- 1Department of Obstetrics and Gynecology of West China Second University Hospital, BMI Center for Biomass Materials and Nanointerfaces, College of Biomass Science and Engineering, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related of Women and Children of Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, China
- 3State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, Sichuan, China
Uterine fibroids (UFs), the most common benign gynecological tumor, can bring severe negative impacts on a woman’s life quality. Vitamin D, is thought to play an important role in regulating cell proliferation and differentiation. In recent years, several studies suggested that higher level of vitamin D has a negative effect on the occurrence of UFs, but the results of studies on the relationship between them are conflicting and further evidence needs to be studied. Here in, we used a two-sample Mendelian Randomization (2SMR) study to explore the causal relationship between genetically predicted vitamin D levels and the risk of UFs. The exposure data comes from a genome-wide association study (GWAS) summary dataset consisting of 441,291 individuals, which includes datasets from United Kingdom Biobank, FinnGen Biobank and the corresponding consortia. Single-nucleotide polymorphisms (SNPs) associated with vitamin D at a significant level of p < 5 × 10−8 and low linkage disequilibrium (LD) level (r2 < 0.01) were selected. The outcome data comes from a GWAS dataset of IEU analysis of United Kingdom Biobank phenotypes consisting of 7,122 UFs cases and 455,811 controls. Our inverse-variance weight (IVW) analysis results support the causal association of genetically predicted vitamin D with the risk of UFs (OR = 0.995,95% CI = 0.990-0.999, p = 0.024). In addition, heterogeneity and pleiotropy were not observed in statistical models. In summary, our results indicate that elevated serum vitamin D levels are in strong relationship with reduction of the risk of UFs, which indicates that the clinical treatment of UFs may have a new and excellent option.
Introduction
Uterine fibroids (UFs), also known as uterine leiomyomas, are benign tumors that negatively affect the function of the uterus in women of childbearing age. The most common symptom of UFs is heavy menstrual bleeding and the resulting anemia and pain (Kempson and Hendrickson, 2000). Other symptoms include pelvic pressure and pain, urinary incontinence and retention, and bowel dysfunction also place a significant burden on patients. UFs may also cause several reproductive problems, such as impaired fertility, pregnancy complications, miscarriage and adverse pregnancy outcomes (Wallach et al., 1981; Zimmermann et al., 2012; Khan et al., 2014; Vercellini and Frattaruolo, 2017). A study pointed out that UFs account for 1/3 to 1/2 of the reasons for hysterectomy and are the most common reason of hysterectomy in the United States (Stewart et al., 2016). Risk factors for UFs have received increasing attention in order to better prevent the occurrence of UFs. Many studies indicated that vitamin D deficiency increases the risk of UFs (Baird et al., 2013; Sabry et al., 2013; Ciebiera et al., 2016). There is a clinical study indicating that vitamin D supplementation has a significant therapeutic effect in patients with small UFs (Ciavattini et al., 2016). However, another clinical trial suggested that vitamin D levels have no significant effect on the occurrence of UFs (Arjeh et al., 2020). Overall, the relationship between vitamin D levels and the risk of UFs is still ambiguous, and further studies are needed to be carried out.
Vitamin D, a fat-soluble vitamin, is a general term for a group of structurally similar sterol derivatives. The target organs of vitamin D are widely distributed, and different type of vitamin D binds to their specific receptors and then play different roles (Lips, 2006). Vitamin D3 and vitamin D2 are the most important members of vitamin D (Ciebiera et al., 2018), and vitamin D3 is the main form of vitamin D in the human body. The level of vitamin D3 in serum can represent the total content of vitamin D in the body and the strength of the effect of vitamin D on the human body (Lips, 2006). Therefore, in the work, we focus on the level of vitamin D3 in serum. vitamin D3 has an anti-proliferative effect and can accelerate the release of tumor necrosis factors from macrophages, which has a broad killing effect on tumor cells (Van Den Bemd et al., 2000; Lips, 2006). Several recent studies pointed out that vitamin D deficiency is an important risk factor for UFs (Baird et al., 2013; Sabry et al., 2013; Ciebiera et al., 2016), and animal experiments have also shown that high doses of vitamin D can decrease the size of UFs (Al-Hendy and Badr, 2014; Ali et al., 2020), but the relationship between vitamin D and the pathogenesis of UFs or a positive and significant treatment effect remains unclear.
