
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 05 January 2022
Sec. Genetics of Common and Rare Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.817974
This article is part of the Research Topic Genetic, Cellular and Molecular Basis of Kidney Diseases View all 7 articles
Nanoparticles (NPs) are differing in particle size, charge, shape, and compatibility of targeting ligands, which are linked to improved pharmacologic characteristics, targetability, and bioavailability. Researchers are now tasked with developing a solution for enhanced renal treatment that is free of side effects and delivers the medicine to the active spot. A growing number of nano-based medication delivery devices are being used to treat renal disorders. Kidney disease management and treatment are currently causing a substantial global burden. Renal problems are multistep processes involving the accumulation of a wide range of molecular and genetic alterations that have been related to a variety of kidney diseases. Renal filtration is a key channel for drug elimination in the kidney, as well as a burgeoning topic of nanomedicine. Although the use of nanotechnology in the treatment of renal illnesses is still in its early phases, it offers a lot of potentials. In this review, we summarized the properties of the kidney and characteristics of drug delivery systems, which affect a drug’s ability should focus on the kidney and highlight the possibilities, problems, and opportunities.
The term nanotechnology was created by Professor N. Taniguchi for the first time in 1974. Drexler designed the term “nanotechnology” and published it in his book “Vehicles of Creation: The Arrival of the Nanotechnology Era” in 1986 (Bayda et al., 2020). Nanoscience is the only field that can collaborate with traditional fields like health discipline, biological chemistry, medicinal remedies, perception, nutriment, maquillage, auto electronic, commodity, and even designed to find new qualities of matter. Nowadays, a well-constructed fusion of applied science has been used to address difficulties in the specialty of biomedical sciences by generating more effective medical management, nanomedicines, and remedial (Bayda et al., 2020; Sahu et al., 2021). The influence of nanotechnology on humans and animals may open new avenues for research and transformation in the field of health science, and it has become an urgent topic for consideration as a therapeutic tool (Sichert et al., 2015). Nanotechnology is a shadowy multidisciplinary field that was created to engineer biological matter like atoms, molecules, and supramolecules (Sichert et al., 2015; Sahu et al., 2021). Nanoparticles (NPs) are one-dimensional objects that are 1–100 nm in size. Nanotechnology refers to the development and production of NP materials on an atomic or molecular size (Ma et al., 2020). Both organic and inorganic materials can be used to make NPs. Organic NPs include polymeric, carbon, liposomes, and dendrimer-based NPs. Quantum dots (qdots) and magnetic iron oxide particles are examples of inorganic NPs (Sung and Yang, 2015). The ability to manipulate and tailor the physiochemistry of materials by the nanometer scale, where molecular reactions occur, which advance to a slew of possibilities in the medicines, including observation of biomarkers in prior stage, focusing cells and tissues, advanced in drug delivery, evaluating end-stage disease, and more (Ratner, 2019). In medical nanotechnology, NPs are used in the depiction, assembly, dominance, and application of curative medications and implements (Contera et al., 2020). In this review, we are focusing on the challenges and perspectives related to nanotechnology in kidney diseases.
Kidney disorders are multistep processes involving the accumulation of a variety of molecular changes. The cellular function of kidney cells and their surroundings is affected by these molecular alterations. Many genetic changes, such as mutations, loss of heterozygosity, deletions, insertions, and aneuploidy, have been linked to various kidney illnesses (Sadikovic et al., 2008; Li et al., 2019). In terms of kidney function progression, kidney diseases are separated into acute kidney injury (AKI), and chronic kidney disease (CKD). AKI is established as a rise of creatinine level in the blood about 0.3 mg/dl in less than 48 h and increased to 1.5 times in less than 7 days, and urine volume of 0.5 ml/kg/h is more than 6 h. Hypovolemia and urinary blockage, drug poisoning is the most common causes. A determined irregularity of structure and function of the kidney (e.g., albuminuria >30 mg per 24 h or glomerular filtration rate (GFR) 60 ml/min/1.73 m2) for more than 3 months is characterized as CKD (Luo and Grams, 2020). The most prevalent causes of CKD include diabetes, hypertension, and primary glomerular disease. In terms of definition, causation, and treatment approaches, they are very different. CKD is a global health concern that affects more than 10% of the population, with a higher prevalence among the elderly (Luo and Grams, 2020). Patients with CKD usually endure a slew of problems and negative outcomes, putting a significant financial strain on both the people affected and society as a whole (Saran et al., 2019). Autosomal dominant polycystic kidney disease (ADPKD) is a typical hereditary dysfunction in humans with a diagnosed prevalence of about 4 in 10,000 implying that approximately 140,000 people in the United States are affected (Ters et al., 2020). The aberrant, cell-autonomous proliferation of cyst epithelial cells in ADPKD is linked to cellular dedifferentiation and overexpression of multiple proliferative factors, and it resembles a cancerous process (Mochizuki et al., 1996). As a result, early detection of CKD and quick disease prevention are becoming a public health priority. There is evidence that nanoparticles might be involved in the creation of renal calculus, polycystic kidney disease, gallstones, and gallbladder inflammation, prostatitis, calciphylaxis, and ovarian cancer, among other diseases and degenerative processes (Grantham, 1990).
Kidney disease is a global issue that affects about 750 million people worldwide, with the severity of the condition varying greatly. The rate of kidney disease and the availability of care for it are largely determined by social, cultural, and political variables, all of which contribute to a major imbalance (Crews et al., 2019). Furthermore, guidelines have been recommended that kidney disease could be recognized as a life-threatening disorder that affects people who require nephrologist’s care, as well as a common disorder with varying severity that requires a concerted public health approach for prevention, early detection, and management (Rettig et al., 2008; Levey and Coresh, 2012). Guidelines have a significant impact on clinical practice, research, and public health, but they have also sparked debate (Eckardt et al., 2009). Complement activation is imbalanced in the kidneys, which have a conspicuous, compartment-dividing membrane, and the glomerular basement membrane, which lacks complement regulators and might be at risk to attack when the cell layer is damaged (Ricklin et al., 2018). More factors, such as high complement component concentrations, pH changes, and disruption or vulnerability of the glycocalyx lining, may increase the susceptibility of the kidney to complement-induced injury (Thurman, 2015). On the other hand, complement-mediated kidney diseases are caused by a wide range of pathogenic mechanisms (Crews et al., 2019). Nanoparticles are currently being used by researchers to develop novel therapeutic medications that can reduce side effects while increasing efficacy. Nanoparticles can also combine numerous functions such as therapeutic and diagnostic capabilities, on a single nanoparticle platform (Chin et al., 2020).
Drug delivery systems using NPs have been developed in recent decades to solve the major problems of ocular drug administration, resulting in a safe and effective system that delivers the medicine to the appropriate region (Qamar et al., 2019). Development of various nanotechnology-based nanomedicines with different novel systems, including liposomes (Urquhart and Eriksen, 2019), solid lipid NPs (Patil et al., 2019), nanostructured lipid carriers (NLA) (Ozdemir et al., 2019), dendrimers (Villanueva et al., 2016), polymeric NPs (Mandal et al., 2017), inorganic NPs, microemulsion, nanosuspension, nanoemulsion, and noisome, which results in increased retention time, the solubility of hydrophobic drugs, bioavailability, enhanced drug penetration, and target specific sites. The encapsulation of the desired medicine in a nanoparticle also helps to protect it from degradation (Qamar et al., 2019).
The most prevalent and industrially used strategies for producing polymer nanoparticles are heterophase polymerization procedures (Demirdogen, 2019). The most common heterogeneous polymerization process is emulsion polymerization. Hydrophobic monomers are composite in an aqueous phase, which is trailed by polymerization in a direct process and oil-in-water emulsions (Jenjob et al., 2018). Surfactants with electrostatic and steric stabilization are commonly used to secure colloidal systems to prevent coagulation throughout chemical reactions (Kurozuka et al., 2017). Submicron polymer particles are produced via emulsion polymerization, with size ranges from 50 to 500 nm (Thickett and Gilbert, 2007). A classic size of the particles includes between 1 and 10,000 macromolecules, each of which is made up of 100–106 monomer units (Chern, 2008). Hydrophilic monomers like acrylamide, acrylic acid, and methacrylic acid are combined and change integrity in a continuous phase (Yamak, 2013). Solid particles from Pickering emulsions, which are emulsions of particle-stabilized emulsions with long-term stability (Jenjob et al., 2019), can be used to stabilize emulsions. Because of their greater stability against coalescence, colloidal particle-stabilized Pickering emulsions have obtained a lot of scrutiny in the current period. Yang et al. have been evaluated the usage of numerous solid particles as Pickering emulsifiers, including silica, clay, magnetic NPs, nanotubes, and as well as their applications in diverse domains (Piao et al., 2015). There are several synthesis methods are used to prepare nanoparticles, which are summarized in Table 1.
The most essential aspects of nanoparticles are their particle size and size distribution, which determine the delivery methods such as in vivo dispersion, biological destiny, toxicity, and their targeting capabilities. They also have an impact on drug loading, drug release, and nanoparticle stability. Nanoparticles have a lot of advantages over microparticles (Yang et al., 2017). One of the most difficult challenges is the characterization of synthesized NPs. The techniques most widely employed to analyze biosynthetic NPs include dynamic light scattering (DLS), transmission electron microscopy (TEM), ultraviolet-visible (UV-vis) spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), and X-ray diffraction (XRD).
For quick concealing of quality control and polydispersity of the samples with low dispersity, DLS is advised, which can be utilized for high-resolution characterization of complicated samples when combined with other technology. During the formulation development, AF4-MALS/DLS has been reported as an image sizing technique (Singh and Lelard, 2009). Number, volume, or mass-based size distributions, polydispersity, and particle concentrations should also be recorded, according to the U.S. Food and Drug Administration (FDA)’s draught advice on nanomaterial-containing formulations (Caputo et al., 2019). The intensity-based size distribution might be used to construct the character and accumulation-based size distributions by using a specific particle shape model, which would otherwise introduce huge inaccuracies when big (Rh > 200 nm) particles are present (U.S. FDA, 2017). As a result, alternative approaches, such as both DLS and microscopy, are advised for reporting intensity- and number-based size distributions, respectively (Wyatt and Weida, 2003). Furthermore, Filipe et al. conducted comprehensive comparative research using DLS and nanoparticle tracking analysis (NTA) to measure particles size ranging 60–1,000 nm and found that NTA recognized polydispersity and big aggregates are better than DLS (Fan et al., 2021).
The particle’s charge profile in relation to a diffusive layer is depicted by the zeta potential, which may be estimated from the electrophoretic potency of particles are measured by phase analysis light scattering (PALS) (Filipe et al., 2010). In order to obtain precise measurements, important medium variables such as nature of the phase, refractive index, viscosity, and temperature, must be pre-decided. Indeed, multiple studies have found that the zeta potential of liposomes is affected by the media’s pH, temperature, and ionic strength (Smith et al., 2017), protein adsorption (Fatouros et al., 2005), and encapsulation of charged active pharmaceutical ingredients (APIs) (Matsumura et al., 1994). The molar % of the ionic lipids included in the liposome had a linear connection with the zeta potential (Filipe et al., 2010), and a value of -30 mV or >30 mV might be generally maintained by sufficient inter-particle repulsion and stable particle suspension (Matos et al., 2004). Even though the polyethylene glycol (PEGylated) lipid is introduced at 0.2 mol percent, PEG modification can protect lipid charges and stabilize liposomes (Filipe et al., 2010). The fluorescence labeling could be able to determine the entire surface potential of liposomes (Wyatt and Weida, 2003).
Several Transmission electron microscopy (TEM) techniques, including negative stain (reagent used are uranyl acetate and uranyl formate), freeze structure, and cryogenic microscopy, are used to detect the size and morphology of the particles (Honary and Zahir, 2013). Recently, cryo-TEM is used to develop images directly after vitrification, giving focus morphology and comprehensive structural facts on lipid layers and encapsulation appliances. Because of the disruptive operations for scanning electron microscopy (SEM), is used an electron beam to scan over the surface of the sample, which used after sample preparation, such as drying, fixing, and imaging under high vacuum are not typically employed for imaging of lipid particles (Severs, 2007). Furthermore, atomic force microscopy (AFM) might be utilized to analyze the three-dimensional structure of liposomes by measuring interactions between the sample surface and a probing tip. Robson et al. compiled a detailed comparison of various imaging techniques for liposomes (Mohammed et al., 2004).
During synthesis, a distinct color change is frequently used as a visual signal of the creation of NPs. Depending on the NPs size, nanoscale gold exhibits a deep purple color. As a result, UV–vis, a general and low-cost method of confirming the existence of NPs. A plasmon resonance exists on every metal surface that corresponds to a certain visible wavelength (Robson et al., 2018). The morphology of particles includes the type of capping agents and the reflecting index of the surrounding medium all influence the position of a resonance band (Aiken and Finke, 1999). Du et al. discovered a faint absorption peak at 535 nm, which corresponded to the creation of gold NPs (AuNPs) in an E. coli solution treated with gold ions for 54 h (Du et al., 2007). After another 120 h of incubation with the same ion concentration is the significantly broader peak, indicating 20–30 nm gold colloids, and particle shape can be seen in UV–vis. He et al. discovered a considerable shift in the absorbance spectrum as a basis of particle structure (He et al., 2008). UV–vis reacted with a characteristic wavelength of 520–550 nm (ruby red), with NPs size 10–20 nm at concentrations as low as 0.25 mM. In the region of 500–900 nm, however, the absorption peak got wide-ranging with the concentration of 0.5 mM. (gray-blue). The development of gold nanowires 50–60 nm in length was blamed for the modifications. For silver NPs (AgNPs), the absorption peak was at 380 nm (deep brown) (Kalathil et al., 2011). Another study discovered a prominent absorption peak for ZnS NPs encapsulated by rhamnolipids at 340 nm (blue) with cationic surfactant, cetyltrimethylammonium bromide (CTAB) is utilized as capping agent, the absorption peak moved to 294 nm (Hazra et al., 2013).
Fourier transform infrared spectroscopy (FTIR) analysis is used extensively to identify binding molecules or functional groups on the surface of NPs generated in a biological matrix. Infrared radiation with a wavelength spanning from 4,000 to 400 cm−1 can be absorbed by biomolecules in the matrix (Davis and Mauer, 2010). In a bacterial matrix of E. coli, a weaker wavelength of 988 cm−1 was observed with rhamnose and a stronger wavelength of 1,385 cm−1 relating to the carboxyl groups, which showed aldehyde group oxidation to carboxyl groups, which led to AgNPs immobilization (Kang et al., 2014). FTIR has been employed by several researchers for similar purposes, because of the complicated sample architectures, the application of FTIR is limited. The complex composition of a biological matrix, combined with the intensity of overlying of infrared absorption bands limits the application of this approach to NPs characterization (Rieppo et al., 2012).
The morphology of NPs can be determined by X-ray diffraction (XRD). To establish the crystal structure of biologically generated NPs, one or other approaches are frequently used. Bragg reflections matching to distinct crystal planes of NPs can be seen on XRD (Kalathil et al., 2011; Pat-Espadas et al., 2014). Selected area electron diffraction (SAED), on the other hand, can be used in conjunction with TEM imaging, which provides a comparable level of study as XRD (Kalathil et al., 2011).
NPs are the factor-dependent dispersion stability with solid core surrounded by suitable surface chemical microenvironment, which influenced the size, polarity, molecules on the surfaces, solvent polarity, surfactants, solvents, and other factors of NPs (Xu et al., 2018). For specific solvents, the polarity and quantity of molecules coated on the surface determine the dispersion stability of NPs. pH, ion concentration, temperature, and storage period are some of the other parameters that affect NPs dispersion stability (Piella et al., 2016). The dispersion stability of NPs is dispersed in the liquid phase has deteriorated, resulting in irregular NP aggregates. The two NP aggregates are homo-aggregation and hetero-aggregation (Huynh et al., 2012). The aggregation of NPs is significant in a variety of applications, including filtration, flotation, medicine, and water purification (Yu and Borkovec, 2002), and the composition, shape, size, and surface charge of NPs vary (Lopez-Lopez et al., 2006). Thermal activation could accelerate and exacerbate the processes of diffusion, relaxation, grain development, and homogenization, resulting in partial or total structural damage of the NPs and performance degradation of the nanodevices (Andrievski, 2003; Andrievski, 2014). When bulk materials are reduced to the nanoscale, the melting point drops dramatically, resulting in poor thermal stability, which severely limits the use of NPs in high-temperature processes. The thermal agitation of atoms during heating results in two common processes: sintering and fusion (Buffat and Borel, 1976; Satterfield, 1991).
Encapsulation is such a common pharmaceutics technique and is unavoidable to overcome a substantial volume of literature describing encapsulated formulations, such as nanomedicines and drug delivery systems (DDSs) (Brayden and Alonso, 2016). The public’s enthusiasm for nanomedicine had heightened the desire to participate in this formulation race. Nowadays, anecdotes and cartoons are abounded to persuade academia, industry, and the general public that nanotech goods will perform miracles and deliver a brighter future for humanity, and it is debatable of its expectations (Park, 2017). Even though it would rather give a critique of the reasons presented to explain the few efficiency phrases, such as entrapment, loading, and encapsulation, which are now widely encountered in encapsulation literature. Encapsulation also has additional loading-related terminology, such as loading efficiency and effective drug loading (Sun et al., 2015). Drug encapsulation efficiency within lipid or polymer particles is low, especially for water-insoluble medicines that must be linked with the vesicle membrane when encapsulated in liposomes (De Cock et al., 2010). Avoiding these phrases while distributing experimental findings is deviating from the norm these days. Entrapment and encapsulation efficiency are used interchangeably in most circumstances because they are calculated using a mathematical equation (Bhattacharjee, 2018).
