- 1The First People's Hospital of Bijie, Bijie, Guizhou, China
- 2Orthopedics, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi, China
- 3Department of Gynecology, Heji Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi
- 4Institute of Evidence-based medicine, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi
Background: Studies have shown that glutathione S-transferase M1 (GSTM1) and. glutathione S-transferase T1 (GSTT1) null genotype may increase the risk of cervical cancer (CC) or ovarian cancer (OC), however, the results of published original studies and meta-analyses are inconsistent.
Objectives: To investigate the association between GSTM1 present/null and GSTT1 present/null polymorphisms, with the risk of cervical cancer or ovarian cancer.
Methods: The odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the association between GSTM1 present/null and GSTT1 present/null polymorphisms and the risk of cervical cancer or ovarian cancer. To assess the confidence of statistically significant associations, we applied false positive reporting probability (FPRP) and bayesian false discovery probability (BFDP) tests.
Results: Overall analysis showed that GSTM1 null was associated with an increased risk of cervical cancer, and subgroup analysis showed a significant increase in cervical cancer risk in Indian and Chinese populations; GSTT1 was not found null genotype are significantly associated with cervical cancer. Overall analysis showed that GSTM1 and GSTT1 null were not associated with the risk of ovarian cancer, subgroup analysis showed that GSTM1 null was associated with an increased risk of OC in East Asia, and GSTT1 null was associated with an increased risk of OC in South America. However, when we used false positive reporting probability and bayesian false discovery probability to verify the confidence of a significant association, all positive results showed “low confidence” (FPRP > .2, BFDP > .8).
Conclusion: Overall, this study strongly suggests that all positive results should be interpreted with caution and are likely a result of missing plausibility rather than a true association.
Introduction
Gynecological cancers have different degrees of negative impact on women’s health around the world. Among them, with CC the highest incidence and OC with the highest mortality have attracted much attention. According to the 2020 global cancer incidence and mortality statistics released by the World Health Organization, about 604,000 women were diagnosed with CC, and about 342,000 women died of CC, witch has become the most common cancer in 23 countries and 36. The number one cause of cancer death in 100 countries. According to the data survey released by the national cancer center of my country, in recent years, the incidence of CC has increased at an average annual rate of 8.7% (Zhao and Song, 2021). According to global statistics in 2020, about 310,000 women were diagnosed with OC, and about 210,000 women died of OC. The analysis of the incidence and death data of OC in the “China cancer registry annual report” shows that from 2005 to 2016, OC in China incidence and mortality are rapidly increasing, and most OC occur in people over the age of 50 (Huang et al., 2022). Although the main pathogenic factors of the two cancers are different, epidemiological studies have shown that the occurrence of both cancers is related to individual genetic susceptibility, and studies have shown that the genetic polymorphism of cancer susceptibility genes is associated with high cancer risk. There may be associations; therefore, finding true gene associations will help people to further understand the pathogenesis of CC and OC, and actively exploring the multi-pathway pathogenesis of CC and OC is of great significance for cancer prevention, diagnosis, and treatment (Ueda et al., 2008).
Glutathione s-transferase system (GSTs: Glutathione s -transferases), as the first line of defense in cell protection, participates in the detoxification process of exogenous toxins in vivo, making reduced glutathione and electrophilic substances combine to convert toxic substances in the body into hydrophilic substances, which are excreted through urine or bile to complete the detoxification process (Board and Menon, 2013; Zou, 2013). Currently, eight glutathione s-transferases have been identified in mammals, including alpha, kappa, mu, omega, pi, sigma, theta, and zeta. Among them, mu (µ)-type GSTM1 and theta (θ)-type GSTT1 is the most studied genes in the relationship between gynecological tumors and glutathione transferase, GSTM1 is located on chromosome 1 (1p13.3), GSTT1 is located on chromosome 22 (p11.23), its function is to link various parent electrochemical compounds (such as drugs, environmental toxins, oxidation chain products, etc.) combine with glutathione to enter the next metabolic step, allowing the toxic substances to be easily excreted from the body. The GST gene has polymorphisms at multiple loci, among which GSTM1 and GSTT1 share a common zero allele. The most common mutation of these two genes is the whole null genotype, and the mutation of the gene will change the activation or inactivation of the corresponding enzyme. The ability to source substrates, thereby affecting the detoxification of carcinogens, exposing cells in the body to toxic substances, causing DNA damage, potentially increasing somatic mutations that increase an individual by 39%, risk of developing tumors (Abbas et al., 2018; Sharma et al., 2019). Therefore individuals with homozygous null genotype polymorphisms are considered potential risk factors for the development of various malignancies in humans. At present, the correlation of GSTM1 and GSTT1 present/null polymorphisms with CC and OC is still unclear. Therefore, studying the glutathione metabolic pathway involving glutathione-s-transferase may be useful for early warning and early warning of gynecological malignancies. Prevention as well as treatment options and prognosis for cancer patients are of great importance.
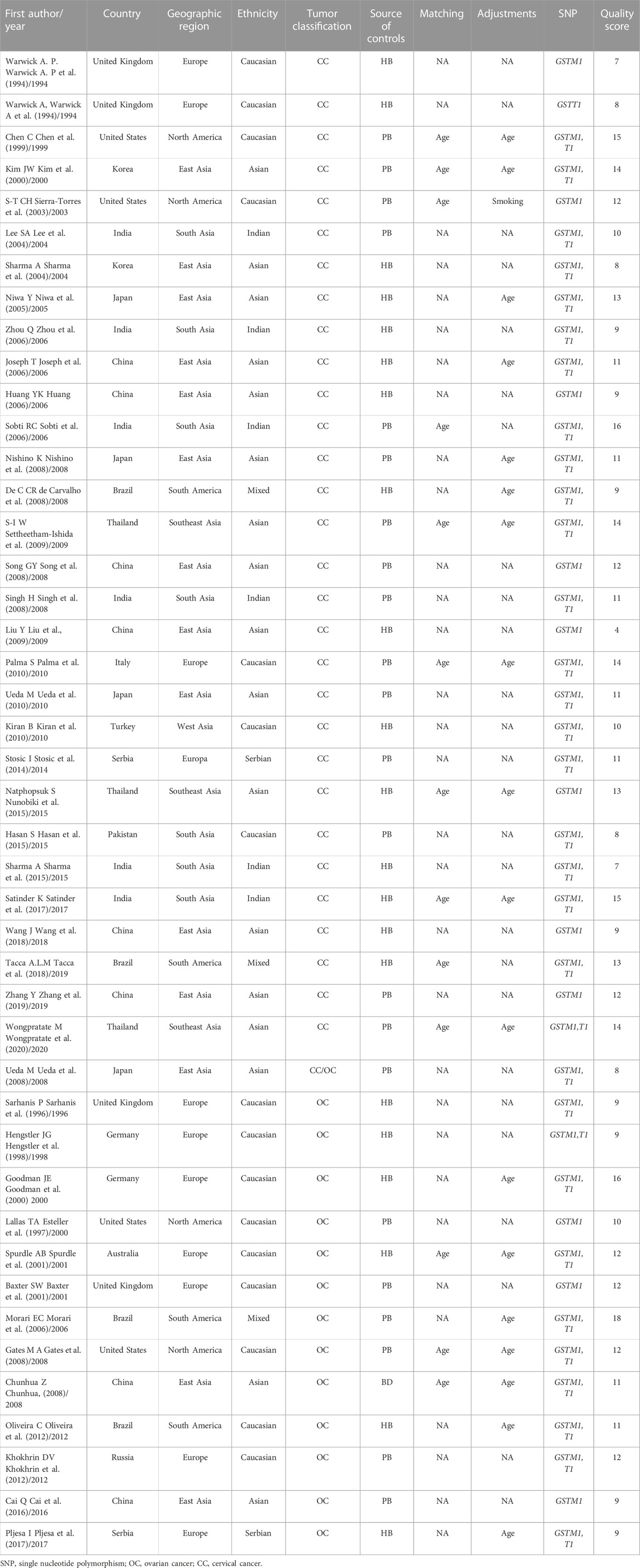
So far, there have been 31 articles (Warwick A. P et al., 1994; Warwick A et al., 1994; Chen et al., 1999; Kim et al., 2000; Sierra-Torres et al., 2003; Lee et al., 2004; Sharma et al., 2004; Niwa et al., 2005; Huang, 2006; Joseph et al., 2006; Sobti et al., 2006; Zhou et al., 2006; de Carvalho et al., 2008; Nishino et al., 2008; Singh et al., 2008; Song et al., 2008; Ueda et al., 2008; Liu et al., 2009; Settheetham-Ishida et al., 2009; Kiran et al., 2010; Palma et al., 2010; Ueda et al., 2010; Stosic et al., 2014; Hasan et al., 2015; Nunobiki et al., 2015; Sharma et al., 2015; Satinder et al., 2017; Tacca et al., 2018; Wang et al., 2018; Zhang et al., 2019; Wongpratate et al., 2020) on the individual and combined effects of GSTM1 and/or GSTT1 present/null polymorphisms and CC risk, and nine meta-analyses (Economopoulos et al., 2010a; Gao et al., 2011; Sui et al., 2011; Wang et al., 2011; Liu and Xu, 2012; Zhang et al., 2012; Zhen et al., 2013; Sun and Song, 2016; Tian et al., 2019) reporting GSTM1 and/or GSTT1 present/null polymorphisms associated with CC risk. 14 articles investigated the individual impact of GSTM1 and/or GSTT1 present/null polymorphisms and OC risk (Sarhanis et al., 1996; Esteller et al., 1997; Hengstler et al., 1998; Goodman et al., 2000; Baxter et al., 2001; Spurdle et al., 2001; Morari et al., 2006; Chunhua, 2008; Gates et al., 2008; Ueda et al., 2008; Khokhrin et al., 2012; Oliveira et al., 2012; Cai et al., 2016; Pljesa et al., 2017), and five meta analyses (Economopoulos et al., 2010b; Yin et al., 2013; Han et al., 2014; Jin and Hao, 2014; Xu et al., 2014) reported individual effects of GSTM1 and/or GSTT1 present/null polymorphisms and OC risk. However, the conclusions of all studies were inconsistent and even contradictory. Furthermore, no study has examined the correlation between the corresponding positive results. Correlations are assessed for reliability. Newer original studies have recently been published investigating these associations, and therefore, an updated meta-analysis should be performed to explore these questions. Two methods FPRP and BFDP tests were used to assess the confidence of these findings. We aim to provide a real link to these questions and to discuss the positive findings identified in terms of biological mechanisms involved in CC and OC.
Material and methods
Literature search strategy
This meta-analysis was conducted based on the priority reported entries of systematic reviews and meta-analyses (PRISMA). Pubmed, Embase, Scopus, Chinese biomedical medical databases (CBM), China national knowledge infrastructure (CNKI), and Wanfang databases and so on in both Chinese and English (up to 15 September 2021) were searched to identify eligible studies that analyzed the GSTM1 present/null and GSTT1 present/null, with CC and OC risk. The following keywords were used: (GSTT1 OR glutathione s-transferase T1 OR GSTM1 OR glutathione s-transferase M1) AND (polymorphism OR variant OR mutation) AND (ovarian cancer OR oophoroma OR carcinoma of ovary OR cervical cancer OR carcinoma of uterine cerxix OR cervical malignancy). The search strategy was designed to be sensitive and broad. We first carefully reviewed the title and abstract of the search results, and then downloaded full articles to identify possible articles. These were evaluated in detail to identify relevant articles. The reference lists of identified articles and reviews was also examined as appropriate. The corresponding author may be contacted by e-mail if only the abstract was available online or the data was incomplete.
