- 1Department of Oncology, The Affiliated Zhuzhou Hospital Xiangya Medical College, Central South University, Zhuzhou, Hunan, China
- 2Department of Ophthalmology, Jiangxi Branch of National Clinical Research Center for Ocular Disease, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 3Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, China
- 4School of Optometry and Vision Science, Cardiff University, Cardiff, United Kingdom
Objective: Lung cancer is a common malignant tumor, characterized by being difficult to detect and lacking specific clinical manifestations. This study aimed to find out the risk factors of mediastinal lymph node metastasis and explore the correlation between serum tumor markers and mediastinal lymph node metastasis and lung cancer prognosis.
Methods: A retrospective study of 3,042 lung cancer patients (330 patients with mediastinal lymph node metastasis and 2,712 patients without mediastinal lymph node metastasis) collected from the First Affiliated Hospital of Nanchang University from April 1999 to July 2020. The patients were divided into two groups, namely, mediastinal lymph node metastasis group and non-mediastinal lymph node metastasis group. Student’s t test, non-parametric rank sum test and chi-square test were used to describe whether there is a significant difference between the two groups. We compared the serum biomarkers of the two groups of patients, including exploring serum alkaline phosphatase (ALP), calcium hemoglobin (HB), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA125, CA-199, CA -153, cytokeratin fragment 19 (CYFRA 21-1), total prostate specific antigen (TPSA), neuron-specific enolase (NSE) levels and the incidence and prognosis of lung cancer mediastinal lymph node metastasis. Binary logistic regression analysis was used to determine its risk factors, and receiver operating curve (ROC) analysis was used to evaluate its diagnostic value for mediastinal lymph node metastasis.
Results: Binary logistic regression analysis showed that carcinoembryonic antigen and CYFRA 21-1 were independent risk factors for mediastinal lymph node metastasis in patients with lung cancer (p < 0.001 and p = 0.002, respectively). The sensitivity and specificity of CEA for the diagnosis of mediastinal lymph node metastasis were 90.2 and 7.6%, respectively; CYFRA 21-1 were 0.6 and 99.0%, respectively.
Conclusion: Serum CEA and CYFRA 21-1 have predictive value in the diagnosis of mediastinal lymph node metastasis in patients with lung cancer.
Introduction
The morbidity and mortality of lung cancer remain high, and the prognosis of lung cancer patients has not been effectively improved. The preferred treatment for lung cancer is surgery. Postoperative radiotherapy, chemotherapy, biological immunotherapy, and the advent of targeted drugs have all led to increases in the survival time of patients. Lymph node metastasis is one of the main metastatic pathways of lung cancer and is an important determinant of lung cancer stage. Lung cancer has no specific symptoms in the early stages. The cancer cells pass through the bronchus and the lymphatic vessels around the pulmonary blood vessels. They first invade the adjacent lung or lymph nodes around the bronchi and then reach the hilar or subcarinal lymph nodes. They may then spread to the mediastinum and paratracheal lymph nodes, and further to the clavicular or cervical lymph nodes. Immune checkpoint inhibitors is a currently widely used tumor treatment method. Through the development of various related clinical trials, immune checkpoint inhibitors have made many breakthroughs in the efficacy, prognosis and disease prediction of lung cancer (Jain et al., 2018; Bai et al., 2020).
Tumor marker detection has become one a routine detection method, but there is still no ideal marker for clinical use as an indicator of lung cancer metastasis and prognosis. Single marker detection systems often suffer from low specificity (Weinberger et al., 2002). The same tumor can contain a variety of tumor markers. Different tissue types of the same tumor can express the same tumor markers or different tumor markers, so combined detection of multiple tumor markers can improve the diagnostic sensitivity of cancer.
A number of studies have identified distinct factors that are associated with lymph node metastasis in lung cancer. Specific substances detected in the lymph nodes or blood can predicts tumor. In this study, the medical records of lung cancer patients from the First Affiliated Hospital of Nanchang University were collected. Based on serological examination of a large number of lung cancer patients, we screened patients with lung cancer mediastinal lymph node metastasis and analyzed their tumor marker content to identify risk factors. We aimed to establish a standard by which to distinguish between mediastinal lymph node metastasis and non-mediastinal lymph node metastasis and to facilitate the development of further targeted anticancer treatment strategies for lung cancer patients.
