
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 28 January 2022
Sec. Human and Medical Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.815270
This article is part of the Research TopicReproductive GenomicsView all 13 articles
 Xiaohan Wang1†
Xiaohan Wang1† Fangting Lu2†
Fangting Lu2† Shun Bai2†
Shun Bai2† Limin Wu2
Limin Wu2 Lingli Huang2
Lingli Huang2 Naru Zhou2
Naru Zhou2 Bo Xu2
Bo Xu2 Yangyang Wan2
Yangyang Wan2 Rentao Jin2*
Rentao Jin2* Xiaohua Jiang2*
Xiaohua Jiang2* Xianhong Tong1*
Xianhong Tong1*Human autologous sperm freezing involves ejaculated sperm, and testicular or epididymal puncture sperm freezing, and autologous sperm freezing is widely used in assisted reproductive technology. In previous studies, researchers have tried to cryopreserve sperm from mammals (rats, dogs, etc.) using a −80°C freezer and have achieved success. It is common to use liquid nitrogen vapor rapid freezing to cryopreserve human autologous sperm. However, the operation of this cooling method is complicated, and the temperature drop is unstable. In this study, we compared the quality of human ejaculation and testicular sperm after liquid nitrogen vapor rapid freezing and −80°C freezing for the first time. By analyzing sperm quality parameters of 93 ejaculated sperm and 10 testicular sperm after liquid nitrogen vapor rapid freezing and −80°C freezing, we found reactive oxygen species (ROS) of sperm of the −80°C freezer was significantly lower than liquid nitrogen vapor rapid freezing. Regression analysis showed that progressive motility, ROS, and DNA fragmentation index (DFI) in post-thaw spermatozoa were correlated with sperm progressive motility, ROS, and DFI before freezing. For the freezing method, the −80°C freezer was positively correlated with the sperm progressive motility. Among the factors of freezing time, long-term freezing was negatively correlated with sperm progressive motility and ROS. Although freezing directly at −80°C freezer had a slower temperature drop than liquid nitrogen vapor rapid freezing over the same period, the curves of the temperature drop were similar, and slight differences in the freezing point were observed. Furthermore, there were no statistically significant differences between the two methods for freezing testicular sperm. The method of direct −80°C freezing could be considered a simplified alternative to vapor freezing for short-term human sperm storage. It could be used for cryopreservation of autologous sperm (especially testicular sperm) by in vitro fertilization centers.
Clinical Trial Registration: (website), identifier (ChiCTR2100050190).
Human sperm cryopreservation has been widely used for human reproduction. Autologous sperm freezing is the general method applied in vitro fertilization (IVF) laboratories in a variety of circumstances ranging from fertility preservation for cancer patients to the clinical management of male infertility (Trottmann et al., 2007).
The first attempt to freeze semen dates can be traced back to 1776 when Abbot et al. (Royere, et al., 1996) reported that snow could store sperm by cooling. Advances in cryobiology seen over the past decades and cryoprotective medium, in particular, have significantly aided sperm freezing-resuscitation technology (Sherman, 1973). A variety of freezing methods have been discovered with the development of cryopreservation technology: slow freezing, liquid nitrogen vapor rapid freezing, and vitrification (Tao et al., 2020). Currently, the mainstream method for semen freezing or freezing a small number of spermatozoa samples is liquid nitrogen vapor rapid freezing (vapor rapid freezing) (Huang et al., 2020). The standard cryopreservation method normally involves freezing human sperm in liquid nitrogen vapor to −80°C and then stored in liquid nitrogen. Previous reports had shown that samples are placed at 1–10 cm above the liquid nitrogen surface for 10–30 min, followed by storage in liquid nitrogen (Gwatkin, 1991; Le et al., 2019). Vapor rapid freezing cannot control the rate of temperature drop caused by the volatilization of liquid nitrogen (Di Santo et al., 2012). Another limitation of liquid nitrogen vapor rapid freezing is that it cannot cool many semen samples simultaneously.
Freezing semen samples by directly placing them in a −80°C freezer is the most practical means because a −80°C freezer is readily available. A few researchers have reported that several mammalian spermatozoa can be successfully frozen by direct placement in a −80°C freezer. For example, Marcello Raspa et al. (2017), Raspa et al. (2018a), Raspa et al. (2018b), reported that mouse spermatozoa can be frozen, transported, shared, and stored at −80°C for a long time without a significant loss of viability. Pezo et al. (2017) demonstrated that semen freezing and storage using a −80°C ultra-freezer is an effective technique for the long-term preservation of canine spermatozoa. Parkes. (1945) indicated that human sperm cryopreservation without cryoprotectants at −79°C offered an advantage over storage at −196°C. In addition, Liu et al. (2016) concluded that storage of neat semen samples at −80°C caused milder damage to sperm DNA than storage at −196°C mixed with cryoprotectants. In 2012, Sanchez et al. (2012) concluded that there were no significant differences in sperm progressive motility, the integrity of mitochondrial membrane potential (MMP) or DNA fragmentation for vitrified swim-up human sperm either at −196°C under liquid nitrogen or at -80°C. However, Vaz et al. (2018) found storage of human sperm at −80°C freezer up to 96 hours was detrimental to sperm viability. Therefore, the impact of −80°C freezer on human sperm needs to be further explored.
The cryopreservation of limited spermatozoa from men afflicted with nonobstructive azoospermia using testicular sperm aspiration (TESA) can avoid repeated surgery and promote the preservation of fertility (Miller et al., 2017). Current research on the freezing of testicular or epididymal sperm is mainly focused on single sperm freezing. In 1997, Cohen et al. (1997) first described a novel cryopreservation technique for single sperm using an empty zona pellucida (ZP). Various single sperm cryopreservation carriers have been proposed in the last 20 years, including ZPs (Hsieh et al., 2000; Just et al., 2004), cryoloops (Desai et al., 2004), culture dishes (Sereni et al., 2008), cell sleepers (Coetzee et al., 2016), cryotops (Endo et al., 2012), and novel sperm vitrification devices (Berkovitz et al., 2018). Each cryopreservation method has limitations; in particular, the usage of these carriers requires experienced technicians to select and capture single sperm, which always results in very few available sperm after this process. Therefore, frozen testicular sperm with single sperm has not become widespread, and an ideal container that can be universally used needs to be developed.
