
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 21 October 2022
Sec. Epigenomics and Epigenetics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.1025306
This article is part of the Research Topic Epigenetics of Metabolism, Immunology and Aging View all 17 articles
Background: Osteosarcoma (OSA), a focus for orthopedic surgeons, always results in severe death due to metastasis. CD146 is severely expressed in several tumors, indicating its potential as a biomarker for OSA.
Method: Two OSA cohorts were enrolled in this study. A Therapeutically Applicable Research to Generate Effective Treatments-Osteosarcoma (TARGET-OS) cohort was used as a training cohort, and GSE21257 was used as the external validation cohort. The R package “limma” was used to discriminate the differentially expressed genes among CD146-high and CD146-low patients and was further annotated by the enriched signaling pathways. The R package MOVICS was used to evaluate immune infiltration and the response to chemotherapy and immunotherapy. All statistical analyses were performed by R version 4.0.2, and p < 0.05 was considered statistically significant.
Result: CD146 plays an important role in promoting the progression, invasion, and metastasis of several tumors. In the current study, we first revealed an integrative unfavorable prognosis in patients with tumors (p < 0.01, HR: 1.10, 95% CI: 1.07-1.14). CD146 is tightly correlated with m5C RNA methylation modification genes in OSA. Furthermore, we revealed that CD146 acts as an oncogene in OSA patients and is linked to poor prognosis in both the TARGET-OS cohort (p = 0.019, HR: 2.61, 95% CI: 1.171-5.834) and the GSE21257 cohort (p = 0.005, HR: 3.61, 95% CI: 1.474-8.855), with a total of 137 patients, regardless of whether they were adjusted for clinical pathological features. Highly-expressed CD146 impacts the signaling pathways of cytokine‒cytokine receptor interactions and is associated with the high infiltration of immunocytes. Moreover, patients with high CD146 expression were more likely to be sensitive to anti-PD-1 immunotherapy, while patients with low expression of CD146 were more likely to be sensitive to cisplatin and doxorubicin chemotherapy.
Conclusion: Overall, CD146 is an independent prognostic factor for OSA patients and can help doctors select clinical treatment strategies.
Osteosarcoma (OSA), which derives from primitive bone-forming mesenchymal cells, is the most common primary bone malignancy (Valery et al., 2015). The incidence rate of OSA changes by age, sex, and race. There are bimodal age peaks of OSA; one is at the age of 0–24 years old, and the other is over 65 years old (Mirabello et al., 2009a; Rojas et al., 2021). It was considered that the incidence of OSA in males was higher than that in females. Moreover, the incidence in females is higher than that in males in the group aged less than 15 years old (Nie and Peng, 2018). In addition, OSA is more likely to be observed in black people (6.8 per million persons per year) and Hispanics (6.5 per million persons per year) than in white people (4.6 per million persons per year) (Mirabello et al., 2009b; Ottaviani and Jaffe, 2009). Many factors can significantly affect the survival outcome of OSA, such as metastatic status, percentage of necrosis, tumor size, Enneking stage, local recurrence, and treatment therapeutic strategy (Pakos et al., 2009). Multidisciplinary approaches are applied for the treatment of patients with OSA—including surgery, radiotherapy, polychemotherapy, and immunomodulation—as 80%–90% of local OSA will develop metastasis (Ritter and Bielack, 2010). Although multidisciplinary treatment strategies have reduced mortality in OSA patients, the prognosis remains poor, and the 5-year and 10-year survival rates are only 56.31% and 22.33%, respectively (Yasin et al., 2020). Therefore, it is crucial to find robust biomarkers and prognostic factors to predict the progression, invasion, and metastasis of OSA.
Melanoma cell adhesion molecule (MCAM/CD146), which was reported in 1987 by Johnson and colleagues, is an integral membrane glycoprotein and belongs to the immunoglobin superfamily. It consists of a signal peptide, an extracellular fragment with five immunoglobin-like domains, a transmembrane region, and a short cytoplasmic tail (Lehmann et al., 1989; Johnson et al., 1993). At present, the pathological processes and physiological function of CD146 have been revealed, for instance, in tissue regeneration, inflammation, infections, signal transduction, cell migration, the cell cycle, mesenchymal stem cell differentiation, angiogenesis, and the immune response (Wang and Yan, 2013). As reported, CD146 is a cell surface receptor and can combine with many ligands involved in proliferation-related signaling pathways (Li et al., 2003). In addition, it can induce tumor angiogenesis (So et al., 2010). These biological characteristics promote tumor progression, invasion, and metastasis, especially in melanoma (Melnikova et al., 2009).
Currently, the focus on OSA has expanded from the tumor cell itself to the tumor microenvironment; the infiltrated immunocytes play an important role in tumor proliferation and migration or are resistant to chemo drugs (Corre et al., 2020). Tumor-associated macrophages account for approximately 50% of tumor volume of OSA (Huang et al., 2021), and M2 type can accelerate the process of tumor metastasis, which can be treated with all-trans retinoic acid to inhibit the polarization of M2 macrophage (Zhou et al., 2017). Yoshida et al. reported that anti-PD-1 therapy can inhibit the infiltration of Tregs with the murine LM8 cell osteosarcoma model, resulting in reduced tumor volume and prolonged survival (Yoshida et al., 2020). CD146 is highly expressed in advanced primary or metastatic melanoma cells but is rarely detected in normal cells, indicating its potential as a biomarker for predicting tumor initialization and prognosis. Beyond melanoma, increased expression of CD146 has been reported in more than ten types of tumors to date (Zabouo et al., 2009; Feng et al., 2012; Zeng et al., 2012; Tian et al., 2013; Wang and Yan, 2013; Li et al., 2014; Liang et al., 2017; Sechler et al., 2017). These studies have demonstrated that CD146 is a novel metastasis biomarker and prognostic factor and that it can also be used as a therapeutic target (Xie et al., 1997; Zabouo et al., 2009; Wu et al., 2012).
Similar results have been reported for OSA and CD146 (Schiano et al., 2012; Westrøm et al., 2016). In the current study, we confirmed the high expression of CD146 in most cancers, and the expression levels were significantly associated with the corresponding prognosis. Univariate and multivariate analyses were performed to reveal that CD146 acts as an independent prognostic factor for OSA patients. The critical pathways were identified by GO enrichment and HALLMARK analysis. Furthermore, we found that CD146 is a biomarker for anti-PD1 therapy. Our goal was to prove the prognostic value of CD146 in OSA and provide a novel tool for predicting immunotherapy efficacy.
