- 1Genetics and Bioinformatics, Dasman Diabetes Institute, Kuwait City, Kuwait
- 2Animal and Imaging Core Facilities, Dasman Diabetes Institute, Kuwait City, Kuwait
- 3Special Services Facility, Dasman Diabetes Institute, Kuwait City, Kuwait
Background: Animal and cell model studies have implicated CAV1 in the pathophysiology of metabolic disorders. Our previous studies demonstrated a potential association of CAV1 rs1997623 C/A variant with pediatric metabolic syndrome (MetS) in Arab children. In the present study, we evaluate whether the CAV1 variant associates with MetS Arab adults as well. The association signal is further examined for ancestry-specific variation by considering cohorts of other ethnicities.
Method: The CAV1 rs1997623 was genotyped in three cohorts of Arab (n = 479), South Asian (n = 660), and South East Asian (n = 362) ethnic adults from Kuwait. MetS status of the individuals was diagnosed using the IDF criteria (presence of central obesity and at least two abnormalities out of: elevated TG, low HDL, hypertension, or T2D). The quantitative measure of MetS was calculated as siMS = 2 × WC/Height + FBG/5.6 + TG/1.7 + SBP/130–HDL/1.02 for males or HDL/1.28 for females. Allelic associations with quantitative and dichotomous MetS traits were assessed using linear and logistic regression models adjusted for age and sex. In addition, empirical p-values (Pemp) were generated using max(T) permutation procedure based on 10,000 permutations.
Results: The CAV1 variant was significantly associated with MetS status (OR = 1.811 [1.25–2.61]; p-value = 0.0015; Pemp = 0.0013) and with siMS (Effect size = 0.206; p-value = 0.0035; Pemp = 0.0028) in the cohort of Arab individuals. The association was weak and insignificant in the South Asian and South East Asian cohorts (OR = 1.19 and 1.11; p-values = 0.25 and 0.67, respectively).
Conclusion: The reported association of CAV1 rs1997623 C/A with MetS in Arab pediatric population is now demonstrated in an adult Arab cohort as well. The weak association signal seen in the Asian cohorts lead us to propose a certain extent of ethnic-specificity in CAV1 rs1997623 association with MetS.
Introduction
Metabolic syndrome (MetS) in adults is defined as a cluster of risk factors for cardiovascular disease and type 2 diabetes mellitus, which includes abdominal obesity, dyslipidemia, glucose intolerance, hypertension, stroke, and atherosclerosis. The global epidemic proportions of MetS are estimated to be around 20–25%, with differences seen across continents–e.g., 12–37% of the Asian population versus 12–26% of the European population are afflicted with MetS (Alberti et al., 2009). The prevalence of MetS in the Arabian Gulf Region is reported as 27.3% (Shin and Jee, 2020). An alarming increase in pediatric MetS has been observed among Arab children and adolescents over 9 years. For instance, in Saudi Arabia, the prevalence of pediatric MetS was 11.8% in 2010 and increased to 20.6% in 2019 (Amer et al., 2021).
MetS is triggered by various factors that include genetic predisposition, aging, obesity, insulin resistance, and physical inactivity. Genetic predisposition is a factor in MetS as prevalence differs among ethnic groups (Ford et al., 2002). Substantial progress has been made over the last decade in identifying the genetic risk factors associated with various traits of MetS (Abou Ziki and Mani, 2016). One of these risk loci is the Caveolin-1 (CAV1) gene, its association with metabolic disorders has been demonstrated in animal models and human cohorts (Baudrand et al., 2016; Chen et al., 2016). CAV1 is an essential structural protein required for the formation of caveolae (Vargas et al., 2002; McMahon et al., 2009), which play key roles in cellular homeostasis including the regulation of influx and efflux of lipid and glucose metabolites in various tissues (Su and Abumrad., 2009; Pilch and Liu, 2011; Grayson et al., 2013). CAV1 has been widely studied in metabolic syndrome disorders such as dyslipidemia and CVD due to signal transduction, trafficking in cholesterol hemostasis, and triacylglycerol metabolism (Jin et al., 2011).
Several studies have indicated that CAV1 genetic variations might interact with other risk factors (Haddad et al., 2020), including dietary intake of fatty acids, suggesting a positive association between CAV1 and hypercholesterolemia (Shyu et al., 2017). A study using whole exome sequencing of a family of European descent with pulmonary arterial hypertension identified a frame-shift mutation in CAV1 (c.474delA, P158PfsX22) among nine family members (Austin et al., 2012). CAV1 rs926198 has been seen associated with increased susceptibility to coronary artery disease and myocardial infarction in Han Chinese population (Chen et al., 2016). Baudrand et al. (2015) found the CAV1 gene variant rs926198 associated with MetS in separate Caucasian and Hispanic cohorts. In 2011, Pojoga et al., 2011 demonstrated the association of CAV1 rs926198 and rs3807989 variants with higher fasting insulin levels and increased homeostatic model assessment for insulin resistance (HOMA-IR) in Caucasian and Hispanic cohorts. Mora-Garcia et al. (2018) found the CAV1 variation rs11773845 to be associated with high serum triglycerides (TG) and MetS in a Latin American cohort.
