
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 04 January 2023
Sec. Human and Medical Genomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.1058261
 Xin Li1†
Xin Li1† Han Kang1†
Han Kang1† Huifeng Yin2†
Huifeng Yin2† Tianjiao Liu1†
Tianjiao Liu1† Qiannan Hou1
Qiannan Hou1 Xiaolan Yu3
Xiaolan Yu3 Yuanlin Guo4
Yuanlin Guo4 Wei Shen1
Wei Shen1 Huisheng Ge1
Huisheng Ge1 Xiaoyan Zeng1
Xiaoyan Zeng1 Kangmu Lu5*
Kangmu Lu5* Ying Xiong1*
Ying Xiong1*Introduction: Though embryonic chromosome abnormalities have been reported to be the most common cause of missed abortions, previous studies have mainly focused on embryonic chromosome abnormalities of missed abortions, with very few studies reporting that of non-missed abortion. Without chromosome studies of normal abortion samples, it is impossible to determine the risk factors of embryo chromosome abnormalities and missed abortion. This study aimed to investigate the maternal and embryonic chromosome characteristics of missed and non-missed abortion, to clarify the questions that how many missed abortions are caused by embryonic chromosomal abnormalities and what are their risk factors.
Material and methods: This study was conducted on 131 women with missed or non-missed abortion from the Longitudinal Missed Abortion Study (LoMAS). Logistic regression analysis was used to identify the association between maternal covariates and embryonic chromosomal abnormalities and missed abortions. Data on the characteristics of women with abortions were collected.
Results: The embryonic chromosome abnormality rate was only 3.9% in non-missed abortion embryos, while it was 64.8% in missed-abortion embryos. Assisted reproductive technology and prior missed abortions increased the risk of embryonic chromosome abnormalities by 1.637 (95% CI: 1.573, 4.346. p = 0.010) and 3.111 (95% CI: 1.809, 7.439. (p < 0.001) times, respectively. In addition, as the age increased by 1 year, the risk of embryonic chromosome abnormality increased by 14.4% (OR: 1.144, 95% CI: 1.030, 1.272. p = 0.012). Moreover, advanced age may lead to different distributions of chromosomal abnormality types.
Conclusion: Nearly two-thirds of missed abortions are caused by embryonic chromosomal abnormalities. Moreover, advanced age, assisted reproductive technology, and prior missed abortions increase the risk of embryonic chromosomal abnormalities.
Missed abortion, also known as overdue abortion, refers to the fact that the embryo or fetus has died and remains in the uterine cavity without natural discharge, before 12 weeks of gestation (Allen et al., 2022; Herkiloglu et al., 2022). As a special type of spontaneous abortion, missed abortions account for 10%–20% of spontaneous abortions, while 25% of women undergo spontaneous abortion (Practice Committee of the American Society for Reproductive, 2012; Shahine and Lathi, 2015; Mohammad-Akbari et al., 2022). The incidence rate of missed abortions has shown an obvious upward trend in recent years (Alnafisah and Alalfy, 2018; Zhao et al., 2021; Torres-Miranda et al., 2022), which has seriously affected the physical and mental health of the patients, family, and social happiness.
Current research shows that missed abortion is mainly caused by four factors: embryonic factors (chromosome abnormalities), maternal factors (systemic diseases, abnormal reproductive organs, endocrine abnormalities, unhealthy lifestyle, and abnormal immune function), paternal factors (sperm chromosome abnormalities), and environmental factors (Segawa et al., 2017; Zhao et al., 2017; Fang et al., 2018; Fu et al., 2018; Yang et al., 2021a; Gong et al., 2021; He et al., 2021; Liu et al., 2022). Though embryonic chromosome abnormalities have been reported to be the most common cause of missed abortions, previous studies have mainly focused on embryonic chromosome abnormalities of missed abortions, with very few studies reporting that of non-missed abortion. In statistical analysis, the risk factors and their odds ratios of missed abortion can be better determined by comparing the patient characteristics of missed abortion and non-missed abortion. Clarifying the cause of missed abortions is conducive to alleviating the psychological burden of patients, and to carry out reasonable treatment and genetic counseling of these patients for the next pregnancy, by predicting the risk of missed abortions in the subsequent pregnancies (Ashaat and Husseiny, 2012; Hu et al., 2015; Li et al., 2018a).