Mendelian randomization (MR) studies are conducted based on Mendel’s laws of inheritance and the use of instrumental variables (IVs). Mendelian laws of inheritance state that genes are randomly assigned and freely selected in the process of inheritance, instrumental variables are related to the risk factors we are interested in but not related to other confounding factors, and its effect on the outcome can only be determined by the exposure factor (Burgess and Thomson, 2015). Therefore, in the two-sample MR study, we use genetic variables to analyze the causal relationship between exposure factors and outcomes, typically single-nucleotide polymorphisms (SNPs). Due to the genes assigned during pregnancy, the direction of the causal relationship can also be determined. The association between serum vitamin D3 levels and the occurrence of UFs has not previously been studied using MR. In this study, we focused on exploring the causal relationship between serum vitamin D3 levels and the occurrence of UFs.
Materials and methods
Study design
In a Mendelian randomization study, to obtain reliable results, genetic variables as instrumental variables must satisfy three assumptions (Figure 1): 1) Genetic variation is associated with exposure factors; 2) Genetic variation is not associated with confounders; 3) Genetic variation only influences the outcome by exposure factors. The dashed line in the figure indicates that the pathway is not allowed, and the solid line indicates the ideal pathway. The second and third assumptions are collectively referred to as independence from pleiotropy. Pleiotropy refers to genetic variation that affects outcomes through pathways independent of risk factors. Investigators need to use sensitivity analysis to make judgments (Emdin et al., 2017).
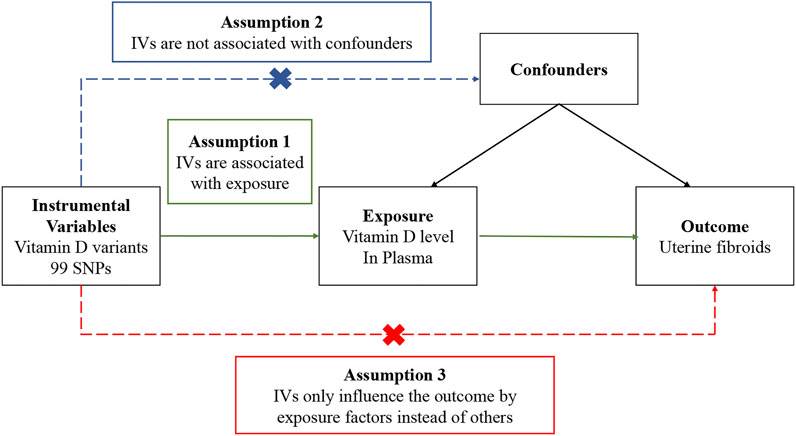
FIGURE 1. Overview of the design and three key assumptions of the Mendelian randomization study. IVs, instrument variables; SNPs, single-nucleotide polymorphisms.
Genetic association dataset for Vitamin D
SNPs associated with vitamin D were selected from a genome-wide association study (GWAS) summary dataset, including 441,291 samples from European populations (Hemani et al., 2018), and the data were derived from research data including the United Kingdom biobank, FinnGen Biobank and other consortia, which were manually collected and organized into a summary dataset for use in MR studies. Independent SNPs that were related to vitamin D at the genome-wide significance level (p < 5 × 10−8) and low linkage disequilibrium (LD) level (r2 < 0.01) were selected. In order to avoid the possible bias caused by weak instrumental variables, we use the F-statistic to judge the strength of the instrumental variable (Burgess et al., 2011). According to experience, the F-statistic should be at least 10 (Staiger and Stock, 1997).
Genetic instrumental vasriables for uterine fibroids
In this MR study, the occurrence of UFs was our outcome. Data for the outcomes were derived from the dataset provided to GWAS by the United Kingdom Biobank (Mitchell et al., 2019), containing 462,933 samples from European populations, of which 7122 were reported as UFs without other cancers. In this study, we extracted the effect estimates and standard errors for each of the 99 vitamin D–related SNPs from the GWAS summary statistics of UFs.