Materials and methods
Ethics statement and study design
All patients in this study volunteered to participate, and the study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University. In this study, patients diagnosed with lung cancer between September 1999 and July 2020 were selected. Patients with mediastinal lymph node metastasis were screened, and their medical records and serological data were compared with those of patients without mediastinal lymph node metastasis. A pathological section obtained by surgical resection or biopsy was used to accurately diagnose the lung cancer of the patient. Computerized tomography (CT) and magnetic resonance imaging (MRI) were used to diagnose mediastinal lymph node metastasis in lung cancer, and data on serum tumor markers were recorded. Patients with primary mediastinal malignancies, benign mediastinal tumors, and secondary mediastinal cancer were excluded. The inclusion criteria for the without mediastinal lymph node metastasis group were patients without organ metastases.
Data collection
We collected various clinical data, including age, gender, time of diagnosis, lesion metastasis, and treatment, from medical records of patients with mediastinal lymph node metastasis and analyzed serum tumor markers, including alkaline phosphatase, serum calcium, HB, alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cytokeratin fragment 19 (CYFRA 21-1), CA-125, CA-153, CA-199, and free prostate-specific antigen (FPSA).
Statistical analyses
We analyzed the differences between tumor markers in the mediastinal lymph node metastasis group and the non-mediastinal lymph node metastasis group by an independent t-test. A binary logistic regression model was then applied to identify independent risk factors for mediastinal lymph node metastasis. A receiver operating curve (ROC) curve was generated, and the area under the curve (AUC) was calculated. Then, we used Microsoft Excel 2010 software (Microsoft corporation,United States) to calculate the cut-off value, sensitivity, and specificity of risk factors. All statistical analyses were performed using SPSS 20.0 (SPSS, IBM, United States) and Excel 2010 software. p values < 0.05 indicates statistical significance.
Results
Demographics and clinical characteristics
In this study, 330 cases of lung cancer with mediastinal lymph node metastasis and 2,712 cases of lung cancers without mediastinal lymph node metastasis were collected. The mean ages of lung cancer patients with and without mediastinal lymph node metastasis were 59.6 ± 10.5 and 60.3 ± 10.8 years, respectively. According to the chi-squared test and Student’s t-test, there were no significant differences in gender or age between the lung cancer groups with and without mediastinal lymph node metastasis (p > 0.05). In contrast, significant differences were noted for the different histopathological types between the two groups (p < 0.05). There was a statistically significant difference in the pathological type between the mediastinal lymph node metastasis group and lung cancers without mediastinal lymph node metastasis (p = 0.012). Furthermore, the incidence of adenocarcinoma was the highest among the different histopathological types. Most patients had been treated with chemotherapy since the onset of the disease. Detailed clinical data of all patients involved in the study are provided in Table 1 and Figures 1–3.
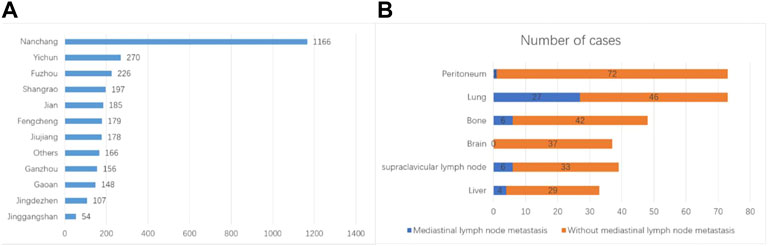
FIGURE 1. Clinical features of the patients. (A) Geographic location of the patients. (B) Anatomical locations of metastasis and corresponding incidences.
