- Department of Hematology, Tohoku University Graduate School of Medicine, Sendai, Japan
Natural killer (NK) cells are the first lymphocytes reconstituted after allogenic hematopoietic stem cell transplantation (HSCT). Especially, in cord blood transplantation (CBT), the increase in the number of NK cells is sustained for a long period. Although there are conflicting results, many studies show that early reconstitution of NK cells is associated with favorable CBT outcomes, suggesting that maximizing NK cell functions could improve the CBT outcome. Killer immunoglobulin-like receptors (KIRs) include inhibitory and stimulatory receptors, which can regulate NK-cell activity. Because some of the KIRs have HLA class I as their ligand, the KIR—ligand interaction on NK cells can be lost in some cases of CBT, which results in the activation of NK cells and alters HSCT outcome. Thus, effects of KIR–ligand mismatch under various conditions have been widely examined; however, the results have been controversial. Among such studies, those using the largest number of CBTs showed that HLA—C2 (KIR2DL1—ligand) mismatches have a favorable effect on the relapse rate and overall survival only when the CBT used methotrexate for graft-versus-host disease prophylaxis. Another study suggested that KIR—ligand mismatch is involved in reducing the relapse of acute myeloid leukemia, mediated by reactivation of cytomegalovirus. These results indicate that activation of NK cells by KIR—ligand mismatch may have favorable effects on CBT outcomes and could help enhance the NK-cell function.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a widely used treatment for relapsed or refractory hematological malignancies. HSCT outcomes have gradually been improving, however they are not yet satisfactory (Auletta et al., 2021), and the main reason for the failure of HSCT is disease relapse (Auletta et al., 2021). Various attempts have been made to reduce relapses after HSCT (Mawad et al., 2013; Yafour et al., 2017; Lee et al., 2019; Kreidieh et al., 2022). In acute myeloid leukemia (AML), the effects of the prophylactic use of donor lymphocyte infusions (DLI) or small molecule compounds, such as tyrosine kinase inhibitors and hypomethylating agents, have been examined and their efficacies have been reported (Burchert et al., 2020; Wei et al., 2020; Xuan et al., 2020). Although these treatments considerably improved HSCT outcomes, their efficacy is limited for some patients and cannot sufficiently reduce the relapse, warranting further development.
Recently, there have been remarkable developments in the treatment of hematological malignancies, mediated by immunological mechanisms, such as bispecific antibodies (Ma et al., 2021) and chimeric antigen receptor T cells (June and Sadelain, 2018). HSCT is one of the immunotherapies that utilizes the strong immune reaction of donor lymphocytes against a patient’s tumor cells. This reaction is called the graft-versus-leukemia effect (GVL effect) (Dickinson et al., 2017; Barisic and Childs, 2022). However, the GVL effect is closely associated with graft-versus-host disease (GVHD), which increases treatment-related mortality due to injury to normal organs (Negrin, 2015). To enhance the GVL effect, DLI has historically been used to treat or prevent post-transplant relapse, although it is associated with the risk of exacerbating GVHD and the benefits of DLI are still under investigation (de Lima et al., 2014; Orti et al., 2017). Under these circumstances, the use of natural killer (NK) cells has been suggested as another promising strategy to elicit GVL effects without exacerbating GVHD (Orti et al., 2017).
To date, the effects of NK cells on HSCT outcomes have mainly been evaluated with regard to two aspects: NK-cell reconstitution and potential interaction between receptors on NK cells and their ligands. Many studies have demonstrated that early NK-cell reconstitution after HSCT results in improved outcomes (Bühlmann et al., 2011; Minculescu et al., 2016; Bejanyan et al., 2018; Ando et al., 2020). The receptor–ligand interaction effect of NK cells after HSCT has predominantly been examined using models that assume an interaction between the killer-immunoglobulin like receptor (KIR) and its ligand (Ruggeri et al., 2007, 2002; Hsu et al., 2006; Cooley et al., 2009; Symons et al., 2010). Although there is some controversy, a few reports suggest that NK-cell activation mediated by KIR has favorable effects on HSCT. Such effects associated with NK cells have mostly been examined in bone marrow (BMT) or peripheral blood stem cell (PBSCT) transplantation, and studies on cord blood transplantation (CBT) are relatively scarce. However, previous studies have demonstrated fast and sustained increases in the number of NK cells and suppression of T-cell functions after CBT compared with that after BMT and PBSCT (Ando et al., 2020), suggesting that NK cells may play a more important role in CBT than in HSCT with other donor sources. This review, therefore, focuses on CBT and summarizes the available data on the role for NK cells.
Effects of NK-cell reconstitution in CBT
The kinetics of NK-cell reconstitution have been examined in various types of HSCTs and compared among donor sources. The NK cells are the lymphocyte subset that is reconstituted most quickly after HSCT (Fry and Mackall, 2005; Ogonek et al., 2016). In CBT, NK-cell recovery at 1 month post-transplantation was almost the same or higher than in BMT and PBSCT. However, in CBT, the NK cells continue to increase and high NK-cell counts persist for approximately 1 year, whereas such predominance of NK cells is not noted in the BM or PBSCT (Rénard et al., 2011; Jacobson et al., 2012; Kanda et al., 2012; Bartelink et al., 2013; Bejanyan et al., 2018; Hattori et al., 2018; Ando et al., 2020). In contrast to the NK cells, the recovery of the T cells in CBT was delayed when compared to that in BMT or PBSCT. The reason for the sustained increase in the number of NK cells is not clear, but it may be associated with the slow T-cell recovery, in view of the fact that the presence of T cells impairs the function and proliferation of NK cells after HSCT (Lowe et al., 2003; Cooley et al., 2005). The NK cells that were reconstituted after CBT showed immature phenotypes that initially expressed NKG2A and bright CD56 (Nguyen et al., 2017). Approximately 3 months after CBT, mature phenotypes with CD16 and CD56dim expression were obtained, which have high cytolytic activity (Tanaka et al., 2009; Kanda et al., 2012; Ando et al., 2020).
