
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 18 October 2022
Sec. Cancer Genetics and Oncogenomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.1001369
Background: Thalassemia is a common inherited hematological disease with genetic disorders characterized by imbalanced synthesis of the globin chains. Due to the improvement of treatment methods, patients with thalassemia can survive for a long time. Therefore, it is not uncommon for patients with thalassemia suffering from malignant tumors. However, there are quite few reports on thalassemia patients complicated with breast cancer. Herein, we try to investigate the prevalence and genetic disorders spectrum of thalassemia in patients with breast cancer.
Methods: Blood routing tests and serum ferritin analysis were conducted in 1887 breast cancer patients treated in the department of radiation oncology during 1 April 2020 and 30 March 2022. The suspected thalassemia carriers with small mean corpuscular volume (MCV), mean corpuscular hemoglobin content (MCH) or mean corpuscular hemoglobin concentration (MCHC) but the concentration of serum ferritin within normal limits were further investigated by polymerase chain reaction (PCR) and flow through hybridization gene chip to detect common mutations of α-globin and β-globin genes using Thalassemia Geno Array Diagnostic Kit. The prevalence and genetic mutation spectrum of thalassemia among breast cancer patients were analyzed.
Results: Four hundred and eighty-nine suspected thalassemia carriers were detected by complete blood cell counts and serum ferritin analysis among 1887 breast cancer patients. One hundred and seven cases (5.7%) were identified as carriers of thalassemia, of which 55 cases (51.4%) were α-thalassemia, 50 cases (46.7%) were β-thalassemia, and 2 cases (1.9%) were co-inheritance of α-thalassemia and β-thalassemia simultaneously. In α-thalassemia, the most prevalent genotype is -SEA/αα; as for β-thalassemia, βIVS−II−654/β is the most common genotype. The degree of anemia is more severe in β-thalassemia than in α-thalassemia.
Conclusion: This is the first comprehensive molecular epidemiological investigation on thalassemia among breast cancer patients. Our data indicated that thalassemia was not uncommon in breast cancer patients. The physicians should have the knowledge to avoid misdiagnosis as iron deficiency anemia.
Thalassemia is one of the most frequently diagnosed inherited monogenic autosomal recessive disease caused by mutations in α-globin or β-globin gene clusters, leading to inadequate or complete loss of the corresponding globin chain synthesis and the imbalanced ratio between α-chain and β-chain in hemoglobin, which was characterized by microcytic hypochromic anemia and showed a wide spectrum of clinical severity from severe anemia to minor or none anemia (Chui et al., 2006; Shafique et al., 2021). Thalassemia can be classified into 2 major types, α-thalassemia and β-thalassemia. Of which, α-thalassemia mainly occurs at the end of the short arm of chromosome 16 α-globin gene cluster, containing α-globin gene deletion or point mutations; β-thalassemia mainly occurs in the short arm of chromosome 11 caused by point mutation within the gene (Muncie and Campbell, 2009; Brancaleoni et al., 2016). The clinical classification of thalassemia includes thalassemia minor, thalassemia intermediate (TI) and thalassemia major (TM) based on their severity of anemia (Viprakasit and Ekwattanakit, 2018). No effective treatment is available for thalassemia patients until now. Severe thalassemia patients need lifelong term blood transfusions to maintain lives, while mild or minor thalassemia has no obvious symptoms, which is usually found during pregnancy examination or annually physical examination. Thalassemia is geographically selective and widely distributed in the Mediterranean countries, Indian subcontinent, the Middle East, North Africa, Southeast Asia and other tropical and subtropical regions, including south China (Viprakasit and Ekwattanakit, 2018). The prevalence of thalassemia also varied significantly in different regions of China. Previous reports suggest a higher frequency of thalassemia in the population of southern China, particularly in the provinces of Guangdong, Guangxi, Yunnan, Fujian and so on (Zhuang et al., 2021). As patients with thalassemia under proper treatment live longer than before, it is not uncommon for thalassemia patients to develop cancer. Some researchers even believe that patients with thalassemia are more likely to develop cancer because of high levels of oxygen free radicals and iron load. However, anemia and other clinical manifestations are often misdiagnosed as iron deficiency anemia (IDA) or cancer related symptoms, which may ignore the diagnosis of thalassemia (Ansari et al., 2013; Hodroj et al., 2019). It is well-known that screening and genetic counseling during early stage pregnant are the most effective ways to prevent newborn with thalassemia, especially severe thalassemia (Jaing et al., 2021). Since people in different geographical regions having completely different mutation spectrum of globin genes, it is vital to fully understand the molecular epidemiological characteristics of incidence rate and distribution of thalassemia in order to formulate appropriate prevention strategies in areas with high incidence of thalassemia. There are quite few reports on the occurrence of malignancies among patients with thalassemia worldwide recently (Poggi et al., 2011; Haghpanah et al., 2020). Breast cancer is one of the most common diagnosed cancers and the primary causes of cancer-specific death among women. The year crude and age adjusted rate of breast cancer incidence were 45/100,000 and 30/100,000, respectively, which was ranking the first in the incidence of cancers in women in China (Lei et al., 2021). The incidence per year remained increasing both in urban areas and in rural areas. Currently, breast cancer patients are managed under multidisciplinary treatment with an approach comprising surgery, chemotherapy, radiation therapy and/or endocrine therapy. The Third Hospital of Nanchang is the largest breast cancer center in Jiangxi Province. The number of annually newly diagnosed breast cancer is over 2000 in our center, accounting nearly one-third of the new cases in Jiangxi province. Annually, about 1000 subjects received radiotherapy in the department of radiation oncology. Quite few studies have reported the occurrence of breast cancer among patients with thalassemia worldwide. After an extensive review of the literatures in PUBMED database, we found a case report on breast cancer in thalassemic patient (Picardo et al., 2015). However, neither the incidence nor the genetic mutation spectrum of thalassemia has been reported yet among breast cancer patients all over the world.
