- 1Graduate College, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Department of Gastroenterology, Anhui Medical University, Hefei, China
- 3Graduate College, Shaanxi University of Traditional Chinese Medicine, Xi’an, China
- 4Department of Chinese Medicine, School of Chinese Medicine Engineering, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background and Purpose: Traditional Chinese medicine (TCM) can regulate intestinal flora so as to affect the occurrence, progression, and prognosis of gastrointestinal cancer. According to clinical studies, TCM oral administration, TCM external treatment, and TCM injections, can adjust intestinal flora disorders in patients with gastrointestinal cancer. This network meta-analysis aims to evaluate the effect of three treatments on the intestinal flora in gastrointestinal cancer patients.
Methods: This meta-analysis was registered in PROSPERO (CRD42022332553). Six electronic databases, namely CNKI, Wanfang, CSTJ, PubMed, Cochrane Library, and EMBASE, were searched from their inception to 1 April 2022. We identified randomized controlled trials (RCT) used to compare the efficacy of three TCM treatment methods—oral administration, external therapy and injections—on the intestinal flora in gastrointestinal cancer patients. The main outcome indicators were Bifidobacteria, Lactobacilli, Escherichia coli, and Enterococci. Stata (15.1) and the Cochrane risk of bias assessment tool were employed.
Results: We identified 20 eligible RCTs with a total of 1,774 patients. According to network meta-analysis results, TCM injection plus common treatment (CT) or oral administration of TCM plus CT was superior to CT alone for supporting Bifidobacterium. In supporting Lactobacillus, TCM injection plus CT demonstrated more obvious effect relative to oral administration of TCM plus CT; TCM injection plus CT was more effective than CT only; and oral administration of TCM plus CT was superior to CT only.The inhibitory effect of TCM injection plus CT on Escherichia coli was better compared with CT only. In terms of inhibiting Enterococci, oral administration of TCM plus CT was superior to CT only.The difference in efficacy among the above treatments was statistically significant. In the SUCRA probability ranking, TCM injection plus CT had the best ranking curve among the three treatments and was the most effective in supporting Bifidobacteria (Sucra = 90.08%), Lactobacilli (Sucra = 96.4%), and regulating Escherichia coli (Sucra = 86.1%) and Enterococci (Sucra = 87.1%).
Conclusion: TCM injections plus CT is the most effective therapy in balancing the intestinal flora of gastrointestinal cancer patients. However, the current results deserve further validation through high-quality research.
Systematic Review Registration: http://www.prisma-statement.org/, identifier 10.1136/bmj.n71.
1 Introduction
Gastrointestinal (GI) cancers, including gastric cancer, colon cancer, and colorectal cancer (Soleimanpour et al., 2020), are among the most common cancers (Wang et al., 2020a), accounting for approximately 26% of total cancer incidence and about 36.4% of cancer-related deaths (Arnold et al., 2020). Recently, the incidence and mortality of GI cancers have been increasing (Bray et al., 2018), so exploring the protective factors and risk factors for the occurrence and development of GI cancers will be conducive to effectively preventing and treating these cancers. Clinically, GI cancers are usually treated by radiotherapy, chemotherapy, surgery, drugs, and immunotherapy, while TCM, generally considered as an adjuvant therapy combined with radiotherapy and chemotherapy, plays an effective anti-tumor role by inducing tumor cell apoptosis and inhibiting tumor angiogenesis (Wang S. et al., 2021). At the same time, it decreases the gastrointestinal reactions caused by radiotherapy and chemotherapy (Zhang et al., 2021). However, with the in-depth study of the relationship between TCM and intestinal flora and gastrointestinal cancers, we found that TCM can adjust intestinal flora, promote beneficial bacteria to produce more Short-chain fatty acids (SCFAs) (Martin-Gallausiaux et al., 2021), mainly including acetate (C2), propionate (C3) and butyrate (C4), and improve the microenvironment of the gastrointestinal tumors, thereby having a certain beneficial impact on the occurrence, development, and prognosis of GI cancers (Sivan et al., 2015). In addition, intestinal flora also has a therapeutic effect on radiation enteritis caused by radiotherapy (Jian et al., 2021). Therefore, we believe that TCM can treat patients with gastrointestinal cancers by regulating intestinal flora in multiple ways.
Human intestinal microbes constitute a complex ecosystem, with around 800 species and more than 7,000 bacterial strains (Ley et al., 2006). In the intestine, symbiotic microorganisms are dynamic, which can maintain intestinal stability and inhibit pathogen colonization. When the balance is broken, the intestinal mucosal barrier and immune function will be undermined, leading to additional pathogenic factors, which are risk factors for colorectal cancer as well (Si et al., 2021).Clinical studies have found significant changes in the structure and characteristics of the intestinal flora in gastrointestinal cancer patients (Ferreira et al., 2018). Additionally, intestinal flora affects the absorption of anticancer drugs and correlates with the prognosis of these patients (Wertman et al., 2021). The pathological mechanisms by which intestinal flora affects colorectal cancer are currently thought to be achieved through multiple pathways, such as the induction of inflammation and immunity (Meng et al., 2018). Notably, intestinal pathogenic bacteria can drive tumorigenesis by shaping the tumor microenvironment or forming biofilms, such as Bacteroides, Escherichia coli, and Clostridium difficile, which can secrete a variety of virulence factors that damage intestinal epithelial cells and trigger chronic inflammatory responses, and develop into colorectal cancers (Hayase and Jenq, 2021). Meanwhile, some intestinal probiotics can directly produce tumor suppressive substances or enhance related antigens to achieve anti-tumor effects (Song et al., 2021). The ferritin produced by Lactobacillus casei ATCC334, for instance, can act as a tumor suppressor through the JNK signaling pathway (Konishi et al., 2016). Therefore, we consider how to balance the environment of intestinal microbiota deserves further exploration.
Among many pathogenic bacteria, Escherichia coli and Enterococcus, belonging to neutral bacteria, are not pathogenic when their population is within a certain range, however, an excessive number of these bacteria may produce Enterotoxin that are highly pathogenic (Wassenaar, 2018; Alhinai et al., 2019). Bifidobacteria and Lactic acid bacteria, as probiotics, produce a large amount of SCFAs, which are beneficial to intestinal health (Zaharuddin et al., 2019). Because of their large number, more in-depth basic research, and easy clinical detection, when intestinal pathology changes, the flora changes significantly, so they are commonly used as clinical indicators for evaluation of intestinal flora (Kuugbee et al., 2016).
In fact, the TCM adjuvant therapy for cancer has achieved a remarkable clinical efficacy (Wang S. et al., 2020c). Nowadays, the main TCM therapies commonly used in the clinics are oral therapy, external therapy and injection therapy (Huang et al., 2018). The classification is based on different routes of administration. Oral treatments of TCM are absorbed through the gastrointestinal tract, external treatments of TCM are absorbed through the skin and mucosa by physical therapy or enema, and TCM injections are the components directly enter the bloodstream. To the best of our knowledge, most of the previous studies have focused more on TCM oral administration, and less on external treatments and TCM injections. It has been shown that TCM can inhibit the development of cancer by regulating intestinal microbes (Chen et al., 2021). XiaoYao decoction (a medicinal diet with Ginseng, Atractylodes and Fushen as the main ingredients), for instance, can increase the abundance of Bacteroides, Lactobacillus, and Proteobacteria, and reduce the abundance of Desulfovibrio and Rickerella (Zhang Z. et al., 2020).
Given that the evidence in the current literature not able to determine which one is the most effective Therefore, we tried to select the best treatment by counting and analyzing the changes of 4 indicators in intestinal flora after the application of three TCM treatments in the previous literature. To date, no meta-analysis has been conducted to compare the effects of CT in combination with each of these three TCM methods on intestinal flora in gastrointestinal cancer patients. We present the paper on the basis of the checklist of the extended PRISMA for network meta-analysis.
