- Department of Urology, Institution of Urology, West China Hospital of Sichuan University, Chengdu, Sichuan, China
Background: The causal relationship between depression and erectile dysfunction (ED) is still uncertain.
Objectives: To identify the genetically predicted causality of depression on ED through Mendelian randomization (MR).
Materials and methods: A comprehensive GWAS meta-analysis comprising 807,553 Europeans provided single-nucleotide polymorphism (SNP) information for depression, and another genome-wide association analysis involving 223,805 European ancestries measured SNPs for ED. The inverse variance weighted (IVW) method was used as the primary MR analysis method to evaluate causal effects. In addition, the maximum likelihood method, MR-Egger, weighted median, robust adjusted contour score (MR.RAPS), and MR pleiotropic residual and outlier (MR-PRESSO) methods were used as supplements for sensitivity analysis.
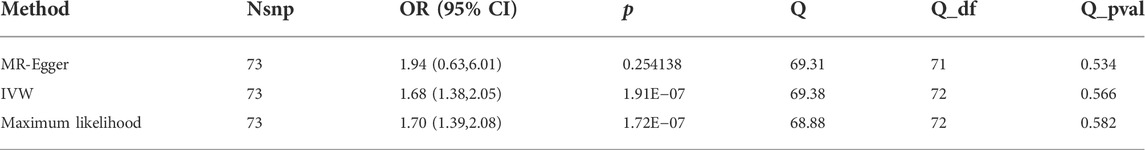
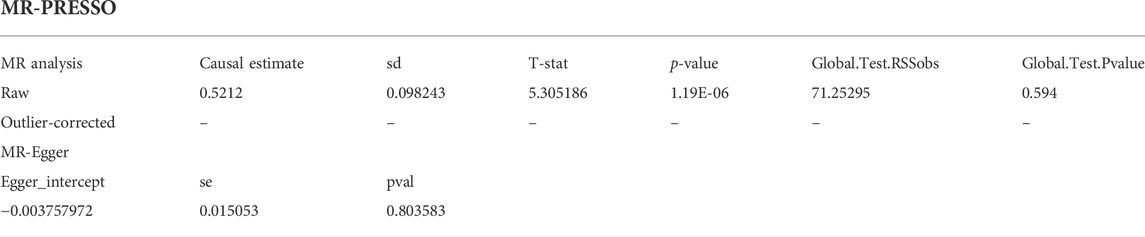
Results: According to the IVW analysis, depression significantly increases the incidence of ED (odds ratio [OR] = 1.68, 95% confidence interval [CI] = 1.38–2.05, p < 0.001). In sensitivity analyses, the ORs for the maximum likelihood method, MR-Egger, weighted median, MR.RAPS, and MR-PRESSO are 1.70 (95% CI = 1.39–2.08, p < 0 .001), 1.94 (95% CI = 0.63–6.01, p > 0 .05), 1.59 (95% CI = 1.21–2.10, p < 0 .001), 1 .70 (95% CI = 1.39–2.08, p < 0 .001), and 1.68 (95% CI = 1.40–2.04, p < 0 .001). There is no clear indication of potential heterogeneity or pleiotropy (p for the MR-Egger intercept = 0.804; p for the global test = 0.594; and p for Cochran’s Q statistics >0.05).
Conclusion: Genetically predicted depression plays a potentially causal role in the occurrence of ED.
Introduction
The inability to generate or sustain an adequate penile erection for satisfying sexual performance is referred to as erectile dysfunction (ED), a traditional male sexual dysfunction that increases with men’s age (NIH Consensus Conference, 1993). As a global problem, it is predicted that there will be 322 million cases of ED by 2025 (Ayta et al., 1999; McKinlay, 2000). The causes of ED are numerous and could be organic (e.g., vascular and neurogenic), psychogenic (e.g., excessive performance anxiety), or mixed (Salonia et al., 2020).
Depression, a common psychiatric disorder, consists of many clinical features, including sensations of melancholy and despair, lack of enthusiasm for enjoyable activities (anhedonia), disruption in appetite, sleep disturbances, fatigue, and difficulty in concentrating (Price, 2004). Clinical depression, excluding bipolar (manic-depressive) illnesses, is typically categorized as a major depressive disorder (MDD) or dysthymia, based on the extent. Both diseases were referred to as depression in this article.
Although depressive illness and ED appeared to have high comorbidity, their causal relationship was still unclear (Seidman and Roose, 2000). There were several proofs suggesting that depression may induce ED. Steiger et al. (1993) reported that the ED symptoms accessed by nocturnal penile tumescence (NPT) in depressed men were diminished after antidepressant treatment. After that, Thase et al. (1988) and Thase et al. (1992) assessed that the NPT time and penile rigidity were significantly impaired in depressed men compared with men without depression. Araujo et al. (1998) demonstrated that those who suffered from depressive symptomatology were 1.82 times more likely to get impotence independent of factors such as income and age. Moreover, some research also disclaimed that the risk of depressive symptoms also elevated among men who presented with moderated or completed erectile dysfunction (Laumann et al., 2008; Takao et al., 2011; Chou et al., 2015). However, these studies were restricted by confounding factors and reverse causation. It was possible to develop both conditions as a result of substance abuse or medical illness, or these conditions might be comorbid simply because they are highly prevalent, especially in older men, regardless of their etiology (Seidman and Roose, 2000).