Statistical analysis
MR analysis of the association between vitamin D and the occurrence of UFs was performed using 99 SNPs associated with 25-hydroxyvitamin D levels as IVs. We primarily performed MR analyses using Inverse-variance Weights (IVW) with random effects to estimate odds ratios (OR) and 95% confidence intervals (CI) for the occurrence of UFs (Burgess and Bowden, 2015).
We then performed a sensitivity analysis to examine heterogeneity and pleiotropy among IVs. MR-Egger regression, weighted median, simple model, and weighted model methods were used to determine whether IVs affected UFs through their effect on vitamin D alone. The slope coefficients of the MR-Egger regressions provided estimates of causal effects, which were used to test for pleiotropic bias (Bowden et al., 2015). Simple medians provide consistent estimates of causal effects if at least 50% of the IVs are valid, but weighted medians provide consistent estimates if at least 50% of the weights come from valid IVs (Bowden et al., 2016). And the weighted mode requires that the largest subset of instruments identifying the same causal effect estimates is contributed by valid IVs so that the result is consistent (Hartwig et al., 2017). We applied MR- Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis method to analyze the pleiotropy of IVs and correct the possible outliers. In addition, we use Q test on the IVW and MR-Egger to estimate the heterogeneity of the IVs. We also used a leave-one-out sensitivity test to test whether the MR outcome was sensitive to its related IV. MR and sensitivity analyses were performed in R (version 4.2.0) using the Two-Sample MR package (version 0.5.6) and the MRPRESSO package (version 1.0).
Results
SNPs used as instrumental variables
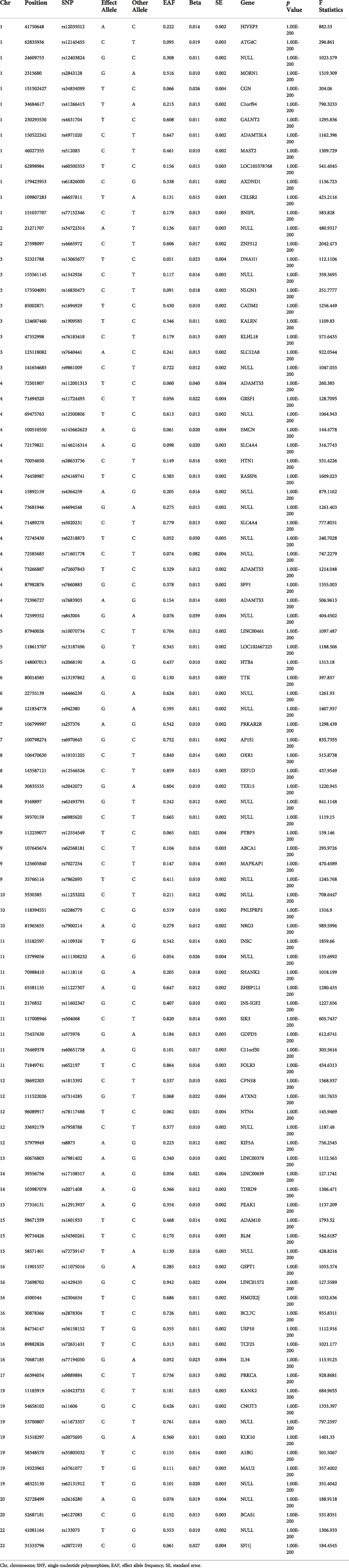
Independent SNPs that were related to 25-hydroxyvitamin D serum levels at the genome-wide significance level (p < 5 × 10−8) and low linkage disequilibrium (LD) level (r2 < 0.01) were selected from the GWAS dataset. Then SNPs with F > 10 were screened from these SNPs and the genes they are in were queried in Pubmed (https://www.ncbi.nlm.nih.gov/), and the genes of SNPs that did not belong to a specific gene were defined as NULL. The remaining 99 SNPs were included to establish the genetic IVs for vitamin D (Table 1).