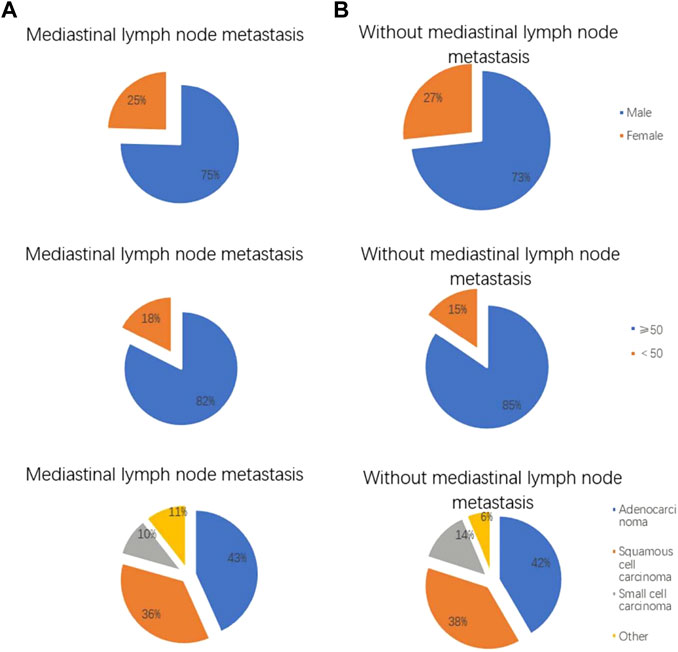
FIGURE 2. Clinical features of lung cancer patients with and without mediastinal lymph node metastasis. The (A) shows lung cancer patients with mediastinal lymph node metastasis, and the (B) shows lung cancer patients without mediastinal lymph node metastasis. Gender distribution of lung cancer patients with and without mediastinal lymph node metastasis. Age distribution of lung cancer patients with and without mediastinal lymph node metastasis. Pathological features of lung cancer patients with and without mediastinal lymph node metastasis.
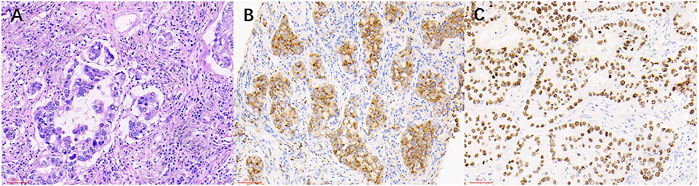
FIGURE 3. The HE staining and IHC images from lung cancer patients with mediastinal lymph node metastasis. (A) Lung cancer (HE × 200). (B) NapsinA (+) (SP × 200). (C) TTF-1 (+) (SP × 200).
Clinical data and risk factors of mediastinal lymph node metastasis in lung cancer
After comparing data on tumor biomarkers in lung cancer patients with and without mediastinal lymph node metastasis, we found that the concentrations of AFP, CEA, and CYFRA 21-1 were significantly higher in patients with mediastinal lymph node metastasis, while HB was higher in patients without mediastinal lymph node metastasis (p < 0.05) (Table 2). There were no significant differences in serum ALP, calcium, CA-125, CA-199, CA-153, TPSA, NSE, between the two groups (p > 0.05) (Table 2). Based on binary logistic regression, CEA and CYFRA 21-1 appear to be independent risk factors for mediastinal lymph node metastasis in lung cancer. More detailed results are shown in Table 3.
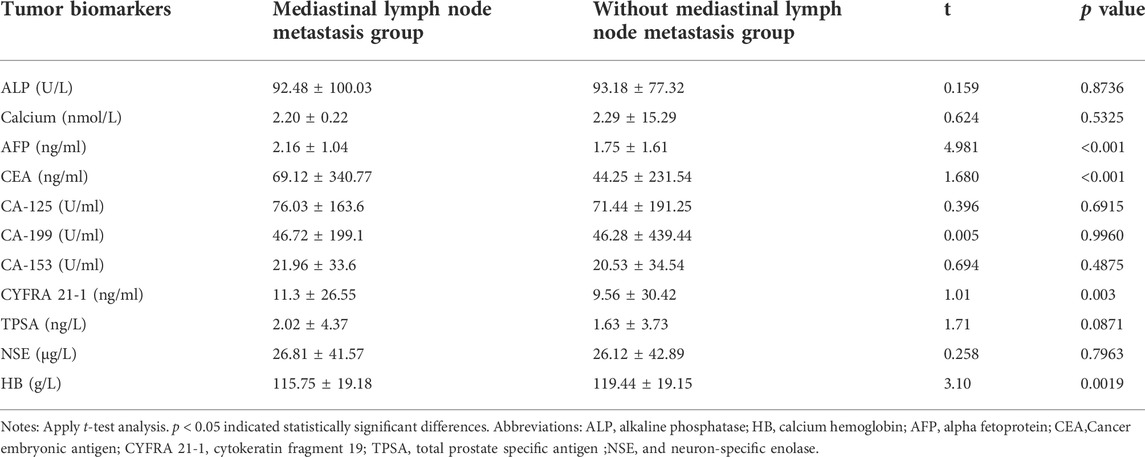
TABLE 2. Differences in tumor biomarkers between lung cancer patients with and without mediastinal lymph node metastasis.