Chromosome analysis techniques were developed from the earliest karyotype analysis, fluorescence in situ hybridization (FISH), chromosome microarray analysis (CMA), and the latest high-throughput sequencing technology (Authors Anonymous, 1988; Dube, 1990; Borgatta et al., 2000; Halder and Fauzdar, 2006; Ashaat and Husseiny, 2012; Segawa et al., 2017; Li et al., 2018b; Dai et al., 2019; Cheng et al., 2021). High-throughput sequencing technology, with its outstanding advantages of high accuracy, throughput, and sensitivity, has been widely used in the field of medical diagnosis (Quintero-Ronderos and Laissue, 2020). In the detection of chromosomal abnormalities in missed abortion villi, high-throughput sequencing technology can detect aneuploidy, large fragment structural abnormalities, chromosome microduplication and microdeletion, and submicroscopic aberrations up to 100 kb, which is relatively superior to other technologies (Ye et al., 2019).
Therefore, in this study, we aimed to investigate the maternal and embryonic chromosome characteristics in missed and non-missed abortion, using high-throughput sequencing technology. Given the findings reported for our cohort, we also aimed to study the impact of maternal characteristics on embryonic chromosomal abnormalities and missed abortions. This data supports the viewpoint that the elimination of altered karyotypes via missed abortion represents a strategy to ensure the integrity of karyotype coding (Ye et al., 2019).
The present study was embedded in the Longitudinal Missed Abortion Study (LoMAS), an ongoing pregnancy and birth cohort study conducted in Chengdu, aiming to determine the relative contributions of genes and the environment to missed abortions (Chinese Clinical Trial Registry: ChiCTR2200060959) approved by the Ethics Committee of the Chengdu Women’s and Children’s Central Hospital (No. 201952). This prospective cohort study was conducted at the Chengdu Women’s and Children’s Central Hospital and included all women with missed abortions as confirmed by ultrasound between March 2021 and December 2021. Written informed consent was obtained from all the participants. This subgroup study included pregnant women who were diagnosed with missed abortions by ultrasound and some matched non-missed abortion women. Non-missed abortions are defined as the normal embryos within 14 weeks of pregnancy terminated pregnancy according to the patient’s requirements and conducted D&C abortions (This is legal in Chinese law). Twin pregnancies were eliminated because it was difficult to separate the villi completely; therefore, women with abortions only in singleton pregnancies were included in this study. Due to the exorbitant rate of abnormal embryos in Perimenopausal women (>45 years old), only women aged 16–45 years were included in the cohort. Women with chromosomal abnormality, chronic metabolic or genetic diseases were not included in the study.
Maternal sociodemographic data (age, height, weight, education, occupation, parity, and mode of conception), lifestyle behaviors before pregnancy (smoking and alcohol use), and preexisting conditions were collected using standardized questionnaires and electronic medical records before D&C abortions. The standardized questionnaire was self-designed for the LoMAS cohort study; detailed information is presented in Supplementary File S1.
With the development and popularization of ultrasonic technology, ultrasonic examination has become a common method for clinical diagnosis of missed abortions. According to the French College of Gynaecologists and Obstetricians (CNGOF) (Delabaere et al., 2014), missed abortion can be diagnosed when ultrasound meets any of the following criteria: First, the embryonic head-hip diameter is greater than or equal to 7 mm, and there is no primitive heart tube pulsation; second, the diameter of the gestational sac is more than 25 mm, and no embryo is found; third, ultrasound examination shows that there is no yolk sac in the gestational sac and there is still no embryo with heartbeat after 2 weeks; fourth, ultrasound examination shows that there is a yolk sac in the gestational sac and there is no embryo with heartbeat after at least 11 days.
Villus samples of missed and non-missed abortion patients who terminated pregnancy in the outpatient operating room were collected under strict aseptic conditions. The specimens were transported to the hospital laboratory under refrigeration, where they were washed with normal saline to obtain clean villus tissues. The villi were dried with sterile gauze and frozen at −80° refrigerators.
After all the samples were collected, the villi were processed as follows: villus DNA was extracted using the Universal Genomic DNA Purification Mini Spin Kit (D0063, Beyotime, China). Agarose gel electrophoresis was used to analyze the degree of DNA degradation and RNA contamination, and Qubit was used to detect the total amount and concentration of DNA (standard: total amount of DNA ≥800 ng, DNA concentration ≥10 ng/μL).
Multiplex fluorescent PCR using short tandem repeat (STR) markers (Guangzhou Darui Biotechnology, GuangZhou, China) was performed to exclude maternal cell contamination. High-throughput sequencing for copy number variations (CNV) was performed as previously described. After library preparation, the samples were sequenced using the pair end 150 bp method (PE150) on the Illumina HiSeq platform (Illumina, San Diego, United States) according to the manufacturer’s instructions. Raw image files were processed using BclToFastq (Illumina) for the base calling and raw data generation. The reads were then mapped to the GRCh37/hg19 human reference genome using BWA software. Candidate CNVs were classified using a five-tiered system according to a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen).