To explore whether freezing sperm at −80°C freezer is feasible for human ejaculate and testicular spermatozoa, we evaluated the effects of freezing sperm at −80°C freezer and vapor rapid freezing techniques on sperm quality, and simplified the procedure and equipment for freezing human ejaculate and testicular spermatozoa.
The procedures in this study were approved by the Ethical Committee of The First Affiliated Hospital of the University of Science and Technology of China (2021-KY-040), and informed consent was obtained from all subjects.
The study included 93 ejaculated sperm from normozoospermic men who sought fertility evaluation at the Reproductive Center of The First Affiliated Hospital of University of Science and Technology of China between March 2021 and June 2021. And ten testicular tissue from patients who were diagnosed with obstructive azoospermia. Based on medical history and seminal examination findings, patients with vasectomy, varicocele, cryptorchidism, or genital infection were excluded from the analysis. Patients receiving any medication or antioxidant supplementation in 3 months before the study were also excluded.
A diagram of the experimental design is shown in Figure 1. The experiments were split into four sections.
Thirty ejaculated sperm from normozoospermic men were analyzed for progressive motility and viability by vapor rapid freezing or direct −80°C freezing. The sperm samples were thawed and sperm parameters were measured 24 h (short-term) or 2 months (long-term) later.
Thirty-three ejaculated sperm from normozoospermic men were analyzed for sperm quality by vapor rapid freezing or direct −80°C freezing, kept for 24 h in a −80°C freezer and then moved into liquid nitrogen. The sperm samples were thawed and sperm parameters were measured 24 h (short-term) or 2 months (long-term) later.
Overall, there were five groups in section 1 and section 2 (Ⅰ) frozen by vapor rapid freezing for short-term (short vapor group); (Ⅱ) frozen by vapor rapid freezing for long-term (long vapor group); (Ⅲ) frozen in -80°C freezers for short-term (short freezer group); (Ⅳ) frozen in −80°C freezers for long-term (long freezer group); (Ⅴ) frozen in −80°C freezers for 24 h and then immersed in liquid nitrogen for 2 months (liquid nitrogen group).
Thirty ejaculated sperm from normozoospermic men were analyzed for acrosome reaction after a week by vapor rapid freezing and direct −80°C freezing.
Vapor rapid freezing and direct −80°C freezing were used to freeze 10 testicular sperm for a week. This section was designed to compare the percentage of motile sperm between vapor rapid freezing and freezing at −80°C.
The liquefied ejaculated semen was diluted with sperm freezing media (ORIGIO, Måløv, Denmark) (ratio 1:1), and 1 ml of the suspension was pipetted into a Nunc cryotube vial (1.8 ml; catalog number: 375418, Thermo Fisher Scientific, Jiangsu, China), which was kept at room temperature (RT) for 10 min and subsequently placed in a horizontal position in the freezer. Some samples were transferred to liquid nitrogen 24 h later. The purpose of placing the container in a horizontal position was to minimize the heat difference between the two ends during freezing (Di Santo et al., 2012).
After 1 ml of suspension was pipetted into a 1.8 ml aseptic cryotube, the samples were kept at RT for 10 min and subsequently placed horizontally 8–10 cm above the liquid nitrogen surface. Fifteen minutes later, it was submerged in liquid nitrogen and stored at −196°C in a tank full of liquid nitrogen for 24 h or 2 months.
Thawing was performed as described previously for a specific sperm freezing medium with some modifications (ORIGIO, Måløv, Denmark). The cryotube was removed from the liquid nitrogen or −80°C freezer and submerged in warm water (37°C) for 10 min. Post-thaw sperm progressive motility, viability, reactive oxygen species (ROS), MMP, DNA fragmentation index (DFI), and high DNA stainability (HDS) were analyzed.
After removing the seminiferous tubules from the testicles, the sample was placed in a dish with 5% 3-(N-Morpholino) propanesulphonic acid with gentamicin (G-MOPSTM, Vitrolife, Sweden) medium and minced with a needle connected to a 1 ml syringe under a dissecting microscope. Then, the sample was examined under an inverted microscope (Olympus, Tokyo, Japan) at 400× magnification. Once spermatozoa (motile or not motile) were observed in the dish, the suspension was mixed by pipetting and transferred to a tube with SpermRinse (Vitrolife, Sweden), and then the tube was placed upright for 5 min. After removing the large sludge at the bottom using a straw, the supernatant was centrifuged at 250 g for 5 min, and approximately 0.5 ml of sediment was reserved. An equal volume of sperm freezing medium (ORIGIO, Måløv, Denmark) was added dropwise onto the sediment, and the solution was carefully mixed after each addition, followed by incubation at RT for 10 min. Each sample was divided into two tubes, which were frozen using vapor rapid freezing or −80°C freezers. After 24 h, the samples frozen in the -80°C freezer were transferred to liquid nitrogen.
The sperm obtained from the testicular puncture and frozen for 1 week were thawed in a 37°C-water bath for 10 min. Then, we used SpermRinse to remove the cryoprotectant in the semen sample and centrifuged it to remove the supernatant. After adding pentoxifylline to the sample for sperm activation, the sample was filled into the sperm counting pool, and the number of sperm and the percentage of motile sperm were counted under a microscope.
The temperature changes during freezing were determined using a portable, multiuse industrial data logger (OM-CP-OCTPRO, Omega Engineering, United States). The temperature sensor uses a thermocouple K matched with the industrial data logger.