Two OSA cohorts were enrolled in this study. The Therapeutically Applicable Research to Generate Effective Treatments (TARGET) osteosarcoma (TARGET-OS) cohort was derived from the UCSC Xena platform (http://xena.ucsc.edu/). It contains 84 samples of gene expression profiles and corresponding clinical information. The GENCODE27 annotation file was applied to annotate the gene symbols of mRNA. Another cohort, GSE21257, which contains 53 OSA patients, was downloaded from the Gene Expression Omnibus platform (GEO, http://www.ncbi.nlm.nih.gov/geo/). The TARGET-OS cohort was used as a training cohort, and GSE21257 was used as the external validation cohort. We conducted log2(TPM+1) processing on the expression data of CD146 to scale it. The median values of CD146 expression were set as threshold values to divide patients into low- and high-expression groups. In addition, we compared the expression of CD146 in normal and tumor tissues based on data from The Cancer Genome Atlas Program (TCGA) project (https://docs.gdc.cancer.gov/) and the GTEx project (https://www.gtexportal.org/home/). The evaluation of the CD146 prognostic value to overall survival (OS) time in pan-cancer was conducted on an online website (http://sangerbox.com/).
We used the R package “limma” to discriminate the differentially expressed genes (DEGs) among CD146-high and CD146-low patients (Ritchie et al., 2015). A fold-change > 0.4 and an adjusted p value <0.01 were set as the cut-off values to filter the DEGs. The packages “org.Hs.eg.db” and “msigdbr” were used to perform Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and HALLMARK enrichment analyses (Ashburner et al., 2000; Liberzon et al., 2015); clusterProfiler was applied to further elucidate potential gene functional interpretation and pathway enrichment (Yu et al., 2012).
To evaluate the immunocyte infiltration status and the difference between the low- and high-expression groups, we collected 28 immunocyte signatures from prior research (Yoshihara et al., 2013). Subsequently, the runGSVA module in MOVICS was used to perform single sample gene set variation analysis (GSVA) to estimate the normalized enrichment score (NES) for the 28 signatures in each OSA patient (Lu et al., 2020).
We applied the comDrugen module in MOVICS to the clinical data of the TARGET-OS cohort to explore the chemotherapy efficacy difference between the low and high CD146-expression groups. Four chemotherapeutic drugs are shown in the violin plots. We also predicted the chemotherapeutic response to EBFs based on the largest publicly available pharmacogenomics database, the Genomics of Drug Sensitivity in Cancer (GDSC), with the use of the GSCA online website (Liu et al., 2018). To compare the likelihood of the CD146 groups with the immunotherapy subgroups, we performed subclass mapping analysis in the samples from patients who had received anti-PD-1 or anti-CTLA4 checkpoint therapy (Wang et al., 2016; Thanindratarn et al., 2019).
All statistical analyses were performed by R version 4.0.2. Student’s t-test was applied to compare the two groups if the data were normally distributed for continuous data; otherwise, the Wilcoxon rank-sum test was used. For categorical data, the chi-square test and Fisher’s exact test were conducted. Kaplan‒Meier curves were generated to compare OS based on the log-rank test. A nomogram was constructed by the R package “regplot” with the results from Cox regression analysis. Calibration curves were plotted to assess the calibration ability of the nomogram, and decision curve analysis (DCA) was performed to show the clinical usefulness of the nomogram. The receiver operating characteristic (ROC) area under the curve (AUC) was calculated to assess the stability of prediction. To identify the independent risk factors, univariate and multivariate analyses were performed. We also established the Cox regression model to calculate the hazard ratio (HR) values and the 95% confidence interval (95% CI). The correlation was determined by the Pearson correlation test, with p < 0.05 being considered statistically significant.
Overexpression of CD146 has been reported in various cancers. However, the comparison of CD146 mRNA levels in different tumors is still unclear. Although all tumors have a certain CD146 mRNA level, the expression levels in sarcoma (SARC) and kidney renal clear cell sarcoma (KIRC) are higher than those in other tumors. The results also demonstrated that CD146 is highly expressed in tumor tissues relative to normal tissues in many tumors, including head and neck squamous cell carcinoma (HNSC), prostate adenocarcinoma (PRAD), kidney renal papillary cell carcinoma (KIRP), KIRC, liver hepatocellular carcinoma (LIHC), thyroid carcinoma (THCA), and pheochromocytoma and paraganglia (PCPG) (Figure 1A). Furthermore, we conducted a meta-analysis to evaluate the connection between CD146 expression and OS time across cancers. The results showed that CD146 acted as an oncogene and was linked to poor prognosis in most tumor types (p < 0.01, HR: 1.10, 95% CI: 1.07-1.14, Figure 1B). To validate the conclusion, we performed a log-rank test via Kaplan‒Meier curves, which was consistent with the meta-analysis (Supplementary Figure S1).
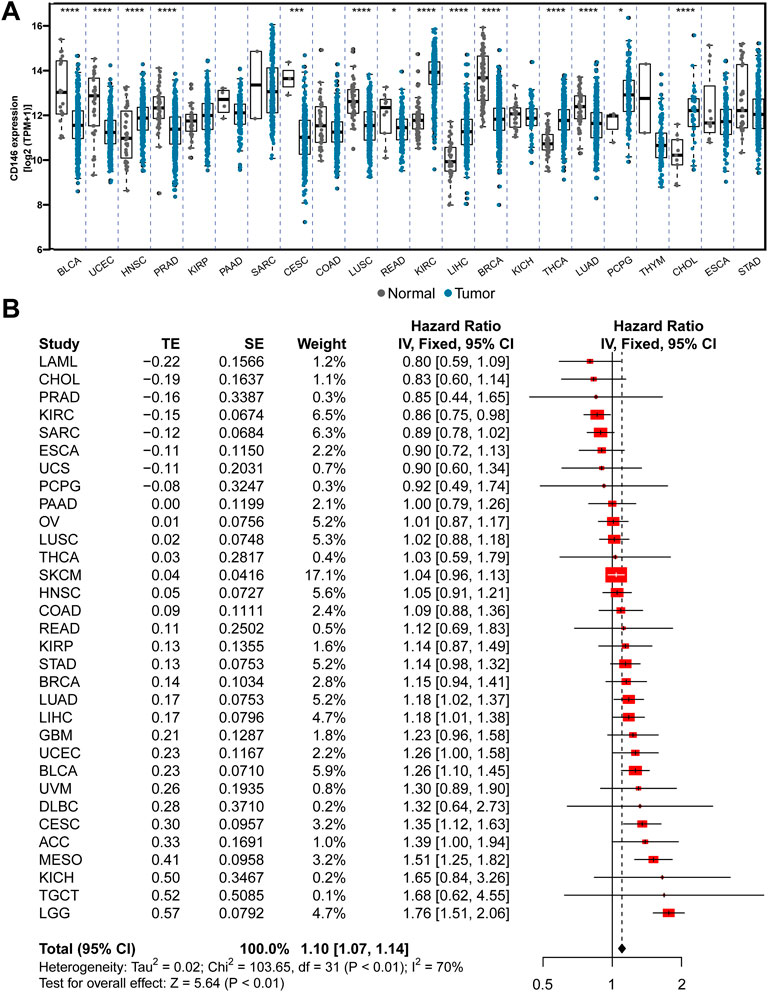
FIGURE 1. CD146 is increased in tumor tissue and predicts poor prognosis among pan-cancer. (A) The differential expression of CD146 in 22 types of tumor and adjacent normal tissues. (B) Meta-analysis revealed the integrative hazard risk of CD146 to pan-cancer.