In the present study, we investigated the genetic mutation profiles of thalassemia among breast cancer patients treated in the department of radiation oncology by polymerase chain reaction (PCR) and flow through hybridization gene chip to detect common mutations of α-globin and β-globin genes using Thalassemia Geno Array Diagnostic Kit (Guangdong Hybribio Biotech Co., Ltd., Yaneng BioSciences, Zeesan Biotech). The purpose of the study is to provide valuable information for medical staff to prevent misdiagnosing thalassemia as IDA and making wrong treatment decision.
A total of 1,887 subjects were enrolled during 1 April 2020 and 30 March 2022, who received radiotherapy in the department of radiation oncology, the Third Hospital of Nanchang. The age of these subjects ranged from 20 to 91 years old, and the male-to-female ratio was 1: 628. The clinicopathological characterisics including age, menopausal status, histological type, grade, TNM stage, estrogen receptor (ER) status, progesterone receptor (PgR) status, HER2 (human epidermal growth factor receptor-2) status, Ki-67 index, molecular subtypes of the patients were listed in Table 1. Written informed consents were obtained from all of the subjects. This study was approved by the Institutional Medical and Ethics Committee of the Third Hospital of Nanchang.
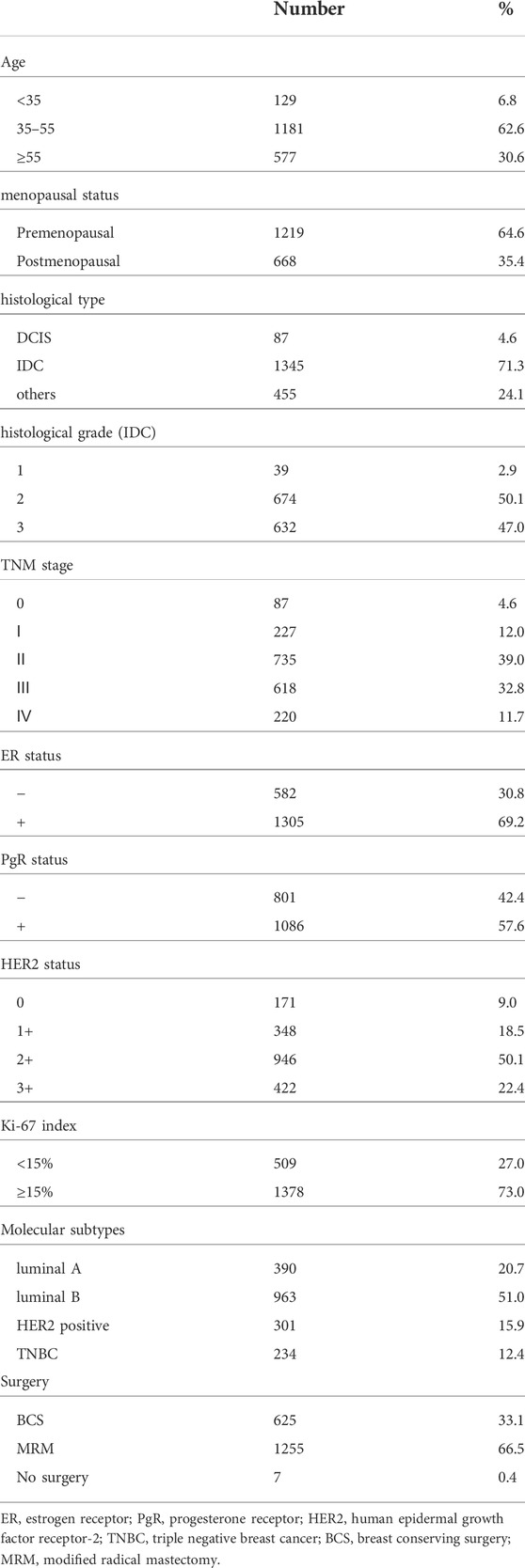
TABLE 1. The clinicopathological characteristics of the breast cancer patients enrolled in this study.