Therefore, drug loading capacity and encapsulation/entrapment efficiency are calculated using the equations:
In vitro, drug release investigations using nanoparticle formulations are more popular and adaptable with a dialysis membrane (DM) than with sampling and separation (SS). Regular dialysis and reverse dialysis are the most common DM treatments. The nanoparticle formulations are held inside the regular dialysis, allowing for simultaneous release and separation of the released API. The dialysis membrane, volume ratios between the sample and release media, and agitation settings are all important characteristics for this procedure (Xu et al., 2012). Unstable particles may collect and clog the membrane, particularly during long-term studies, or the released medication may be adsorbed on the membrane, causing drug release profiles to be underestimated in both cases (Yu et al., 2019). The continuous flow (CF) technique makes use of the United States Pharmacopeia (USP) IV (4) apparatus to circulate and modify the release media in a dynamic manner. Regular dialysis could be utilized to hold nanoparticle formulations on top of the glass beads in the sample chamber for usage with nanoparticle formulations. The combination DM-CF strategy better-distinguished dexamethasone release profiles from liposomes with varied lipid compositions than the traditional dialysis sac (DS) method (D’Souza, 2014).
Although drug release can be measured using a variety of ways, the main purpose of all these tests is to predict formulation pharmacokinetics in-vivo using in-vitro-in-vivo correlations (IVIVC), hence reducing the burden of research for generic product development. It would be preferable to have a linear or non-linear point-to-point correspondence between in-vitro and in-vivo release kinetics (Bhardwaj and Burgess, 2010). Therefore, the equation of in vitro drug release is:
NPs are only biodistributed after injection, and they are carried by the bloodstream and distributed throughout organs and other tissue systems. During blood transfer, the nanoparticles are bound with serum proteins termed opsonins (Mirshafiee et al., 2016; Wei et al., 2018). Due to macrophages, the injected doses may result in a significant reduction in nanoparticle removal. The capability of lone circulation in the formation of nanoparticles could reduce the serum protein binding (Li and Huang, 2008). Glomerular filtration and tubular secretion are the processes of renal excretion (Krediet, 2017). The wall of the glomerular capillary has an intrinsic permeability property, which can filter substances such as lesser than 5.5 nm size or proteins weighing less than 3 KDa (Liu et al., 2013). Normally, the NP’s charge, shape, and size lesser than 100 nm could be easily filtered (Liu et al., 2013). The spleen is a big lymphoid organ with high vascularity, which produces lymphocytes by storing blood, disintegrating old blood cells, and filtering foreign materials out of the blood (Hoshyar et al., 2016). According to research, the size of 150 nm with spherical shape and larger particles with 200–250 nm are especially susceptible to filtration at the spleen’s interendothelial cell slits (Moghimi et al., 2012). The liver has several sinusoidal endothelial cells (LSECs), which are highly specialized endothelial cells that serve as a permeable barrier between blood cells and hepatocytes/hepatic stellate cells (Hang et al., 2012). Fenestrae allows nanoparticles to move from the sinusoidal lumen to the surfaces of hepatocytes, which results in increased absorption and retention in the liver. According to a study, the diameter of fenestrae in persons without liver disease is 107 ± 1.5 nm (Wisse et al., 2008). Another study found that NPs can be eliminated by the liver hepatobiliary system, which enters the bile duct via the bile canaliculi, gather inside the gallbladder close to the common bile duct, and then be expelled into the duodenum (Zhang et al., 2016). The enhanced permeability and retention (EPR) effect allows for a wide spectrum of NPs movement, which is dependent on their fenestrations and the lack of good lymphatic drainage for drugs and NPs accumulation (Orbay et al., 2016). Furthermore, endothelial cells use an antibody- and caveolae-dependent transcytosis pathway to transport particles from the blood into tumors, and the size of particles transferred into tumors is limited to 50–100 nm (Wang, 2014). In addition, the elimination of NPs has been documented in many different organs, rather than blood, kidneys, liver, and targeted tissues, including the NPs circulation station, capillary vessel borders in the lung, which may detain some NPs larger than 1,000 nm (Moghimi et al., 2012). The clearance of nanoparticles from the bloodstream is also aided by lymph nodes and skin (Larraneta et al., 2016). Most target receptors lack small-molecule ligands that engage strongly and specifically with their extracellular domains. The lead compound for small molecule targeting ligand development is a natural substrate, a small molecule inhibitor, or a transition state analog of the target receptors (Friedman et al., 2013). Synthetic small molecule targeting ligands, which are unable to generate small molecules, bind with high affinity and specificity to the targeted extracellular domains. Multivalent targeting, which is utilized to boost the bonding strength between two molecules, is a potential (Friedman et al., 2013).
Nanotechnology is a major interdisciplinary field, which combines biology, chemistry, medicine, and engineering to promote and develop effective bio-medical tools. These approaches provide a new perspective and dimension to clinical practice and surgery. Nanomedicine incorporates a remarkable clash on drug delivery systems, tissue regeneration programs, medical diagnostics, detection, and introducing new materials (Chandarana et al., 2018). The US-FDA has approved 51 nanomedicines, with another 77 in the pre-clinical/clinical stage. Some of the commercially available nanomedicines are liposomes, proteins, polymers, micelles, emulsions, nanocapsules, and dendrimers (Mozafari, 2018). Nanotechnology offers numerous possibilities for disease perception, precaution, treatment, and preservation. The potential of nanotechnology in health care and medicine has yet to be completely explored, therefore research and development are required to aid people with serious medical illnesses such as cancer, cardiovascular disease, and chronic kidney disease (Bhardwaj and Burgess, 2010; Ma et al., 2020). We summarize the drug delivery system of nanoparticles in the treatment of various diseases (Table 2). The sizes of the nanoparticles were varying with the disease based on targeting receptor.
A successful intervention therapy could be used to prevent CKD and decrease consequences including infection, hypertension, anemia, and heart failure. On the other hand, traditional diagnostic approaches have several drawbacks, including being inconsiderate and inconvenient. (Ma et al., 2020). Albuminuria is a risk factor for both incident and progressive CKD (Bobo et al., 2016). If the concentration of urinary albumin was greater than 30 mg/dl, it shows positive results with the routine urine analysis dipstick. Microalbuminuria can be detected using specific urinary albumin dipsticks or specific antibody methods (Reichel et al., 2020). Surface-enhanced Raman scattering (SERS) with NPs is the commercially accessible emission technology, which involves the inelastic scattering concerning incident laser energy. It undergoes great sensitivity, simple sample processing, and rapid analysis (Mosier-Boss, 2017). According to one report, the detection of urine albumin levels does not need pre-processing of the samples and the method was quite quick (McNay et al., 2011). Commercial instruments are currently available that allow for microalbuminuria screening at the point of care (Mosier-Boss, 2017). The instruments have been proven beneficial for diagnosing early diabetic kidney disease by comparing the results with devices and laboratory tested, and it could be utilized in remote underdeveloped countries in the future (McNay et al., 2011). Cystatin C (CysC), N-acetyl-d-glucosaminidase (NAG), and kidney injury molecule-1 (KIM-1) are found to be effective predictors of CKD (Kumar et al., 2018). An increasing amount of evidence suggests that NPs could be employed to boost an immunosensor in response to the observation of these indicators (Wang et al., 2016; Lopes et al., 2019). KIM-1 is a biomarker of pre-stage renal injury and indicates the action of renal injury and its recovery has also been determined using an electrochemiluminescence (ECL) biosensor. The electron transfer efficiency was then improved by using platinum (Pt) NPs (Zhang et al., 2018). GFR is a measurement of kidney function, and physicians use the CKD-EPI as a formula to evaluate GFR and are referred to as eGFR (Yang et al., 2018). Furthermore, tubular cells can absorb and secrete a small amount of creatinine, which has been found to be ineffective to detect the CKD in the pre-or post-stages, whereas fluorescent NPs such as qdots, gold, and silica NPs have been found to be providing advantages over current methods to detect the GFR (Takeda et al., 2002).
Fluorescent semiconductor nanocrystals, which are widely used in the biological area and are commercially available (Huang and Gretz, 2017). Moreover, near-infrared fluorescence imaging, which is highly sensitive and affordable, has been widely used in the investigation of various disorders (Wang et al., 2019). Non-invasive fluorescence imaging of renal insufficiency and staging are still in the pre-clinical stage of development (Penna et al., 2011). To measure kidney function, Yu et al. employed the renal clearable near infrared-emitting glutathione-coated gold NPs (GS-AuNPs) as a contrast agent in fluorescence imaging of kidneys. They discovered that this nanotechnology may be used to noninvasively measure kidney disease, describe the stages of malfunction even disclose an adaptive function in mice with unilateral obstructive nephropathy (UUO) (Yu et al., 2016). Furthermore, they were successful in identifying kidney malfunction that corresponded to renal damage as determined by pathological results [134]. Many renal-clearable NPs such as AuNPs, copper NPs (CuNPs), iron oxide NPs, silica NPs (SiNPs), carbon dots, and palladium nanosheets, are now available, allowing NPs to be used in noninvasive renal imaging (Shirai et al., 2012; Huang et al., 2013; Zhou et al., 2013; Tang et al., 2014; Yang et al., 2015). In animal models of UUO, SiNPs with the fluorescent probe anti-CD11b have been used as an imaging tool for measuring inflammation and fibrosis at a high intensity (Shirai et al., 2012). On the other hand, nano-magnetic resonance imaging (nano-MRI) could overcome the limits of traditional modalities and represent the next step in renal biopsy (Khosroshahi et al., 2017). Furthermore, it is non-invasive and examines the status of kidneys, which makes it a useful follow-up test. Iron oxide NPs is possibly an alternative to nephrotoxic gadolinium-based MRI contrast agent in the development of nanoscale sensing technologies (Stabi Bendz, 2011). Before and after 24 h injection of iron oxide, the signal strength of each kidney was assessed by several conditions of nephropathies. In rats with nephrotoxic nephritis, the cortical signal intensity reduced considerably. In the obstructive nephropathy model, the signal intensity is decreased in renal compartments, which is responsible for the diffuse interstitial lesions, suggesting that nano-MRI could aid in the diagnosis of various nephropathies (Hauger et al., 2000; Stabi Bendz, 2011). Nephropathies could be recognized and discriminated against by evaluating the strength and area of an MRI signal. Though the long-term safety of iron oxide NPs is uncertain, their preclinical pharmacokinetics and safety appear to be suitable in terms of application possibilities in a single dose of a diagnostic agent for human MRI (Hauger et al., 2000).
Renal function is lost as a result of CKD, and the condition can progress to renal failure. Unlike more acute inflammatory glomerulonephritis, though immunosuppression may be curable, there are currently no treatments available to reverse the loss of renal function in CKD (Bourrinet et al., 2006). To date, there are just a few therapeutic options for slowing the progression of CKD. Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), as well as other conservative medicines, are examples of supportive care (Breyer and Susztak, 2016). NPs are significant because they serve as a kidney-targeted transport route for various medicines and nucleic acids (Ma et al., 2020). The characterized NPs target the ligands that can be tailored to target specific cells or tissues in NPs (Ma et al., 2020). The size of NPs has a big impact on cellular absorption, circulation half-life, and targeting (Sharma et al., 2011). The half-life of NPs with 100 nm is longer than the NPs with smaller or larger diameters. Fewer particles were more likely to pass through the kidneys, although those smaller than 10 nm are more likely to be eliminated via renal excretion and phagocytosis (Sharma et al., 2011).
It has been reported that the larger NPs (>100 nm) were unable to penetrate the renal mesangium due to its size limitation imposed by fenestrations of glomerular endothelial cells (GECs) (Hoshyar et al., 2016). Targeting the renal tubule has also been studied, with NPs (less than 10 nm) engineered to pass through the glomerular filtration barrier and internalized by the epithelial cells (Choi et al., 2011; Sancey et al., 2015). Another study revealed that 5 nm sizes of dextran-based NPs and dendrimer NPs are filtered and absorbed by tubular epithelial cells in a time-or dose-dependent manner in vivo (Nair et al., 2015). NPs with 400 nm, which were significantly larger than the glomerular basement membrane’s (GBM) fenestrations, were discovered to selectively target proximal tubules. The data revealed that the NPs are internalized at the basal side by proximal tubule epithelial cells through peritubular capillaries [143]. NPs must be properly delivered and bonded to their specified target, and the shape of NPs has a substantial impact on their performance and biological distribution in vivo.
Several shapes have been constructed like cubic, spherical, hexagonal, helical, and rod; even the cylindrical shape is altered by blood flow (Blanco et al., 2015). When compared to other shapes, the rod structure has shown to be more effective at penetrating tumors (Chauhan et al., 2011). Because of the aspect ratio and dimension, spherical NPs clear more slowly than non-spherical NPs; yet they migrate to vessel walls less efficiently (Gentile et al., 2008).
A study suggested that carbon nanotubes are delivered therapeutic siRNA to proximal tubular cells reducing injury in cisplatin-induced kidney injury models (Alidori, et al., 2016). Coating liposomes with an anti-E-selectin antibody effectively suppressed E-selectin expression in GECs and reduced albuminuria (Asgeirsdottir et al., 2008). NPs carrying the anti-inflammatory peptide Ac2-26 have been demonstrated to bind with collagen IV and targets subendothelial collagen IV (Kamaly et al., 2013). Targeting medications of proximal tubular cells may be a promising way for the treatment of tubulointerstitial fibrosis, as it reduces the likelihood of negative side effects while increasing the potency of antifibrotic therapies (Dolman et al., 2012). Megalin is the multi-ligand receptor, which is expressed in the apical membrane of proximal tubular epithelial cells and plays a key function in the cell’s endocytosis (Wicher et al., 2006). Several studies have investigated whether the megalin receptor can transport pharmaceuticals to the kidneys specifically based on targeting active sites (Gao et al., 2014; Oroojalian et al., 2017).
NPs may cause cellular toxicity through oxidative stress, inflammation, and interactions with the cell membrane (Prow, 2010; Occhiutto et al., 2020). On the other hand, nano-toxicity is determined by the purity of the molecule and the drug concentrations, as well as other features such as nanoparticle size, surface form, and ionic charge (Voigt et al., 2014). Particle, excipient, contaminant, and inflammatory toxicity are all examples of toxicity that could be caused by independent drug delivery (De Matteis and Rinaldi, 2018). In vitro and in vivo models should be used to study each toxicity separately. There is a lack of specific literature on the toxicity of therapeutic NPs (Prow, 2010). Nano-toxicity is a critical topic that must be investigated in connection with each NP’s therapeutic potential, especially for those that require multiple doses (De Jong et al., 2013; El-Ansary et al., 2013; De Matteis and Rinaldi, 2018). Before receiving full regulatory approval, all nanoparticles that are suggested to be used for therapeutic reasons must be thoroughly examined in terms of local and systemic toxicity.
The structure of kidneys and features of drug delivery devices has been extensively investigated in order to successfully deliver medications to the kidney (Kamaly et al., 2016; Chen et al., 2020). The delivery strategy of kidney-targeted drug delivery systems might be discussed in detail in the following sections, with emphasis on key targets such as GECs, GBM, podocytes, mesangial cells (MCs), and proximal tubules, which play critical roles in the progressions of kidney diseases (Chen et al., 2020).
GECs, which are found in the glomerular capillary wall, are the first portion of the glomerular filtration barrier. Fenestrations and transcellular pores (60–160 nm) are packed with the endothelial glycocalyx that distinguishes them (Kamaly et al., 2016). GECs and their surface of glycocalyx play a key role in the plasma component filtering (Ichimura et al., 2008). Renal failure, proteinuria, and glomerulosclerosis are all illnesses associated with endothelial filtration abnormalities (Satchell and Braet, 2009). A second glomerular filtration barrier is formed by GBM, a unique extracellular matrix linked to GECs. According to the research, GBM is discovered to be a reticular connective tissue, which is made up of proteoglycan, collagen IV, and the laminin is released by the GECs and the podocytes (Suleiman et al., 2013). Meshes of a diameter of 4–10 nm are found on the surface of GBM, which could become larger in the presence of renal illness (Gagliardini et al., 2010). Heparin sulfate proteoglycan, which has a large negative charge, is the major component of proteoglycans, which causes GBM with negatively charged to produce repulsion for blood and impede plasma albumin filtration (Yu et al., 2019). As a result of the electrostatic adsorption of proteoglycan, the cationic carrier can be employed in drug delivery systems, which might be target GBM. Zuckerman and colleagues found that siRNA nanoparticles made of cationic polymer could reach the kidney quickly. After 6 min of injection, the siRNA signal in GBM had accumulated to roughly 20% of the typical dose (Zuckerman et al., 2012).