All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). The chi-squared or Fisher’s exact test was used to assess categorical data, which were reported as counts and percentages. The means and standard deviations of continuous variables were calculated using the Student’s t-test, one-way analysis of variance, or the non-parametric test. Binary logistic regression analysis was used to detect the influence of women’s characteristics on missed abortions and embryonic chromosome abnormalities. Covariates were selected according to the different variables in univariate analysis and the factors reported in previous studies that would affect missed abortion or embryonic chromosome abnormality. All tests were two-tailed, and statistical significance was set at p < 0.05.
The selection process of the study population is shown in Figure 1. A total of 171 women with missed or non-missed abortion were initially recruited into this subgroup study as part of the LoMAS study. After excluding patients who did not match the inclusion criteria and cases of failure to extract embryonic DNA, the final analysis included 131 women with missed or non-missed abortion. Descriptive data of the study participants are shown in Table 1. The average patient age at delivery was 28.34 ± 5.48 years, and the average gestational age was 8.93 ± 1.85 weeks. Furthermore, 19.1% of patients conceived via assisted reproductive technology (ART), and 96.2% of patient parity was less than or equal to two due to the Chinese previous two-child policy. Finally, 54 (41.2%) missed abortions and 77 (58.8%) normal abortion cases were included in the analysis.
In the patients with missed abortions, nearly two-third of the patients whose embryos were accompanied by chromosome abnormality, 25.9% had conceived via ART, and 13.0% had a previous missed abortion; this group with higher age and pre-pregnancy BMI had a higher incidence of antenatal bleeding but less parity. Specific abnormal chromosomal types are shown in Figure 2. The top five prevalent chromosomal abnormalities were as follows: 22.86% X monosomy, 22.86% trisomy 16, 11.43% trisomy 22, 8.57% trisomy 2, 8.57% trisomy 15. These five types of chromosomal abnormalities account for three-quarters of all chromosomal abnormalities (Tables 2,3).
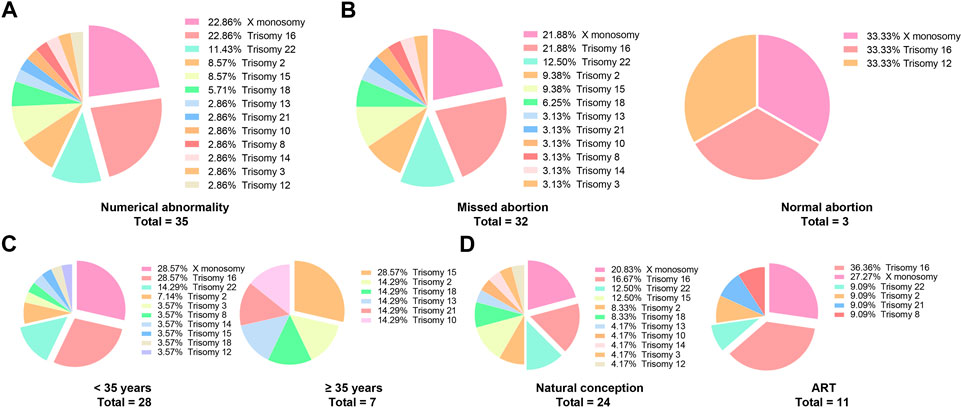
FIGURE 2. The types of specific chromosomal abnormalities. (A) It shows the types of all the chromosomal numerical abnormalities of the aborted embryos; (B–D) According to missed abortion, maternal age and ART, the types of chromosome abnormalities were displayed.
A significant difference in the patient’s age, pre-pregnancy BMI, mode of conception parity, prior missed abortion, and embryonic chromosome abnormality was found between the missed and normal abortion groups (Tables 2,3). Binary logistic regression showed that missed abortions were significantly associated with age, mode of conception, and parity (OR: 0.691, 95% CI: 0.500, 0.955. p = 0.025), prior missed abortions, and embryonic chromosomal abnormalities, but were not found to be correlated with BMI, smoking, conception season, gravidity, D&C abortions. Notably, due to too few samples in falling ill, taking special drugs, and exposure to hazardous substances during pregnancy, we cannot clearly infer their relationship with missed abortion. ART, prior missed abortions, and embryonic chromosome abnormalities increased the risk of missed abortions by 2.110 (95% CI: 1.395, 5.598. p = 0.034), 3.040 (95% CI: 1.068, 8.654. p < 0.001) and 16.352 (95% CI: 11.230, 40.409. p < 0.001) times, respectively. Moreover, as the age increased by 1 year, the risk of missed abortion increased by 15% (OR: 1.150, 95% CI: 1.055, 1.254. p < 0.001) (Table 4).