Cut-off value, sensitivity, and specificity of CEA and CYFRA 21-1 for the diagnosis of mediastinal lymph node metastasis in lung cancer
Table 4 shows that the cut-off values for CEA and CYFRA 21-1 are 1.005 ng/ml and 135.31 ng/ml, respectively, and the area under the curve (AUC) of CYFRA 21-1 is the highest. The sensitivity and specificity for the diagnosis of mediastinal lymph node metastasis were CEA, 90.2 and 7.6%, respectively; CYFRA 21-1 was 0.6 and 99.0%, respectively. Figure 4A shows the receiver operating curve (ROC) curves for CEA and CYFRA 21-1, each as a single factor. We then tested the combination of these two risk factors in pairs, and Figure 4B shows the ROC curve for the CEA + CYFRA 21-1 combination. We found that the combination of CEA + CYFRA 21-1 has a higher AUC value of 0.585. The sensitivity and specificity of CEA + CYFRA 21-1 are shown in Table 4, and results were statistically significant (p < 0.05).
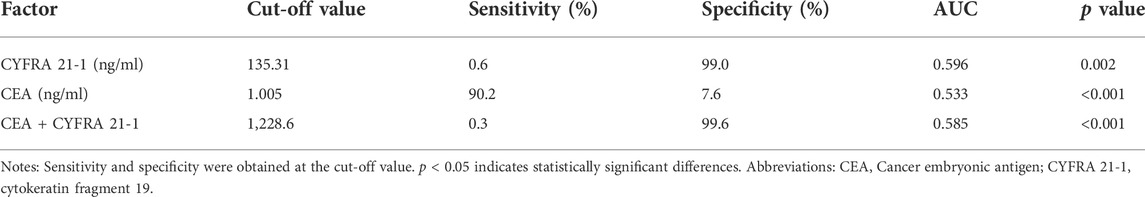
TABLE 4. Critical value, sensitivity, specificity and AUC of CEA and CYFRA 21-1 in lung cancer patients with mediastinal lymph node metastasis.
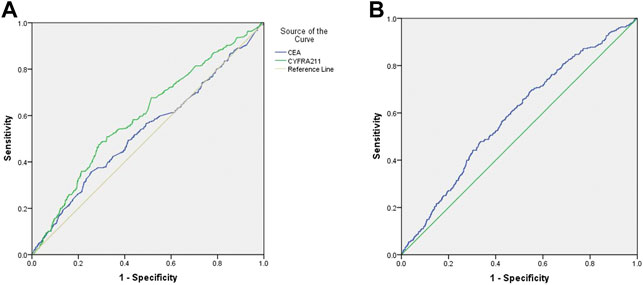
FIGURE 4. (A)ROC curve of CEA and CYFRA 21-1 levels in lung cancer patients with mediastinal lymph node metastasis. (B)ROC curve of CEA + CYFRA 21-1 in lung cancer patients with mediastinal lymph node metastasis.
Discussion
Studies have shown that lymphatic metastasis occurs in papillary thyroid cancer. (Wang et al., 2019; Wen et al., 2019). Another scholar found that (Shimazu et al., 2019) used a one-step nucleic acid amplification test to detect lymph node metastasis of breast cancer, and in our study, serological tests were also found to predict lymph node metastasis of lung cancer. Studies have shown that (Han et al., 2019) lung cancer is prone to various types of metastasis, such as non-small cell lung cancer will occur bone metastasis.
Marchi et al. (Marchi et al., 2008). Used proteomics technology to screen the serum markers of lung cancer brain metastasis and found that patients with lung cancer brain metastasis had higher levels of ProApolipoprotein A1 and S100beta than those with lung cancer and cerebral ischemia. Roberts et al. (Roberts et al., 2002) first discovered the relationship between abnormal expression of retinol binding protein (RBP) and malignant metastasis of ovarian cancer epithelial cells, and speculated that due to the down-regulation and loss of RBP expression, it destroyed the metabolism of retinol and the production of retinoic acid. Promotes gene damage, leading to malignant transformation of ovarian epidermal cells.