Approximately 3 ml of anticoagulated peripheral blood of each subject was collected into EDTA-containing vacutainers used for blood routing test. We performed complete blood cell counting on all of the subjects according to standard laboratory procedures using an automated hematology cell counter followed the standard operating procedures provided by Sysmex XN350 blood analyzer (Sysmex XN350; Sysmex Co., Ltd., Kobe, Japan). Patients with mean corpuscular volume (MCV), mean corpuscular hemoglobin content (MCH) and/or mean corpuscular hemoglobin concentration (MCHC) below lower limits of the normal threshold were further analyzed by serum ferritin test. The serum ferritin test was performed with immunochromatographic result interpretation recorder (NS7001, Tianjin, China). Patients with serum ferritin <20 ng/ml (lower limits of the normal threshold) were considered IDA and received iron supplementation. Patients with serum ferritin >20 ng/ml (lower limits of the normal threshold) and any of MCV <82 fl, MCH <27 pg and/or an MCHC <320 g/L regarded as suspected thalassemia carriers were subjected to further thalassemia genetic testing.
We collected a further 2 ml of peripheral blood from every patient with abnormal small corpuscular volume red blood cells but normal concentration of serum ferritin for the molecular analysis of common α-thalassemia and β-thalassemia. Automatic blood DNA extraction kits (Guangdong Hybribio Biotech Co., Ltd., Yaneng BioSciences, Zeesan Biotech) was used to extract the genomic DNA of the subjects according to the manufacturer’s protocol. The DNA concentration and purity were determined by UV spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States) at the wavelength of 260 nm.
Blood DNA was used to amplify α-thalassemia and β-thalassemia related genes with the PCR amplification Mix (Guangdong Hybribio Biotech Co., Ltd., Yaneng BioSciences, Zeesan Biotech), as previously described (Peng et al., 2021). Briefly, 5 μl DNA extraction was mixed with 45 μl reaction mixture system containing 100 pmol of α-globin or β-globin primers (Hybribio Biotechnology PCR Kit). Blood DNA was amplified in an Applied Biosystems Automated Thermal Cycler (Thermo Fisher Scientific Inc.), the amplification conditions were 95°C for 15 min, followed by denaturation for 50 s at 97°C, annealing for 60 s at 60°C, and extension at 72°C for 120 s for a total of 35 cycles. Amplification was followed by a 19 min terminal extension step at 72°C, then the products were hold on ice for further flow-through hybridization. Genotypes of thalassemia were done by flow-through hybridization and gene chip (Guangdong Hybribio Biotech Co., Ltd., Yaneng BioSciences, Zeesan Biotech). The chip could identify three common deletional α-thalassemia mutations (-SEA, -α3.7, -α4.2) and three common non-deletional α-thalassemia mutations (αCS, αQS, αWS) for α-thalassemia. The 17 common β-thalassemia mutations detected were as follows: CD41-42, IVS-II-654, –28, CD17, CD71-72, βE, –29, CD43, CD31, –32, CD14-15, CD27-28, IVS I-1, IVS I-5, Int, Cap, –30. The kit of the gene chip was approved by Chinese State Food and Drug Administration. All experimental operations were performed strictly following the manufacturers’ protocols (ADICON Clinical laboratories, Inc., Nanchang, China). The final results were detected and read by colorimetric change on the chip under direct visualization (Figure 1).