It is widely reported that posttranscriptional modification plays a key role in tumorigenesis, including the methylation modification methods of m1A, m5C, and m6A. Zhang et al. (2022) reported that METTL3 upregulates COPS5 expression by promoting COPS5 methylation in an m6A-related manner and results in the promotion of OSA progression. Li et al. (2022) also demonstrated the METTL14-mediated epitranscriptome modification of MN1 mRNA and the promotion of OSA tumorigenicity. Therefore, we assessed the correlation between RNA methylation modification genes and CD146 levels. We observed that the CD146 expression levels in most types of tumor positively correlated with RNA methylation modification based on the pan-cancer data from the TCGA project, especially for lymphoid neoplasms, diffuse large B-cell lymphoma, cholangiocarcinoma, ovarian serous cystadenocarcinoma, and thymoma (Figure 2A). For RNA methylation genes in OSA, we evaluated their correlation with CD146 in the TARGET-OS and GSE21257 cohorts and found similar results, especially for the m5C genes DNMT3B, NSUN2, NUSU5, NSUN6, and DNMT1 (Figure 2B).

FIGURE 2. CD146 is regulated by RNA methylation. (A) Correlation of CD144 expression and RNA methylation regulators in m1A, m5C, and m6A among pan-cancer. (B) Correlation of CD144 expression and RNA methylation regulators among pan-cancer OSA datasets.
To explore the association between CD146 and OSA, we divided the TARGET-OS cohort into two subgroups: high CD146 expression (HEXP) and low CD146 expression (LEXP). The clinical feature distribution in HEXP and LEXP showed no difference, except for the first tumor event, and HEXP patients experienced more relapse (p = 0.014, Table 1). The Kaplan‒Meier curve for OS time is shown in Figure 3A. We found a statistically significant difference in OS time among patients in the HEXP and LEXP subgroups (p = 1.019, HR = 2.61, 95% CI: 1.71—5.834). As the 1-year, 3-year, and 5-year AUCs were 0.573, 0.668, and 0.730, respectively, we confirmed that CD146 is responsible for the poor prognosis in OSA (Figure 3B). Univariate analysis showed that the metastatic-diagnosis (p < 0.001, HR: 4.764, 95% CI: 2.221-10.221) and CD146 subgroups (p = 0.019, HR: 2.614, 95% CI: 1.171-5.834) were related to the OS time of OSA (Figure 3C). Multivariable analysis revealed that the same variables were related to poor prognosis, which proved that CD146 is an independent prognostic factor for OSA (p = 0.020, HR: 2.639, 95% CI: 1.166-5.972, Figure 3D). At present, the impact of age and sex on the prognosis of OSA is controversial (Pan et al., 2019; Ding et al., 2020). It is worth noting that we validated the impact of age and sex on prognosis to be small.
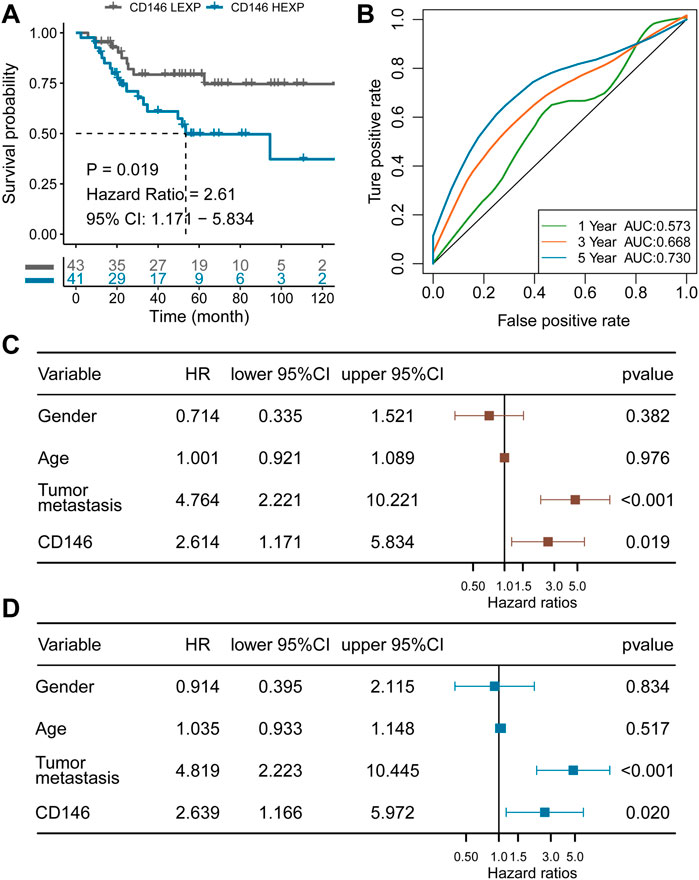
FIGURE 3. CD146 is an independent prognostic factor for OSA identified in the TARGET-OS cohort. (A) K-M plot showing the diverse clinical overall survival outcomes of the CD146 low and high groups; (B) ROC curve showing the prognostic value of the CD146 expression level; (C) results from univariate Cox regression analysis of the CD146 group and clinical features; (D) results from multivariate Cox regression analysis of the CD146 group and clinical features.
Furthermore, we also constructed a prediction nomogram with the clinical features (Figure 4A). We revealed that the CD146 expression group acted as an assistor that enhanced the traditional pathological method. The total AUC value increased to 0.765 (95% CI: 0.660-0.871, Figure 4B), indicating the preferable prognostic value of the nomogram—also confirmed by the calibration curve. There was no significant difference between the nomogram-predicted survival probability and the actual survival probability (p = 0.243 for 3-year, p = 0.416 for 5-year, Figure 4C). We also observed that the nomogram performed with better prediction effectiveness than single metastasis or CD146 expression (Figure 4D). We calculated the prognostic C-index value of clinical features and observed that the nomogram contained the highest value of 0.781, as compared with age (C-index: 0.490), gender (C-index: 0.561), and metastasis status (C-index: 0.696) (Figure 4E). Moreover, we randomly selected a portion of patients (n = 60) to be re-assessed for the prognostic stability of nomogram ten times and observed that all the 10 C-indexes were higher than 0.75 (Figure 4F), indicating that the nomogram is accurate and stable.