The association between NK-cell recovery and HSCT outcomes has been examined in many studies (Dunbar et al., 2008; Bühlmann et al., 2011; Bartelink et al., 2013; Pical-Izard et al., 2015; Minculescu et al., 2016; Nguyen et al., 2017; Bejanyan et al., 2018; Hattori et al., 2018; Russo et al., 2018; Willem et al., 2019; Ando et al., 2020; Zhao et al., 2022). Among these studies, those including CBT cases are shown in Table 1 (Bartelink et al., 2013; Nguyen et al., 2017; Bejanyan et al., 2018; Hattori et al., 2018; Ando et al., 2020). In two of these studies, the effect of NK-cell recovery could not be detected, whereas in others, favorable impact on HSCT outcomes was observed. This discrepancy may be due to differences in patient background or in the transplantation methods. In addition, Ando et al. (2020) reported that only increases in the NK cell subpopulation, but not in the NK cell bulk population, has significant effects. These results, such as improvement in progression-free survival and relapse rate, suggest that NK cells are responsible for the antitumor effects during the period of reduced T cell function after CBT.
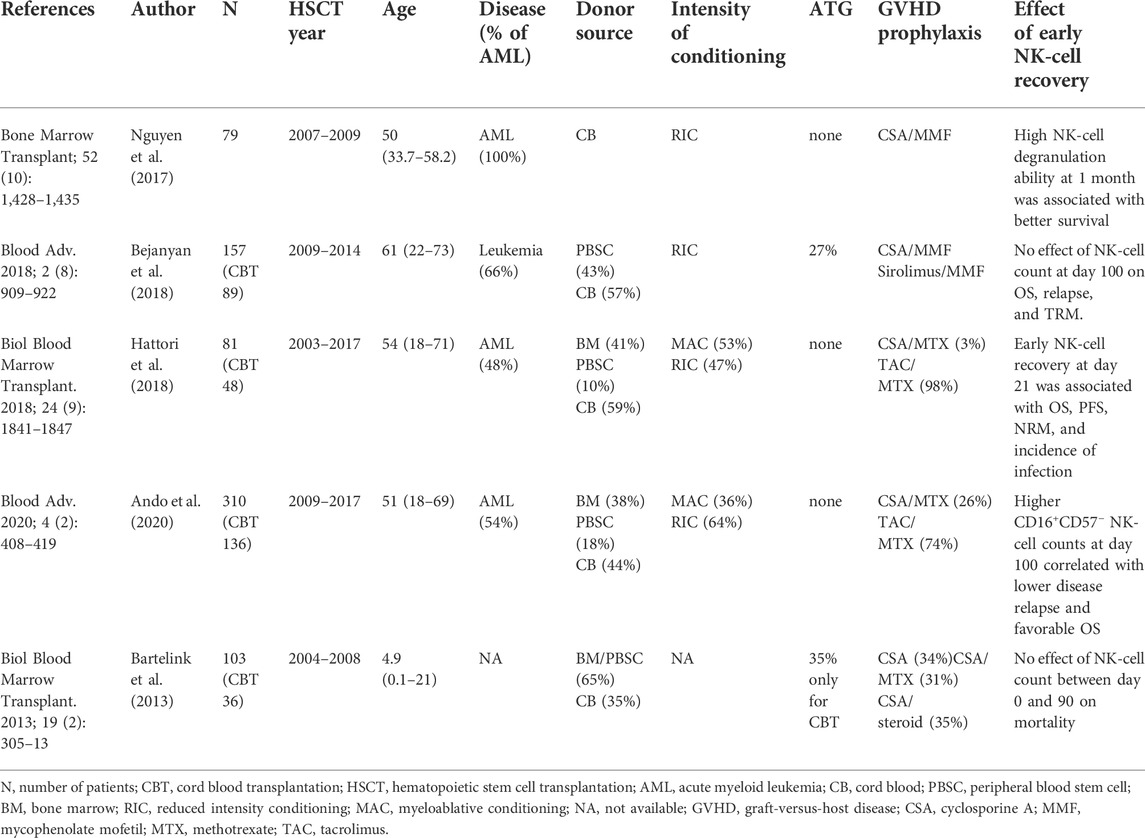
TABLE 1. Effects of early natural killer (NK)-cell recovery in the cohort that includes patients undergoing cord blood transplantation.
NK-cell activation models mediated by the interaction between KIR and its ligand
KIR is one of the major receptors involved in regulating the activity and differentiation of NK cells (Leung, 2011; Dubreuil et al., 2021) There are two major subtypes of KIR—stimulatory and inhibitory receptors. Some KIRs can recognize HLA class I as a ligand. Among these KIRs, KIR2DL1/2DS1, KIR2DL2/3, KIR3DL1, and KIR3DL2 have been well examined (Leung, 2011). Although there are a few exceptions, KIR2DL1/2DS1 recognizes HLA-C with Lys80 (group C2) and KIR2DL2/3 recognizes HLA-C with Asp80 (group C1). Similarly, KIR3DL1 recognizes HLA-Bw4 and KIR3DL2 recognizes HLA-A3/A11. In certain settings of HLA-mismatched HSCT, interactions between KIR and its ligand can be altered, resulting in the activation of NK cells.
The effect of KIR-mediated NK-cell activation on HSCT outcomes was first examined by Ruggeri et al. (2007), Ruggeri et al. (2002), and Ruggeri et al. (1999). In their haploidentical transplantation analysis, they demonstrated that the presence of KIR2DL1, KIR2DL2/3, and KIR3DL1 ligand mismatch (HLA-C1, C2, and Bw4 mismatch) between the donor and recipient could result in the loss of KIR-mediated NK-cell suppression and could activate NK-cell function, which improves HSCT outcomes. Furthermore, they also examined the effects in mouse models and showed that the involvement of NK cells in reducing disease relapse, GVHD, and in improving engraftment had favorable effects on HSCT (Ruggeri et al., 2002). The KIR—ligand mismatch model was then verified in various settings of the HSCT cohort. However, the results were controversial and the favorable effects were suggested to be predominantly associated with T-cell depleted haploidentical transplantation (Giebel et al., 2003; Farag et al., 2006; Morishima et al., 2007; Sun et al., 2007).