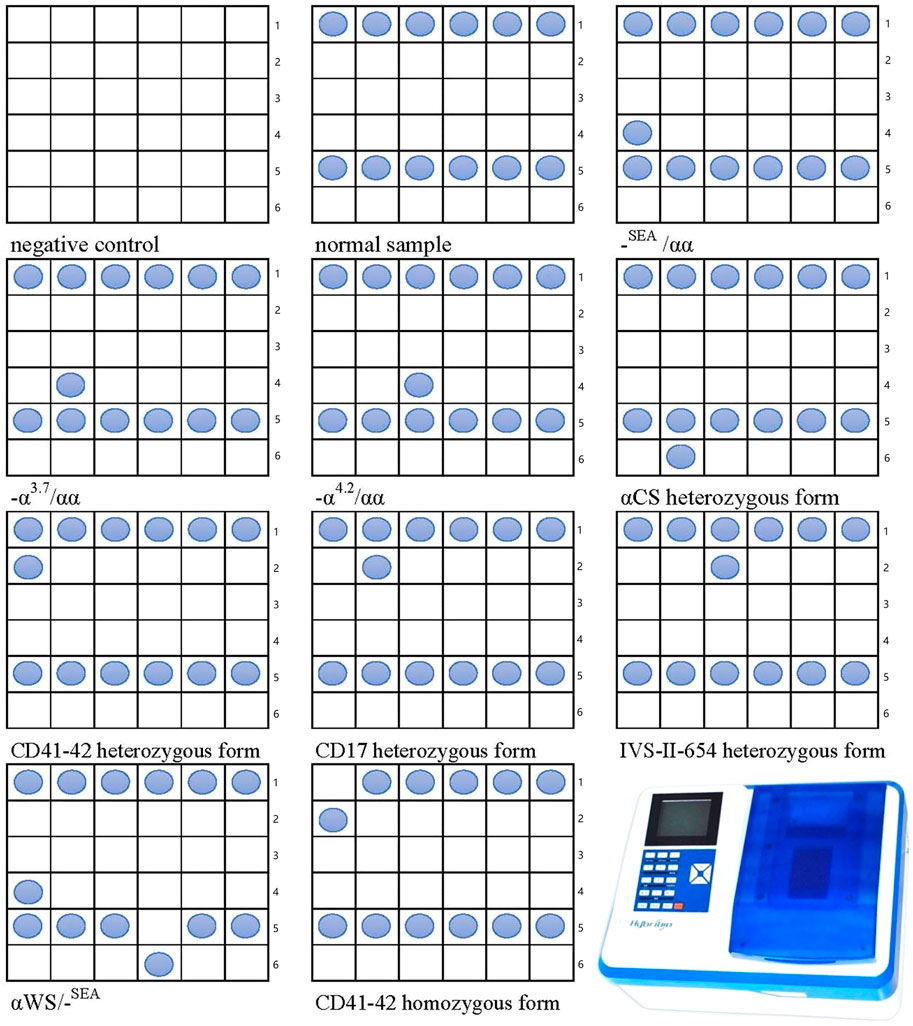
FIGURE 1. The Nucleotide template in each cell and the establishment of the genotyping of thalassemia using flow-through hybridization and gene chip. Line 1:41–42N, 17N, 654N, 71–72N, −28N, βEN; line 2: 41–42M, 17M, 654M, 71–72M, -28M, βEM; line 3: 43M, 14–15M, −30M, −32M, −29M, CapM; line 4: SEA, α3.7, α4.2, 31M, IVSⅠ-5M, IntM; line 5: NP, CSN, QSN, WSN, IVSⅠ-1N, 27–28N; line 6: blank, CSM, QSM, WSM, IVSⅠ-1M. N, normal; M, mutation.
The statistical analysis was conducted using the SPSS version 22. Results were expressed as mean ± SD values, and differences among groups were compared for each hematological index using independent sample Student’s t test or chi-square test to compare the detection rates between the groups. A p-value < 0.05 was considered statistically significant.
Five hundred and sixty-three cases with microcytic hypochromia were detected among 1887 breast cancer patients. The definition of microcytic hypochromia is mean corpuscular volume (MCV) values below 80 fl, mean corpuscular hemoglobin (MCH) values below 27 pg, according to the lower limits of the normal threshold in our central lab. Seventy-four (13.1%) of the patients with microcytic hypochromia would be due to iron deficiency as serum ferritin level below 20 ng/ml, the lower limits of the normal threshold in our central lab. The rest 489 suspected thalassemia carriers underwent further thalassemia genetic testing.