Literature inclusion and exclusion criteria
Inclusion criteria were as listed below: 1) articles on the GSTM1 present/null and GSTT1 present/null, with the risk of CC or OC. 2) The diagnostic criteria for CC and OC meet histological or pathological criteria. 3) case-control studies or cohort studies where the language of the literature is limited to Chinese or English. 4) sufficient genotype data to calculate ORs and 95% CIs. Exclusion criteria were as listed below: 1) no raw data. 2) no control. 3) review articles, case reports, editorials, or animal research. 4) duplicate and insufficient data.
Extraction information
Two investigators independently extracted data using excel. Any disagreement was solved by iteration, discussion, and consensus. The details of the data extraction form included the following: first author, year of publication, country, geographical region, ethnicity, control source, control type, matching, adjusted OR, SNP, sample size, each locus, the number of genotypes, and the literature quality score. Of these, the literature quality score needs to be obtained by calculation.
Quality score assessment
The quality of all studies was assessed independently by two researchers. We supplemented and improved the quality assessment criteria from relevant guidelines and previous meta-analysis, combined with NOS criteria (Aerssens et al., 2000; Moher et al., 2009; Thakkinstian et al., 2011), Supplementary Table S1 lists the quality assessment scales for studies of the association of CC or OC risk. Studies were considered to be of low quality if the quality score was less than 9, whereas in the Meta-analysis, scores ≥ 11 were considered to be of high quality, and studies with scores between 9 and 11 were considered to be of moderate quality. Supplementary Table S1 lists the scoring scale for assessing the quality of the literature with the following entries: 1) source of the experimental group; 2) source of the control group. 3) diagnostic criteria for patients with CC and OC. 4) inclusion criteria for the control group. 5) whether the experimental and control groups were matched. 6) genotype testing. 7) samples used to determine genotype. 8) assessment of the association between genotype and OC and CC. 9) size of sample size.
Statistical analysis
We applied the crude ratio (OR) and its 95% confidence interval (CI) to assess the association effect of the GSTM1 present/null and GSTT1 present/null, with the risk of CC or OC. Q-tests were used to assess heterogeneity between selected studies and statistically, significant heterogeneity was considered if p < .10 and/or I2 > 50%, using a random-effects model (Mantel and Haenszel, 1959), and if heterogeneity was not significant (I2 ≤ 50%), a fixed-effects model (Der Simonian and Laird, 2015) was considered, followed by a search for sources of heterogeneity based on meta-regression analysis. Subgroup analyses were performed for HPV infection, smoking, geographic region, and ethnicity according to CC epidemiology, and for ethnicity and geographic region according to OC epidemiology. Two methods were used to conduct sensitivity analyses: one was to exclude one study at a time. The second was to conduct statistical analyses after excluding low-quality and small-sample studies. Publication bias was confirmed according to Begg’s funnel plot (Begg and Mazumdar, 1994) and Egger’s test (considered significant publication bias if p < .05) (Egger et al., 1997) and if publication bias was observed, non-parametric pruning and padding methods were applied to identify missing studies (Dual and Tweedie, 2000). To assess the confidence of statistically significant associations in the current and previous meta-analyses, we applied the FPRP (Wacholder et al., 2004) and the BFDP test (Ioannidis et al., 2008), and the FPRP was estimated using the excel spreadsheet appendix. All statistical analyses were calculated using Stata version 12.0 (STATA Corporation, college station, TX).
Results
Literature search results
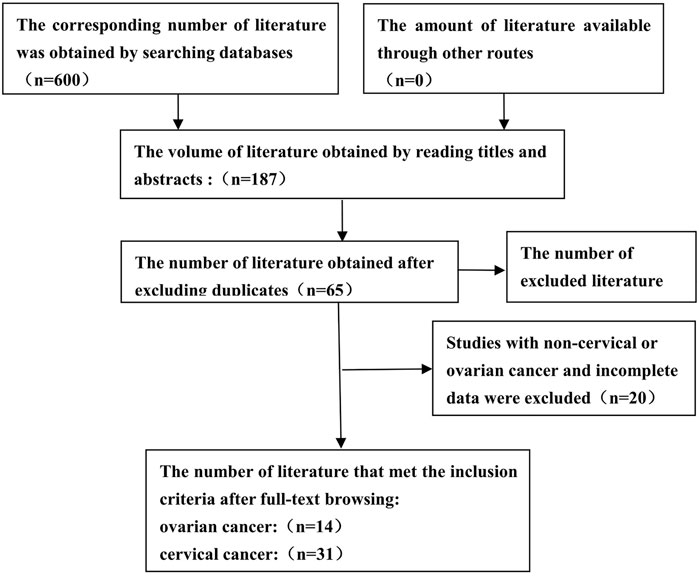
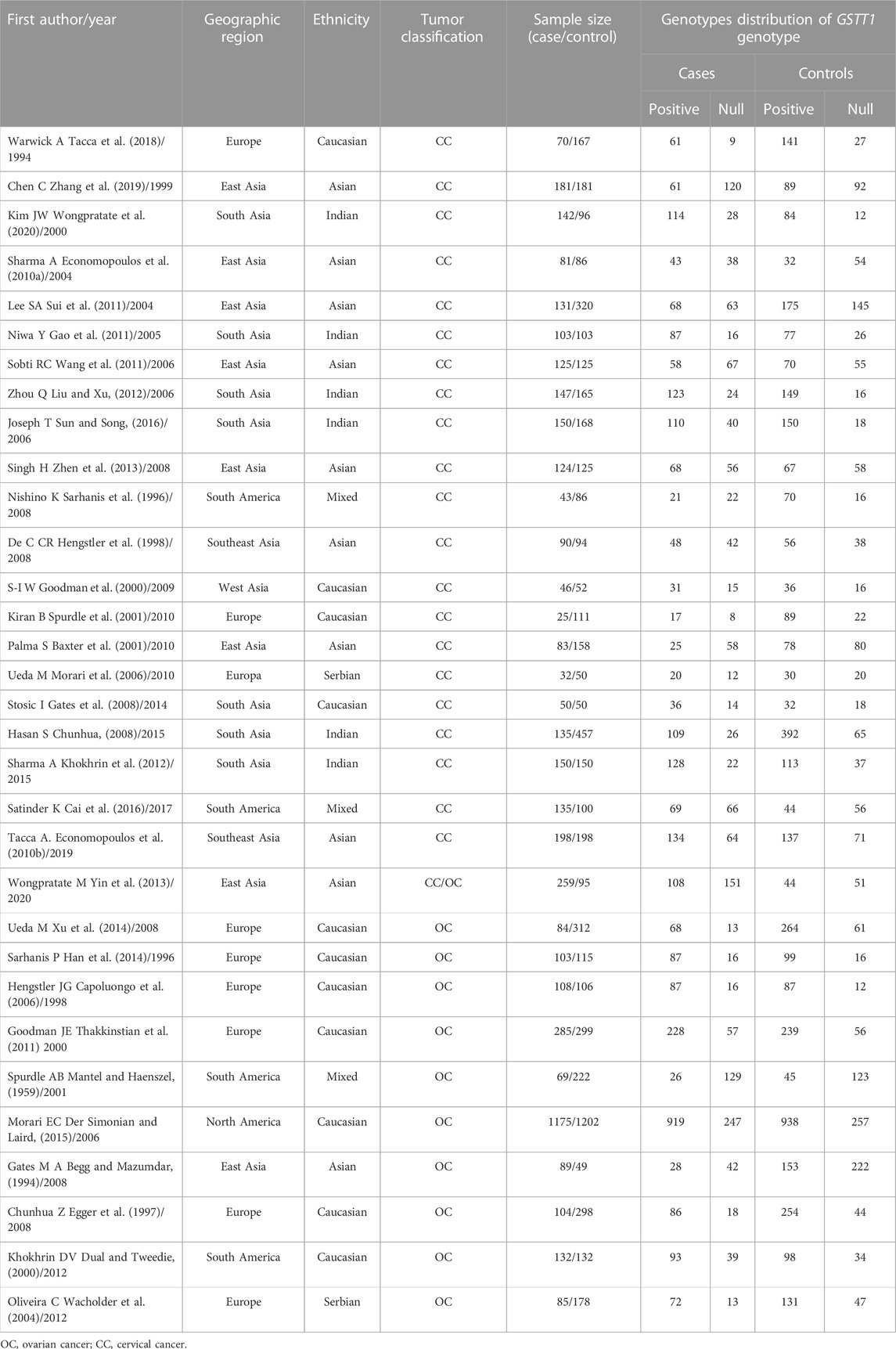
A total of 600 articles were searched (Figure 1). After reading the topic, 413 articles inconsistent with this study (including other genotype studies, reviews, case reports, meta-analyses, and letters) were excluded, 122 duplicate articles were excluded after further reading of the title and abstract, and the remaining articles were read in full of the 66 articles, 22 studies for which complete data were not available were excluded, and the final 44 original articles were included in this study. 31 studies related to CC were included (including 30 for GSTM1 and 22 for GSTT1) (Warwick A. P et al., 1994; Warwick A et al., 1994; Chen et al., 1999; Kim et al., 2000; Sierra-Torres et al., 2003; Lee et al., 2004; Sharma et al., 2004; Niwa et al., 2005; Huang, 2006; Joseph et al., 2006; Sobti et al., 2006; Zhou et al., 2006; de Carvalho et al., 2008; Nishino et al., 2008; Singh et al., 2008; Song et al., 2008; Ueda et al., 2008; Liu et al., 2009; Settheetham-Ishida et al., 2009; Kiran et al., 2010; Palma et al., 2010; Ueda et al., 2010; Stosic et al., 2014; Hasan et al., 2015; Nunobiki et al., 2015; Sharma et al., 2015; Satinder et al., 2017; Tacca et al., 2018; Wang et al., 2018; Zhang et al., 2019; Wongpratate et al., 2020), 14 studies related to OC (including 14 GSTM1 and 11 GSTT1) (Sarhanis et al., 1996; Esteller et al., 1997; Hengstler et al., 1998; Goodman et al., 2000; Baxter et al., 2001; Spurdle et al., 2001; Morari et al., 2006; Chunhua, 2008; Gates et al., 2008; Ueda et al., 2008; Khokhrin et al., 2012; Oliveira et al., 2012; Cai et al., 2016; Pljesa et al., 2017). Table 1 shows the general characteristics of the studies included in this meta-analysis. Among the studies on CC risk, there were 30 articles on GSTM1 present/null polymorphisms (including 3,484 cases and 4,208 controls, see Table 2), 22 articles on GSTT1 present/null polymorphisms (including 2,500 cases), and 3,148 control cases, see Table 3). Among OC risk studies, there were 14 articles on GSTM1 present/null polymorphisms (including 3,035 cases and 3,422 controls, see Table 2), 11 articles on GSTT1 present/null polymorphisms (including 2,543 cases and 3,275 controls, see Table 3). Finally, according to the quality assessment of molecular association studies, among the studies on the association of GSTM1 present/null polymorphisms with CC risk, there were 13 high-quality, 7 medium-quality, and 10 low-quality studies. Among studies on the association between polymorphisms and CC risk, there were 9 high-quality, 7 moderate-quality and 7 low-quality studies, Among the studies on the association between GSTM1 present/null and OC risk, there were 6 high-quality studies. High-quality, 3 moderate-quality, and 5 low-quality studies, among the studies on the association of GSTT1 present/null polymorphisms with OC risk, there were 5 high-quality, 2 moderate-quality, and 4 low-quality studies.