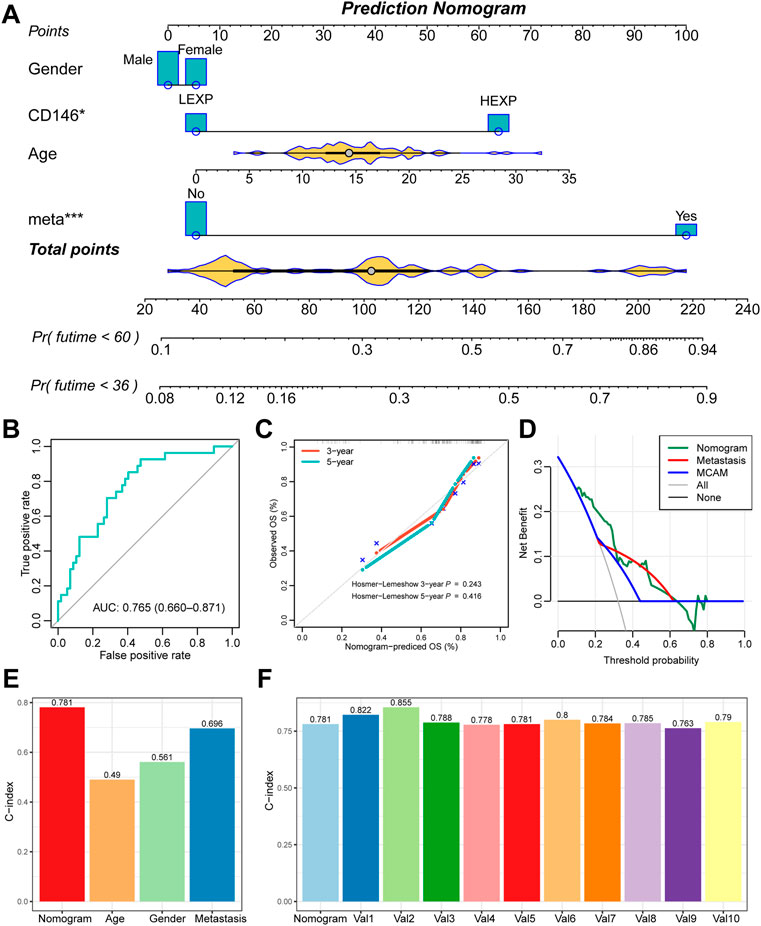
FIGURE 4. The nomogram further enhanced the prognostic value of CD146 in OSA. (A) Prognostic nomogram of 3-year and 5-year overall survival of OSA patients; (B) ROC curves for the nomogram of overall death events; (C) calibration plot to compare the nomogram prediction and the actual death events; (D) DCA curve for the nomogram, metastasis, and CD146 expression group; (E) C-index of the nomogram, age, gender, and metastasis status; (F) 10-times randomization C-index test to assess the stability of the prognostic nomogram.
Enrichment analysis was carried out to investigate the potential mechanism by which CD146 impacts OSA. We first obtained 580 DEGs between the HEXP and LEXP subgroups along with the preset cut-off values mentioned above. The 580 DEGs were further enriched and annotated in different biological pathways. According to the biological process (BP) GO terms, pathways involving vasculature development and positive anion transport were the most relevant to CD146. In molecular function (MF) analysis, CD146 was obviously relevant to signaling receptor activator activity, receptor-ligand activity, cytokine receptor binding, and cytokine activity (Figure 5A). In KEGG analysis, numerous DEGs were enriched in the neuroactive ligand-receptor interaction and cytokine‒cytokine receptor interaction pathways (Figure 5B). For HALLMARK analysis, complement and TNFA signaling via NFKB had the most impact on CD146 (Figure 5C). The above enriched signaling pathways indicated that CD146 played an important role in the immune system in OSA. Therefore, we were concerned about the link between immunocytes and CD146 expression levels. As shown in Figure 5D, 11 types of immunocytes, including activated dendritic cells, eosinophils, gamma cells, immature dendritic cells, mast cells, monocytes, neutrophils, regulatory T cells, T follicular helper cells, type-17 T helper cells, and myeloid-derived suppressor cells were more highly infiltrative in the HEXP group.
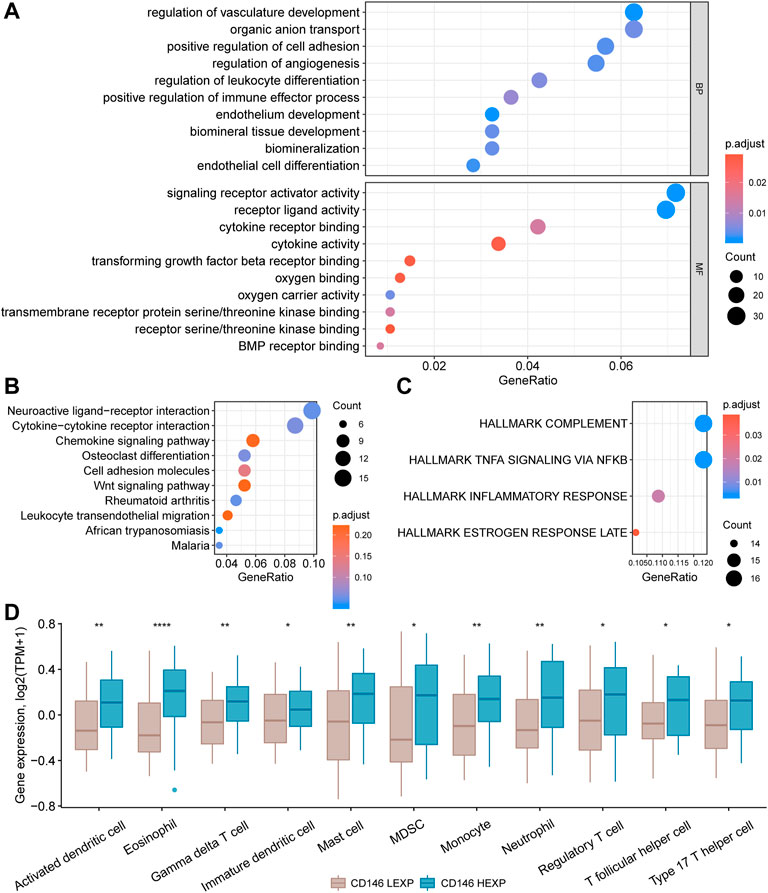
FIGURE 5. Enrichment of signaling pathways and differential infiltration of immunocytes in the CD146 high and low groups. Enrichment of 580 DEGs between the HEXP and LEXP subgroups by GO terms (A), KEGG terms (B), and HALLMARK terms (C). (D) Differential infiltration of 12 immunocytes between the CD146 high and low groups.