Routine semen analyses were performed using computer-assisted semen analysis (CASA) to determine progressive motility. We analyzed a minimum of six fields of view per chamber and, at least 200 sperm were evaluated in each chamber according to World Health Organization (WHO) guidelines (WHO, 2010).
Sperm viability was evaluated by eosin-nigrosine staining (Ankebio, China). At least 200 spermatozoa were analyzed with an optical microscope (magnification 1000×). Sperm with red heads were considered nonviable (membrane-damaged), whereas sperm showing no color were considered alive (membrane-intact) (Rarani et al., 2019).
Sperm Chromatin Structure Assay (SCSA) was measured by flow cytometry according to the protocol based on Evenson et al. (1980). A commercial kit (Cellpro, China) was used for the evaluation of SCSA. Damaged chromatin in the sperm nucleus after acid treatment forms a single chain and emits red or orange fluorescence upon binding the dye acridine orange; normal sperm chromatin in the nucleus maintains the integrity of the double-stranded structure after acid treatment and emits green fluorescence when combined with acridine orange. At least 5,000 cells were counted per sample. The SCSA parameters include DFI defined as the percentage of denatured sperm DNA and HDS defined as the percentage of spermatozoa with abnormally high DNA stainability.
Reactive oxygen species (ROS) were determined using a Sperm Reactive Oxygen Species Detection Kit (Ankebio, China), flow cytometry was used for detection, and each sample contained at least 5,000 cells (Mahfouz et al., 2009).
To measure the mitochondrial membrane potential (MMP) of the sperm, a sperm mitochondrial staining kit (JC-1 fluorescent staining method, Ankebio, China) was used. The MMP in sperm cells can be labeled with fluorescent dyes. The fluorescent probes gather in the mitochondria and emit red fluorescence when there is high MMP. At least 5,000 cells were counted per sample.
According to the WHO guidelines, the acrosome reaction (AR) was assessed by Fluorescein isothiocyanate-Pisum sativum agglutinin (FITC-PSA, Sigma-Aldrich, St. Louis, America) staining. After rinsing sperm with phosphate buffer saline, sperm were fixed with 4% (w/v) paraformaldehyde or 10 min, mounted on slides, then air-dried and incubated 2 h in the dark at 4°C with 25 mg/L FITC-PSA. Sperm were washed with PBS and examined by fluorescence Nikon Eclipse 80i microscopy (n b 200 sperm/ sample) (Nikon Inc., Tokyo, Japan).
The results were analyzed by using the program Statistical Product and Service Solutions (SPSS) Statistics 23.0 (SPSS Inc., Chicago, IL, United States). Data are expressed as the means ± standard deviation (SD). The comparison between the two groups was performed by paired-samples t-test. The percentage of motile sperm was expressed as proportion, and p-value was derived from the chi-square test. Regression analysis was used to compare the correlation between sperm parameters. A statistical value of p < 0.05 was considered statistically significant.
A total of ninety-three specimens from patients attending the reproductive center were enrolled. The characteristics of the study population are shown in Table 1. Among the 93 participants, the mean age was 32.23 ± 4.96 years and the mean semen volume was 3.85 ± 1.31 ml. The mean of sperm parameters, such as sperm concentration, viability, and progressive motility, were all above the reference established by the WHO (2010).
The sample was placed in liquid nitrogen vapor for 5 min, and the cooling rate was −18°C/min down from RT to −65°C. The drop from RT to −80°C took approximately 410 s (Figure 2, Supplementary Table 1). In the −80°C freezer, the sample was placed in liquid nitrogen vapor for 5 min, the cooling rate was approximately -12°C/min down from RT to −40°C, and the drop from RT to −80°C took approximately 1990 s (Figure 2, Supplementary Table 1). Although freezing at −80°C has a slower temperature drop than vapor rapid freezing during the same period, the curves of the temperature drop are similar between the two methods. Notably, slight differences in the temperature were observed at the freezing point (Figure 2B, Supplementary Table 1).
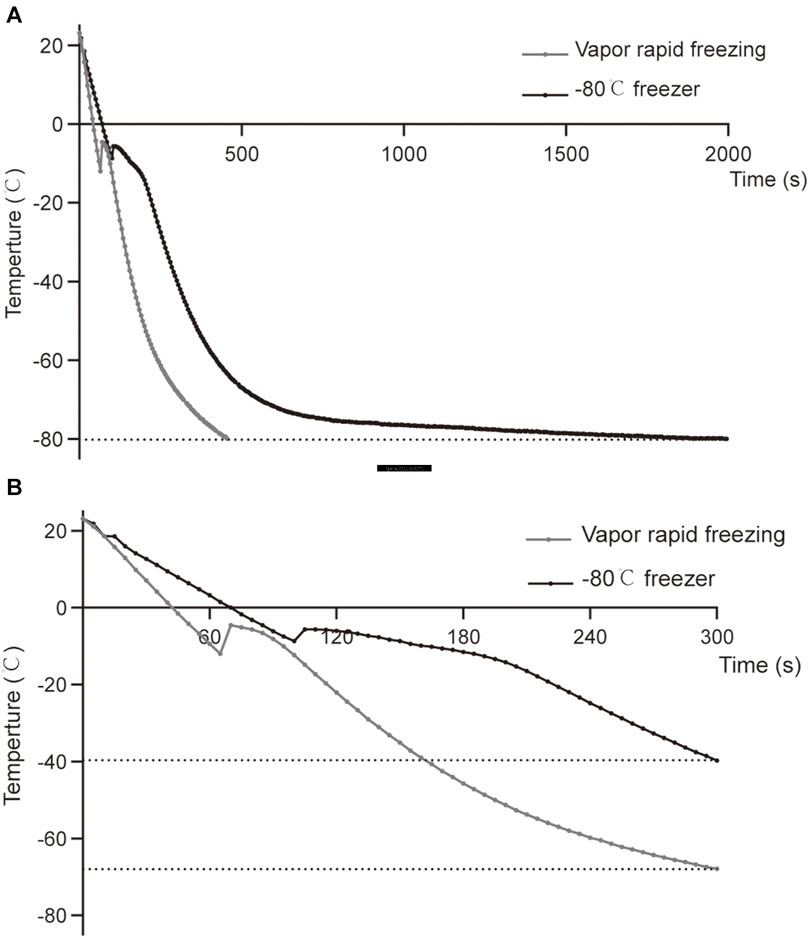
FIGURE 2. Comparison of temperature curves of vapor rapid freezing and −80°C freezer. (A) The temperature drop curve of sperm samples from RT to −80°C in liquid nitrogen vapor rapid freezing and −80°C freezer. (B) The temperature drop curve of the sperm samples in the first 300 s of liquid nitrogen vapor rapid freezing and −80°C freezer.