Quantitative synthesis
Association of GSTM1 present/null with the risk of CC development
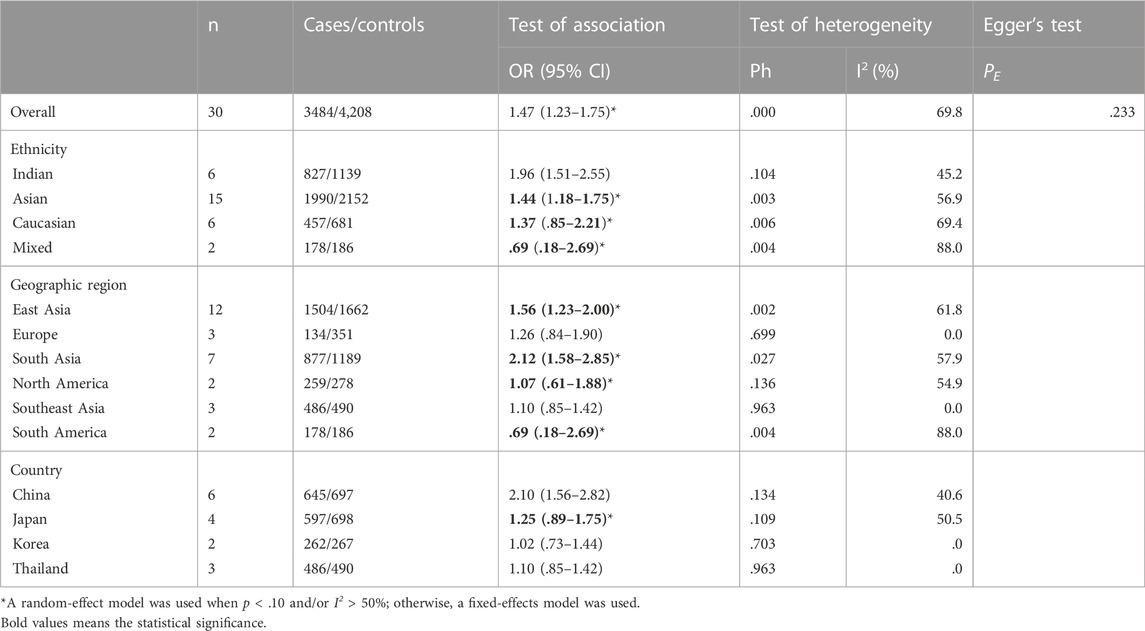
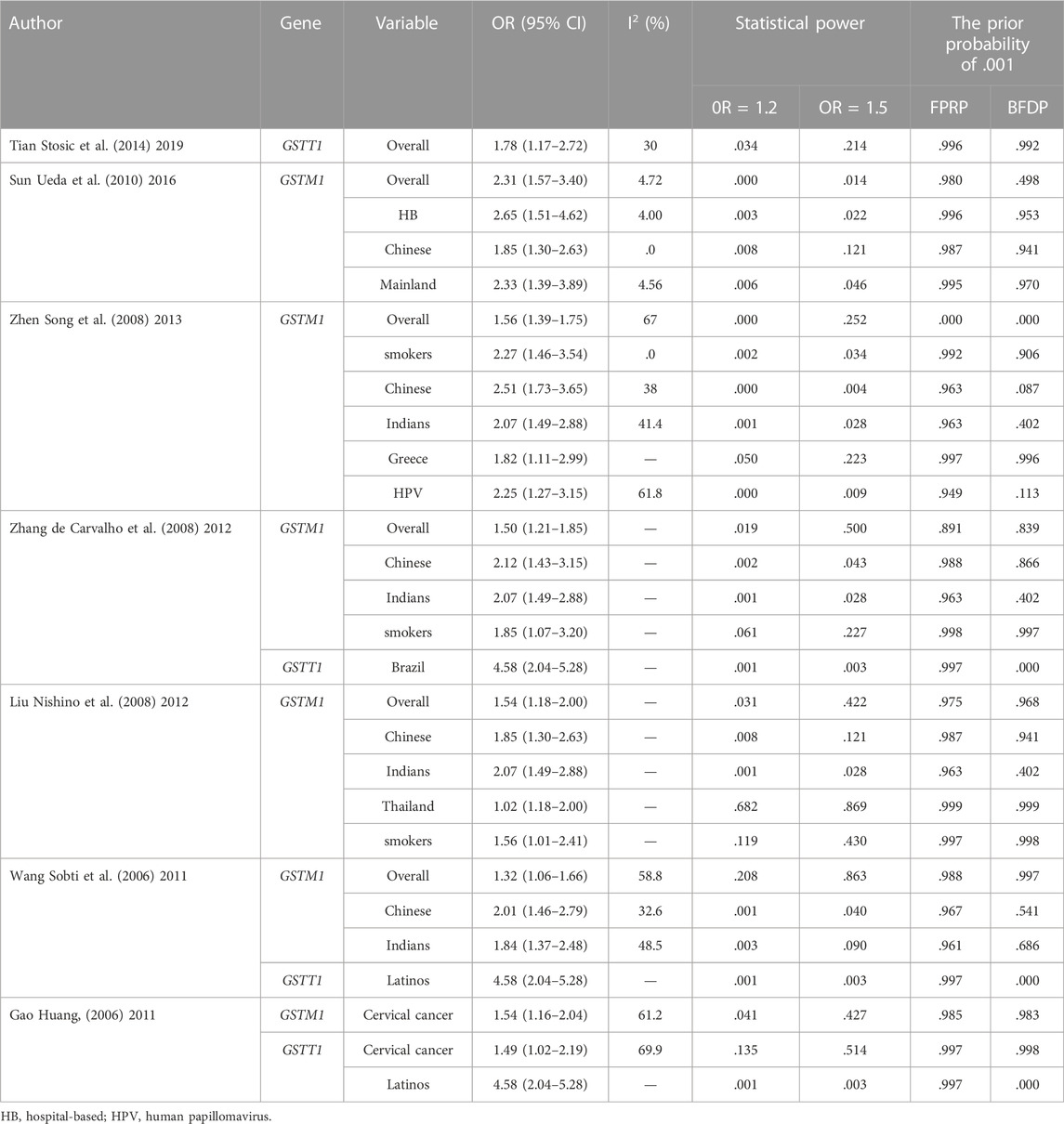
A total of 30 studies on GSTM1 present/null polymorphisms and the risk of CC were included. Regarding the comparison of the distribution of positive vs. null in the case group and the control group, the heterogeneity test results showed that the Q test p = .000 and I2 = 69.8%, the random effect model is used, and the forest diagram: OR [95% CI] is 1.47 (1.23–1.75), see Figure 2 and Table 4 shows the results of the association between GSTM1 present/null polymorphisms and CC risk. In the overall analysis, individuals with GSTM1 null genotype had a significantly increased risk of CC (OR = 1.47, 95% CI:1.23–1.75). Further subgroup analysis for race, country and geographical region showed that a significantly increased risk of CC was observed in Indians (OR = 1.96, 95% CI:1.51–2.55) and Asians (OR = 1.44, 95% CI:1.18–1.75), a significantly increased risk of CC was observed in East Asia (OR = 1.56, 95% CI:1.23–2.00) and South Asia (OR = 2.12, 95% CI: 1.58–2.85), a subgroup analysis of Asian countries showed that a significantly increased risk of CC was observed only in the Chinese population (OR = 2.10, 95% CI: 1.56–2.82).
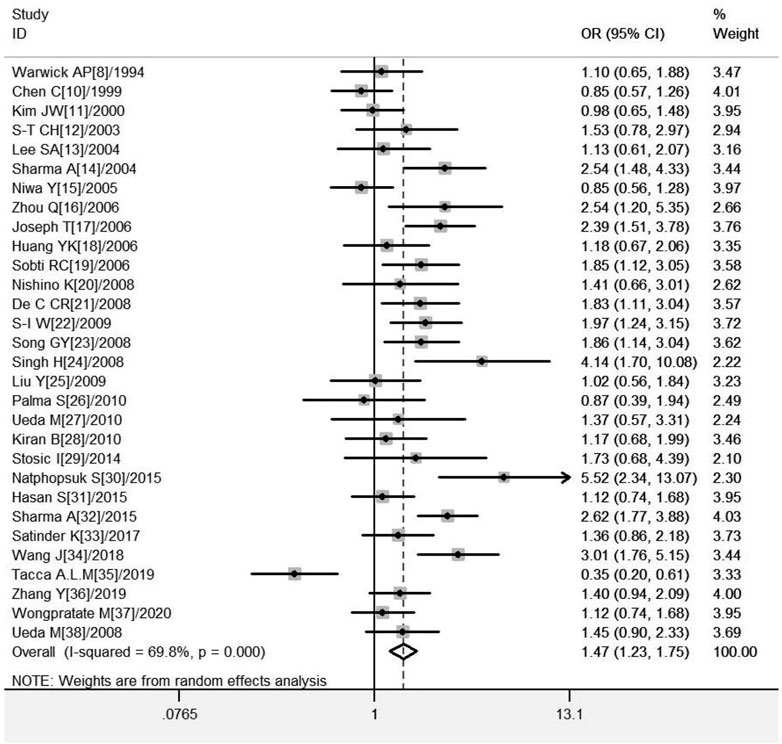
FIGURE 2. Forest plot of meta-analysis of the relationship between GSTM1 gene polymorphisms and cervical cancer risk.
Association of GSTT1 present/null with the risk of CC development
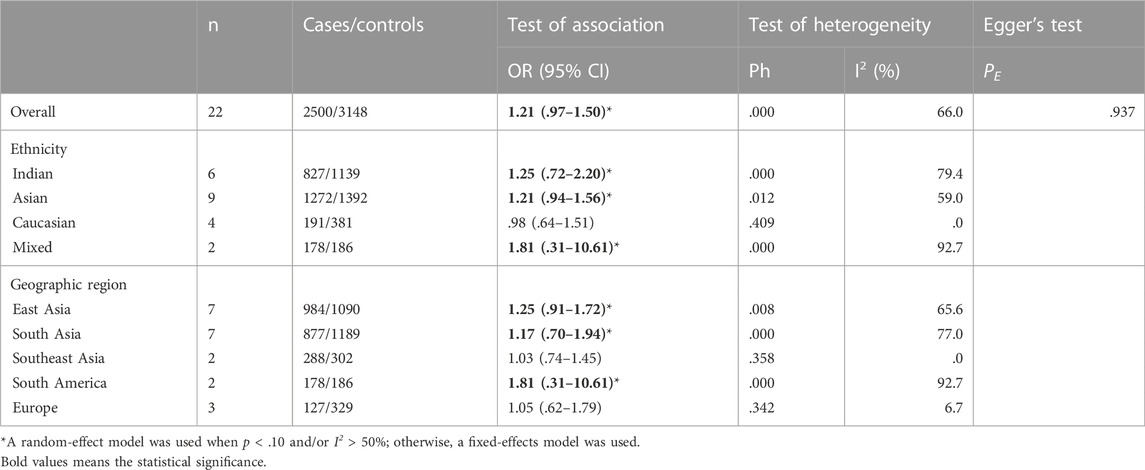
A total of 22 studies on GSTT1 present/null polymorphisms and risk of CC were included, and the heterogeneity test showed Q-test p = .000 and I2 = 66.0%, and the random-effects model was selected, and the forest plot showed that the OR [95% CI] was 1.21 (.97–1.50), as shown in Figure 3. Table 5 shows the results of the association between GSTT1 present/null polymorphisms and CC risk. In the overall analysis, no association was observed between GSTT1 null genotype and CC risk, and no association with CC risk was observed in further subgroup analysis.