Moreover, microdeletions and microduplications of embryonic chromosomes were analyzed in our cohort using high-throughput sequencing technology. Although the embryonic chromosome microduplication rate in the missed abortion group (9.3%) was higher than that in the normal abortion group (5.2%), there was no significant difference between the two groups (Table 3). The likely pathogenic chromosomal deletions and duplications in this study are shown in Table 5.
Considering that nearly two-thirds of the embryos were accompanied by chromosomal abnormalities in women with missed abortions, binary logistic regression was used to analyze the factors influencing embryonic chromosome abnormalities. The results showed that embryonic chromosome abnormalities were significantly associated with age, mode of conception, and parity (OR: 0.754, 95% CI: 0.591, 0.962. p = 0.023) and prior missed abortions. ART and prior missed abortions increased the risk of embryonic chromosome abnormalities by 1.637 (95% CI: 1.573, 4.346. p = 0.010) and 3.111 (95% CI: 1.809, 7.439. p < 0.001) times, respectively. In addition, as age increased by 1 year, the risk of embryonic chromosome abnormality increased by 14.4% (OR: 1.144, 95% CI: 1.030, 1.272. p = 0.012) (Figure 3A).
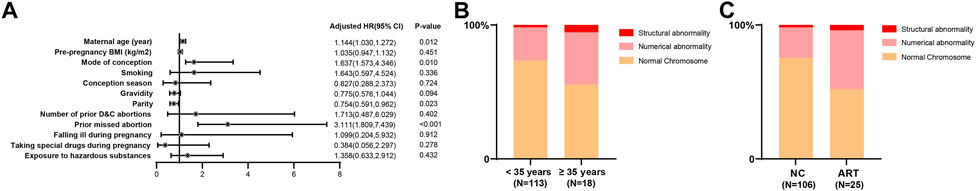
FIGURE 3. The maternal risk factors for embryonic chromosome abnormalities. (A) Binary logistic regression showed that ART and prior missed abortion increased the risk of embryonic chromosome abnormality by 1.6 and 3.1 times, respectively. Besides, with an increase in age by 1 year, the risk of embryonic chromosome abnormality increased by 14.4%; (B) 28 (24.8%) women had embryonic chromosomal numerical abnormalities and two (1.8%) had embryonic structural abnormalities in the normal age group, while seven (38.9%) had embryonic chromosomal numerical abnormalities and one (5.6%) had embryonic structural abnormalities in the advanced maternal age group; (C) 24 (22.6%) women had embryonic chromosomal numerical abnormalities and two (1.9%) had embryonic structural abnormalities in the natural conception group, whereas 11 (44.0%) had embryonic chromosome numerical abnormalities and one (4.0%) had embryonic structural abnormalities in the ART group.
In our cohort, 28 (24.8%) women had embryonic chromosomal numerical abnormalities and two (1.8%) had embryonic structural abnormalities in the normal age group, while seven (38.9%) had embryonic chromosomal numerical abnormalities and one (5.6%) had embryonic structural abnormalities in the advanced maternal age group (Figure 3B). Moreover, 24 (22.6%) women had embryonic chromosomal numerical abnormalities and two (1.9%) had embryonic structural abnormalities in the natural conception group, whereas 11 (44.0%) had embryonic chromosome numerical abnormalities and one (4.0%) had embryonic structural abnormalities in the ART group (Figure 3C).
Considering that the incidence of embryonic chromosomal abnormalities is significantly increased in the advanced maternal age and ART groups, further analysis focused on the effects of age and ART on the types of embryonic chromosome abnormalities. Surprisingly, the distribution and proportion of chromosome abnormalities in the ART group were not significantly different from those in the natural conception group, while there was an evidently different type and distribution of chromosome abnormalities between the normal and advanced maternal age groups (Figures 2B–D).
In this prospective preliminary study, we investigated the maternal and embryonic chromosome characteristics in missed and non-missed abortion using high-throughput sequencing technology. Given the findings reported for our cohort, we found effects of maternal age, ART, prior missed abortion on embryonic chromosome abnormality, and missed abortion.