2 Materials and methods
This meta-analysis was registered in PROSPERO (CRD42022332553).
2.1 Search strategies
We searched three English databases (PubMed, Cochrane Library and Embase) and three Chinese electronic databases (CNKI, Wanfang and Chinese Science and Technology Journal Database). The search period started from the establishment of the database until 1 April 2022.
Our search strategy contains comprehensive terms in the English database as follows: (Medical, Chinese traditional or Chinese medicine) and (gastric or colorectal or colorectal or gastrointestinal tumors) or (intestinal flora or gut microbes or Bifidobacterium or Lactobacillus or Escherichia coli or Enterococcus). A comprehensive search with subject terms, joint keywords and free words was conducted according to different databases to ensure the systematization and integrity of the search.
2.2 Inclusion standards
(1) The symptoms and clinical indicators of patients were in accordance with the newly compiled guideline The Diagnostic Criteria of Gastrointestinal Tumors.
(2) Randomized controlled trial.
(3) The control group was treated with CT, and the treatment group with one of three TCM intervention methods, namely CT + Oral administration of TCM, CT + external therapy of TCM (e.g., enema of TCM, acupoint catgut embedding, cutaneous scraping therapy, acupuncture, and moxibustion), and CT + TCM injection.
(4) The observation indicators are the numbers of Bifidobacterium, Lactobacillus, Escherichia coli and Enterococcus. At least one result was available in the literature.
(5) The intestinal microbiota numbers in fecal samples of patients can only be analyzed by 16SrDNA sequencing.
2.3 Exclusion standards
(1) Literature review, animal experiment, experience summary and other types of literature are excluded.
(2) Patients with non-simple gastrointestinal cancer.
(3) The treatment group did not meet the requirements of combined TCM and common treatment or did not use one of the 3 treatment methods of TCM, or the control group was treated with TCM.
(4) Articles with multiple publications and those with full text unavailable or with incomplete data were excluded.
(5) None of the four selected indicators of intestinal flora (Bifidobacteria, Lactobacilli, Escherichia coli, and Enterococci) was found in the outcome indicators of RCT.
2.4 Types of outcome measures
The outcome indicators of this study were determined based on the frequency of outcome indicators in the articles involved and the 2020 AGA clinical practice guidelines. The main outcome indicators are as follows: 1) Bifidobacteria and Lactobacilli (increased number) and 2) Escherichia coli and Enterococci (decreased number).
2.5 Literature screening and data extraction
According to the search strategy, relevant literature was found in the database and the bibliography was exported. Duplicate literature was excluded using Endnotex9 software.The literature that met the inclusion criteria were downloaded for comparison, and the full text was ultimately read for exclusion.
Two reviewers (Niran Feng and Kunyang Li) independently searched the database and the selected articles. If there was any disagreement between them, a third party (Shurui Wang) would participate in the discussion and propose a solution to resolve their differences. Furthermore, the references in the selected studies were examined to incorporate literature missing from the main studies.
Data extraction criteria included: first author, publication year, country, title, number of cases, treatment duration, intervention measures in both the experimental group and the control group, and treatment results.
2.6 Quality assessment
Two researchers (Niran Feng and Zixin Xu) independently assessed the literature according to the inclusion and exclusion criteria. A third party participated in the discussion and decided whether there was any objection. We used the Revman software 5.2 and the “bias risk assessment” tool recommended by the Cochrane manual as the evaluation index for the quality assessment for all included studies. We evaluated the content of the literature with high risk, low risk, and unknown risk. In the case of incomplete data during the evaluation process, we obtained data by contacting the authors.
2.7 Statistical investigation
Considering the data of the four intestinal microbiota as continuous variables, the weighted mean difference (WMD) and 95% CI were used as effect size indicators for continuous variables. The difference was considered statistically significant, when the confidence interval (CI) was set to 95% and 0 was excluded. The data extracted from the article were ranked for efficacy and ranked cumulative probabilities using stata15.0 (Stata Corporation, College Station, TX, United States). Heterogeneity was assessed using funnel plots, where I2 values greater than 50% represented considerable statistical heterogeneity. In addition, data processing, network link graph, forest graph and surface under the curve ranking (Sucra) were completed sequentially.
3 Results
3.1 Literature search of the included studies
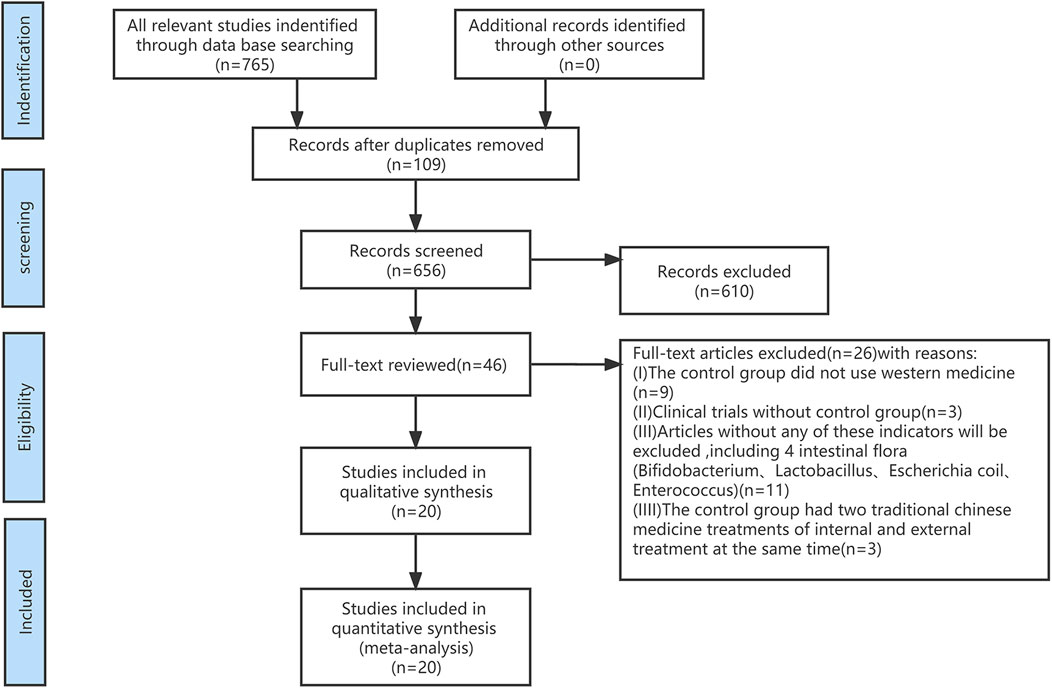
First, we screened out 765 articles and eliminated 109 duplicates according to the search criteria. Next, after reading the titles and abstracts, another 610 references were excluded. Finally, the remaining 46 articles were read and 20 eligible RCTs were included (Figure 1). The 20 RCTs comprised a total of 1,774 patients, including 888 in the treatment group and 886 in the control group. All included studies were conducted in China, with a sample size range of 30–109 entries. The duration of medication varied from 7 days to 3 months. 20 studies were RCTs, and 2 studies had no Enterococci-related data.