Mendelian randomization test results and data visualization
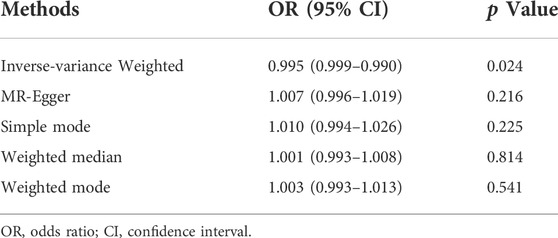
In the work, the IVW method was used to test for causal effects firstly. We found that a one-SD increase in vitamin D levels was associated with a decreased risk of UFs [odds ratio (OR): 0.995, 95% CI: 0.990-0.999, p = 0.024]. The result reveals the causal relationship between vitamin D levels and the risk of UFs in the European population. Then we adopted four different models to test and verify the causal relationship between serum vitamin D3 levels and UFs. All of the MR-Egger regression, weighted median, simple model, and weighted model results were opposite to IVW analysis (Table 2). Nevertheless, according to the judgment method of MR test results (Burgess and Thomson, 2015), our results are still able to draw the same conclusion. In addition, there has no bias value between our IVs in the scatter plot of correlation analysis (Figure 2) and the results of the leave-one-out sensitivity test (Figure 3), it illustrates that the causal relationship between vitamin D levels and the risk of UFs is highly reliable.
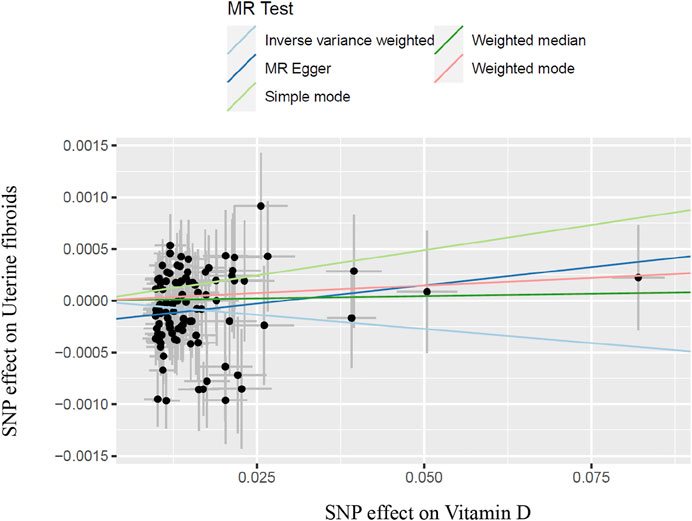
FIGURE 2. Scatter plot of SNPs with vitamin D and uterine fibroids and results of different test models; SNP, single-nucleotide polymorphism.

FIGURE 3. A leave one-out pleiotropy test was performed for each SNP to determine the robustness of the MR test; SNP, single-nucleotide polymorphism.
Heterogeneity and pleiotropy test results
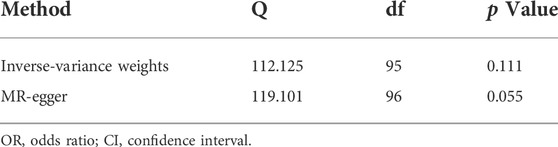
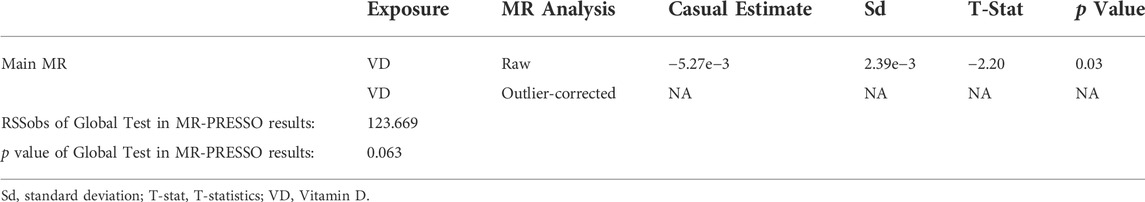
To remove the possible bias of instrumental variables, heterogeneity test and horizontal pleiotropy test was conducted in the MR study. In sensitivity analysis, there have no heterogeneity was detected in the IVW method or the MR-egger method between IVs (p > 0.05) (Table 3). This means that our results are not confounded by other factors between populations grouped by IVs. But the intercept obtained by the MR-egger method was too far from 0, suggesting that there may be horizontal pleiotropy between the IVs (p < 0.05) (Table 4). Therefore, we performed multiple operations with MR-PRESSO and found no offset in IVs and no pleiotropy (p > 0.05). Furthermore, there have no outliers and horizontal pleiotropy were found after 2000 simulations using MR-PRESSO (Table 5). This indicates that the IVs used in this work impacted the risk of UFs only by affecting serum vitamin D3 levels.