As shown in Table 2, no significant difference was observed in progressive motility and viability across the different freezing methods (p > 0.05).

TABLE 2. Comparison of sperm motility and viability between liquid nitrogen vapor rapid freezing and −80°C freezer.
The ROS of sperm of the freezer group was significantly lower than that of the vapor group (Table 3). However, the HDS of sperm in the long freezer group was significantly higher than that of sperm in the long vapor group (p < 0.05). There was no significant difference in the other parameters between the different methods (p > 0.05).
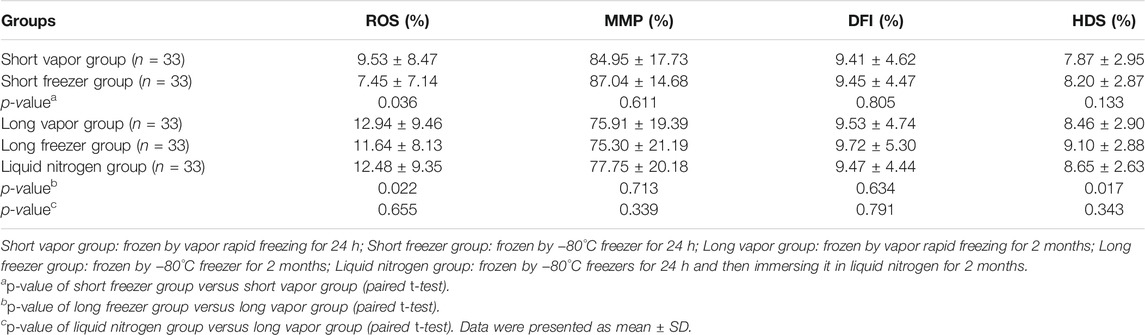
TABLE 3. Comparison of sperm function between liquid nitrogen vapor rapid freezing and −80°C freezer.
We compared the state of the sperm after freezing and found that progressive motility and viability were significantly decreased (Figures 3A,B). Specifically, compared with short-term freezing, long-term freezing significantly decreased sperm motility. There was no significant difference in ROS before and after freezing (Figure 3C), while long-term freezing decreased MMP (Figure 3D). Moreover, long-term storage of sperm had a similar DFI with short-term storage (Figure 3E). In addition, freezing also resulted in a higher HDS in the sperm (Figure 3F).
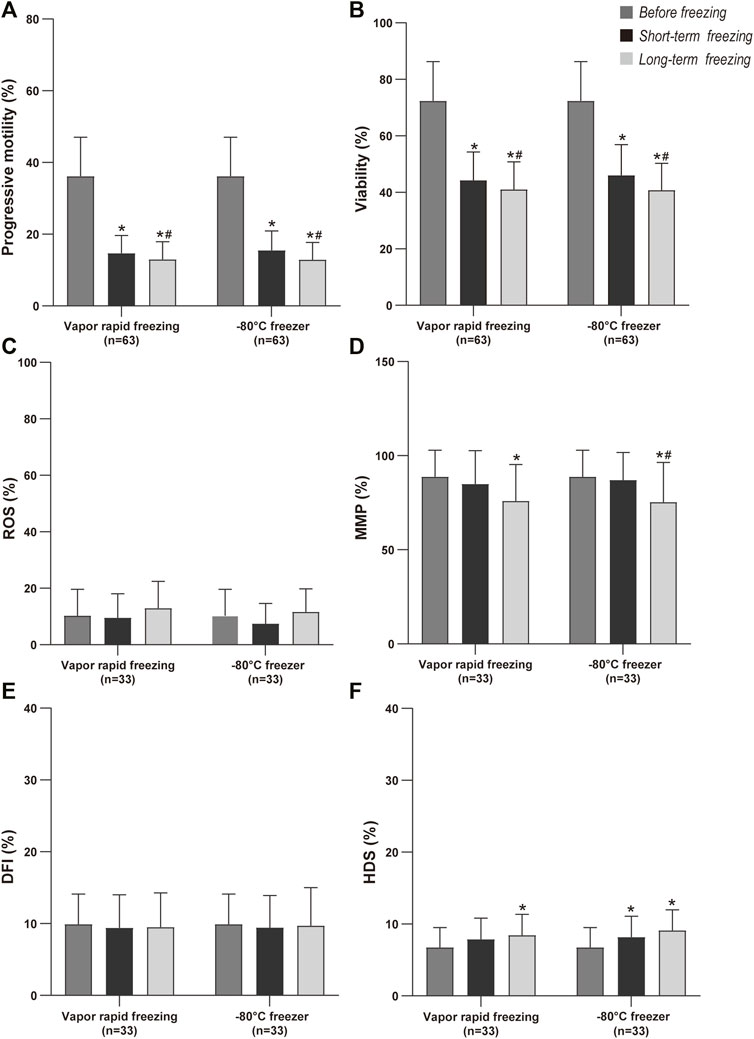
FIGURE 3. Comparison of sperm quality by cryopreservation time before freezing, short term and long-term freezing. Values are mean and bar are SD; *p-value < 0.05 compare with before freezing (paired t-test); #p-value < 0.05 compare with short-term freezing (paired t-test).