One hundred and seven patients were identified as thalassemia carriers, of which, 55 cases were α-thalassemia, 50 cases were β-thalassemia, and 2 cases were co-inheritance of α-thalassemia and β-thalassemia simultaneously. The overall prevalence rate of thalassemia was 5.7% among breast cancer patients in our breast cancer institute. The top six common genotypes were -SEA/αα, βIVS−II−654/β, βCodons 41/42/β, -α3.7/αα, β−28/β and βCodon17/β with frequencies of 43.0%, 22.4%, 13.1%, 4.7%, 2.8%, and 2.8%, respectively (Figure 2). The prevalence rate of α-thalassemia was slightly higher than that of β-thalassemia, and the ratio was 51.4% vs. 46.7%. What’s more, we found two carriers with both α-thalassemia and β-thalassemia, and the rate was 1.9%. Consistent with previous reports, the most prevalent genotype is -SEA/αα in α-thalassemia, accounting for 83.6% of all α-thalassemia genotypes (Figure 3) (Lai et al., 2017; He et al., 2021; Peng et al., 2021; Zhuang et al., 2021). As for β-thalassemia, βIVS−II−654/β is the most common genotype, accounting for 48% of all β-thalassemia genotypes (Figure 4).
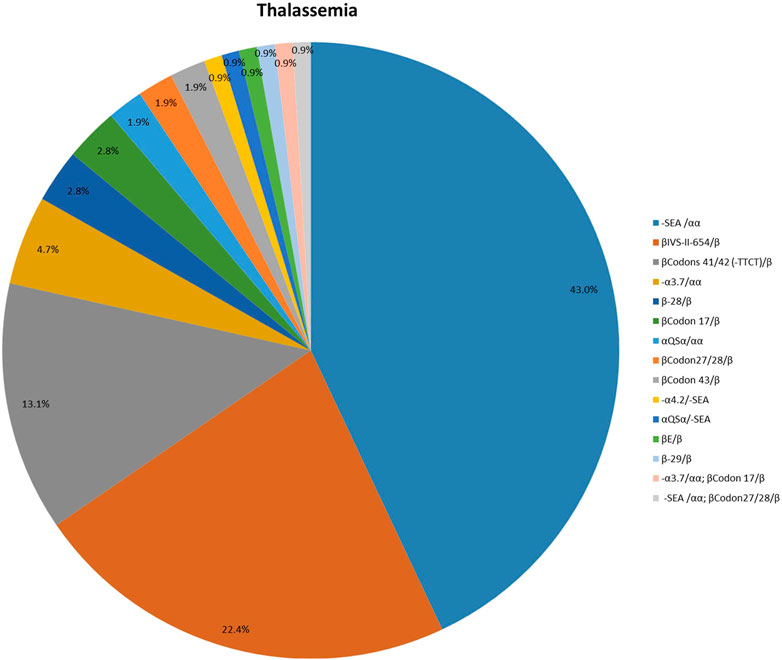
FIGURE 2. The distribution of molecular genetics spectrum of thalassemia among breast cancer patients in Jiangxi province. The -SEA/αα, βIVS−II−654/β, βCodons 41/42/β, -α3.7/αα, β−28/β and βCodon17/β were the top six common genotypes with frequencies of 43.0%, 22.4%, 13.1%, 4.7%, 2.8%, and 2.8%, respectively.
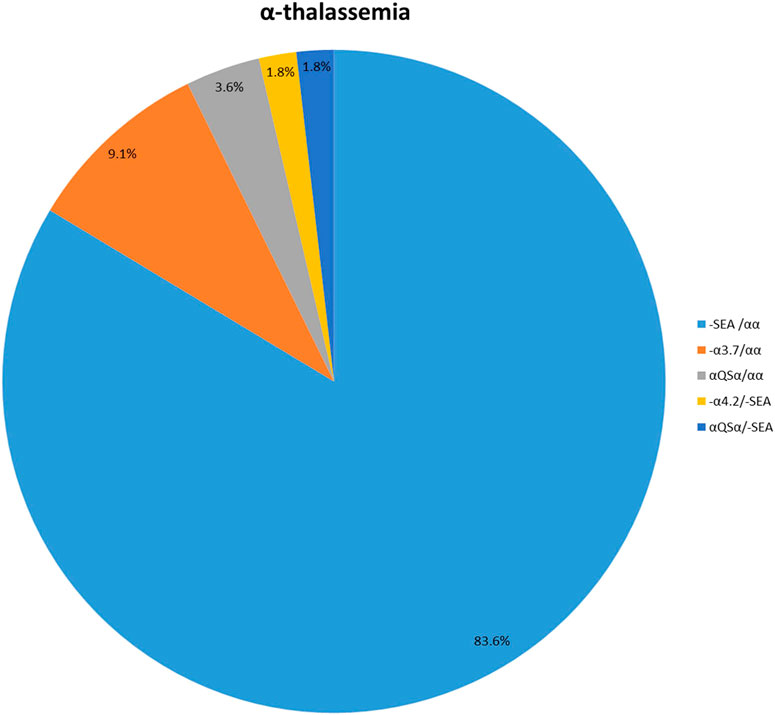
FIGURE 3. The prevalence of molecular genetics spectrum among α-thalassemia breast cancer patients in Jiangxi province. The most prevalent genotype of α-thalassemia was -SEA/αα, accounting for 83.6% of all α-thalassemia genotypes.