Other than the KIR–ligand mismatch model, which does not consider the donor KIR genotype, some NK-cell activation models mediated by KIR have also been proposed. The receptor—ligand model considers the matching between the donor inhibitory KIR genotype and the recipient KIR ligand type (Leung et al., 2004; Hsu et al., 2005). Hsu et al. (2005) analyzed the T-cell depleted BMT cases with the receptor–ligand model and found that patients who did not have one or more ligand for donor KIR showed superior disease free and overall survival. Similar to this receptor–ligand model, a missing-ligand model that only considers the recipient’s KIR-ligand interaction was also reported. If the patient’s KIR-ligand group is homozygous, such as HLA-C1/C1 or C2/C2, inhibitory KIR without the ligand would facilitate NK-cell activation in contrast to the heterozygous group (Hsu et al., 2006; Miller et al., 2007; Arima et al., 2018).
There is another well-tested model that considers the presence of the donor’s stimulatory KIR. The KIR gene cluster is located on chromosome 19 and its haplotypes are classified into two major groups (Leung, 2011; Dubreuil et al., 2021). Haplotype group A have only one stimulatory KIR, KIR2DS4, in addition to the inhibitory KIRs, whereas group B have several other stimulatory KIRs. This suggests that donors with the group B haplotype (A/B or B/B) are more susceptible to NK-cell activation than donors with only the group A haplotype (A/A). The benefit of the KIR haplotype B donor was first demonstrated by Cooley et al. (2009). They analyzed myeloablative HLA-matched BMT/PBSCT and found improved relapse-free survival rates in patients undergoing transplantation with the KIR B haplotype donor. The effect of this KIR B haplotype has also been examined in other studies (Cooley et al., 2010; Zhou et al., 2014) Recently, the KIR-mediated NK-cell activation model was evaluated, taking into account genetic polymorphisms of KIR and HLA (Gagne et al., 2009; Boudreau et al., 2017; Shaffer et al., 2021).
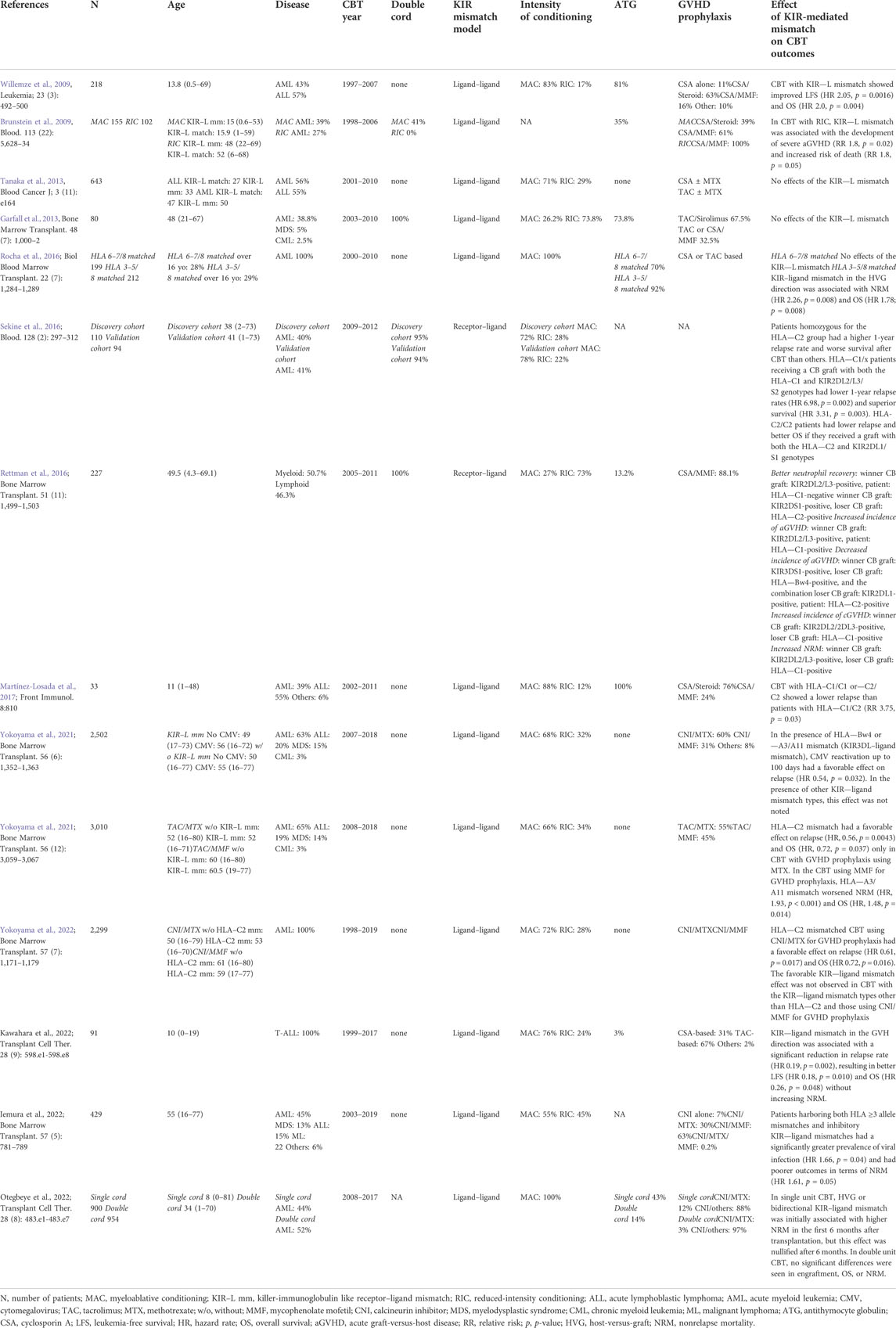
Effects of KIR–ligand mismatch on CBT
In CBT, the early recovery of NK cells and their long-term sustained increase has been reported, along with delayed recovery of T-cell function (Jacobson et al., 2012; Kanda et al., 2012; Bejanyan et al., 2018; Hattori et al., 2018; Ando et al., 2020). Based on these findings, theoretically, it is assumed that NK-cell function may be more clearly detected in CBT than in BMT/PBSCT. As of date, the effects of KIR–ligand interaction models in CBT have been examined in several studies, albeit not sufficiently, compared with those in BMT/PBSCT (Brunstein et al., 2009; Willemze et al., 2009; Garfall et al., 2013; Tanaka et al., 2013; Rettman et al., 2016; Rocha et al., 2016; Sekine et al., 2016; Martínez-Losada et al., 2017). As shown in Table 2, some results suggest an improvement in CBT outcomes (Willemze et al., 2009), whereas others show no effect (Tanaka et al., 2013) or increases in the nonrelapse mortality (NRM) (Brunstein et al., 2009). The reason for the inconsistency is unknown; however, it may be attributed to the differences in patient age, disease type, or GVHD prophylaxis. It has been reported that the frequency of viral infection, such as with cytomegalovirus (CMV), is different between children and adults (Yokoyama et al., 2019), which may cause the inconsistency in results. In addition, more distinctive effect of KIR–ligand mismatch has been shown in AML than in acute lymphoblastic leukemia (Ruggeri et al., 2002). Furthermore, because T- and NK-cell functions are known to be antagonistic (Lowe et al., 2003; Cooley et al., 2005), the use of antithymocyte globulin (ATG) for GVHD prophylaxis, that is, in-vivo T-cell depletion, can indirectly affect the NK-cell activity. In addition, the use of mycophenolate mofetil (MMF) for GVHD prophylaxis may cause a weak T-cell suppression compared with that cause by methotrexate (MTX) (Terakura et al., 2017a; 2017b), whereas it elicits a stronger inhibitory effect on the NK-cell function (Ohata et al., 2011). Another issue that may impact the KIR–ligand interaction effect in CBT is the method for determining the KIR–ligand mismatch. Because activation intensity and rate of expression along NK-cell maturation are different for each KIR (Foley et al., 2012a; Nguyen et al., 2017), it would be preferable to examine each KIR subtype mismatch individually rather than to analyze them as a bulk.