3.2 Characteristics of included literature
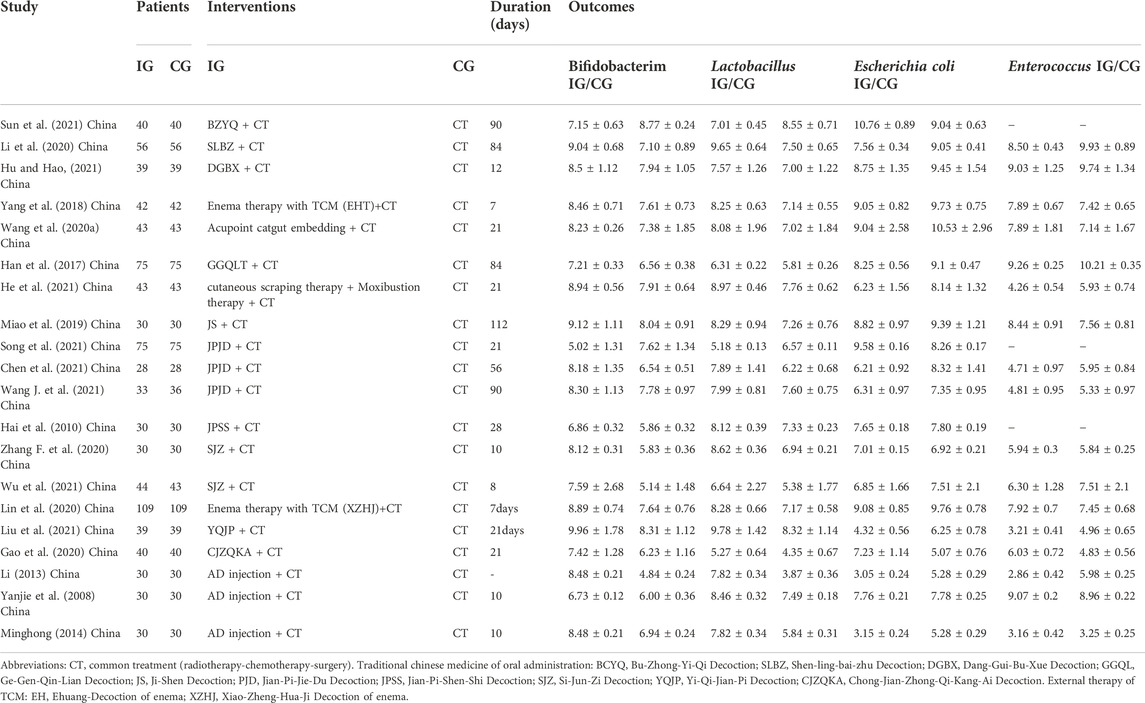
A total of 20 studies (Yanjie et al., 2008; Hai et al., 2010; Li et al., 2013; Minghong, 2014; Han et al., 2017; Yang et al., 2018; Miao et al., 2019; Wang et al., 2020b; Chen et al., 2020; Gao et al., 2020; Li et al., 2020; Lin et al., 2020; Zhang F. et al., 2020; He et al., 2021; Hu and Hao, 2021; Liu et al., 2021; Song, 2021; Sun et al., 2021; Wang S. et al., 2021; Wu et al., 2021) were included, and 14 RCT experiments of oral medicine use 10 TCM prescriptions: Buzhong yiqi Decoction (BZYQ), Shenling bai zhu Decoction (SLBZ), Danggui buxue Decoction (DGBX), Gegen qinlian Decoction (GGQL), Jishen Decoction (JS), Jianpi jiedu Decoction (JPJD), Jianpi shenshi Decoction (JPSS), Sijunzi Decoction (SJZ), Yiqi jianpi Decoction (YQJP), and Chongjian zhongqi kangai Decoction (CJZQKA). Three RCT experiments used external treatment methods, including Ehuang Decoction (EH) of enema, Xiaozheng Huaji Decoction (XZHJ) of enema, acupoint catgut embedding, skin scraping therapy and ginger separated moxibustion. Three RCT experiments involved TCM injection: Aidi injection. The treatment duration ranged from 7 to 90 days. Table 1 gives the basic information about the involved literature.
3.3 Risk of basis
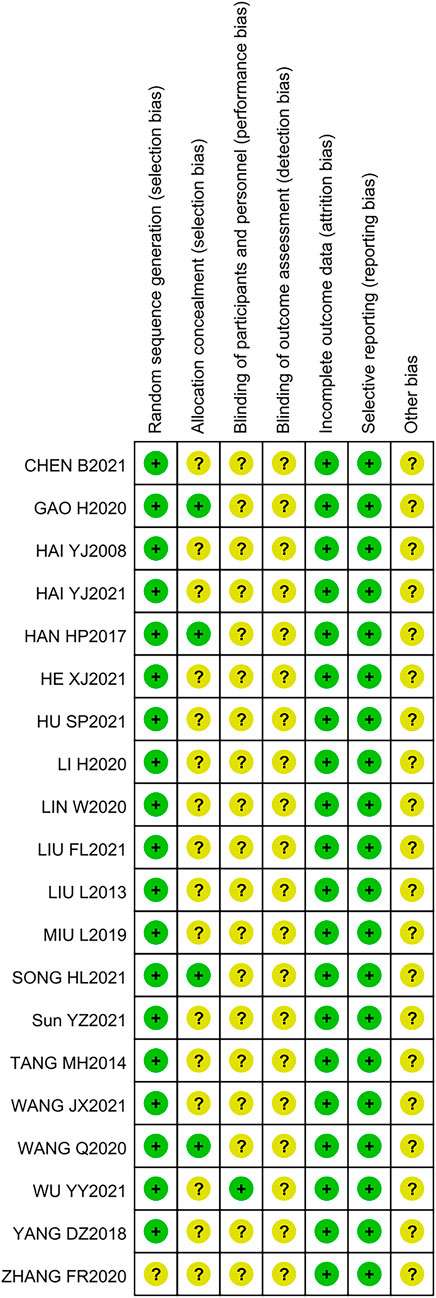
The results of the quality assessment are presented in Figure 2, which shows that the risks of a large proportion of the studies were unclear and low. However, the overall quality of the 20 RCTs was acceptable.
3.4 Outcome indicators
3.4.1 Data analysis
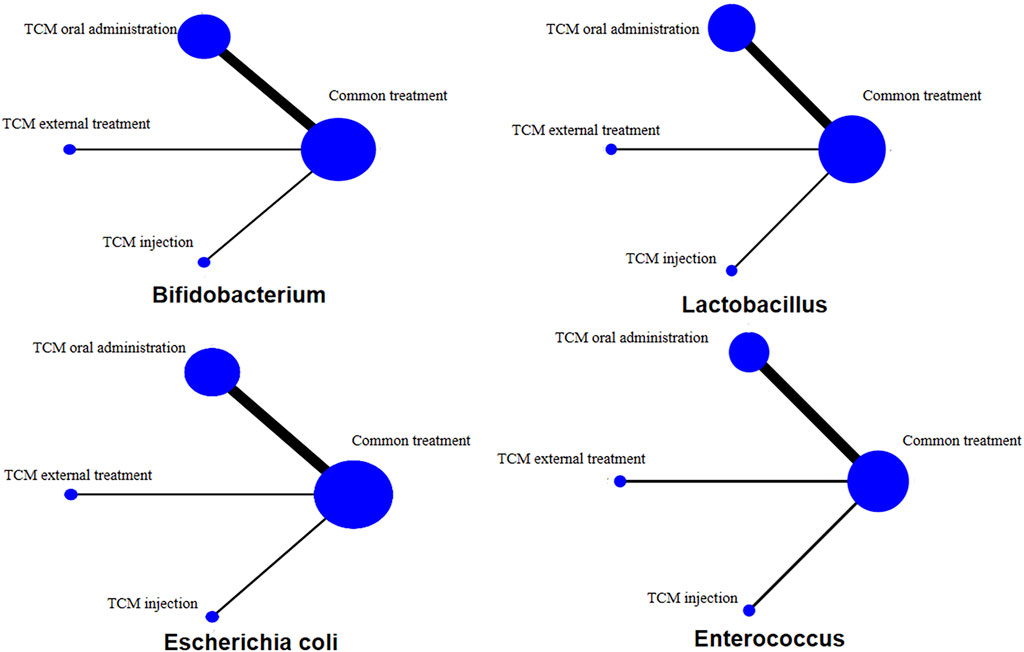
The network diagram includes 20 RCTs. The line between two points indicates the evidence for direct comparison between the two methods. There is no closed loop between interventions; that is, there is no direct comparison between interventions (Figure 3). Among the four bacteria, the three types of TCM treatment measures (oral administration, external treatment and injection) plus CT are directly compared with CT only, and the thickness of the line indicates the number of RCTs. This shows that the number of treatment methods using oral Chinese medicine is the largest, followed by external treatment and injection. All pairwise comparisons between interventions were from indirect comparisons. Therefore, statistical analysis can be performed directly under the consistency model.