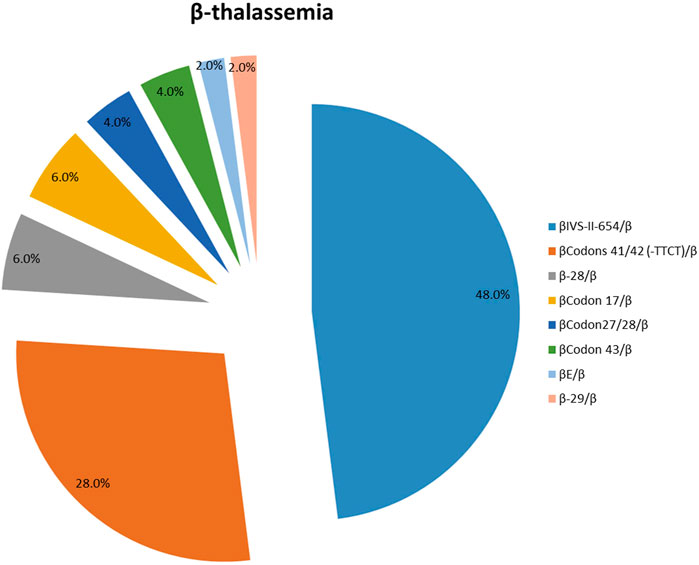
FIGURE 4. The prevalence of molecular genetics spectrum among β-thalassemia breast cancer patients in Jiangxi province. βIVS−II−654/β was the most common genotype, accounting for 48% of all β-thalassemia genotypes.
We further explored the prevalence and mutation spectrum of thalassemia among different molecular subtypes of breast cancer. However, we found no significant difference in the prevalence and mutation spectrum of thalassemia among luminal A, luminal B, HER2 positive and triple negative breast cancer.
There are quite few reports available on the hematological parameters characterization of patients with different genotypes of thalassemia. To further analyze the hematological phenotype among different genotypes of thalassemia, we performed a comparison of the hematological parameters. As Table 2 illustrating, there were significant differences of hematological parameters including Hb, MCV and MCH between α-thalassemia and β-thalassemia, while no difference in RBC count and MCHC. The percentage of clinically silent thalassemia (Hb > 120 g/L) in α-thalassemia and β-thalassemia was 21.6% and 8%, respectively. While the rate of patients with moderate degree of anemia in α-thalassemia and β-thalassemia was 2% and 12% respectively. What’s more, we detected one patient with severe anemia in β-thalassemia. No patients with extremely severe anemia was found in this population studied (Table 3).
Thalassemia is an inherited autosomal recessive disorder with the traits of microcytic hypochromic anemia. However, the clinical manifestation varied significantly, from almost normal blood test to a lethal hemolytic anemia. It is reported that the incidence of thalassemia was very high in southern China population. Lin reported that a higher frequency (9.49%) of thalassemia in Ganzhou (the south of Jiangxi province), whereas the frequency was 3.90% in Xinyu (the middle of Jiangxi province) and 2.63% in Nanchang (the north of Jiangxi province, also the capital of the province) (Lin et al., 2014).
Over the past three decades, there were several reports on the malignancies complicated with thalassemia. Some scholars even believed that patients with thalassemia would be more likely to suffer from cancer. E. Picardo et al. first reported a unique patient of breast cancer with thalassemia intermediate receiving regular red blood cell transfusions since she was two years old and began desferrioxamine after 10 transfusions (Picardo et al., 2015). The occurrence of breast cancer in patients with thalassemia may not be a pure coincidence. Some recently researches showed that there are specific mammogram patterns among thalassemic patients, nearly one-third of the patients had high density breast in mammograms, which indicated the risk of breast cancer among thalassemic patients (Rund and Rachmilewitz, 2005).
As the prevalence of thalassemia is relatively high in our province, the patients with microcytic hypochromia in complete blood cell counts receiving radiotherapy for breast cancer are not uncommon. Consequently, we conducted this study to see the distribution of thalassemia in breast cancer patients. To our knowledge, this is the first study performed to reveal the prevalence and molecular characteristics of thalassemia in the patients with breast cancer. Consistent with common population, -SEA/αα was also the most common thalassemia mutation in patients with breast cancer, while βIVS−II−654/β was the most frequent mutation in β-thalassemia.