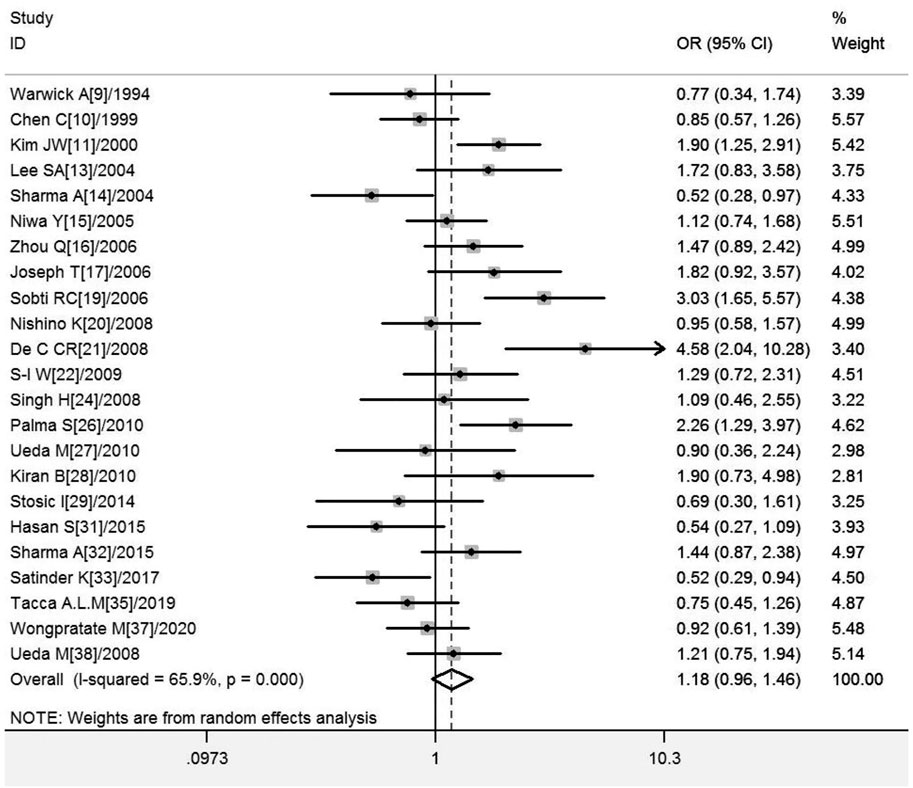
FIGURE 3. Forest plot of meta-analysis of the relationship between GSTT1 gene polymorphisms and cervical cancer risk.
Association of GSTM1 present/null with the risk of OC development
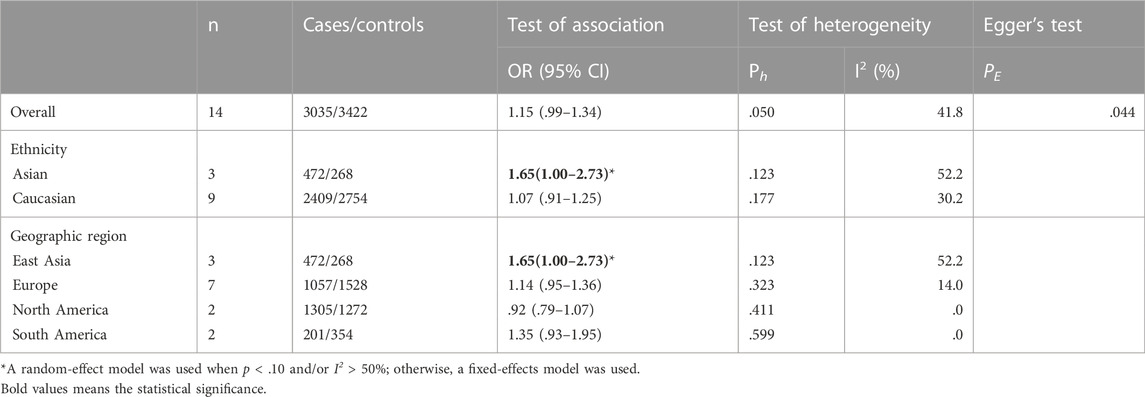
A total of 14 studies on GSTM1 present/null polymorphisms and risk of OC were included. The heterogeneity test showed Q-test p = .050 and I2 = 41.8%, and a fixed-effects model was selected, and the forest plot showed that the OR [95% CI] was 1.15 (.99–1.34), as shown in Figure 4 and Table 6 shows the results of the association between GSTM1 present/null polymorphisms and OC risk. In the overall analysis, GSTM1 null was not significantly associated with increased OC risk, and further subgroup analysis showed that GSTM1 null genotype was associated with increased OC risk in East Asia (OR = 1.65, 95% CI:1.00–2.73).
FIGURE 4. Forest plot of meta-analysis of the relationship between GSTM1 gene polymorphisms and ovarian cancer risk.
Association of GSTT1 present/null with the risk of OC development
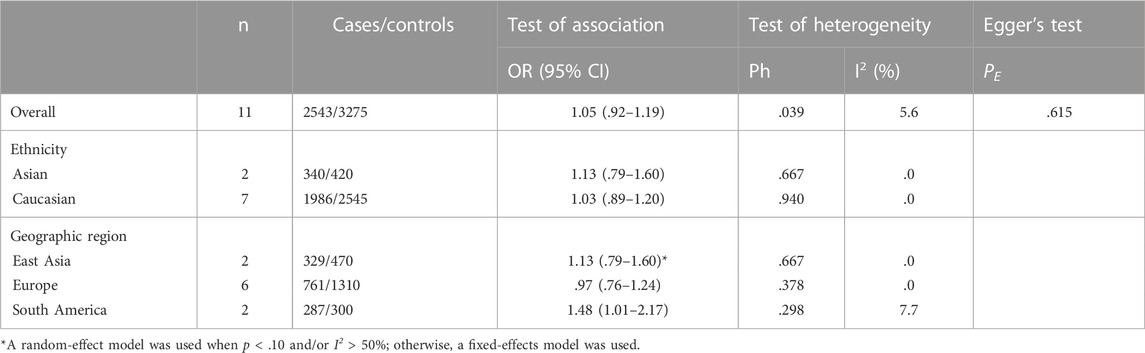
A total of 11 studies were included regarding the GSTT1 present/null polymorphisms and the risk of OC, and the results of the heterogeneity test showed Q-test p = .039 and I2 = 5.6%, and the fixed-effects model was chosen, and the forest plot showed that the OR [95% CI] was 1.05 (.92–1.19), as shown in Figure 5 and Table 7 shows the results of the association between GSTT1 present/null polymorphisms and OC risk. In the overall analysis, The GSTT1 null genotype was not significantly associated with OC risk, but subgroup analysis showed that the GSTT1 null genotype was associated with an increased risk of OC in South America (OR = 1.48, 95% CI:1.01–2.17).
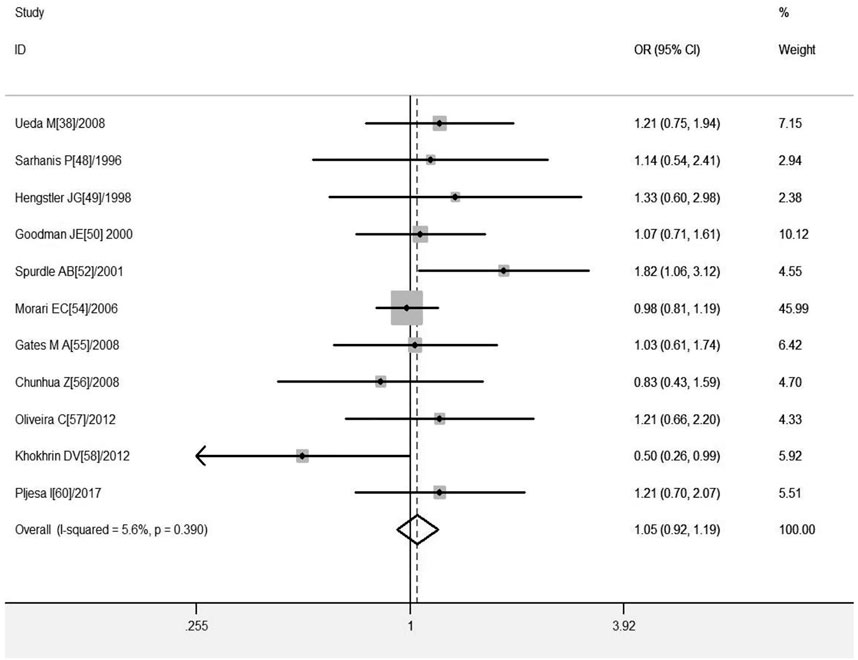
FIGURE 5. Forest plot of meta-analysis of the relationship between GSTT1 gene polymorphisms and ovarian cancer risk.
Heterogeneity test
Due to the sources of potential heterogeneity in the individual original studies, we applied meta-regression analysis to test for heterogeneity, as shown in Table 8. In the study of GSTM1 present/null polymorphisms and CC risk, there was heterogeneity in control matching and literature quality (p < .05), where matching explained 27.93% of the sources of heterogeneity and literature quality explained 18.96% of the sources of heterogeneity (not specifically reported), considering that the two types of covariates may be the main source of heterogeneity in the relevant studies. In the study of GSTM1 present/null polymorphisms and OC risk, there was heterogeneity in sample size (p < .05), showing that it could explain 31.75% of the sources of heterogeneity (not specifically reported), considering that sample size could be the main source of heterogeneity in the relevant studies. No covariates were identified as a source of heterogeneity in studies of GSTT1 present/null and risk of CC or OC.
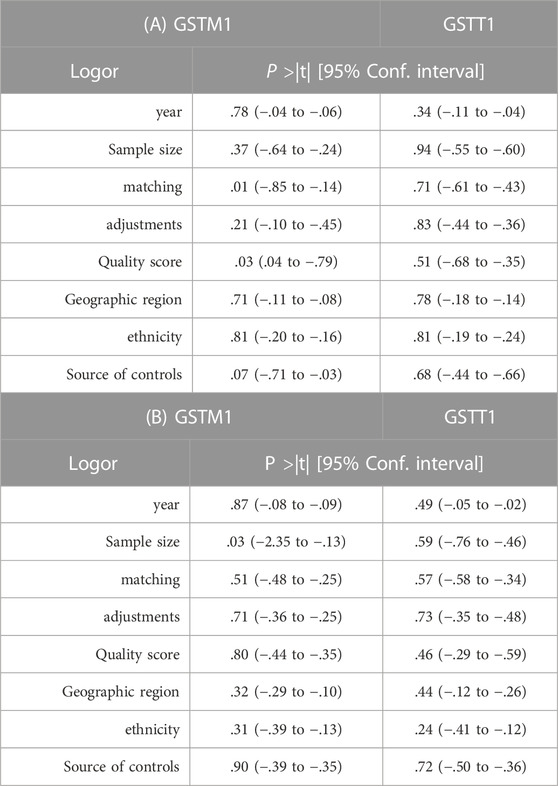
TABLE 8. A) Meta-regression analysis of GSTM1, GSTT1 gene polymorphisms, and risk of cervical cancer. (B) Meta-regression analysis of GSTM1, GSTT1 gene polymorphisms, and risk of ovarian cancer.
Sensitivity analysis
Sensitivity analysis was performed using two methods for meta-analysis. First, in evaluating the stability of the current meta-analysis, the results of each study were not changed after deleting them. Second, considering that studies with low quality and small sample size may be more likely to have positive results, we performed sensitivity analysis after excluding low-quality and small sample studies, and the results showed that GSTM1 null was not associated with CC risk in the overall study (OR = 1.24, 95% CI:0.99–1.57), GSTT1 null genotype was associated with CC risk in East Asia (OR = 1.45, 95% CI:1.07–1.96), GSTM1 null genotype was not significantly associated with OC risk in East Asia, and the remaining results were not significantly changed (as shown in Tables 9).