3.4.2 Publication bias
Publication bias was assessed by the comparative-adjusted funnel method. Comparative correction charts were prepared for the included studies to evaluate the small sample effects. As shown in Figure 4, the RCTs with Lactobacillus and Bifidobacterium as outcome indicators in this study are roughly symmetrically distributed on both sides of the midline, indicating that the possibility of a small sample effect is low, and RCTs with Escherichia coli and Enterococcus as outcome indicators are not symmetrically distributed on both sides of the midline, indicating that the possibility of a small sample effect is high.

FIGURE 4. Funnel chart for comparison and correction of bifidobacteria, Lactobacillus, Escherichia coli and Enterococcus. (A) conventional treatment, (B) conventional treatment + TCM oral administration, (C) conventional treatment + TCM external treatment, (D) conventional treatment + TCM injection.
3.4.3 Network meta-analysis
In the comparison of pairwise methods, a total of 6 groups are meaningful (Table 2). In the bifidobacteria group, there were 2 pairs of comparison with statistically significant differences. CT only was compared with TCM injection in combination with CT, which the MD is 1.97 [MD = 1.97, 95% CI (0.48, 3.46)]. CT only was compared with oral administration of TCM in combination with CT, which MD is 0.83 [MD = 0.83, 95% CI (0.13,1.53)]. In Lactobacillus, 3 pairs of comparison showed statistically significant differences. TCM injection plus CT was compared with oral administration of TCM plus CT, which the MD is 1.55 [MD = 1.55 95% CI (0.20, 2.89)]. TCM injection combined with CT only was compared with CT, which the MD is 2.3 [MD = 2.30, 95% CI (1.08, 3.51)]. Oral administration of TCM combined with CT was compared with CT, which the MD is 0.75 [MD = 0.75, 95% CI (0.18, 1.32)]. In Escherichia coli, there is a statistically significant difference in one pair of comparison, the curative effect of TCM injection combined with CT was compared with CT only, which the MD is -1.46 [MD = -1.46, 95% CI (- 2.88, - 0.03)]. Among enterococci, one pair of comparison indicated a statistically significant difference. Oral administration combined with CT of TCM Compare with Common treatment, which the MD is -0.66 [MD = -0.66, 95% CI (- 1.31, - 0.01)].
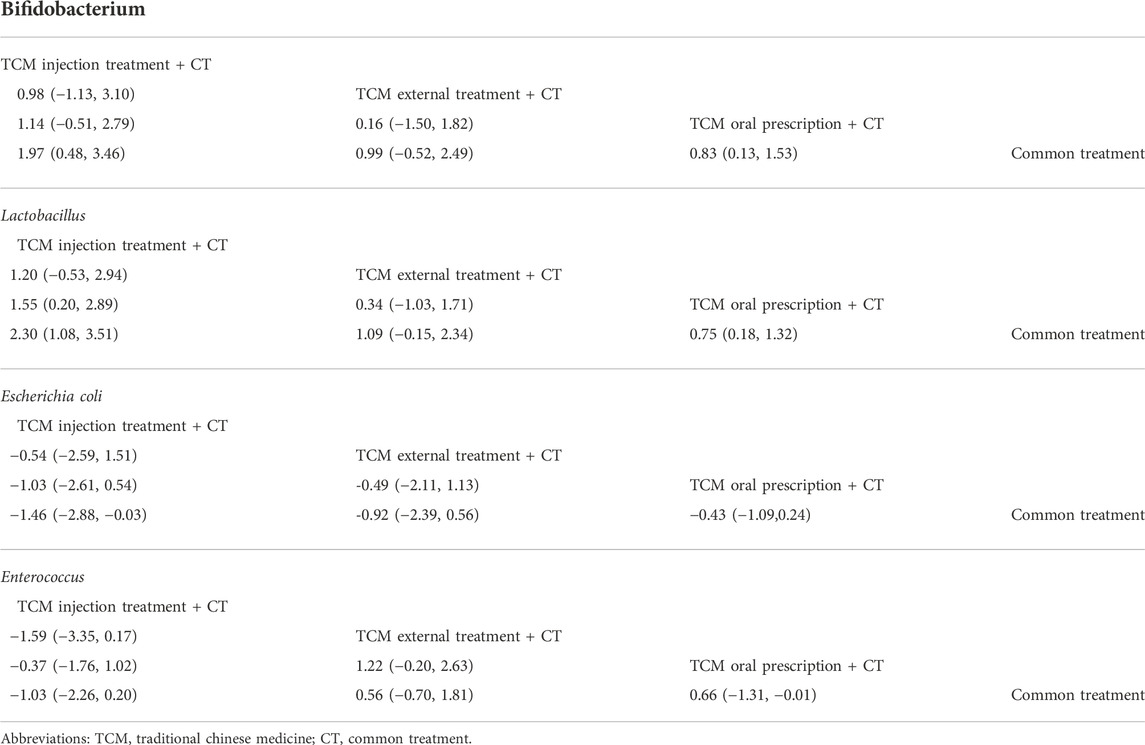
TABLE 2. Network meta-analysis matrix of results Comparison of treatments: Mean difference (95% confidence intervals).
3.4.4 SUCRA probability ranking
The cumulative ranking of the four methods is shown in Figure 5. High SUCRA values are correlated with good efficacy of this treatment for this type of gut microbiota. According to Sucra values, the total ranking of the four methods (A, Common Treatment; B, Common treatment plus TCM oral prescription; C, Common treatment plusTCM external treatment; D, Common treatment + TCM injection treatment) in supporting bifidobacteria was: D (Sucra = 90.08) > C (Sucra = 55.9) > B (Sucra = 49.7) > A (Sucra = 3.6); in supporting Lactobacillus, the total ranking of four methods was: D (Sucra = 96.4) > C (Sucra = 58.4) > B (Sucra = 43.7) > A (Sucra = 1.5); for Escherichia coli, the total ranking was: D (Sucra = 86.1) > C (Sucra = 63.2) > B (Sucra = 42.4) >A (Sucra = 8.3); and in inhibiting Enterococcus, the total ranking was: D (Sucra = 87.1) > B (Sucra = 74.3) > A (Sucra = 29.6) > C (Sucra = 8.9); To sum up, CT plus TCM injection can increase the probiotics (bifidobacteria and lactic acid bacteria) and reduce the pathogens (Escherichia coli and Enterococcus) in the intestinal tract of patients with gastric cancer. Thus, it is the best choice.
