- Department of Pathology, Chifeng Municipal Hospital, Chifeng, China
Breast cancer and colorectal cancer are two of the most common malignant tumors worldwide. They cause the leading causes of cancer mortality. Many researches have demonstrated that long noncoding RNAs (lncRNAs) have close linkages with the occurrence and development of the two cancers. Therefore, it is essential to design an effective way to identify potential lncRNA biomarkers for them. In this study, we developed a computational method (LDA-RWLMF) by integrating random walk with restart and Logistic Matrix Factorization to investigate the roles of lncRNA biomarkers in the prognosis and diagnosis of the two cancers. We first fuse disease semantic and Gaussian association profile similarities and lncRNA functional and Gaussian association profile similarities. Second, we design a negative selection algorithm to extract negative LncRNA-Disease Associations (LDA) based on random walk. Third, we develop a logistic matrix factorization model to predict possible LDAs. We compare our proposed LDA-RWLMF method with four classical LDA prediction methods, that is, LNCSIM1, LNCSIM2, ILNCSIM, and IDSSIM. The results from 5-fold cross validation on the MNDR dataset show that LDA-RWLMF computes the best AUC value of 0.9312, outperforming the above four LDA prediction methods. Finally, we rank all lncRNA biomarkers for the two cancers after determining the performance of LDA-RWLMF, respectively. We find that 48 and 50 lncRNAs have the highest association scores with breast cancer and colorectal cancer among all lncRNAs known to associate with them on the MNDR dataset, respectively. We predict that lncRNAs HULC and HAR1A could be separately potential biomarkers for breast cancer and colorectal cancer and need to biomedical experimental validation.
1 Introduction
Breast cancer is the second leading cause of cancer-related death in women worldwide and the most common malignant tumor among US woman (Sun et al., 2017; DeSantis et al., 2019; Yang et al., 2013; Waks and Winer, 2019). During the past 25 years, breast cancer mortality rate showed a substantial increase in the world (Garrido-Castro et al., 2019). This increasing rate is one threaten to health for women in the world, in particular women from developing and low-income regions. More than 1.5 million women were diagnosed to breast cancer every year, which accounts for 25% among all women with cancers (Sun et al., 2017). In 2018, breast cancer accounts for approximately 24% of new cancer cases and approximately 15% of cancer deaths in women (Heer et al., 2020). In 2019, it is estimated that about 268,600 new patients suffer from invasive breast cancer and 48,100 patients suffer from ductal carcinoma in situ among US women. Moreover, 41,760 women may die from breast cancer in the same year (DeSantis et al., 2019). About 13% of women may suffer from invasive breast cancer in lifetime (DeSantis et al., 2019). The incident rate of breast cancer will increase by more than 46% by 2040 (Heer et al., 2020). Consequently, breast cancer has been one essential problem to be solved around the world.
However, the precise mechanisms of breast cancer remain unclear (Barzaman et al., 2020). Systemic treatment of breast cancer patients mainly consists of chemotherapy, endocrine treatment, and targeted therapy (Campos-Parra et al., 2018). In spite of rapid progress in different treatment strategies, accumulating patients show recurrence of the disease and decreased survival because of therapy resistance, which increases metastasis rates (Sledge et al., 2014). Once the metastasis occurs, the 5-year overall survival rate may be below 25% (Siegel et al., 2013).
Colorectal cancer is the third most frequent cancer and the second most death-caused cancer. It is estimated that there are about 1.9 million new cases and 0.9 million death cases worldwide in 2020 (Xi and Xu, 2021). Of new diagnose cases, 20% of patients have metastases and another 25% with localized disease may later develop metastases (Biller and Schrag, 2021). Its incidence is high in developed countries and is increasing in low- and middle-income countries, which poses a challenge to global public health (Biller and Schrag, 2021; Xi and Xu, 2021).
In this situation, it is essential to discover novel molecular biomarkers that can characterize therapy response for breast cancer and colorectal cancer. We can extend the overall survival rates of patients and delay or prevent the two cancers from metastases based on molecular biomarkers (Campos-Parra et al., 2018). Consequently, screening reliable biomarker is a research hotspot on the diagnosis and treatment of cancer including breast cancer and colorectal cancer (Huang et al., 2019; Yang et al., 2020; Peng et al., 2022a).
A substantial number of evidence suggest that over 80% of the human genome can be transcribed into non-coding RNAs, such as microRNAs (Peng et al., 2017; Peng et al., 2018; Chen et al., 2019; Huang et al., 2021), circle RNAs (Zhao et al., 2019; Lan et al., 2022), and long non-coding RNAs (lncRNAs) (Zhang et al., 2021a; Peng et al., 2021a; Peng et al., 2022b; Zhou et al., 2021a; Zhou et al., 2021b). In particular, lncRNAs obtain emerging interest as diagnostic biomarkers and therapeutic targets (Chandra Gupta and Nandan Tripathi, 2017; Guo et al., 2022). Differential expression of lncRNAs forms specific patterns to various complex diseases including cancer (Wahlestedt C, 2013). Once the regulation effects of lncRNAs are detected, they are promising therapeutic targets.
LncRNAs are closely related to breast cancer and colorectal cancer. For example, lncRNA BCRT1, MaTAR25, DSCAM-AS1, and CDC6 can promote breast cancer progression (Niknafs et al., 2016; Kong et al., 2019a; Chang et al., 2020; Liang et al., 2020), BCRT4 can induce signaling transduction in breast cancer (Xing et al., 2015), LINC00673 can promote cell proliferation of breast cancer (Qiao et al., 2019), and BORG can cause breast cancer metastasis and disease recurrence (Gooding et al., 2017). SNHG11, FEZF1-AS1, RP11, and DLEU1 have been reported to novel biomarkers of colorectal cancer (Bian et al., 2018; Liu et al., 2018; Wu et al., 2019; Xu et al., 2020). Thus, many computational models have been developed to discover lncRNA biomarkers for cancers (Peng et al., 2020a; Shen et al., 2022; Sun et al., 2022), for instance, rotation forest (Guo et al., 2019), KATZ measure (Chen, 2015), collaborative deep learning (Lan et al., 2020), matrix factorization (Fu et al., 2018; Wang et al., 2021a), network consistency projection (Li et al., 2019), and graph autoencoder (Shi et al., 2021).
In this manuscript, inspired by the association prediction method provided by Peng et al. (2020b), we develop a computational method, LDA-RWLMF, to predict LncRNA-Disease Associations (LDAs). LDA-RWLMF integrates random walk and Logistic Matrix Factorization to discover the roles of lncRNA biomarkers in the prognosis and diagnosis for breast cancer and colorectal cancer. First, we compute disease similarity and lncRNA similarity. Second, we first use random walk to extract negative LDAs. Third, we explored a logistic matrix factorization model to predict possible LDAs. The results from 5-fold cross validation show that LDA-RWLMF computes the best AUC value of 0.9312 on the MNDR dataset. Finally, we rank all lncRNA biomarkers for breast cancer and colorectal cancer after determining the performance of LDA-RWLMF.