To overcome these problems in the analysis of the KIR–ligand mismatch effect, a study with the largest number of CBT cases was conducted (Yokoyama et al., 2021a). A total of 3,010 cases of adult single unit CBT were classified based on GVHD prophylaxis (tacrolimus/MTX or tacrolimus/MMF), and the effects of the KIR2DL1-, KIR3DL1-, and KIR3DL2–ligand mismatches (HLA-C2, Bw4, and A3/A11 mismatches, respectively) were examined (Table 2). The HLA–C2 mismatch had a favorable effect on relapse [hazard ratio (HR), 0.56, p = 0.0043] and OS (HR, 0.72, p = 0.037) only in the CBT, with GVHD prophylaxis using MTX, whereas these effects were not noted in the other types of KIR–ligand mismatch. In the CBT using MMF for GVHD prophylaxis, the HLA–A3/A11 mismatch had a negative effect on the NRM (HR, 1.93, p < 0.001) and OS (HR, 1.48, p = 0.014). After further accumulation of cases, we conducted a similar analysis focusing on 3,627 patients with AML and found that only in the group using MTX for GVHD prophylaxis, the presence of HLA–C2 mismatch resulted in favorable effects, such as reduced incidence of relapse (HR 0.61, p = 0.017) and improved OS (HR 0.72, p = 0.016) (Yokoyama et al., 2022).
NK-cell maturation induced by CMV reactivation after HSCT and the effects of the KIR–ligand mismatch
The percentage of mature NK cells has been shown to rapidly increase after CMV reactivation in various settings of HSCT (Foley et al., 2012b, 2012a; Li et al., 2019; Rashidi et al., 2019; Zaghi et al., 2021). Most of these cells have CD56 dim, NKG2A-negative, KIR-positive, and NKG2C-positive phenotypes, and are classified as memory-like NK cells (Foley et al., 2012b). CMV reactivation has been suggested to reduce AML relapse (Elmaagacli et al., 2011; Green et al., 2013; Manjappa et al., 2014; Jang et al., 2015; Takenaka et al., 2015; Ramanathan et al., 2016; Teira et al., 2016; Yokoyama et al., 2020), and the involvement of these mature NK cells has been postulated as a possible underlying mechanism. Despite some negative reports (Takenaka et al., 2015; Teira et al., 2016) and controversial results, the cooperative effects of KIR–ligand mismatch in reducing AML relapse by CMV reactivation have been reported (Yokoyama et al., 2021b). It was reported that in the presence of the HLA—Bw4 or—A3/A11 mismatch (KIR3DL—ligand mismatch), CMV reactivation for up to 100 days had a favorable effect on the relapse rate (HR 0.54, p = 0.032), whereas in the presence of other KIR–ligand mismatch types, these effects were not noted.
Conclusion
The effects of KIR–ligand interactions on NK cells in CBT remain unclear. This might be partially due to the difficulty in demonstrating the complex mechanisms of NK-cell activation in a model that only considers a single receptor, such as KIR. It might, therefore, be necessary to make a model that can consider multiple mechanisms of NK-cell activation, or to analyze a homogeneous population extracted from many cases. Despite such complex situations, attempts are being made to construct a more reliable model of NK-cell activation (Dhuyser et al., 2022). These findings will not only be useful for donor selection in CBT, but would also be useful for adoptive NK-cell therapy to maximize their efficacy in the future.
Author contributions
HY wrote the manuscript.
Funding
This work was supported by JSPS KAKENHI, Grant Number JP21K08363.
Acknowledgments
I would like to thank all the co-authors associated with this work.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ando, T., Tachibana, T., Tanaka, M., Suzuki, T., Ishiyama, Y., Koyama, S., et al. (2020). Impact of graft sources on immune reconstitution and survival outcomes following allogeneic stem cell transplantation. Blood Adv. 4, 408–419. doi:10.1182/bloodadvances.2019001021
Arima, N., Kanda, J., Tanaka, J., Yabe, T., Morishima, Y., Kim, S.-W., et al. (2018). Homozygous HLA-C1 is associated with reduced risk of relapse after HLA-matched transplantation in patients with myeloid leukemia. Biol. Blood Marrow Transpl. 24, 717–725. doi:10.1016/j.bbmt.2017.11.029
Auletta, J. J., Kou, J., Chen, M., and Shaw, B. E. (2021). Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides.