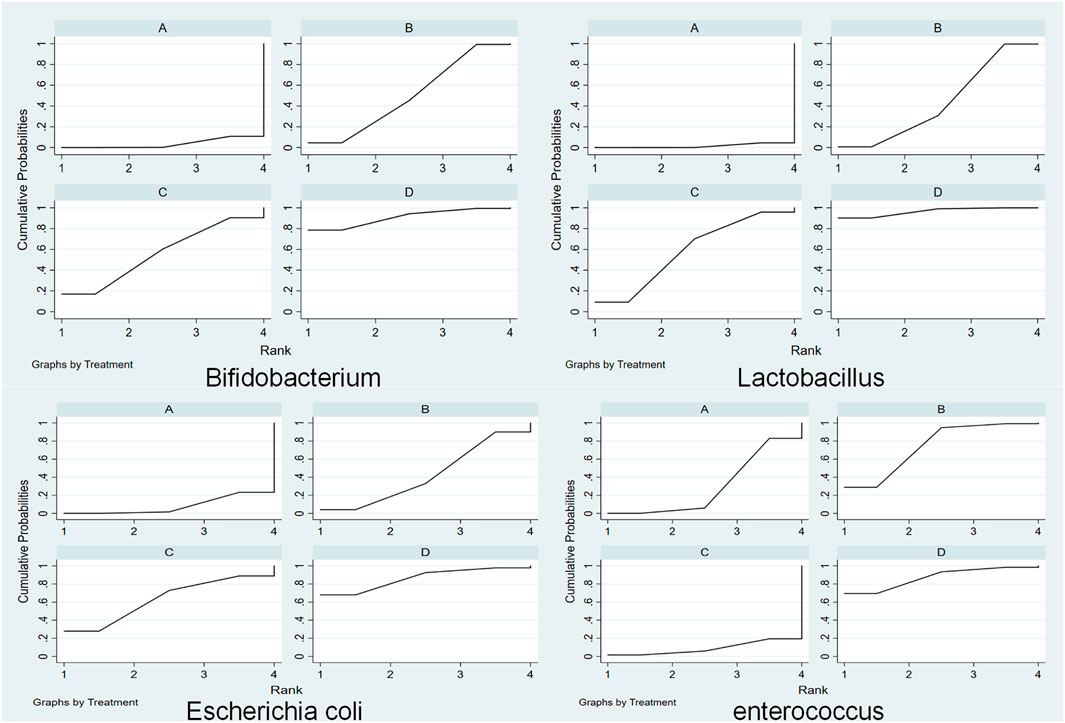
FIGURE 5. Cumulative probability of total effective rate (Abbreviations: (A) traditional therapy; (B) traditional therapy + traditional Chinese medicine (TCM) oral prescription; (C) common treatment + TCM external treatment; (D) common treatment + TCM injection treatment).
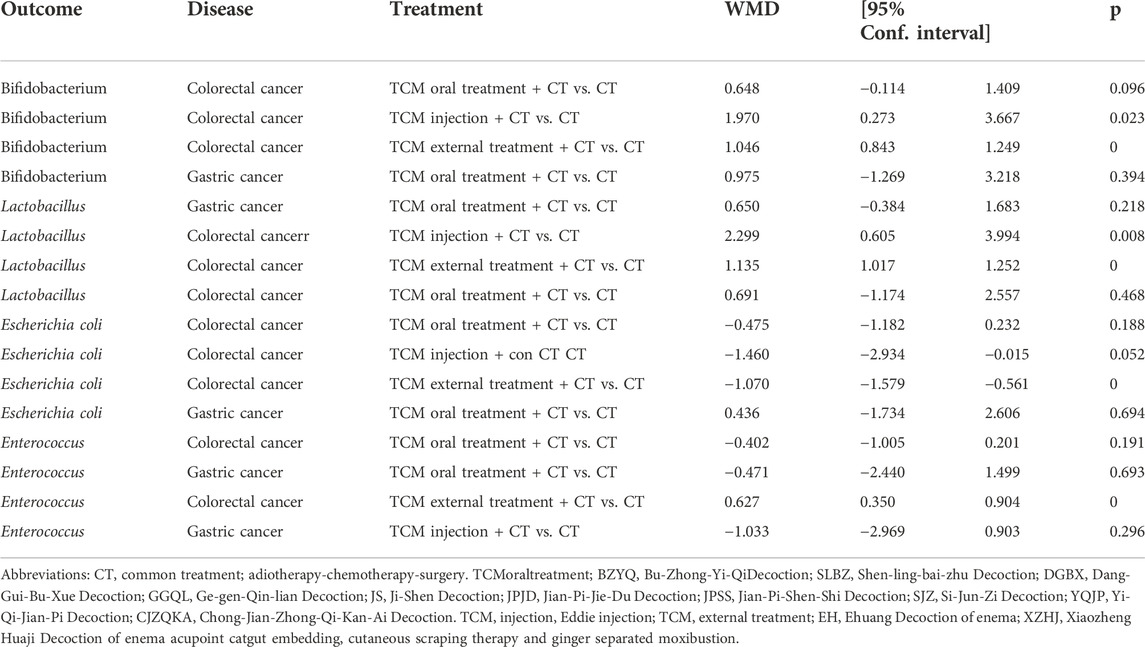
3.4.5 Comparative effect of colorectal cancer and gastric cancer
To make the results more stable and credible, we performed a meta-analysis supplementing the intestinal flora of colorectal and gastric cancers (Table 3). The random-effects model shows that TCM injection plus CT (WMD = 1.97, 95% CI (0.273, 3.667), p < 0.05] or TCM external treatment plus CT (WMD = 1.046, 95% CI (0.843,1.249), p < 0.05] is compared with CT, the amount of Bifidobacteria and Lactobacillus in feces of colorectal cancer patients are higher than those of the control group. For Escherichia coli, the amount of Escherichia coli in colorectal cancer patients treated with external treatment plus CT (WMD = -1.070, 95% CI (- 1.579, - 0.561), p < 0.05] is lower than that in the control group. As compared to CT only, colorectal cancer patients treated with TCM external therapy plus CT (WMD = 0.627,95% CI (0.35,0.904), p < 0.05] have significant differences in Enterococcus in the treatment group. Conclusion: compared with CT only, the combination of TCM injection or external treatment with CT is more effective for supporting the number of Bifidobacterium and Lactobacillus in colorectal cancer. Similarly, as compared to CT, the effect of external treatment of TCM combined with CT is better for inhibiting the number of Enterococcus and Escherichia coli in intestinal cancer.
4 Discussion
A total of 20 RCTs with 1774 patients was included in this paper. Through the comparison of SUCRA results, Aidi injection is the most effective in increasing the number of Bifidobacteria and Lactobacillus, and in inhibiting the number of Escherichia coli and Enterococcus. In the pairwise comparison of three TCM treatments, injection of TCM plus CT or oral Chinese medicine plus CT are effective for Bifidobacteria and Lactobacillus. For Escherichia coli, TCM injection plus CT takes effect. For Enterococcus, TCM oral treatment plus CT is practical.
There are three treatments of TCM, including internal treatment (oral absorption), external treatment (physical therapy or skin mucosal absorption), and injection (direct blood injection). Internal treatment mainly uses oral Chinese medicine decoction, the preparation of which is to soak the traditional Chinese medicine in boiling water or hot water to produce an aqueous extract containing a mixture of chemical components (Zhou et al., 2016; Deng et al., 2019). Specifically, after oral administration of TCM into the colon, intestinal microbiota converts carbohydrates, proteins, lipids and small non-nutritive compounds from TCM into chemical metabolites that may have beneficial or adverse effects on human health (Wang et al., 2013). For example, the continuous digestion of polysaccharides and carbohydrates (PS) produces many short-chain oligosaccharides, which can promote the growth of probiotics such as Bifidobacteria and Bacteroides. Shorter PSs are digested to form monosaccharides, which can be continuously catabolized to form short-chain fatty acids (SCFA) (e.g., formate, acetate, propionate, butyrate), lactic acid, hydrogen, carbon dioxide and other metabolites. Valproic acid, a kind of SCFAs, has antitumor activity, and its main mechanism is to inhibit histone deacetylase (Gurvich et al., 2004). These metabolites may directly affect the host intestinal environment and improve the microenvironment of gastrointestinal cancer (Vernocchi et al., 2016; Feng et al., 2019).