Mendelian randomization (MR) was an epidemiological study design that could be applied to investigate the potential causality between depression and ED (Smith and Ebrahim, 2003). In Mendelian randomization, single-nucleotide polymorphisms (SNPs) served as proxy indicators of instrumental variables (IVs) of exposure. These genetic variants were used to evaluate exposure’s causal effect on outcome variables at the genetic level. Mendelian randomization has the following desirable features: 1) genetic variant measurements were highly accurate and can capture long-term exposure effects, thus avoiding estimation bias caused by the measurement error (Haycock et al., 2016). 2) The alleles were randomly segregated when forming a zygote and such segregation is independent of postnatal confounders, making the results less vulnerable to potential confounders and reverse causation (Smith and Ebrahim, 2003). Therefore, MR analysis imitated a naturally emerged controlled trial (RCT) to evaluate causal estimates.
Up to now, no study has elucidated the causes of the connections between depression and ED. Hence, using two-sample Mendelian randomization, our study investigated whether depression may be causally related to the onset of ED at the genetic level.
Materials and methods
MR assumptions
This study was conducted under the guideline of the reporting MR study, and the STORBE-MR checklist is provided in the Supplementary Material (Skrivankova et al., 2021a). There were three main assumptions needed to be satisfied in the MR study: (A-I) the instrument variables (IVs) are associated with exposure; (A-II) the IVs are not correlated with confounding factors; and (A-III) the IVs influence the outcome only through exposure (Skrivankova et al., 2021a; Skrivankova et al., 2021b).
Data sources
The GWAS performed by Bovijn et al. (2019), the largest GWAS of ED at present, was obtained as the summary-level dataset of ED. This comprehensive study recruited 223,805 European males (6,175 cases and 217,630 controls) from the hospital-recruited Partners HealthCare Biobank cohort, the United Kingdom Biobank (UKB) cohort, and the Estonian Genome Center of the University of Tartu cohort. ED cases were identified by self-report, doctor-diagnosed, taking oral ED drugs, or surgery history for ED.
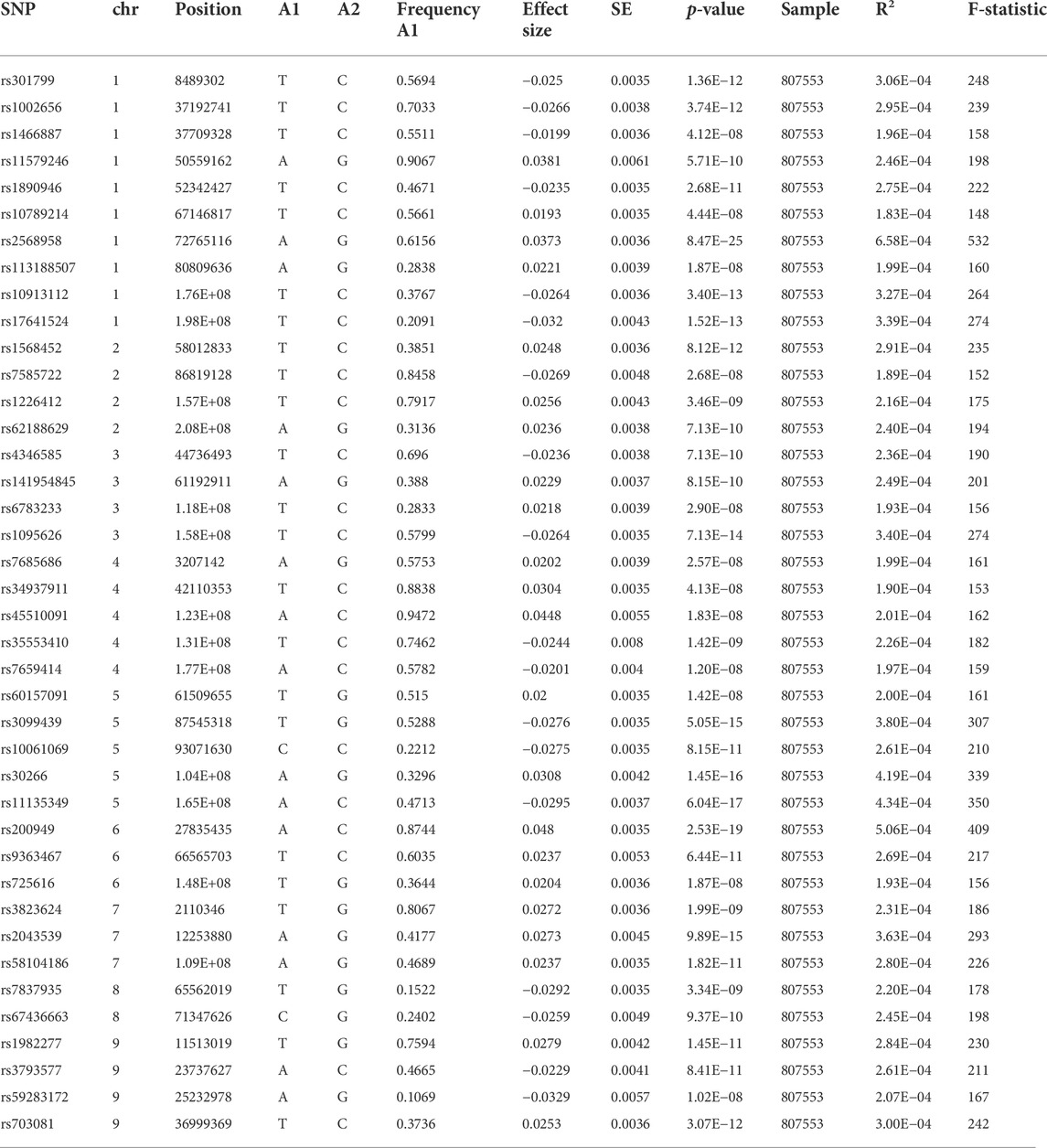
For the SNPs of depression, we extracted from the comprehensive GWAS meta-analysis comprising 807,553 Europeans (246,363 cases and 561,190 controls) and consisted of 23andMe_307k, United Kingdom Biobank, and PGC_139k cohorts (Howard et al., 2019). The depression phenotype definition ranged from “broad depression” (self-reported seeking assistance for issues with stress, anxiety, or sadness), self-reported depressive symptoms with accompanying disability and depression diagnosed clinically, and MDD clinically diagnosed. Further detailed information on these two phenotypes was obtained through previous publications (Bovijn et al., 2019; Howard et al., 2019).
