- 1Department of Genetics and Genomics, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
- 2Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates
- 3Department of Pediatrics, Tawam Hospital, Al Ain, United Arab Emirates
- 4Division of Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom
We reported a 22-year-old Emirati male with autosomal recessive primary hypertrophic osteoarthropathy caused by a possibly pathogenic homozygous non-synonymous variant in the SLCO2A1 gene (NM_005630.3: c.289C>T, p. Arg97Cys) presenting with joint swelling, forehead furrowing, and significant clubbing in all fingers and toes. Currently, no standard treatments are approved for this disease; medical care is palliative and includes non-steroidal anti-inflammatory drugs, corticosteroids, tamoxifen, retinoids, and risedronate. Colchicine may be helpful for the pain due to subperiosteal new bone formation. Our patient was treated with etoricoxib 60 mg once daily and showed a significant clinical improvement at the 6-month mark that was reversed upon the withdrawal of this medication. This case report highlights the importance of placing etoricoxib among first-line therapy recommendations for cases with confirmed primary hypertrophic osteoarthropathy diagnosis. To the best of our knowledge, this is the only case of primary hypertrophic osteoarthropathy from the Middle Eastern population of Arab ethnicity that has responded to non-steroidal anti-inflammatory drug therapy.
Introduction
Hypertrophic osteoarthropathy (PHO) is an orphan syndrome characterized by abnormal proliferation of the skin and osseous tissues at the distal parts of the extremities. The main clinical features are a peculiar bulbous deformity of the tips of the digits conventionally described as “clubbing,” periosteal proliferation of the tubular bones, and synovial effusions (Martínez-Lavín, 2020). PHO is classified as either primary or secondary, as shown in the following paragraphs. Secondary PHO is more common and always associated with a primary cause (such as cardiac or pulmonary disease), while the primary form of PHO is a rare genetic disease and is further classified based on the range of tissues involved and divided into three subtypes: 1) the complete form, presenting with pachydermia, digital clubbing, and periostosis; 2) the fruste form, with pachydermia and minimal skeletal changes; and 3) the incomplete form, with periostosis and no pachydermia (Zhang et al., 2013).
PHO is characterized by digital clubbing, which is present in almost all cases, arthralgia, or arthritis, commonly affecting the knee, the ankle, and the wrist present in about 20–40% of cases, and periostosis of long bones (Zhang et al., 2013). There is gradual thickening and furrowing of the skin on the face and scalp, resulting in prominent forehead skin folds (cutis verticis gyrata), seborrhea, and blephartosis due to sebaceous gland hypertrophy, and hyperhidrosis due to hypertrophy of sweat glands (Zhang et al., 2013). The precise incidence and prevalence of PHO is still unknown (Zhang et al., 2013). The age of the disease onset has a bimodal distribution, peaking during the first year of life and at puberty. Males are affected predominantly more than females with a ratio of 9:1 (Diggle et al., 2012). Both autosomal recessive (PHOAR) and autosomal dominant (PHOAD) forms of PHO have been described in the literature (Li et al., 2017). Genetic studies have identified the impaired prostaglandin (PGE2) metabolism as the leading cause of PHO, specifically due to biallelic mutations in the HPGD gene (MIM*606188) encoding 15-hydroxyprostaglandin dehydrogenase (15-PGDH) or the SLCO2A1 gene (MIM*601460) encoding the prostaglandin transporter (PGT) (Li et al., 2017). Monoallelic mutations in SLCO2A1 were also reported to cause PHOAD with incomplete penetrance in heterozygous carriers.
Here, we present the first Emirati patient with a confirmed diagnosis of PHOAR who was treated successfully with etoricoxib for 6 months and showed clinical improvement in symptoms. This report adds more evidence to the existing literature of using etoricoxib as a treatment of choice for PHO disease in different ethnicities.