Recently, we reported an association between the CAV1 rs1997623 variant and MetS in a cohort of Kuwaiti children (Nizam et al., 2018). Evaluation of this association has not yet been performed in adults. Cardiovascular risk factors, comprising MetS, can appear in childhood and progress through adulthood, leading to higher cardiovascular morbidity in adults (Varda and Gregoric, 2009). In this study, we assess whether the association signals observed between the CAV1 rs1997623 variant and pediatric MetS are also observed in adult MetS. We further evaluate the association signal involving the rs1997623 variant for ethnic variation by analyzing additional South Asian and South East Asian cohorts.
Materials and methods
The studies involving human participants were reviewed and approved by Ethical Review Committee, Dasman Diabetes Institute, Kuwait. The patients/participants provided their written informed consent to participate in this study.
Type of study
The study is of transverse observational type.
Recruitment of participants and study cohort
Adult individuals of Arab ethnicity from Kuwait were recruited as study subjects for the Arab cohort; of South Asian ethnicity for the South Asian cohort; and of South East Asian ethnicity for the South East Asian cohort. Individuals aged >18 years and residents of Kuwait were included. Pregnant women and individuals with Mendelian or rare genetic disorders, cognitive deficiencies, or chronic disorders (such as cancer) were excluded. The Arab cohort comprised 479 Arab subjects; the South Asian cohort comprised 660 South Asian individuals; and the South East Asian cohort comprised 362 South East Asian individuals. For each participant upon enrolment, data on demographics, health disorders (obesity, diabetes, and hypertension), baseline characteristics (such as height, weight, waist circumference, and blood pressure), and use of medication (such as those for lowering lipid levels or treating diabetes and hypertension) were recorded.
Derivation of MetS status and siMS score
We adopted the adult diagnosis criteria defined by the International Diabetes Federation (Alberti et al., 2006) to derive the MetS status of every study participant. To diagnose the patients with MetS, they should have central obesity and two or more of the following: high TG, low high-density lipoprotein (HDL), hypertension, or raised fasting plasma glucose (FPG). An individual was considered to have 1) central obesity if the waist circumference (WC) was ≥94 cm in the case of men or ≥80 cm in the case of women, 2) high TG if the serum TG level was ≥1.7 mmol/L (151 mg/dl) or was taking medication to lower TG; 3) low HDL if the serum HDL cholesterol level was < 1.03 mmol/L (40 mg/dl) in the case of men or < 1.29 mmol/L (50 mg/dl) in the case of women or was taking medication to restore HDL levels; 4) hypertension if systolic blood pressure was ≥130 mm Hg, diastolic blood pressure was ≥85 mm Hg, or was having treatment for previously diagnosed hypertension; and 5) diabetes if FPG was ≥5.6 mmol/L (100 mg/dl), or was previously diagnosed for type 2 diabetes. Furthermore, we calculated the siMS score, which is a simple and accurate method for quantifying MetS in adults, using the formula described in (Soldatovic et al., 2016): siMS score = 2*WC/Height + FBG/5.6 + TG/1.7 + SBP/130-HDL/1.02 for men or HDL/1.28 for women.
Blood sample collection and processing
After confirming that the participant had fasted overnight, blood samples were collected in EDTA-treated tubes. DNA was extracted using Gentra Puregene kit (Qiagen, Valencia, CA, United States) and was quantified using Quant-iT™ PicoGreen dsDNA Assay Kit (Life Technologies, Grand Island, NY, United States) and Epoch Microplate Spectrophotometer (BioTek Instruments). We checked absorbance values at 260–280 nm for adherence to an optical density range of 1.8–2.1 as previously described (Nizam et al., 2018).
Targeted genotyping of the CAV1 study variant rs1997623
Candidate SNP genotyping was performed using the TaqMan Genotyping Assay kit (C_2972973_10 from Thermo Fisher Scientific Inc, United States) on ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). 10 ng of DNA and 5 × FIREPol Master Mix (Solis BioDyne, Estonia) was mixed with 1 µl of 20 × TaqMan SNP Genotyping Assay to form the polymerase chain reaction sample. Thermal cycling conditions were set at 60°C for 1 min and at 95°C for 15 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Sanger sequencing was performed, using the BigDye Terminator v3.1 Cycle Sequencing on Applied Biosystems 3730xl DNA Analyzer, for selected cases of homozygotes and heterozygotes to validate genotypes determined by the above techniques (Nizam et al., 2018).
Quality procedures for SNP and trait measurements
SNP quality and statistical associations with traits were assessed using PLINK (version1.9) (Purcell et al., 2007). Minor allele frequency (MAF) was calculated in the study cohort and compared with those reported for ethnic populations in the 1000 Genomes Project (Auton et al., 2015). Hardy–Weinberg equilibrium was calculated for the studied variant. Quantitative trait values < Q1- 1.5 * IQR or > Q3+1.5 * IQR were treated as outliers and excluded from further analysis.
Association tests and empirical p-values to ascertain statistical significance
Allelic association tests for the studied variant with quantitative traits (such as siMS score) and dichotomous traits (such as MetS status) were performed using linear and logistic regression, respectively. The tests were performed under a genetic model based on the additive mode of inheritance. The tests were adjusted for regular corrections towards age and sex. We also adjusted for diabetes medication and lipid-lowering medication. Empirical p-values (Pemp) were generated using the max(T) permutation procedure available in PLINK, based on 10,000 permutations. A threshold of < 0.05 was set for both the p-value and Pemp value to assess the statistical significance of the association signal.