Previous studies have shown that the incidence of embryonic chromosome abnormalities in missed abortion was 50%–60% (Delabaere et al., 2014; Yang et al., 2021b; Voorhies et al., 2022), which is consistent with the 64.8% reported in our study. In addition, we characterized embryonic chromosomes from non-missed abortion, which were not included in previous studies. This is crucial because the chromosomal abnormality rate of missed abortions is 64.8%, which cannot be attributed to embryonic chromosome abnormalities alone. The “cause and effect” relationship between missed abortion and embryonic chromosome abnormality is undefined when the rate of embryonic chromosome abnormalities in normal abortion has not been detected. In our cohort, the chromosome abnormality rate was only 3.9% in non-missed abortion embryos, while it was 64.8% in missed abortion embryos. Embryonic chromosome abnormality increased the risk of missed abortion by 16.352 times. Based on this result, it can be concluded that embryonic chromosomal abnormalities are the main cause of missed abortions. Recently, the concept of karyotype coding is proposed to illustrate the importance of the normal karyotype, as any altered karyotype can alter the genomic network, some of which is closely associated with disease conditions (Ye et al., 2019). Thus, the high rate of missed abortion can effectively eliminate the altered genome systems (Gorelick and Heng, 2011).
Previous studies have shown that conception with the help of ART may result in a higher rate of missed abortions and embryonic chromosome abnormalities (Askerov, 2016; Li et al., 2018b), which is consistent with our study. After the implementation of Chinese two-child and three-child policies (Chen et al., 2022; Long et al., 2022), many women who cannot get pregnant naturally due to previous tubal ligation or advanced age get pregnant by ART. Further research is necessary to clarify the relationship between ART and embryonic chromosome abnormalities to promote the development of ART technology.
In our cohort, with an increase in maternal age, the rate of missed abortions and embryonic chromosome abnormalities gradually increased (Yang et al., 2021a; Salman et al., 2021; Allen et al., 2022), which is consistent with previous studies. However, although ART and advanced age increase the incidence of chromosomal abnormalities, the types of chromosomal abnormalities are different. The distribution and proportion of chromosomal abnormalities in the ART group were not significantly different from those in the natural conception group, while there was an evidently different type and distribution of chromosomal abnormalities between the normal age and advanced maternal age groups. However, due to the relatively limited sample size in this study, it cannot be concluded that advanced age may lead to different distributions of chromosomal abnormalities. Further research is needed to determine the effect of advanced age on the localization of embryonic chromosomal abnormalities.
Based on our study, previous missed abortions may increase the risk of missed abortions in subsequent pregnancies. To a certain extent, this is consistent with the lower risk of missed abortions in women who have successfully delivered in the past. Embryo chromosome abnormalities arise mainly due to sperm or egg chromosome abnormalities or the influence of external environmental factors in early pregnancy (Del Carmen Nogales et al., 2017; Shen et al., 2019; Wang et al., 2021). Some of these factors are similar in two pregnancies in the same woman, which means that if a woman has two or more missed abortions, both partners need to have a more detailed pre-pregnancy examination before the next pregnancy. Moreover, although some microdeletions and microduplications of embryonic chromosomes have been reported to lead to missed abortion (Cui et al., 2021; Familiari et al., 2021; Kokkonen et al., 2021; Baba et al., 2022; Yue et al., 2022), and the chromosome microduplication rate of the missed abortion group was higher than that of the normal abortion group in our cohort, there was no significant difference between the two groups.
The merits of our study include the inclusion of a specialized study population. Study participants were screened using strict inclusion and exclusion criteria. Non-singleton pregnant women were excluded because it was difficult to separate the villi completely. Additionally, we collected maternal sociodemographic data and carried out chromosome analysis of both missed- and non-missed abortion women, resulting in a comprehensive study design. Because of the difficulty in obtaining normal aborted embryo villi and the high cost of high-throughput sequencing technology, obtaining embryonic villi specimens and detecting them is time-consuming and costly. It is relatively challenging to recruit participants, collect specimens, detect embryonic chromosomes, and follow up for 1 year.
This preliminary study has significantly contributed to our understanding of the impact of maternal characteristics on embryonic chromosome abnormalities and missed abortions, but also has several limitations that need to be acknowledged. First, compared to some epidemiological surveys, the sample size in this study was relatively modest. Second, the 1 year follow-up of their next pregnancy in women with missed abortions is still ongoing; these data will be reported after finishing the follow-up. Third, due to China’s geomorphic and ethnic diversity, missed abortion-related variables, such as living region altitude and nationality, were limited. To further understand the relevant maternal characteristics of embryonic chromosome abnormalities and missed abortions in China, a large-scale study involving more regions and nationalities, conducted in multiple centers, is required.
In conclusion, this study suggests that nearly two-thirds of missed abortions are indeed caused by embryonic chromosomal abnormalities. Moreover, advanced age, ART, and prior missed abortions increase the risk of embryonic chromosomal abnormalities.