Immunotherapy and chemotherapy have developed essential treatment strategies for excellent therapeutic effects on malignant tumors (Furue, 2003; Rodig et al., 2018). However, the current evidence shows that most checkpoint inhibitors have little effect on OSA and that chemotherapeutic drug resistance increases each year for multiple causes (Chatterjee and Bivona, 2019; Chen et al., 2021). Therefore, it is crucial to validate predictive biomarkers to optimize the choice of treatment strategy. In this study, we set the half maximal inhibitory concentration (IC50) as the observation index and compared the therapeutic effect of four commonly-used chemotherapy items in the LEXP and HEXP subgroups. We found a significant difference in the IC50 value of LEXP and HEXP to cisplatin (Figure 6A, p = 0.002) and that LEXP patients can respond to doxorubicin treatment (p = 0.054) but not to etoposide (p = 0.21) or bleomycin (p = 0.44) (Figure 6A). Furthermore, we also tried to select potential chemotherapy drugs from the GSCA online website and revealed that patients with high levels of CD146 were sensitive to chemotherapy with PLX4720, dabrfenib, SE590885, staurosporine, BRD-K99006945, vemurafenib, and several other proposed drugs (Table 2). To predict the immunotherapy response for each patient with OSA, we subsequently conducted SubMap analysis with an OSA cohort which included both patients receiving and not receiving anti-PD-1 or anti-CTLA4 therapy. There was no significant difference in CTAL-4 treatment response between the LEXP and HEXP subgroups; however, HEXP patients presented a better treatment response to anti-PD1 therapy than LEXP (Figure 6B, Bonferroni corrected p < 0.05).
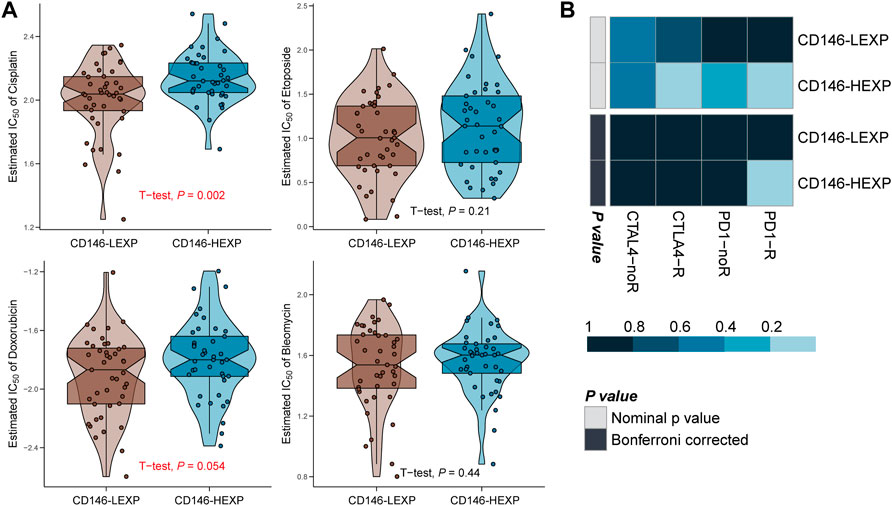
FIGURE 6. The CD146 level reflected the response to immunotherapy and chemotherapy. (A) The response of OSA patients to four common chemotherapy drugs, cisplatin, etoposide, doxorubicin, and bleomycin. (B) The response of OSA patients to anti-PD-1 and anti-CTAL4 immunotherapy.
To validate the value of prognostic prediction, the GSE21257 cohort containing 53 OSA patients was enrolled. We set the median value of CD146 expression as a cut-off value and divided the 53 OSA patients into HEXP (n = 26) and LEXP (n = 27) subgroups. The clinicopathological features among the two subgroups showed no difference, except a little among the metastasis status of tumor patients with high CD146 expression who met more tumor metastasis whether at or after diagnosis (p = 0.042, Table 3). Consistent with the results of the training cohort, the LEXP group showed higher OS, and the HEXP group had a 3.61-fold HR compared to the LEXP group (Figure 7A, 95% CI: 1.474-8.855, p = 0.005). The 1-year, 2-year, and 5-year AUCs were 0.763, 0.801, and 0.734, respectively, indicating reliability (Figure 7B). After removing the interference of confounding factors, we confirmed that CD146 was an independent prognostic factor (Figures 7C,D). For the prognostic nomogram generated in the TARGET-OS cohort, we calculated the point for each patient in the GSE21257 cohort; the ROC curve showed a high AUC value at 0.868 (95% CI: 0.770-0.966, Figure 7E). There was no significant difference between the nomogram-predicted survival probability and the actual survival probability (p = 0.316, Figure 7F).
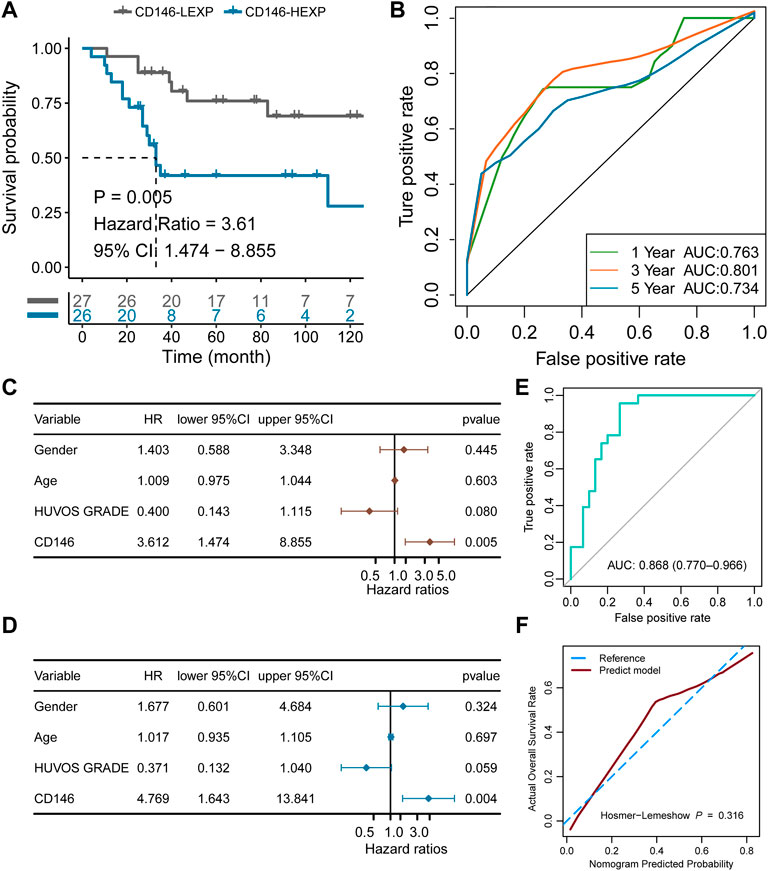
FIGURE 7. Validation of the independent prognostic value of CD146 in the external GSE21257 cohort. (A) K-M plot showing the diverse clinical overall survival outcomes of the CD146 low and high groups. (B) ROC curve showing the prognostic value of the CD146 expression level. (C) Results from univariate Cox regression analysis of the CD146 group and clinical features. (D) Results from multivariate Cox regression analysis of the CD146 group and clinical features. (E) Validation of the nomogram by ROC curve in GSE21257 cohort. (F) Validation of the nomogram by calibration curve in GSE21257 cohort.