Instrument variable selection
First, 102 single-nucleotide polymorphisms (SNPs) with genome-wide statistical significance ≥ 5 × 10−8 of depression were identified by Howard et al. (2019). F-statistics were used to calculate each SNP’s strength using the following formula:
Statistical analyses
To obtain MR estimates of depression on erectile dysfunction, we performed the inverse variance weighting (IVW) approach as the main result of MR analysis. For each
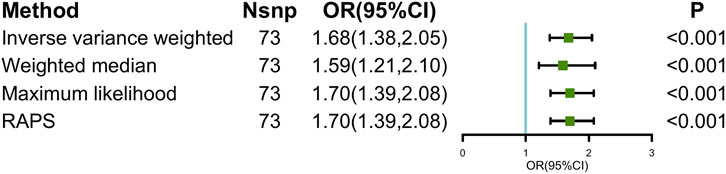
FIGURE 1. Causal estimates of depression on ED in MR. Abbreviations: ED, erectile dysfunction; MR, Mendelian randomization; Nsnp, number of single-nucleotide polymorphism used in MR analysis; OR, odds ratio; CI, confidence interval; RAPS, robust adjusted profile score.
We conducted sensitivity analysis mainly under three aspects: the heterogeneity test, the pleiotropy test, and the leave-one-out sensitivity test. The MR-Egger and maximum likelihood approaches were used to test the heterogeneity. Cochran’s Q statistic was utilized to quantify the heterogeneity. In particular, heterogeneity was identified if the Cochran Q test’s p value <0.05. Many sensitivity studies were carried out to test and lessen the impact of potential pleiotropy on the results, including the weighted median, MR-Egger, robust adjusted profile score (MR.RAPS), and MR Pleiotropy Residual Sum and Outlier (MR-PRESSO). The weighted median demands that variables from reliable instruments receive 50% of the total weight (Bowden et al., 2016). Even if all SNPs are invalid, the MR-Egger approach can still produce unbiased estimates (Bowden et al., 2015). In addition, the intercept term taken from MR-Egger could be adopted to quantify the directional pleiotropy. MR.RAPS could produce reliable causal estimates by running a linear model while accounting for the profile likelihood of the summary data, even when weak IVs existed (Zhao et al., 2019). In order to find the horizontal pleiotropic outliers, MR-PRESSO (MR-PRESSO) methods were applied. Outlying SNPs were then eliminated, and the effect estimates were re-evaluated (Verbanck et al., 2018). In addition, we used the leave-one-out method to determine which IVs had a significant impact on the estimates. This method utilized the IVW method to repeat MR analysis after excluding IV in turn.
An online tool (https://shiny.cnsgenomics.com/mRnd/) is used to assess the statistical power to identify the difference. The statistical power of depression on ED is 100% when the type I error rate is 0.05 (Brion et al., 2013).
All analyses and figures were made by the packages TwoSampleMR (version 0.5.6), MRPRESSO (version 1.0), and mr.raps (version 0.2) in R (version 4.2.0). p < 0.05 (two-sided) was considered statistically significant (Wald, 1940).