Case presentation
A 22-year-old male presented complaints of symmetrical pain in his wrists, hand proximal interphalangeal (PIP) joints, ankles, and feet. This was associated with joint swelling, furrowing of the forehead, and significant clubbing in all fingers and toes. The patient denies any family history of similar symptoms or parent relatedness (Figure 1A). Immunological work-up was negative including the anti-nuclear antibody, rheumatoid factor, anticyclic citrullinated peptide, HLA-B27, and a double-stranded DNA. The C-reactive protein was 15.44 mg/L. Magnetic resonance imaging (MRI) of the right ankle and foot showed an ankle effusion with a healing sprain of the posterior inferior tibiofibular ligament. There were effusions noted in the calcaneocuboid, naviculocuneiform, first tarsometatarsal (TMT), first metatarsophalangeal (MTP) joints, and the second–fifth distal interphalangeal (IP) joints and associated non-specific multifocal marrow edema. The subcortical edema and hyperostosis with mild periostitis of the distal tibia and fibula were concerning for an underlying metabolic bone problem.
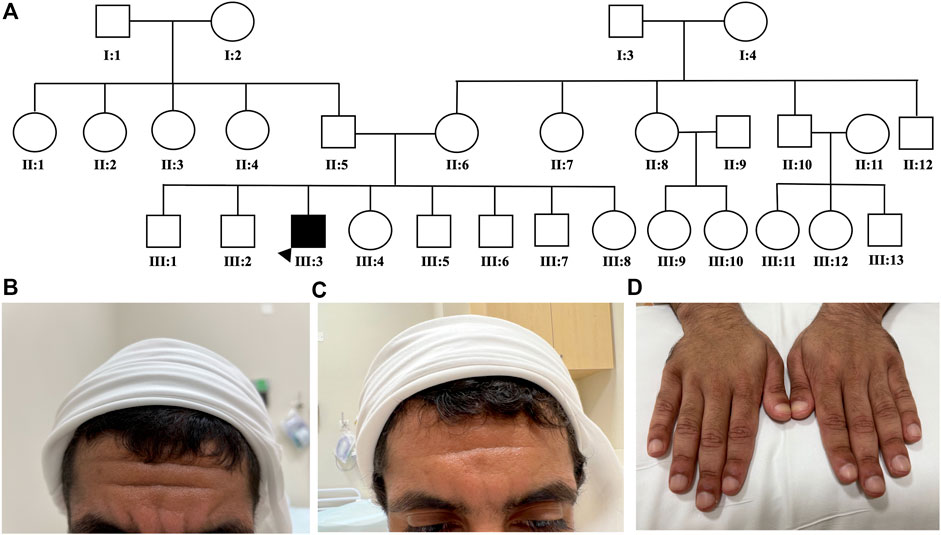
FIGURE 1. Patients’ family pedigree and characteristics. Patient’s family pedigree showing a negative history of similar conditions (A). Severity of patient’s forehead furrowing is shown before (B), and after (C) etoricoxib treatment. A significant improvement in digital clubbing after etoricoxib treatment was observed (D).
The initial working diagnosis was seronegative rheumatoid arthritis, and the patient was treated with intramuscular Kenalog injection (triamcinolone acetonide, 40 mg/ml). However, the patient denied any improvement of his joint pain or swelling and was non-compliant to the methotrexate 12.5-mg tablets weekly that he was commenced on. The patient was seen by several physicians. Two years later, the patient was re-referred to rheumatology to assess for possible inflammatory arthritis after presenting with ongoing arthralgias. Autoimmune serology was negative with a mildly elevated erythrocyte sedimentation rate of 29 and a C-reactive protein of 19.13 mg/L. On musculoskeletal examination, the tender and swollen joint counts were 4/28 (bilateral ankles and knees). An ultrasound of bilateral ankles and knees showed no active synovitis or erosions. Clubbing with red periungual discoloration on all the patient’s fingers and toes was noted. A dermatological examination showed peeling on the lateral aspects of the soles of the feet but no evidence of psoriasis, mucocutaneous or skin ulcers, livedo, malar, or discoid rash. Due to the clinical picture, the lack of synovitis on joint ultrasounds, and the lack of response to IM corticosteroids, the top differential diagnosis was PHO.
Genetic investigations
Using whole-exome sequencing and unbiased mutational analysis of rare (<1%MAF) pathogenic variants in coding and splicing regions, we detected a homozygous variant on chromosome 3 (GRCh37, Chr3:133692615G>A) that is not found in a homozygous state in gnomAD genomes or exomes, residing in a strongly conserved position. This rare variant is predicted to be a likely pathogenic non-synonymous substitution in exon 3 of the SLCO2A1 gene (NM_005630.3: c.289C>T, p. Arg97Cys) by 10 pathogenic predictors, namely, BayesDel_addAF, DANN, DEOGEN2, EIGEN, FATHMM-MKL, LIST-S2, M-CAP, MutationAssessor, MutationTaster, and SIFT in VarSome (http://varsome.com). Additionally, this variant was reported as a second hit in a patient with PHO (Li et al., 2017). No pathogenic variant was detected in the HPGD gene.