Osteosarcoma (OSA) is one of the focuses of orthopedic surgeons. Although it is the most frequent bone cancer, the incidence rate among all tumor types is rare, with an annual incidence of 3–4 patients per million (Smeland et al., 2019). The metaphyses of long bones are vulnerable to OSA, including the distal femur, proximal tibia, and proximal humerus. Once OSA develops, patients typically present with swelling, pain, localized enlargement, and pathologic fracture. As a result of the low incidence rate and remarkable heterogeneity, the pathogenesis mechanism of OSA and prognostic factors remain unclear (Yang and Zhang, 2013). However, most patients are diagnosed at an advanced stage and die due to metastasis. Therefore, signatures with high accuracy and sensitivity for detecting metastasis and predicting survival are needed.
CD146 has been reported to be highly expressed in a variety of cancers in prior studies. CD146 plays an important role in promoting the progression, invasion, and metastasis of melanoma, gallbladder adenocarcinoma, and breast cancer. It has been confirmed as a predictor of poor survival in gastric cancer, lung adenocarcinoma, malignant pleural mesothelioma, and non-small cell lung cancer (Kristiansen et al., 2003; Sato et al., 2010; Liu et al., 2012; Jiang et al., 2016). Our study analyzed the expression of CD146 in 22 types of tumor and adjacent normal tissues and the correlation between CD146 mRNA levels and 32 types of tumor. We discovered that the mRNA level of CD146 was correlated with the corresponding prognosis. A high CD146 mRNA concentration indicated a poor survival time, especially in brain lower-grade glioma (LGG), testicular germ cell tumors (TGCT), kidney chromophobe (KICH), ACC, cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), and uveal melanoma (UVM)— consistent with previous studies. In addition, the expression levels of CD146 on OSA cell lines have been observed to be higher than those on normal osteoblast cells (OST) via confocal images (Schiano et al., 2012), implying its predictive value in OSA. We found that high CD146 expression was related to poor clinical outcomes in the training cohort: this result was validated in the GSE21257 cohort. Thus, we speculated that CD146 can act as a diagnostic biomarker to distinguish OSA from benign lesions. It is important to explore the mechanism of CD146 function in OSA. Lei et al. (2021) reported that CD146 promoted OSA growth by regulating angiogenesis and nourishment, endothelial cell proliferation, permeability, migration, vascular number, and diameter, which were similar to other tumors. However, the underlying mechanisms require further research. We enriched 580 DEGs in different pathways; as a result, vascular development, organic anion transport, cell adhesion, and ligand‒receptor interactions are likely involved in the development of OSA. Immunocyte infiltration has been reported in many cancers and is the signature of hot tumors, also named “immune-inflamed tumors”, which are more sensitive to immune checkpoint inhibitors (ICIs) than cold tumors (Meng et al., 2019). The most prominent characteristic of hot tumors is high T-cell infiltration (Liu and Sun, 2021). According to our study, OSA patients with high CD146 expression show abundant and diverse T-cell infiltration. Thus, we speculate that high CD146 expression is responsible for the sensitivity to immunotherapy. The hypothesis was confirmed through SubMap analysis. OSA patients with high CD146 expression were more vulnerable to anti-PD1 therapy. T-cell infiltration has also been reported as a prognostic factor. T follicular helper cells were proven to be capable of predicting pathological complete response after chemotherapy in breast cancer (Gu-Trantien et al., 2013). Nevertheless, we found that low CD146 was related to low T-cell infiltration but was also related to a high response to cisplatin therapy. More research is required in this field. Therefore, the high expression of CD146 groups represent subgroups with high immunocyte infiltration, low response to cisplatin but high response to anti-PD1 therapy, and poor prognosis; the low expression of CD146 groups represents subgroups with low immunocyte infiltration, high response to cisplatin but low response to anti-PD1 therapy, and good prognosis.
Previous studies have found many related factors affecting the prognosis of OSA patients. Age and sex were controversial in different studies. Some studies showed that the 5-year OS at 0–14 years old was lower than that in other age groups, while a meta-analysis showed no statistical correlation between age and the survival of OSAAs for sex. Some studies reported that the prognosis of male patients was worse than that of female patients, whereas some studies showed that sex had no correlation with prognosis. (Mirabello et al., 2009a; Joo et al., 2015; Ding et al., 2020). By performing robust statistical analysis, our study showed no correlation between age and the survival of OSA or sex. Multivariate analysis revealed that the CD146 mRNA level was an independent prognostic factor for OSA.
In summary, the role of CD146 in OSA was systematically analyzed. CD146 can act as a novel biomarker in predicting the prognosis of OSA and as an independent prognostic factor. We revealed the mechanisms by which CD146 promotes the growth, development, and metastasis of OSA. Contrary to cisplatin therapy, patients with high CD146 expression were more likely to be sensitive to anti-PD-1 therapy. The evidence above and prior research prove the potential value in individual survival prediction, in checkpoint therapy guidance, and as a novel therapeutic target (Stalin et al., 2017).
However, there are several limitations to our study. The samples we collected in the training and validation cohorts were insufficient. CD146 is a reliable prognosis-related factor but its role in the early diagnosis of OSA remains unclear. CD146 mRNA overexpression has been found in many cancers; therefore, the specificity for detection is poor. More research is needed in this field.
We reveal that CD146 acts as an oncogene in the prognosis of several types of tumor and illustrate its function well in OSA. CD146 is an independent prognostic factor for OSA patients after adjusting for age, sex, and metastatic status at diagnosis. In addition, the expression of CD146 also indicates clinical treatment strategies. Patients with low expression of CD146 are more suitable for chemotherapy with cisplatin and doxorubicin, while patients with high expression of CD146 respond better to anti-PD-1 immunotherapy.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
JW, ZW, and XX outlined the study design. JW, XZ, and MZ performed implementation and data collection. JW, MZ, and SY contributed to the data analysis. JW and XX contributed to both the draft and final versions of the manuscript. All authors read and approved the final manuscript.