Discussion
The Mendelian randomization study performed an analysis of the causal relationship between vitamin D and UFs based on a summary dataset from the United Kingdom Biobank including 462,933 individuals using multiple SNPs as instrumental variables. Our results revealed a causal relationship between serum vitamin D3 levels and the occurrence of UFs that the reduction of vitamin D levels will increase the risk of UFs.
Our findings are consistent with numerous previous studies. Early animal experiments found that vitamin D supplementation can significantly reduce the volume of UFs (Halder et al., 2010). A subsequent observational experiment showed that differences in serum vitamin D levels were significantly associated with the risk of developing UFs (Sabry et al., 2013), patients with lower vitamin D levels having a higher risk of developing UFs. These studies provide a potentially excellent therapeutic approach for the clinical treatment of UFs, which inspired researchers to further explore the phenomenon.
In the early 2000s, several studies revealed the mechanism of UFs—excessive secretion of compounds from extracellular matrix (ECM) such as collagen and fibers can cause UFs—and recent studies have also confirmed this (Sozen I and Arici, 2002; Rafique et al., 2017). The symptoms of ECM accumulation during the occurrence of UFs is similar to inflammation, which manifested as massive exudation of intracellular material and accumulation of ECM. Some researchers have proposed the possible involvement of inflammation in the development of UFs, and these processes are closely related to the function of vitamin D in vivo (Protic et al., 2016).
Vitamin D is a natural active substance, and its receptors are widely distributed in vivo and play different functions. Anti-inflammatory and anti-tumor effects are representative functions of Vitamin D (Van Den Bemd et al., 2000; Lips, 2006). Vitamin D generally exerts its biological function by regulating the level of growth factors through various signaling pathways. For example, Vitamin D is involved in the regulation of Wnt/β-catenin and TGF-β pathways (Ciebiera et al., 2017), which play important roles in the anti-inflammation and regulation of cell proliferation (Protic et al., 2016). Overexpression of TGF-β can lead to excessive secretion of ECM by stimulating the synthesis of collagen, proteoglycans, and other ECM compounds, which further induces the occurrence of UFs (Leppert et al., 2004; Ciebiera et al., 2017). Other studies also pointed out that increased vitamin D levels can suppress the cell proliferation and slow down the development of UFs by inhibiting Wnt/β-catenin and TGF-β pathways in the process of culturing UFs in vitro (Al-Hendy et al., 2016).
There are a series of factors participate in the regulation of cell proliferation and apoptosis, such as proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 1 (CDK1), M-phase promoting factor and catechol-O-methyltransferase (COMT), etc., Overexpression of these factors can promote the development of UFs by stimulating cell proliferation, which showing that vitamin D compounds can significantly inhibit the activation of enzymes that regulate factor expression and down-regulate the expression of them (Sharan et al., 2011). Furthermore, a study found that vitamin D can inhibit the expression of estrogen and progesterone receptors, and then suppress estrogen and progesterone perform endocrine functions (Al-Hendy et al., 2015). All of these evidences suggest that vitamin D play an important role in the development of UFs.
The researchers further conducted randomized clinical trials (RCT) to evaluate the effect of vitamin D supplementation on UFs. A clinical study, conducted on patients with small UFs and published in 2016, had reported a positive effect of vitamin D on fibroid volume reduction (Ciavattini et al., 2016). It indicated that vitamin D supplementation can significantly reduce the volume of UFs, but this is completely opposite to the results of another RCT (Arjeh et al., 2020). Interference of confounding factors and heterogeneity are also unavoidable in clinical trials. Thus, it is necessary to perform a MR study for further research. High-quality MR studies use Mendel’s law of random assignment and use SNPs as instrumental variables to minimize the influence of confounding factors and ensure that there is no heterogeneity in the study subjects, which would make the findings more convincing. Our MR study showed that low levels of vitamin D is associated with the increasing risk of UFs, which is consistent with the results of the former RCT (Ciavattini et al., 2016). We speculate that this is because the exposure simulated by the MR study is lifetime exposure, and the outcome is caused by chronically low serum vitamin D3 levels. On the other hand, vitamin D treatment within the RCT period is hardly produce enough therapeutic effects on normal-sized UFs compared to the life cycle, but it has better therapeutic effect on smaller-sized UFs.