Treatment and follow-up
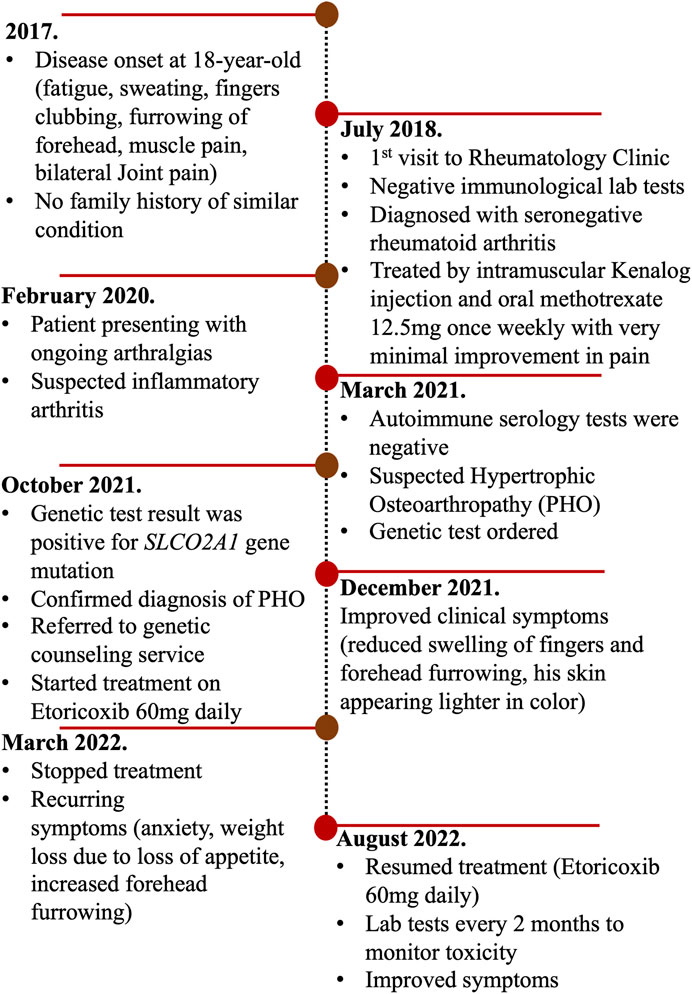
After establishing the diagnosis of primary hypertrophic osteoarthropathy, we reviewed the literature and identified a handful of reports proposing etoricoxib as a treatment for PHO disease (Table 1). We started the patient on a trial of etoricoxib 60 mg once daily, and a clinical improvement was observed within 2 months of treatment on the skin color, sweating, clubbing, weight loss, joint pain, and forehead furrowing, as shown in Figures 1B–D. Treatment with etoricoxib over 6 months was well tolerated, and the patient denied any gastric upset, esophageal reflux symptoms, or diarrhea. The patient and his family were happy with the response to treatment. He also exhibited increased energy levels and self-esteem, and the overall quality of life has improved (Supplementary Table S1). He developed recurring symptoms after discontinuing treatment, which included fatigue, weight loss, clubbing, joint pain, sweating, and furrowing. Six months later, the patient was retreated with the same medication and the same dose (etoricoxib 60 mg once daily) and showed a remarkable improvement in symptoms. To assess treatment safety, regular laboratory tests were conducted every 3 months including a renal function test (eGFR and creatinine), liver enzymes (ALT and AST), and basic metabolic panel. A timeline of the patient’s journey and care was designed to provide a simple and interactive way to review the patient’s medical history using a time-based visualization (Figure 2).