Aidi injection is mainly composed of ginseng, Astragalus membranaceus, canthatis, and acanthopanax senticosus. The active components are ginsenoside, astragalus polysaccharide, astragalus saponin, cantharidin and Acanthopanax Senticosus Polysaccharide (Quirke et al., 2007). Astragalus polysaccharides can increase the number of lactic acid bacteria and Bifidobacteria, thereby reducing pro-inflammatory factors, such as interleukin-6 and tumor necrosis factor-α. Therefore, as an inflammatory response inhibitor, it can also reduce the inflammatory response by reducing Salmonella typhi in the intestine (Tang et al., 2021). Ginsenoside-rb3 and ginsenoside Rd can promote the growth of beneficial bacteria, such as Bifidobacterium, Lactobacillus, Acidophilus and Anisoid, and can also reduce a number of cancer-related pathogens and Helicobacter pylori spp to prevent the development of colorectal cancer (CRC) (Huang et al., 2017). There are few studies on other drugs. We attribute the better effect of Aidi injection to its high bioavailability compared with the other two methods. When it comes to the cold and hot nature of the drug, all four drugs in the prescription are warm products. Therefore, it can be inferred that Aidi injection is hot and may be more suitable for the body of cancer patients undergoing radiotherapy and chemotherapy. External treatments include enema, acupoint embedding, skin scratching and ginger moxibustion. These methods are rarely studied in the field of intestinal flora research.
Many studies have shown that there is a causal relationship between changes in the intestinal flora and colorectal cancer. Patients with colorectal cancer have poor nutritional status and low systemic and partial resistance, which inhibit the growth of intestinal dominant bacteria, such as Lactobacillus and Bifidobacterium, resulting in the imbalance of the intestinal microenvironment. Meanwhile, intestinal flora imbalance will decrease the immune function of the body, and the decline of immune function will aggravate the flora imbalance, thus forming a vicious circle. In addition, chemotherapy drugs further reduce the immunity of patients and interfere with the proportion of normal intestinal flora. Moreover, the more obvious the imbalance is before chemotherapy, the more serious the imbalance is after chemotherapy. Therefore, the anti-cancer research of intestinal flora is of great significance. Lactic acid bacteria and Bifidobacterium strains induce dendritic cell (DC) to mature (Hickey et al., 2021) and produce IFN- γ (IFN- γ), enhancing the cytolytic potential of NK cells (Zhou et al., 2019). Probiotics induce apoptosis by inhibiting the expression of COX-2, NF KB, and MAPK, suppressing the inactivation of inflammatory bodies, and activating Caspase-3 (Iyer et al., 2008). It also induces cell death through autophagy (Engevik et al., 2019). Bifidobacterium can increase anti-PD-L1 and inhibit tumor volume by inducing anti-inflammatory activity of macrophages and dendritic cells (Xu et al., 2020). Probiotics such as Lactobacillus and Bifidobacterium inhibit the growth of colorectal cancer by suppressing inflammation and angiogenesis, and enhance the intestinal barrier function by secreting short-chain fatty acids (SCFAs) (Koh et al., 2016). Escherichia coli is more prevalent in colorectal cancer tissues (Buc et al., 2013). Enterococcus faecalis produces enterotoxins (e.g., tartary buckwheat glucoside) and reactive oxygen species, which can lead to DNA oxidative damage and intestinal epithelial cell inflammation (Baldassarri et al., 2005). Enterococcus faecalis is responsible for producing reactive oxygen species and superoxide anions, resulting in DNA damage and genomic instability in colorectal cancer (Geravand et al., 2019). Fecal Escherichia coli induces mucosal macrophages to produce DNA damage inducers (Goodwin et al., 2011), such as 4-hydroxy-2-nonyl, through COX-2 (Yang et al., 2013).
We did a general meta-analysis, separating the patients with gastric cancer and colorectal cancer, to compare the differences between CT only and the three methods combined with CT. For colorectal cancer patients, in Bifidobacteria and Lactobacillus, external treatment plus CT or injection of TCM plus CT, are more effective than CT. For Escherichia coli, the TCM external treatment plus CT and the TCM injection plus CT are more effective than CT only. For Enterococci, We prefer TCM injection plus CT because it exhibits better effect than CT only.
5 Limitations
The following limitations should be considered in this study. The methodological quality of the effect of TCM on intestinal flora of gastrointestinal cancers is subject to some risk deviation, such as insufficient sample size and short duration. In addition, gastrointestinal cancers are subdivided into gastric and colorectal cancers. Because the number of articles is too small to perform a heterogeneity testing, we did a general meta-analysis to assist the results. TCM is divided into three categories. The external treatment includes enema, the effect of which overlaps with that of oral TCM. However, considering its direct effect on intestinal flora and its short duration, it is placed in the external treatment.
6 Conclusion
In this study, Bifidobacteria and Lactobacilli, Escherichia coli and Enterococci were used as the main therapeutic indicators for comprehensive evaluation. Overall, TCM injection may be the best treatment, followed by TCM external treatment. TCM plays a certain role in the intestinal flora of patients with gastrointestinal cancers through multi-targeted comprehensive intervention. Clinically, it can be used in combination with other therapies depending on the actual situation of patients, and is suitable for the whole treatment process for gastrointestinal cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
All authors contributed to the study conception and design. NF and SW: Conceptualization, Methodology, Software, Writing—Original draft, Data curation, Visualization were performed; CL, ZX, and ZS: Methodology, Software, Writing—Original draft were performed; KL and ZY: Concetualization, Supervision, Project administration, Funding acquisition were performed. All authors read and approved the final manuscript.
Funding
This overview was funded by the Special Support Plan for National 12th Five Year Plan Project (No. 2012BAI25B05).