2 Datasets
2.1 LncRNA-disease associations
Human LDA dataset was collected from the MNDR database (Cui et al., 2018; Fan et al., 2020) (http://www.rna-society.org/mndr/index.html). There are 1,529 LDAs between 89 diseases and 190 lncRNAs after preprocessing. For an LDA matrix between
2.2 Disease semantic similarity
We use the method provided by Fan et al. (2020) to compute disease semantic similarity based on the MeSH descriptors. Disease semantic similarity method provided by Fan et al. (2020) was based on LNCSIM1 and LNCSIM2 provided by Chen (2015). For a disease
where
The above equation demonstrates that terms at the same layer from
In this case, we compute the second semantic contribution of term
where
where
where
Furthermore, the contribution of all terms in
Finally, the semantic similarity between two diseases (A and B) can be computed by Eq. 7:
2.3 LncRNA functional similarity
We use the method provided by Fan et al. (Fan et al., 2020) to compute lncRNA functional similarity. Let that DG(u) [or DG(v)] indicate diseases linking to lncRNA
Similarly, the similarity between
And the similarity of
And similarity of
The similarity between lncRNAs
where
3 Methods
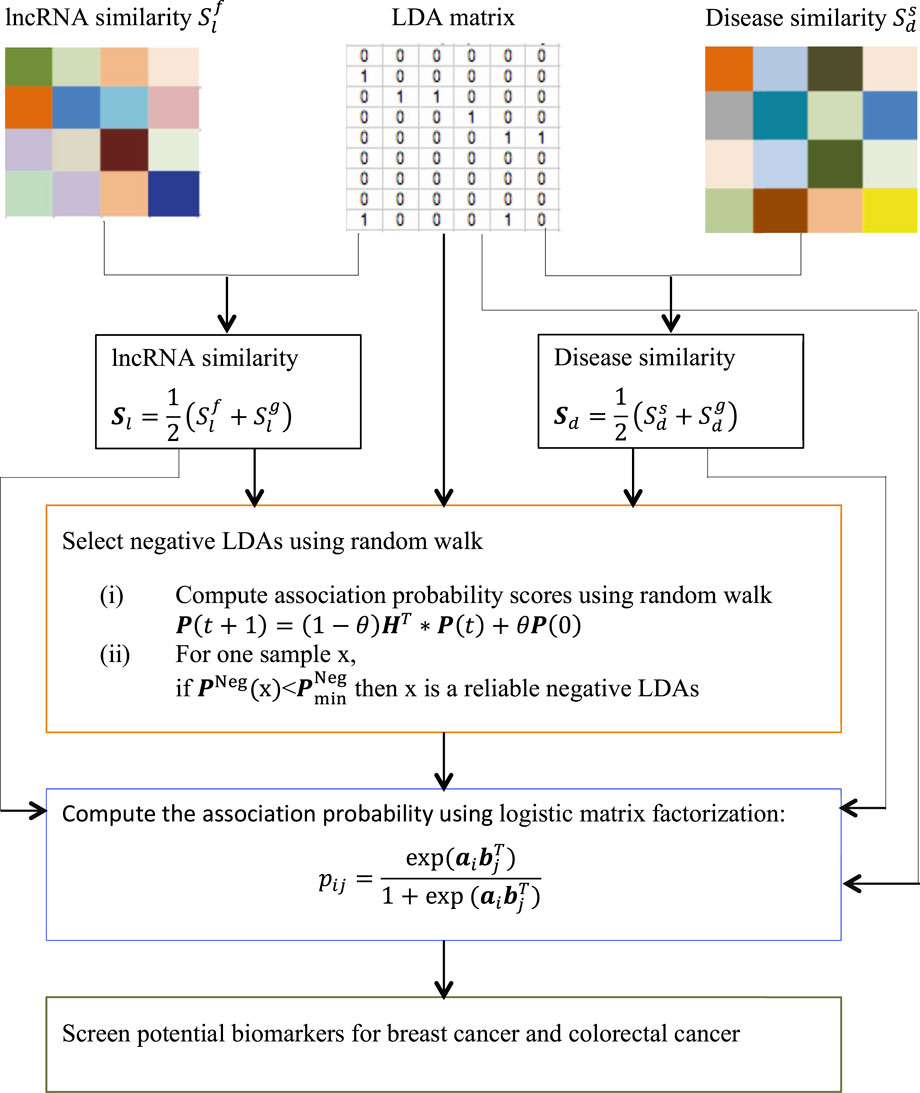
We want to compute association probability for each lncRNA-disease pair based on disease semantic similarity and lncRNA functional similarity. The pipeline is shown in Figure 1.
3.1 Gaussian association profile similarity and similarity fusion
In this section, we use Gaussian Association Profile (GAP) to compute the GAP similarity of diseases and lncRNAs. For a lncRNA
where
Similarly, the disease GAP similarity
3.2 Screening negative LDAs
There are not negative LDAs in the MNDR dataset. Credible negative LDAs help improve LDA prediction performance and further more effectively find potential lncRNA biomarkers for breast cancer and colorectal cancer. Peng et al. (2021b) developed a random walk with restart-based virus-drug association prediction method and obtained better performance. Inspired by the method provided by Peng et al. (2021b), we first compute association probability for each lncRNA-disease pair through random walk with restart and then screen credible negative LDAs.
We first constructed a heterogeneous network composed of lncRNA similarity network, disease similarity network, and LDA network. lncRNA similarity matrix
where
We then compute transition probability on the heterogeneous graph. Suppose that
The
or jump to a disease
Similarly, the
or jump to an lncRNA
At the
where
In the second step, we consider known LDAs as positive sample set
Step 1. Randomly screening positive sample subset
Step 2. Adding
Step 3. Considering
Step 4. Obtaining LDA score matrix
Step 5. Ranking lncRNA-disease pairs in
Step 6. For every lncRNA-disease pair
3.3 LDA prediction based on logistic matrix factorization
Logistic matrix factorization has been applied to multiple areas (Liu et al., 2020; Tang et al., 2021; Tian et al., 2022). Inspired by the approaches, we develop a logistic matrix factorization-based LDA prediction method, LDA-RWLMF.
Assume that both lncRNAs and diseases are mapped to
The latent vector matrix of all lncRNAs or diseases can be represented as
Model (21) can be optimized based on the Bayesian distribution by Eq. 23:
where
where
We compute
Finally, lncRNA-disease association score
4 Results
4.1 Experimental settings
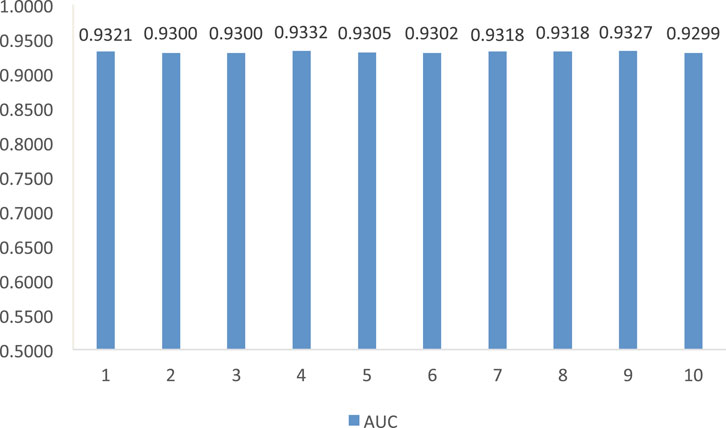
We conduct 5-fold cross validation for 10 times to investigate the performance of LDA-RWLMF. AUC is used to evaluate the prediction accuracy of LDA identification models. AUC is the area under the true positive rate (TPR)-false positive rate (FPR) curve, where TPR and FPR are defined by Eqs 26, 27:
where TP, FP, TN, FN represent the number of true positives, false positives, true negatives, false negatives, respectively. Higher AUC is, better the prediction performance is. In addition, parameters in LDA-RWLMF are set to defaults provided by Peng et al. (2020b). And parameters in the other four comparison LDA prediction methods (LNCSIM1, LNCSIM2, ILNCSIM, and IDSSIM) are set to the same values provided by corresponding methods.