Discussion
We reported a patient exhibiting PHOAR2 (MIM#614441) caused by biallelic mutations in the SLCO2A1 gene, leading to a defect in the PGE2 metabolism pathway (Zhang et al., 2012). PGE2 is a lipid molecule that promotes hormone-like effects in the body. Derived from arachidonic acid in the cellular membrane, the production of PGE2 is dependent on the cyclooxygenase enzymes 1 and 2 (COX1/2), often elevated in regions of inflammation. Therefore, circulating levels are very low in healthy individuals. PGE2 inactivation, including a cellular uptake by PGT and degradation by 15-PGDH, is important to control bioactive prostaglandin levels at a physiological range. Studies indicated that PHO patients have an elevated circulating PGE2 level and urinary level (Zhang et al., 2012). It has been suggested that increased levels of PGE2 might be responsible for the secondary overexpression of the vascular endothelial growth factor (VEGF) (Martínez-Lavín, 2020). PGE2 induces transcription of the VEGF in osteoblasts and stimulates bone formation, activates endothelial cells that increase transcription of VEGF promoting local angiogenesis, increases capillary permeability, and promotes stimulation and migration of osteoblasts, new bone formation, and edema, all of which account for the characteristic findings in PHO (Zhang et al., 2013), as shown in Figure 3.
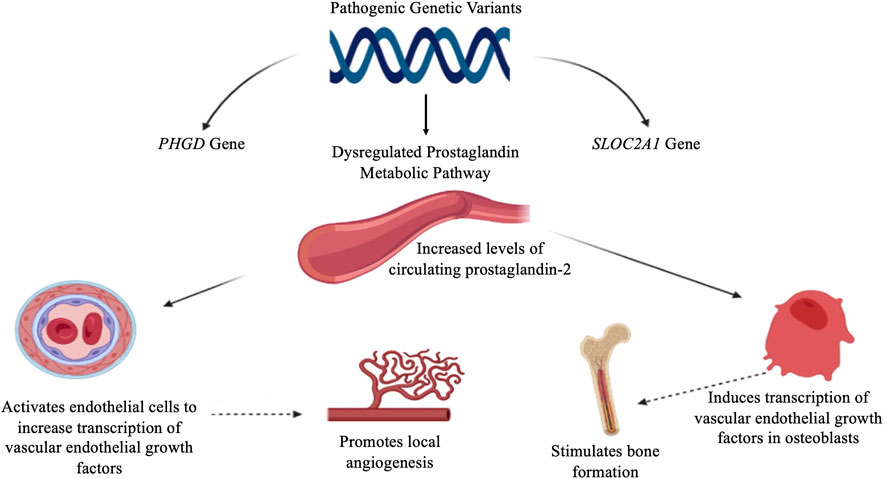
FIGURE 3. Proposed pathophysiology of primary hypertrophic osteoarthropathy. PGE2 induces the transcription of VEGF in osteoblasts and promotion of endothelial cells, leading to local angiogenesis and a new bone formation. PGE2: prostaglandin E2, VEGF: vascular endothelial growth factor.
Currently, no standard treatment is approved for PHO, and there are no clinical trials except some case reports with varying therapeutic responses (Shakya et al., 2018) (Li et al., 2017). Given the accepted pathophysiology of PGE2-driven overproduction of VEGF, COX-2 inhibitors [non-steroid anti-inflammatory drugs (NSAIDs)], monoclonal anti-VEGF antibodies, steroids, colchicine, tamoxifen, retinoids, and risedronate to alleviate the painful polyarthritis/osteoarthropathy, as well as bisphosphonates (a potent inhibitor of osteoclastic bone resorption), are currently the treatments of choice (Zhang et al., 2013).