The current study is supported by the Key Research and Development Project in Anhui Province (202104j07020026).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.1025306/full#supplementary-material
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., et al. (2000). Gene ontology: tool for the unification of biology. The gene Ontology consortium. Nat. Genet. 25 (1), 25–29. doi:10.1038/75556
Chatterjee, N., and Bivona, T. G. (2019). Polytherapy and targeted cancer drug resistance. Trends Cancer 5 (3), 170–182. doi:10.1016/j.trecan.2019.02.003
Chen, C., Xie, L., Ren, T., Huang, Y., Xu, J., and Guo, W. (2021). Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 500, 1–10. doi:10.1016/j.canlet.2020.12.024
Corre, I., Verrecchia, F., Crenn, V., Redini, F., and Trichet, V. (2020). The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells 9 (4), E976. doi:10.3390/cells9040976
Ding, W. Z., Liu, K., Li, Z., and Chen, S. R. (2020). A meta-analysis of prognostic factors of osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 24 (8), 4103–4112. doi:10.26355/eurrev_202004_20989
Feng, G., Fang, F., Liu, C., Zhang, F., Huang, H., and Pu, C. (2012). CD146 gene expression in clear cell renal cell carcinoma: a potential marker for prediction of early recurrence after nephrectomy. Int. Urol. Nephrol. 44 (6), 1663–1669. doi:10.1007/s11255-012-0255-4
Furue, H. (2003). Chemotherapy cancer treatment during the past sixty years. Gan Kagaku Ryoho. 30 (10), 1404–1411.
Gu-Trantien, C., Loi, S., Garaud, S., Equeter, C., Libin, M., de Wind, A., et al. (2013). CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123 (7), 2873–2892. doi:10.1172/jci67428
Huang, Q., Liang, X., Ren, T., Huang, Y., Zhang, H., Yu, Y., et al. (2021). The role of tumor-associated macrophages in osteosarcoma progression - therapeutic implications. Cell. Oncol. 44 (3), 525–539. doi:10.1007/s13402-021-00598-w
Jiang, G., Zhang, L., Zhu, Q., Bai, D., Zhang, C., and Wang, X. (2016). CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 35, 38. doi:10.1186/s13046-016-0313-3
Johnson, J. P., Rothbächer, U., Sers, C., and Rothbacher, U. (1993). The progression associated antigen MUC18: a unique member of the immunoglobulin supergene family. Melanoma Res. 3 (5), 337–340. doi:10.1097/00008390-199310000-00006
Joo, M. W., Shin, S. H., Kang, Y. K., Kawai, A., Kim, H. S., Asavamongkolkul, A., et al. (2015). Osteosarcoma in asian populations over the age of 40 Years: A multicenter study. Ann. Surg. Oncol. 22 (11), 3557–3564. doi:10.1245/s10434-015-4414-6
Kristiansen, G., Yu, Y., Schlüns, K., Sers, C., Dietel, M., and Petersen, I. (2003). Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal. Cell. Pathol. 25 (2), 77–81. doi:10.1155/2003/574829
Lehmann, J. M., Riethmüller, G., and Johnson, J. P. (1989). MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. U. S. A. 86 (24), 9891–9895. doi:10.1073/pnas.86.24.9891
Lei, X., Wang, K., Wang, W., Jin, H., Gu, W., Chen, Z., et al. (2021). Recognize the role of CD146/MCAM in the osteosarcoma progression: an in vitro study. Cancer Cell Int. 21 (1), 300. doi:10.1186/s12935-021-02006-7
Li, G., Kalabis, J., Xu, X., Meier, F., Oka, M., Bogenrieder, T., et al. (2003). Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene 22 (44), 6891–6899. doi:10.1038/sj.onc.1206819
Li, H. B., Huang, G., Tu, J., Lv, D. M., Jin, Q. L., Chen, J. K., et al. (2022). METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine 82, 104142. doi:10.1016/j.ebiom.2022.104142
Li, Y., Yu, J. M., Zhan, X. M., Liu, L. L., Jin, N., and Zhang, Y. X. (2014). Correlation of CD146 expression and clinicopathological characteristics in esophageal squamous cell carcinoma. Oncol. Lett. 8 (2), 859–863. doi:10.3892/ol.2014.2227
Liang, Y. K., Zeng, D., Xiao, Y. S., Wu, Y., Ouyang, Y. X., Chen, M., et al. (2017). MCAM/CD146 promotes tamoxifen resistance in breast cancer cells through induction of epithelial-mesenchymal transition, decreased ERα expression and AKT activation. Cancer Lett. 386, 65–76. doi:10.1016/j.canlet.2016.11.004
Liberzon, A., Birger, C., Thorvaldsdóttir, H., Ghandi, M., Mesirov, J. P., and Tamayo, P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1 (6), 417–425. doi:10.1016/j.cels.2015.12.004
Liu, C. J., Hu, F. F., Xia, M. X., Han, L., Zhang, Q., and Guo, A. Y. (2018). GSCALite: a web server for gene set cancer analysis. Bioinformatics 34 (21), 3771–3772. doi:10.1093/bioinformatics/bty411
Liu, W. F., Ji, S. R., Sun, J. J., Zhang, Y., Liu, Z. Y., Liang, A. B., et al. (2012). CD146 expression correlates with epithelial-mesenchymal transition markers and a poor prognosis in gastric cancer. Int. J. Mol. Sci. 13 (5), 6399–6406. doi:10.3390/ijms13056399
Liu, Y. T., and Sun, Z. J. (2021). Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 11 (11), 5365–5386. doi:10.7150/thno.58390
Lu, X., Meng, J., Zhou, Y., Jiang, L., and Yan, F. (2020). MOVICS: an R package for multi-omics integration and visualization in cancer subtyping. Bioinformatics 36, 5539–5541. doi:10.1093/bioinformatics/btaa1018
Melnikova, V. O., Balasubramanian, K., Villares, G. J., Dobroff, A. S., Zigler, M., Wang, H., et al. (2009). Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. J. Biol. Chem. 284 (42), 28845–28855. doi:10.1074/jbc.M109.042150
Meng, J., Liu, Y., Guan, S., Fan, S., Zhou, J., Zhang, M., et al. (2019). The establishment of immune infiltration based novel recurrence predicting nomogram in prostate cancer. Cancer Med. 8 (11), 5202–5213. doi:10.1002/cam4.2433
Mirabello, L., Troisi, R. J., and Savage, S. A. (2009a). International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 125 (1), 229–234. doi:10.1002/ijc.24320
Mirabello, L., Troisi, R. J., and Savage, S. A. (2009b). Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results Program. Cancer 115 (7), 1531–1543. doi:10.1002/cncr.24121
Nie, Z., and Peng, H. (2018). Osteosarcoma in patients below 25 years of age: An observational study of incidence, metastasis, treatment and outcomes. Oncol. Lett. 16 (5), 6502–6514. doi:10.3892/ol.2018.9453
Ottaviani, G., and Jaffe, N. (2009). The epidemiology of osteosarcoma. Cancer Treat. Res. 152, 3–13. doi:10.1007/978-1-4419-0284-9_1