Power calculation
We used the Quanto software tool (University of Southern California, Los Angeles, CA, United States) to calculate the power of the study cohorts and its ability to delineate quantitative trait variability at a given power (which was set at 80%). We considered additive mode as the underlying genetic model with “gene only” hypothesis at type 1 error, p ≤ 0.05. Genetic effect that accounts for at least 0.1%–5% variance in the trait was detected by assuming RG2 (estimate for marginal genetic effect) values in the range of 0.001–0.05 in step of 0.005. We considered the populations (mean ± standard deviation) of the quantitative trait and MAF in these calculations.
Results
Characteristics of the study cohorts and study variant
Participants with MetS in our study cohorts differed significantly from those free of the syndrome in the levels of metabolic traits (except in height, TC, and LDL) and in the status for metabolic disorders (obesity, diabetes, and hypertension) in each of the cohorts of Arabs, South Asians, and South East Asians (Table 1). Sub-cohorts of MetS individuals and MetS-free individuals, though did not differ in sex, differed significantly in age; the results of association tests were subsequently adjusted for age and sex.
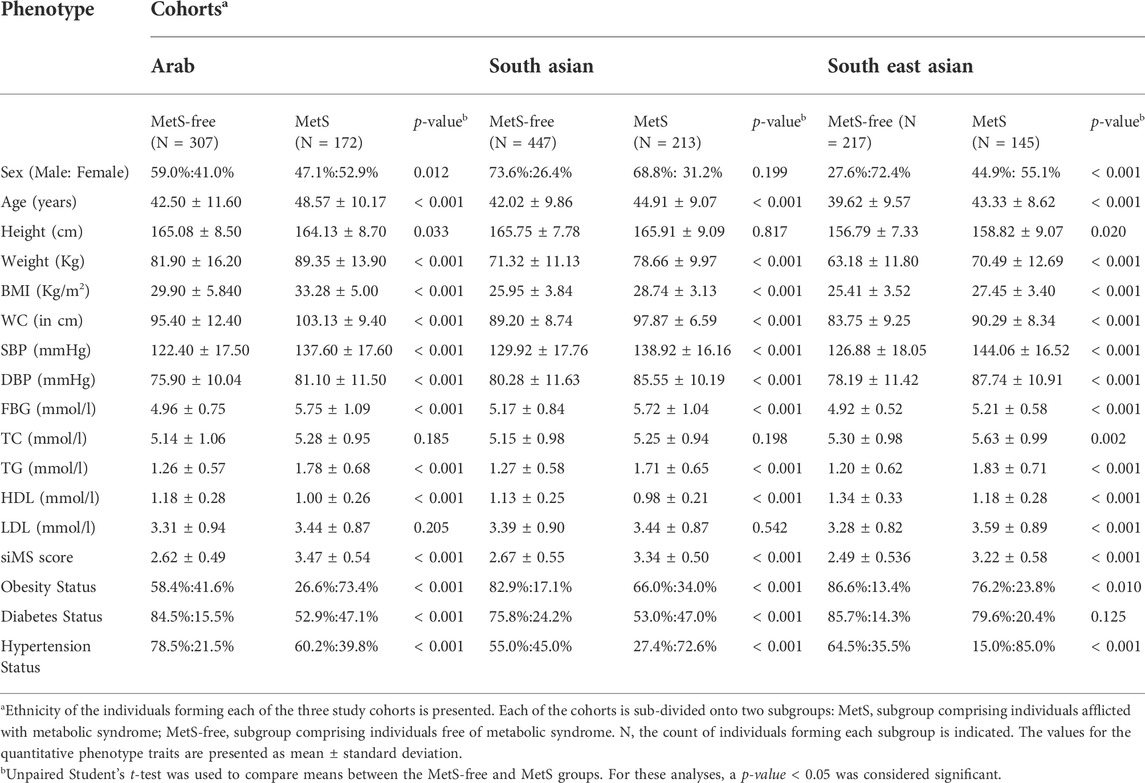
TABLE 1. Descriptive statistics for MetS and MetS-free individuals in the study cohorts of Arab, South Asian and South East Asian individuals from Kuwait.
The CAV1 rs1997623 variant passed the quality control for HWE >10–3 in each of the three cohorts (Table 2). The minor allele frequency (MAF) varied across the three study cohorts (16,17% in Arabs and South Asians versus 10% in South East Asians). The sub-cohorts of MetS and MetS-free individuals from each of the three study cohorts had a varied MAF (21% versus 14% in sub-cohorts of Arab individuals). The 1000 Genomes Project Phase 3 allele frequencies for the minor allele A also showed significant variations across continental populations: Africans (31%), admixed Americans (11%), East Asians (7%), Europeans (14%), and South Asians (13%).