At present, most clinicians’ method of clinical staging of lung cancer is a multidisciplinary diagnosis method based on chest CT scan (Liam et al., 2015), but chest CT is not specific for the diagnosis of intrapulmonary lymph node metastasis of lung cancer, and accuracy is not accurate. Cannot be used as a basis for surgical clearance (Zhang et al., 2019). Xu et al. (Xu et al., 2003) reported that the sensitivity, specificity and accuracy of PET in the diagnosis of mediastinal lymph node metastasis were 100, 93 and 94%, respectively, and the number and location of positive lymph nodes were completely consistent with pathological results.
The relationship between tumor size and invasion and lymph node metastasis is currently reported. Li Yu et al. (Li et al., 2000) summarized 386 cases of pathological data and thought that with the increase of tumor and increased invasion, the chance of lymph node metastasis increased significantly. Min Kong et al. (Kong et al., 2017) analyzed 1,156 patients, and the test results showed that there is some correlation between lymph node metastasis and the size of the primary tumor.
The intrathoracic lymphatic drainage route of lung cancer is usually performed according to a certain rule, that is, from the near to the far side, from the top to the bottom, from the lung to the mediastinum to the mediastinum, (Ndiaye et al., 2016), regardless of the location and severity, most cases are one station. In some cases, the transfer order of each station can change, or even jump. This situation has been reported in the literature at home and abroad. (Yoshino et al., 1996; Celikoglu et al., 2010).
According to the original location of the tumor, Watanabe et al. (Watanabe et al., 1990) divided the mediastinal lymph nodes into upper and lower parts according to the tracheal bifurcation. The incidence of lung cancer in the lower lobe was 22%, and the incidence of lung cancer in the upper lobe was 8%. Advocate extensive mediastinal lymph node dissection. However, extensive lymph node dissection can directly affect the patient’s survival. Funatsu et al. (Funatsu et al., 1994) found that the difference in survival rate may be related to the low immunity caused by mediastinal lymph node dissection. Additionally, the higher the Topography; Lymph Node; Metastasis (TNM)stage, the higher the serum CEA level. Some studies have found that the preoperative CEA level is related to non-small cell carcinoma survival. There have reported important factors related to independence (Okada et al., 2003; Yu et al., 2014).
CYFRA 21-1 is currently considered to be the main tumor marker used for the diagnosis of lung cancer. It is primarily distributed throughout the cytoplasm of the stratified tumor epithelium. When a cell dies, CYFRA 21-1 is released into the blood as a lysed fragment, resulting in an increase in serum levels. Pujol et al. reported that CYFRA 21-1 is an independent prognostic factor in lung cancer (Pujol et al., 2004).
In our study, we collected serum and assessed ALP, calcium, HB, AFP, CEA, CA-125, CA-199, CA-153, CYFRA 21-1, TPSA, and NSE levels. Relative to lung cancer patients without mediastinal lymph node metastasis, the concentrations of AFP, CEA, and CYFRA 21-1 in lung cancer patients with mediastinal lymph node metastasis were found to be extremely high, while HB was found to be lower (p < 0.05). Based on previous studies, we chose CEA and CYFRA 21-1 as independent risk factors for lung cancer patients with mediastinal lymph node metastasis (p < 0.01 and p = 0.002, respectively). Furthermore, we assessed the cut-off, sensitivity, specificity, and AUC of CEA and CYFRA 21-1 levels. Finally, we conclude that CEA, and CYFRA 21-1 are risk factors for mediastinal lymph node metastasis in lung cancer.
By using the final ROC curve of these serum biomarkers to provide reliable clinical indicators, we can conclude that CEA and CYFRA 21-1 have cut-off values of 1.005 ng/ml and 135.31 ng/ml, respectively, in lung cancer patients with mediastinal lymph node metastasis. CYFRA 21-1 had the highest AUC, demonstrating that it had the highest accuracy in distinguishing between lung cancer patients with and without mediastinal lymph node metastasis. On this basis, we utilized further detailed diagnostic techniques to diagnose or rule out mediastinal lymph node metastasis without providing a basis for follow-up treatment. Unlike previous studies, this study showed that the combination of CEA + CYFRA 21-1 had a higher AUC value of 0.585. Therefore, we believe that the combination of CEA + CYFRA 21-1 can also be used as a predictor of mediastinal lymph node metastasis in lung cancer (the higher the level of CEA + CYFRA 21-1, the greater the likelihood of mediastinal lymph node metastasis in lung cancer patients). We also summarized the risk factors for lung cancer metastasis in previous studies (Table 5).