The mechanism of action of vitamin D in the body is complex (Lips, 2006). This study found that some genetic variants can affect the risk of UFs through vitamin D3 serum levels. Genetic factors can affect vitamin D through multiple pathways (Hoffman et al., 2004), some non-vitamin D-related genes and their signaling pathways have been shown to play a role in promoting the development of uterine fibroids (Leppert et al., 2006), and this study can only explain some of the effects of vitamin D-related genetic variants on UFs. Therefore, vitamin D-related variants can only explain part of the risk of uterine fibroids, and other signaling pathways need to be analyzed to better understand other risk factors for uterine fibroids.
In the present work, we provide a scientific basis for further research on whether insufficient vitamin D is a causative risk factor for UFs, which may have important public health implications. Vitamin D is a natural component with high safety, relatively small side effects, high economic feasibility, and great research value. Further investigation of randomized clinical trials is needed to be constructed to actively explore the potential role of vitamin D or combination with other drugs on the treatment of UFs. This may help scientists develop a new generation of UFs treatment option (Al-Hendy and Badr, 2014).
One of the major strengths of study is the use of the MR study design, which can reduce the interference of confounders and determine the direction of causality. In addition, the study had large sample size, allowing us to examine more reliable causal association. Furthermore, we evaluate the consistency of the association through different methods to support the robustness of our results. However, several limitations in the study are also worth considering. First, our analysis is based on GWAS data from European populations, and the genetic variation among different races did not satisfy Mendel’s law of inheritance, so the obtained results may be difficult to extrapolate to the whole population. Second, our study did not investigate the therapeutic effect of vitamin D on UFs, although it established a causal relationship between vitamin D levels and UFs. Thirdly, The study examined serum vitamin D3 levels only through genetic pathways, and these genetic variants only play a role in specific contexts, given the complex biological role of the vitamin (Lips, 2006).
Conclusion
In the MR study, we found that a one-SD decrease in serum vitamin D levels was associated with higher risk of UFs, consistent with previous studies describing a critical biological role for vitamin D in the development of UFs. Our study also implies the importance of adequate daily intake of vitamin D, which has positive effects on the prevention of UFs. However, due to the limited availability of evidence from clinical studies, further clinical studies are needed to explore the utility of vitamin D for the treatment of UFs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: JG and YZ; data curation: WG and ZZ; MR analysis: WG; funding acquisition: JG and YZ; software and visualization: MD and MC; writing—original draft: WG; writing—review and editing: YZ and GG. WG and SZ have verified the underlying data. All the authors approved the final version of the manuscript.
Funding
National Natural Science Foundation of China (YZ, Grant No. 82001496), project of Chengdu Science and Technology Bureau, (YZ, Grant No. 2021-YF05-02110-SN), China Postdoctoral Science Foundation (YZ, Grant Nos 2020M680149, 2020T130087ZX). The Fundamental Research Funds for the Central Universities (SCU2020D4132).