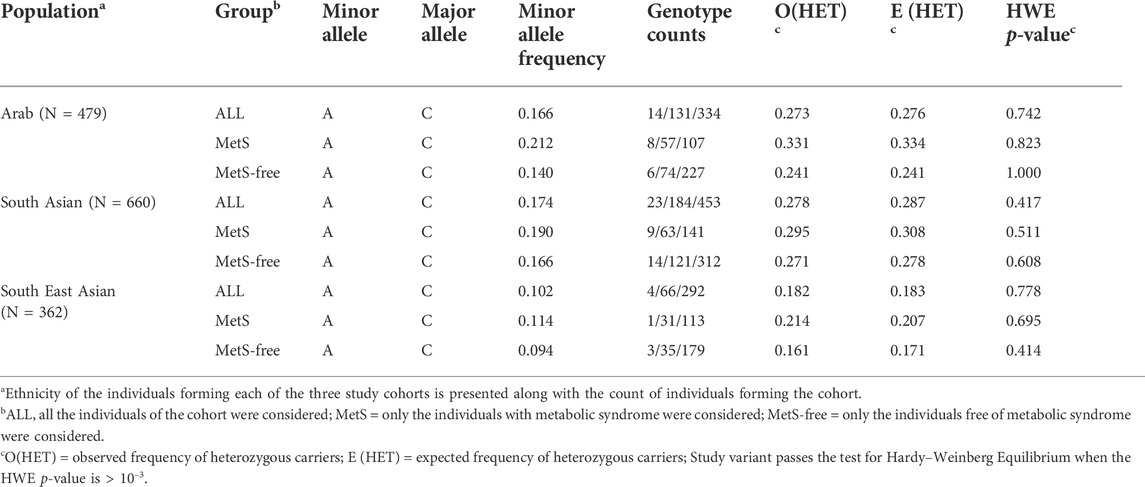
TABLE 2. Assessment of Hardy–Weinberg Equilibrium (HWE) deviation for rs1997623 genotypes across sample sets of MetS, MetS- free and All individuals among Arab, South Asian and South East Asian cohorts.
CAV1 rs1997623 C >A variant is significantly associated with siMS score and MetS status in arab adults
Linear regression tests adjusted for age and sex revealed significant associations from the study variant with three of the tested 12 quantitative metabolic traits albeit only in the Arab cohort (Table 3); such associations were with increased levels of siMS (effect = 0.206; p-value = 0.0035; Pemp = 0.0028); systolic blood pressure (effect = 4.029; p-value = 0.023; Pemp = 0.022) and diastolic blood pressure (effect = 2.669; p-value = 0.019; Pemp = 0.018). Of these, the p-value for the association with siMS score at 0.0035 passed the Bonferroni-corrected p-value threshold of 0.0042 (=0.05/12). Upon correcting the linear regression model for the confounding factor “drug treatment,” such as diabetes, hypertension, and lipids-lowering medications, the association with siMS score remained significant (effect = 0.18; p-value = 0.035; Pemp = 0.035).
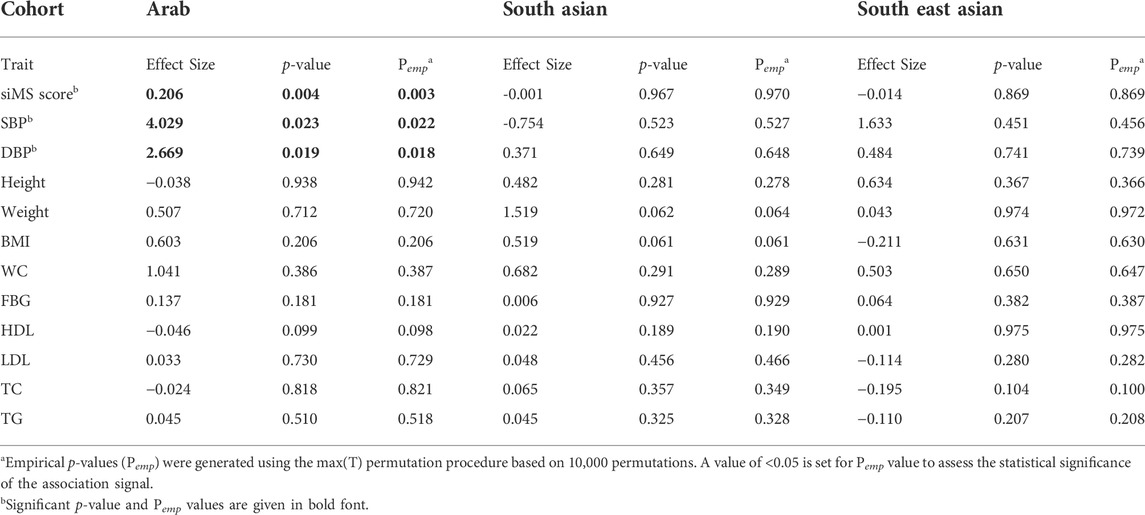
TABLE 3. Summary statistics of quantitative trait association with rs1997623_A in Arab, South Asian and South East Asian cohorts using linear regression adjusted for Age and Sex.
Logistic regression analysis adjusted for age and sex revealed significant association with presence of MetS (Odds ratio = 1.811 [1.25–2.61]; p-value = 0.0015; Pemp = 0.0013) albeit only in the Arab cohort (Table 4). Associations with diabetes, obesity, or hypertension status were not seen at statistically significant levels in any of the three cohorts.