Barisic, S., and Childs, R. W. (2022). Graft-versus-solid-tumor effect: From hematopoietic stem cell transplantation to adoptive cell therapies. Stem Cells 40, 556–563. doi:10.1093/stmcls/sxac021
Bartelink, I. H., Belitser, S. V., Knibbe, C. A. J., Danhof, M., de Pagter, A. J., Egberts, T. C. G., et al. (2013). Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol. Blood Marrow Transpl. 19, 305–313. doi:10.1016/j.bbmt.2012.10.010
Bejanyan, N., Brunstein, C. G., Cao, Q., Lazaryan, A., Luo, X., Curtsinger, J., et al. (2018). Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2, 909–922. doi:10.1182/bloodadvances.2017014464
Boudreau, J. E., Giglio, F., Gooley, T. A., Stevenson, P. A., Le Luduec, J.-B., Shaffer, B. C., et al. (2017). KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J. Clin. Oncol. 35, 2268–2278. doi:10.1200/JCO.2016.70.7059
Brunstein, C. G., Wagner, J. E., Weisdorf, D. J., Cooley, S., Noreen, H., Barker, J. N., et al. (2009). Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood 113, 5628–5634. doi:10.1182/blood-2008-12-197467
Bühlmann, L., Buser, A. S., Cantoni, N., Gerull, S., Tichelli, A., Gratwohl, A., et al. (2011). Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transpl. 46, 1357–1362. doi:10.1038/bmt.2010.306
Burchert, A., Bug, G., Fritz, L. V., Finke, J., Stelljes, M., Röllig, C., et al. (2020). Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J. Clin. Oncol. 38, 2993–3002. doi:10.1200/JCO.19.03345
Cooley, S., McCullar, V., Wangen, R., Bergemann, T. L., Spellman, S., Weisdorf, D. J., et al. (2005). KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood 106, 4370–4376. doi:10.1182/blood-2005-04-1644
Cooley, S., Trachtenberg, E., Bergemann, T. L., Saeteurn, K., Klein, J., Le, C. T., et al. (2009). Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 113, 726–732. doi:10.1182/blood-2008-07-171926
Cooley, S., Weisdorf, D. J., Guethlein, L. A., Klein, J. P., Wang, T., Le, C. T., et al. (2010). Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116, 2411–2419. doi:10.1182/blood-2010-05-283051
de Lima, M., Porter, D. L., Battiwalla, M., Bishop, M. R., Giralt, S. A., Hardy, N. M., et al. (2014). Proceedings from the national cancer institute's second international workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: Part III. Prevention and treatment of relapse after allogeneic transplantation. Biol. Blood Marrow Transpl. 20, 4–13. doi:10.1016/j.bbmt.2013.08.012
Dhuyser, A., Aarnink, A., Pérès, M., Jayaraman, J., Nemat-Gorgani, N., Rubio, M. T., et al. (2022). KIR in allogeneic hematopoietic stem cell transplantation: Need for a unified paradigm for donor selection. Front. Immunol. 13, 821533. doi:10.3389/fimmu.2022.821533
Dickinson, A. M., Norden, J., Li, S., Hromadnikova, I., Schmid, C., Schmetzer, H., et al. (2017). Graft-versus-Leukemia effect following hematopoietic stem cell transplantation for leukemia. Front. Immunol. 8, 496. doi:10.3389/fimmu.2017.00496
Dubreuil, L., Chevallier, P., Retière, C., and Gagne, K. (2021). Relevance of polymorphic KIR and HLA class I genes in NK-Cell-Based immunotherapies for adult leukemic patients. Cancers 13, 3767–3827. doi:10.3390/cancers13153767
Dunbar, E. M., Buzzeo, M. P., Levine, J. B., Schold, J. D., Meier-Kriesche, H.-U., and Reddy, V. (2008). The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica 93, 1852–1858. doi:10.3324/haematol.13033
Elmaagacli, A. H., Steckel, N. K., Koldehoff, M., Hegerfeldt, Y., Trenschel, R., Ditschkowski, M., et al. (2011). Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: Evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 118, 1402–1412. doi:10.1182/blood-2010-08-304121
Farag, S. S., Bacigalupo, A., Eapen, M., Hurley, C., Dupont, B., Caligiuri, M. A., et al. (2006). The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: A report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol. Blood Marrow Transpl. 12, 876–884. doi:10.1016/j.bbmt.2006.05.007
Foley, B., Cooley, S., Verneris, M. R., Curtsinger, J., Luo, X., Waller, E. K., et al. (2012a). Human cytomegalovirus (CMV)-Induced memory-like NKG2C + NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 189, 5082–5088. doi:10.4049/jimmunol.1201964
Foley, B., Cooley, S., Verneris, M. R., Pitt, M., Curtsinger, J., Luo, X., et al. (2012b). Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119, 2665–2674. doi:10.1182/blood-2011-10-386995
Fry, T. J., and Mackall, C. L. (2005). Immune reconstitution following hematopoietic progenitor cell transplantation: Challenges for the future. Bone Marrow Transpl. 35 (1), S53–S57. doi:10.1038/sj.bmt.1704848
Gagne, K., Busson, M., Bignon, J.-D., Balère-Appert, M.-L., Loiseau, P., Dormoy, A., et al. (2009). ARS2000 FRM and FGM groupDonor KIR3DL1/3DS1 gene and recipient Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 15, 1366–1375. doi:10.1016/j.bbmt.2009.06.015
Garfall, A., Kim, H. T., Sun, L., Ho, V. T., Armand, P., Koreth, J., et al. (2013). KIR ligand incompatibility is not associated with relapse reduction after double umbilical cord blood transplantation. Bone Marrow Transpl. 48, 1000–1002. doi:10.1038/bmt.2012.272
Giebel, S., Locatelli, F., Lamparelli, T., Velardi, A., Davies, S., Frumento, G., et al. (2003). Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 102, 814–819. doi:10.1182/blood-2003-01-0091