Results
MR estimates of depression on ED
The main causal relationship estimates of depression on ED revealed that depression could elevate the incidence of ED (IVW: OR = 1.68, 95% confidence intervals [CI]: 1.38–2.05, p < 0.001) (Figure 1 and Table 2). In addition, the ORs for the maximum likelihood method, MR-Egger, weighted median, MR.RAPS, and MR-PRESSO were 1.70 (95% CI = 1.39–2.08, p < 0 .001), 1.94 (95% CI = 0.63–6.01, p > 0 .05), 1.59 (95% CI = 1.21–2.10, p < 0 .001), 1.70 (95% CI = 1.39–2.08, p < 0 .001), and 1.68 (95% CI = 1.40–2.04, p < 0 .001), respectively (Table 2 and Table 3). The forest maps in Figure 2 indicated each SNP’s effect for depression on ED and their whole estimates. As shown in Figure 3, the risk of ED correspondingly increased as the significance of IVs on depression increased. The Cochran’s Q statistics in IVW, maximum likelihood method, and MR-Egger were 69.38 (p = 0.566), 68.88 (p = 0.582), and 69.31 (p = 0.534), respectively (Table 2), indicating a scanty demonstration of heterogeneity. The funnel plot displayed in Figure 4 visualized the heterogeneity. No directional pleiotropy was detected in the MR-Egger test (intercept = −0.0038, p = 0.804) and MR-PRESSO test (global test p = 0.594). No outlier SNPs were detected in MR-PRESSO analysis, suggesting limited evidence of pleiotropic bias. In addition, according to the leave-one-out analysis, no significant SNPs were driving the relationship between depression and ED (Figure 5).
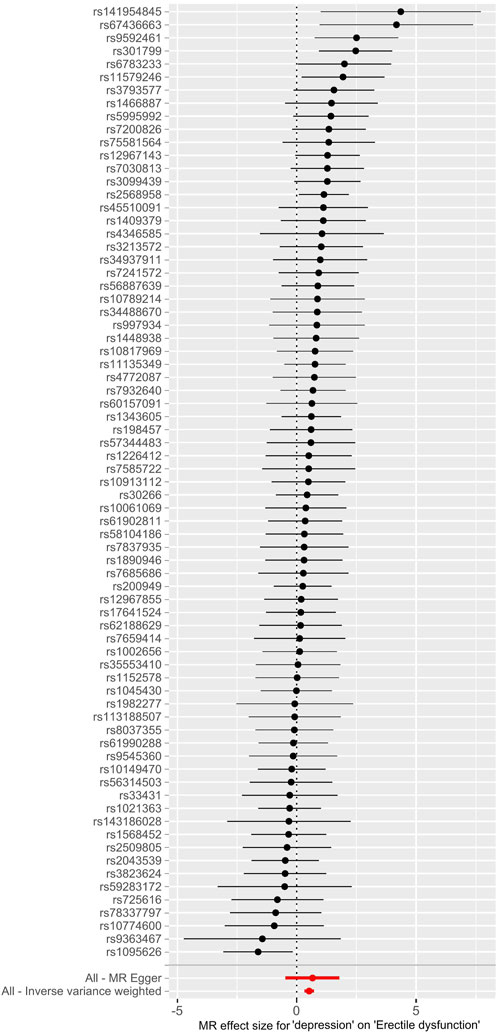
FIGURE 2. Forest maps of each SNP’s effect for depression on ED and all estimates. Abbreviations: SNP, single-nucleotide polymorphism; ED, erectile dysfunction.
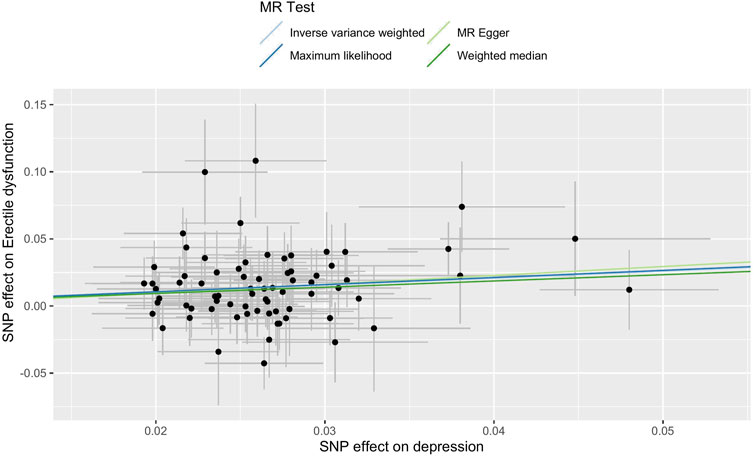
FIGURE 3. Scatter plot of the effect size of each SNP on depression and ED in MR. Abbreviations: SNP, single-nucleotide polymorphism; ED, erectile dysfunction; MR, Mendelian randomization.