Acknowledgments
Data in the European population on uterine fibroids and serum 25-hydroxyvitamin D levels are available through the United Kingdom Biobank and data analysis is available through the GWAS database. The authors thank these researchers for their selfless sharing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Hendy, A., Diamond, M. P., Boyer, T. G., and Halder, S. K. (2016). Vitamin D3 inhibits wnt/β-catenin and mTOR signaling pathways in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 101 (4), 1542–1551. doi:10.1210/jc.2015-3555
Al-Hendy, A., Diamond, M. P., El-Sohemy, A., and Halder, S. K. (2015). 1, 25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 100 (4), E572–E582. doi:10.1210/jc.2014-4011
Al-Hendy, A. M. B., and Badr, M. (2014). Can vitamin D reduce the risk of uterine fibroids. Women’s Health 10 (4), 353–358. doi:10.2217/whe.14.24
Ali, M., Prince, L., and Al-Hendy, A. (2020). Vitamin D and uterine fibroids: Preclinical evidence is in; time for an overdue clinical study. Fertil. Steril. 113 (1), 89–90. doi:10.1016/j.fertnstert.2019.10.015
Arjeh, S., Darsareh, F., Asl, Z. A., and Azizi Kutenaei, M. (2020). Effect of oral consumption of vitamin D on uterine fibroids: A randomized clinical trial. Complement. Ther. Clin. Pract. 39, 101159. doi:10.1016/j.ctcp.2020.101159
Baird, D. D., Hill, M. C., Schectman, J. M., and Hollis, B. W. (2013). Vitamin d and the risk of uterine fibroids. Epidemiology 24 (3), 447–453. doi:10.1097/EDE.0b013e31828acca0
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44 (2), 512–525. doi:10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 (4), 304–314. doi:10.1002/gepi.21965
Burgess, S., and Bowden, J. (2015). Integrating summarized data from multiple genetic variants in Mendelian randomization bias and coverage properties of inverse-variance weighted methods. arXiv preprint arXiv:. Available from: http://151204486 (Accessed Nov 27, 2015).
Burgess, S., Thompson, S. G., and Collaboration, C. C. G. (2011). Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40 (3), 755–764. doi:10.1093/ije/dyr036
Burgess, S., and Thomson, S. (2015). Mendelian randomization: Methods for using genetic variants in causal estimation. Florida, United States: CRC Press.
Ciavattini, A., Delli Carpini, G., Serri, M., Vignini, A., Sabbatinelli, J., Tozzi, A., et al. (2016). Hypovitaminosis D and "small burden" uterine fibroids: Opportunity for a vitamin D supplementation. Med. Baltim. 95 (52), e5698. doi:10.1097/MD.0000000000005698
Ciebiera, M., Wlodarczyk, M., Ciebiera, M., Zareba, K., Lukaszuk, K., and Jakiel, G. (2018). Vitamin D and uterine fibroids-review of the literature and novel concepts. Int. J. Mol. Sci. 19 (7), E2051. doi:10.3390/ijms19072051
Ciebiera, M., Wlodarczyk, M., Slabuszewska-Jozwiak, A., Nowicka, G., and Jakiel, G. (2016). Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 106 (7), 1787–1792. doi:10.1016/j.fertnstert.2016.09.007
Ciebiera, M., Wlodarczyk, M., Wrzosek, M., Meczekalski, B., Nowicka, G., Lukaszuk, K., et al. (2017). Role of transforming growth factor beta in uterine fibroid biology. Int. J. Mol. Sci. 18 (11), E2435. doi:10.3390/ijms18112435
Emdin, C. A., Khera, A. V. S. K., and Kathiresan, S. (2017). Mendelian randomization. JAMA J. Am. Med. Assoc. 318 (19), 1925–1926. doi:10.1001/jama.2017.17219
Halder, S. K., Sharan, C., and Al-Hendy, A. (2010). Vitamin D treatment induces dramatic shrinkage of uterine leiomyomas growth in the Eker rat model. Fertil. Steril. 94 (4), S75–S76. doi:10.1016/j.fertnstert.2010.07.293
Hartwig, F. P., Davey Smith, G., and Bowden, J. (2017). Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46 (6), 1985–1998. doi:10.1093/ije/dyx102
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408. doi:10.7554/eLife.34408
Hoffman, P. J., Milliken, D. B., Gregg, L. C., Davis, R. R., and Gregg, J. P. (2004). Molecular characterization of uterine fibroids and its implication for underlying mechanisms of pathogenesis. Fertil. Steril. 82 (3), 639–649. doi:10.1016/j.fertnstert.2004.01.047
Kempson, R. L, R. H. M., and Hendrickson, M. R. (2000). Smooth muscle, endometrial stromal, and mixed Müllerian tumors of the uterus. Mod. Pathol. 13 (3), 328–342. doi:10.1038/modpathol.3880055
Khan, A. T., Shehmar, M., and Gupta, J. K. (2014). Uterine fibroids: Current perspectives. Int. J. Womens Health 6, 95–114. doi:10.2147/IJWH.S51083
Leppert, P. C., Baginski, T., Prupas, C., Catherino, W. H., Pletcher, S., and Segars, J. H. (2004). Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil. Steril. 82, 1182–1187. doi:10.1016/j.fertnstert.2004.04.030
Leppert, P. C., Catherino, W. H., and Segars, J. H. (2006). A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am. J. Obstet. Gynecol. 195 (2), 415–420. doi:10.1016/j.ajog.2005.12.059
Lips, P. (2006). Vitamin D physiology. Prog. Biophys. Mol. Biol. 92 (1), 4–8. doi:10.1016/j.pbiomolbio.2006.02.016
Mitchell, R. E., Elsworth, B. L., Mitchell, R., Raistrick, C. A., Paternoster, L., Hemani, G., et al. (2019). MRC IEU UK Biobank GWAS pipeline version 2. Bristol, UK: University of Bristol.