Podocytes connected to the outside of the GBM and represent the final barrier of glomerular filtration. The foot processes and slit diaphragm are distinctive structural features of podocytes, with pore sizes ranging from 30 to 40 nm. In between podocyte foot processes, the glomerulus filtration capacity may be controlled, and big molecules in the blood cannot get through, which is important for the kidney’s filtration barrier (Lal et al., 2015). Due to its highly differentiated characteristics and limited ability to repair or regenerate, Podocytes are easily damaged (Brinkkoetter et al., 2013). Podocytes are involved in glomerulosclerosis, glomerulonephritis, membranous nephropathy, and diabetic nephropathy, among other kidney illnesses (Pavenstadt et al., 2003). The upregulation of the integrin αvβ3 receptor in diabetic podocytes could be the target for the medication delivery system (Mallipattu and He, 2016). In a separate study, inorganic nanoparticles treated with an integrin v3 antibody were found to be more selectively taken up by podocytes than unmodified inorganic nanoparticles (Pollinger et al., 2012). On the other hand, the preceding examples of targeted podocytes are all done out in vitro in cell models. The drugs must have a diameter of at least 10 nm in order to pass through GECs and GBM and reach podocytes in vivo. FcRn receptors were discovered on podocytes, and they were determined to be responsible for IgG internalization (Akilesh et al., 2008). MCs are responsible for expanding and balancing the glomerular matrix, regulating the filtration surface area, and eliminating immune complexes, as well as maintaining the glomerular microvascular bed’s structural integrity (Wu et al., 2017). As a result, medications administered to MCs for the treatment of numerous renal illnesses are beneficial (Zhao et al., 2019). Nanoparticles with smaller sizes than GEC pores (60–160 nm) can be delivered to the MCs due to the lack of GBM and podocytes on the side of the glomerular capillary closest to the MCs (Scindia et al., 2008). As a result, the size of the nanoparticle is a critical component in establishing a direct link between the mesangial region and circulation. Choi and colleagues found that the gold nanoparticles with 75 ± 25 nm diameter accumulated successfully in the mesangial area of mice, which indicates that nanoparticles with a diameter of 50–100 nm could be effective in targeting MCs (Choi et al., 2011). Another study found that the combination of albumin with celastrol NPs to target MCs could pass through fenestrated endothelium, accumulate in MCs, and improve renal pathological morphology in nephritis model rats with the results showing that the albumin-celastrol NPs with 95 nm size could pass through fenestrated endothelium and accumulate in MCs, which improve renal pathological morphology (Guo et al., 2017). Furthermore, Shimizu et al. discovered that the mitogen-activated protein kinase 1 (MAPK1) siRNA NPs with 10–20 nm size delivered by poly (ethylene glycol) (PEG)-poly (l-lysine) copolymer-based delivery vehicles successfully penetrated the pores of GECs into the mesangial region, decreased MAPK1 mRNA, TGF-1, and fibronectin expression (Shimizu et al., 2010). Targeted alteration happens according to the particle size, which can improve nanoparticle uptake by MCs, which express the mannose receptor in the kidney, which can mediate nanoparticle uptake in vivo study (Zhang et al., 2011).
Proximal tubular cells, which are responsible for the active movement of endogenous and foreign substances between blood and urine, are the most active cells in renal physiological metabolism. They can boost the tubulointerstitial inflammatory response, as well as the development and progression of fibrosis, by presenting antigens and producing cytokines (Ramos et al., 2015). As a result, medication delivery to the proximal tubular cells is extremely beneficial for lowering tubulointerstitial fibrosis, inflammation, and boosting renal tubular regeneration (Falke et al., 2015). The ability of drug delivery methods to reach proximal tubular cells is largely determined by their characteristics and the anatomical structure of the kidney. The drug delivery methods can reach proximal tubular cells in two ways. The apical side of the proximal tubule is the first place to look for proximal tubular cells (Kaissling et al., 1996). To reach the proximal tubular cells, drug delivery methods must have a particle size of at least 10 nm. The second approach to get to proximal tubular cells is through the proximal tubule’s basolateral side (Kaissling et al., 1996). To begin, the particles must pass via the renal peritubular capillaries, which contain endothelial fenestrations measuring 60–70 nm in diameter (Satchell and Braet, 2009). A diaphragm with 3–5 nm thickness is roughly closed these fenestrations (Nico et al., 2009). The study demonstrated that the positively charged particles can easily pass through the fenestrations because they contain negatively charged heparin sulfate (Satchell and Braet, 2009).
We summarize the potential drug carriers to proximal tubules (Figure 1), and some advantages and disadvantages of nanocarriers are shown in Table 3. Low molecular weight proteins (LMWP) have been extensively studied for delivering drugs to proximal tubular cells. The molecular weight and charge of LMWP determine its capacity to permeate the glomerular filtration barrier and the degree of reabsorption by the proximal tubular cells. Lysozyme (LMZ) is the best researched LMWP protein with a molecular weight of 14 KDa and the capacity to flow nearly easily past the glomerular filtration barrier before being reabsorbed by the megalin receptors of proximal tubular cells, lysozyme (LMZ) is the best researched LMWP protein (Christensen et al., 2009).
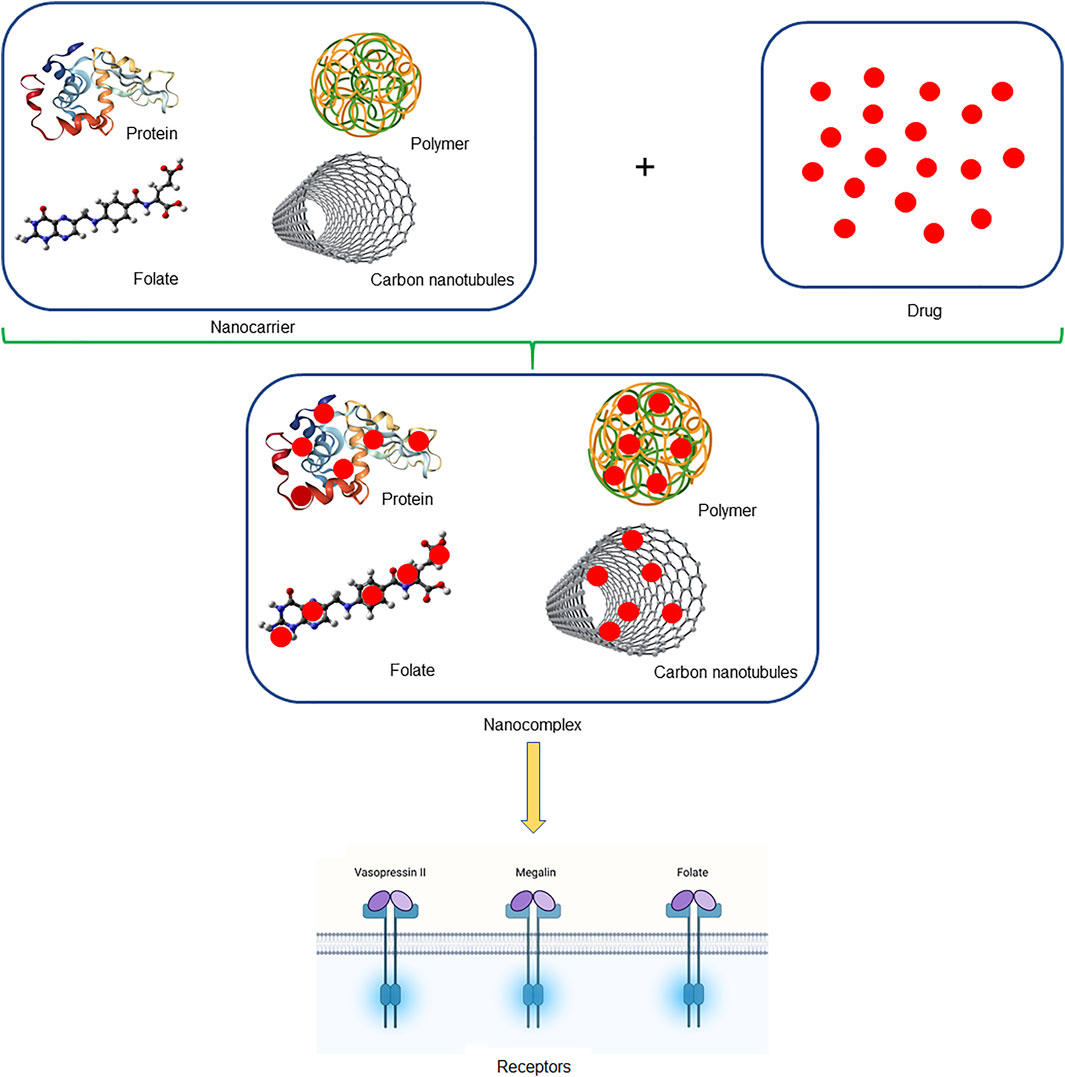
FIGURE 1. Schematic illustration of nanocomplex. The figure mentioned briefly the nanocarriers which were used to carry drugs in the form of nanocomplex, which tends to deliver drugs safely to the kidney.
By connecting lysozyme to a sunitinib analog, Dolman et al. created the kidney-targeted conjugated LMZ-17864 particles (Dolman et al., 2012). After a single dose, LMZ-17864 immediately accumulated in the kidney, which persisted for 3 days. When compared to free sunitinib, the drug level in the kidneys was boosted 28 times and no clear harmful side effects were seen. These conjugates can target the kidney efficiently and quickly, halting the progression of kidney disease (Zhang et al., 2009). Peptides could be employed as a drug carrier to target proximal tubular cells in addition to LMWP (Wang et al., 2017). Both kidneys had a 25 and 46% accumulation content of radiolabeled gastrin and glucagonlike peptide 1 (Gotthardt et al., 2007). According to Geng et al., a combination of G3-C12 peptide and a small molecule model medication captopril (CAP) to form G3-C12-captopril (G3-C12-CAP) (Geng et al., 2012). Fluorescence imaging revealed that G3-C12-CAP accumulated swiftly and precisely in the kidneys of mice, owing to proximal tubular cell reabsorption. G3-C12-CAP demonstrated a 2.7-fold increase in the kidney when compared to free CAP. MCLPVAS is a seven-amino-acid peptide that targets the kidneys (Geng et al., 2012). An elastin-like polypeptide (ELP) is a non-immunogenic protein transporter, which can connect tiny molecules and peptides in a stable manner (Cho et al., 2016).
Anionic polyvinylpyrrolidone (PVP) has been shown to be a potential kidney-targeted carrier. Approximately 30% of the administered dose accumulated in the kidneys when mice were given carboxylated PVP intravenously for 3 h (Kodaira et al., 2004). Liu et al. created the panel of 25-kDa p (OEGMA-co-MAA) copolymers made up of different ratios of neutral methyl glycol methacrylate (OEGMA) to anionic methacrylic acid (MAA) (1:0, 1:1, 1:4). The polymers are accumulated in the kidney, and the amount accumulated increases as the quantity of anionic monomer (Liu et al., 2018). According to Yamamoto et al., polyvinylpyrrolidone-co-dimethyl maleic acid (PVD) is a drug carrier with strong kidney-targeting ability and safety. Renal accumulation of PVD with the molecular weight of 6–8 kDa has the highest renal level (Yamamoto et al., 2004). As a kidney-targeted drug carrier, Kamada and colleagues created polyvinylpyrrolidone-co-dimethyl maleic anhydride (PVDn). At high doses, roughly 80% of PVDn was selectively deposited in the kidney after 24 h of intravenous administration to mice and did not produce kidney or other tissue damage (Kamada et al., 2003). Low molecular weight chitosan (LMWC) is a biocompatible and degradable polymer that can be utilized by targeting kidneys (He et al., 2012). Yuan et al. used a chemical reaction to link 50% of the N-acetylated LMWC with a molecular weight of 19 kDa to the model medication prednisolone (Yuan et al., 2007). In mice, 14% of LMWC conjugates accumulated in the kidney within 15 min of treatment, and the prednisone levels are boosted by 13 times in the kidney. Liang et al. formulated AZT-COS by the combination of chitosan oligomers (COS) and zidovudine (AZT). The average retention period of AZT-COS in the body is raised by 2.5 times, and the cumulative amount in the kidney is increased by 5.6 times when compared to free AZT (Liang et al., 2012). Yuan and others found that LMWC uptake was considerably reduced in megalin-shedding animals, implying that the LMWC might be entered into the proximal tubular cells via the megalin receptor (Yuan et al., 2009). Wang et al. have investigated that chitosan nanoparticles (CS-NP) promising as drug delivery platforms for oral delivery in kidney disease (Wang J et al., 2021).
The antibodies with a higher molecular weight of around 150 kDa cannot pass into the glomerular filtration barrier and are challenging to drug carriers for proximal tubular cell targeting (Akizawa et al., 2008). According to the study, the antibody fragments with less than 50 kDa molecular weight can pass through the glomerular filtration barrier and reabsorb by the proximal tubular cells. Radiolabeled monoclonal antibody fragments could clump together in the proximal tubules of the kidney (Grunberg et al., 2005). As a result, targeting proximal tubular cells with antibody fragments as a drug carrier could be a realistic strategy.
A prodrug is a drug derivative that can be digested or activated at a specific target site, allowing active medicines to be released or produced (Kratz et al., 2008). Prodrugs-based folate and glycosyl have been shown to target tubules in the studies. Folate is a nutrient that is often used in the delivery of anti-cancer drugs (Cheng et al., 2017; Abazari et al., 2019). Folate can be used as a ligand to transport drugs to proximal tubular cells because the folate receptor on these cells can be reabsorbed (Mathias et al., 2000). After a 5-min intravenous administration of the radiolabeled DTPA-folate conjugate [99mTc]DTPA-folate in mice. The radiolabeled DTPA-folate conjugate [99mTc]DTPA-folate was quickly absorbed by the kidney, according to Mathias et al. The kidney collected roughly 21% of the injected dose per Gram tissue after 4 h of intravenous administration. The level of [99mTc]DTPA-folate in the kidney reduced to 2.3 percent when free folic acid was pre-injected (Trump et al., 2002). Similar investigations found that after intravenous injection of [99mTc](CO)3-DTPA-folate for 4 h, the accumulation in the kidney is approximately 47% of the injected dose per Gram tissue (Trump et al., 2002). As a knowledge, folate could be the effective vehicle for drug delivery to the kidneys.
Carbon nanotubes (CNTs) are the new delivery vehicle in medicinal and diagnostic applications due to their unique intrinsic physical, chemical, and optical capabilities. According to McDevitt, CNTs with a length of 100–500 nm and a diameter of 1–2 nm are filtered through the glomerulus and reabsorbed in the proximal tubule (McDevitt et al., 2007). After a 20-min injection of CNTs in mice, around 65% of CNTs were eliminated by the kidney and about 15% were reabsorbed in the proximal tubules, even though CNTs were much larger than glomerular filtration (Ruggiero et al., 2010). The study discovered that CNT-mediated siRNA was preferentially delivered to proximal tubular cells and successfully shut off the expression of multiple target genes to minimize kidney injury in model mice with acute renal injury (Alidori et al., 2016). Another study found that CNTs might pass through the glomerulus and enter the renal capsule in a direction perpendicular to the GBM (Lacerda et al., 2008).
The clearance rate of medications and therapeutic materials from the body is one of the most important elements influencing their pharmacokinetics. NPs enter the systemic circulation after absorption, disperse and interact with the body before being removed by the reticuloendothelial system (RES) or the kidney (Moss and Siccardi, 2014). The kidneys receive 1–1.2 L of blood per minute or 20–25% of cardiac output. The basic functional unit of the kidney, the nephron, has an average of one million nephrons in each kidney. The renal corpuscle, or glomerulus, as well as the proximal and distal tubules, make up each nephron. The primary filtrate is transmitted through fenestrated capillaries into the Bowman’s capsule, which encloses the glomerulus and collects the filtrate, via the renal vasculature to the glomeruli. The filtrate is subsequently passed via the proximal and distal renal tubules, where nutrients, water, and ions are reabsorbed, and waste products are released (Hauser et al., 2021).
When constructing nanomedicine, the size, shape, and charge of nanoparticles must be considered, since these will determine whether they are cleared or accumulated by the kidneys. A positively charged nanomedicine, for example, may be able to filter through a negatively charged GBM and podocytes more efficiently than neutral or negatively charged substances (Hauser et al., 2021). Interaction sites and probable uptake sites of NPs, which are extracted or employed to target the kidney. NPs enter the kidney via the renal artery and are carried to the afferent arteriole, where they remain in the bloodstream or are subjected to renal filtration from the blood in the glomerular capillaries, depending on particle properties. Renal components such as the glycocalyx, endothelial cells, and the glomerular basement membrane can be tailored to help select NPs for filtration is shown in Figure 2. NPs can interact with podocytes in the Bowman’s lumen after filtering. The filtrates NPs are carried to the proximal tubule, where they interact with proximal epithelial cells and may be reabsorbed. Pro-drug NPs can be triggered in proximal tubular cells lysosomes. After being delivered from the efferent arteriole to the peritubular network, NPs that are not selected for renal filtration can interact with the renal tubular compartment (Hauser et al., 2021). Targeting was done in two ways in the drug delivery system, which are active and passive targeting. Active targets used the ligand-receptor approach to locate the ultimate target, whereas passive targeting uses the enhanced permeability (EPR) effect to locate specific spots of the receptor (Hossen et al., 2019). The vasopressin II receptor (V2R) is a G protein-coupled receptor (GPCR) with seven transmembrane domains that is expressed in the basolateral membrane of epithelial cells that line the distal tubule, connecting tubule, and collecting ducts (Robben et al., 2004), which might be useful for targeted drug delivery through the active target. In renal fibrosis, megalin receptor-mediated endosomal absorption of various medicines and safe release of the active drug from endosomes (Nastase et al., 2018). Although characterized NPs might allow for passive accumulation in the kidneys, active targeting nanoparticles, such as peptides and antibodies, are also being investigated to increase kidney targeting (Huang et al., 2021).