The original contributions presented in the study are included in Supplementary Data Sheet S2. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Chengdu Women’s and Children’s Central Hospital (No. 201952). The patients/participants provided their written informed consent to participate in this study.
XL and HK designed the research protocol; HY and TL conducted the study; QH and XY analyzed the data; YG and WS drafted the manuscript; HG and XZ critically revised the manuscript; KL and YX provided funding resources. All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Financial support of this work was provided by Chengdu Science and Technology Bureau (2021-YF09-00048-SN), and Sichuan Provincial Science and Technology Bureau (23ZDYF1365 and 23ZDYF1360). The funding agencies did not have any role in the design of the study, collection, analysis, and interpretation of data, and in writing the manuscript.
The authors would like to thank all the participants and researchers who contributed to this cohort study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.1058261/full#supplementary-material
Allen, R., Lee, A., Hanuscin, C., and Gleyzer, A. (2022). Missed abortion with negative biomarkers: A case report. Am. J. Emerg. Med. 57, 236.e5–236236.e6. doi:10.1016/j.ajem.2022.04.028
Alnafisah, F., and Alalfy, S. A. (2018). Unilateral uterine and ovarian arterial ligations in a case of missed abortion at 12 Weeks of gestation with undiagnosed placenta accreta. Cureus 10 (11), e3562. doi:10.7759/cureus.3562
Ashaat, N., and Husseiny, A. (2012). Correlation between missed abortion and insertional translocation involving chromosomes 1 and 7. Iran. J. Reprod. Med. 10 (1), 15–22.
Askerov, R. (2016). Embryoscopic diagnostics of endometrial defects in missed abortion after IVF. Gynecol. Endocrinol. 32 (2), 62–63. doi:10.1080/09513590.2016.1232903
Baba, N., Lengyel, A., Pinti, E., Yapici, E., Schreyer, I., Liehr, T., et al. (2022). Microdeletions in 1q21 and 8q12.1 depict two additional molecular subgroups of Silver-Russell syndrome like phenotypes. Mol. Cytogenet 15 (1), 19. doi:10.1186/s13039-022-00596-z
Borgatta, L., French, A., Vragovic, O., and Burnhill, M. (2000). Early medical abortion with methotrexate. Outcome and satisfaction among women aged 15-20 years. J. Pediatr. Adolesc. Gynecol. 13 (2), 87–88. doi:10.1016/s1083-3188(00)00014-0
Chen, F., Wang, W., Wang, Y., and Wu, Y. (2022). How two-child policy affects China's energy consumption: The mediating role of lifestyle. Front. Public Health 10, 866324. doi:10.3389/fpubh.2022.866324
Cheng, Z., Cheng, D., Li, J., Guo, L., Zhang, W., Zhang, C., et al. (2021). Polymorphisms within DNA double-strand breaks repair-related genes contribute to structural chromosome abnormality in recurrent pregnancy loss. Front. Genet. 12, 787718. doi:10.3389/fgene.2021.787718
Cui, Y., Xiao, J., Zhao, L., Yang, L., Tang, Y., Tao, H., et al. (2021). Prenatal diagnosis and genetic counseling in 19 cases with 22q11.2 microduplications. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 38 (12), 1180–1184. doi:10.3760/cma.j.cn511374-20200921-00679
Dai, R., Xi, Q., Wang, R., Zhang, H., Jiang, Y., Li, L., et al. (2019). Chromosomal copy number variations in products of conception from spontaneous abortion by next-generation sequencing technology. Med. Baltim. 98 (47), e18041. doi:10.1097/MD.0000000000018041
Del Carmen Nogales, M., Bronet, F., Basile, N., Martinez, E. M., Linan, A., Rodrigo, L., et al. (2017). Type of chromosome abnormality affects embryo morphology dynamics. Fertil. Steril. 107 (1), 229–235. doi:10.1016/j.fertnstert.2016.09.019
Delabaere, A., Huchon, C., Lavoue, V., Iraola, E., Nedellec, S., Gallot, V., et al. (2014). Definition of pregnancy losses: Standardization of terminology from the French national College of Obstetricians and Gynecologists (CNGOF). J. Gynecol. Obstet. Biol. Reprod. Paris. 43 (10), 756–763. doi:10.1016/j.jgyn.2014.09.010