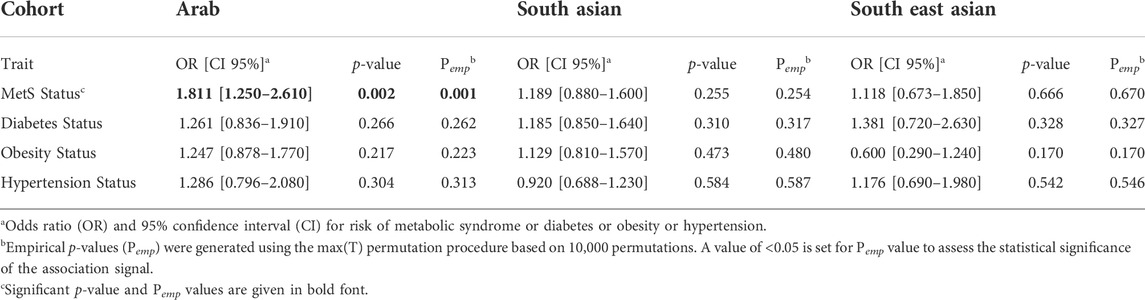
TABLE 4. Summary statistics of dichotomous traits association with rs1997623_A in Arab, South Asian and South East Asian cohorts using logistic regression adjusted for Age and Sex.
Results from power calculation are presented in Table 5. Results of power calculation indicated that the study cohorts had 80% power to detect associations with the CAV1 variant (MAF = 16.6% in Arab, 17.4% in South Asian, and 10.2% in South East Asian cohorts) and could explain 1.7% variance in the quantitative traits of siMS score, SBP and DBP of the Arab cohort (1.3% and 2.5% variance in the cases of South Asian and South East Asian cohorts, respectively). The observed effect sizes of 0.206, 4.03 and 2.67 in the Arab cohort for the association of the CAV1 variant with siMS score, SBP and DBP, respectively (see Table 3) were less than the expected effect sizes of 0.225, 4.69 and 2.73, respectively.
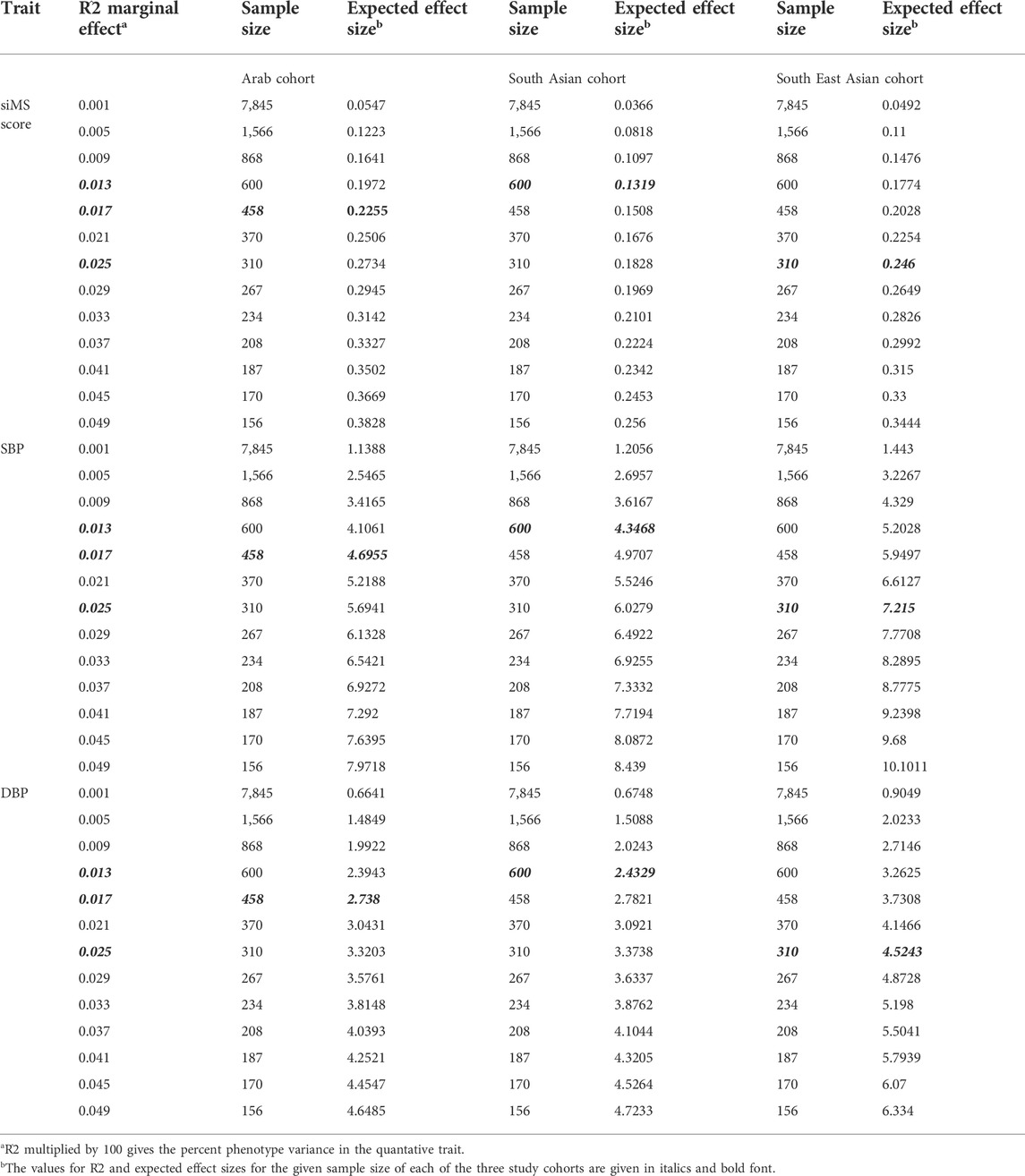
TABLE 5. Power Calculation for the Arab, South Asian and South East Asian cohorts for the traits of siMS score, SBP and DBP found associated in the Arab cohort.