4.2 Performance comparison with other methods
To measure the performance of the proposed LDA-RWLMF method, we compare it with four other representative LDA inference approaches on the MNDR dataset. That is, LNCSIM1 (Chen, 2015), LNCSIM2 (Chen, 2015), ILNCSIM (Huang et al., 2016), and IDSSIM (Fan et al., 2020). LNCSIM1 and LNCSIM2 used Laplacian regularized least squares to predict possible LDAs based on disease DAGs and the information content, respectively. ILNCSIM first combined the hierarchical structure of disease DAG and the information content to compute disease similarity and then used Laplacian regularized least squares to infer new LDAs. IDSSIM designed a weighted K nearest neighbor approach to identify potential associations between lncRNAs and diseases by integrating disease semantic similarity and lncRNA functional similarity. Table 1 gives the AUC values of the four LDA identification methods and our proposed LDA-RWLMF on the MNDR dataset.
The results from Table 1 demonstrate that LDA-RWLMF computes the highest AUC compared to LNCSIM1, LNCSIM2, ILNCSIM, and IDSSIM on the MNDR dataset. Figure 2 gives the results of LDA-RWLMF from 10 time cross validation. From Figure 2, we can find that AUC obtain by LDA-RWLMF is relatively steady during 10 time cross validation.
4.3 Case study
4.3.1 lncRNA biomarker identification for breast cancer
Breast cancer is the commonest life-threatening cancer in women (Key et al., 2001; Sharma, et al., 2010). lncRNAs play important roles in epigenetic regulation, transcriptional regulation and post-transcriptional regulation and have been potential biomarkers of many diseases. Substantial publications have reported that lncRNAs affect proliferation and apoptosis, invasion and metastasis, and cancer stemness of breast cancer. For example, LSINCT5 and Zfas one can promote the proliferation of breast cancer, HOTAIR suppresses invasion and migration of breast cancer, SOX2OT induces SOX2 expression in breast cancer, and SRA is the expression activator of breast cancer (Sun et al., 2017). We want to conduct case analyses to find possible lncRNA biomarkers for breast cancer based on the proposed LDA-RWLMF model.
In the MNDR dataset, there are 89 lncRNAs that may associate with breast cancer, where 54 lncRNAs have been experimentally validated to associate with the cancer and 35 lncRNAs have unknown associations with it. We use the proposed LDA-RWLMF method to rank the 89 lncRNAs for breast cancer. The results are shown in Tables 2, 3. Table 2 demonstrates the ranking results of the predicted top 48 lncRNAs according to the computed association score with breast cancer on the MNDR dataset. These 48 lncRNAs are known to link to breast cancer on the MNDR dataset and are ranked as top 48.
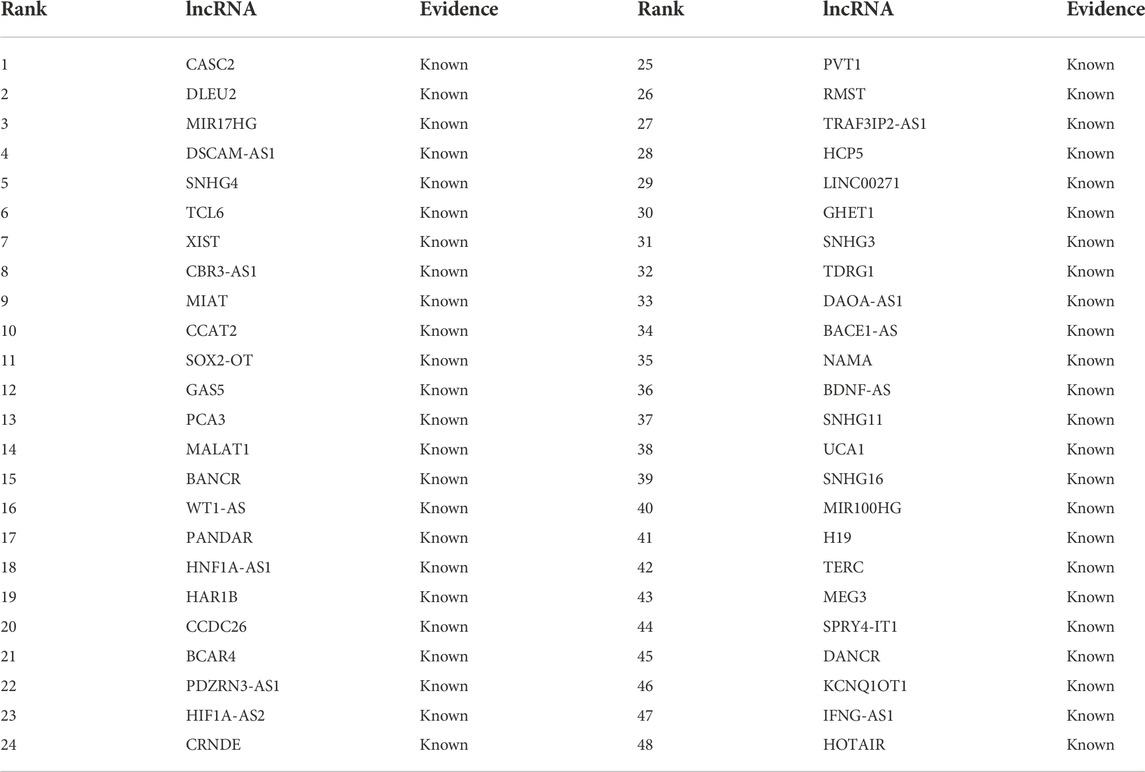
TABLE 2. The rankings of the predicted top 48 lncRNAs according to association with breast cancer on the MNDR dataset.
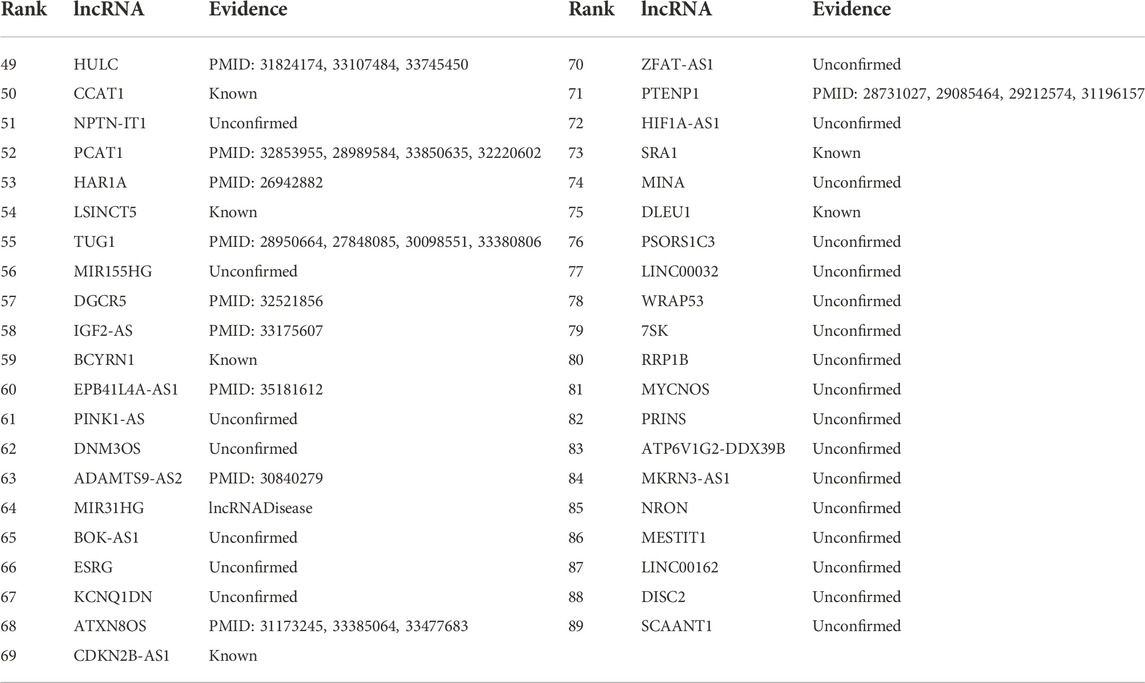
TABLE 3. The rankings of the remaining 41 lncRNAs according to association with breast cancer on the MNDR dataset.