In summary, the high expression of serum CEA and CYFRA 21-1 may be related to the occurrence of mediastinal lymph node metastasis in lung cancer patients. At the same time, assessment of the combination of CEA + CYFRA 21-1 can aid in the diagnosis of mediastinal lymph node metastasis in lung cancer patients. The positive expression of serum CEA + and CYFRA 21-1 is associated with the prognosis of patients with mediastinal lymph node metastasis.
However, there are some limitations, because of the large individual differences in some patients, and the small number of samples in this study, the statistical significance is not very significant, and the difference between the minimum and maximum values of CEA in patients is large, which leads to the standard deviation is too high, also The error and statistical difference that would cause the experiment are not significant, and the minimum and maximum values with obvious individual differences can be removed while ensuring the number of samples, thereby reducing the error.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The study methods and protocols were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (Nanchang, China) and followed the principles of the Declaration of Helsinki. All subjects were notified of the objectives and content of the study and latent risks, and then provided written informed consent to participate.
Author contributions
JT and TS conceived and designed the present study. MK, H-YS, and TS were responsible for acquiring the data, designing the figures and tables and drafting the manuscript. X-LL and PY contributed to the acquisition of the data and interpreting the results. JZ, QL, and Y-XW assisted in the acquisition of the data and drafting the manuscript. TS, HW, and YS assisted in the acquisition and analysis of the data with constructive discussion.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AFP, alpha fetoprotein; ALP, alkaline phosphatase; AUC, area under the curve; CEA, Cancer embryonic antigen; CT,Computerized tomography; CYFRA 21-1, cytokeratin fragment 19; HB, calcium hemoglobin; MRI, magnetic resonance imaging; NSE, and neuron-specific enolase; ROC, receiver operating curve; TPSA, total prostate specific antigen.
References
Bai, R., Li, L. Y., Chen, X., Chen, N., Song, W., and Cui, J. (2020). Neoadjuvant and adjuvant immunotherapy: Opening new horizons for patients with early-stage non-small cell lung cancer. Front. Oncol. 9, 575472. doi:10.3389/fonc.2020.575472
Cabreraalarcon, J. L., Carrillovico, A., Santotoribio, J. D., CArrillo-Vico, A., Leon-Justel, A., GonzAlez-CAstro, A., et al. (2011). CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin. Lab. 57 (11-12), 1011–1014.
Cedrés, S., Nuñez, I., Longo, M., Martinez, P., Checa, E., Torrejon, D., et al. (2011). Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin. Lung Cancer 12 (3), 172–179. doi:10.1016/j.cllc.2011.03.019
Celikoglu, F., Celikoglu, S. I., and Goldberg, E. P. (2010). Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J. Pharm. Pharmacol. 62 (3), 287–295. doi:10.1211/jpp.62.03.0001
Chen, F., Yan, C. E., Li, J., Han, X. H., Wang, H., and Qi, J. (2015). Diagnostic value of CYFRA 21-1 and CEA for predicting lymph node metastasis in operable lung cancer. Int. J. Clin. Exp. Med. 8 (6), 9820–9824.
Chen, Y., Peng, W., Huang, Y., Chen, J., Su, G., Jiang, C., et al. (2015). Significance of serum neuron-specificenolase before treatment in predicting brain metastases and prognosis of advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 37 (7), 508–511.
Chu, Y., Lai, Y-H., Lee, M-C., Yeh, Y. J., Wu, Y. K., Tsao, W., et al. (2017). Calsyntenin-1, clusterin and neutrophil gelatinase-associated lipocalin are candidate serological biomarkers for lung adenocarcinoma. Oncotarget 8 (64), 107964–107976. doi:10.18632/oncotarget.22438
Funatsu, T., Matsubara, Y., Ikeda, S., Hatakenaka, R., Hanawa, T., and IsHida, H. (1994). Preoperative mediastinoscopic assessment of N factors and the need for mediastinal lymph node dissection in T1 lung cancer. J. Thorac. Cardiovasc. Surg. 108 (2), ∶321–328. doi:10.1016/s0022-5223(94)70014-1
Han, S., Wang, T., Chen, Y. M., Han, Z., Guo, L., Wu, Z., et al. (2019). High CCL7 expression is associated with migration, invasion and bone metastasis of non-small cell lung cancer cells. Am. J. Transl. Res. 11 (1), 442–452.