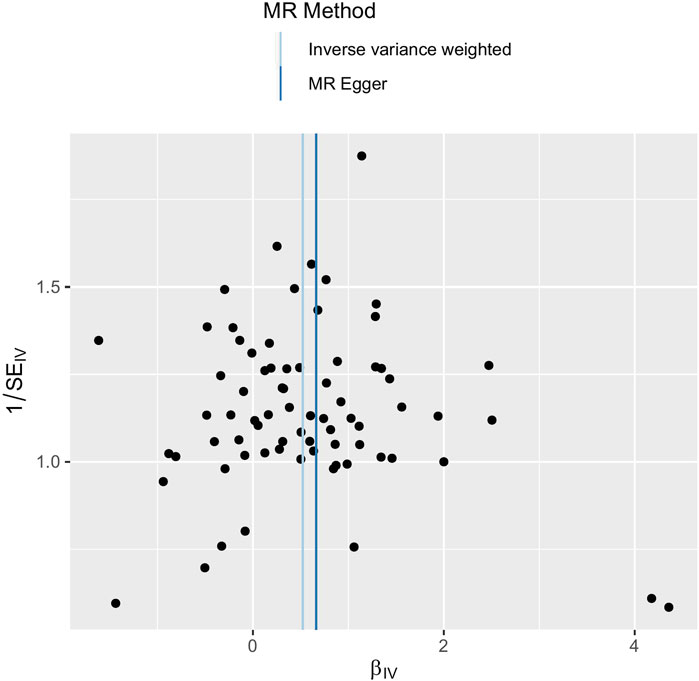
FIGURE 4. Funnel plot of SNPs used in MR of depression on ED. Abbreviations: SNP, single-nucleotide polymorphism; β, the effect size; SE, the standard error of the effect size; IVs, instrumental variables; ED, erectile dysfunction; MR, Mendelian randomization.
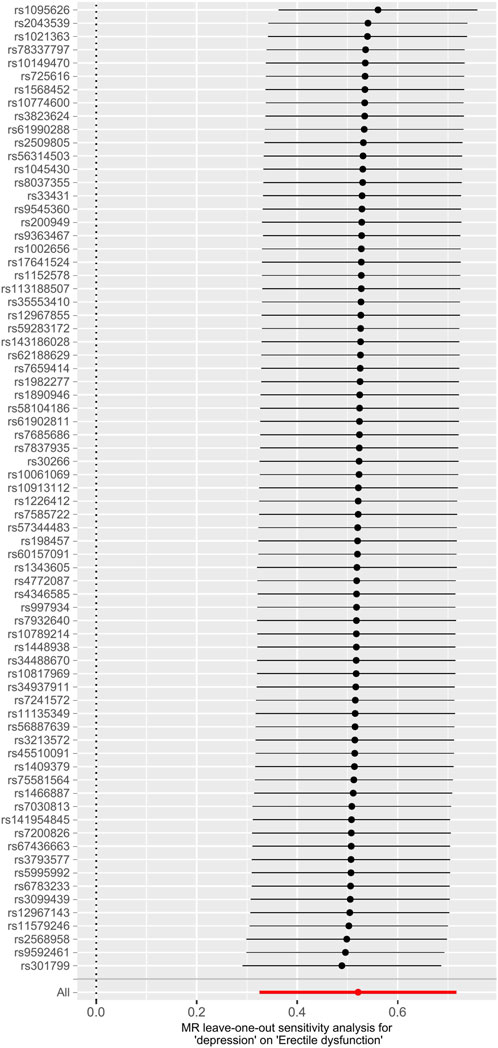
FIGURE 5. Leave-one-out analysis of depression on ED. Abbreviations: ED, erectile dysfunction; MR, Mendelian randomization.
Additionally, an online tool (https://sb452.shinyapps.io/overlap/) is used to calculate the overlap bias. The bias value is 0.001 with a type I error rate of 0.05 and a 100% overlap proportion assumption, which suggests that the demographic overlap is less likely to skew the results.
Discussion
It was challenging to determine the causal link between depression and ED without long-term prospective research and tightly controlled randomized trials. This study offered a genetically potential causal proof that depressed patients have a greater risk of ED within the scope of the MR design.
Earlier observational studies had described the association between depression and ED (Araujo et al., 1998; Shabsigh et al., 1998; Seftel et al., 2004; Shiri et al., 2007; Nwakanma and Ofoedu, 2016). Men who had untreated depression in the historic Male Massachusetts Aging Study (MMAS) were nearly twice as likely to report ED compared to males without depression after adjusting the ages (Araujo et al., 1998). Similar findings may be derived from the Multinational Men’s Attitudes to Life Events and Sexuality research, where depressive symptoms were found in 25% of men with ED and only 13% of those without ED (Rosen et al., 2004). Nevertheless, other studies discovered no correlation between the prevalence of ED and depressive symptoms (Kantor et al., 2002; Mak et al., 2002; Wong et al., 2009; Habibi et al., 2011; Kim et al., 2015). Of the 334 patients sampled, Kantor et al. (2002) indicated that present depressed symptoms were not linked to mild or severe erectile dysfunction. In addition, psychiatrists might be hesitant to probe a patient regarding ED in depth (ClaytonMcGarvey et al., 2001).