Green, M. L., Leisenring, W. M., Xie, H., Walter, R. B., Mielcarek, M., Sandmaier, B. M., et al. (2013). CMV reactivation after allogeneic HCT and relapse risk: Evidence for early protection in acute myeloid leukemia. Blood 122, 1316–1324. doi:10.1182/blood-2013-02-487074
Hattori, N., Saito, B., Sasaki, Y., Shimada, S., Murai, S., Abe, M., et al. (2018). Status of natural killer cell recovery in day 21 bone marrow after allogeneic hematopoietic stem cell transplantation predicts clinical outcome. Biol. Blood Marrow Transpl. 24, 1841–1847. doi:10.1016/j.bbmt.2018.05.007
Hsu, K. C., Gooley, T., Malkki, M., Pinto-Agnello, C., Dupont, B., Bignon, J.-D., et al. and International Histocompatibility Working Group (2006). KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol. Blood Marrow Transpl. 12, 828–836. doi:10.1016/j.bbmt.2006.04.008
Hsu, K. C., Keever-Taylor, C. A., Wilton, A., Pinto, C., Heller, G., Arkun, K., et al. (2005). Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 105, 4878–4884. doi:10.1182/blood-2004-12-4825
Jacobson, C. A., Turki, A. T., McDonough, S. M., Stevenson, K. E., Kim, H. T., Kao, G., et al. (2012). Immune reconstitution after double umbilical cord blood stem cell transplantation: Comparison with unrelated peripheral blood stem cell transplantation. Biol. Blood Marrow Transpl. 18, 565–574. doi:10.1016/j.bbmt.2011.08.018
Jang, J. E., Kim, S. J., Cheong, J.-W., Hyun, S. Y., Kim, Y. D., Kim, Y. R., et al. (2015). Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann. Hematol. 94, 275–282. doi:10.1007/s00277-014-2190-1
June, C. H., and Sadelain, M. (2018). Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73. doi:10.1056/NEJMra1706169
Kanda, J., Chiou, L.-W., Szabolcs, P., Sempowski, G. D., Rizzieri, D. A., Long, G. D., et al. (2012). Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 18, 1664–1676. doi:10.1016/j.bbmt.2012.06.005
Kreidieh, F., Abou Dalle, I., Moukalled, N., El-Cheikh, J., Brissot, E., Mohty, M., et al. (2022). Relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: An overview of prevention and treatment. Int. J. Hematol. 116, 330–340. doi:10.1007/s12185-022-03416-7
Lee, C. J., Savani, B. N., Mohty, M., Gorin, N. C., Labopin, M., Ruggeri, A., et al. (2019). Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: Expert review from the acute leukemia working party of the European society for blood and marrow transplantation. Bone Marrow Transpl. 54, 519–530. doi:10.1038/s41409-018-0286-2
Leung, W., Iyengar, R., Turner, V., Lang, P., Bader, P., Conn, P., et al. (2004). Determinants of antileukemia effects of allogeneic NK cells. J. Immunol. 172, 644–650. doi:10.4049/jimmunol.172.1.644
Leung, W. (2011). Use of NK cell activity in cure by transplant. Br. J. Haematol. 155, 14–29. doi:10.1111/j.1365-2141.2011.08823.x
Li, L., Chen, H., Marin, D., Xi, Y., Miao, Q., Lv, J., et al. (2019). A novel immature natural killer cell subpopulation predicts relapse after cord blood transplantation. Blood Adv. 3, 4117–4130. doi:10.1182/bloodadvances.2019000835
Lowe, E. J., Turner, V., Handgretinger, R., Horwitz, E. M., Benaim, E., Hale, G. A., et al. (2003). T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br. J. Haematol. 123, 323–326. doi:10.1046/j.1365-2141.2003.04604.x
Ma, J., Mo, Y., Tang, M., Shen, J., Qi, Y., Zhao, W., et al. (2021). Bispecific antibodies: From research to clinical application. Front. Immunol. 12, 626616. doi:10.3389/fimmu.2021.626616
Manjappa, S., Bhamidipati, P. K., Stokerl-Goldstein, K. E., DiPersio, J. F., Uy, G. L., Westervelt, P., et al. (2014). Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol. Blood Marrow Transpl. 20, 46–52. doi:10.1016/j.bbmt.2013.10.003
Martínez-Losada, C., Martín, C., Gonzalez, R., Manzanares, B., García-Torres, E., and Herrera, C. (2017). Patients lacking a KIR-ligand of HLA group C1 or C2 have a better outcome after umbilical cord blood transplantation. Front. Immunol. 8, 810. doi:10.3389/fimmu.2017.00810
Mawad, R., Lionberger, J. M., and Pagel, J. M. (2013). Strategies to reduce relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Curr. Hematol. Malig. Rep. 8, 132–140. doi:10.1007/s11899-013-0153-6
Miller, J. S., Cooley, S., Parham, P., Farag, S. S., Verneris, M. R., McQueen, K. L., et al. (2007). Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood 109, 5058–5061. doi:10.1182/blood-2007-01-065383
Minculescu, L., Marquart, H. V., Friis, L. S., Petersen, S. L., Schiødt, I., Ryder, L. P., et al. (2016). Early natural killer cell reconstitution predicts overall survival in T cell-replete allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 22, 2187–2193. doi:10.1016/j.bbmt.2016.09.006
Morishima, Y., Yabe, T., Matsuo, K., Kashiwase, K., Inoko, H., Saji, H., et al. (2007). Japan Marrow Donor ProgramEffects of HLA allele and killer immunoglobulin-like receptor ligand matching on clinical outcome in leukemia patients undergoing transplantation with T-cell-replete marrow from an unrelated donor. Biol. Blood Marrow Transpl. 13, 315–328. doi:10.1016/j.bbmt.2006.10.027
Negrin, R. S. (2015). Graft-versus-host disease versus graft-versus-leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2015, 225–230. doi:10.1182/asheducation-2015.1.225
Nguyen, S., Achour, A., Souchet, L., Vigouroux, S., Chevallier, P., Furst, S., et al. (2017). Clinical impact of NK-cell reconstitution after reduced intensity conditioned unrelated cord blood transplantation in patients with acute myeloid leukemia: Analysis of a prospective phase II multicenter trial on behalf of the société française de Greffe de Moelle osseuse et thérapie cellulaire and eurocord. Bone Marrow Transpl. 52, 1428–1435. doi:10.1038/bmt.2017.122