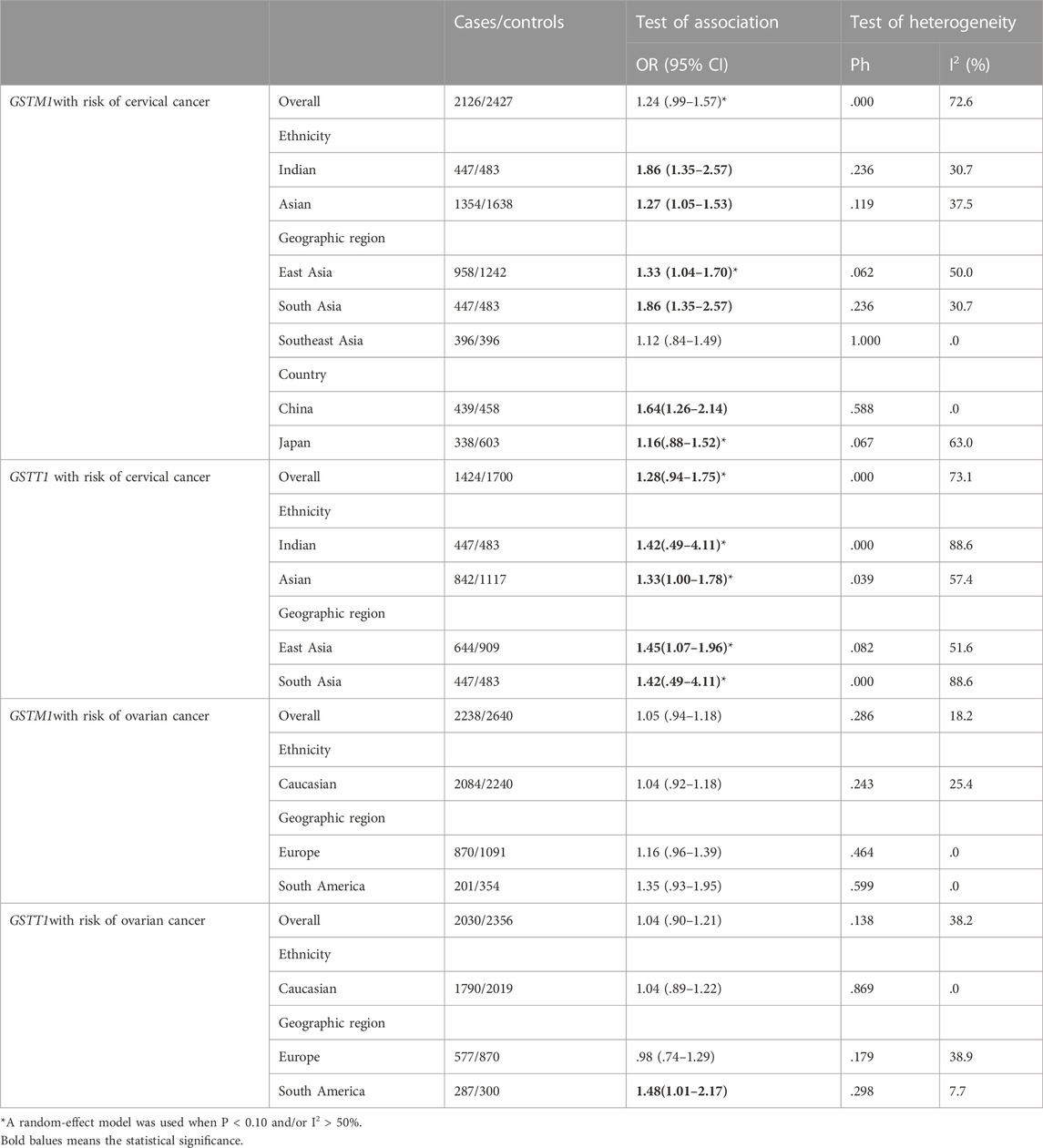
TABLE 9. Pooled estimates of the association of GSTM1, GSTT1 polymorphism with risk of cervical cancer or ovarian cancer. Exclude low-quality and small sample-studies.
Publication bias
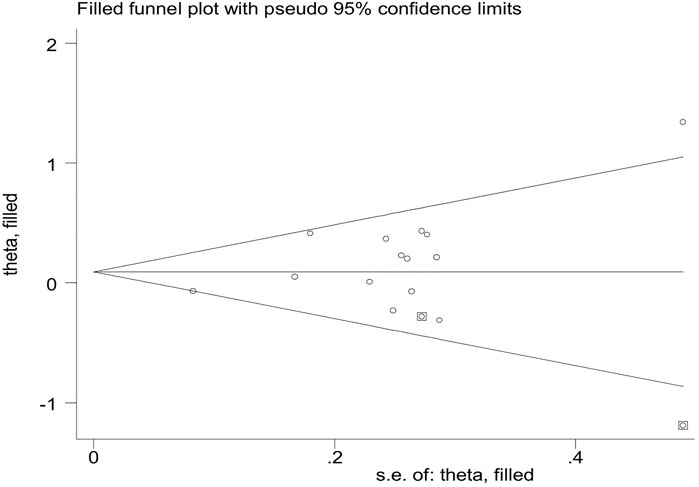
Publication bias was assessed by Begg’s funnel plot and Egger’s test, which showed no evidence of publication bias in the studies of both the GSTM1 present/null and GSTT1 present/null, with the CC risk (see Figure 6). No data showed publication bias between GSTT1 present/null polymorphisms and OC risk (see Figure 7B). The data analysis showed a bias between GSTM1 present/null polymorphisms and OC risk (p = .044), as shown in Figure 7A. Further adjusted for publication bias using a non-parametric “trim and fill” approach, the results remained the same (as shown in Figure 8), indicating that the addition of studies does not affect the overall combined results.
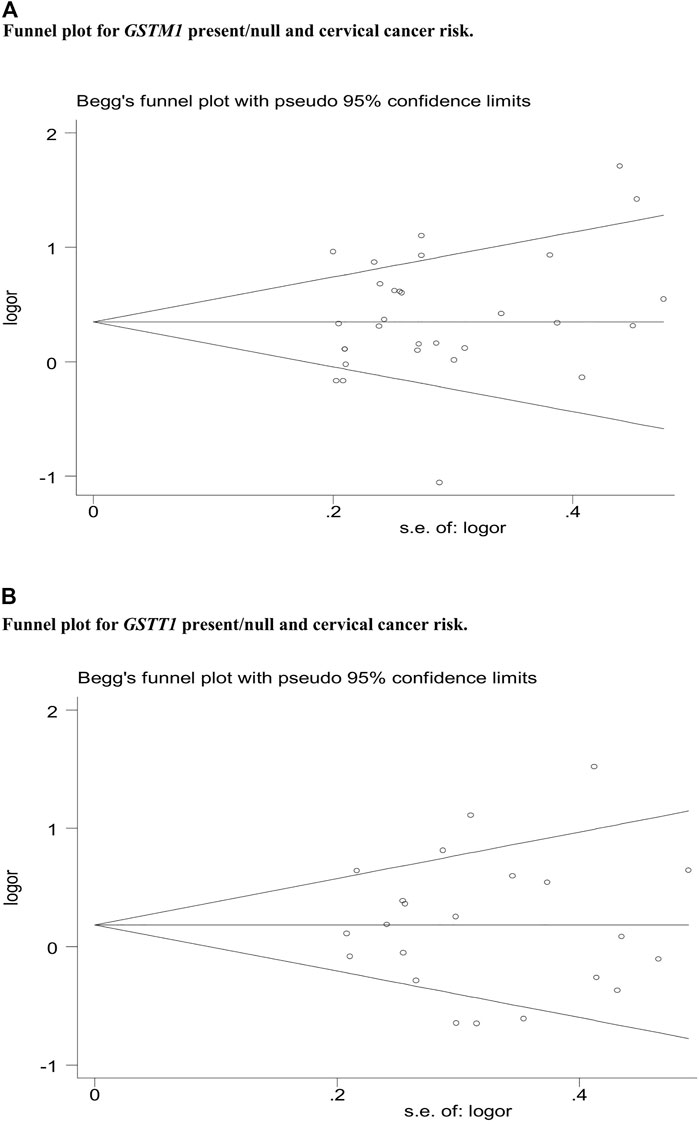
FIGURE 6. (A) Funnel plot for GSTM1 present/null and cervical cancer risk. (B) Funnel plot for GSTT1 present/null and cervical cancer risk.
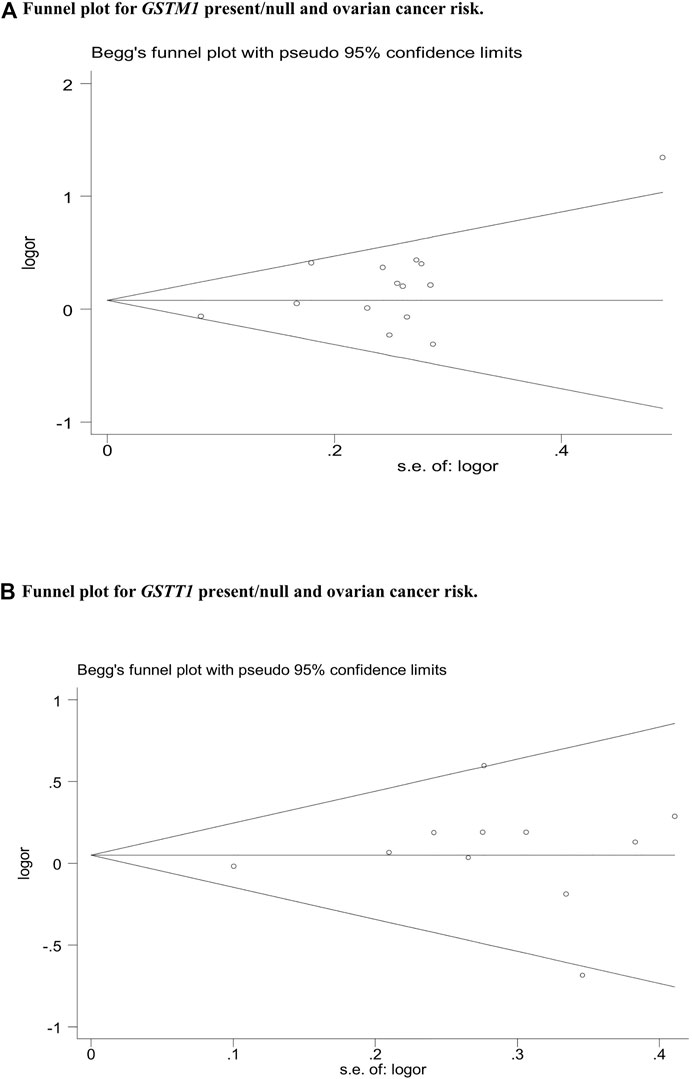
FIGURE 7. (A) Funnel plot for GSTM1 present/null and ovarian cancer risk. (B) Funnel plot for GSTT1 present/null and ovarian cancer risk.
Reliability of positive results of current and previous meta-analyses
FPRP and BFDP can assess the likelihood of a genuine association between genetic associations and disease. We, therefore, used FPRP and BFDP to validate the credibility of the current and previous meta-analyses. An excel spreadsheet was applied to calculate FPRP and BFDP. critical values of .2 and .8 for FPRP and BRDP, respectively, were used to assess whether they were significantly associated. We determined that significant associations meeting the following statistical criteria were classified as “positive results” (Theodoratou et al., 2012): 1) p < .05 was observed in at least one of the two genetic models (individual the GSTM1 present/null and GSTT1 present/null polymorphisms, with the risk of CC or OC did not need to meet this condition, as they were only used null vs. present). 2) FPRP < .2 and BFDP < .8 at a p-value level of .05. 3) statistical efficacy > .8 and 4) I2 < 50%. If the above criteria were not met, the association was considered a “positive result with low confidence”. Tables 10, 11, present the statistical significance associations, I2 values, statistical efficacy, and FPRP and BFDP values for the current and previous meta-analyses, respectively. Based on these criteria, the results show that the positive results in the current study and the positive results of the previous meta-analysis showed “low confidence” (FPRP > .2 and BFDP > .8).