Familiari, A., Boito, S., Rembouskos, G., Ischia, B., Accurti, V., Fabietti, I., et al. (2021). Cell-free DNA analysis of maternal blood in prenatal screening for chromosomal microdeletions and microduplications: A systematic review. Prenat. Diagn 41 (10), 1324–1331. doi:10.1002/pd.5928
Fang, J., Xie, B., Chen, B., Qiao, C., Zheng, B., Luan, X., et al. (2018). Biochemical clinical factors associated with missed abortion independent of maternal age: A retrospective study of 795 cases with missed abortion and 694 cases with normal pregnancy. Med. Baltim. 97 (50), e13573. doi:10.1097/MD.0000000000013573
Fu, M., Mu, S., Wen, C., Jiang, S., Li, L., Meng, Y., et al. (2018). Wholeexome sequencing analysis of products of conception identifies novel mutations associated with missed abortion. Mol. Med. Rep. 18 (2), 2027–2032. doi:10.3892/mmr.2018.9201
Gong, G., Yin, C., Huang, Y., Yang, Y., Hu, T., Zhu, Z., et al. (2021). A survey of influencing factors of missed abortion during the two-child peak period. J. Obstet. Gynaecol. 41 (6), 977–980. doi:10.1080/01443615.2020.1821616
Gorelick, R., and Heng, H. H. (2011). Sex reduces genetic variation: A multidisciplinary review. Evolution 65 (4), 1088–1098. doi:10.1111/j.1558-5646.2010.01173.x
Halder, A., and Fauzdar, A. (2006). Skewed sex ratio and low aneuploidy in recurrent early missed abortion. Indian J. Med. Res. 124 (1), 41–50.
He, J., Chang, K., Liu, S., Ji, J., Liu, L., Feng, Y., et al. (2021). Phthalate levels in urine of pregnant women and their associated missed abortion risk. Reprod. Biol. 21 (1), 100476. doi:10.1016/j.repbio.2020.100476
Herkiloglu, D., Gokce, S., and Cevik, O. (2022). Relationship of interferon regulator factor 5 and interferon-gamma with missed abortion. Exp. Ther. Med. 23 (5), 356. doi:10.3892/etm.2022.11283
Hu, H., Yang, H., Yin, Z., and Zhao, L. (2015). Chromosome examination of missed abortion patients. Zhonghua Yi Xue Za Zhi 95 (35), 2837–2840. doi:10.3760/cma.j.issn.0376-2491
Kokkonen, H., Siren, A., Maatta, T., Kamila Kadlubowska, M., Acharya, A., Nouel-Saied, L. M., et al. (2021). Identification of microduplications at Xp21.2 and Xq13.1 in neurodevelopmental disorders. Mol. Genet. Genomic Med. 9 (12), e1703. doi:10.1002/mgg3.1703
Li, G., Jin, H., Niu, W., Xu, J., Guo, Y., Su, Y., et al. (2018). Effect of assisted reproductive technology on the molecular karyotype of missed abortion tissues. Biosci. Rep. 38 (5), BSR20180605. doi:10.1042/BSR20180605
Li, Z. Y., Liu, X. Y., Peng, P., CheNN., , Ou, J., HaoN., , et al. (2018). Role of BoBs technology in early missed abortion chorionic villi. Zhonghua Fu Chan Ke Za Zhi 53 (5), 308–312. doi:10.3760/cma.j.issn.0529-567x.2018.05.005
Liu, S., Han, M., Zhang, J., Ji, J., Wu, Y., and Wei, J. (2022). Interactions between Benzo(a)pyrene exposure and genetic polymorphisms of AhR signaling pathway on missed abortion. Int. J. Environ. Health Res., 1–13. doi:10.1080/09603123.2022.2064436
Long, Q., Zhang, Y., Zhang, J., Tang, X., and Kingdon, C. (2022). Changes in caesarean section rates in China during the period of transition from the one-child to two-child policy era: Cross-sectional national household health services surveys. BMJ Open 12 (4), e059208. doi:10.1136/bmjopen-2021-059208
Mohammad-Akbari, A., Mohazzab, A., Tavakoli, M., Karimi, A., Zafardoust, S., Zolghadri, Z., et al. (2022). The effect of low-molecular-weight heparin on live birth rate of patients with unexplained early recurrent pregnancy loss: A two-arm randomized clinical trial. J. Res. Med. Sci. 27, 78. doi:10.4103/jrms.jrms_81_21
Practice Committee of the American Society for Reproductive, M. (2012). Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 98 (5), 1103–1111. doi:10.1016/j.fertnstert.2012.06.048
Quintero-Ronderos, P., and Laissue, P. (2020). Genetic variants contributing to early recurrent pregnancy loss etiology identified by sequencing approaches. Reprod. Sci. 27 (8), 1541–1552. doi:10.1007/s43032-020-00187-6
Salman, A. F., Alrawi, S., Abdulrahman Hadi, B. A., and Nori, W. (2021). Maternal platelets in missed abortion; from a clinical perspective. J. Pak Med. Assoc. 71 (912), S43–S46.