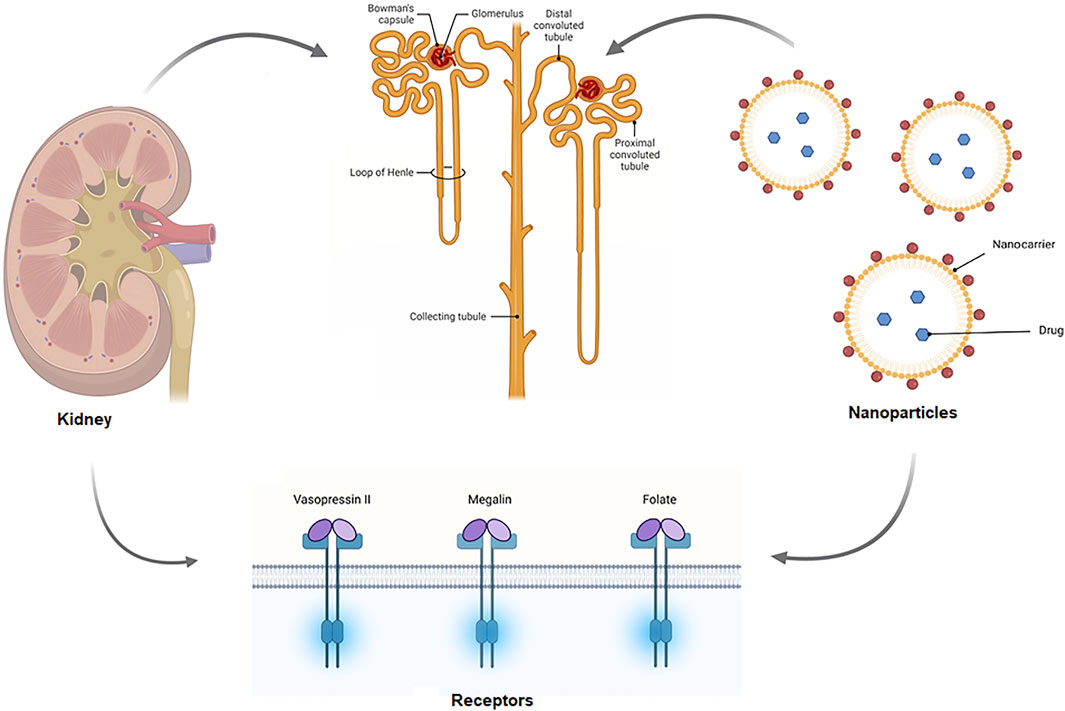
FIGURE 2. Schematic of the mechanism of targeted drug delivery in the kidney. Drug carrying nanoparticles enter the kidney via the renal artery and are carried to the afferent arteriole, where they remain in the bloodstream or are subjected to renal filtration from the blood in the glomerular capillaries. Renal components such as the glycocalyx, endothelial cells, and the glomerular basement membrane can be tailored to help select NPs for filtration. NPs can interact with podocytes in the Bowman’s lumen after filtering. The filtrates NPs are carried to the proximal tubule, where they interact with proximal epithelial cells and might be reabsorbed.
Kidney disease is becoming a major epidemiologic problem over the world. Despite significant research achievements, the pathophysiologic processes involved in the progression of many kidney disorders are still unknown. Some nanomaterials have been used in clinical therapy as a result of the rapid development of nanomedicine. The goal of creating kidney-targeted drugs is to increase medication levels and therapeutic efficacy while reducing drug toxicity and side effects. Altering the characteristic features of nanoparticles of the drug delivery system is based on the physiological or pathological characteristics of the kidney can help achieve the goal of targeting the kidney. Although nanotechnology has shown some benefits in kidney illness, there are several difficulties to be handled and improved, including limited manufactured products, expensive, need in vivo steadiness, less selective regulation, and possible lesions to nontarget organs. The current focus of research on kidney-targeted drug delivery systems is on finding acceptable porters and boosting targeted productivity when the release profile and the metabolic processes of drug delivery systems after they penetrate targeted cells are still being studied. Based on the knowledge, our future research will focus on building nanoparticles containing therapeutic drug-based kidney-targeted drug delivery systems.
JPJM wrote the initial draft of the manuscript. XL have reviewed and edited the manuscript.
This research was funded by National Institutes of Health R01 DK 129241 and R01 DK126662 grants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ag, Silver; Au, Gold; CKD, Chronic kidney disease; CS, Chitosan; DLS, Dynamic light scattering; FDA, Food and Drug Administration; FT-IR, Fourier-transform infrared spectroscopy; GBM, Glomerular basement membrane; GECs, Glomerular endothelial cells; MCs; Mesangial cells; NPs, Nanoparticles; PLGA, Poly (lactide-co-glycolide); TEM, Transmission electron microscopy; UV-Vis, Ultraviolet-visible spectroscopy; XRD, X-ray diffraction.
Abazari, R., Ataei, F., Morsali, A., Slawin, A. M. Z., and L. Carpenter-Warren, C. (2019). A Luminescent Amine-Functionalized Metal-Organic Framework Conjugated with Folic Acid as a Targeted Biocompatible pH-Responsive Nanocarrier for Apoptosis Induction in Breast Cancer Cells. ACS Appl. Mater. Inter. 11 (49), 45442–45454. doi:10.1021/acsami.9b16473
Abdelkader, D. H., Tambuwala, M. M., Mitchell, C. A., Osman, M. A., El-Gizawy, S. A., and Faheem, A. M. (2018). Enhanced Cutaneous Wound Healing in Rats Following Topical Delivery of Insulin-Loaded Nanoparticles Embedded in Poly(vinyl Alcohol)-Borate Hydrogels. Drug Deliv. Transl. Res. 8, 1053–1065. doi:10.1007/s13346-018-0554-0
Ahangarpour, A., Oroojan, A. A., Khorsandi, L., Kouchak, M., and Badavi, M. (20182018). Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid Med. Cel Longev, 7496936. doi:10.1155/2018/7496936
Aiken, J. D., and Finke, R. G. (1999). A Review of Modern Transition-Metal Nanoclusters: Their Synthesis, Characterization, and Applications in Catalysis. J. Mol. Catal. A. Chem. 145 (1-2), 1–44. doi:10.1016/S1381-1169(99)00098-9
Akilesh, S., Huber, T., Wu, H., Wang, G., Hartleben, B., Kopp, J., et al. (2008). Podocytes Use FcRn to clear IgG from the Glomerular Basement Membrane. Proc. Natl. Acad. Sci. U. S. A. 105, 967–972. doi:10.1073/pnas.0711515105
Akizawa, H., Uehara, T., and Arano, Y. (2008). Renal Uptake and Metabolism of Radiopharmaceuticals Derived from Peptides and Proteins. Adv. Drug Deliv. Rev. 60 (12), 1319–1328. doi:10.1016/j.addr.2008.04.005
Akolade, J. O., Oloyede, H. O. B., and Onyenekwe, P. C. (2017). Encapsulation in Chitosan-Based Polyelectrolyte Complexes Enhances Antidiabetic Activity of Curcumin. J. Funct. Foods 35, 584–594. doi:10.1016/j.jff.2017.06.023
Alidori, S., Akhavein, N., Thorek, D. L., Behling, K., Romin, Y., Queen, D., et al. (2016). Targeted Fibrillar Nanocarbon RNAi Treatment of Acute Kidney Injury. Sci. Transl. Med. 8 (331), 331ra339. doi:10.1126/scitranslmed.aac9647
Almutairi, F. M., ElRabey, H. A., Tayel, A. A., Alalawy, A. I., Al-Duais, M. A., Sakran, M. I., et al. (2020). Augmented Anticancer Activity of Curcumin Loaded Fungal Chitosan Nanoparticles. Int. J. Biol. Macromol. 155, 861–867. doi:10.1016/j.ijbiomac.2019.11.207
Amjadi, S., Abbasi, M., Shokouhi, B., Ghorbani, M., and Hamishehkar, H. (2019). Enhancement of Therapeutic Efficacy of Betanin for Diabetes Treatment by Liposomal Nanocarriers. J. Funct. Foods 59, 119–128. doi:10.1016/j.jff.2019.05.015
Andrievski, R. A. (2014). Review of thermal Stability of Nanomaterials. J. Mater. Sci. 49, 1449–1460. doi:10.1007/s10853-013-7836-1
Andrievski, R. A. (2003). Review Stability of Nanostructured Materials. J. Mater. Sci. 38, 1367–1375. doi:10.1023/A:1022988706296
Asanuma, H., Sanada, S., Yoshitomi, T., Sasaki, H., Takahama, H., Ihara, M., et al. (2017). Novel Synthesized Radical-Containing Nanoparticles Limit Infarct Size Following Ischemia and Reperfusion in Canine Hearts. Cardiovasc. Drugs Ther. 31, 501–510. doi:10.1007/s10557-017-6758-6
Asgeirsdottir, S. A., Zwiers, P. J., Morselt, H. W., Moorlag, H. E., Bakker, H. I., Heeringa, P., et al. (2008). Inhibition of Proinflammatory Genes in Anti-GBM Glomerulonephritis by Targeted Dexamethasone-Loaded AbEsel Liposomes. Am. J. Physiol. Ren. Physiol. 294 (3), F554–F561. doi:10.1152/ajprenal.00391.2007
Asl, S. S., Amiri, I., Samzadeh-kermani, A., Abbasalipourkabir, R., Gholamigeravand, B., and Shahidi, S. (2021). Chitosan-coated Selenium Nanoparticles Enhance the Efficiency of Stem Cells in the Neuroprotection of Streptozotocin-Induced Neurotoxicity in Male Rats. Int. J. Biochem. Cel Biol. 141, 106089. doi:10.1016/j.biocel.2021.106089
Bayda, B., Adeel, M., Tuccinardi, T., Cordani, M., and Rizzolio, F. (2020). The History of Nanoscience and Nanotechnology: from Chemical-Physical Applications to Nanomedicine. Molecules 25, 112–127. doi:10.3390/molecules25010112
Bei, W., Jing, L., and Chen, N. (2020). Cardio Protective Role of Wogonin Loaded Nanoparticle against Isoproterenol Induced Myocardial Infarction by Moderating Oxidative Stress and Inflammation. Colloids Surf. B. 185, 110635. doi:10.1016/j.colsurfb.2019.110635
Bhardwaj, U., and Burgess, D. J. (2010). A Novel USP Apparatus 4 Based Release Testing Method for Dispersed Systems. Int. J. Pharm. 388, 287–294. doi:10.1016/j.ijpharm.2010.01.009
Bhattacharjee, S. (2018). How Efficient Are the Efficiency Terms of Encapsulation? Ther. Deliv. 9 (4), 237–239. doi:10.4155/tde-2017-0119
Bitar, A., Zafar, N., Valour, J. P., Agusti, G., Fessi, H., Humbert, P., et al. (2015). Elaboration of Sponge-like Particles for Textile Functionalization and Skin Penetration. Colloid Polym. Sci. 293, 2967–2977. doi:10.1007/s00396-015-3704-7
Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 33 (9), 941–951. doi:10.1038/nbt.3330
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., and Corrie, S. R. (2016). Nanoparticle-based Medicines: a Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 33 (10), 2373–2387. doi:10.1007/s11095-016-1958-5
Bourrinet, P., Bengele, H. H., Bonnemain, B., Dencausse, A., Idee, J. M., Jacobs, P. M., et al. (2006). Preclinical Safety and Pharmacokinetic Profile of Ferumoxtran-10, Anultrasmall Superparamagnetic Iron Oxide Magnetic Resonance Contrast Agent. Investig. Radiol. 41 (3), 313–324. doi:10.1097/01.rli.0000197669.80475.dd
Brayden, D. J., and Alonso, M-J. (2016). Oral Delivery of Peptides: Opportunities and Issues for Translation. Adv. Drug Deliv. Rev. 106 (Part B), 193–195. doi:10.1016/j.addr.2016.10.005
Breyer, M. D., and Susztak, K. (2016). The Next Generation of Therapeutics for Chronic Kidney Disease. Nat. Rev. Drug Discov. 15 (8), 568–588. doi:10.1038/nrd.2016.67
Brinkkoetter, P. T., Ising, C., and Benzing, T. (2013). The Role of the Podocyte in Albumin Filtration. Nat. Rev. Nephrol. 9 (6), 328–336. doi:10.1038/nrneph.2013.78
Buffat, P., and Borel, J. P. (1976). Size Effect on the Melting Temperature of Gold Particles. Phys. Rev. A: Mol. Opt. Phys. 13, 2287−2298. doi:10.1103/PhysRevA.13.2287
Caban-Toktas, S., Sahin, A., Lule, S., Esendagli, G., and Vural, I. (2020). Combination of Paclitaxel and R-Flurbiprofen Loaded PLGA Nanoparticles Suppresses Glioblastoma Growth on Systemic Administration. Int. J. Pharm. 578, 119076. doi:10.1016/j.ijpharm.2020.119076
Campos, E. V. R., de Melo, N. F. S., de Paula, E., Rosa, A. H., and Fraceto, L. F. (2013). Screening of Conditions for the Preparation of Poly(-Caprolactone) Nanocapsules Containing the Local Anesthetic Articaine. J. Colloid Sci. Biotechnol. 2, 106–111. doi:10.1166/jcsb.2013.1040
Cao, X., Hou, D., Wang, L., Li, S., Sun, S., Ping, Q., et al. (2016). Effects and Molecularmechanism of Chitosan-Coated Levodopa Nanoliposomes on Behavior of Dyskinesia Rats. Biol. Res. 49 (1), 32. doi:10.1186/s40659-016-0093-4
Caputo, F., Clogston, J., Calzolai, L., Rosslein, M., and Prina-Mello, A. (2019). Measuring Particle Size Distribution of Nanoparticle Enabled Medicinal Products, the Joint View of EUNCL and NCI-NCL. A Step by Step Approach Combining Orthogonal Measurements with Increasing Complexity. J. Control Release 299, 31–43. doi:10.1016/j.jconrel.2019.02.030
Chandarana, M., Curtis, A., and Hoskins, C. (2018). The Use of Nanotechnology in Cardiovascular Disease. Appl. Nanosci. 8 (7), 1607–1619. doi:10.1007/s13204-018-0856-z
Chang, M. Y., Yang, Y. J., Chang, C. H., Tang, A. C., Liao, W. Y., Cheng, F. Y., et al. (2013). Functionalized Nanoparticles Provide Early Cardioprotection after Acute Myocardial Infarction. J. Control Release 170, 287–294. doi:10.1016/j.jconrel.2013.04.022
Chauhan, V. P., Popovic, Z., Chen, O., Cui, J., Fukumura, D., Bawendi, M. G., et al. (2011). Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-dependent Tumor Penetration. Angew. Chem. Int. Ed. 50 (48), 11417–11420. doi:10.1002/anie.201104449
Chen, Z., Peng, H., and Zhang, C. (2020). Advances in Kidney-Targeted Drug Delivery Systems. Int. J. Pharm. 587, 119679. doi:10.1016/j.ijpharm.2020.119679
Cheng, W., Nie, J., Xu, L., Liang, C., Peng, Y., Liu, G., et al. (2017). PH-sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopaminemodified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Inter. 9 (22), 18462–18473. doi:10.1021/acsami.7b02457
Chern, C-S. (2008). Principles and Applications of Emulsion Polymerization. John Wiley & Sons. doi:10.1002/9780470377949
Chin, D. D., Poon, C., Trac, N., Wang, J., Cook, J., Joo, J., et al. (2020). Collagenase-cleavable Peptide Amphiphile Micelles as a Novel Theranostic Strategy in Atherosclerosis. Adv. Ther. (Weinh) 3 (3), 1900196. doi:10.1002/adtp.201900196
Chitkara, D., Nikalaje, S. K., Mittal, A., Chand, M., and Kumar, N. (2012). Development of Quercetin Nanoformulation and In Vivo Evaluation Using Streptozotocin Induced Diabetic Rat Model. Drug Deliv. Transl. Res. 2 (2), 112–123. doi:10.1007/s13346-012-0063-5
Cho, S., Dong, S., Parent, K. N., and Chen, M. (2016). Immune-tolerant Elastin-like Polypeptides (iTEPs) and Their Application as CTL Vaccine Carriers. J. Drug Target. 24 (4), 328–339. doi:10.3109/1061186X.2015.1077847
Choi, C. H., Zuckerman, J. E., Webster, P., and Davis, M. E. (2011). Targeting Kidney Mesangium by Nanoparticles of Defined Size. Proc. Natl. Acad. Sci. U. S. A. 108 (16), 6656–6661. doi:10.1073/pnas.1103573108
Choi, D. G., Venkatesan, J., and Shim, M. S. (2019). Selective Anticancer Therapy Using Pro-oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles. Int. J. Mol. Sci. 20, 3220. doi:10.3390/ijms20133220
Christensen, E. I., Verroust, P. J., and Nielsen, R. (2009). Receptor-mediated Endocytosis in Renal Proximal Tubule. Pflugers Arch. 458 (6), 1039–1048. doi:10.1007/s00424-009-0685-8
Chu, L., Wang, A., Ni, L., Yan, X., Song, Y., Zhao, M., et al. (2018). Nose-to-brain Delivery of Temozolomide-Loaded PLGA Nanoparticles Functionalized with Anti-EPHA3 for Glioblastoma Targeting. Drug Deliv. 25 (1), 1634–1641. doi:10.1080/10717544.2018.1494226
Contera, S., de la Serna, J. B., and Tetley, T. D. (2020). Biotechnology, Nanotechnology and Medicine. Emerging Top. Life Sci. 4, 551–554. doi:10.1042/ETLS20200350
Crews, D. C., Bello, A. K., and Saadi, G. (2019). World Kidney Day Steering CommitteeBurden, Access and Disparities in Kidney Disease. J. Ren. Care 45 (1), 4–8. doi:10.1111/jorc.12269
Davis, R., and Mauer, L. J. (2010). Fourier Transform Infrared (FT-IR) Spectroscopy: a Rapid Tool for Detection and Analysis of Foodborne Pathogenic Bacteria. Curr. Res. Tech. Edu. Top. Appl. Microbiol. Microb. Biotechnol. 2, 1582–1594. 10.1.1.455.3195
De Cock, L. J., De Koker, S., De Geest, B. G., Grooten, J., Vervaet, C., Remon, J. P., et al. (2010). Polymeric Multilayer Capsules in Drug Delivery. Angew. Chem. Int. Ed. Engl. 49 (39), 6954–6973. doi:10.1002/anie.200906266