A total of 30 men were included and significant differences (9.23 ± 4.61 vs. 14.62 ± 6.86 vs. 14.38 ± 6.05, p < 0.05) were observed for acrosome reaction (AR) within before and freeze-thawed spermatozoa (Figure 4). To be noted, there was no significant difference in the AR between vapor rapid freezing and −80°C freezer (14.62 ± 6.86 vs. 14.38 ± 6.05, p > 0.05).
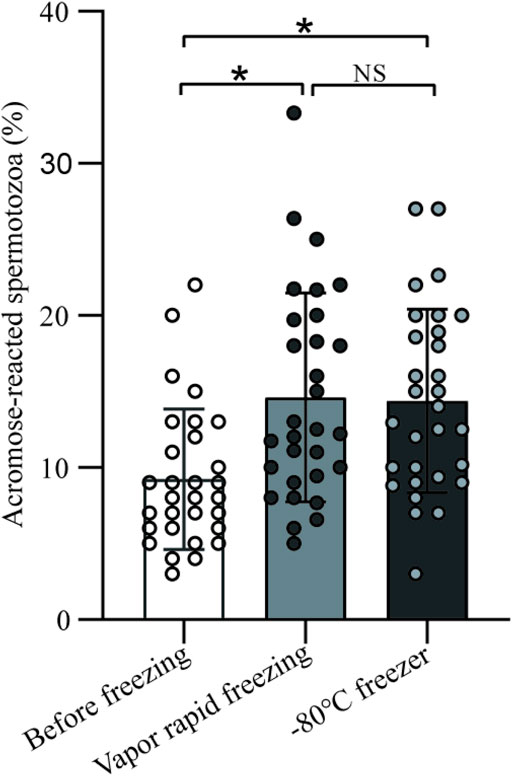
FIGURE 4. The impact on sperm acrosome of vapor rapid freezing and −80°C freezer. Sperm treated by freezing had significant higher acrosome reaction than before freezing (p-value < 0.05). There was no significant difference in the acrosome reaction between vapor rapid freezing and −80°C freezer (p-value > 0.05).
In regression analysis, we found that progressive motility in post-thaw spermatozoa was positively correlated with both progressive motility before freezing and the direct −80°C freezing methods but negatively correlated with ROS, DFI before freezing, and storage time (p < 0.05, Table 4). ROS in post-thaw spermatozoa was positively correlated with ROS before freezing and storage time (p < 0.05). DFI in post-thaw spermatozoa was positively correlated with DFI before freezing but negatively correlated with ROS before freezing (p < 0.05).
To analyze the outcomes of freeze-thawed spermatozoa collected by TESA, we compared the parameters of sperm conserved by vapor rapid freezing and stored at −80°C (Table 5). We counted a total of 62 motile sperm, accounting for 16.89% of the total sperm, when vapor rapid freezing was used. A total of 65 motile sperm, accounting for 17.02% of the total sperm, were counted when freezing at −80°C. There was no statistically significant difference between the two methods for freezing testicular sperm (p = 0.965).
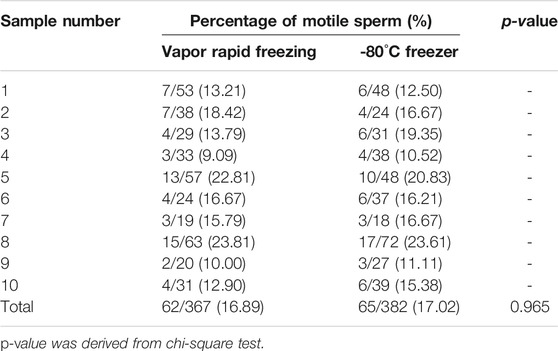
TABLE 5. Motile sperm percentage of testicular sperm samples after cryopreservation with vapor rapid freezing and -80°C freezer.
This study compared the effects of a −80°C freezer and vapor rapid freezing on sperm quality, and we found that there was no significant difference in progressive motility and viability across the different freezing methods. Correlation analysis showed that progressive motility, ROS, and DFI in the post-thaw spermatozoa were correlated with sperm characteristics before freezing, methods and storage time.
Some studies have reported that freezing-thawing decreases sperm quality and function (Satirapod et al., 2012; Lusignan et al., 2018; Le et al., 2019). For example, proteomic analysis of sperm showed significant changes in proteins related to motility, viability, and acrosomal integrity of sperm compared with the fresh state (Wang et al., 2014). It has also been suggested that rapid changes in intracellular ice crystal formation and osmolarity during the cryopreservation process may lead to changes in the carbohydrate composition and the membrane proteins, which can disrupt membrane structures and reduce sperm viability (Pedersen and Lebech, 1971). The production of ROS and lower antioxidant enzyme activity in sperm induce apoptotic pathways, which can lead to a reduction in sperm viability (Di Santo et al., 2012). In our study, we found the same results as previous research that showed that after freezing-thawing, sperm viability and progressive motility were both decreased significantly. ROS increased and MMP continued to decrease as the freezing time extended. However, compared with vapor rapid freezing, there was no significant difference in progressive motility, viability, MMP or DFI of sperm frozen in a −80°C freezer. Previously, Rahana et al. (2011) also reported similar results that there was no significant difference in human sperm motility and DFI between -85°C and conventional liquid nitrogen cryopreservation. Previous studies observed a decrease in AR after the freezing-thawing process (Gomez-Torres et al., 2017; Rahiminia et al., 2017). Here we also observed that the acrosome status was vulnerable to the freezing-thawing process, but the percentages of AR decrease for different cryopreservation methods was similar, which further confirmed the efficiency of −80°C freezer.