Protic, O., Toti, P., Islam, M. S., Occhini, R., Giannubilo, S. R., Catherino, W. H., et al. (2016). Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 364 (2), 415–427. doi:10.1007/s00441-015-2324-3
Rafique, S., Segars, J. H., and Leppert, P. C. (2017). Mechanical signaling and extracellular matrix in uterine fibroids. Semin. Reprod. Med. 35 (6), 487–493. doi:10.1055/s-0037-1607268
Staiger, D., and Stock, J. H. (1997). Instrumental variables regression with weak instruments. Econometrica 65, 557–586. doi:10.2307/2171753
Sabry, M., Halder, S. K., Allah, A. S., Roshdy, E., Rajaratnam, V., and Al-Hendy, A. (2013). Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 5, 93–100. doi:10.2147/IJWH.S38800
Sharan, C., Halder, S. K., Thota, C., Jaleel, T., Nair, S., and Al-Hendy, A. (2011). Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 95 (1), 247–253. doi:10.1016/j.fertnstert.2010.07.1041
Sozen I, A. A., and Arici, A. (2002). Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 78 (1), 1–12. doi:10.1016/s0015-0282(02)03154-0
Stewart, E. A., Laughlin-Tommaso, S. K., Catherino, W. H., Lalitkumar, S., Gupta, D., and Vollenhoven, B. (2016). Uterine fibroids. Nat. Rev. Dis. Prim. 2, 16043. doi:10.1038/nrdp.2016.43
Van Den Bemd, G. J., Pols, H. A., and Leeuwen Van, J. P. (2000). Anti-tumor effects of 1, 25-dihydroxyvitamin D3 and vitamin D analogs. Curr. Pharm. Des. 6 (7), 717–732. doi:10.2174/1381612003400498
Vercellini, P., and Frattaruolo, M. P. (2017). Uterine fibroids: From observational epidemiology to clinical management. BJOG 124 (10), 1513. doi:10.1111/1471-0528.14730
Wallach, E. E., Buttram, V. C., and Reiter, R. C. (1981). Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 36 (4), 433–445. doi:10.1016/s0015-0282(16)45789-4
Keywords: vitamin D, uterine fibroids, SNP, mendelian randomization, GWAS
Citation: Guo W, Dai M, Zhong Z, Zhu S, Gong G, Chen M, Guo J and Zhang Y (2022) The association between vitamin D and uterine fibroids: A mendelian randomization study. Front. Genet. 13:1013192. doi: 10.3389/fgene.2022.1013192
Received: 06 August 2022; Accepted: 22 August 2022;
Published: 21 September 2022.
Edited by:
Shengqian Xia, The University of Chicago, United StatesReviewed by:
Xi Xia, Shenzhen Hospital, Peking University, ChinaYue Zhao, Peking University Third Hospital, China
Copyright © 2022 Guo, Dai, Zhong, Zhu, Gong, Chen, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaoyao Zhang, eWFveWFvemhhbmdAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Weijie Guo1,2†
Weijie Guo1,2† Junling Guo
Junling Guo Yaoyao Zhang
Yaoyao Zhang