Segawa, T., Kuroda, T., Kato, K., Kuroda, M., Omi, K., Miyauchi, O., et al. (2017). Cytogenetic analysis of the retained products of conception after missed abortion following blastocyst transfer: A retrospective, large-scale, single-centre study. Reprod. Biomed. Online 34 (2), 203–210. doi:10.1016/j.rbmo.2016.11.005
Shahine, L., and Lathi, R. (2015). Recurrent pregnancy loss: Evaluation and treatment. Obstet. Gynecol. Clin. North Am. 42 (1), 117–134. doi:10.1016/j.ogc.2014.10.002
Shen, J. D., Sun, F. X., Qu, D. Y., Xie, J. Z., Gao, L., Qiu, Q., et al. (2019). Chromosome abnormality rate and related factors of spontaneous abortion in early pregnancy. Zhonghua Fu Chan Ke Za Zhi 54 (12), 797–802. doi:10.3760/cma.j.issn.0529-567x.2019.12.002
Torres-Miranda, M. D., Duro Gomez, J., Pena Lobo-Goncalves, S., De la Torre Gonzalez, A. J., and Castelo-Branco, C. (2022). Intravaginal misoprostol versus uterine curettage for missed abortion: A cost-effectiveness analysis. J. Obstet. Gynaecol. Res. 48 (5), 1110–1115. doi:10.1111/jog.15201
Voorhies, M., Cohen, S., Shea, T. P., Petrus, S., Munoz, J. F., Poplawski, S., et al. (2022). Chromosome-level genome assembly of a human fungal pathogen reveals synteny among geographically distinct species. mBio 13, e0257421. doi:10.1128/mbio.02574-21
Wang, W., Shao, S., Chen, W., Chuai, Y., Li, Y., Guo, Y., et al. (2021). Electrofusion stimulation is an independent factor of chromosome abnormality in mice oocytes reconstructed via spindle transfer. Front. Endocrinol. (Lausanne) 12, 705837. doi:10.3389/fendo.2021.705837
Yang, Y., Wu, J., Wang, X., Yao, J., Lao, K. S., Qiao, Y., et al. (2021). Circulating fibroblast growth factor 21 as a potential biomarker for missed abortion in humans. Fertil. Steril. 116 (4), 1040–1049. doi:10.1016/j.fertnstert.2021.05.098
Yang, Y., Wu, L., Weng, Z., Wu, X., Wang, X., Xia, J., et al. (2021). Chromosome genome assembly of cromileptes altivelis reveals loss of genome fragment in cromileptes compared with Epinephelus species. Genes (Basel). 12 (12), 1873. doi:10.3390/genes12121873
Ye, C. J., Stilgenbauer, L., Moy, A., Liu, G., and Heng, H. H. (2019). What is karyotype coding and Why is genomic Topology important for cancer and evolution? Front. Genet. 10, 1082. doi:10.3389/fgene.2019.01082
Yue, F., Xi, Q., Zhang, X., Jiang, Y., Zhang, H., and Liu, R. (2022). Molecular cytogenetic characterization of 16p11.2 microdeletions with diverse prenatal phenotypes: Four cases report and literature review. Taiwan J. Obstet. Gynecol. 61 (3), 544–550. doi:10.1016/j.tjog.2022.03.027
Zhao, R., Wu, Y., Zhao, F., Lv, Y., Huang, D., Wei, J., et al. (2017). The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: A case control study in Chinese women. Med. Baltim. 96 (51), e9388. doi:10.1097/MD.0000000000009388
Keywords: missed abortion, chromosome abnormality, assisted reproductive technology, advanced age, gene
Citation: Li X, Kang H, Yin H, Liu T, Hou Q, Yu X, Guo Y, Shen W, Ge H, Zeng X, Lu K and Xiong Y (2023) How many missed abortions are caused by embryonic chromosomal abnormalities and what are their risk factors?. Front. Genet. 13:1058261. doi: 10.3389/fgene.2022.1058261
Received: 30 September 2022; Accepted: 08 December 2022;
Published: 04 January 2023.
Edited by:
Henry H. Heng, Wayne State University, United StatesCopyright © 2023 Li, Kang, Yin, Liu, Hou, Yu, Guo, Shen, Ge, Zeng, Lu and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kangmu Lu, a2FuZ21vbHVAcXEuY29t; Ying Xiong, NDY5NzYxNDQ3QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.