Acknowledgments
Thanks to the authors of the included studies to provide primary data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhinai, E. A., Walton, G. E., and Commane, D. M. (2019). The role of the gut microbiota in colorectal cancer causation. Int. J. Mol. Sci. 20 (21), E5295. doi:10.3390/ijms20215295
Arnold, M., Abnet, C. C., Neale, R. E., Vignat, J., Giovannucci, E. L., McGlynn, K. A., et al. (2020). Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 159 (1), 335–349.e315. doi:10.1053/j.gastro.2020.02.068
Baldassarri, L., Bertuccini, L., Creti, R., Filippini, P., Ammendolia, M. G., Koch, S., et al. (2005). Glycosaminoglycans mediate invasion and survival of Enterococcus faecalis into macrophages. J. Infect. Dis. 191 (8), 1253–1262. doi:10.1086/428778
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Buc, E., Dubois, D., Sauvanet, P., Raisch, J., Delmas, J., Darfeuille-Michaud, A., et al. (2013). High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 8 (2), e56964. doi:10.1371/journal.pone.0056964
Chen, B., Liang, F., Yuan, X., Wan, G., Yu, H., and Xie, M. (2020). Effects of modified Jianpi Jiedu formula on intestinal flora and immune function in advanced colorectal cancer chemotherapy with spleen Qi deficiency syndrome. J. traditional Chin. Med. 61 (05), 423–427. doi:10.13288/j.11-2166/r.2020.05.013
Chen, Y. Z., Yuan, M. Y., Chen, Y. L., Zhang, X., Xu, X. T., Liu, S. L., et al. (2021). The gut microbiota and traditional Chinese medicine: A new clinical frontier on cancer. Curr. Drug Targets 22 (11), 1222–1231. doi:10.2174/1389450122666210412141304
Deng, Z., Jing, W. G., Wang, S. H., Jiao, M. J., Zhang, Q., Zhou, H. Y., et al. (2019). Discussion and research progress in standard decoction of medicinal slices. Zhongguo Zhong Yao Za Zhi 44 (2), 242–248. doi:10.19540/j.cnki.cjcmm.20181108.005
Engevik, M. A., Luk, B., Chang-Graham, A. L., Hall, A., Herrmann, B., Ruan, W., et al. (2019). Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio 10 (3), e01087-19. doi:10.1128/mBio.01087-19
Feng, W., Ao, H., Peng, C., and Yan, D. (2019). Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 142, 176–191. doi:10.1016/j.phrs.2019.02.024
Ferreira, R. M., Pereira-Marques, J., Pinto-Ribeiro, I., Costa, J. L., Carneiro, F., Machado, J. C., et al. (2018). Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67 (2), 226–236. doi:10.1136/gutjnl-2017-314205
Gao, H., Yin, D., Xin, X., and Zhou, L. (2020). Adverse reactions of postoperative chemotherapy of chongjian zhongqi kang'ai decoction I in the treatment of gastric cancer. ACTA Chin. Med. 35 (03), 637–641. doi:10.16368/j.issn.1674-8999.2020.03.144
Geravand, M., Fallah, P., Yaghoobi, M. H., Soleimanifar, F., Farid, M., Zinatizadeh, N., et al. (2019). Investigation of enterococcus faecalis population in patients with polyp and colorectal cancer in comparison of healthy individuals. Arq. Gastroenterol. 56 (2), 141–145. doi:10.1590/s0004-2803.201900000-28
Goodwin, A. C., Destefano Shields, C. E., Wu, S., Huso, D. L., Wu, X., Murray-Stewart, T. R., et al. (2011). Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 108 (37), 15354–15359. doi:10.1073/pnas.1010203108
Gurvich, N., Tsygankova, O. M., Meinkoth, J. L., and Klein, P. S. (2004). Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64 (3), 1079–1086. doi:10.1158/0008-5472.can-03-0799
Hai, Y., Lu, L., and Ding, Y. (2010). Study on Intestinal flora of Jianpishenshi decoction in patients advanced colorectal cancer. Chin. Pharm. 13 (11), 1545–1547. doi:10.3969/j.issn.1005-376X.2008.04.029
Han, H., Zhang, L., Dong, J., Yingchun, L., and Fang, W. (2017). Effect of compound glutamin entersoluble capsule combined with Gegen Qinlian Decoction on prevention and treatment of advanced colorectal cancer with FOLFIRI chemotherapy related diarrhea and its influence on intestinal permeability and immune cytokine. Mod. J. Integr. Traditional Chin. West. Med. 26 (33), 3667–3670+3739.
Hayase, E., and Jenq, R. R. (2021). Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 13 (1), 107. doi:10.1186/s13073-021-00923-w
He, X., Qin, M., Tang, X., Jie, C., and Lin, Y. (2021). Clinical study on scraping therapy combined with ginger-separated moxibustion for improve immune function after chemotherapy of colorectal cancer. J. Sichuan Traditional Chin. Med. 39 (12), 217–220.
Hickey, A., Stamou, P., Udayan, S., Ramón-Vázquez, A., Esteban-Torres, M., Bottacini, F., et al. (2021). Bifidobacterium breve exopolysaccharide blocks dendritic cell maturation and activation of CD4(+) T cells. Front. Microbiol. 12, 653587. doi:10.3389/fmicb.2021.653587
Hu, S., and Hao, Y. (2021). Effect of Danggui buxue decoction on intestinal flora and immune function of perioperative patients with colorectal cancer. Guangming J. Chin. Med. 36 (14), 2362–2364.
Huang, G., Khan, I., Li, X., Chen, L., Leong, W., Ho, L. T., et al. (2017). Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in Apc(Min/+) mice. Sci. Rep. 7 (1), 12552. doi:10.1038/s41598-017-12644-5
Huang, Y., Zhang, L., and Tang, E. (2018). Reflections on classification of TCM treatment methods. J. Nanjing Univ. traditional Chin. Med. 34 (05), 436–439+479. doi:10.14148/j.issn.1672-0482.2018.0436
Iyer, C., Kosters, A., Sethi, G., Kunnumakkara, A. B., Aggarwal, B. B., and Versalovic, J. (2008). Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell. Microbiol. 10 (7), 1442–1452. doi:10.1111/j.1462-5822.2008.01137.x
Jian, Y., Zhang, D., Liu, M., Wang, Y., and Xu, Z. X. (2021). The impact of gut microbiota on radiation-induced enteritis. Front. Cell. Infect. Microbiol. 11, 586392. doi:10.3389/fcimb.2021.586392
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165 (6), 1332–1345. doi:10.1016/j.cell.2016.05.041
Konishi, H., Fujiya, M., Tanaka, H., Ueno, N., Moriichi, K., Sasajima, J., et al. (2016). Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 7, 12365. doi:10.1038/ncomms12365
Kuugbee, E. D., Shang, X., Gamallat, Y., Bamba, D., Awadasseid, A., Suliman, M. A., et al. (2016). Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 61 (10), 2908–2920. doi:10.1007/s10620-016-4238-7
Ley, R. E., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124 (4), 837–848. doi:10.1016/j.cell.2006.02.017
Li, L. (2013). Aidi’s effect on intestinal flora after radiotherapy for colorectal cancer. Henan Tradit. Chin. Med. 33, 126.
Li, H., Li, G., Han, B., Lei, L., and Li, L. (2020). Regulation of shenling baizhu powder on intestinal microflora, intestinal barrier and immune function in gastric cancer patients with chemotherapy after operation. Anti-tumor Pharm. 10 (04), 477–482.
Li, L., Hu, J., Huang, H., and He, Y. (2013). Effect of Jinlong capsule on proliferation and apoptosis of human pancreatic cancer cells BxPC-3. J. Traditional Chin. Med. 33 (0), 205–210. doi:10.1016/s0254-6272(13)60126-0
Lin, W., Qiu, Z., Chen, J., Li, Z., and Zhao, T. (2020). Effect of Xiaozheng Huaji recipe enema combined with Bifidobacterium on intestinal microecology and mucosal immune function in patients with colorectal cancer after surgery. Oncol. Progress. 18 (11), 1163–1166.
Liu, F., Ma, L., Song, R., and Yan, H. (2021). Effect of Yiqi Jianpi Decoction on immune function of patients with spleen deficiency syndrome after colorectal cancer operation flora, and intestinal flora. J. Sichuan Traditional Chin. Med. 39 (11), 102–105.
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P., and Lapaque, N. (2021). Scfa: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80 (1), 37–49. doi:10.1017/s0029665120006916
Meng, C., Bai, C., Brown, T. D., Hood, L. E., and Tian, Q. (2018). Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinforma. 16 (1), 33–49. doi:10.1016/j.gpb.2017.06.002
Miao, X., Tao, Y., Gu, X., and Shen, S. (2019). Jisheng decoction combined with postoperative adjuvant chemotherapy in treating patients undergoing chemotherapy post II period colorectal cancer operation and its effect on intestinal flora. Chin. J. Surg. Oncol. 11 (05), 346–349.
Minghong, T. (2014). The effect of Aidi injection on intestinal flora of patients with colorectal cancer after radiotherapy. J. Clin. Med. Pract. 18 (0), 107–108.