In regression analysis, compared with vapor rapid freezing, −80°C freezing was positively correlated with sperm progressive motility. Our study also indicated that freezing in a −80°C freezer resulted in lower ROS than vapor rapid freezing, which confirmed that a slow temperature drop would result in lower ROS damage. During freezing, the cooling rate of vapor rapid freezing was quicker than that of the −80°C freezer, and thus vapor rapid freezing took less time to reach the freezing point and had a lower freezing point temperature than the −80°C freezer (Supplementary Table 1). In the freezing process, water tends to chill beyond its freezing point without forming ice, which is known as supercooling. Studies have confirmed that super-cooling can cause damages of sperm in mice and human (Check et al., 1995; Mazur and Koshimoto, 2002). Compared with vapor rapid freezing, the samples frozen in a −80°C freezer have a higher freezing point that is closer to −6°C (Supplementary Table 1). The WHO (2010) first recommended that a human sperm freezing program decline from RT (22–25°C) to −6°C, and we speculated that −6°C was the freezing point of semen with cryoprotectant. Therefore, samples in a −80°C freezer were less supercooled than vapor rapid freezing, which may result in less damage. Notably, the HDS of sperm frozen in a −80°C freezer for a long time was higher than that in liquid nitrogen. Compared with vapor rapid freezing, the −80°C freezer has a more stable and slower cooling rate and can meet the demand for sperm freezing so that the freezing step is simplified. Thus, although liquid nitrogen storage is irreplaceable and is still the first choice for long-term sperm cryopreservation at a low temperature of −196°C, a −80°C freezer can be an alternative method for short-term sperm storage.
In this study, we used 1.8 ml cryotubes and a −80°C freezer to simplify the steps of freezing testicular sperm. We have proven that −80°C freezers and vapor rapid freezing have similar effects on testicular sperm. Previously, the method of freezing single sperm captured under a microscope using intracytoplasmic sperm injection (ICSI) pipettes equipped with a micromanipulator is widely used for testicular sperm (Coetzee et al., 2016). However, more sperm can be obtained after freezing all the testicular sperm in a freezing tube and a −80°C freezer compared with the freezing of single sperm. Using cryotubes will provide enough sperm after freezing-thawing, and it is better to select motile sperm for ICSI. Furthermore, the efficiency of single sperm freezing is low and cannot meet the requirements of storing numerous samples. The freezing of testicular sperm in a −80°C freezer in a freezer tube ensures that all sperm in the testicular tissue are captured, it only takes a short time and simplifies the process, and several samples can be processed simultaneously.
The present study was based on a limited sample size and normal semen meeting the WHO standard, and further studies of −80°C freezers in clinical applications with sperm samples from oligo-astheno-teratozoospermia are warranted. This was a preliminary study to improve the freezing of testicular sperm, and it was necessary to freeze tremendous testicular sperm samples to avoid instability. In vitro fertilization tests, follow-up embryo development, and implantation are also the focus of future research.
In conclusion, this study demonstrated that short-term storage of sperm at −80°C freezer could be a viable alternative to liquid nitrogen vapor rapid freezing at −196°C due to their comparable post-thaw results and lower ROS. During long-term freezing, the −80°C freezer is expected to be a cooling process that can provide an option except for liquid nitrogen vapor rapid freezing. An improved sperm freezing process was also preliminarily explored in this study. Additional study is necessary to confirm the clinical value of the freezing testicular sperm method.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of The First Affiliated Hospital of the University of Science and Technology of China. The patients/participants provided their written informed consent to participate in this study.
XW and FL contributed to sample preparation, sample analysis, data analysis, and wrote the manuscript. SB and XJ contributed to manuscript preparation, supervised the analysis, and critically revised the manuscript. LW, LH, NZ and RJ recruited study subjects, participated in sample collection, and provided clinical information. BX and YW recruited study subjects and controls. SB and XJ performed the statistical analysis. XT and XJ conceptualized and designed the study, supervised the analysis, critically revised the manuscript, edited the paper, and gave final approval. All authors read and approved the final manuscript.
This work was supported by National Key R&D Program of China (2018YFC1003900), National Natural Science Foundation of China (No.81971446), Open Project Fund from Key Laboratory of Reproduction Regulation of NHC (KF 2020-07) and Natural Science Foundation of Qinghai (2019-HZ-823).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the teacher and staff of Reproductive Center of the First Affiliated Hospital of University of Science and Technology of China. We also thank the technicians and research staff of the Institute of graduate, Anhui Medical University.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.799504/full#supplementary-material
DFI, DNA fragmentation index; FITC-PSA, fluorescein isothiocyanate-pisum sativum agglutinin; HDS, high DNA stainability; ICSI, intracytoplasmic sperm injection; IVF, in vitrofertilization; MMP, mitochondrial membrane potential; RT, room temperature; ROS, reactive oxygen species; SCSA, the sperm chromatin structure assay; CASA, computer-assisted semen analysis; SPSS, statistical product and service solutions; SD, standard deviations; TESA, testicular sperm aspiration; ZP, zona pellucida, AR, acrosome reaction.