Ogonek, J., Kralj Juric, M., Ghimire, S., Varanasi, P. R., Holler, E., Greinix, H., et al. (2016). Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 7, 507. doi:10.3389/fimmu.2016.00507
Ohata, K., Espinoza, J. L., Lu, X., Kondo, Y., and Nakao, S. (2011). Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: A possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol. Blood Marrow Transpl. 17, 205–213. doi:10.1016/j.bbmt.2010.08.014
Orti, G., Barba, P., Fox, L., Salamero, O., Bosch, F., and Valcarcel, D. (2017). Donor lymphocyte infusions in AML and MDS: Enhancing the graft-versus-leukemia effect. Exp. Hematol. 48, 1–11. doi:10.1016/j.exphem.2016.12.004
Pical-Izard, C., Crocchiolo, R., Granjeaud, S., Kochbati, E., Just-Landi, S., Chabannon, C., et al. (2015). Reconstitution of natural killer cells in HLA-matched HSCT after reduced-intensity conditioning: Impact on clinical outcome. Biol. Blood Marrow Transpl. 21, 429–439. doi:10.1016/j.bbmt.2014.11.681
Ramanathan, M., Teira, P., Battiwalla, M., Barrett, J., Ahn, K. W., Chen, M., et al. (2016). Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transpl. 51, 1113–1120. doi:10.1038/bmt.2016.89
Rashidi, A., Luo, X., Cooley, S., Anasetti, C., Waller, E. K., Brunstein, C. G., et al. (2019). The association of CMV with NK-cell reconstitution depends on graft source: Results from BMT CTN-0201 samples. Blood Adv. 3, 2465–2469. doi:10.1182/bloodadvances.2019000298
Rénard, C., Barlogis, V., Mialou, V., Galambrun, C., Bernoux, D., Goutagny, M. P., et al. (2011). Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br. J. Haematol. 152, 322–330. doi:10.1111/j.1365-2141.2010.08409.x
Rettman, P., Malard, F., Legrand, N., Avinens, O., Eliaou, J.-F., Picard, C., et al. (2016). Impact of KIR/HLA genetic combinations on double umbilical cord blood transplantation outcomes. Results of a French multicentric retrospective study on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) and the Société Francophone d'Histocompatibilité et d'Immunogénétique (SFHI). Bone Marrow Transpl. 51, 1499–1503. doi:10.1038/bmt.2016.151
Rocha, V., Ruggeri, A., Spellman, S., Wang, T., Sobecks, R., Locatelli, F., et al. (2016). Killer cell immunoglobulin-like receptor-ligand matching and outcomes after unrelated cord blood transplantation in acute myeloid leukemia. Biol. Blood Marrow Transpl. 22, 1284–1289. doi:10.1016/j.bbmt.2016.04.007
Ruggeri, L., Capanni, M., Casucci, M., Volpi, I., Tosti, A., Perruccio, K., et al. (1999). Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 94, 333–339. doi:10.1182/blood.v94.1.333.413a31_333_339
Ruggeri, L., Capanni, M., Urbani, E., Perruccio, K., Shlomchik, W. D., Tosti, A., et al. (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100. doi:10.1126/science.1068440
Ruggeri, L., Mancusi, A., Capanni, M., Urbani, E., Carotti, A., Aloisi, T., et al. (2007). Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: Challenging its predictive value. Blood 110, 433–440. doi:10.1182/blood-2006-07-038687
Russo, A., Oliveira, G., Berglund, S., Greco, R., Gambacorta, V., Cieri, N., et al. (2018). NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 131, 247–262. doi:10.1182/blood-2017-05-780668
Sekine, T., Marin, D., Cao, K., Li, L., Mehta, P., Shaim, H., et al. (2016). Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood 128, 297–312. doi:10.1182/blood-2016-03-706317
Shaffer, B. C., Le Luduec, J.-B., Park, S., Devlin, S., Archer, A., Davis, E., et al. (2021). Prospective KIR genotype evaluation of hematopoietic cell donors is feasible with potential to benefit patients with AML. Blood Adv. 5, 2003–2011. doi:10.1182/bloodadvances.2020002701
Sun, J. Y., Dagis, A., Gaidulis, L., Miller, M. M., Rodriguez, R., Parker, P., et al. (2007). Detrimental effect of natural killer cell alloreactivity in T-replete hematopoietic cell transplantation (HCT) for leukemia patients. Biol. Blood Marrow Transpl. 13, 197–205. doi:10.1016/j.bbmt.2006.09.009
Symons, H. J., Leffell, M. S., Rossiter, N. D., Zahurak, M., Jones, R. J., and Fuchs, E. J. (2010). Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol. Blood Marrow Transpl. 16, 533–542. doi:10.1016/j.bbmt.2009.11.022
Takenaka, K., Nishida, T., Asano-Mori, Y., Oshima, K., Ohashi, K., Mori, T., et al. (2015). Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: The Japan society for hematopoietic cell transplantation transplantation-related complication working group. Biol. Blood Marrow Transpl. 21, 2008–2016. –16. doi:10.1016/j.bbmt.2015.07.019
Tanaka, J., Morishima, Y., Takahashi, Y., Yabe, T., Oba, K., Takahashi, S., et al. (2013). Effects of KIR ligand incompatibility on clinical outcomes of umbilical cord blood transplantation without ATG for acute leukemia in complete remission. Blood Cancer J. 3, e164. doi:10.1038/bcj.2013.62
Tanaka, J., Sugita, J., Asanuma, S., Arita, K., Shono, Y., Kikutchi, M., et al. (2009). Increased number of CD16(+)CD56(dim) NK cells in peripheral blood mononuclear cells after allogeneic cord blood transplantation. Hum. Immunol. 70, 701–705. doi:10.1016/j.humimm.2009.06.002