Table 3 gives the rankings of the remaining 41 lncRNAs according to the association scores with breast cancer on the MNDR dataset. Among all lncRNAs unknown to associate with breast cancer on the MNDR dataset, lncRNA HULC is predicted to link to breast cancer with the highest association scores. Shi et al. (2016) observed that HULC can act as an oncogene biomarker in triple-negative breast cancer and as an independent possible poor prognostic factor in patients suffered from triple-negative breast cancer. Wang et al. (2019) found that HULC can promote the development of breast cancer through regulating the expression of LYPD1. Gavgani et al. (2020) investigated that the HULC knockdown can induce apoptosis and suppress cellular migration in breast cancer cells.
PCAT1 may link to breast cancer with the ranking of three among all lncRNAs unknown to associate with breast cancer on the MNDR dataset. Several studies have reported that PCAT1 can associate with breast cancer although its association with the cancer on the MNDR dataset is unobserved. Abdollahzadeh et al. (2020) reported that the altered regulation of PCAT1 may play crucial roles in the development and pathogenesis of breast cancer. Sarrafzadeh et al. (2017) assessed the expression of PCAT-1 through real-time reverse transcription polymerase chain reaction in breast tumor samples from 47 breast cancer patients and found that PCAT-1 may involve in the pathogenesis of breast cancers. Wang et al. (2021a) observed that PCAT-1 can facilitate breast cancer progression by binding to RACK1 and thus boosting oxygen-independent stability of HIF-1α. Tang et al. (2022) detect that PCAT1 can regulate the expression of PITX2 in breast cancer.
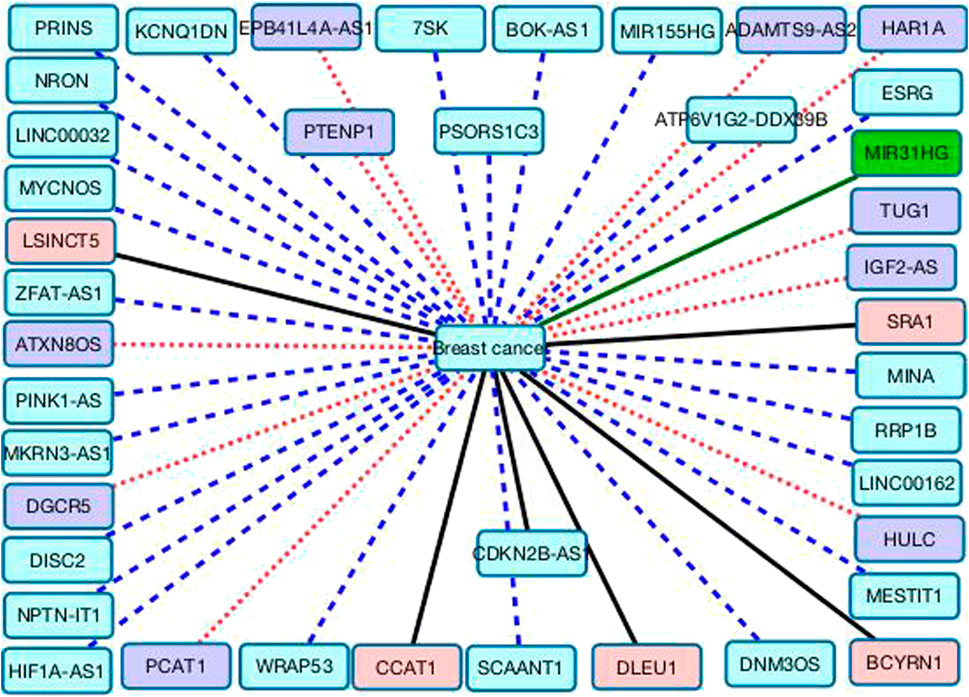
In addition, we predict that nephronectin intronic transcript 1 (NPTN-IT1, also known as lncRNA-LET) may have relationship with breast cancer. NPTN-IT1 has been reported to associate with bladder cancer through attenuating the expression of the target of miR-145 and ILF3 in bladder cancer (Zhang et al., 2021b). It was significantly down-regulated in multiple tumor tissues of colorectal cancer. It also has a regulation role in hypoxia signaling of hepatocellular carcinoma (Sun et al., 2013) and was highly expressed in HepG2 cells (Kong et al., 2019b). We hope that association between three lncRNAs (HULC, NPTN-IT1, and PCAT1) and breast cancer can be validated through wet experiments. Figure 3 shows the associations between the 41 lncRNAs that are ranked as the last 41 and breast cancer. Black solid lines represent known LDAs in the MNDR database. Green solid lines represent LDAs that can be observed in the lncRNA disease database. Red dots lines represent LDAs that are predicted to be potential lncRNA biomarkers of breast cancer and can be confirmed by related publications. Blue equal dash lines represent unknown LDAs.
4.3.2 lncRNA biomarker identification for colorectal cancer
Colorectal cancer is a heterogeneous disease. It has high morbidity and mortality. lncRNAs demonstrate dense associations with colorectal cancer. In this study, we conduct case analyses to identify possible lncRNA biomarkers for colorectal cancer based on LDA-RWLMF. In the MNDR dataset, 89 lncRNAs possibly associate with colorectal cancer, where 55 lncRNAs have been validated to be the biomarkers of the cancer and remaining 34 lncRNAs have not been validated. We use LDA-RWLMF to compute the association scores between all 89 lncRNAs and colorectal cancer and rank the 89 lncRNAs for colorectal cancer. The results are shown in Tables 4, 5. Table 4 shows the rankings of the identified top 50 lncRNAs according to the computed association score with colorectal cancer on the MNDR dataset. The 50 lncRNAs are known to associate with colorectal cancer on the MNDR dataset and are ranked as top 50.
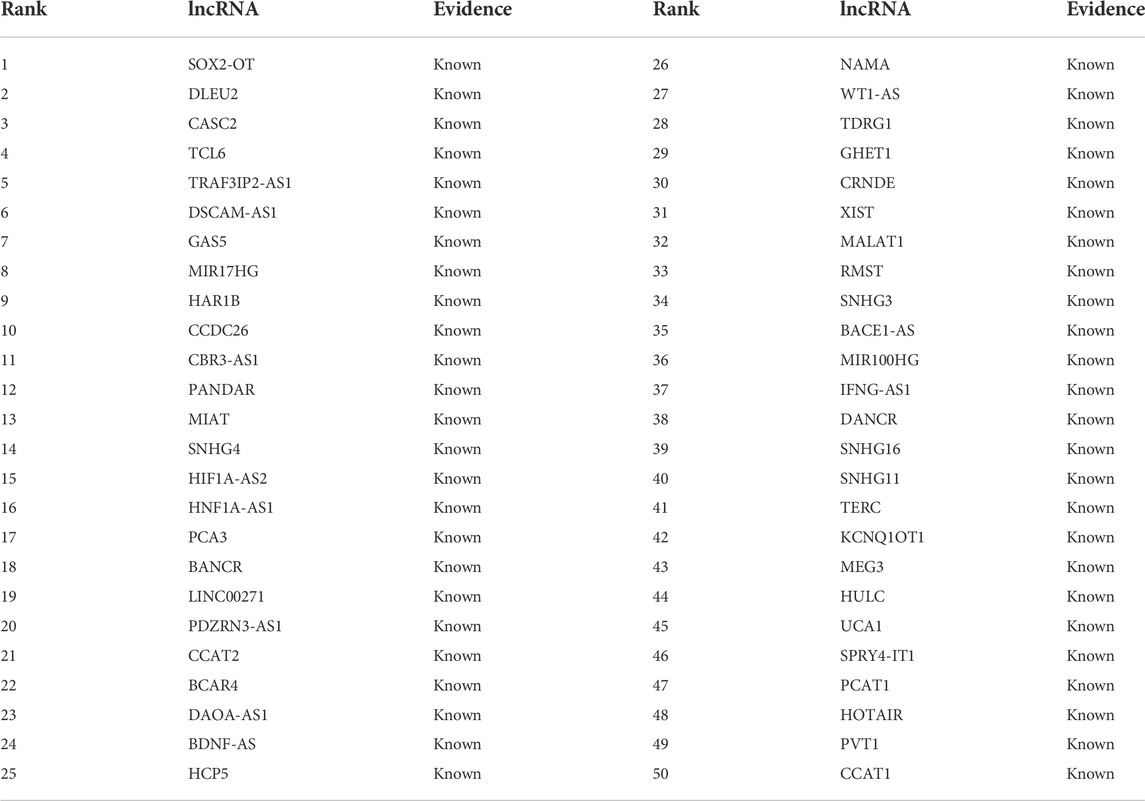
TABLE 4. The rankings of the identified top 50 lncRNAs associated with colorectal cancer on the MNDR dataset.