Discussion
In the present study, we show that the CAV1 rs1997623-A variant is significantly associated with metabolic syndrome, represented as a dichotomous trait (MetS) and as a quantitative trait of continuous metabolic syndrome score (siMS), in adult Arabs from Kuwait. The statistical significance of association was indicated by p-values passing the Bonferroni corrected thresholds and by significant empirical p-values. These observed associations complement the findings from our previous study on pediatric MetS in Arabs (Nizam et al., 2018).
We present for the first time the rs1997623 from CAV1 as a risk variant for MetS. At the most, this variant is only in a weak LD with two other variants, namely rs11773845 and rs926198, associated with MetS by previously researchers as enumerated below: The CAV1 rs11773845 was found to be consistently associated with high serum TG and MetS in Latin Americans (Mora-García et al., 2018), and the CAV1 rs926198 was found to be correlated with MetS in Caucasians and Hispanics (Baudrand et al., 2015). Caucasians harboring the minor allele at rs926198 displayed higher odds of insulin resistance and low HDL. Our study variant CAV1 rs1997623 is not in particularly strong LD with the above-discussed two variants rs11773845 and rs926198 (R2 = 0.2281 and 0.2952, respectively). Thus, the presented study variant rs1997623 is a newly proposed risk variant of CAV1 known for its association with MetS.
Genome annotation systems, such as Ensembl (Cunningham et al., 2022), annotate the studied variant to overlap as many as nine transcript isoforms of CAV1 and five protein isoforms of different lengths. Depending on the transcript and protein isoform, rs1997623 can be a missense (leading to amino acid change) or synonymous (leading to no change in amino acid) or an intronic or an upstream gene variation. The Ensembl genome annotation system annotates the variant as part of a regulatory region within the promoter.
It is intriguing that we do not find the association signal between the study variant and MetS in the South Asian and South East Asian individuals. The study variant, CAV1 rs1997623, displays considerable variation (7%–31%) in allele frequency across the continents. The frequency of the rs1997623_A allele across the populations is 7% in East Asians, 11% in admixed Americans, 13% in South Asians, 14% in Europeans, and 31% in Africans. The frequency of the A-allele in our study cohort of Arab individuals is 16.6%, with the sub-cohort of individuals with MetS exhibiting a higher value of 21% compared to 14% exhibited by the sub-cohort of MetS-free individuals. Considerable regional and global variation in the estimates for MetS burden on both children (Noubiap et al., 2022) and adults (Ranasinghe et al., 2017; Moreira et al., 2020) have also been reported. These variations in the prevalence of MetS across the populations are probably consistent with variations in the effect allele frequencies and with the association signals across the populations. The prevalence rate of MetS is particularly high in Middle East countries and it is a noticeable cause for stroke, coronary heart disease, and cardiovascular disease in these countries (Ansarimoghaddam et al., 2018). Genetics of the Arab population in the Peninsula has been largely driven by consanguinity and inbreeding. A combination of inherent genetic predispositions and rapid lifestyle changes in the rich post-oil era has shaped the observed high prevalence of metabolic disorders in the region. Generalizing metabolic risk loci established in global populations to the Arab population and vice versa is an open topic and has been discussed, in our previous study (Hebbar et al., 2021), in the context of differences in allele frequencies, linkage disequilibrium, effect sizes, and heritability), and phenotype variance.
Interestingly, apart from targeted SNP association studies, there has been no large-scale GWA study performed implicating CAV1 variants in the context of metabolic diseases in any ethnic group. Even the targeted SNP association studies, as mentioned earlier, examined only few SNPs from CAV1 in the context metabolic traits and disorders. Therefore, our data, by way of showing that the association signal involving the studied variant is seen only in the Kuwaiti cohort and not in the cohorts of South Asians and South East Asians, demonstrates for the first time the ethnic-specific variations in CAV1 impacting MetS.
As a limitation, the study cohorts are of moderate sample sizes at 479 for the Arab cohort, 660 for the South Asian cohort, and 362 for the South East Asian cohort. However, the reported associations in the Arab adults’ cohort are supported by significant empirical p-values and they replicate the findings from our studies on cohorts of Arab children. Considering that the study variant is a “common” variant with MAF values of 17% in Arab and South Asian cohorts and 10% in East Asian cohort, the above-mentioned sample sizes give sufficient power at 80% to the study to detect upto 1.7%, 1.3% and 2.5% phenotypic variance in siMS score of Arab, South Asian and South East Asian cohort, respectively. The study variant association in Arabs which is not observed in South Asian and South East Asian individuals living in Kuwait suggest a unique life style or environmental factor interaction with CAV1 in Arabs mediating MetS. Nevertheless, it is fair to mention that future studies to replicate the reported ethnic-specificity may benefit from large multi-ethnicity cohorts with deep phenotyping relating to life style and environmental factors.