Our study supported the previous research that depression increased the prevalence of erectile dysfunction at the genetic level. A clear causal direction could facilitate clinical decision-making. Clinicians and decision-makers should pay attention to the ED symptoms of depression patients to improve overall patient care.
Although the causal relationship between depression and ED has been studied in detail, more research into the precise molecular pathways is still required. In general, two theories existed regarding how depression causes ED: the “behavior-based” model and the “biologic-based” one. According to the behavior-based model, depressed individuals exhibited behaviors or beliefs that led to performance anxiety, which negatively impacted erectile function. Meisler and Carey (1991) showed that mood might alter sexual desire, which supported the behavior model. The biological hypothesis was well articulated by Goldstein (2000), who noted that ED and inadequate cavernosal muscle relaxation were both caused by excess catecholamine production, which was caused by depression. In addition, the dopamine system and the dopaminergic synapse signaling pathway were dysfunctional in the depression rat model (Hong et al., 2022).
This study included several strengths and limitations. The primary advantage was that the MR analysis reduced endogeneity and bias caused by confounding variables. Considering the challenge of implementing RCTs, this study offered a genetic proof that depression causes impotence. Additionally, SNP data sources were restricted to people of European individuals, which limited the applicability of our findings to other races while avoiding population structure bias. Additionally, there might be some crossover between the samples of depression and ED, which could cause the models to be overfitted and reduce the strength of causal inference (Burgess et al., 2016). Despite this, the bias might not be very noticeable based on our study’s use of robust IVs (F-statistics > 10). Additionally, the non-linear relationship between depression and ED cannot be investigated because of the binary evaluation of depression and the absence of precise individual statistics (Burgess et al., 2016). Lastly, several confounding factors may also influence the causal associations between depression and ED. There were genetically complex associations between depression and other traits, such as insomnia, cardiovascular diseases (CVDs), and neuroticism. Previous studies demonstrated that insomnia and cardiovascular diseases shared multiple genetical variants with depression, which were also the risk factors of erectile dysfunction (Zhang et al., 2021a; Baranova et al., 2022). Short sleep duration was a risk factor for CVD in spite of observational studies or MR analysis (Ai et al., 2021; Wang et al., 2022). In addition, neuroticism was genetically associated with depression and showed substantial genetic overlaps with CVD, making it a potential confounder for our study (Zhang et al., 2021b; Zhang et al., 2022).
Conclusion
In conclusion, this study offered a genetic proof that depression may contribute to the development of ED. For more thorough and careful treatment of ED patients, medical therapies should be considered for patients with depression.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found at: https://gwas.mrcieu.ac.uk/.
Author contributions
KM, PS, and QD conceived and designed the analysis; KM, JZ, and JC extracted and checked the data; KM, ZL, LY, and LW performed the analysis; KM and PS wrote the manuscript; and QD reviewed the manuscript.
Funding
This work was supported by a Key Project of the National Natural Science Foundation of China; Grant ID: 8177060452 and Project of Science and Technology Department of Sichuan Province, Grant ID: 2021YFS0117 and 2021YF0500717SN.
Acknowledgments
The authors would like to express their gratitude to the participants and researchers of the database they used for this investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.1026227/full#supplementary-material
References
Ai, S., Zhang, J., Zhao, G., Wang, N., Li, G., So, H. C., et al. (2021). Causal associations of short and long sleep durations with 12 cardiovascular diseases: Linear and nonlinear mendelian randomization analyses in UK Biobank. Eur. Heart J. 42, 3349–3357. doi:10.1093/eurheartj/ehab170
Araujo, A. B., Durante, R., Feldman, H. A., Goldstein, I., and McKinlay, J. B. (1998). The relationship between depressive symptoms and male erectile dysfunction: Cross-sectional results from the Massachusetts male aging study. Psychosom. Med. 60, 458–465. doi:10.1097/00006842-199807000-00011
Ayta, I. A., McKinlay, J. B., and Krane, R. J. (1999). The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 84, 50–56. doi:10.1046/j.1464-410x.1999.00142.x
Baranova, A., Cao, H., and Zhang, F. (2022). Shared genetic liability and causal effects between major depressive disorder and insomnia. Hum. Mol. Genet. 31, 1336–1345. doi:10.1093/hmg/ddab328
Bovijn, J., Jackson, L., Censin, J., Chen, C. Y., Laisk, T., Laber, S., et al. (2019). GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am. J. Hum. Genet. 104, 157–163. doi:10.1016/j.ajhg.2018.11.004
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. doi:10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi:10.1002/gepi.21965
Brion, M. J., Shakhbazov, K., and Visscher, P. M. (2013). Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501. doi:10.1093/ije/dyt179
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. doi:10.1002/gepi.21758
Burgess, S., Davies, N. M., and Thompson, S. G. (2016). Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40, 597–608. doi:10.1002/gepi.21998
Burgess, S., and Thompson, S. G. (2011). Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40, 755–764. doi:10.1093/ije/dyr036
Chou, P. S., Chou, W. P., Chen, M. C., Lai, C. L., Wen, Y. C., Yeh, K. C., et al. (2015). Newly diagnosed erectile dysfunction and risk of depression: A population-based 5-year follow-up study in taiwan. J. Sex. Med. 12, 804–812. doi:10.1111/jsm.12792
Clayton, A. H., McGarvey, E. L., Abouesh, A. I., and Pinkerton, R. C. (2001). Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J. Clin. Psychiatry 62 (3), 185–190. doi:10.4088/jcp.v62n0309
Goldstein, I. (2000). The mutually reinforcing triad of depressive symptoms, cardiovascular disease, and erectile dysfunction. Am. J. Cardiol. 86, 41F–45f. doi:10.1016/s0002-9149(00)00892-4
Habibi, A., Kalbasi, S., Saadatjoo, S. A., and Arefi, M. G. (2011). Evaluation of erectile dysfunction and associated factors in type-II diabetic patients in birjand, Iran in 2008-2009. J. Res. Health Sci. 11, 97–102.