Jain, P., Jain, C., and Velcheti, V. (2018). Role of immune-checkpoint inhibitors in lung cancer. Ther. Adv. Respir. Dis. 12, 1753465817750075. doi:10.1177/1753465817750075
Kong, M., Jiang, J., Cai, X., Shen, J., Ma, D., Ye, M., et al. (2017). Characteristics of lymph node metastasis in resected adenosquamous lung cancer. Med. Baltim. 96 (48), e8870. doi:10.1097/MD.0000000000008870
Lee, D. S., Kim, Y. S., Jung, S. L., Lee, K. Y., Kang, J. H., Park, S., et al. (2012). The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol. 33 (4), 1065–1073. doi:10.1007/s13277-012-0344-0
Li, Y., Li, H. W., and Tong, Y. J. (2000). Clinical study of lymph node metastasis in lung cancer. Chin. J. Thorac. Cardiovasc Surg. 16 (1), 1012.
Liam, C-K., Andarini, S., Lee, P., Ho, J. C. M., Chau, N. Q., and Tscheikuna, J. (2015). Lung cancer staging now and in the future. Respirology 20 (4), 526–534. doi:10.1111/resp.12489
Marchi, N., Peter, M., Fazio, V., Mekhail, T., Masaryk, T., and Janigro, D. (2008). ProApolipoprotein A1: A serum marker of brain metastases in lung cancer patients. Cancer 112 (6), 1313–1324. doi:10.1002/cncr.23314
Morita, S., Suda, T., Oda, C., Kobayashi, M., Hoshi, T., Kanefuji, T., et al. (2019). The value of 18F-fdg PET in the diagnosis of intertrabecular vertebral metastasis in a small cell lung cancer patient with a high serum CEA level. Intern. Med. 58 (3), 415–418. doi:10.2169/internalmedicine.1394-18
Ndiaye, A., Di-Marino, V., Ba, P. S., Gaye., M., and Nazarian, S. (2016). Anatomical variations in lymphatic drainage of the right lung: Applications in lung cancer surgery. Surg. Radiol. Anat. 38 (10), 1143–1151. doi:10.1007/s00276-016-1685-y
Nikliúski, J., Furman, M., Laudanski, J., and Kozhowski, M. (1992). Evaluation of squamous cell carcinoma antigen (SCC-Ag) in the diagnosis and follow-up of patients with non-small cell lung carcinoma. Neoplasma 39 (5), 279–282.
Okada, M., Sakamoto, T., Nishio, W., Uchino, K., and Tsubota, N. (2003). Characteristics and prognosis of patients after resection of non-small cell lung carcinoma measuring 2 cm or less in greatest dimension. Cancer 98 (3), 535–541. doi:10.1002/cncr.11530
Oshiro, Y., Takada, Y., Enomoto, T., Fukao, K., Ishikawa, S., and Iijima, T. (2004). A resected case of metachronous liver metastasis from lung cancer producing alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II). Hepatogastroenterology. 51 (58), 1144–1147.
Pollan, M., Varela, G., Torres, A., de la Torre, M., Ludena, M. D., Ortega, M. D., et al. (2003). Clinical value of p53, c-erbB-2, CEA and CA125 regarding relapse, metastasis and death in resectable non-small cell lung cancer. Int. J. Cancer 107 (5), 781–790. doi:10.1002/ijc.11472
Pujol, J. L., Molinier, O., Ebert, W., Daurès, J. P., Barlesi, F., Buccheri, G., et al. (2004). CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: Results of a meta-analysis in 2063 patients. Br. J. Cancer 90 (11), 2097–2105. doi:10.1038/sj.bjc.6601851
Roberts, D., Williams, S. J., Cvetkovic, D., Weinstein, J. K., Godwin, A. K., Johnson, S. W., et al. (2002). Decreased expression of retinol-binding proteins is associated with malignant transformation of the ovarian surface epithelium. DNA Cell Biol. 21 (1), 11–19. doi:10.1089/10445490252810276
Shimazu, K., Tanei, T., Tamaki, Y., Saeki, T., Osaki, A., Hasebe, T., et al. (2019). Performance of a new system using a one-step nucleic acid amplification assay for detecting lymph node metastases in breast cancer. Med. Oncol. 36, 54–58. doi:10.1007/s12032-019-1277-x