Conclusion
The present study builds on our first discovery that demonstrated a significant association between rs1997623-A and pediatric MetS (Nizam et al., 2018). Here we replicated the same association in Kuwaiti adults. The study is the first to show the association of the CAV1 rs1997623-A variant with MetS status and increased siMS score. Combined with our previous study, the association signals are seen in pediatric and adult MetS. We demonstrate the ethnic variation in the association signal—the association is seen only in the Kuwaiti Arab cohort and not in South Asian or South East Asian cohorts.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving human participants was reviewed and approved by Ethical Review Committee, Dasman Diabetes Institute, Kuwait. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AA, FA-M, and TAT conceived the study design, data interpretation, and wrote the manuscript. PH, RN, and DH performed the genetic analysis and contributed to manuscript writing. MM and MA-F performed the genotyping of the studied variant in the study cohorts and validated the genotype calls. All authors contributed to the drafting and critical review of the manuscript. All authors agreed to the final manuscript’s content and submission to the journal.
Acknowledgments
The authors would like to thank the Kuwait Foundation for the Advancement of Sciences (KFAS) for the institutional financial support (Study project number: RA CB-2021-007). We acknowledge the Dasman Diabetes Institute Biobank for processing the samples. We thank Fawaz Alzaid for his critical review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abou Ziki, M. D., and Mani, A. (2016). Metabolic syndrome: Genetic insights into disease pathogenesis. Curr. Opin. Lipidol. 27, 162–171. doi:10.1097/MOL.0000000000000276
Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
Alberti, K. G., Zimmet, P., and Shaw, J. (2006). Metabolic syndrome--a new worldwide definition. A consensus statement from the international diabetes federation. Diabet. Med. 23, 469–480. doi:10.1111/j.1464-5491.2006.01858.x
Amer, O. E., Sabico, S., Khattak, M. N. K., Alnaami, A. M., Aljohani, N. J., Alfawaz, H., et al. (2021)., Increasing prevalence of pediatric metabolic syndrome and its components among Arab youth: A time-series study from 2010-2019 Children 8. Children (Basel, Switzerland), 1129. doi:10.3390/children8121129
Ansarimoghaddam, A., Adineh, H. A., Zareban, I., Iranpour, S., HosseinZadeh, A., and Kh, F. (2018). Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab. Syndr. 12, 195–201. doi:10.1016/j.dsx.2017.11.004
Austin, E. D., Ma, L., LeDuc, C., Berman Rosenzweig, E., Borczuk, A., Phillips, J. A., et al. (2012). Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 5, 336–343. doi:10.1161/CIRCGENETICS.111.961888
Auton, A., Brooks, L. D., Durbin, R. M., Garrison, E. P., Kang, H. M., Korbel, J. O., et al. (2015). A global reference for human genetic variation. Nature 526, 68–74. doi:10.1038/nature15393
Baudrand, R., Goodarzi, M. O., Vaidya, A., Underwood, P. C., Williams, J. S., Jeunemaitre, X., et al. (2015). A prevalent caveolin-1 gene variant is associated with the metabolic syndrome in Caucasians and Hispanics. Metabolism. 64, 1674–1681. doi:10.1016/j.metabol.2015.09.005
Baudrand, R., Gupta, N., Garza, A. E., Vaidya, A., Leopold, J. A., Hopkins, P. N., et al. (2016). Caveolin 1 modulates aldosterone-mediated pathways of glucose and lipid homeostasis. J. Am. Heart Assoc. 5, e003845. doi:10.1161/JAHA.116.003845
Chen, S., Wang, X., Wang, J., Zhao, Y., Wang, D., Tan, C., et al. (2016). Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis 246, 148–156. doi:10.1016/j.atherosclerosis.2016.01.008
Cunningham, F., Allen, J. E., Allen, J., Alvarez-Jarreta, J., Amode, M. R., Armean, I. M., et al. (2022). Ensembl 2022. Nucleic Acids Res. 50, D988–D995. doi:10.1093/nar/gkab1049
Ford, E. S., Giles, H. G., and Dietz, W. H. (2002). Prevalence of the metabolic syndrome among US adults: Findings from the third national health and nutrition examination survey. JAMA 287, 356–359. doi:10.1001/jama.287.3.356
Grayson, T. H., Chadha, P. S., Bertrand, P. P., Chen, H., Morris, M. J., Senadheera, S., et al. (2013). Increased caveolae density and caveolin-1 expression accompany impaired NO-mediated vasorelaxation in diet-induced obesity. Histochem. Cell Biol. 139, 309–321. doi:10.1007/s00418-012-1032-2
Haddad, D., Al Madhoun, A., Nizam, R., and Al-Mulla, F. (2020). Role of caveolin-1 in diabetes and its complications. Oxid. Med. Cell. Longev. 2020, 9761539. doi:10.1155/2020/9761539