Haycock, P. C., Burgess, S., Wade, K. H., Bowden, J., Relton, C., and Davey Smith, G. (2016). Best (but oft-forgotten) practices: The design, analysis, and interpretation of mendelian randomization studies. Am. J. Clin. Nutr. 103, 965–978. doi:10.3945/ajcn.115.118216
Hong, Z. M., Chen, Z. L., Feng, J. L., Wang, S. J., Qiu, J. F., Zeng, Y. L., et al. (2022). Mechanistic analysis of erectile dysfunction in a depression rat model. J. Int. Med. Res. 50, 030006052211003. doi:10.1177/03000605221100334
Howard, D. M., Adams, M. J., Clarke, T. K., Hafferty, J. D., Gibson, J., Shirali, M., et al. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352. doi:10.1038/s41593-018-0326-7
Kantor, J., Bilker, W. B., Glasser, D. B., and Margolis, D. J. (2002). Prevalence of erectile dysfunction and active depression: An analytic cross-sectional study of general medical patients. Am. J. Epidemiol. 156, 1035–1042. doi:10.1093/aje/kwf142
Kim, M., Kim, S. Y., Rou, W. S., Hwang, S. W., and Lee, B. S. (2015). Erectile dysfunction in patients with liver disease related to chronic Hepatitis B. Clin. Mol. Hepatol. 21, 352–357. doi:10.3350/cmh.2015.21.4.352
Laumann, E. O., Kang, J. H., Glasser, D. B., Rosen, R. C., and Carson, C. C. (2008). Lower urinary tract symptoms are associated with depressive symptoms in white, black and Hispanic men in the United States. J. Urol. 180, 233–240. doi:10.1016/j.juro.2008.03.055
Mak, R., De Backer, G., Kornitzer, M., and De Meyer, J. M. (2002). Prevalence and correlates of erectile dysfunction in a population-based study in Belgium. Eur. Urol. 41, 132–138. doi:10.1016/s0302-2838(01)00029-x
McKinlay, J. B. (2000). The worldwide prevalence and epidemiology of erectile dysfunction. Int. J. Impot. Res. 12 (4), S6–s11. doi:10.1038/sj.ijir.3900567
Meisler, A. W., and Carey, M. P. (1991). Depressed affect and male sexual arousal. Arch. Sex. Behav. 20, 541–554. doi:10.1007/BF01550953
NIH Consensus conference (1993). Impotence. NIH Consensus development panel on impotence. Jama 270, 83–90.
Nwakanma, N. C., and Ofoedu, J. N. (2016). Depressive symptoms and marital adjustment among primary care patients with erectile dysfunction in Umuahia, Nigeria. S. Afr. J. Psychiatr. 22, 979. doi:10.4102/sajpsychiatry.v22i1.979
Rosen, R. C., Fisher, W. A., Eardley, I., Niederberger, C., Nadel, A., Sand, M., et al. (2004). The multinational men's Attitudes to Life Events and sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr. Med. Res. Opin. 20, 607–617. doi:10.1185/030079904125003467
Salonia, A., Bettocchi, C., Carvalho, J., Corona, G., Jones, T., Kadioglu, A., et al. (2020). EAU guidelines on sexual and reproductive health. AvaliableAt: https://uroweb.org/guidelines/sexual-and-reproductive-health.