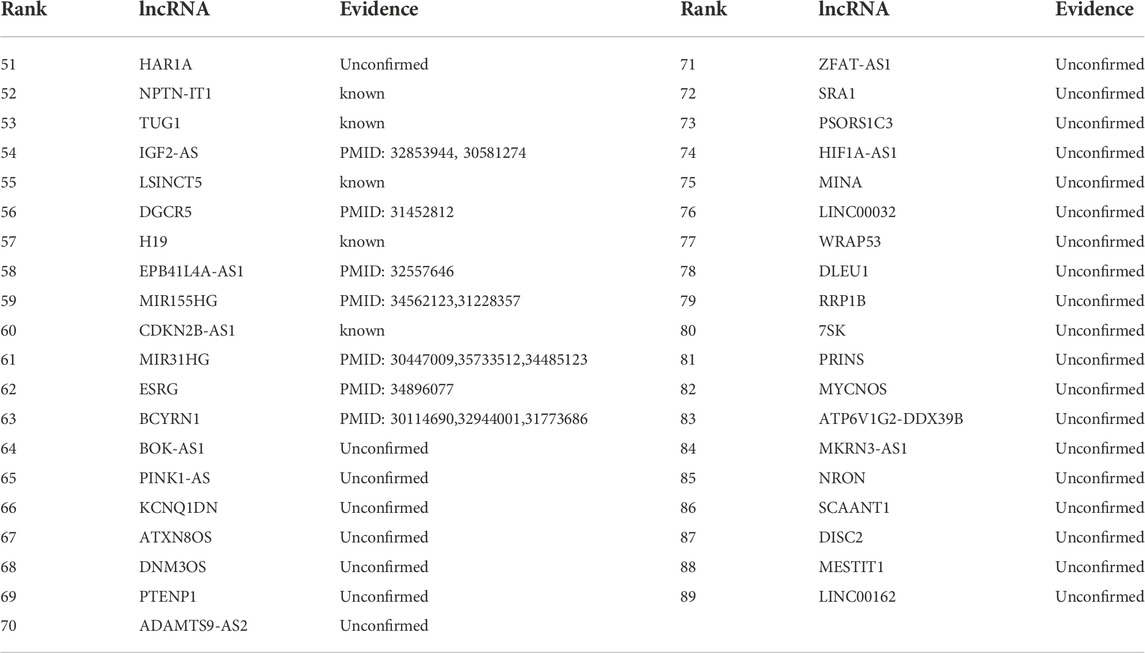
TABLE 5. The rankings of the remaining 41 lncRNAs according to association with breast cancer on the MNDR dataset.
Table 5 gives the rankings of the remaining 39 lncRNAs according to the association scores with colorectal cancer on the MNDR dataset. Among all lncRNAs unknown association with colorectal cancer on the MNDR dataset, lncRNA HAR1A is inferred to link to colorectal cancer with the highest association scores. HAR1A is a favorable prognostic biomarker for patients. Shi et al. (2019) analyzed the expression profiles of HAR1A using RT-qPCR and found its expression level was significantly lower in hepatocullular cancer. Chen et al. (2020) have still reported that the HAR1A expression levels were reduced in hepatocellular carcinoma tissues.
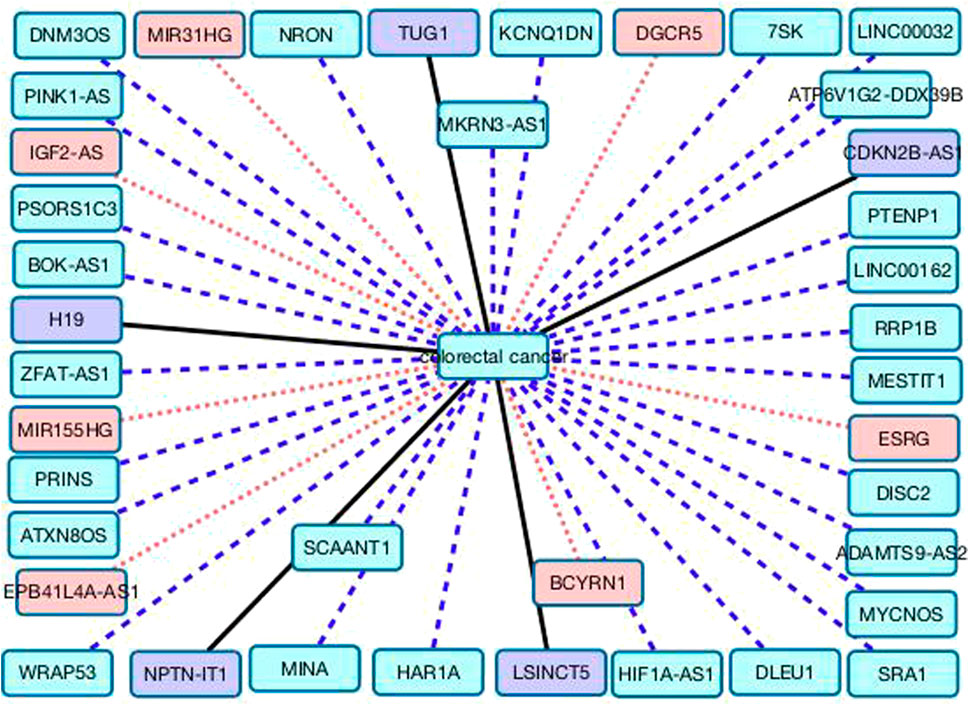
Figure 4 gives the associations between the remaining 39 lncRNAs and colorectal cancer. Black solid lines represent known LDAs in the MNDR database. Red dots lines represent LDAs that are predicted to be potential lncRNA biomarkers of breast cancer and can be confirmed by related publications. Blue equal dash lines represent unknown LDAs.
5 Discussion and conclusion
Breast cancer and colorectal cancer are the most frequent cancers with high mortality rates. They demonstrate very high heterogeneity at molecular and clinical levels. With the fast development of next generation sequencing technologies, we can more accurately characterize the human genome. lncRNAs act mainly as gene expression regulators. The dysregulation of lncRNAs may destroy the normal transcriptional landscape and thus cause malignant transformation. In addition, their highly specific expression and functional tertiary structure force them to be as promising diagnostic biomarkers and potential targets for various diseases including breast cancer and colorectal cancer.