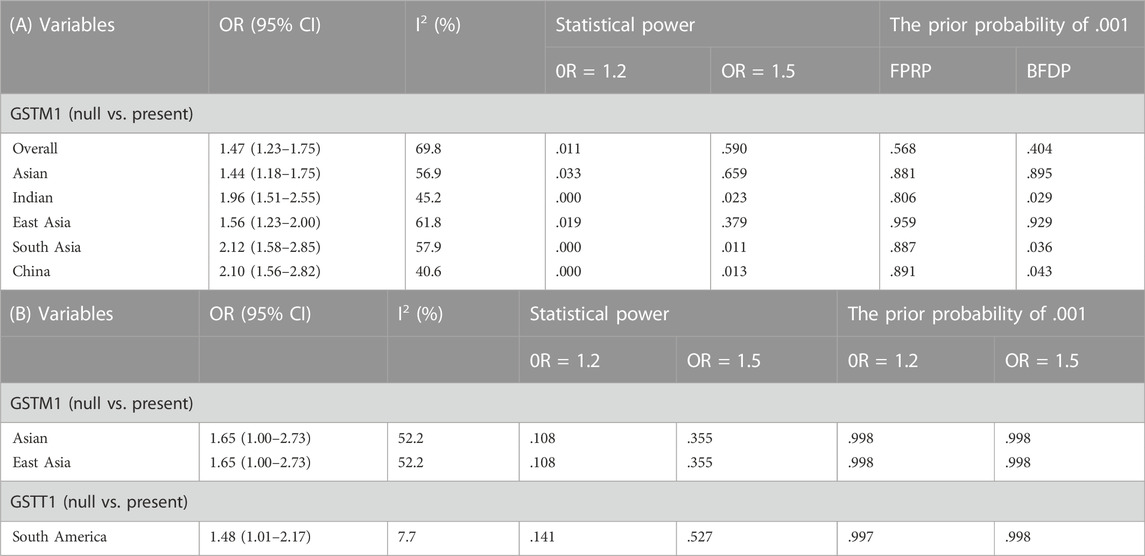
TABLE 10. (A) Cervical cancer false-positive report probability values for the current meta-analysis. (B) Ovarian cancer false-positive report probability values for the current meta-analysis.
Discussion
CC and OC, as common gynecological cancers, not only impose a heavy physical and psychological burden on women worldwide but also an economic burden on their families and society. Research on genetic susceptibility in their pathogenesis has been long-standing, glutathione transferase, as one of the phase II detoxification enzymes, can catalyze the binding of glutathione to a variety of exogenous organisms and increase the water solubility and excretion of the molecule, and this detoxification ability plays a crucial role in the detoxification of glutathione S-transferase into drugs, carcinogens and reactive oxygen species. Both GSTM1 and GSTT1 have null genotype, which can lead to the deletion of their expression and loss of enzymatic activity, which may impair the ability of individuals to inactivate carcinogens and increase the risk of cancer. However, the results of studies related to the risk of CC or OC by GSTM1 and GSTT1 are inconsistent or even contradictory, so we performed a new statistical analysis of previous and newly published studies to obtain more accurate evidence-based medical conclusion.
Overall, in the current meta-analysis, statistically significant null of the GSTM1 increased the risk of CC, and based on the biochemical characteristics of GSTM1 present/null polymorphisms. We estimated that individual effects of these genes were associated with an increased risk of CC in all ethnic groups. However, the risk was not consistent across populations, and studies showed that only in Indian and Chinese populations was the risk of CC significantly the increased risk was observed only in Indian and Chinese populations, and no risk correlation was observed in Caucasian and mixed populations, etc., Which may be due to the association of CC development with environmental factors. In addition, in studies related to OC risk, GSTM1 null was shown to be associated with an increased risk of OC in East Asia. GSTT1 null genotype was associated with an increased risk of OC in South America; while no correlation was found in other regions and populations. These results suggest, that the same genes may play different roles in cancer susceptibility across ethnicities and geographic regions. Because cancer is a complex polygenic disease and different genetic backgrounds and environmental factors (economic conditions or lifestyle) may contribute to such differences. Furthermore, random errors and biases are often found in some small-sample, low-quality studies in control groups, so the results of these original studies are not credible, especially in studies of genetic polymorphisms and disease susceptibility. In addition, small sample studies with positive results may be more likely to be reported, however, when they tend to achieve positive results, their studies may be less rigorous and often of lower quality (Attia et al., 2003). Therefore, we assessed the sensitivity analysis to see if there was any variation in the results by including only high-quality and large sample studies, and finally used FPRP and BFDP tests to assess the association between the positive findings from the current meta-analysis and the results of previous relevant studies, as FPRP is considered an appropriate method to assess the probability of significant results in multiple hypothesis testing of genetic polymorphisms and disease susceptibility studies, and In turn, Wacholder et al. (2004) provided a more precise genetic test, and the two methods together further strengthen the confidence of the conclusions, the results of the test on the current study showed that in GSTM1 null may be associated with an increased risk of CC and GSTM1 and GSTT1 null may be associated with an increased risk of OC, but the associated positive results showed “low confidence” (FPRP > .2, BFDP > .8).
A total of nine previous studies have been published on the association between individual GSTM1 and/or GSTT1 present/null polymorphisms and CC risk (Economopoulos et al., 2010a; Gao et al., 2011; Sui et al., 2011; Wang et al., 2011; Liu and Xu, 2012; Zhang et al., 2012; Zhen et al., 2013; Sun and Song, 2016; Tian et al., 2019), Economopoulos et al. (2010a) published a meta-analysis showing that GSTM1 null increases the risk of CC in non-Chinese, while Sui et al. (2011) showed in a published study that GSTM1, GSTT1 null was not associated with CC risk, Gao et al. (2011) suggested in a published study that individual GSTM1 and GSTT1 null increased the risk of CC in the entire study population, in a meta-analysis published by Wang et al. (2011), Liu and Xu (2012), Zhang et al. (2012), Zheng et al. (2013) and Sun and Song (2016) all concluded that GSTM1 null increased the risk of CC in the overall study, smokers, Indians and Chinese, but not in Koreans, while in the Japanese population or other ethnic groups, such as Caucasians, Wang et al. (2011), and Zhang et al. (2012) also performed a combined analysis of GSTT1 null genotype and CC risk, and all results showed no significant association with CC risk. Although the results of the latest meta-analysis published by Tian et al. (2019) were not fully consistent with the previous results, the analysis of results observed that a single GSTM1 null genotype was not associated with an increased risk of CC, whereas GSTT1 null increased the risk of CC in the whole study. Five previous papers have summarized the association between individual GSTM1 and/or GSTT1 present/null polymorphisms and OC risk, concluding that none of the studies observed any association with OC risk except for the finding by Jin et al. (Xu et al., 2014) showing that GSTT1 null increases OC risk in Asian populations. In addition, previously published studies had several shortcomings, I2 values were not shown in two meta-analyses (Liu and Xu, 2012; Zhang et al., 2012). Ten meta-analyses did not assess the quality of eligible studies (Capoluongo et al., 2006; Economopoulos et al., 2010a; Gao et al., 2011; Sui et al., 2011; Wang et al., 2011; Liu and Xu, 2012; Yin et al., 2013; Han et al., 2014; Jin and Hao, 2014; Xu et al., 2014), all meta-analyses did not look for sources of heterogeneity, and the probability and statistical significance of false positive reports were not assessed (Capoluongo et al., 2006; Economopoulos et al., 2010a; Gao et al., 2011; Sui et al., 2011; Wang et al., 2011; Liu and Xu, 2012; Zhang et al., 2012; Yin et al., 2013; Zhen et al., 2013; Han et al., 2014; Jin and Hao, 2014; Xu et al., 2014; Sun and Song, 2016; Tian et al., 2019). Therefore, by assessing the degree of association between positive results, the results showed that their meta-analysis results may not be credible (all meta-analyses FPRP> .2, BFDP> .8) (as shown in Table 11).
Compared with previous meta-analyses, this meta-analysis has several advantages: First, in addition to the inclusion of newly published original studies, the sample size was larger, including 30 studies of GSTM1 gene polymorphism (3,484 cases and 4,208 controls) and 22 studies of GSTT1 present/null polymorphisms (2,500 cases and 3,148 controls) associated with the risk of CC, and OC risk included 14 studies of GSTM1 present/null polymorphisms (3,035 cases and 3,422 controls) and 11 studies of GSTT1 present/null polymorphisms (2,543 cases and 3,275 controls). Second, we performed a quality assessment of the included eligible studies. Third, we applied FPRP and BFDP tests to assess false positive associations to estimate positive findings from this meta-analysis and previous relevant studies. Fourth, meta-regression analysis was applied to explore the sources of heterogeneity. Fifth, important sensitivity analyses were performed for studies with high-quality and large samples. However, our meta-analysis has some limitations: First, some potential covariates were not controlled for, such as age. Second, in the subgroup analysis, although some population studies showed positive results, for example, in the study on the association between GSTM1 and/or GSTT1 present/null polymorphisms and CC risk, the results on South American countries showed that GSTT1 null genotype reduced the risk of CC, and in studies on the association between GSTM1 and/or GSTT1 null genotype and OC risk, GSTT1 null genotype was found to increase the risk of OC in mixed ethnic and Serbian populations. However, the positive results of the above studies corresponded to only one study each (not specifically reported) and the sample size was small enough to explore the true association between them and confirm the validity of their results, so a large sample size and sufficiently large studies would help to validate our findings. Third, the current meta-analysis included only published articles, so there may be publication bias, as shown in Figure 8; known positive results are more likely to be published than negative results, so the genetic effect of GSTM1 and GSTT1 null genotype may be underestimated. Fourth, we did not consider whether the genotype distribution in the controls was in Hardy–Weinberg equilibrium (HWE). Under normal circumstances, the HWE in the meta-analysis of genetic polymorphisms must be calculated to assess the quality, genotyping errors, and selection bias in the study (Hosking et al., 2004; Thakkinstian et al., 2011). However, we cannot calculate or extract the relevant data in the original studies. Fifth, for CC, data on other risk factors such as HPV infection, age and smoking were not extracted, while for ovarian cancer, data on age, obesity and tumor pathological classification were not extracted.
Conclusion
The results of this meta-analysis study suggest that the positive results of GSTM1 null genotype associated with increased risk of CC, and GSTM1 and GSTT1 null genotype associated with increased risk of OC in Chinese and Indian populations may be results with missing credibility rather than true associations, and therefore we should interpret these positive results with caution. In conclusion, due to the small sample size of the relevant studies and the limitations of this study, the GSTM1 present/null and/or GSTT1 present/null polymorphisms with risk of CC or OC still needs to be further explored in depth, and we need more original studies with larger samples for validation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
JY: designed research, performed research, collected data, analyzed data, wrote paper. Y-YM: check and analyzed the data. JW and X-FH: designed research and revised article.