De Jong, W. H., Van Der Ven, L. T. M., Sleijffers, A., Part, M. V. D. Z., Jansen, E. H. J. M., Loveren, H. V., et al. (2013). Systemic and Immunotoxicity of Silver Nanoparticles in an Intravenous 28 Days Repeated Dose Toxicity Study in Rats. Biomaterials 34 (33), 8333–8343. doi:10.1016/j.biomaterials.2013.06.048
De Matteis, V., and Rinaldi, R. (2018). Toxicity Assessment in the Nanoparticle Era. Adv. Exp. Med. Biol. 1048, 1–19. doi:10.1007/978-3-319-72041-8_1
Demirdogen, B. C. (2019). Potential Role of Calcifying Nanoparticles in the Etiology of Multiple Sclerosis. Med. Hypotheses. 128, 25–27. doi:10.1016/j.mehy.2019.05.005
Destree, C., George, S., Champagne, B., Guillaume, M., Ghijsen, J. B., and Nagy, J. (2007). J-complexes of Retinol Formed within the Nanoparticles Prepared from Microemulsions. Colloid Polym. Sci. 286, 15–30. doi:10.1007/s00396-007-1679-8
Dolman, E., Harmsen, S., Pieters, E., Sparidans, R., Lacombe, M., Szokol, B., et al. (2012). Targeting of a Platinum-Bound Sunitinib Analog to Renal Proximal Tubular Cells. Int. J. Nanomed 7, 417–433. doi:10.2147/IJN.S26485
Dong, Z., Guo, J., Xing, X., Zhang, X., Du, Y., and Lu, Q. (2017). RGD Modified and PEGylated Lipid Nanoparticles Loaded with Puerarin: Formulation, Characterization and Protective Effects on Acute Myocardial Ischemia Model. Biomed. Pharmacother. 89, 297–304. doi:10.1016/j.biopha.2017.02.029
D’Souza, S. (20142014). A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm., 304757. doi:10.1155/2014/304757
Du, L., Jiang, H., Liu, X., and Wang, E. (2007). Biosynthesis of Gold Nanoparticles Assisted by Escherichia coli DH5 Alpha and its Application on Direct Electrochemistry of Hemoglobin. Electrochem. Commun. 9 (5), 1165–1170. doi:10.1016/j.elecom.2007.01.007
Duan, T., Xu, Z., Sun, F., Wang, Y., and Zhang, J. (2019). HPA Aptamer Functionalized Paclitaxel-Loaded PLGA Nanoparticles for Enhanced Anticancer Therapy through Targeted Effects and Microenvironment Modulation. Biomed. Pharmacother. 117, 109121. doi:10.1016/j.biopha.2019.109121
Dudhipala, N., and Veerabrahma, K. (2016). Candesartan Cilexetil Loaded Solid Lipid Nanoparticles for Oral Delivery: Characterization, Pharmacokinetic and Pharmacodynamic Evaluation. Drug Deliv. 23 (2), 395–404. doi:10.3109/10717544.2014.914986
Eckardt, K. U., Berns, J. S., Rocco, M. V., and Kasiske, B. L. (2009). Definition and Classification of CKD: the Debate Should Be about Patient Prognosis—A Position Statement from KDOQI and KDIGO. Am. J. Kidney Dis. 53, 915–920. doi:10.1053/j.ajkd.2009.04.001
El-Ansary, A., Al-Daihan, S., Bacha, A. B., and Kotb, M. (2013). Toxicity of Novel Nanosized Formulations Used in Medicine. Methods Mol. Biol. 1028, 47–74. doi:10.1007/978-1-62703-475-3_4
Elbialy, N. S., Fathy, M. M., and Khalil, W. M. (2015). Doxorubicin Loaded Magnetic Gold Nanoparticles for In Vivo Targeted Drug Delivery. Int. J. Pharm. 490, 190–199. doi:10.1016/j.ijpharm.2015.05.032
Elsewedy, H. S., Al Dhubiab, B. E., Mahdy, M. A., and Elnahas, H. M. (2020). Development, Optimization, and Evaluation of PEGylated Brucine-Loaded PLGA Nanoparticles. Drug Deliv. 27 (1), 1134–1146. doi:10.1080/10717544.2020.1797237
Falke, L. L., Gholizadeh, S., Goldschmeding, R., Kok, R. J., and Nguyen, T. Q. (2015). Diverse Origins of the Myofibroblast-Implications for Kidney Fibrosis. Nat. Rev. Nephrol. 11 (4), 233–244. doi:10.1038/nrneph.2014.246
Fan, Y., Marioli, M., and Zhang, K. (2021). Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 192, 113642. doi:10.1016/j.jpba.2020.113642
Fatouros, D. G., Klepetsanis, P., Ioannou, P. V., and Antimisiaris, S. G. (2005). The Effect of pH on the Electrophoretic Behaviour of a New Class of Liposomes: Arsonoliposomes. Int. J. Pharm. 288 (1), 151–156. doi:10.1016/j.ijpharm.2004.09.016
Filipe, V., Hawe, A., and Jiskoot, W. (2010). Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 27 (5), 796–810. doi:10.1007/s11095-010-0073-2
Fleischmann, D., Harloff, M., Figueroa, S. M., Schlossmann, J., and Goepferich, A. (2021). Targeted Delivery of Soluble Guanylate Cyclase (sGC) Activator Cinaciguat to Renal Mesangial Cells via Virus-Mimetic Nanoparticles Potentiates Anti-fibrotic Effects by cGMP-Mediated Suppression of the TGF-β Pathway. Int. J. Mol. Sci. 22, 2557. doi:10.3390/ijms22052557
Friedman, A. D., Claypool, S. E., and Liu, R. (2013). The Smart Targeting of Nanoparticles. Curr. Pharm. Des. 19 (35), 6315–6329. doi:10.2174/13816128113199990375
Gagliardini, E., Conti, S., Benigni, A., Remuzzi, G., and Remuzzi, A. (2010). Imaging of the Porous Ultrastructure of the Glomerular Epithelial Filtration Slit. J. Am. Soc. Nephrol. 21 (12), 2081–2089. doi:10.1681/ASN.2010020199
Gao, S., Hein, S., Dagnaes-Hansen, F., Weyer, K., Yang, C., Nielsen, R., et al. (2014). Megalin-mediated Specific Uptake of chitosan/siRNA Nanoparticles in Mouse Kidney Proximal Tubule Epithelial Cells Enables AQP1 Gene Silencing. Theranostics 4 (10), 1039–1051. doi:10.7150/thno.7866
Gao, Y., Gu, W., Chen, L., Xu, Z., and Li, Y. (2008). The Role of Daidzein-Loaded Sterically Stabilized Solid Lipid Nanoparticles in Therapy for Cardio-Cerebrovascular Diseases. Biomaterials 29, 4129–4136. doi:10.1016/j.biomaterials.2008.07.008
Geng, Q., Sun, X., Gong, T., and Zhang, Z. R. (2012). Peptide-drug Conjugate Linked via a Disulfide Bond for Kidney Targeted Drug Delivery. Bioconjug. Chem. 23 (6), 1200–1210. doi:10.1021/bc300020f
Gentile, F., Chiappini, C., Fine, D., Bhavane, R. C., Peluccio, M. S., Cheng, M. M., et al. (2008). The Effect of Shape on the Margination Dynamics of Nonneutrally Buoyant Particles in Two-Dimensional Shear Flows. J. Biomech. 41 (10), 2312–2318. doi:10.1016/j.jbiomech.2008.03.021
Gotov, O., Battogtokh, G., and Ko, Y. T. (2018). Docetaxel-loaded Hyaluronic acid−Cathepsin B-Cleavable-Peptide−gold Nanoparticles for the Treatment of Cancer. Mol. Pharm. 15, 4668–4676. doi:10.1021/acs.molpharmaceut.8b00640
Gotthardt, M., van Eerd-Vismale, J., Oyen, W. J., de Jong, M., Zhang, H., Rolleman, E., et al. (2007). Indication for Different Mechanisms of Kidney Uptake of Radiolabeled Peptides. J. Nucl. Med. 48 (4), 596–601. doi:10.2967/jnumed.106.036020
Gouda, W., Hafiz, N. A., Mageed, L., Alazzouni, A. S., Khalil, W. K. B., Afify, M., et al. (2019). Effects of Nano-Curcumin on Gene Expression of Insulin and Insulin Receptor. Bull. Nat. Res. Centre 43, 128. doi:10.1186/s42269-019-0164-0
Grantham, J. J. (1990). Polycystic Kidney Disease: Neoplasia in Disguise. Am. J. Kidney Dis. 15, 110–116. doi:10.1016/s0272-6386(12)80507-5
Grunberg, J., Novak-Hofer, I., Honer, M., Zimmermann, K., Knogler, K., Blauenstein, P., et al. (2005). In Vivo evaluation of 177Lu- and 67/64Cu-Labeled Recombinant Fragments of Antibody chCE7 for Radioimmunotherapy and PET Imaging of L1-CAM-Positive Tumors. Clin. Cancer Res. 11, 5112–5120. doi:10.1158/1078-0432.CCR-05-0227
Guo, L., Luo, S., Du, Z., Zhou, M., Li, P., Fu, Y., et al. (2017). Targeted Delivery of Celastrol to Mesangial Cells Is Effective against Mesangioproliferative Glomerulonephritis. Nat. Commun. 8 (1), 878. doi:10.1038/s41467-017-00834-8
Han, S., Li, M., Liu, X., Gao, H., and Wu, Y. (2013). Construction of Amphiphilic Copolymer Nanoparticles Based on Gelatin as Drug Carriers for Doxorubicin Delivery. Colloids Surf. B Biointerfaces 102, 833–841. doi:10.1016/j.colsurfb.2012.09.010
Hanafy, A. S., Farid, R. M., Helmy, M. W., and ElGamal, S. S. (2016). Pharmacological, Toxicological and Neuronal Localization Assessment of Galantamine/chitosan Complex Nanoparticles in Rats: Future Potential Contribution in Alzheimer’s Disease Management. Drug Deliv. 23 (8), 3111–3122. doi:10.3109/10717544.2016.1153748
Hang, T-C., Lauffenburger, D. A., Griffith, L. G., and Stolz, D. B. (2012). Lipids Promote Survival, Proliferation, and Maintenance of Differentiation of Rat Liver Sinusoidal Endothelial Cells. Vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 302 (3), G375–G388. doi:10.1152/ajpgi.00288.2011
Hauger, O., Delalande, C., Deminiere, C., Fouqueray, B., Ohayon, C., Garcia, S., et al. (2000). Nephrotoxic Nephritis and Obstructive Nephropathy: Evaluation with MR Imaging Enhanced with Ultrasmall Superparamagnetic Iron Oxide-Preliminary Findings in a Rat Model. Radiology 217 (3), 819–826. doi:10.1148/radiology.217.3.r00dc04819
Hauser, P. H., Chang, H-M., Yanagawa, N., and Hamon, M. (2021). Nanotechnology, Nanomedicine, and the Kidney. Appl. Sci. 11, 7187. doi:10.3390/app11167187
Hazra, C., Kundu, D., Chaudhari, A., and Jana, T. (2013). Biogenic Synthesis: Characterization, Toxicity and Photocatalysis of Zinc Sulfide Nanoparticles Using Rhamnolipids from Pseudomonas aeruginosa BS01 as Capping and Stabilizing Agent. J. Chem. Technol. Biotechnol. 88 (6), 1039–1048. doi:10.1002/jctb.3934
He, S., Zhang, Y., Guo, Z., and Gu, N. (2008). Biological Synthesis of Gold Nanowires Using Extract of. Rhodopseudomonas Capsulata. Biotechnol. Prog. 24 (2), 476–480. doi:10.1021/bp0703174
He, X. K., Yuan, Z. X., Wu, X. J., Xu, C. Q., and Li, W. Y. (2012). Low Molecular Weight Hydroxyethyl Chitosan-Prednisolone Conjugate for Renal Targeting Therapy: Synthesis, Characterization and In Vivo Studies. Theranostics 2 (11), 1054–1063. doi:10.7150/thno.3705
Honary, S., and Zahir, F. (2013). Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems - a Review (Part 2). Trop. J. Pharm. Res. 12 (2), 265–273. doi:10.4314/tjpr.v12i2.20
Hoshyar, N., Gray, S., Han, H., and Bao, G. (2016). The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine (Lond). 11 (6), 673–692. doi:10.2217/nnm.16.5
Hossen, S., Hossain, M. K., Basher, M. K., Mia, M. N. H., Rahman, M. T., and Uddin, M. J. (2019). Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 15, 1–18. doi:10.1016/j.jare.2018.06.005
Hu, H., and Steinmetz, N. F. (2020). Cisplatin Prodrug-Loaded Nanoparticles Based on Physalis Mottle Virus for Cancer Therapy. Mol. Pharm. 17 (12), 4629–4636. doi:10.1021/acs.molpharmaceut.0c00834
Hu, J., Huang, S., Zhu, L., Huang, W., Zhao, Y., and Jin, K. (2018). Tissue Plasminogen Activator-Porous Magnetic Microrods for Targeted Thrombolytic Therapy after Ischemic Stroke. ACS Appl. Mater. Inter. 10, 32988–32997. doi:10.1021/acsami.8b09423
Huang, J., and Gretz, N. (2017). Light-emitting Agents for Noninvasive Assessment of Kidneyfunction. ChemistryOpen 6 (4), 456–471. doi:10.1002/open.201700065
Huang, X., Zhang, F., Zhu, L., Choi, K. Y., Guo, N., Guo, J., et al. (2013). Effect of Injection Routes on the Biodistribution, Clearance, and Tumor Uptake of Carbon Dots. ACSNano 7 (7), 5684–5693. doi:10.1021/nn401911k
Huang, Y., Wang, J., Jiang, K., and Chung, E. J. (2021). Improving Kidney Targeting: The Influence of Nanoparticle Physicochemical Properties on Kidney Interactions. J. Control Release 334, 127–137. doi:10.1016/j.jconrel.2021.04.016
Huynh, K. A., McCaffery, J. M., and Chen, K. L. (2012). Heteroaggregation of Multiwalled Carbon Nanotubes and Hematite Nanoparticles: Rates and Mechanisms. Environ. Sci. Technol. 46, 5912–5920. doi:10.1021/es2047206
Ibrahim, W. N., Rosli, L. M. B. M., and Doolaanea, A. A. (2020). Formulation, Cellular Uptake and Cytotoxicity of Thymoquinone-Loaded PLGA Nanoparticles in Malignant Melanoma Cancer Cells. Int. J. Nanomedicine 15, 8059–8074. doi:10.2147/IJN.S269340
Ichimura, K., Stan, R. V., Kurihara, H., and Sakai, T. (2008). Glomerular Endothelial Cells Form Diaphragms during Development and Pathologic Conditions. J. Am. Soc. Nephrol. 19 (8), 1463–1471. doi:10.1681/ASN.2007101138
Ikeda, G., Matoba, T., Nakano, Y., Nagaoka, K., Ishikita, A., Nakano, K., et al. (2016). Nanoparticle-mediated Targeting of Cyclosporine a Enhances Cardioprotection against Ischemia-Reperfusion Injury through Inhibition of Mitochondrial Permeability Transition Pore Opening. Sci. Rep. 6, 20467. doi:10.1038/srep20467
Jenjob, R., Phakkeeree, T., Seidi, F., Theerasilp, M., and Crespy, D. (2019). Emulsion Techniques for the Production of Pharmacological Nanoparticles. Macromol. Biosci. 19 (6), e1900063. doi:10.1002/mabi.201900063
Jenjob, R., Seidi, F., and Crespy, D. (2018). Recent Advances in Polymerizations in Dispersed media. Adv. Colloid Interf. Sci. 260, 24–31. doi:10.1016/j.cis.2018.08.002
Kaissling, B., Hegyi, I., Loffing, J., and Hir, M. (1996). Morphology of Interstitial Cells in the Healthy Kidney. Anat. Embryol. 193, 303–318. doi:10.1007/BF00186688
Kalathil, S., Lee, J., and Cho, M. H. (2011). Electrochemically Active Biofilm-Mediated Synthesis of Silver Nanoparticles in Water. Green. Chem. 13 (6), 1482–1485. doi:10.1039/C1GC15309A
Kamada, H., Tsutsumi, Y., Sato-Kamada, K., Yamamoto, Y., Yoshioka, Y., and Okamoto, T. (2003). Synthesis of a Poly(vinylpyrrolidone-Codimethyl Maleic Anhydride) Co-polymer and its Application for Renal Drug Targeting. Nat. Biotechnol. 21 (4), 399–404. doi:10.1038/nbt798
Kamaly, N., Fredman, G., Subramanian, M., Gadde, S., Pesic, A., Cheung, L., et al. (2013). Development and In Vivo Efficacy of Targeted Polymeric Inflammation-Resolving Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 110 (16), 6506–6511. doi:10.1073/pnas.1303377110
Kamaly, N., He, J. C., Ausiello, D. A., and Farokhzad, O. C. (2016). Nanomedicines for Renal Disease: Current Status and Future Applications. Nat. Rev. Nephrol. 12 (12), 738–753. doi:10.1038/nrneph.2016.156
Kang, F., Alvarez, P. J., and Zhu, D. (2014). Microbial Extracellular Polymeric Substances Reduce Ag+ to Silver Nanoparticles and Antagonize Bactericidal Activity. Environ. Sci. Technol. 8 (1), 316–322. doi:10.1021/es403796x