In this study, we proposed a computational lncRNA-disease association method (LDA-RWLMF) to identify potential biomarkers for breast cancer and colorectal cancer. First, a random walk with restart method was designed to extract negative LDAs. Second, a logistic matrix factorization model was explored to infer possible associations between lncRNAs and diseases. Finally, all lncRNAs are ranked according to association scores with breast cancer and colorectal cancer on the MNDR dataset.
We conduct 5-fold cross validation for 10 times to compare LDA-RWLMF with state-of-the-art LDA prediction models on the MNDR dataset, that is, LNCSIM1, LNCSIM2, ILNCSIM, and IDSSIM. The results show that LDA-RWLMF computes the best AUC values of 0.9312. We predict that lncRNAs (HULC, NPTN-IT1, and PCAT1) may be possible biomarkers of breast cancer and colorectal cancer.
Our proposed LDA-RWLMF method has two disadvantages. First, it extracted credible negative LDA samples. In the area of association prediction, there are no negative association samples because of the limitation of biomedical experiments, which causes relatively poor performance. Thus, we designed a negative LDA extraction method based on PU learning. Second, the logistic matrix factorization model can effectively discover possible associations between two biological entities. Thus, we used the model to identify new LDAs. In addition, diseases and lncRNAs exhibit abundant biological features. In this study, we failed to consider these diverse features. In the future, we will further integrate more biological information to improve LDA prediction.
In the future, we will further design more effective negative sample screening method based on positive-unlabeled learning. In addition, we will also develop deep learning model for LDA prediction. We anticipate that the proposed LDA-RWLMF method can help design therapeutic regimens for personalized treatment of breast cancer and colorectal cancer and thus opportunely inhibit its recurrence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: SL, MC, and LT; Methodology: SL, MC, YW, MW, and FW; Project administration: SL; Software: SL and MC; Writing-original draft: SL; Writing-review and editing: SL, MC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahzadeh, R., Mansoori, Y., Azarnezhad, A., Daraei, A., Paknahad, S., Mehrabi, S., et al. (2020). Expression and clinicopathological significance of AOC4P, PRNCR1, and PCAT1 lncRNAs in breast cancer. Pathol. Res. Pract. 216 (10), 153131. doi:10.1016/j.prp.2020.153131
Barzaman, K., Karami, J., Zarei, Z., Hosseinzadeh, A., Kazemi, M. H., Moradi-Kalbolandi, S., et al. (2020). Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 84, 106535. doi:10.1016/j.intimp.2020.106535
Bian, Z., Zhang, J., Min, L., Feng, Y., Xue, W., Jia, Z., et al. (2018). LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin. Cancer Res. 24 (19), 4808–4819. doi:10.1158/1078-0432.CCR-17-2967
Biller, L. H., and Schrag, D. (2021). Diagnosis and treatment of metastatic colorectal cancer: A review. Jama 325 (7), 669–685. doi:10.1001/jama.2021.0106
Campos-Parra, A. D., López-Urrutia, E., Orozco Moreno, L. T., Lopez-Camarillo, C., Meza-Menchaca, T., Figueroa Gonzalez, G., et al. (2018). Long non-coding RNAs as new master regulators of resistance to systemic treatments in breast cancer. Int. J. Mol. Sci. 19 (9), 2711. doi:10.3390/ijms19092711
Chandra Gupta, S., and Nandan Tripathi, Y. (2017). Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 140 (9), 1955–1967. doi:10.1002/ijc.30546
Chang, K. C., Diermeier, S. D., Yu, A. T., Brine, L. D., and Spector, D. L. (2020). MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression[J]. Nat. Commun. 11 (1), 1–19.
Chen, G., Wang, Z., Wang, D., Qiu, C., Liu, M., Chen, X., et al. (2012). LncRNADisease: A database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 41 (D1), D983–D986. doi:10.1093/nar/gks1099
Chen, X. (2015). Katzlda: KATZ measure for the lncRNA-disease association prediction. Sci. Rep. 5 (1), 16840–16911. doi:10.1038/srep16840
Chen, X., Xie, D., Zhao, Q., and You, Z. H. (2019). MicroRNAs and complex diseases: From experimental results to computational models. Brief. Bioinform. 20 (2), 515–539. doi:10.1093/bib/bbx130
Chen, Y., Guo, Y., Chen, H., and Ma, F. (2020). Long non-coding RNA expression profiling identifies a four-long non-coding RNA prognostic signature for isocitrate dehydrogenase mutant glioma. Front. Neurol. 11, 573264. doi:10.3389/fneur.2020.573264
Cui, T., Zhang, L., Huang, Y., Yi, Y., Tan, P., Zhao, Y., et al. (2018). MNDR v2.0: An updated resource of ncRNA-disease associations in mammals Nucleic Acids Res. 46 (D1), D371–D374. doi:10.1093/nar/gkx1025
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A., et al. (2019). Breast cancer statistics. Ca. Cancer J. Clin. 69 (6), 438–451. doi:10.3322/caac.21583
Duffy, M. J., Synnott, N. C., and Crown, J. (2018). Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 170 (2), 213–219. doi:10.1007/s10549-018-4753-7
Fan, W., Shang, J., Li, F., Sun,, Y., and Liu, J. X. (2020). Idssim: An lncRNA functional similarity calculation model based on an improved disease semantic similarity method[J]. BMC Bioinforma. 21 (1), 1–14.