Pakos, E. E., Nearchou, A. D., Grimer, R. J., Koumoullis, H. D., Abudu, A., Bramer, J. A., et al. (2009). Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur. J. Cancer 45 (13), 2367–2375. doi:10.1016/j.ejca.2009.03.005
Pan, Y., Chen, D., Hu, T., Lv, G., and Dai, Z. (2019). Characteristics and prognostic factors of patients with osteosarcoma older than 60 Years from the SEER database. Cancer Control 26 (1), 1073274819888893. doi:10.1177/1073274819888893
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47. doi:10.1093/nar/gkv007
Ritter, J., and Bielack, S. S. (2010). Osteosarcoma. Ann. Oncol. 21, vii320–325. doi:10.1093/annonc/mdq276
Rodig, S. J., Gusenleitner, D., Jackson, D. G., Gjini, E., Giobbie-Hurder, A., Jin, C., et al. (2018). MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 10 (450), eaar3342. doi:10.1126/scitranslmed.aar3342
Rojas, G. A., Hubbard, A. K., Diessner, B. J., Ribeiro, K. B., and Spector, L. G. (2021). International trends in incidence of osteosarcoma (1988-2012). Int. J. Cancer 149 (5), 1044–1053. doi:10.1002/ijc.33673
Sato, A., Torii, I., Okamura, Y., Yamamoto, T., Nishigami, T., Kataoka, T. R., et al. (2010). Immunocytochemistry of CD146 is useful to discriminate between malignant pleural mesothelioma and reactive mesothelium. Mod. Pathol. 23 (11), 1458–1466. doi:10.1038/modpathol.2010.134
Schiano, C., Grimaldi, V., Casamassimi, A., Infante, T., Esposito, A., Giovane, A., et al. (2012). Different expression of CD146 in human normal and osteosarcoma cell lines. Med. Oncol. 29 (4), 2998–3002. doi:10.1007/s12032-012-0158-3
Sechler, M., Parrish, J. K., Birks, D. K., and Jedlicka, P. (2017). The histone demethylase KDM3A, and its downstream target MCAM, promote Ewing Sarcoma cell migration and metastasis. Oncogene 36 (29), 4150–4160. doi:10.1038/onc.2017.44
Smeland, S., Bielack, S. S., Whelan, J., Bernstein, M., Hogendoorn, P., Krailo, M. D., et al. (2019). Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American osteosarcoma study) cohort. Eur. J. Cancer 109, 36–50. doi:10.1016/j.ejca.2018.11.027
So, J. H., Hong, S. K., Kim, H. T., Jung, S. H., Lee, M. S., Choi, J. H., et al. (2010). Gicerin/Cd146 is involved in zebrafish cardiovascular development and tumor angiogenesis. Genes Cells 15 (11), 1099–1110. doi:10.1111/j.1365-2443.2010.01448.x
Stalin, J., Nollet, M., Dignat-George, F., Bardin, N., and Blot-Chabaud, M. (2017). Therapeutic and diagnostic antibodies to CD146: Thirty years of research on its potential for detection and treatment of tumors. Antibodies (Basel) 6 (4), E17. doi:10.3390/antib6040017
Thanindratarn, P., Dean, D. C., Nelson, S. D., Hornicek, F. J., and Duan, Z. (2019). Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 15, 100221. doi:10.1016/j.jbo.2019.100221
Tian, B., Zhang, Y., and Li, N. (2013). CD146 protein as a marker to predict postoperative liver metastasis in colorectal cancer. Cancer Biother. Radiopharm. 28 (6), 466–470. doi:10.1089/cbr.2012.1426
Valery, P. C., Laversanne, M., and Bray, F. (2015). Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control 26 (8), 1127–1139. doi:10.1007/s10552-015-0607-3
Wang, S. D., Li, H. Y., Li, B. H., Xie, T., Zhu, T., Sun, L. L., et al. (2016). The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int. Immunopharmacol. 38, 81–89. doi:10.1016/j.intimp.2016.05.016
Wang, Z., and Yan, X. (2013). CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 330 (2), 150–162. doi:10.1016/j.canlet.2012.11.049
Westrøm, S., Bønsdorff, T. B., Abbas, N., Bruland Ø, S., Jonasdottir, T. J., Mælandsmo, G. M., et al. (2016). Evaluation of CD146 as target for radioimmunotherapy against osteosarcoma. PLoS One 11 (10), e0165382. doi:10.1371/journal.pone.0165382
Wu, Z., Wu, Z., Li, J., Yang, X., Wang, Y., Yu, Y., et al. (2012). MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumour Biol. 33 (5), 1619–1628. doi:10.1007/s13277-012-0417-0
Xie, S., Luca, M., Huang, S., Gutman, M., Reich, R., Johnson, J. P., et al. (1997). Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res. 57 (11), 2295–2303.
Yang, J., and Zhang, W. (2013). New molecular insights into osteosarcoma targeted therapy. Curr. Opin. Oncol. 25 (4), 398–406. doi:10.1097/CCO.0b013e3283622c1b
Yasin, N. F., Abdul Rashid, M. L., and Ajit Singh, V. (2020). Survival analysis of osteosarcoma patients: A 15-year experience. J. Orthop. Surg. 28 (1), 2309499019896662. doi:10.1177/2309499019896662
Yoshida, K., Okamoto, M., Sasaki, J., Kuroda, C., Ishida, H., Ueda, K., et al. (2020). Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer 20 (1), 25. doi:10.1186/s12885-019-6499-y
Yoshihara, K., Shahmoradgoli, M., Martínez, E., Vegesna, R., Kim, H., Torres-Garcia, W., et al. (2013). Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612. doi:10.1038/ncomms3612
Yu, G., Wang, L. G., Han, Y., and He, Q. Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16 (5), 284–287. doi:10.1089/omi.2011.0118
Zabouo, G., Imbert, A. M., Jacquemier, J., Finetti, P., Moreau, T., Esterni, B., et al. (2009). CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 11 (1), R1. doi:10.1186/bcr2215
Zeng, Q., Li, W., Lu, D., Wu, Z., Duan, H., Luo, Y., et al. (2012). CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. U. S. A. 109 (4), 1127–1132. doi:10.1073/pnas.1111053108
Zhang, C., Wan, J., Liu, Q., Long, F., Wen, Z., and Liu, Y. (2022). METTL3 upregulates COPS5 expression in osteosarcoma in an m(6)A-related manner to promote osteosarcoma progression. Exp. Cell Res. 420 (2), 113353. doi:10.1016/j.yexcr.2022.113353
Keywords: osteosarcoma, prognosis, microenvironment, immunotherapy, personalized treatment
Citation: Wang J, Wu Z, Zheng M, Yu S, Zhang X and Xu X (2022) CD146 is closely associated with the prognosis and molecular features of osteosarcoma: Guidance for personalized clinical treatment. Front. Genet. 13:1025306. doi: 10.3389/fgene.2022.1025306
Received: 22 August 2022; Accepted: 29 September 2022;
Published: 21 October 2022.
Edited by:
Xiaofan Lu, INSERM U964 Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), FranceReviewed by:
Dan Zhang, Institute of Genetics and Developmental Biology (CAS), ChinaCopyright © 2022 Wang, Wu, Zheng, Yu, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XinZhong Xu, eHV4aW56aG9uZ0BhaG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.