Quirke, P., Williams, G. T., Ectors, N., Ensari, A., Piard, F., and Nagtegaal, I. (2007). The future of the TNM staging system in colorectal cancer: Time for a debate? Lancet. Oncol. 8 (7), 651–657. doi:10.1016/s1470-2045(07)70205-x
Si, H., Yang, Q., Hu, H., Ding, C., Wang, H., and Lin, X. (2021). Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin. Cancer Biol. 70, 3–10. doi:10.1016/j.semcancer.2020.05.004
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-Michaels, K., Earley, Z. M., et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350 (6264), 1084–1089. doi:10.1126/science.aac4255
Soleimanpour, S., Hasanian, S. M., Avan, A., Yaghoubi, A., and Khazaei, M. (2020). Bacteriotherapy in gastrointestinal cancer. Life Sci. 254, 117754. doi:10.1016/j.lfs.2020.117754
Song, H. (2021). Effects of Jianpi Jiedu decoction on clinical symptoms, intestinal flora and T lymphocyte sub- sets in patients with colorectal cancer. Med. J. Liaoning 35 (06), 44–47.
Song, P., Wang, Q. B., Liang, B., and Jiang, S. J. (2021). Advances in research on the relationship between the gut microbiome and cancer. Eur. Rev. Med. Pharmacol. Sci. 25 (16), 5104–5112. doi:10.26355/eurrev_202108_26521
Sun, Y., Li, B., and Chen, L. (2021). Efficacy ang safety of modified Buzhong yiqi decoction on patients with spleen and stomach qi deficiency syndrome after grastric cancer chemotherapy. World J. Integr. Traditional West. Med. 16 (12), 2308–2313+2319. doi:10.13935/j.cnki.sjzx.211230
Tang, S., Liu, W., Zhao, Q., Li, K., Zhu, J., Yao, W., et al. (2021). Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 264, 113280. doi:10.1016/j.jep.2020.113280
Vernocchi, P., Del Chierico, F., and Putignani, L. (2016). Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health. Front. Microbiol. 7, 1144. doi:10.3389/fmicb.2016.01144
Wang, J., Cao, L., Han, J., and Shen, Q. (2021). Clinical efficacy of Jianpi Jiedu formula combined with FOLFOX4 regimen in patients with colorectal cancer undergoing chemotherapy. Chin. Tradit. Pat. Med. 43 (11), 3258–3261.
Wang, Q., Geng, W., Guo, H., Wang, Z., Xu, K., Chen, C., et al. (2020a). Emerging role of RNA methyltransferase METTL3 in gastrointestinal cancer. J. Hematol. Oncol. 13 (1), 57. doi:10.1186/s13045-020-00895-1
Wang, Q., Kuang, H., Su, Y., Sun, Y., Feng, J., Guo, R., et al. (2013). Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 146 (1), 9–39. doi:10.1016/j.jep.2012.12.013
Wang, Q., Luo, M., Li, W., Jiwen, Z., and Weizhong, S. (2020b). Effect of Ehuang Decoction Retention enema combined with acupoint catgut embedding at Zusanli on postoperative short-term rehabilitation and quality of life in patients with colorectal cancer. Chin. J. Clin. Oncol. Rehabilitation 27 (01), 48–51. doi:10.13455/j.cnki.cjcor.2020.01.14
Wang, S., Fu, J. L., Hao, H. F., Jiao, Y. N., Li, P. P., and Han, S. Y. (2021). Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol. Res. 170, 105728. doi:10.1016/j.phrs.2021.105728
Wang, S., Long, S., Deng, Z., and Wu, W. (2020c). Positive role of Chinese herbal medicine in cancer immune regulation. Am. J. Chin. Med. 48 (7), 1577–1592. doi:10.1142/s0192415x20500780
Wassenaar, T. M. (2018). E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 44 (5), 619–632. doi:10.1080/1040841x.2018.1481013
Wertman, J. N., Dunn, K. A., and Kulkarni, K. (2021). The impact of the host intestinal microbiome on carcinogenesis and the response to chemotherapy. Future Oncol. 17 (32), 4371–4387. doi:10.2217/fon-2021-0087
Wu, Y., Yang, J., and Zhou, W. (2021). Clinical study on Sijunzi decoction combined enteral nutrition in the treatment of gastric cancer with spleen deficiency syndrome. World Chin. Med. 16 (07), 1104–1108.
Xu, D., Zou, W., Luo, Y., Gao, X., Jiang, B., Wang, Y., et al. (2020). Feasibility between bifidobacteria targeting and changes in the acoustic environment of tumor tissue for synergistic HIFU. Sci. Rep. 10 (1), 7772. doi:10.1038/s41598-020-64661-6
Yang, D., Junming, H., Yong, J., Fu, T., Ming, D., Yuanwei, L., et al. (2018). Effect of ehuang decoction retention enema on intestinal flora and intestinal mucosal permeability after colorectal cancer surgery. Guid. J. Traditional Chin. Med. 24 (10), 46–49. doi:10.13862/j.cnki.cn43-1446/r.2018.10.012
Yang, Y., Wang, X., Huycke, T., Moore, D. R., Lightfoot, S. A., and Huycke, M. M. (2013). Colon macrophages polarized by commensal bacteria cause colitis and cancer through the bystander effect. Transl. Oncol. 6 (5), 596–606. doi:10.1593/tlo.13412
Yanjie, H., Baoling, J., and Lin, L. (2008). Study on intestinal flora and immune function of Aidi injection combined with chemotherapy in colorectal cancer patients. Chin. J. Microecology 20 (4), 400–401.
Zaharuddin, L., Mokhtar, N. M., Muhammad Nawawi, K. N., and Raja Ali, R. A. (2019). A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 19 (1), 131. doi:10.1186/s12876-019-1047-4
Zhang, F., Xie, A., Zhang, J., Cai, X., Chen, J., and Li, W. (2020). Clinical study on the influence of Sijunzi decoction on gut microbiota and immune function of postoperative patients with colorectal cancer chemotherapy. J. Shantou Univ. Med. Coll. 33 (04), 206–208. doi:10.13401/j.cnki.jsumc.2020.04.004
Zhang, X., Qiu, H., Li, C., Cai, P., and Qi, F. (2021). The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 15 (5), 283–298. doi:10.5582/bst.2021.01318
Zhang, Z., Shao, S., Zhang, Y., Jia, R., Hu, X., Liu, H., et al. (2020). Xiaoyaosan slows cancer progression and ameliorates gut dysbiosis in mice with chronic restraint stress and colorectal cancer xenografts. Biomed. Pharmacother. 132, 110916. doi:10.1016/j.biopha.2020.110916
Zhou, S. S., Xu, J., Zhu, H., Wu, J., Xu, J. D., Yan, R., et al. (2016). Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 6, 22474. doi:10.1038/srep22474
Keywords: gastrointestinal cancers, intestinal flora, systematic review, treatment, traditional Chinese medicine (TCM)
Citation: Feng N, Wang S, Liu C, Xu Z, Song Z, Li K and Yu Z (2022) A network meta-analysis to evaluate the efficacy of traditional Chinese medicine on intestinal flora in patients with gastrointestinal cancer. Front. Genet. 13:1069780. doi: 10.3389/fgene.2022.1069780
Received: 14 October 2022; Accepted: 10 November 2022;
Published: 28 November 2022.
Edited by:
Simin Li, Southern Medical University, ChinaReviewed by:
Zhinuan Hong, Fujian Medical University Union Hospital, ChinaJu Wang, Sapienza University of Rome, Italy
Copyright © 2022 Feng, Wang, Liu, Xu, Song, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunyang Li, MTU1Njc1OTIxMTVAMTYzLmNvbQ==; Zhifeng Yu, WVV6ZjE5ODZAdGp1dGNtLmVkdS5jbg==
 Niran Feng1
Niran Feng1 Shurui Wang
Shurui Wang Chengjiang Liu
Chengjiang Liu Kunyang Li
Kunyang Li Zhifeng Yu
Zhifeng Yu