Fu, G., Wang, J., Domeniconi, C., and Yu, G. (2018). Matrix factorization-based data fusion for the prediction of lncRNA-disease associations. Bioinformatics 34 (9), 1529–1537. doi:10.1093/bioinformatics/btx794
Garrido-Castro, A. C., Lin, N. U., and Polyak, K. (2019). Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9 (2), 176–198. doi:10.1158/2159-8290.CD-18-1177
Gavgani, R. R., Babaei, E., Hosseinpourfeizi, M. A., Fakhrjou, A., and Montazeri, V. (2020). Study of long non-coding RNA highly upregulated in liver cancer (HULC) in breast cancer: A clinical & in vitro investigation. Indian J. Med. Res. 152 (3), 244–253. doi:10.4103/ijmr.IJMR_1823_18
Gooding, A. J., Zhang, B., Jahanbani, F. K., Gilmore, H. L., Chang, J. C., Valadkhan, S., et al. (2017). The lncRNA BORG drives breast cancer metastasis and disease recurrence. Sci. Rep. 7 (1), 1–18. doi:10.1038/s41598-017-12716-6
Guo, Z. H., You, Z. H., Wang, Y. B., Yi, H. C., and Chen, Z. H. (2019). A learning-based method for LncRNA-disease association identification combing similarity information and rotation forest. IScience 19, 786–795. doi:10.1016/j.isci.2019.08.030
Guo, Z., Hui, Y., Kong, F., and Lin, X. (2022). Finding lung-cancer-related lncRNAs based on laplacian regularized least squares with unbalanced Bi-random walk. Front. Genet. 13, 933009. doi:10.3389/fgene.2022.933009
Heer, E., Harper, A., Escandor, N., Sung, H., McCormack, V., and Fidler-Benaoudia, M. M. (2020). Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet. Glob. Health 8 (8), e1027–e1037. doi:10.1016/S2214-109X(20)30215-1
Huang, F., Yue, X., Xiong, Z., Yu, Z., Liu, S., and Zhang, W. (2021). Tensor decomposition with relational constraints for predicting multiple types of microRNA-disease associations. Brief. Bioinform. 22 (3), bbaa140. doi:10.1093/bib/bbaa140
Huang, Y. A., Chen, X., You, Z. H., Huang, D. S., and Chan, K. C. C. (2016). Ilncsim: Improved lncRNA functional similarity calculation model. Oncotarget 7 (18), 25902–25914. doi:10.18632/oncotarget.8296
Huang, Z., Shi, J., Gao, Y., Cui, C., Zhang, S., Li, J., et al. (2019). HMDD v3.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 47 (D1), D1013–D1017. doi:10.1093/nar/gky1010
Key, T. J., Verkasalo, P. K., and Banks, E. (2001). Epidemiology of breast cancer. Lancet. Oncol. 2 (3), 133–140. doi:10.1016/S1470-2045(00)00254-0
Kong, J., Qiu, Y., Li, Y., Zhang, H., and Wang, W. (2019b). TGF-β1 elevates P-gp and BCRP in hepatocellular carcinoma through HOTAIR/miR-145 axis. Biopharm. Drug Dispos. 40 (2), 70–80. doi:10.1002/bdd.2172
Kong, X., Duan, Y., Sang, Y., Li, Y., Zhang, H., Liang, Y., et al. (2019a). LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J. Cell. Physiol. 234 (6), 9105–9117. doi:10.1002/jcp.27587
Lan, W., Dong, Y., Chen, Q., Zheng, R., Liu, J., Pan, Y., et al. (2022). Kgancda: Predicting circRNA-disease associations based on knowledge graph attention network. Brief. Bioinform. 23 (1), bbab494. doi:10.1093/bib/bbab494
Lan, W., Lai, D., and Chen, Q. (2020). Ldicdl: LncRNA-disease association identification based on collaborative deep learning[J]. IEEE/ACM Trans. Comput. Biol. Bioinforma. 19, 1715–1723. doi:10.1109/TCBB.2020.3034910
Li, G., Luo, J., Liang, C., Xiao, Q., Ding, P., and Zhang, Y. (2019). Prediction of LncRNA-disease associations based on network consistency projection. Ieee Access 7, 58849–58856. doi:10.1109/access.2019.2914533
Liang, Y., Song, X., Li, Y., Chen, B., Zhao, W., Wang, L., et al. (2020). Retraction note to: LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer 19 (1), 131–220. doi:10.1186/s12943-022-01576-y
Liang, Y., Wu, Y., and Zhang, Z. (2022a). Hyb4mC: A hybrid DNA2vec-based model for DNA N4-methylcytosine sites prediction[J]. BMC Bioinforma. 23 (1), 1–18. doi:10.1186/s12859-022-04789-6
Liang, Y., Zhang, Z. Q., Liu, N. N., Wu, Y. N., Gu, C. L., and Wang, Y. L. (2022b). Magcnse: Predicting lncRNA-disease associations using multi-view attention graph convolutional network and stacking ensemble model. BMC Bioinforma. 23 (1), 1–22. doi:10.1186/s12859-022-04715-w
Liu, C., Wei, D., Xiang, J., Ren, F., Huang, L., Lang, J., et al. (2020). An improved anticancer drug-response prediction based on an ensemble method integrating matrix completion and ridge regression. Mol. Ther. Nucleic Acids 21, 676–686. doi:10.1016/j.omtn.2020.07.003
Liu, T., Han, Z., Li, H., Zhu, Y., Sun, Z., and Zhu, A. (2018). LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol. Cancer 17 (1), 1–13. doi:10.1186/s12943-018-0873-2
Niknafs, Y. S., Han, S., Ma, T., Speers, C., Zhang, C., Wilder-Romans, K., et al. (2016). The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1 in breast cancer progression. Nat. Commun. 7 (1), 12791–12813. doi:10.1038/ncomms12791
Peng, L. H., Chen, Y. Q., Ma, N., and Chen, X. (2017). Narrmda: Negative-aware and rating-based recommendation algorithm for miRNA-disease association prediction. Mol. Biosyst. 13 (12), 2650–2659. doi:10.1039/c7mb00499k
Peng, L. H., Sun, C. N., Guan, N. N., Qiang, J., and Chen, X. (2018). Hnmda: Heterogeneous network-based miRNA-disease association prediction. Mol. Genet. Genomics 293 (4), 983–995. doi:10.1007/s00438-018-1438-1
Peng, L. H., Tian, X. F., Shen, L., Kuang, M., Li, T. B., Tian, G., et al. (2020a). Identifying effective antiviral drugs against SARS-CoV-2 by drug repositioning through virus-drug association prediction. Front. Genet. 11, 577387. doi:10.3389/fgene.2020.577387
Peng, L., Shen, L., Liao, L., Liu, G., and Zhou, L. (2020b). Rnmfmda: A microbe-disease association identification method based on reliable negative sample selection and logistic matrix factorization with neighborhood regularization. Front. Microbiol. 11, 592430. doi:10.3389/fmicb.2020.592430
Peng, L., Wang, C., Tian, X., Zhou, L., and Li, K. (2021). Finding lncRNA-protein interactions based on deep learning with dual-net neural architecture[J]. IEEE/ACM Trans. Comput. Biol. Bioinforma. doi:10.1109/TCBB.2021.3116232
Peng, L. H., Shen, L., Xu, J. L., Tian, X. F., Liu, F. X., Wang, J. J., et al. (2021b). Prioritizing antiviral drugs against SARS-CoV-2 by integrating viral complete genome sequences and drug chemical structures[J]. Sci. Rep. 11 (1), 1–11.
Peng, L., Wang, F., Wang, Z., Tan, J., Huang, L., Tian, X., et al. (2022a). Cell-cell communication inference and analysis in the tumour microenvironments from single-cell transcriptomics: Data resources and computational strategies. Brief. Bioinform. 23 (4), bbac234. doi:10.1093/bib/bbac234
Peng, L. H., Tan, J. W., Tian, X. F., and Zhou, L. Q. (2022b). EnANNDeep: An ensemble-based lncRNA–protein interaction prediction framework with adaptive k-nearest neighbor classifier and deep models[J]. Interdiscip. Sci. Comput. Life Sci., 1–24. doi:10.1007/s12539-021-00483-y
Qiao, K., Ning, S., Wan, L., Wu, H., Wang, Q., Zhang, X., et al. (2019). LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4/Hippo signaling pathway. J. Exp. Clin. Cancer Res. 38 (1), 418–515. doi:10.1186/s13046-019-1421-7
Sarrafzadeh, S., Geranpayeh, L., and Ghafouri-Fard, S. (2017). Expression analysis of long non-coding PCAT-1in breast cancer. Int. J. Hematol. Oncol. Stem Cell Res. 11 (3), 185–191.
Sharma, G. N., Dave, R., Sanadya, J., Sharma, P., and Sharma, K. K. (2010). Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 1 (2), 109–126.
