J. J. (1997). Mutations in Myostatin (GDF8) in Double-Muscled Belgian Blue and Piedmontese Cattle. Genome Res. 7 (9), 910–915. doi:10.1101/gr.7.9.910
Kolkman, I., De Vliegher, S., Hoflack, G., Van Aert, M., Laureyns, J., Lips, D., et al. (2007). Protocol of the Caesarean Section as Performed in Daily Bovine Practice in Belgium. Reprod. Domest. Anim. 42 (6), 583–589. doi:10.1111/j.1439-0531.2006.00825.x
Leroy, J. L. M. R., Opsomer, G., De Vliegher, S., Vanholder, T., Goossens, L., Geldhof, A., et al. (2005). Comparison of Embryo Quality in High-Yielding Dairy Cows, in Dairy Heifers and in Beef Cows. Theriogenology 64 (9), 2022–2036. doi:10.1016/j.theriogenology.2005.05.003
Liang, Y.-C., Yeh, J.-Y., and Ou, B.-R. (2007). Effect of Maternal Myostatin Antibody on Offspring Growth Performance and Body Composition in Mice. J. Exp. Biol. 210 (Pt 3), 477–483. doi:10.1242/jeb.02665
Luo, Z. B., Luo, Q. R., Xuan, M. F., Han, S. Z., Wang, J. X., Guo, Q., et al. (2019). Comparison of Internal Organs between Myostatin Mutant and Wild‐type Piglets. J. Sci. Food Agric. 99 (15), 6788–6795. doi:10.1002/jsfa.9962
Matika, O., Robledo, D., Pong-Wong, R., Bishop, S. C., Riggio, V., Finlayson, H., et al. (2019). Balancing Selection at a Premature Stop Mutation in the Myostatin Gene Underlies a Recessive Leg Weakness Syndrome in Pigs. Plos Genet. 15 (1), e1007759. doi:10.1371/journal.pgen.1007759
McPherron, A. C., Lawler, A. M., and Lee, S. J. (1997). Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 387 (6628), 83–90. doi:10.1038/387083a0
McPherron, A. C., and Lee, S.-J. (1997). Double Muscling in Cattle Due to Mutations in the Myostatin Gene. Proc. Natl. Acad. Sci. 94 (23), 12457–12461. doi:10.1073/pnas.94.23.12457
Monin, G., Boccard, R., Vernin, P., Talmant, A., and Nicolas, N. (1974). Caractéristiques physiologiques respiratoires des bovins culards. Genet. Selection Evol. 6 (2), 187–193. doi:10.1186/1297-9686-6-2-187
Mosher, D. S., Quignon, P., Bustamante, C. D., Sutter, N. B., Mellersh, C. S., Parker, H. G., et al. (2007). A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. Plos Genet. 3 (5), e79. doi:10.1371/journal.pgen.0030079
Ortigues, I., and Doreau, M. (1995). Responses of the Splanchnic Tissues of Ruminants to Changes in Intake: Absorption of Digestion End Products, Tissue Mass, Metabolic Activity and Implications to Whole Animal Energy Metabolism. Annales De Zootechnie 44 (4), 321–346. doi:10.1051/animres:19950401
Patterson, D. J., Bellows, R. A., and Burfening, P. J. (1981). Effects of Caesarean Section, Retained Placenta and Vaginal or Uterine Prolapse on Subsequent Fertility in Beef Cattle. J. Anim. Sci. 53 (4), 916–921. doi:10.2527/jas1981.534916x
Qian, L., Tang, M., Yang, J., Wang, Q., Cai, C., Jiang, S., et al. (2015). Targeted Mutations in Myostatin by Zinc-finger Nucleases Result in Double-Muscled Phenotype in Meishan Pigs. Sci. Rep. 5, 14435. doi:10.1038/srep14435
Rao, S., Fujimura, T., Matsunari, H., Sakuma, T., Nakano, K., Watanabe, M., et al. (2016). Efficient Modification of the Myostatin Gene in Porcine Somatic Cells and Generation of Knockout Piglets. Mol. Reprod. Dev. 83 (1), 61–70. doi:10.1002/mrd.22591
Schuelke, M., Wagner, K. R., Stolz, L. E., Hübner, C., Riebel, T., Kömen, W., et al. (2004). Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 350 (26), 2682–2688. doi:10.1056/NEJMoa040933
Sharma, M., Kambadur, R., Matthews, K. G., Somers, W. G., Devlin, G. P., Conaglen, J. V., et al. (1999). Myostatin, a Transforming Growth Factor-? Superfamily Member, Is Expressed in Heart Muscle and Is Upregulated in Cardiomyocytes after Infarct. J. Cel. Physiol. 180 (1), 1–9. doi:10.1002/(sici)1097-4652(199907)180:1<1::aid-jcp1>3.0.co;2-v
Soybel, D. I. (2005). Anatomy and Physiology of the Stomach. Surg. Clin. North America 85 (5), 875–894. doi:10.1016/j.suc.2005.05.009(
Wang, K., Ouyang, H., Xie, Z., Yao, C., Guo, N., Li, M., et al. (2015). Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 5, 16623. doi:10.1038/srep16623
Wang, K., Tang, X., Xie, Z., Zou, X., Li, M., Yuan, H., et al. (2017). CRISPR/Cas9-mediated Knockout of Myostatin in Chinese Indigenous Erhualian Pigs. Transgenic Res. 26 (6), 799–805. doi:10.1007/s11248-017-0044-z
Keywords: myostatin, pigs, heterozygote, visceral, histomorphology
Citation: Pei Y, Fan Z, Song Y, Chen C, Mu Y, Li B, Feng Z, Li H and Li K (2022) Viscera Characteristics of MSTN-Edited Heterozygous Pigs. Front. Genet. 13:764965. doi: 10.3389/fgene.2022.764965
Received: 26 August 2021; Accepted: 08 February 2022;
Published: 01 March 2022.
Edited by:
James Reecy, Iowa State University, United StatesReviewed by:
Martha Nchang Bemji, Federal University of Agriculture, Abeokuta, NigeriaCopyright © 2022 Pei, Fan, Song, Chen, Mu, Li, Feng, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kui Li, bGlrdWlAY2Fhcy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.