Wang, Y. J., Guan, Q., and Xiang, J. (2019). Nomogram for predicting level V lymph node metastases in papillary thyroid carcinoma with clinically lateral lymph node metastases: A large retrospective cohort study of 1037 patients from fduscc. J. Cancer 10, 772–778. doi:10.7150/jca.28527
Watanabe, Y., Shimizu, J., Tsubota, M., and Iwa, T. (1990). Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest 97 (5), 1059–1065. doi:10.1378/chest.97.5.1059
Weinberger, S. R., Dalmasson, E. A., and Fung, E. T. (2002). Current achievements using protein chip array technology. Curr. Opin. Chem. Biol. 6, 86–91. doi:10.1016/s1367-5931(01)00282-4
Wen, X. Z., Wang, B., Jin, Q. M., Zhang, W., and Qiu, M. (2019). Thyroid antibody status is associated with central lymph node metastases in papillary thyroid carcinoma patients with hashimoto’s thyroiditis. Ann. Surg. Oncol. 26, 1751–1758. doi:10.1245/s10434-019-07256-4
Wu, L., Hu, B., Zhao, B., Liu, Y., Yang, Y., Zhang, L., et al. (2017). Circulating microRNA-422a is associated with lymphatic metastasis in lung cancer. Oncotarget 8 (26), 42173–42188. doi:10.18632/oncotarget.15025
Xu, B. X., Liu, Y. L., Yao, S. L., Shao, M., Chen, Y., Wang, K., et al. (2003). Value of FDG PET for mediastinal lymph node staging in non-small cell lung cancer. Chin. J. Lung Cancer 6 (3), 198–200. doi:10.3779/j.issn.1009-3419.2003.03.09
Yoshino, I., Yokoyama, H., Yano, T., Ueda, T., Takai, E., Mizutani, K., et al. (1996). Skip metastasis to the mediastinal lymph nodes in non-small cell lung cancer. Ann. Thorac. Surg. 62 (4), 1021–1025. doi:10.1016/0003-4975(96)00470-5
Yu, Z., Chen, X. Z., Cui, L. H., Si, H. Z., Lu, H. J., and Liu, S. H. (2014). Prediction of lung cancer based on serum biomarkers by gene expression programming methods. Asian pac. J. Cancer Prev. 15 (21), 9367–9373. doi:10.7314/apjcp.2014.15.21.9367
Zhang, D. G., Chen, X. C., Zhu, D., Qin, C., Dong, J., Qiu, X., et al. (2019). Intrapulmonary lymph node metastasis is common in clinically staged IA adenocarcinoma of the lung. Thorac. Cancer 10 (2), 123–127. doi:10.1111/1759-7714.12908
Zhang, L., Liu, D., Li, L., Pu, D., Zhou, P., Jing, Y., et al. (2017). The important role of circulatingCYFRA21-1 in metastasis diagnosis and prognostic value compared with carcinoembryonic antigen and neuron-specific enolase in lung cancer patients. BMC Cancer 17, 96. doi:10.1186/s12885-017-3070-6
Keywords: lung cancer, mediastinal lymph node metastasis potential indicators, tumor blood markers, cancer, risk factors
Citation: Tang J, Shu H-Y, Sun T, Zhang L-J, Kang M, Ying P, Ling Q, Zou J, Liao X-L, Wang Y-X, Wei H and Shao Y (2022) Potential factors of cytokeratin fragment 21-1 and cancer embryonic antigen for mediastinal lymph node metastasis in lung cancer. Front. Genet. 13:1009141. doi: 10.3389/fgene.2022.1009141
Received: 01 August 2022; Accepted: 24 August 2022;
Published: 13 September 2022.
Edited by:
Qian Wang, Tai’an City Central Hospital, ChinaReviewed by:
Zhenhao Zhang, Nanjing Medical University, ChinaHe Wang, Xuzhou Medical University, China
Copyright © 2022 Tang, Shu, Sun, Zhang, Kang, Ying, Ling, Zou, Liao, Wang, Wei and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Shao, ZnJlZWJlZTk5QDE2My5jb20=
†These authors have contributed equally to this work
 Jing Tang1†
Jing Tang1† Hui-Ye Shu
Hui-Ye Shu Tie Sun
Tie Sun Li-Juan Zhang
Li-Juan Zhang Min Kang
Min Kang Ping Ying
Ping Ying Jie Zou
Jie Zou Xu-Lin Liao
Xu-Lin Liao Yi-Xin Wang
Yi-Xin Wang Hong Wei
Hong Wei Yi Shao
Yi Shao