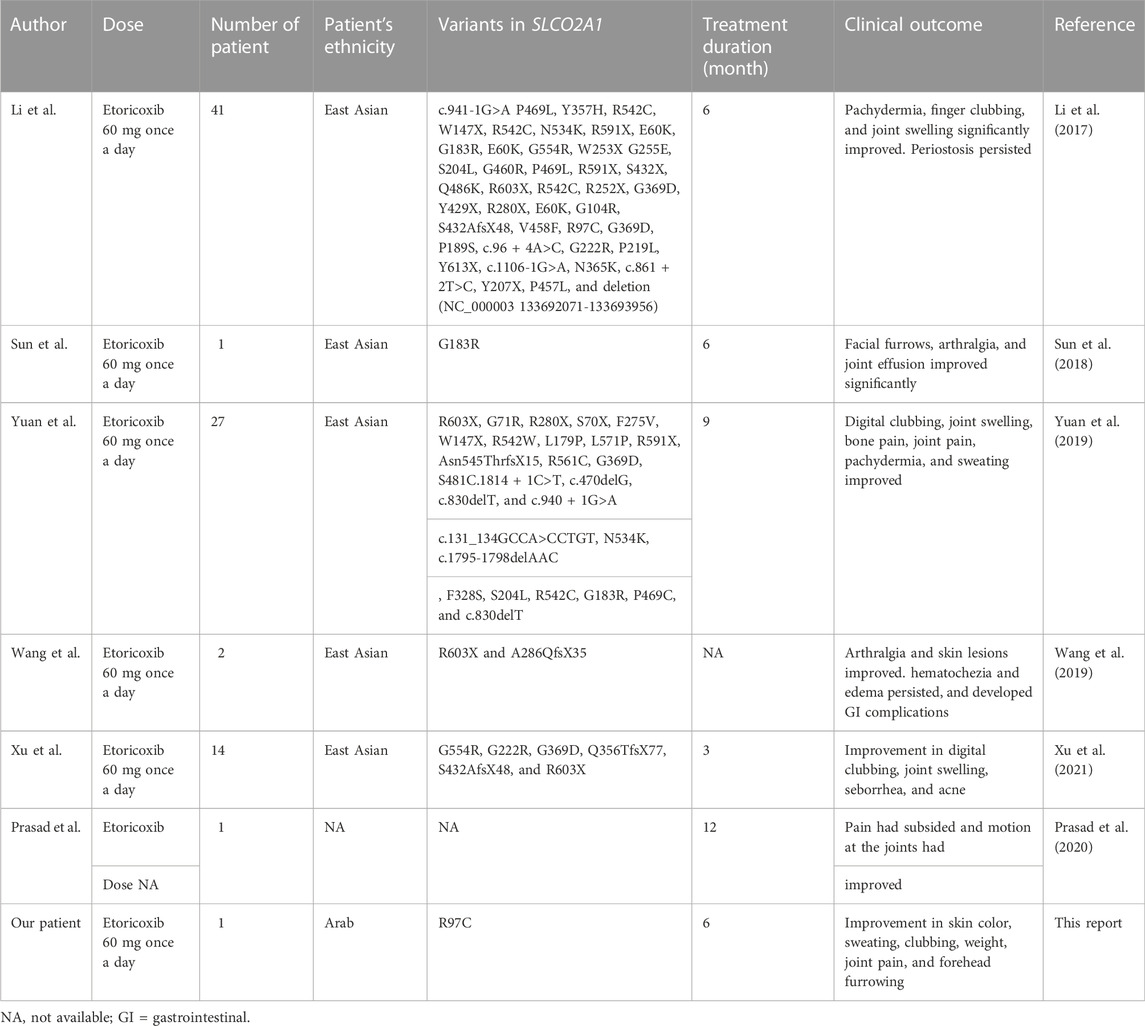
There have been various case reports of a symptomatic relief of PHO with the use of bisphosphonates (pamidronate or zoledronic acid) (Jayakar et al., 2011). Steroids can produce hematological improvement but have no effects on the skin or digital clubbing (Giancane et al., 2015). NSAIDs appear to be the best option for improving the musculocutaneous manifestations. Indeed, a systematic review for using NSAIDs among PHO patients concluded that NSAIDs are effective in reducing the arthralgia or arthritis symptoms among PHO patients (Shakya et al., 2018). Etoricoxib is a selective COX-2 inhibitor, approved in Europe for the symptomatic treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and acute gouty arthritis, with the potential advantages of convenient once-daily administration and superior gastrointestinal tolerability compared with traditional NSAIDs (Escudero-Contreras et al., 2007). We reviewed the literature and studies that mentioned etoricoxib as treatment for PHO disease and are listed in table 1. Li et al. reported the effect of treatment with etoricoxib on seven patients with PHOAR1 (MIM#259100) and 33 patients with PHOAR2 (Li et al., 2017). A significant reduction in urinary PGE2 levels was observed in the majority of patients during the 6 months of treatment (Li et al., 2017). Moreover, there was a notable improvement in pachydermia, finger clubbing, and joint swelling. However, there was no visible evidence of a positive effect of etoricoxib on periostosis. Xu et al. reviewed 3-month etoricoxib clinical trial results on four patients with PHOAD (MIM#167100) and 33 patients with PHOAR2 and observed an effective response similar to those reported previously by Li et al. (Xu et al., 2021). Additionally, Yuan et al. conducted a single-arm intervention trial to treat 27 PHO patients with etoricoxib 60 mg once daily for a period of 9 months and found that most patients have symptoms improvement during the course of treatment including digital clubbing, joint swelling, bone and joint pain, pachydermia, and sweating (Yuan et al., 2019). However, in that study, one patient discontinued the treatment after 12 months of therapy and developed recurrent symptoms including pachydermia and joint swelling within 1 month.