As the largest breast cancer center in Jiangxi province, the Third Hospital of Nanchang treated about one-third of breast cancer patients in Jiangxi province. The prevalence and mutation spectrum of thalassemia among breast cancer patients received radiotherapy in the department of radiation oncology could reflect the whole situation of Jiangxi Province. As far as I am concerned, this is the first large scaled epidemiological investigation and spectrum analysis on the mutations and hematological characteristics of thalassemia among breast cancer patients. The findings of this study may provide reference for our medical colleagues with caution of breast cancer patients complicated with thalassemia. What’s more, the correlation of genotype and anemia manifestation in this study might help the clinicians to have rapid primary impression of thalassemia.
Different from previous reports, none of the subjects in this study received regular red blood cell transfusions for anemia. Tumor hypoxia has been intensively reported to be associated with decreased sensitivity to radiotherapy, previously (Wiechec et al., 2022; Yan et al., 2022; Eisenbrey et al., 2018). Anemia is thought to worsen intramural hypoxia, whose presence before or during radiation treatment adversely influences tumor radiosensitivity and is independently correlated with poor locoregional disease control and overall survival (Harrison and Blackwell, 2004; Tanaka et al., 2021). Consequently, the relationship between thalassemia and prognosis of patients with breast cancer needs further study.
Thalassemia was not uncommon in breast cancer patients. The physicians should have the knowledge to avoid misdiagnosing as IDA. Since anemia may cause cell hypoxia, while hypoxic cells showing low sensitivity to radiotherapy, whether the prognosis of breast cancer patients with thalassemia is poorer after radiotherapy remains to be further studied.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Medical and Ethics Committee of the Third Hospital of Nanchang. The patients/participants provided their written informed consent to participate in this study.
YG designed the study. JD wrote the manuscript. JD and ZH performed data preparation and analysis. ZH, XJ, QL, YC, and YG revised and polished the manuscript. All authors have read and approved the final version of the manuscript.
This study was supported partly by the Natural Science Foundation of Jiangxi province (No. 20171BAB205057) and the program of the health commission of Jiangxi province (20184023).
We thank Dr. Qingxiang Li in the department of clinical laboratory and Dr. Yongbo Yu in nuclear medicine department for their help in hematological analysis and serum ferritin test. We also thank the staff of ADICON Clinical laboratory (Nanchang, China) for their help in optimizing the experimental design and excellent technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ansari, S., Azarkivan, A., and Halagi, F. (2013). Incidence of hepatocellular carcinoma in patients with thalassemia who had hepatitis C. Acta Med. Iran. 51 (6), 404–407.
Brancaleoni, V., Di Pierro, E., Motta, I., and Cappellini, M. D. (2016). Laboratory diagnosis of thalassemia. Int. J. Lab. Hematol. 38 (1), 32–40. doi:10.1111/ijlh.12527
Chui, D. H., Cunningham, M. J., Luo, H. Y., Wolfe, L. C., Neufeld, E. J., and Steinberg, M. H. (2006). Screening and counseling for thalassemia. Blood 107 (4), 1735–1737. doi:10.1182/blood-2005-09-3557
Eisenbrey, J. R., Shraim, R., Liu, J. B., Li, J., Stanczak, M., Oeffinger, B., et al. (2018). Sensitization of hypoxic tumors to radiation therapy using ultrasound-sensitive oxygen microbubbles. Int. J. Radiat. Oncol. Biol. Phys. 101 (1), 88–96. doi:10.1016/j.ijrobp.2018.01.042
Haghpanah, S., Pishdad, P., Zarei, T., Shahsavani, A., Amirmoezi, F., Ilkhanipoor, H., et al. (2020). Frequency of thyroid nodules in patients with β-thalassemias in Southern Iran. Acta Endocrinol. 16 (1), 68–73. doi:10.4183/aeb.2020.68
Harrison, L., and Blackwell, K. (2004). Hypoxia and anemia: Factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist 9 (5), 31–40. doi:10.1634/theoncologist.9-90005-31
He, S., Li, D., Yi, S., Huang, X., Zhou, C., Chen, B., et al. (2021). Molecular characterization of α- and β-thalassaemia among children from 1 to 10 Years of age in Guangxi, A multi-ethnic region in southern China. Front. Pediatr. 9, 724196. doi:10.3389/fped.2021.724196
Hodroj, M. H., Bou-Fakhredin, R., Nour-Eldine, W., Noureldine, H. A., Noureldine, M., and Taher, A. T. (2019). Thalassemia and malignancy: An emerging concern? Blood Rev. 37, 100585. doi:10.1016/j.blre.2019.06.002
Jaing, T., Chang, T., Chen, S., Lin, C., Wen, Y., and Chiu, C. (2021). Molecular genetics of β-thalassemia: A narrative review. Medicine 100 (45), e27522. doi:10.1097/MD.0000000000027522
Lai, K., Huang, G., Su, L., and He, Y. (2017). The prevalence of thalassemia in mainland China: Evidence from epidemiological surveys. Sci. Rep. 7 (1), 920. doi:10.1038/s41598-017-00967-2
Lei, S., Zheng, R., Zhang, S., Chen, R., Wang, S., Sun, K., et al. (2021). Breast cancer incidence and mortality in women in China: Temporal trends and projections to 2030. Cancer Biol. Med. 18, 900–909. doi:10.20892/j.issn.2095-3941.2020.0523
Lin, M., Zhong, T. Y., Chen, Y. G., Wang, J. Z., Wu, J. R., Lin, F., et al. (2014). Molecular epidemiological characterization and health burden of thalassemia in Jiangxi Province, P. R. China. PLoS One 9 (7), e101505. doi:10.1371/journal.pone.0101505
Muncie, H. J., and Campbell, J. (2009). Alpha and beta thalassemia. Am. Fam. Physician. 80 (4), 339–344.