Hebbar, P., Abu-Farha, M., Abubaker, J., Channanath, A. M., Al-Mulla, F., and Thanaraj, T. A. (2021). Generalizability of GWA-identified genetic risk variants for metabolic traits to populations from the arabian Peninsula. Genes 12, 1637. doi:10.3390/genes12101637
Jin, Y., Lee, S-J., Minshall, R. D., and Choi, A. M. K. (2011). Caveolin-1: A critical regulator of lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L151–L160. doi:10.1152/ajplung.00170.2010
McMahon, K-A., Zajicek, H., Li, W-P., Peyton, M. J., Minna, J. D., Hernandez, V. J., et al. (2009). SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 28, 1001–1015. doi:10.1038/emboj.2009.46
Mora-Garcia, G., Gomez-Camargo, D., Alario, A., and Gomez-Alegria, C. (2018). A common variation in the caveolin 1 gene is associated with high serum triglycerides and metabolic syndrome in an admixed Latin American population. Metab. Syndr. Relat. Disord. 16, 453–463. doi:10.1089/met.2018.0004
Moreira, N. C. d. V., Hussain, A., Bhowmik, B., Mdala, I., Siddiquee, T., Fernandes, V. O., et al. (2020). Prevalence of Metabolic Syndrome by different definitions, and its association with type 2 diabetes, pre-diabetes, and cardiovascular disease risk in Brazil. Diabetes Metab. Syndr. 14, 1217–1224. doi:10.1016/j.dsx.2020.05.043
Nizam, R., Al-Ozairi, E., Goodson, J. M., Melhem, M., Davidsson, L., Alkhandari, H., et al. (2018). Caveolin-1 variant is associated with the metabolic syndrome in Kuwaiti children. Front. Genet. 9, 689. doi:10.3389/fgene.2018.00689
Noubiap, J. J., Nansseu, J. R., Lontchi-Yimagou, E., Nkeck, J. R., Nyaga, U. F., Ngouo, A. T., et al. (2022). Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet. Child. Adolesc. Health 6, 158–170. doi:10.1016/S2352-4642(21)00374-6
Pilch, P. F., and Liu, L. (2011). Fat caves: Caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metab. 22, 318–324. doi:10.1016/j.tem.2011.04.001
Pojoga, L. H., Underwood, P. C., Goodarzi, M. O., Williams, J. S., Adlet, G. K., Jeunemaitre, X., et al. (2011). Variants of the caveolin-1 gene: A translational investigation linking insulin resistance and hypertension. J. Clin. Endocrinol. Metab. 96, E1288–E1292. doi:10.1210/jc.2010-2738
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). Plink: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi:10.1086/519795
Ranasinghe, P., Mathangasinghe, Y., Jayawardena, R., Hills, A. P., and Misra, A. (2017). Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 17, 101. doi:10.1186/s12889-017-4041-1
Shin, S., and Jee, H. (2020). Prevalence of metabolic syndrome in the Gulf cooperation council countries: meta-analysis of cross-sectional studies. J. Exerc. Rehabil. 16, 27–35. doi:10.12965/jer.1938758.379
Shyu, H-Y., Chen, M-H., Hsieh, Y-H., Shieh, J-C., Yen, L-R., Wang, H-W., et al. (2017). Association of eNOS and Cav-1 gene polymorphisms with susceptibility risk of large artery atherosclerotic stroke. PLoS One 12, e0174110. doi:10.1371/journal.pone.0174110
Su, X., and Abumrad, N. A. (2009). Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol. Metab. 20, 72–77. doi:10.1016/j.tem.2008.11.001
Varda, N. M., and Gregoric, A. (2009). Metabolic syndrome in the pediatric population: A short overview. Pediatr. Rep. 1, e1. doi:10.4081/pr.2009.e1
Keywords: caveolin-1, rs1997623, MetS, metabolic syndrome, ethnicity, Arab, South Asian, Southeast Asian
Citation: Al Madhoun A, Hebbar P, Nizam R, Haddad D, Melhem M, Abu-Farha M, Thanaraj TA and Al-Mulla F (2022) Caveolin-1 rs1997623 variant and adult metabolic syndrome—Assessing the association in three ethnic cohorts of Arabs, South Asians and South East Asians. Front. Genet. 13:1034892. doi: 10.3389/fgene.2022.1034892
Received: 02 September 2022; Accepted: 05 October 2022;
Published: 21 October 2022.
Edited by:
Ranajit Das, Yenepoya University, IndiaReviewed by:
Patricia Canto, Universidad Nacional Autónoma de México, MexicoMartha Guevara-Cruz, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico
Copyright © 2022 Al Madhoun, Hebbar, Nizam, Haddad, Melhem, Abu-Farha, Thanaraj and Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thangavel Alphonse Thanaraj, YWxwaG9uc2UudGhhbmdhdmVsQGRhc21hbmluc3RpdHV0ZS5vcmc=; Fahd Al-Mulla, ZmFoZC5hbG11bGxhQGRhc21hbmluc3RpdHV0ZS5vcmc=
†These authors have contributed equally to this work
 Ashraf Al Madhoun
Ashraf Al Madhoun Prashantha Hebbar
Prashantha Hebbar Rasheeba Nizam
Rasheeba Nizam Dania Haddad
Dania Haddad Motasem Melhem
Motasem Melhem Mohamed Abu-Farha
Mohamed Abu-Farha Thangavel Alphonse Thanaraj
Thangavel Alphonse Thanaraj Fahd Al-Mulla
Fahd Al-Mulla