Consistent with the literature, our patient also responded to etoricoxib treatment for 6 months and documented a notable clinical symptom improvement including the skin, furrowing, diaphoresis, weight loss, and digital clubbing. We also showed the effect of discontinuation of the etoricoxib treatment after the 6-month treatment period on the patient who developed recurrent symptoms. This report adds to the literature as most of the reported patients so far were of East Asian ethnicity, while our Emirati patient is of Arab ethnicity.
Having said that, there are potential side effects for etoricoxib treatment as with all NSAIDs (Reginster et al., 2007), yet the potential gastrointestinal (GI) and cardiovascular risks of treatment with etoricoxib should be weighed against the potential benefits in individual patients. In this context, several clinical trials have been carried out to assess the efficacy and safety of etoricoxib use in chronic conditions, such as osteoarthritis (OA), for example, a study conducted in 617 patients with OA treated with etoricoxib at doses ranging from 30 to 90 mg over 52 weeks demonstrated etoricoxib clinical efficacy in patients with OA and was generally well tolerated (Curtis et al., 2005). Another clinical trial in OA patients treated with etoricoxib 60 mg once daily over a 138-week treatment period demonstrated a long-term clinical efficacy of etoricoxib for the treatment of OA (Reginster et al., 2007).
In summary, etoricoxib is an important treatment option for the management of PHO disease with demonstrated efficacy and safety. Further studies should be conducted to assess the long-term impact of etoricoxib on PHO.
Data availability statement
The datasets presented in this article are not readily available because of federal law restrictions within the UAE. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Abu Dhabi Health Services Co., Abu Dhabi, United Arab Emirates. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AA assisted in data collection and wrote the draft manuscript; MA collected clinical data; SD and FA carried out the clinical assessment and follow-up of the patient and reviewed the manuscript; and NA assisted in data collection, analysis of laboratory results, and wrote and reviewed the final manuscript.
Funding
This project is funded by the United Arab Emirates University (grant number 12M106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.1053999/full#supplementary-material
References
Curtis, S. P., Bockow, B., Fisher, C., Olaleye, J., Compton, A., Ko, A. T., et al. (2005). Etoricoxib in the treatment of osteoarthritis over 52-weeks: A double-blind, active-comparator controlled trial [NCT00242489]. BMC Musculoskelet. Disord. 6, 58. doi:10.1186/1471-2474-6-58
Diggle, C. P., Parry, D. A., Logan, C. V., Laissue, P., Rivera, C., Restrepo, C. M., et al. (2012). Prostaglandin transporter mutations cause pachydermoperiostosis with myelofibrosis. Hum. Mutat. 33 (8), 1175–1181. doi:10.1002/humu.22111
Escudero-Contreras, A., Cervantes, J. V. M., and Collantes-Estevez, E. (2007). Update on the clinical pharmacology of etoricoxib, a potent cyclooxygenase-2 inhibitor. Future Rheumatol. 2 (6), 545–565. doi:10.2217/17460816.2.6.545
Giancane, G., Diggle, C. P., Legger, E. G., Tekstra, J., Prakken, B., Brenkman, A. B., et al. (2015). Primary hypertrophic osteoarthropathy: An update on patient features and treatment. J. Rheumatol.Journal Rheumatology 42 (11), 2211–2214. doi:10.3899/jrheum.150364
Jayakar, B. A., Abelson, A. G., and Yao, Q. (2011). Treatment of hypertrophic osteoarthropathy with zoledronic acid: Case report and review of the literature. Semin. Arthritis Rheum. 41 (2), 291–296. doi:10.1016/j.semarthrit.2011.01.007