Khalil, N. M., Nascimento, T. C. F., do Casa, D. M., Dalmolin, L. F., Mattos, A. C., de Hoss, I., et al. (2013). Pharmacokinetics of Curcumin-Loaded PLGA and PLGA–PEG Blend Nanoparticles after Oral Administration in Rats. Colloids Surf. B Biointerfaces 101, 353–360. doi:10.1016/j.colsurfb.2012.06.024
Khosroshahi, H. T., Abedi, B., Daneshvar, S., Sarbaz, Y., and Bavil, S. (2017). Future Ofthe Renal Biopsy: Time to Change the Conventional Modality Using Nanotechnology. Int. J. Biomed. Imaging 6141734. doi:10.1155/2017/6141734
Kodaira, H., Tsutsumi, Y., Yoshioka, Y., Kamada, H., Kaneda, Y., Yamamoto, Y., et al. (2004). The Targeting of Anionized Polyvinylpyrrolidone to the Renal System. Biomaterials 25 (18), 4309–4315. doi:10.1016/j.biomaterials.2003.10.097
Kou, C-H., Han, J., Han, X-L., Zhuang, H-J., and Zhao, Z-M. (2017). Preparation and Characterization of the Adriamycin-Loaded Amphiphilic Chitosan Nanoparticles and Their Application in the Treatment of Liver Cancer. Oncol. Lett. 17, 7833–7841. doi:10.3892/ol.2017.7210
Kozuka, C., Shimizu-Okabe, C., Takayama, C., Nakano, K., Morinaga, H., Kinjo, A., et al. (2017). Marked Augmentation of PLGA Nanoparticle-Induced Metabolically Beneficial Impact of Gamma-Oryzanol on Fuel Dyshomeostasis in Genetically Obese-Diabetic Ob/ob Mice. Drug Deliv. 24 (1), 558–568. doi:10.1080/10717544.2017.1279237
Kratz, F., Müller, I., Ryppa, C., and Warnecke, A. (2008). Prodrug Strategies in Anticancer Chemotherapy. ChemMedChem 3, 20–53. doi:10.1002/cmdc.200700159
Krediet, R. T. (2017). Preservation of Residual Kidney Function and Urine Volume in Patients on Dialysis. Clin. J. Am. Soc. Nephrol. 12 (3), 377–379. doi:10.2215/CJN.00330117
Kumar, V., Hebbar, S., Bhat, A., Panwar, S., Vaishnav, M., Muniraj, K., et al. (2018). Application of a Nanotechnology-Based, point-of-care Diagnostic Device in Diabetic Kidney Disease. Kidney Int. Rep. 3 (5), 1110–1118. doi:10.1016/j.ekir.2018.05.008
Kurozuka, A., Onishi, S., Nagano, T., Yamaguchi, K., Suzuki, T., and Minami, H. (2017). Emulsion Polymerization with a Biosurfactant. Langmuir 33 (23), 5814–5818. doi:10.1021/acs.langmuir.7b00851
Lacerda, L., Herrero, M. A., Venner, K., Bianco, A., Prato, M., and Kostarelos, K. (2008). Carbonnanotube Shape and Individualization Critical for Renal Excretion. Small 4 (8), 1130–1132. doi:10.1002/smll.200800323
Lal, M. A., Young, K. W., and Andag, U. (2015). Targeting the Podocyte to Treat Glomerular Kidney Disease. Drug Discov. Today 20 (10), 1228–1234. doi:10.1016/j.drudis.2015.06.003
Larraneta, E., Mccrudden, M. T., Courtenay, A. J., and Donnelly, R. F. (2016). Microneedles: a New Frontier in Nanomedicine Delivery. Pharm. Res. 33 (5), 1055–1073. doi:10.1007/s11095-016-1885-5
Lee, S-Y., and Shieh, M-J. (2020). Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Inter. 12 (4), 4254–4264. doi:10.1021/acsami.9b18855
Levey, A. S., and Coresh, J. (2012). Chronic Kidney Disease. Lancet 379, 165–180. doi:10.1016/S0140-6736(11)60178-5
Li, F., Xu, Y., Li, X., Wang, X., Yang, Z., Li, W., et al. (2021). Triblock Copolymer Nanomicelles Loaded with Curcumin Attenuates Inflammation via Inhibiting the NF-Κb Pathway in the Rat Model of Cerebral Ischemia. Int. J. Nanomedicine 16, 3173–3183. doi:10.2147/IJN.S300379
Li, L. X., Agborbesong, E., Zhang, L., and Li, X. (2019). Investigation of Epigenetics in Kidney Cell Biology. Methods Cel Biol 153, 255–277. doi:10.1016/bs.mcb.2019.04.015
Li, S. D., and Huang, L. (2008). Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 5 (4), 496–504. doi:10.1021/mp800049w
Liang, Z., Gong, T., Sun, X., Tang, J. Z., and Zhang, Z. (2012). Chitosan Oligomers as Drug Carriers for Renal Delivery of Zidovudine. Carbohydr. Polym. 87 (3), 2284–2290. doi:10.1016/j.carpol.2011.10.06010.1016/j.carbpol.2011.10.060
Liu, G. W., Prossnitz, A. N., Eng, D. G., Cheng, Y., Subrahmanyam, N., Pippin, J. W., et al. (2018). Glomerular Disease Augments Kidney Accumulation of Synthetic Anionic Polymers. Biomaterials 178, 317–325. doi:10.1016/j.biomaterials.2018.06.001
Liu, J., Yu, M., Ning, X., Zhou, C., Yang, S., and Zheng, J. (2013). PEGylation and Zwitterionization: Pros and Cons in the Renal Clearance and Tumor Targeting of Near-IR-Emitting Gold Nanoparticles. Angew. Chem. Int. Ed. Engl. 52 (48), 12572–12576. doi:10.1002/anie.201304465
Liu, J., Yu, M., Zhou, C., and Zheng, J. (2013). Renal Clearable Inorganic Nanoparticles: a New Frontier of Bionanotechnology. Mat.Today. 16 (12), 477–486. doi:10.1016/j.mattod.2013.11.003
Lopes, P., Costa-Rama, E., Beirao, I., Nouws, H. P. A., Santos-Silva, A., and Delerue-Matos, C. (2019). Disposable Electrochemical Immunosensor for Analysis of Cystatin C, a CKD Biomarker. Talanta 201, 211–216. doi:10.1016/j.talanta.2019.04.006
Lopez-Lopez, J. M., Schmitt, A., Moncho-Jorda, A., and Hidalgo-lvarez, R. (2006). Stability of Binary Colloids: Kinetic and Structural Aspects of Heteroaggregation Processes. Soft Matter 2, 1025–1042. doi:10.1039/b608349h
Lu, W., Xie, Z., Tang, Y., Bai, L., Yao, Y., Fu, C., et al. (2015). Photoluminescent Mesoporous Silicon Nanoparticles with siCCR2 Improve the Effects of Mesenchymal Stromal Cell Transplantation after Acute Myocardial Infarction. Theranostics 5, 1068–1082. doi:10.7150/thno.11517
Luo, S., and Grams, M. E. (2020). Epidemiology Research to foster Improvement in Chronic Kidney Disease Care. Kidney Int. 97, 477–486. doi:10.1016/j.kint.2019.11.010
Ma, Y., Cai, F., Li, Y., Chen, J., Han, F., and Lin, W. (2020). A Review of the Application of Nanoparticles in the Diagnosis and Treatment of Chronic Kidney Disease. Bioact. Mater. 5, 732–743. doi:10.1016/j.bioactmat.2020.05.002
Mallipattu, S., and He, J. (2016). Podocyte as a Direct Target for Treatment of Glomerular Disease? Am. J. Physiol. Ren. Physiol. 311 (1), F46–F51. doi:10.1152/ajprenal.00184.2016
Mandal, A., Bisht, R., Rupenthal, I. D., and Mitra, A. K. (2017). Polymeric Micelles for Ocular Drug Delivery: From Structural Frameworks to Recent Preclinical Studies. J. Control. Rel. 248 (248), 96–116. doi:10.1016/j.jconrel.2017.01.012
Mao, Y., Koga, J. I., Tokutome, M., Matoba, T., Ikeda, G., Nakano, K., et al. (2017). Nanoparticle-mediated Delivery of Pitavastatin to Monocytes/macrophages Inhibits Left Ventricular Remodeling after Acute Myocardial Infarction by Inhibiting Monocyte-Mediated Inflammation. Int. Heart J. 58, 615–623. doi:10.1536/ihj.16-457
Mathias, C., Hubers, D., Low, P., and Green, M. (2000). Synthesis of [99m Tc]DTPA-Folate and its Evaluation as a Folate-Receptor-Targeted Radiopharmaceutical. Bioconjug. Chem. 11, 253–257. doi:10.1021/bc9901447
Matos, C., de Castro, B., Gameiro, P., Lima, J. L., and Reis, S. (2004). Zeta-potential Measurements as a Tool to Quantify the Effect of Charged Drugs on the Surface Potential of Egg Phosphatidylcholine Liposomes. Langmuir 20 (2), 369–377. doi:10.1021/la034780b
Matsumura, H., Mori, F., Kawahara, K., Obata, C., and Furusawa, K. (1994). Effect of Amino-Acids, Polypeptides and Proteins on Electrophoretic Mobilities of Phospholipid Liposomes. Colloids Surf. 92, 87–93. doi:10.1016/0927-7757(94)02785-4
McDevitt, M. R., Chattopadhyay, D., Jaggi, J. S., Finn, R. D., Zanzonico, P. B., Villa, C., et al. (2007). PET Imaging of Soluble Yttrium-86-Labeled Carbon Nanotubes in Mice. PLoS ONE 2 (9), e907. doi:10.1371/journal.pone.0000907
McNay, G., Eustace, D., Smith, W. E., Faulds, K., and Graham, D. (2011). Surface-enhanced Raman Scattering (SERS) and Surface-Enhanced Resonance Raman Scattering (SERRS): a Review of Applications. Appl. Spectrosc. 65 (8), 825–837. doi:10.1366/11-06365
Md, S., Ali, M., Baboota, S., Sahni, J. K., Bhatnagar, A., and Ali, J. (2014). Preparation, Characterization, In Vivo Biodistribution and Pharmacokinetic Studies of Donepezil-Loaded PLGA Nanoparticles for Brain Targeting. Drug Dev. Ind. Pharm. 40 (2), 278–287. doi:10.3109/03639045.2012.758130
Merlin, J. P. J., Prasad, N. R., Shibli, S. M. A., and Sebeela, M. (2012). Ferulic Acid Loaded Poly-D,l-Lactide-Co-Glycolide Nanoparticles: Systematic Study of Particle Size, Drug Encapsulation Efficiency and Anticancer Effect in Non-small Cell Lung Carcinoma Cell Line In Vitro. Biomed. Prev. Nutr. 2, 69–76. doi:10.1016/j.bionut.2011.12.007
Merlin, J. P. J., Venkadesh, B., Rajan, S. S., and Subramanian, P. (2017). Multidrug Resistance for Cancer Treatment: Delivery of Ursolic Acid and Caffeine by Poly ([actic-Co-Glycolic Acid) Nanoparticles. J. Can. Sci. Res. 3, 10. doi:10.4172/2576-1447.1000S2-010
Miller, S. A., Dykes, D. D., and Polesky, H. F. (1988). A Simple Salting Out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 16, 1215. doi:10.1093/nar/16.3.1215
Mirshafiee, V., Kim, R., Park, S., Mahmoudi, M., and Kraft, M. L. (2016). Impact of Protein Pre-coating on the Protein corona Composition and Nanoparticle Cellular Uptake. Biomaterials 75, 295–304. doi:10.1016/j.biomaterials.2015.10.019
Mishra, S. B., Malaviya, J., and Mukerjee, A. (2015). Attenuation of Oxidative Stress and Glucose Toxicity by Lutein Loaded Nanoparticles from Spinacia Oleracea Leaves. J. Pharm. Sci. Pharmacol. 2 (3), 242–249. doi:10.1166/jpsp.2015.1067
Mittal, G., Carswell, H., Brett, R., Currie, S., and Kumar, M. N. V. R. (2011). Development and Evaluation of Polymer Nanoparticles for Oral Delivery of Estradiol Torat Brain in a Model of Alzheimer's Pathology. J. Control Release 150, 220–228. doi:10.1016/j.jconrel.2010.11.013
Mochizuki, T., Wu, G., Hayashi, T., Veldhuisen, B., Saris, J. J., Reynolds, D. M., et al. (1996). PKD2, a Gene for Polycystic Kidney Disease that Encodes an Integral Membrane Protein. Science 272 (5266), 1339–1342. doi:10.1126/science.272.5266.1339
Moghimi, S. M., Hunter, A. C., and Andresen, T. L. (2012). Factors Controlling Nanoparticle Pharmacokinetics: an Integrated Analysis and Perspective. Annu. Rev. Pharmacol. Toxicol. 52, 481–503. doi:10.1146/annurev-pharmtox-010611-134623
Mohammed, A. R., Weston, N., Coombes, A. G., Fitzgerald, M., and Perrie, Y. (2004). Liposome Formulation of Poorly Water Soluble Drugs: Optimization of Drug Loading and ESEM Analysis of Stability. Int. J. Pharm. 285, 23–34. doi:10.1016/j.ijpharm.2004.07.010
Mohseni, R., ArabSadeghabadi, Z., Ziamajidi, N., Abbasalipourkabir, R., and RezaeiFarimani, A. (2019). Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes. Nanoscale Res. Lett. 14 (1), 227. doi:10.1186/s11671-019-3042-7
Mosier-Boss, P. A. (2017). Review of SERS Substrates for Chemical Sensing. Nanomaterials (Basel) 7 (6), 142. doi:10.3390/nano7060142
Moss, D. M., and Siccardi, M. (2014). Optimizing Nanomedicine Pharmacokinetics Using Physiologically Based Pharmacokinetics Modelling. Br. J. Pharm. 171, 3963–3979. doi:10.1111/bph.12604
Mozafari, M. (2018). Nanotechnology in Wound Care: One Step Closer to the Clinic. Mol. Ther. 26 (9), 2085–2086. doi:10.1016/j.ymthe.2018.08.008
Muhamad, N., Plengsuriyakarn, T., Chittasupho, C., and Na-Bangchang, K. (2020). The Potential of Atractylodin-Loaded PLGA Nanoparticles as Chemotherapeutic for Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 21 (4), 935–941. doi:10.31557/APJCP.2020.21.4.935
Mukhopadhyay, P., Maity, S., Mandal, S., Chakraborti, A. S., Prajapati, A. K., and Kundu, P. P. (2018). Preparation, Characterization and In Vivo Evaluation of pH Sensitive, Safe Quercetin-Succinylated Chitosan-Alginate Core-Shell-corona Nanoparticle for Diabetes Treatment. Carbohydr. Polym. 182, 42–51. doi:10.1016/j.carbpol.2017.10.098
Nagaoka, K., Matoba, T., Mao, Y., Nakano, Y., Ikeda, G., Egusa, S., et al. (2015). A New Therapeutic Modality for Acute Myocardial Infarction: Nanoparticle Mediated Delivery of Pitavastatin Induces Cardioprotection from Ischemia Reperfusion Injury via Activation of PI3K/Akt Pathway and Anti-inflammation in a Rat Model. PLoS One 10, e0132451. doi:10.1371/journal.pone.0132451
Nair, A. V., Keliher, E. J., Core, A. B., Brown, D., and Weissleder, R. (2015). Characterizing the Interactions of Organic Nanoparticles with Renal Epithelial Cells In Vivo. ACS Nano 9 (4), 3641–3653. doi:10.1021/acsnano.5b00428
Nakano, Y., Matoba, T., Tokutome, M., Funamoto, D., Katsuki, S., Ikeda, G., et al. (2016). Nanoparticle-mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte Mediated Inflammation. Sci. Rep. 6, 29601. doi:10.1038/srep29601
Nastase, M. V., Zeng-Brouwers, J., Wygrecka, M., and Schaefer, L. (2018). Targeting Renal Fibrosis: Mechanisms and Drug Delivery Systems. Adv. Drug Deliv. Rev. 129, 295–307. doi:10.1016/j.addr.2017.12.019
Negro, S., Boeva, L., Slowing, K., Fernandez-Carballido, A., Garcia-Garcia, L., and Barcia, E. (2017). Efficacy of Ropinirole-Loaded PLGA Microspheres for the Reversion Ofrotenone- Induced Parkinsonism. Curr. Pharm. Des. 23 (23), 3423–3431. doi:10.2174/1381612822666160928145346
Nico, B., Crivellato, E., and Ribatti, D. (2009). The Importance of Electron Microscopy in the Study of Capillary Endothelial Cells: an Historical Review. Endothelium 14 (6), 257–264. doi:10.1080/10623320701746289
Occhiutto, M. L., Maranhao, R. C., Costa, V. P., and Konstas, A. G. (2020). Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 37, 155–199. doi:10.1007/s12325-019-01163-6
Oduk, Y., Zhu, W., Kannappan, R., Zhao, M., Borovjagin, A. V., Oparil, S., et al. (2018). VEGF Nanoparticles Repair the Heart after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 314, H278–H284. doi:10.1152/ajpheart.00471.2017