Peng, Q., Zhang, Z., Li, S., Cheng, C., Li, W., Rao, C., et al. (2021). Molecular epidemiological and hematological profile of thalassemia in the dongguan region of Guangdong province, southern China. J. Clin. Lab. Anal. 35 (2), e23596. doi:10.1002/jcla.23596
Picardo, E., Mitidieri, M., Minniti, E., Ambroggio, S., D Addato, F., Benedetto, C., et al. (2015). The first case of breast cancer in thalassemic patient: Case report and review of literature. Gynecol. Endocrinol. 31 (5), 345–348. doi:10.3109/09513590.2014.998646
Poggi, M., Sorrentino, F., Pascucci, C., Monti, S., Lauri, C., Bisogni, V., et al. (2011). Malignancies in beta-thalassemia patients: First description of two cases of thyroid cancer and review of the literature. Hemoglobin 35 (4), 439–446. doi:10.3109/03630269.2011.588355
Rund, D., and Rachmilewitz, E. (2005). Beta-thalassemia. N. Engl. J. Med. 353 (11), 1135–1146. doi:10.1056/NEJMra050436
Shafique, F., Ali, S., Almansouri, T., Van Eeden, F., Shafi, N., Khalid, M., et al. (2021). Thalassemia, a human blood disorder. Braz. J. Biol. 83, e246062. doi:10.1590/1519-6984.246062
Tanaka, H., Ono, T., Manabe, Y., Kajima, M., Fujimoto, K., Yuasa, Y., et al. (2021). Anemia is a prognostic factor for overall survival rate in patients with non-small cell lung cancer treated with stereotactic body radiation therapy. Cancer Manag. Res. 13, 7447–7453. doi:10.2147/CMAR.S336044
Viprakasit, V., and Ekwattanakit, S. (2018). Clinical classification, screening and diagnosis for thalassemia. Hematol. Oncol. Clin. North Am. 32 (2), 193–211. doi:10.1016/j.hoc.2017.11.006
Wiechec, E., Matic, N., Ali, A., and Roberg, K. (2022). Hypoxia induces radioresistance, epithelialmesenchymal transition, cancer stem celllike phenotype and changes in genes possessing multiple biological functions in head and neck squamous cell carcinoma. Oncol. Rep. 47 (3), 58. doi:10.3892/or.2022.8269
Yan, D., Cai, S., Bai, L., Du, Z., Li, H., Sun, P., et al. (2022). Integration of immune and hypoxia gene signatures improves the prediction of radiosensitivity in breast cancer. Am. J. Cancer Res. 12 (3), 1222–1240.
Keywords: anemia, thalassemia, breast cancer, serum ferritin, molecular mutation spectrum
Citation: Ding J, Huang Z, Jiang X, Li Q, Cao Y and Guo Y (2022) The prevalence and genetic disorders spectrum of thalassemia among breast cancer patients in Jiangxi province, China. Front. Genet. 13:1001369. doi: 10.3389/fgene.2022.1001369
Received: 23 July 2022; Accepted: 04 October 2022;
Published: 18 October 2022.
Edited by:
Anupam Basu, National Institute of Biomedical Genomics (NIBMG), IndiaReviewed by:
Bahador Bagheri, Semnan University of Medical Sciences, IranCopyright © 2022 Ding, Huang, Jiang, Li, Cao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Guo, NDA0NzE3NDM0QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.