Seftel, A. D., Sun, P., and Swindle, R. (2004). The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J. Urol. 171, 2341–2345. doi:10.1097/01.ju.0000125198.32936.38
Seidman, S. N., and Roose, S. P. (2000). The relationship between depression and erectile dysfunction. Curr. Psychiatry Rep. 2, 201–205. doi:10.1007/s11920-996-0008-0
Shabsigh, R., Klein, L. T., Seidman, S., Kaplan, S. A., Lehrhoff, B. J., and Ritter, J. S. (1998). Increased incidence of depressive symptoms in men with erectile dysfunction. Urology 52, 848–852. doi:10.1016/s0090-4295(98)00292-1
Shiri, R., Koskimäki, J., Tammela, T. L., Häkkinen, J., Auvinen, A., and Hakama, M. (2007). Bidirectional relationship between depression and erectile dysfunction. J. Urol. 177, 669–673. doi:10.1016/j.juro.2006.09.030
Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Davies, N. M., Swanson, S. A., VanderWeele, T. J., et al. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ Clin. Res. ed.) 375, n2233. doi:10.1136/bmj.n2233
Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Yarmolinsky, J., Davies, N. M., Swanson, S. A., et al. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. Jama 326, 1614–1621. doi:10.1001/jama.2021.18236
Smith, G. D., and Ebrahim, S. (2003). Mendelian randomization': Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22. doi:10.1093/ije/dyg070
Steiger, A., Holsboer, F., and Benkert, O. (1993). Studies of nocturnal penile tumescence and sleep electroencephalogram in patients with major depression and in normal controls. Acta Psychiatr. Scand. 87, 358–363. doi:10.1111/j.1600-0447.1993.tb03387.x
Takao, T., Tsujimura, A., Okuda, H., Yamamoto, K., Fukuhara, S., Matsuoka, Y., et al. (2011). Lower urinary tract symptoms and erectile dysfunction associated with depression among Japanese patients with late-onset hypogonadism symptoms. Aging Male 14, 110–114. doi:10.3109/13685538.2010.512374
Thase, M. E., Reynolds, C. F., Jennings, J. R., Frank, E., Garamoni, G. L., Nofzinger, E. A., et al. (1992). Diminished nocturnal penile tumescence in depression: A replication study. Biol. Psychiatry 31, 1136–1142. doi:10.1016/0006-3223(92)90158-v
Thase, M. E., Reynolds, C. F., Jennings, J. R., Frank, E., Howell, J. R., Houck, P. R., et al. (1988). Nocturnal penile tumescence is diminished in depressed men. Biol. Psychiatry 24, 33–46. doi:10.1016/0006-3223(88)90119-9
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi:10.1038/s41588-018-0099-7
Wald, A. (1940). The fitting of straight lines if both variables are subject to error. J. Ann. Math. Stat. 11, 284–300.
Wang, S., Li, Z., Wang, X., Guo, S., Sun, Y., Li, G., et al. (2022). Associations between sleep duration and cardiovascular diseases: A meta-review and meta-analysis of observational and mendelian randomization studies. Front. Cardiovasc. Med. 9, 930000. doi:10.3389/fcvm.2022.930000
Wong, S. Y., Leung, J. C., and Woo, J. (2009). Sexual activity, erectile dysfunction and their correlates among 1, 566 older Chinese men in Southern China. J. Sex. Med. 6, 74–80. doi:10.1111/j.1743-6109.2008.01034.x
Zhang, F., Baranova, A., Zhou, C., Cao, H., Chen, J., Zhang, X., et al. (2021). Causal influences of neuroticism on mental health and cardiovascular disease. Hum. Genet. 140, 1267–1281. doi:10.1007/s00439-021-02288-x
Zhang, F., Cao, H., and Baranova, A. (2022). Genetic variation mediating neuroticism's influence on cardiovascular diseases. J. Psychopathol. Clin. Sci. 131, 278–286. doi:10.1037/abn0000744
Zhang, F., Cao, H., and Baranova, A. (2021). Shared genetic liability and causal associations between major depressive disorder and cardiovascular diseases. Front. Cardiovasc. Med. 8, 735136. doi:10.3389/fcvm.2021.735136
Keywords: depression, erectile dysfunction, causal estimates, Mendelian randomization, single-nucleotide polymorphisms
Citation: Ma K, Song P, Liu Z, Yang L, Wang L, Zhou J, Chen J and Dong Q (2022) Genetic evidence suggests that depression increases the risk of erectile dysfunction: A Mendelian randomization study. Front. Genet. 13:1026227. doi: 10.3389/fgene.2022.1026227
Received: 23 August 2022; Accepted: 26 September 2022;
Published: 14 October 2022.
Edited by:
Fuquan Zhang, Nanjing Medical University, ChinaReviewed by:
Can Yang, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaSizhi Ai, The First Affiliated Hospital of Xinxiang Medical University, China
Copyright © 2022 Ma, Song, Liu, Yang, Wang, Zhou, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Dong, ZHFpYW5nNjY2QDE2My5jb20=
†These authors have contributed equally to this work
 Kai Ma
Kai Ma Pan Song
Pan Song Zhenghuan Liu
Zhenghuan Liu Qiang Dong
Qiang Dong