Orbay, H., Li, Y., Xiao, W., Cherry, S. R., Lam, K., and Sahar, D. E. (2016). Developing a Nanoparticle-Delivered High-Efficacy Treatment for Infantile Hemangiomas Using a Mouse Hemangioendothelioma Model. Plast. Reconstr. Surg. 138 (2), 410–417. doi:10.1097/PRS.0000000000002403
Oroojalian, F., Rezayan, A. H., Mehrnejad, F., Nia, A. H., Shier, W. T., and Abnous, K. (2017). Efficient Megalin Targeted Delivery to Renal Proximal Tubular Cells Mediated Bymodified-Polymyxin B-Polyethylenimine Based Nano-Gene-Carriers. Mater. Sci. Eng. C. 79, 770–782. doi:10.1016/j.msec.2017.05.068
Ozdemir, S., Celik, B., and Under, M. (2019). Properties and Therapeutic Potential of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Promising Colloidal Drug Delivery Systems. Biomed. Mater. Eng. 457–505. doi:10.1016/B978-0-12-816913-1.00015-5
Pandey, P., Dua, K., and Dureja, H. (2019). Erlotinib Loaded Chitosan Nanoparticles: Formulation, Physicochemical Characterization and Cytotoxic Potential. Int. J. Biol. Macromol. 139, 1304–1316. doi:10.1016/j.ijbiomac.2019.08.084
Pandya, A. D., Overbye, A., Sahariah, P., Gaware, V. S., Hogset, H., Masson, M., et al. (2020). Drug-loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 21, 1489–1498. doi:10.1021/acs.biomac.0c00061
Panwar, R., Raghuwanshi, N., Srivastava, A. K., Sharma, A. K., and Pruthi, V. (2018). In-vivo Sustained Release of Nanoencapsulated Ferulic Acid and its Impact in Induced Diabetes. Mater. Sci. Eng. C Mater. Biol. Appl. 92, 381–392. doi:10.1016/j.msec.2018.06.055
Park, K. (2017). The Drug Delivery Field at the Inflection point: Time to Fight its Way Out of the Egg. J. Control Release 267 (Suppl. C), 2–14. doi:10.1016/j.jconrel.2017.07.030
Park, T., Lee, S., Amatya, R., Cheong, H., Moon, C., Kwak, H. D., et al. (2020). ICG-loaded PEGylated BSA-Silver Nanoparticles for Effective Photothermal Cancer Therapy. Int. J. Nanomedicine 15, 5459–5471. doi:10.2147/IJN.S255874
Pat-Espadas, A. M., Razo-Flores, E., Rangel-Mendez, J. R., and Cervantes, F. J. (2014). Direct and Quinone-Mediated Palladium Reduction by Geobacter Sulfurreducens: Mechanisms and Modeling. Environ. Sci. Technol. 48 (5), 2910–2919. doi:10.1021/es403968e
Patil, P., Harak, K., and Saudagar, R. (2019). The Solid Lipid Nanoparticles A Review. J. Drug Deliv. Ther. 9 (3), 525–530. doi:10.22270/jddt.v9i5.3560
Paulis, L. E., Geelen, T., Kuhlmann, M. T., Coolen, B. F., Schafers, M., Nicolay, K., et al. (2012). Distribution of Lipid-Based Nanoparticles to Infarcted Myocardium with Potential Application for MRI-Monitored Drug Delivery. J. Control Release 162, 276–285. doi:10.1016/j.jconrel.2012.06.035
Pavenstadt, H., Kriz, W., and Kretzler, M. (2003). Cell Biology of the Glomerular Podocyte. Physiol. Rev. 83, 253–307. doi:10.1152/physrev.00020.2002
Penna, F. J., Chow, J. S., Minnillo, B. J., Passerotti, C. C., Barnewolt, C. E., Treves, S. T., et al. (2011). Identifying Ureteropelvic junction Obstruction by Fluorescence Imaging: a Comparative Study of Imaging Modalities to Assess Renal Function and Degree of Obstruction in a Mouse Model. J. Urol. 185 (6), 2405–2413. doi:10.1016/j.juro.2011.02.015
Pereverzeva, E., Treschalin, I., Treschalin, M., and Arantseva, D. (2019). Toxicological Study of Doxorubicin-Loaded PLGA Nanoparticles for the Treatment of Glioblastoma. Int. J. Pharm. 554, 161–178. doi:10.1016/j.ijpharm.2018.11.014
Petro, M., Jaffer, H., Yang, J., Kabu, S., Morris, V. B., and Labhasetwar, V. (2016). Tissueplasminogen Activator Followed by Antioxidant-Loaded Nanoparticle Delivery Promotes Activation/mobilization of Progenitor Cells in Infarcted Rat Brain. Biomaterials 81, 169–180. doi:10.1016/j.biomaterials.2015.12.009
Piao, S. H., Kwon, S. H., Zhang, W. L., and Choi, H. J. (2015). Celebrating Soft Matter's 10th Anniversary: Stimuli-Responsive Pickering Emulsion Polymerized Smart Fluids. Soft Matter 11 (4), 646–654. doi:10.1039/c4sm02393e
Piella, J., Bastus, N. G., and Puntes, V. (2016). Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 28, 1066–1075. doi:10.1021/acs.chemmater.5b04406
Pollinger, K., Hennig, R., Breunig, M., Tessmar, J., Ohlmann, A., Tamm, E. R., et al. (2012). Kidney Podocytes as Specific Targets for cyclo(RGDfC)-Modified Nanoparticles. Small 8 (21), 3368–3375. doi:10.1002/smll.201200733
Prow, T. W. (2010). Toxicity of Nanomaterials to the Eye. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2 (4), 317–333. doi:10.1002/wnan.65
Qamar, Z., Qizilbash, F. F., Iqubal, M. K., Ali, A., Narang, J. K., Ali, J., et al. (2019). Nano-based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 13 (4), 246–254. doi:10.2174/1872211314666191224115211
Qu, Y., Shi, H., Liu, M., Zhang, M., Wang, H., Pang, L., et al. (2021). In Vivo insulin Peptide Autoantigen Delivery by Mannosylated Sodium Alginate Nanoparticles Delayed but Could Not Prevent the Onset of Type 1 Diabetes in Nonobese Diabetic Mice. Mol. Pharm. 18, 1806–1818. doi:10.1021/acs.molpharmaceut.1c00054
Ramos, A., Guerrero, C. G., Sanz, A., Sanchez-Nino, M., Rodríguez-Osorio, L., Martin-Cleary, C., et al. (2015). Designing Drugs that Combat Kidney Damage. Expert Opin. Drug Discov. 10 (5), 541–556. doi:10.1517/17460441.2015.1033394
Ratner, B. D. (2019). Biomaterials: Been There, Done that, and Evolving into the Future. Annu. Rev. Biomed. Eng. 21, 171–191. doi:10.1146/annurev-bioeng-062117-120940
Reichel, H., Zee, J., Tu, C., Young, E., Pisoni, R. L., Stengel, B., et al. (2020). Chronic Kidney Disease Progression and Mortality Risk Profiles in Germany: Results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol. Dial. Transpl. 35 (5), 803–810. doi:10.1093/ndt/gfz260
Rettig, R. A., Norris, K., and Nissenson, A. R. (2008). Chronic Kidney Disease in the United States: a Public Policy Imperative. Clin. J. Am. Soc. Nephrol. 3, 1902–1910. doi:10.2215/CJN.02330508
Ricklin, D., Mastellos, D. C., Reis, E. S., and Lambris, J. D. (2018). The Renaissance of Complement Therapeutics. Nat. Rev. Nephrol. 14 (1), 26–47. doi:10.1038/nrneph.2017.156
Rieppo, L., Saarakkala, S., Narhi, T., Helminen, H. J., Jurvelin, J. S., and Rieppo, J. (2012). Application of Second Derivative Spectroscopy for Increasing Molecular Specificity of Fourier Transform Infrared Spectroscopic Imaging of Articular Cartilage. Osteoarthr. Cartil. 20 (5), 451–459. doi:10.1016/j.joca.2012.01.010
Robben, J. H., Knoers, N. V. A. M., and Deen, P. M. T. (2004). Regulation of the Vasopressin V2 Receptor by Vasopressin in Polarized Renal Collecting Duct Cells. Mol. Biol. Cel 15 (12), 5693–5699. doi:10.1091/mbc.e04-04-0337
Robson, A. L., Dastoor, P. C., Flynn, J., Plamer, W., Martin, A., Smith, D. W., et al. (2018). Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 9, 80. doi:10.3389/fphar.2018.00080
Ruggiero, A., Villa, C. H., Bander, E., Rey, D. A., Bergkvist, M., Batt, C. A., et al. (2010). Paradoxical Glomerular Filtration of Carbon Nanotubes. Proc. Natl. Acad. Sci. U. S. A. 107 (27), 12369–12374. doi:10.1073/pnas.0913667107
Sabaeifard, P., Abdi-Ali, A., Soudi, M. R., Gamazo, C., and Irache, J. M. (2016). Amikacin Loaded PLGA Nanoparticles against Pseudomonas aeruginosa. Eur. J. Pharm. Sci. 93, 392–398. doi:10.1016/j.ejps.2016.08.049
Saberi, A. H., and McClements, D. J. (2015). Fabrication of Protein Nanoparticles and Microparticles within Water Domains Formed in Surfactant-Oil-Water Mixtures: Phase Inversion Temperature Method. Food Hydrocolloids 51, 441–448. doi:10.1016/j.foodhyd.2015.06.001
Sadikovic, B., Al-Romaih, K., Squire, J. A., and Zielenska, M. (2008). Cause and Consequences of Genetic and Epigenetic Alterations in Human Cancer. Curr. Genomics 9 (6), 394–408. doi:10.2174/138920208785699580
Sahu, T., Ratre, Y. K., Chauhan, S., Bhaskar, L. V. K. S., Nair, M. P., and Verma, H. K. (2021). Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science. J. Drug Deliv. Sci. Technol. 63, 102487. doi:10.1016/j.jddst.2021.102487
Salanki, N., Mehta, M., Chellappan, D. K., Gupta, G., Hansbro, N. G., Tambuwala, M. M., et al. (2020). Antiproliferative Effects of Boswellic Acid-Loaded Chitosan Nanoparticles on Human Lung Cancer Cell Line A549. Future Med. Chem. 12 (22), 2019–2034. doi:10.4155/fmc-2020-0083
Sancey, L., Kotb, S., Truillet, C., Appaix, F., Marais, A., Thomas, E., et al. (2015). Tillement, Long-Term In Vivo Clearance of Gadoliniumbased AGuIX Nanoparticles and Their Biocompatibility after Systemic Injection. ACS Nano 9 (3), 2477–2488. doi:10.1021/acsnano.5b00552
Saran, R., Robinson, B., Abbott, K. C., Agodoa, Y. C. L., Bragg-Gresham, J., Balkrishnan, R., et al. (2019). US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 73, A7–A8. doi:10.1053/j.ajkd.2019.01.001
Satchell, S. C., and Braet, F. (2009). Glomerular Endothelial Cell Fenestrations: an Integral Component of the Glomerular Filtration Barrier. Am. J. Physiol. Ren. Physiol. 296 (5), F947–F956. doi:10.1152/ajprenal.90601.2008
Satchell, S. (2013). The Role of the Glomerular Endothelium in Albumin Handling. Nat. Rev. Nephrol. 9 (12), 717–725. doi:10.1038/nrneph.2013.197
Satterfield, C. N. (19911991). Heterogeneous Catalysis in Industrial Practice. New York: McGraw Hill Book Co.
Scindia, Y., Deshmukh, U., Thimmalapura, P. R., and Bagavant, H. (2008). Anti-alpha8 Integrin Immunoliposomes in Glomeruli of Lupus-Susceptible Mice: a Novel System for Delivery of Therapeutic Agents to the Renal Glomerulus in Systemic Lupus Erythematosus. Arthritis Rheum. 58 (12), 3884–3891. doi:10.1002/art.24026
Severs, N. J. (2007). Freeze-fracture Electron Microscopy. Nat. Protoc. (3), 547–576. doi:10.1038/nprot.2007.55
Shandiz, S. A. S., Ardestani, M. S., Shahbazzadeh, D., Assadi, A., Cohan, R. A., and Asgary, V. (2017). Novel Imatinib-Loaded Silver Nanoparticles for Enhanced Apoptosis of Human Breast Cancer MCF-7 Cells. Artif. Cell Nanomed. Biotechnol 45 (6), 1–10. doi:10.1080/21691401.2016.1202257
Shao, M., Yang, W., and Han, G. (2017). Protective Effects on Myocardial Infarction Model: Delivery of Schisandrin B Using Matrix Metalloproteinase-Sensitive Peptide-Modified, PEGylated Lipid Nanoparticles. Int. J. Nanomed. 12, 7121–7130. doi:10.2147/IJN.S141549
Sharma, A. K., Gupta, L., Sahu, H., Qayum, A., Singh, S. K., Nakhate, K. T., et al. (2018). Chitosan Engineered PAMAM Dendrimers as Nano Constructs for the Enhanced Anti-cancer Potential and Improved In Vivo Brain Pharmacokinetics of Temozolomide. Pharm. Res. 35 (1), 9. doi:10.1007/s11095-017-2324-y
Sharma, P., Blackburn, R. C., Parke, C. L., McCullough, K., Marks, A., and Black, C. (2011). Angiotensin-converting Enzyme Inhibitors and Angiotensin Receptor Blockers Foradults with Early (Stage 1 to 3) Non-diabetic Chronic Kidney Disease. Cochrane Database Syst. Rev. 5 (10), Cd007751. doi:10.1002/14651858.CD007751.pub2
Shi, A., Li, D., Wang, L., Li, B., and Adhikari, B. (2011). Preparation of Starch-Based Nanoparticles through High-Pressure Homogenization and Miniemulsion Cross-Linking: Influence of Various Process Parameters on Particle Size and Stability. Carbohydr. Polym. 83, 1604–1610. doi:10.1016/j.carbpol.2010.10.011
Shimizu, H., Hori, Y., Kaname, S., Yamada, K., Nishiyama, N., Matsumoto, S., et al. (2010). siRNA-based Therapy Ameliorates Glomerulonephritis. J. Am. Soc. Nephrol. JASN. 21, 622–633. doi:10.1681/ASN.2009030295
Shirai, T., Kohara, H., and Tabata, Y. (2012). Inflammation Imaging by Silica Nanoparticles with Antibodies Orientedly Immobilized. J. Drug Target. 20 (6), 535–543. doi:10.3109/1061186X.2012.693500
Sichert, J. A., Tong, Y., Mutz, N., Vollmer, M., Fischer, S., Milowska, K. Z., et al. (2015). Quantum Size Effect in Organometal Halide Perovskite Nanoplatelets. Nano Lett. 15 (10), 6521–6527. doi:10.1021/acs.nanolett.5b02985
Singh, A. K., Pandey, H., Ramteke, P. W., and Mishra, S. B. (2019). Nano-suspension of Ursolic Acid for Improving Oral Bioavailability and Attenuation of Type II Diabetes: A Histopathological Investigation. Biocatal. Agric. Biotechnol. 2, 22. doi:10.1016/j.bcab.2019.101433
Singh, R., and Lillard, J. W. (2009). Nanoparticle-based Targeted Drug Delivery. Exp. Mol. Pathol. 86 (3), 215–223. doi:10.1016/j.yexmp.2008.12.004
Smith, M. C., Crist, R. M., Clogston, J. D., and McNeil, S. E. (2017). Zeta Potential: a Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 409 (24), 5779–5787. doi:10.1007/s00216-017-0527-z
Somasuntharam, I., Boopathy, A. V., Khan, R. S., Martinez, M. D., Brown, M. E., Murthy, N., et al. (2013). Delivery of Nox2-NADPH Oxidase siRNA with Polyketal Nanoparticles for Improving Cardiac Function Following Myocardial Infarction. Biomaterials 34, 7790–7798. doi:10.1016/j.biomaterials.2013.06.051
Sridhar, V., Gaud, R., Bajaj, A., and Wairkar, S. (2018). Pharmacokinetics Andpharmacodynamics of Intranasally Administered Selegiline Nanoparticles with Improved Brain Delivery in Parkinson ’s Disease. Nanomed. Nanotechnol. Biol. Med. 14 (8), 2609–2618. doi:10.1016/j.nano.2018.08.004
Stabi, K. L., and Bendz, L. M. (2011). Ferumoxytol Use as an Intravenous Contrast Agent for Magnetic Resonance Angiography. Ann. Pharmacother. 45 (12), 1571–1575. doi:10.1345/aph.1Q431
Suleiman, H., Zhang, L., Roth, R., Heuser, J. E., Miner, J. H., Shaw, A. S., et al. (2013). Nanoscale Protein Architecture of the Kidney Glomerular Basement Membrane. eLife 2, e01149. doi:10.7554/eLife.01149
Sun, S. B., Liu, P., Shao, F. M., and Miao, Q. L. (2015). Formulation and Evaluation of PLGA Nanoparticles Loaded Capecitabine for Prostate Cancer. Int. J. Clin. Exp. Med. 8 (10), 19670–19681.