Acknowledgments
We would like to acknowledge the authors of all the original studies included in the meta-analysis. At the same time, I would like to thank X-FH and JW for their guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.1074570/full#supplementary-material
References
Abbas, M., Kushwaha, V. S., Srivastava, K., Raza, S. T., and BanerjeeM., (2018). Impact of GSTM1, GSTT1 and GSTP1 genes polymorphisms on clinical toxicities and response to concomitant chemoradiotherapy in cervical cancer. Br. J. Biomed. Sci. 75 (4), 169–174. doi:10.1080/09674845.2018.1482734
Aerssens, J., Dequeker, J., Peeters, J., Breemans, S., Broos, P., and Boonen, S. (2000). Polymorphisms of the VDR, ER and COLIA1 genes and osteoporotic hip fracture in elderly postmenopausal women. Osteoporos. Int. 11, 583–591. doi:10.1007/s001980070079
Attia, J., Thakkinstian, A., and D’Este, C. (2003). Meta-analyses of molecular association studies: Methodologic lessons for genetic epidemiology. J. Clin. Epidemiol. 56, 297–303. doi:10.1016/s0895-4356(03)00011-8
Baxter, S. W., Thomas, E. J., and Campbell, I. G. (2001). GSTM1 null polymorphism and susceptibility to endometriosis and ovarian cancer. Carcinogenesis 22, 63–65. doi:10.1093/carcin/22.1.63
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi:10.2307/2533446
Board, P. G., and Menon, D. (2013). Glutathione transferases regulators of cellular metabolism and physiology. Biochimica Biophysica Acta 830, 3267–3288. doi:10.1016/j.bbagen.2012.11.019
Cai, Q., Jiang, L., and Chen, X. (2016). Relationship between GSTM1 and GSTP1 gene polymorphisms and the risk of ovarian cancer[J]. Chin. Maternal Child Health Study 27 (08), 933–936. doi:10.3969/j.issn.1673-5293.2016.08.010
Capoluongo, E., Pitocco, D., Concolino, P., SantonoCito, C., Di Stasio, E., d'Onofrio, G., et al. (2006). Slight association between type 1 diabetes and "ff" VDR FokI genotype in patients from the Italian Lazio Region. Lack of association with diabetes complications. Clin. Biochem. 39 (9), 888–892. doi:10.1016/j.clinbiochem.2006.05.006
Chen, C., Madeleine, M. M., Weiss, N. S., and Daling, J. R. (1999). Glutathione S-transferase M1 genotypes and the risk of squamous carcinoma of the cervix: A population-based case-control study. Am. J. Epidemiol.150 (6), 568–572. doi:10.1093/oxfordjournals.aje.a010054
Chunhua, Z. (2008). Clinic research for gene polymorphism of GSTT1 and susceptibility of ovarian cancer. China: JilinUniversity. [Article in Chinese].
de Carvalho, C. R., da Silva, I. D. C. G., Pereira, J. S., de Souza, N. C. N., Focchi, G. R. d. A., and Ribalta, J. C. L. (2008). Polymorphisms of p53, GSTM1, GSTT1, and HPV in uterine cervix adenocarcinoma. Eur. J. Gynaecol. Oncol. 29, 590–593.
Der Simonian, R., and Laird, N. (2015). Meta-analysis in clinical trials revisited. Contemp. Clin. trials 45, 139–145. doi:10.1016/j.cct.2015.09.002
Dual, S., and Tweedie, R. (2000). A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 95, 89–98. doi:10.2307/2669529
Economopoulos, K. P., Choussein, S., Vlahos, N. F., and Sergentanis, T. N. (2010a). GSTM1 polymorphism, GSTT1polymorphism, and cervical cancer risk: A meta-analysis. Int. J. Gynecol. Cancer 20 (9), 1576–1580. doi:10.1111/IGC.0b013e3181ca1dfc
Economopoulos, K. P., Sergentanis, T. N., and Vlahos, N. F. (2010b). Glutathione S-transferase M1, T1, and P1polymorphisms and ovarian cancer risk: A meta-analysis. Int. J. Gynecol. Cancer 20 (5), 732–737. doi:10.1111/IGC.0b013e3181dedeb5
Egger, M., Smith, D. G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. doi:10.1136/bmj.315.7109.629
Esteller, M., García, A., Martínez-Palones, J. M., Xercavins, J., Reventós, J., and GarciA, A. (1997). Susceptibility to endometrial cancer: Influence of allelism at p53, glutathione S-transferase (GSTM1 and GSTT1) and cytochrome P-450 (CYP1A1) loci. Br. J. Cancer 75, 1385–1388. doi:10.1038/bjc.1997.235
Gao, L. B., Pan, X. M., Li, L. J., Liang, W. B., Bai, P., Rao, L., et al. (2011). Null genotypes of GSTM1 and GSTT1 contribute to the risk of cervical neoplasia: An evidence-based meta-analysis. PLoS One 6 (5), e20157. doi:10.1371/journal.pone.0020157
Gates, M. A., Tworoger, S. S., Terry, K. L., Titus-Ernstoff, L., Rosner, B., De Vivo, I., et al. (2008). Talc use, variants of the GSTM1, GSTT1, and NAT2 genes, and risk of epithelial ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 17, 2436–2444. doi:10.1158/1055-9965.EPI-08-0399
Goodman, J. E., Lavigne, J., Hengstler, J. G., Tanner, B., Helzlsouer, K. J., and Yager, J. D. (2000). Catechol-O-methyltransferase polymorphism is not associated with ovarian cancer risk. Cancer Epidemiol. Biomarkers Pre 9, 1373–1376.
Han, L. Y., Liu, K., Lin, X. L., Zou, B. B., and Zhao, J. S. (2014). Lack of any association of GST genetic polymorphisms with susceptibility to ovarian cancer--a meta-analysis. Asian Pac J. Cancer Prev. 15 (15), 6131–6136. doi:10.7314/apjcp.2014.15.15.6131
Hasan, S., Hameed, A., Saleem, S., Shahid, S. M., Haider, G., and Azhar, A. (2015). The association of GSTM1 and GSTT1 polymorphisms with squamous cell carcinoma of the cervix in Pakistan. Tumour Biol. 36, 5195–5199. doi:10.1007/s13277-015-3175-y
Hengstler, J. G., Kett, A., Arand., M., Oesch-Bartlomowicz, B., OeschF., , PilcH, H., et al. (1998). GlutathioneS-transferase T1 and M1 gene defects in ovarian carcinoma. Cancer Lett. 130, 43–48. doi:10.1016/s0304-3835(98)00123-2
Hosking, L., Lumsden, S., Lewis, K., Yeo, A., McCarthy, L., Bansal, A., et al. (2004). Detection of genotyping errors by Hardy–Weinberg equilibrium testing. Eur. J. Hum. Genet. 12, 395–399. doi:10.1038/sj.ejhg.5201164
Huang, H., Chen, S., and Xu, G. (2022). Research on the incidence and mortality trend of ovarian cancer in Chinese women from 2005 to 2016 [J]. Chin. General Med. 25 (08), 990–994.
Huang, Y-K. (2006). Genetic polymorphisms of phase I and phase II xenobiotic enzymes in human papillomavirus related lesion and cancer of the uterine cervix. Tzu Chi Med. J. 18 (4), 8–12.
Ioannidis, J. P., Boffetta, P., Little, J., O'Brien, T. R., Uitterlinden, A. G., Vineis, P., et al. (2008). Assessment of cumulative evidence on genetic associations: Interim guidelines. Int. J. Epidemiol. 37 (1), 120–132. PMID: 17898028. doi:10.1093/ije/dym159
Jin, Y., and Hao, Z. (2014). Polymorphisms of glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) in ovarian cancer risk. Tumour Biol. 35 (6), 5267–5272.
Joseph, T., Chacko, P., Wesley, R., Jayaprakash, P. G., James, F. V., and Pillai, M. R. (2006). Germline genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes in Indian cervical cancer: Associations with tumor progression, age, and human papillomavirus infection. Gynecol. Oncol. 101, 411–417. doi:10.1016/j.ygyno.2005.10.033
Khokhrin, D. V., Khrunin, A. V., Moiseev, A. A., Gorbunov, V. A., and Limborskaia, S. A. (2012). Association of polymorphisms in glutathioneS-transferase and DNA repair genes with ovarian cancer risk in the Russian population. Genetika 48, 764–766. doi:10.1134/s1022795412050110
Kim, J. W., Lee, C. G., Park, Y. G., Kim, K. S., Kim, I. K., Sohn, Y. W., et al. (2000). Combined analysis of germline polymorphisms of p53, GSTM1, GSTT1, CYP1A1, and CYP2E1: Relation to the incidence rate of cervical carcinoma. Cancer 88, 2082–2091. doi:10.1002/(sici)1097-0142(20000501)88:9<2082::aid-cncr14>3.0.co;2-d
Kiran, B., Karkucak, M., Ozan, H., Yakut, T., Ozerkan, K., Sag, S., et al. (2010). GST (GSTM1, GSTT1, and GSTP1) polymorphisms in the genetic susceptibility of Turkish patients to cervical cancer. J. Gynecol. Oncol. 21 (21), 169–173. doi:10.3802/jgo.2010.21.3.169
Lee, S. A., Kim, J. W., Roh, J. W., Choi, J. Y., Lee, K. M., Yoo, K. Y., et al. (2004). Genetic polymorphisms of GSTM1, p21, p53 and HPV infection with cervical cancer in Korean women. Gynecol. Oncol. 93, 14–18. doi:10.1016/j.ygyno.2003.11.045
Liu, Y., Ma, W., and Liu, Q. (2009). Association between genetic polymorphism of GSTM1, CYP2E1 and susceptibility to cervical cancer and its precancerous lesions in Uighur women in Xinjiang. Prog. Obstet. Gynecol. 18, 840–843. doi:10.13283/j.cnki.xdfckjz.2009.11.019
Liu, Y., and Xu, L. Z. (2012). Meta-analysis of the association between GSTM1 gene polymorphism and cervical cancer. Asian Pac J. Trop. Med. 5 (6), 480–484. doi:10.1016/S1995-7645(12)60083-2
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. Natl. Cancer Inst. 22, 719–748. doi:10.1093/jnci/22.4.719
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 62 (10), 1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Morari, E. C., Lima, A. B. C., Bufalo, N. E., Leite, J. L., Granja, F., and Ward, L. S. (2006). Role of glutathione-S-transferase and codon 72 of P53 genotypes in epithelial ovarian cancer patients. J. Cancer Res. Clin. Oncol. 132, 521–528. doi:10.1007/s00432-006-0099-3
Nishino, K., Sekine, M., Kodama, S., Sudo, N., Aoki, Y., Seki, N., et al. (2008). Cigarette smoking and glutathione S-transferase M1 polymorphism associated with risk for uterine cervical cancer. J. Obstet. Gynaecol. Res. 34, 994–1001. doi:10.1111/j.1447-0756.2008.00798.x
Niwa, Y., Hirose, K., Nakanishi, T., Nawa, A., Kuzuya, K., Tajima, K., et al. (2005). Association of the NAD(P)H: Quinone oxidoreductase C609T polymorphism and the risk of cervical cancer in Japanese subjects. Gynecol. Oncol. 96, 423–429. doi:10.1016/j.ygyno.2004.10.015
Nunobiki, S., Ueda, M., Akise, H., Izuma, S., Torii, K., Okamoto, Y., et al. (2015). GSTM1, GSTT1, and NQO1 polymorphisms in cervical carcinogenesis. Hum. Cell 28, 109–113. doi:10.1007/s13577-015-0111-9
Oliveira, C., Lourenco, G. J., Sagarra, R. A., Derchain, S. F. M., Segalla, J. G., and Lima, C. S. P. (2012). Polymorphisms of glutathione S-transferase Mu 1(GSTM1), Theta 1 (GSTT1), and Pi 1 (GSTP1) genes and epithelial ovarian cancer risk. Dis. Markers 33, 155–159. doi:10.1155/2012/497692
Palma, S., Novelli, F., Padua, L., Venuti, A., Prignano, G., Mariani, L., et al. (2010). Interaction between glutathione-S-transferase polymorphisms, smoking habit, and HPV infection in cervical cancer risk. J. Cancer Res. Clin. Oncol. 136 (7), 1101–1109. doi:10.1007/s00432-009-0757-3
Pljesa, I., Berisavac, M., Simic, T., Pekmezovic, T., Coric, V., Suvakov, S., et al. (2017). Polymorphic expression of glutathione transferases A1, M1, P1 and T1 in epithelial ovarian cancer: A Serbian case-control study. J. BUON 22 (1), 72–79.