Teira, P., Battiwalla, M., Ramanathan, M., Barrett, A. J., Ahn, K. W., Chen, M., et al. (2016). Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 127, 2427–2438. doi:10.1182/blood-2015-11-679639
Terakura, S., Kuwatsuka, Y., Yamasaki, S., Wake, A., Kanda, J., Inamoto, Y., et al. (2017a). GvHD prophylaxis after single-unit reduced intensity conditioning cord blood transplantation in adults with acute leukemia. Bone Marrow Transpl. 52, 1261–1267. doi:10.1038/bmt.2017.116
Terakura, S., Wake, A., Inamoto, Y., Murata, M., Sakai, R., Yamaguchi, T., et al. (2017b). Exploratory research for optimal GvHD prophylaxis after single unit CBT in adults: Short-term methotrexate reduced the incidence of severe GvHD more than mycophenolate mofetil. Bone Marrow Transpl. 52, 423–430. doi:10.1038/bmt.2016.255
Wei, A. H., Döhner, H., Pocock, C., Montesinos, P., Afanasyev, B., Dombret, H., et al. (2020). QUAZAR AML-001 trial InvestigatorsOral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N. Engl. J. Med. 383, 2526–2537. doi:10.1056/NEJMoa2004444
Willem, C., Makanga, D. R., Guillaume, T., Maniangou, B., Legrand, N., Gagne, K., et al. (2019). Impact of KIR/HLA incompatibilities on NK cell reconstitution and clinical outcome after T cell-replete haploidentical hematopoietic stem cell transplantation with posttransplant cyclophosphamide. J. Immunol. 202, 2141–2152. doi:10.4049/jimmunol.1801489
Willemze, R., Rodrigues, C. A., Labopin, M., Sanz, G., Michel, G., Socié, G., et al. (2009). Eurocord-Netcord and Acute Leukaemia Working Party of the EBMTKIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia 23, 492–500. doi:10.1038/leu.2008.365
Xuan, L., Wang, Y., Huang, F., Fan, Z., Xu, Y., Sun, J., et al. (2020). Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: An open-label, multicentre, randomised phase 3 trial. Lancet. Oncol. 21, 1201–1212. doi:10.1016/S1470-2045(20)30455-1
Yafour, N., Beckerich, F., Bulabois, C. E., Chevallier, P., Daguindau, E., Dumesnil, C., et al. (2017). How to prevent relapse after allogeneic hematopoietic stem cell transplantation in patients with acute leukemia and myelodysplastic syndrome. Curr. Res. Transl. Med. 65, 65–69. doi:10.1016/j.retram.2017.06.001
Yokoyama, H., Hirayama, M., Takahashi, Y., Uchida, N., Tanaka, M., Onizuka, M., et al. (2021a). Altered effect of killer immunoglobulin-like receptor-ligand mismatch by graft versus host disease prophylaxis in cord blood transplantation. Bone Marrow Transpl. 56, 3059–3067. doi:10.1038/s41409-021-01469-6
Yokoyama, H., Kanaya, M., Iemura, T., Hirayama, M., Yamasaki, S., Kondo, T., et al. (2022). Improved outcomes of single-unit cord blood transplantation for acute myeloid leukemia by killer immunoglobulin-like receptor 2DL1-ligand mismatch. Bone Marrow Transpl. 57, 1171–1179. doi:10.1038/s41409-022-01700-y
Yokoyama, H., Kanda, J., Kato, S., Kondo, E., Maeda, Y., Saji, H., et al. (2019). HLA Working Group of the Japan Society for Hematopoietic Cell TransplantationEffects of HLA mismatch on cytomegalovirus reactivation in cord blood transplantation. Bone Marrow Transpl. 54, 1004–1012. doi:10.1038/s41409-018-0369-0
Yokoyama, H., Kanda, J., Kawahara, Y., Uchida, N., Tanaka, M., Takahashi, S., et al. (2021b). Reduced leukemia relapse through cytomegalovirus reactivation in killer cell immunoglobulin-like receptor-ligand-mismatched cord blood transplantation. Bone Marrow Transpl. 56, 1352–1363. doi:10.1038/s41409-020-01203-8
Yokoyama, H., Takenaka, K., Nishida, T., Seo, S., Shinohara, A., Uchida, N., et al. (2020). Japan society for hematopoietic cell transplantation transplantation-related complication working GroupFavorable effect of cytomegalovirus reactivation on outcomes in cord blood transplant and its differences among disease risk or type. Biol. Blood Marrow Transpl. 26, 1363–1370. doi:10.1016/j.bbmt.2020.04.002
Zaghi, E., Calvi, M., Puccio, S., Spata, G., Terzoli, S., Peano, C., et al. (2021). Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT. JCI insight 6, 146973. doi:10.1172/jci.insight.146973
Zhao, F., Shi, Y., Chen, X., Zhang, R., Pang, A., Zhai, W., et al. (2022). Higher dose of CD34+ cells promotes early reconstitution of natural killer cells and is associated with better outcomes after unmanipulated hematopoietic stem cell transplantation for myeloid malignancies. Transpl. Cell. Ther. 000, 589.e1–589589.e10. doi:10.1016/j.jtct.2022.06.007
Zhou, H., Bao, X., Wu, X., Tang, X., Wang, M., Wu, D., et al. (2014). Donor selection for killer immunoglobulin-like receptors B haplotype of the centromeric motifs can improve the outcome after HLA-identical sibling hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 20, 98–105. doi:10.1016/j.bbmt.2013.10.017
Keywords: NK cell, cord blood transplantation (CBT), killer cell immunoglobulin-like receptor (KIR), GvHD prophylaxis, cytomegalovirus
Citation: Yokoyama H (2022) Role of NK cells in cord blood transplantation and their enhancement by the missing ligand effect of the killer-immunoglobulin like receptor. Front. Genet. 13:1041468. doi: 10.3389/fgene.2022.1041468
Received: 10 September 2022; Accepted: 04 October 2022;
Published: 18 October 2022.
Edited by:
Daniel Fürst, German Red Cross Blood Transfusion Service, GermanyReviewed by:
Antonio Balas, Transfusion Center of the Community of Madrid, SpainCopyright © 2022 Yokoyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hisayuki Yokoyama, aGlzYXl1a2kueW9rb3lhbWEuYTFAdG9ob2t1LmFjLmpw
 Hisayuki Yokoyama
Hisayuki Yokoyama