Sung, H. J., and Yang, J. W. (2015). Current Progress in Nanotechnology Applications for Diagnosis and Treatment of Kidney Diseases. Adv. Healthc. Mater. 4, 2037–2045. doi:10.1002/adhm.201500177
Takeda, M., Khamdang, S., Narikawa, S., Kimura, H., Kobayashi, Y., Yamamoto, T., et al. (2002). Human Organic Anion Transporters and Human Organic Cation Transporters Mediate Renal Antiviral Transport. J. Pharmacol. Exp. Ther. 300 (3), 918–924. doi:10.1124/jpet.300.3.918
Tang, S., Chen, M., and Zheng, N. (2014). Sub-10-nm Pd Nanosheets with Renal Clearance for Efficient Near-Infrared Photothermal Cancer Therapy. Small 10 (15), 3139–3144. doi:10.1002/smll.201303631
Ters, M. E., Zhou, X., Lepping, R. J., Lu, P., Karcher, R. T., Mahnken, J. D., et al. (2020). Biological Efficacy and Safety of Niacinamide in Patients with ADPKD. Kidney Int. Rep. 5, 1271–1279. doi:10.1016/j.ekir.2020.06.002
Thickett, S. C., and Gilbert, R. G. (2007). Emulsion Polymerization: State of the Art in Kinetics and Mechanisms. Polymer 48 (24), 6965–6991. doi:10.1016/j.polymer.2007.09.031
Thurman, J. M. (2015). Complement in Kidney Disease: Core Curriculum. Am. J. Kidney Dis. 65, 156–168. doi:10.1053/j.ajkd.2014.06.035
Trump, D., Mathias, C., Yang, Z., Low, P., Marmion, M., and Green, M. (2002). Synthesis and Evaluation of 99mTc(CO)3-DTPA-folate as a Folate-Receptor-Targeted Radiopharmaceutical. Nucl. Med. Biol. 29, 569–573. doi:10.1016/s0969-8051(02)00310-4
Urquhart, A. J., and Eriksen, A. Z. (2019). Recent Developments in Liposomal Drug Delivery Systems for the Treatment of Retinal Diseases. Drug Discov. Today 24 (8), 1660–1668. doi:10.1016/j.drudis.2019.04.004
U.S. Food, Drug Administration (2017). Drug Products, Including Biological Products, that Contain Nanomaterials-Guidance for Industry. Rockville, MD: Center for Drug Evaluation and Research.
Villanueva, J. R., Navarro, M. G., and Villanueva, R. L. (2016). Dendrimers as a Promising Tool in Ocular Therapeutics: Latest Advances and Perspectives. Int. J. Pharm. 511 (1), 359–366. doi:10.1016/j.ijpharm.2016.07.031
Voigt, N., Henrich-Noack, P., Kockentiedt, S., Hintz, W., Tomas, J., and Sabel, B. A. (20142014). Toxicity of Polymeric Nanoparticles In Vivo and In Vitro. J. Nanopart. Res. 16 (6), 2379. doi:10.1007/s11051-014-2379-1
Wang, A., Wang, L., Sun, K., Liu, W., Sha, C., and Li, Y. (2012). Preparation of Rotigotine-Loaded Microspheres and Their Combination Use with L-DOPA to Modify Dyskinesias in 6-OHDA-Lesioned Rats. Curr. Pharm. Res. 29, 2367–2376. doi:10.1007/s11095-012-0762-0
Wang, H., Mu, Q., Wang, K., Revia, R. A., Yen, C., Gu, X., et al. (2019). Nitrogen and boron Dual-Doped Graphene Quantum Dots for Near-Infrared Second Window Imaging and Photothermal Therapy. Appl. Mater. Today 14, 108–117. doi:10.1016/j.apmt.2018.11.011
Wang, H., Yuan, Y., Zhuo, Y., Chai, Y., and Yuan, Y. (2016). Self-enhanced Electrochemiluminescence Nanorods of Tris(bipyridine) Ruthenium(II) Derivative and Itssensing Application for Detection of N-Acetyl-Beta-D-Glucosaminidase. Anal. Chem. 88 (4), 2258–2265. doi:10.1021/acs.analchem.5b03954
Wang, J., Chin, D., Poon, C., Mancino, V., Pham, J., Li, H., et al. (2021). Oral Delivery of Metformin by Chitosan Nanoparticles for Polycystic Kidney Disease. J. Control. Release 329, 1198–1209. doi:10.1016/j.jconrel.2020.10.047
Wang, J., Masehi-Lano, J. J., and Chung, E. J. (2017). Peptide and Antibody Ligands for Renal Targeting: Nanomedicine Strategies for Kidney Disease. Biomater. Sci. 5 (8), 1450–1459. doi:10.1039/c7bm00271h
Wang, J., Seo, M. J., Deci, M. B., Weil, B. R., Canty, J. M., and Nguyen, J. (2018). Effect of CCR2 Inhibitor-Loaded Lipid Micelles on Inflammatory Cell Migration and Cardiac Function after Myocardial Infarction. Int. J. Nanomed. 13, 6441–6451. doi:10.2147/IJN.S178650
Wang X, X., Yang, L., Zhang, H., Tian, B., Li, R., Hou, X., et al. (2018). Fluorescent Magnetic PEI-PLGA Nanoparticles Loaded with Paclitaxel for Concurrent Cell Imaging, Enhanced Apoptosis and Autophagy in Human Brain Cancer. Colloids Surf. B: Biointerfaces 172, 707–717. doi:10.1016/j.colsurfb.2018.09.033
Wang, Y., Wu, Y., Long, L., Yang, L., Fu, D., Hu, C., et al. (2021). Inflammation-responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Inter. 13, 33584–33599. doi:10.1021/acsami.1c09889
Wang, Z. (2014). Caveolae-mediated Delivery of Therapeutic Nanoparticles across Blood-Endothelial Barrier. Austin J. Anal. Pharm. Chem. 1 (4), 1018–1011.
Wang, Z. H., Wang, Z. Y., Sun, C. S., Wang, C. Y., Jiang, V., and Wang, S. L. (2010). Trimethylated Chitosan-Conjugated PLGA Nanoparticles for the Delivery of Drugs to the Brain. Biomaterials 31 (5), 908–915. doi:10.1016/j.biomaterials.2009.09.104
Wang, Z., Wu, J., Zhou, Q., Wang, Y., and Chen, T. (2015). Berberine Nanosuspension Enhances Hypoglycemic Efficacy on Streptozotocin Induced Diabetic C57BL/6 Mice. Evid. Based Complement. Alternat. Med. 2015, 239749. doi:10.1155/2015/239749
Wei, Y., Quan, L., Zhou, C., and Zhan, Q. (2018). Factors Relating to the Biodistribution & Clearance of Nanoparticles & Their Effects on In Vivo Application. Nanomedicine (Lond). 13 (12), 1495–1512. doi:10.2217/nnm-2018-0040
Wicher, G., Larsson, M., Svenningsen, A. F., Gyllencreutz, E., Rask, L., and Aldskogius, H. (2006). Low Density Lipoprotein Receptor-Related Protein-2/megalin Is Expressed in Oligodendrocytes in the Mouse Spinal Cord white Matter. J. Neurosci. Res. 83 (5), 864–873. doi:10.1002/jnr.20774
Wieland-Berghausen, S., Schote, U., Frey, M., and Schmidt, F. (2002). Comparison of Microencapsulation Techniques for the Water-Soluble Drugs Nitenpyram and Clomipramine HCl. J. Control Release 85, 35–43. doi:10.1016/s0168-3659(02)00269-9
Williams, R. M., Shah, J., Ng, B. D., Minton, D. R., Gudas, L. J., Park, C. Y., et al. (2015). Mesoscale Nanoparticles Selectively Target the Renal Proximal Tubule Epithelium. Nano Lett. 15 (4), 2358–2364. doi:10.1021/nl504610d
Wisse, E., Jacobs, F., Topal, B., Frederik, P., and Geest, B. D. (2008). The Size of Endothelial Fenestrae in Human Liver Sinusoids: Implications for Hepatocyte-Directed Gene Transfer. Gene Ther. 15 (17), 1193–1199. doi:10.1038/gt.2008.60
Wu, G., Wang, C., Tan, Z., and Zhang, H. (2011). Effect of Temperature on Emulsion Polymerization of N-Butyl Acrylate. Proced. Eng 18, 353–357. doi:10.1016/j.proeng.2011.11.056
Wu, L., Chen, M., Mao, H., Wang, N., Zhang, B., Zhao, X., et al. (2017). Albumin-based Nanoparticles as Methylprednisolone Carriers for Targeted Delivery towards the Neonatal Fc Receptor in Glomerular Podocytes. Int. J. Mol. Med. 39 (4), 851–860. doi:10.3892/ijmm.2017.2902
Wyatt, P. J., and Weida, M. J. (2003). Method and Apparatus for Determining Absolute Number Densities of Particles in Suspension. Santa Barbara, CA: U.S. Patent.
Xu, L., Liang, H-W., Yang, Y., and Yu, S-H. (2018). Stability and Reactivity: Positive and Negative Aspects for Nanoparticle Processing. Chem. Rev. 118, 3209–3250. doi:10.1021/acs.chemrev.7b00208
Xu, X., Khan, M. A., and Burgess, D. J. (2012). A Two-Stage Reverse Dialysis In Vitro Dissolution Testing Method for Passive Targeted Liposomes. Int. J. Pharm. 426, 211–218. doi:10.1016/j.ijpharm.2012.01.030
Xue, M., Yang, M-X., Zhang, W., Li, X-M., Gao, D-H., Ou, Z-M., et al. (2013). Characterization, Pharmacokinetics, and Hypoglycemic Effect of Berberine Loaded Solid Lipid Nanoparticles. Int. J. Nanomedicine 8, 4677–4687. doi:10.2147/IJN.S51262
Xue, X., Shi, X., Dong, H., You, S., Cao, H., Wang, K., et al. (2018). Delivery of microRNA-1 Inhibitor by Dendrimer-Based Nanovector: an Early Targeting Therapy for Myocardial Infarction in Mice. Nanomedicine 14, 619–631. doi:10.1016/j.nano.2017.12.004
Yamak, H. B. (2013). Emulsion Polymerization: Effects of Polymerization Variables on the Properties of Vinyl Acetate Based Emulsion Polymers. Polym. Sci. doi:10.5772/51498
Yamamoto, Y., Tsutsumi, Y., Yoshioka, Y., Kamada, H., Sato-Kamada, K., Okamoto, T., et al. (2004). Poly(vinylpyrrolidone-codimethyl Maleic Acid) as a Novel Renal Targeting Carrier. J. Control Release 95 (2), 229–237. doi:10.1016/j.jconrel.2003.11.017
Yang, H., Wang, H., Xiong, C., Chai, Y., and Yuan, R. (2018). Highly Sensitive Electrochemiluminescence Immunosensor Based on ABEI/H2O2 System with PFO Dots as Enhancer for Detection of Kidney Injury Molecule-1. Biosens. Bioelectron. 116, 16–22. doi:10.1016/j.bios.2018.05.032
Yang, J., Brown, M. E., Zhang, H., Martinez, M., Zhao, Z., Bhutani, S., et al. (2017). High-throughput Screening Identifies microRNAs that Target Nox2 and Improve Function after Acute Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 312, H1002–H1012. doi:10.1152/ajpheart.00685.2016
Yang, J., Gong, X., Fang, L., Fan, Q., Cai, L., Qiu, X., et al. (2017). Potential of CeCl3@mSiO2nanoparticles in Alleviating Diabetic Cataract Development and Progression. Nanomed.: Nanotechnol. Biol. Med. 13, 1147–1155. doi:10.1016/j.nano.2016.12.021
Yang, S., Sun, S., Zhou, C., Hao, G., Liu, J., Ramezani, S., et al. (2015). Renal Clearance and Degradation of Glutathione-Coated Copper Nanoparticles. Bioconjug. Chem. 26 (3), 511–519. doi:10.1021/acs.bioconjchem.5b00003
Yang, Y., Fang, Z., Chen, X., Zhang, W., Xie, Y., Chen, Y., et al. (2017). An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 8, 287. doi:10.3389/fphar.2017.00287
Yegin, B. A., Benoit, J. P., and Lamprecht, A. (2006). Paclitaxel-loaded Lipid Nanoparticles Prepared by Solvent Injection or Ultrasound Emulsification. Drug Dev. Ind. Pharm. 32, 1089–1094. doi:10.1080/03639040600683501
Yin, Q., Pei, Z., Wang, H., and Zhao, Y. (2014). Cyclosporine A-Nanoparticles Enhance the Therapeutic Benefit of Adipose Tissue-Derived Stem Cell Transplantation in a Swine Myocardial Infarction Model. Int. J. Nanomed. 9, 17–26. doi:10.2147/IJN.S52005
Yu, F., Li, Y., Chen, Q., He, Y., Wang, H., Yang, L., et al. (2016). Monodisperse Microparticles Loaded with the Self-Assembled Berberine-Phospholipid Complex-Based Phytosomes for Improving Oral Bioavailability and Enhancing Hypoglycemic Efficiency. Eur. J. Pharm. Biopharm. 10 (3), 136–148. doi:10.1016/j.ejpb.2016.03.019
Yu, H., Lin, T., Chen, W., Cao, W., Zhang, C., Wang, T., et al. (2019). Size and Temporal- Dependent Efficacy of Oltipraz-Loaded PLGA Nanoparticles for Treatment of Acute Kidney Injury and Fibrosis. Biomaterials 219, 119368. doi:10.1016/j.biomaterials.2019.119368
Yu, M., Yuan, W., Li, D., Schwendeman, A., and Schwendeman, S. P. (2019). Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control Release 315, 23–30. doi:10.1016/j.jconrel.2019.09.016
Yu, M., Zhou, J., Du, B., Ning, X., Authement, C., Gandee, L., et al. (2016). Noninvasive Staging of Kidney Dysfunction Enabled by Renal-Clearable Luminescent Gold Nanoparticles. Angew. Chem. Int. Ed. Engl. 55 (8), 2787–2791. doi:10.1002/anie.201511148
Yu, W. L., and Borkovec, M. (2002). Distinguishing Heteroaggregation from Homoaggregation in Mixed Binary Particle Suspensions by Multiangle Static and Dynamic Light Scattering. J. Phys. Chem. B. 106, 13106–13110. doi:10.1021/jp021792h
Yuan, Z.-X., Zhang, Z.-R., Zhu, D., Sun, X., Gong, T., Liu, J., et al. (2009). Specific Renal Uptake of Randomly 50% N-Acetylated Low Molecular Weight Chitosan. Mol. Pharm. 6 (1), 305–314. doi:10.1021/mp800078a
Yuan, Z. X., Sun, X., Gong, T., Ding, H., Fu, Y., and Zhang, Z. R. (2007). Randomly 50% Nacetylated Low Molecular Weight Chitosan as a Novel Renal Targeting Carrier. J. Drug Target. 15 (4), 269–278. doi:10.1080/10611860701289875
Zhang, F., Zhong, H., Lin, Y., Chen, M., Wang, Q., Lin, Y., et al. (2018). A Nanohybridcomposed of Prussian Blue and Graphitic C3N4 Nanosheets as the Signal-Generating Tag in an Enzyme-free Electrochemical Immunoassay for the Neutrophil Gelatinaseassociated Lipocalin. Microchimica Acta 185 (7), 327. doi:10.1007/s00604-018-2865-8
Zhang, X. S., Brondyk, W., Lydon, J. T., Thurberg, B. L., and Piepenhagen, P. A. (2011). Biotherapeutic Target or Sink: Analysis of the Macrophage Mannose Receptor Tissue Distribution in Murine Models of Lysosomal Storage Diseases. J. Inherit. Metab. Dis. 34 (3), 795–809. doi:10.1007/s10545-011-9285-9
Zhang, Y-N., Poon, W., Tavares, A. J., Mcgilvray, I. D., and Chan, W. C. W. (2016). Nanoparticle-liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control Rel. 240, 332–348. doi:10.1016/j.jconrel.2016.01.020
Zhang, Z., Zheng, Q., Han, J., Gao, G., Liu, J., Gong, T., et al. (2009). The Targeting of 14-succinate Triptolide-Lysozyme Conjugate to Proximal Renal Tubular Epithelial Cells. Biomaterials 30 (7), 1372–1381. doi:10.1016/j.biomaterials.2008.11.035
Zhao, J. H. (2019). Mesangial Cells and Renal Fibrosis. Adv. Exp. Med. Biol. 1165, 165–194. doi:10.1007/978-981-13-8871-2_9
Zhao, X., Wang, W., Zu, Y., Zhang, Y., Li, Y., Sun, W., et al. (2014). Preparation and Characterization of Betulin Nanoparticles for Oral Hypoglycemic Drug by Antisolvent Precipitation. Drug Deliv. 21 (6), 467–479. doi:10.3109/10717544.2014.881438
Zhao, Y., Zhao, W., Lim, W. C., and Liu, T. (2019). Salinomycin-loaded Gold Nanoparticles for Treating Cancer Stem Cells by Ferroptosis-Induced Cell Death. Mol. Pharm. 16, 2532–2539. doi:10.1021/acs.molpharmaceut.9b00132
Zhou, Z., Wang, L., Chi, X., Bao, J., Yang, L., Zhao, W., et al. (2013). Engineered Iron-Oxide-Based Nanoparticles as Enhanced T1 Contrast Agents for Efficient Tumor Imaging. ACS Nano 7 (4), 3287–3296. doi:10.1021/nn305991e
Zhu, D., Tao, W., Zhang, H., Liu, G., Wang, T., Zhang, L., et al. (2016). Docetaxel (DTX)-loaded Polydopamine-Modified TPGS-PLA Nanoparticles as a Targeted Drug Delivery System for the Treatment of Liver Cancer. Acta Biomater. 30, 144–154. doi:10.1016/j.actbio.2015.11.031
Keywords: nanotechnology, nanoparticles, nanomedicine, drug delivery, kidney disease
Citation: Merlin JPJ and Li X (2022) Role of Nanotechnology and Their Perspectives in the Treatment of Kidney Diseases. Front. Genet. 12:817974. doi: 10.3389/fgene.2021.817974
Received: 18 November 2021; Accepted: 06 December 2021;
Published: 05 January 2022.
Edited by:
Ratnakar Tiwari, Northwestern University, United StatesReviewed by:
Eun Ji Chung, University of Southern California, United StatesCopyright © 2022 Merlin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaogang Li, bGkueGlhb2dhbmdAbWF5by5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.