Li, S. S., He, J. W., Fu, W. Z., Liu, Y. J., Hu, Y. Q., and Zhang, Z. L. (2017). Clinical, biochemical, and genetic features of 41 han Chinese families with primary hypertrophic osteoarthropathy, and their therapeutic response to etoricoxib: Results from a six-month prospective clinical intervention. J. Bone Min. Res. 32 (8), 1659–1666. doi:10.1002/jbmr.3157
Martínez-Lavín, M., and Paiva, E. S. (2020). Hypertrophic osteoarthropathy best practice and research: Clinical rheumatology. Best. Pract. Res. Clin. Rheumatol. 34 (3), 101561. doi:10.1016/j.berh.2020.101561
Prasad, A., Shahi, P., Sehgal, A., and Bhagirathi Mallikarjunaswamy, M. (2020). Incomplete primary hypertrophic osteoarthropathy. BMJ Case Rep. 13 (5), e236034. doi:10.1136/bcr-2020-236034
Reginster, J. Y., Malmstrom, K., Mehta, A., Bergman, G., Ko, A. T., Curtis, S. P., et al. (2007). Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomised studies of patients with osteoarthritis. Ann. Rheum. Dis. 66 (7), 945–951. doi:10.1136/ard.2006.059162
Shakya, P., Pokhrel, K. N., Mlunde, L. B., Tan, S., Ota, E., and Niizeki, H. (2018). Effectiveness of non-steroidal anti-inflammatory drugs among patients with primary hypertrophic osteoarthropathy: A systematic review. J. Dermatol. Sci. 90 (1), 21–26. doi:10.1016/j.jdermsci.2017.12.012
Sun, F., Guo, L., and Ye, S. (2018). Primary hypertrophic osteoarthropathy with SLCO2A1 mutation in a Chinese patient successfully treated with etoricoxib. J. Clin. Rheumatol. 24 (3), 164–167. doi:10.1097/RHU.0000000000000647
Wang, Q., Li, Y. H., Lin, G. L., Li, Y., Zhou, W. X., Qian, J. M., et al. (2019). Primary hypertrophic osteoarthropathy related gastrointestinal complication has distinctive clinical and pathological characteristics: Two cases report and review of the literature. Orphanet J. Rare Dis. 14 (1), 297. doi:10.1186/s13023-019-1264-5
Xu, Y., Zhang, Z., Yue, H., Li, S., and Zhang, Z. (2021). Monoallelic mutations in SLCO2A1 cause autosomal dominant primary hypertrophic osteoarthropathy. J. Bone Min. Res. 36 (8), 1459–1468. doi:10.1002/jbmr.4310
Yuan, L., Liao, R. X., Lin, Y. Y., Jiang, Y., Wang, O., Li, M., et al. (2019). Safety and efficacy of cyclooxygenase-2 inhibition for treatment of primary hypertrophic osteoarthropathy: A single-arm intervention trial. J. Orthop. Transl. 18, 109–118. doi:10.1016/j.jot.2018.10.001
Zhang, Z., Xia, W., He, J., Zhang, Z., Ke, Y., Yue, H., et al. (2012). Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am. J. Hum. Genet. 90 (1), 125–132. doi:10.1016/j.ajhg.2011.11.019
Keywords: autosomal recessive primary hypertrophic osteoarthropathy, SLCO2A1 gene, etoricoxib, PGE2, digital clubbing
Citation: Albawa'neh A, Al Mansoori MG, Diab S, Al Jasmi F and Akawi N (2022) Etoricoxib as a treatment of choice for patients with SLCO2A1 mutation exhibiting autosomal recessive primary hypertrophic osteoarthropathy: A case report. Front. Genet. 13:1053999. doi: 10.3389/fgene.2022.1053999
Received: 26 September 2022; Accepted: 28 November 2022;
Published: 13 December 2022.
Edited by:
Rajendra Awasthi, University of Petroleum and Energy Studies, IndiaReviewed by:
Emilia Severin, Carol Davila University of Medicine and Pharmacy, RomaniaEmanuele Micaglio, IRCCS San Donato Polyclinic, Italy
Copyright © 2022 Albawa'neh, Al Mansoori, Diab, Al Jasmi and Akawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatma Al Jasmi, YWxqYXNtaWZAdWFldS5hYy5hZQ==; Nadia Akawi, bmFkaWEuYWthd2lAdWFldS5hYy5hZQ==
 Areej Albawa'neh1
Areej Albawa'neh1 Fatma Al Jasmi
Fatma Al Jasmi Nadia Akawi
Nadia Akawi