Shen, L., Liu, F. X., Huang, L., Liu, G. Y., Zhou, L. Q., and Peng, L. H. (2022). VDA-RWLRLS: An anti-SARS-CoV-2 drug prioritizing framework combining an unbalanced bi-random walk and Laplacian regularized least squares. Comput. Biol. Med. 140, 105119. doi:10.1016/j.compbiomed.2021.105119
Shi, F., Xiao, F., Ding, P., Qin, H., and Huang, R. (2016). Long noncoding RNA highly up-regulated in liver cancer predicts unfavorable outcome and regulates metastasis by MMPs in triple-negative breast cancer. Arch. Med. Res. 47 (6), 446–453. doi:10.1016/j.arcmed.2016.11.001
Shi, Z., Luo, Y., Zhu, M., Zhou, Y., Zheng, B., Wu, D., et al. (2019). Expression analysis of long non-coding RNA HAR1A and HAR1B in HBV-induced hepatocullular carcinoma in Chinese patients. Lab. Med. 50 (2), 150–157. doi:10.1093/labmed/lmy055
Shi, Z., Zhang, H., Jin, C., Quan, X., and Yin, Y. (2021). A representation learning model based on variational inference and graph autoencoder for predicting lncRNA-disease associations. BMC Bioinforma. 22 (1), 136–220. doi:10.1186/s12859-021-04073-z
Siegel, R., and Naishadhamjemal, D. A. (2013). Cancer statistics, 2013. Ca. Cancer J. Clin. 63, 11–30. doi:10.3322/caac.21166
Sledge, G. W., Mamounas, E. P., Hortobagyi, G. N., Burstein, H. J., Goodwin, P. J., and Wolff, A. C. (2014). Past, present, and future challenges in breast cancer treatment. J. Clin. Oncol. 32 (19), 1979–1986. doi:10.1200/JCO.2014.55.4139
Sun, F., Sun, J., and Zhao, Q. (2022). A deep learning method for predicting metabolite-disease associations via graph neural network. Brief. Bioinform. 23 (4), bbac266. doi:10.1093/bib/bbac266
Sun, W., Wu, Y., Yu, X., Liu, Y., Song, H., Xia, T., et al. (2013). Decreased expression of long noncoding RNA AC096655.1-002 in gastric cancer and its clinical significance. Tumour Biol. 34 (5), 2697–2701. doi:10.1007/s13277-013-0821-0
Sun, Y. S., Zhao, Z., Yang, Z. N., Xu, F., Lu, H. J., Zhu, Z. Y., et al. (2017). Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 13 (11), 1387–1397. doi:10.7150/ijbs.21635
Tang, W., Lu, G., and Ji, Y. (2022). Long non-coding RNA PCAT1 sponges miR-134-3p to regulate PITX2 expression in breast cancer[J]. Mol. Med. Rep. 25 (3), 1–10.
Tang, X., Cai, L., Meng, Y., Xu, J., Lu, C., and Yang, J. (2021). Indicator regularized non-negative matrix factorization method-based drug repurposing for COVID-19. Front. Immunol. 11, 3824. doi:10.3389/fimmu.2020.603615
Tian, X., Shen, L., Gao, P., Huang, L., Liu, G., Zhou, L., et al. (2022). Discovery of potential therapeutic drugs for COVID-19 through logistic matrix factorization with kernel diffusion. Front. Microbiol. 13, 13. doi:10.3389/fmicb.2022.740382
Wahlestedt, C. (2013). Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat. Rev. Drug Discov. 12 (6), 433–446. doi:10.1038/nrd4018
Waks, A. G., and Winer, E. P. (2019). Breast cancer treatment: A review. Jama 321 (3), 288–300. doi:10.1001/jama.2018.19323
Wang, D., Wang, J., Lu, M., Song, F., and Cui, Q. (2010). Inferring the human microRNA functional similarity and functional network based on microRNA-associated diseases. Bioinformatics 26 (13), 1644–1650. doi:10.1093/bioinformatics/btq241
Wang, J., Chen, X., Hu, H., Yao, M., Song, Y., Yang, A., et al. (2021b). PCAT-1 facilitates breast cancer progression via binding to RACK1 and enhancing oxygen-independent stability of HIF-1α. Mol. Ther. - Nucleic Acids 24, 310–324. doi:10.1016/j.omtn.2021.02.034
Wang, M. N., You, Z. H., Wang, L., Li, L. P., and Zheng, K. (2021a). Ldgrnmf: LncRNA-disease associations prediction based on graph regularized non-negative matrix factorization. Neurocomputing 424, 236–245. doi:10.1016/j.neucom.2020.02.062
Wang, N., Zhong, C., Fu, M., Li, L., Wang, F., Lv, P., et al. (2019). Long non-coding RNA HULC promotes the development of breast cancer through regulating LYPD1 expression by sponging miR-6754-5p. Onco. Targets. Ther. 12, 10671–10679. doi:10.2147/OTT.S226040
Wu, Y., Yang, X., and Chen, Z. (2019). m6A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1[J]. Mol. cancer 18 (1), 1–16.
Xi, Y., and Xu, P. (2021). Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 14 (10), 101174. doi:10.1016/j.tranon.2021.101174
Xing, Z., Park, P. K., Lin, C., and Yang, L. (2015). LncRNA BCAR4 wires up signaling transduction in breast cancer. RNA Biol. 12 (7), 681–689. doi:10.1080/15476286.2015.1053687
Xu, W., Zhou, G., Wang, H., Liu, Y., Chen, B., Chen, W., et al. (2020). Circulating lncRNA SNHG11 as a novel biomarker for early diagnosis and prognosis of colorectal cancer. Int. J. Cancer 146 (10), 2901–2912. doi:10.1002/ijc.32747
Yang, J., Grünewald, S., and Wan, X. F. (2013). Quartet-net: A quartet-based method to reconstruct phylogenetic networks[J]. Mol. Biol. 30 (5), 1206–1217.
Yang, J., Peng, S., and Zhang, B. (2020). Human geroprotector discovery by targeting the converging subnetworks of aging and age-related diseases[J]. Geroscience 42 (1), 353–372.
Zhang, H., Jiang, L., Zhong, S., Li, J., Sun, D., Hou, J., et al. (2021b). The role of long non-coding RNAs in drug resistance of cancer. Clin. Genet. 99 (1), 84–92. doi:10.1111/cge.13800
Zhang, L., Yang, P., Feng, H., Zhao, Q., and Liu, H. (2021a). Using network distance analysis to predict lncRNA–miRNA interactions. Interdiscip. Sci. Comput. Life Sci. 13 (3), 535–545. doi:10.1007/s12539-021-00458-z
Zhang, W., Li, Z., Guo, W., Yang, W., and Huang, F. (2019b). A fast linear neighborhood similarity-based network link inference method to predict MicroRNA-disease associations. IEEE/ACM Trans. Comput. Biol. Bioinform. 18 (2), 405–415. doi:10.1109/tcbb.2019.2931546
Zhang, W., Jing, K., Huang, F., Chen, Y., Li, B., Li, J., et al. (2019a). Sflln: A sparse feature learning ensemble method with linear neighborhood regularization for predicting drug-drug interactions. Inf. Sci. (N. Y). 497, 189–201. doi:10.1016/j.ins.2019.05.017
Zhao, Q., Yang, Y., Ren, G., and Fan, C. (2019). Integrating bipartite network projection and KATZ measure to identify novel CircRNA-disease associations. IEEE Trans. Nanobioscience 18 (4), 578–584. doi:10.1109/TNB.2019.2922214
Zhou, L., Duan, Q., Tian, X., Tang, J., and Peng, L. H. (2021a). LPI-HyADBS: A hybrid framework for lncRNA-protein interaction prediction integrating feature selection and classification[J]. BMC Bioinforma. 22 (1), 1–31.
Keywords: breast cancer, colorectal cancer, lncRNA, biomarker, lncRNA-disease association, random walk, logistic matrix factorization
Citation: Li S, Chang M, Tong L, Wang Y, Wang M and Wang F (2023) Screening potential lncRNA biomarkers for breast cancer and colorectal cancer combining random walk and logistic matrix factorization. Front. Genet. 13:1023615. doi: 10.3389/fgene.2022.1023615
Received: 20 August 2022; Accepted: 10 October 2022;
Published: 20 January 2023.
Edited by:
Lihong Peng, Hunan University of Technology, ChinaReviewed by:
Guanghui Li, East China Jiaotong University, ChinaLi Zejun, Professional Services Review, Australia
Copyright © 2023 Li, Chang, Tong, Wang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijun Li, Y2ZsaXNoaWp1bjY1ODhAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Shijun Li
Shijun Li Miaomiao Chang†
Miaomiao Chang† Ling Tong
Ling Tong