Berkovitz, A., Miller, N., Silberman, M., Belenky, M., and Itsykson, P. (2018). A Novel Solution for Freezing Small Numbers of Spermatozoa Using a Sperm Vitrification Device. Hum. Reprod. 33 (11), 1975–1983. doi:10.1093/humrep/dey304
Check, D. J., Katsoff, D., and Check, J. H. (1995). Effect of an Intermediate Hold with Vapor Freezing on Subsequent Hypoosmotic Swelling in Thawed Sperm. Arch. Androl. 35 (1), 79–81. doi:10.3109/01485019508987857
Coetzee, K., Ozgur, K., Berkkanoglu, M., Bulut, H., and Isikli, A. (2016). Reliable Single Sperm Cryopreservation in Cell Sleepers for Azoospermia Management. Andrologia 48 (2), 203–210. doi:10.1111/and.12434
Cohen, J., Garrisi, G. J., Congedo-Ferrara, T. A., Kieck, K. A., Schimmel, T. W., and Scott, R. T. (1997). Cryopreservation of Single Human Spermatozoa. Hum. Reprod. 12 (5), 994–1001. doi:10.1093/humrep/12.5.994
Desai, N., Culler, C., and Goldfarb, J. (2004). Cryopreservation of Single Sperm from Epididymal and Testicular Samples on Cryoloops: Preliminary Case Report. Fertil. Sterility 82, S264–S265. doi:10.1016/j.fertnstert.2004.07.706
Di Santo, M., Tarozzi, N., Nadalini, M., and Borini, A. (2012). Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012, 1–12. doi:10.1155/2012/854837
Endo, Y., Fujii, Y., Kurotsuchi, S., Motoyama, H., and Funahashi, H. (2012). Successful Delivery Derived from Vitrified-Warmed Spermatozoa from a Patient with Nonobstructive Azoospermia. Fertil. Sterility 98 (6), 1423–1427. doi:10.1016/j.fertnstert.2012.07.1128
Evenson, D. P., Darzynkiewicz, Z., and Melamed, M. R. (1980). Relation of Mammalian Sperm Chromatin Heterogeneity to Fertility. Science 210 (4474), 1131–1133. doi:10.1126/science.7444440
Gomez-Torres, M. J., Medrano, L., Romero, A., Fernández-Colom, P. J., and Aizpurúa, J. (2017). Effectiveness of Human Spermatozoa Biomarkers as Indicators of Structural Damage during Cryopreservation. Cryobiology 78, 90–94. doi:10.1016/j.cryobiol.2017.06.008
Gwatkin, R. B. L. (1991). “Handbook of the Laboratory Diagnosis and Treatment of Infertility,” in Molecular Reproduction and Development. Editors B. Keel, and B. Webster. (Boca Raton, FL: CRC Press), 29, 431. doi:10.1002/mrd.1080290117
Hsieh, Y.-Y., Tsai, H.-D., Chang, C.-C., and Lo, H.-Y. (2000). Cryopreservation of Human Spermatozoa within Human or Mouse Empty Zona Pellucidae. Fertil. Sterility 73 (4), 694–698. doi:10.1016/s0015-0282(99)00612-3
Huang, W.-J., Zhang, D., Hong, Z.-W., Chen, Z.-B., Dong, L.-H., Zhang, Y., et al. (2020). Sequential Interval Micro-droplet Loading in Closed Hemi-Straw Carrier System: A Convenient and Efficient Method for Ultra-rapid Cryopreservation in Extreme Oligozoospermia. Cryobiology 93, 75–83. doi:10.1016/j.cryobiol.2020.02.005
Just, A., Gruber, I., Wöber, M., Lahodny, J., Obruca, A., and Strohmer, H. (2004). Novel Method for the Cryopreservation of Testicular Sperm and Ejaculated Spermatozoa from Patients with Severe Oligospermia: A Pilot Study. Fertil. Sterility 82 (2), 445–447. doi:10.1016/j.fertnstert.2003.12.050
Le, M. T., Nguyen, T. T. T., Nguyen, T. T., Nguyen, V. T., Nguyen, T. T. A., Nguyen, V. Q. H., et al. (2019). Cryopreservation of Human Spermatozoa by Vitrification versus Conventional Rapid Freezing: Effects on Motility, Viability, Morphology and Cellular Defects. Eur. J. Obstet. Gynecol. Reprod. Biol. 234, 14–20. doi:10.1016/j.ejogrb.2019.01.001
Liu, T., Gao, J., Zhou, N., Mo, M., Wang, X., Zhang, X., et al. (2016). The Effect of Two Cryopreservation Methods on Human Sperm DNA Damage. Cryobiology 72 (3), 210–215. doi:10.1016/j.cryobiol.2016.04.004
Lusignan, M. F., Li, X., Herrero, B., Delbes, G., and Chan, P. T. K. (2018). Effects of Different Cryopreservation Methods on DNA Integrity and Sperm Chromatin Quality in Men. Andrology 6 (6), 829–835. doi:10.1111/andr.12529
Mahfouz, R., Sharma, R., Lackner, J., Aziz, N., and Agarwal, A. (2009). Evaluation of Chemiluminescence and Flow Cytometry as Tools in Assessing Production of Hydrogen Peroxide and Superoxide Anion in Human Spermatozoa. Fertil. Sterility 92 (2), 819–827. doi:10.1016/j.fertnstert.2008.05.087
Mazur, P., and Koshimoto, C. (2002). Is Intracellular Ice Formation the Cause of Death of Mouse Sperm Frozen at High Cooling Rates? Biol. Reprod. 66, 1485–1490. doi:10.1095/biolreprod66.5.1485
Miller, N., Biron-Shental, T., Pasternak, Y., Belenky, M., Shefi, S., Itsykson, P., et al. (2017). Fertility Outcomes after Extended Searches for Ejaculated Spermatozoa in Men with Virtual Azoospermia. Fertil. Sterility 107 (6), 1305–1311. doi:10.1016/j.fertnstert.2017.04.005
Parkes, A. S. (1945). Preservation of Spermatozoa at Low Temperatures. Bmj 2 (4415), 212–213. doi:10.1136/bmj.2.4415.212
Pedersen, H., and Lebech, P. E. (1971). Ultrastructural Changes in the Human Spermatozoon after Freezing for Artificial Insemination. Fertil. Sterility 22 (2), 125–133. doi:10.1016/S0015-0282(16)38048-7
Pezo, F., Cheuquemán, C., Salinas, P., and Risopatrón, J. (2017). Freezing Dog Semen Using −80 °C Ultra-freezer: Sperm Function and In Vivo Fertility. Theriogenology 99, 36–40. doi:10.1016/j.theriogenology.2017.05.007
Rahana, A. R., Ng, S. P., Leong, C. F., and Rahimah, M. D. (2011). Comparison between Mechanical Freezer and Conventional Freezing Using Liquid Nitrogen in Normozoospermia. Singapore Med. J. 52 (10), 734–737. PubMed (nih.gov).