Sarhanis, P., Redman, C., Perrett, C., Brannigan, K., Clayton, R. N., Hand, P., et al. (1996). Epithelial ovarian cancer: Influence of polymorphism at the glutathioneS-transferase GSTM1 and GSTT1 loci onp53expression. Br. J. Cancer 74, 1757–1761. doi:10.1038/bjc.1996.626
Satinder, K., Sobti, R. C., and Pushpinder, K. (2017). Impact of single nucleotide polymorphism in chemical metabolizing genes and exposure to wood smoke on risk of cervical cancer in North-Indian women. Exp. Oncol. 39, 69–74. doi:10.31768/2312-8852.2017.39(1):69-74
Settheetham-Ishida, W., Yuenyao, P., Kularbkaew, C., Settheetham, D., and Ishida, T. (2009). Glutathione S-transferase (GSTM1 and GSTT1) polymorphisms in cervical cancer in Northeastern Thailand. Asian Pac J. Cancer Prev. 10, 365–368.
Sharma, A., Gupta, S., Sodhani, P., Singh, V., Sehgal, A., Sardana, S., et al. (2015). Glutathione S-transferase M1 andT1 polymorphisms, cigarette smoking and HPV infection in precancerous and cancerous lesions of the uterine cervix. Asian Pac J. Cancer Prev. 16, 6429–6438. doi:10.7314/apjcp.2015.16.15.6429
Sharma, A., Murthy, N. S., and Mitra, A. B. (2004). Polymorphisms at GSTM1 and GSTT1 gene loci and susceptibility to cervical cancer in Indian population. Neoplasma 51, 12–16.
Sharma, T., Banerjee, B. D., Thakur, G. K., Guleria, K., and Mazumdar, D. (2019). Polymorphism of xenobiotic metabolizing gene and susceptibility of epithelial ovarian cancer with reference to organochlorine pesticides exposure. Exp. Biol. Med. (Maywood). 244 (16), 1446–1453. doi:10.1177/1535370219878652
Sierra-Torres, C. H., Au, W. W., Arrastia, C. D., Cajas-Salazar, N., Robazetti, S. C., Payne, D. A., et al. (2003). Polymorphisms for chemical metabolizing genes and risk for cervical neoplasia. Environ. Mol. Mutagen. 41, 69–76. doi:10.1002/em.10132
Singh, H., Sachan, R., Devi, S., Pandey, S. N., and Mittal, B. (2008). Association of GSTM1, GSTT1, and GSTM3 gene polymorphisms and susceptibility to cervical cancer in a North Indian population. Am. J. Obstet. Gynecol. 198, e1. e6. doi:10.1016/j.ajog.2007.09.046
Sobti, R. C., Kaur, S., Kaur, P., Singh, J., Gupta, I., Jain, V., et al. (2006). Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet. Cytogenet 166, 117–123. doi:10.1016/j.cancergencyto.2005.10.001
Song, G. Y., Song, Z. Y., and Xu, J. P. (2008). Association of single nucleotide polymorphism in glutathione S-transferase-M1 with susceptibility to cervical cancer in Shanxi Province. Chin. J. Cancer Prev. Treat. 15, 1054–1056. doi:10.3969/j.issn.1673-5269.2008.14.004
Spurdle, A. B., Webb, P. M., Purdie, D. M., Chen, X., Green, A., and Chenevix-Trench, G. (2001). Polymorphisms at the glutathione S-transferase GSTM1, GSTT1 and GSTP1 loci: Risk of ovarian cancer by histological subtype. Carcinogenesis 22, 67–72. doi:10.1093/carcin/22.1.67
Stosic, I., Grujicic, D., Arsenijevic, S., Brkic, M., and Milosevic-Djordjevic, O. (2014). Glutathione S-transferase T1 and M1 polymorphisms and risk of uterine cervical lesions in women from central Serbia. Asian Pac J. Cancer Prev. 15, 3201–3205. doi:10.7314/apjcp.2014.15.7.3201
Sui, Y., Han, W., Yang, Z., Jiang, M., and Li, J. (2011). Association of glutathione S-transferase M1 and T1 null polymorphisms with the development of cervical lesions: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 159 (2), 443–448. doi:10.1016/j.ejogrb.2011.09.012
Sun, P., and Song, W. Q. (2016). GSTM1 null genotype and susceptibility to cervical cancer in the Chinese population: An updated meta-analysis. J. Cancer Res. Ther. 12 (2), 712–715. doi:10.4103/0973-1482.154004
Tacca, A. L. Ã. M., Lopes, A. K., Vilanova-Costa, C. A. S. T., Cordeiro Silva, A. M. T., Nascente Costa, S. H., Ribeiro, A. A., et al. (2018). Null polymorphisms in GSTT1 and GSTM1 genes and their associations with smoking and cervical cancer[J]. Genet. Mol. Res. 17 (2), 18067. doi:10.4238/gmr18067
Thakkinstian, A., McKay, G. J., McEvoy, M., Chakravarthy, U., Chakrabarti, S., Silvestri, G., et al. (2011). Systematic review and meta-analysis of the association between complement component 3 and age-related macular degeneration: A HuGE review and meta-analysis. Am. J. Epidemiol. 173, 1365–1379. doi:10.1093/aje/kwr025
Theodoratou, E., Montazeri, Z., Hawken, S., Allum, G. C., Gong, J., Tait, V., et al. (2012). Systematic meta-analyses and field synopsis of genetic association studies in colorectal cancer. J. Natl. Cancer Inst. 104, 1433–1457. doi:10.1093/jnci/djs369
Tian, S., Yang, X., Zhang, L., Zhao, J., Pei, M., Yu, Y., et al. (2019). Polymorphic variants conferring genetic risk to cervical lesions support GSTs as important associated loci. Med. Baltim. 98 (41), e17487. doi:10.1097/MD.0000000000017487
Ueda, M., Toji, E., Nunobiki, O., Izuma, S., Okamoto, Y., Torii, K., et al. (2008). Germline polymorphism of cancer susceptibility genes in gynecologic cancer. Hum. Cell 21 (4), 95–104. doi:10.1111/j.1749-0774.2008.00058.x
Ueda, M., Toji, E., Nunobiki, O., Sato, N., Izuma, S., Torii, K., et al. (2010). Germline polymorphisms of glutathione-S-transferase GSTM1, GSTT1 and p53 codon 72 in cervical carcinogenesis. Hum. Cell 23 (4), 119–125. doi:10.1111/j.1749-0774.2010.00089.x
Wacholder, S., Chanock, S., Garcia-Closas, M., El Ghormli, L., and Rothman, N. (2004). Assessing the probability that a positive report is false: An approach for molecular epidemiology studies. J. Natl. Cancer Inst. 96, 434–442. doi:10.1093/jnci/djh075
Wang, D., Wang, B., Zhai, J. X., and Sun, G. G. (2011). Glutathione S-transferase M1 and T1 polymorphisms and cervical cancer risk: A meta-analysis. Neoplasma 58 (4), 352–359. doi:10.4149/neo_2011_04_352
Wang, J., Wang, T., and Song, J. (2018). The relationship between glutathione S-transferase M1 polymorphism, HPV16 infection, and cervical cancer. Chin. J. Gerontol. 38, 1606–1608. doi:10.3969/j.issn.1005-9202.2018.07.031
Warwick A. P, A. P., Redman, C. W., Jones, P. W., Fryer, A. A., Gilford, J., Alldersea, J., et al. (1994). Progression of cervical intraepithelial neoplasia to cervical cancer: Interactions of cytochrome P450 CYP2D6 EM and glutathione S-transferase GSTM1 null genotypes and cigarette smoking. Br. J. Cancer 70, 704–708. doi:10.1038/bjc.1994.378
Warwick, A., Redman, C., Pemble, S., Taylor, J. B., Ketterer, B., Alldersea, J., et al. (1994). Theta class glutathione S-transferase GSTT1 genotypes and susceptibility to cervical neoplasia: Interactions with GSTM1, CYP2D6, and smoking. Carcinogenesis 15, 2841–2845. doi:10.1093/carcin/15.12.2841
Wongpratate, M., Phuthong, S., Natphopsuk, S., and Ishida, T. (2020). Genetic polymorphism of glutathione S-transferase and cervical cancer susceptibility in northeastern Thailand. Asian Pac. J. Cancer Biol. 5 (2), 35–41. doi:10.31557/apjcb.2020.5.2.35-41
Xu, C., Chen, S., Wang, T., Gao, H., Zhao, K., You, X., et al. (2014). Quantitative assessment of the influence of glutathione S-transferase M1 null variant on ovarian cancer risk. J. Cancer Res. Ther. 10, C201–C205. doi:10.4103/0973-1482.145872
Yin, Y., Feng, L., and Sun, J. (2013). Association between glutathione S-transferase M 1 null genotype and risk of ovarian cancer: A meta-analysis. Tumour Biol. 34 (6), 4059–4063. doi:10.1007/s13277-013-0995-5
Zhang, Y., Xu, Y., and Cheng, X-L. (2019). Association of GSTMl and CYP1A1 cycle polymorphisms with cervical cancer occurrence and progression[J]. Mod. Oncol. Med. 27 (18), 3297–3301. doi:10.3969/j.issn.1672-4992.2019.12.036
Zhang, Z. Y., Jin, X. Y., Wu, R., Wu, L. N., Xing, R., Yang, S. J., et al. (2012). Meta-analysis of the association between GSTM1 and GSTT1 gene polymorphisms and cervical cancer. Asian Pac J. Cancer Prev. 13 (3), 815–819. doi:10.7314/apjcp.2012.13.3.815
Zhao, C., and Song, S. (2021). Epidemiology and etiology of cervical cancer in my country [J]. Med. Inf. 34 (05), 6–8. doi:10.1007/s13277-014-1685-7
Zhen, S., Hu, C. M., and Bian, L. H. (2013). Glutathione S-transferase polymorphism interactions with smoking status and HPV infection in cervical cancer risk: An evidence-based meta-analysis. PLoS One 8 (12), e83497. doi:10.1371/journal.pone.0083497
Zhou, Q., Wang, J., and Shao, S. (2006). The study of the relationship between glutathione S-transferase M1, T1 genotypes, and the risk of cervical cancer. Modem Prev. Med. 33, 269–271. doi:10.3969/j.issn.1007-6611.2006.04.013
Keywords: GSTT1, GSTM1, cervical cancer, ovarian cancer, BFDP, FPRP
Citation: Ye J, Mu Y-Y, Wang J and He X-F (2023) Individual effects of GSTM1 and GSTT1 polymorphisms on cervical or ovarian cancer risk: An updated meta-analysis. Front. Genet. 13:1074570. doi: 10.3389/fgene.2022.1074570
Received: 19 October 2022; Accepted: 29 December 2022;
Published: 12 January 2023.
Edited by:
Mohammed S. Mustak, Mangalore University, IndiaReviewed by:
Rocio Gomez, Center for Research and Advanced Studies (CINVESTAV), MexicoRasa Ugenskiene, Lithuanian University of Health Sciences, Lithuania
Copyright © 2023 Ye, Mu, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Feng He, MzkzMTIwODIzQHFxLmNvbQ==
 Jing Ye
Jing Ye Yi-Yang Mu2
Yi-Yang Mu2 Xiao-Feng He
Xiao-Feng He