Rahiminia, T., Hosseini, A., Anvari, M., Ghasemi-Esmailabad, S., and Talebi, A. R. (2017). Modern Human Sperm Freezing: Effect on DNA, Chromatin and Acrosome Integrity. Taiwanese J. Obstet. Gynecol. 56 (4), 472–476. doi:10.1016/j.tjog.2017.02.004
Rarani, F. Z., Golshan-Iranpour, F., and Dashti, G. R. (2019). Correlation between Sperm Motility and Sperm Chromatin/DNA Damage before and after Cryopreservation and the Effect of Folic Acid and Nicotinic Acid on post-thaw Sperm Quality in Normozoospermic Men. Cell Tissue Bank 20 (3), 367–378. doi:10.1007/s10561-019-09775-6
Raspa, M., Fray, M., Paoletti, R., Montoliu, L., Giuliani, A., and Scavizzi, F. (2018b). A New, Simple and Efficient Liquid Nitrogen Free Method to Cryopreserve Mouse Spermatozoa at −80 °C. Theriogenology 119, 52–59. doi:10.1016/j.theriogenology.2018.06.020
Raspa, M., Fray, M., Paoletti, R., Montoliu, L., Giuliani, A., and Scavizzi, F. (2018a). Long Term Maintenance of Frozen Mouse Spermatozoa at −80 °C. Theriogenology 107, 41–49. doi:10.1016/j.theriogenology.2017.10.036
Raspa, M., Guan, M., Paoletti, R., Montoliu, L., Ayadi, A., Marschall, S., et al. (2017). Dry Ice Is a Reliable Substrate for the Distribution of Frozen Mouse Spermatozoa: A Multi-Centric Study. Theriogenology 96, 49–57. doi:10.1016/j.theriogenology.2017.04.003
Royere, D., Barthelemy, C., Hamamah, S., and Lansac, J. (1996). Cryopreservation of Spermatozoa: a 1996 Review. Hum. Reprod. Update 2 (6), 553–559. doi:10.1093/humupd/2.6.553
Sanchez, R., Risopatrón, J., Schulz, M., Villegas, J. V., Isachenko, V., and Isachenko, E. (2012). Vitrified Sperm banks: the New Aseptic Technique for Human Spermatozoa Allows Cryopreservation at −86 °C. Andrologia 44 (6), 433–435. doi:10.1111/j.1439-0272.2012.01314.x
Satirapod, C., Treetampinich, C., Weerakiet, S., Wongkularb, A., Rattanasiri, S., and Choktanasiri, W. (2012). Comparison of Cryopreserved Human Sperm from Solid Surface Vitrification and Standard Vapor Freezing Method: on Motility, Morphology, Vitality and DNA Integrity. Andrologia 44 (s1), 786–790. doi:10.1111/j.1439-0272.2011.01267.x
Sereni, E., Bonu, M., Fava, L., Sciajno, R., Serrao, L., Preti, S., et al. (2008). Freezing Spermatozoa Obtained by Testicular fine Needle Aspiration: a New Technique. Reprod. BioMedicine Online 16 (1), 89–95. doi:10.1016/S1472-6483(10)60560-3
Sherman, J. K. (1973). Synopsis of the Use of Frozen Human Semen since 1964: State of the Art of Human Semen Banking. Fertil. Sterility 24 (5), 397–412. doi:10.1016/S0015-0282(16)39678-9
Tao, Y., Sanger, E., Saewu, A., and Leveille, M.-C. (2020). Human Sperm Vitrification: the State of the Art. Reprod. Biol. Endocrinol. 18 (1), 17. doi:10.1186/s12958-020-00580-5
Trottmann, M., Becker, A. J., Stadler, T., Straub, J., Soljanik, I., Schlenker, B., et al. (2007). Semen Quality in Men with Malignant Diseases before and after Therapy and the Role of Cryopreservation. Eur. Urol. 52 (2), 355–367. doi:10.1016/j.eururo.2007.03.085
Vaz, C. R., Lamim, T., Salvador, R. A., Batschauer, A. P. B., Amaral, V. L. L., and Til, D. (2018). Could Cryopreserved Human Semen Samples Be Stored at -80°C? JBRA Assist. Reprod. 22 (2), 108–112. doi:10.5935/1518-0557.20180016
Wang, S., Wang, W., Xu, Y., Tang, M., Fang, J., Sun, H., et al. (2014). Proteomic Characteristics of Human Sperm Cryopreservation. Proteomics 14 (2-3), 298–310. doi:10.1002/pmic.201300225
Keywords: human sperm, cryopreservation, −80°C freezer, testicular sperm, liquid nitrogen vapor rapid freezing
Citation: Wang X, Lu F, Bai S, Wu L, Huang L, Zhou N, Xu B, Wan Y, Jin R, Jiang X and Tong X (2022) A Simple and Efficient Method to Cryopreserve Human Ejaculated and Testicular Spermatozoa in −80°C Freezer. Front. Genet. 12:815270. doi: 10.3389/fgene.2021.815270
Received: 15 November 2021; Accepted: 27 December 2021;
Published: 28 January 2022.
Edited by:
Mengcheng Luo, Wuhan University, ChinaReviewed by:
Jinmin Gao, Shandong Normal University, ChinaCopyright © 2022 Wang, Lu, Bai, Wu, Huang, Zhou, Xu, Wan, Jin, Jiang and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Jiang, YmlvanhoQHVzdGMuZWR1LmNu; Xianhong Tong, dG9uZzY4eGlhbmhvbmdAMTYzLmNvbQ==, Rentao Jin, anJ0YW8yMDAxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.