
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 03 January 2022
Sec. Genetics of Common and Rare Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.809256
This article is part of the Research TopicGenetics of Familial Hypercholesterolemia: New Insight - Volume IIView all 17 articles
 Antoine Rimbert1†
Antoine Rimbert1† Hinda Daggag2†
Hinda Daggag2† Peter Lansberg1
Peter Lansberg1 Adam Buckley2
Adam Buckley2 Martijn Viel3
Martijn Viel3 Roan Kanninga3
Roan Kanninga3 Lennart Johansson3
Lennart Johansson3 Robin P. F. Dullaart4
Robin P. F. Dullaart4 Richard Sinke3
Richard Sinke3 Alia Al Tikriti2
Alia Al Tikriti2 Jan Albert Kuivenhoven1*‡
Jan Albert Kuivenhoven1*‡ Maha Taysir Barakat2‡
Maha Taysir Barakat2‡Background: Programs to screen for Familial hypercholesterolemia (FH) are conducted worldwide. In Western societies, these programs have been shown to be cost-effective with hit/detection rates of 1 in 217–250. Thus far, there is no published data on genetic FH in the Gulf region. Using United Arab Emirates as a proxy for the Gulf region, we assessed the prevalence of genetically confirmed FH in the Emirati population sample.
Materials and Methods: We recruited 229 patients with LDL-C >95th percentile and employed a customized next generation sequencing pipeline to screen canonical FH genes (LDLR, APOB, PCSK9, LDLRAP1).
Results: Participants were characterized by mean total cholesterol and low-density lipoprotein cholesterol (LDL-c) of 6.3 ± 1.1 and 4.7 ± 1.1 mmol/L respectively. Ninety-six percent of the participants were using lipid-lowering medication with mean corrected LDL-c values of 10.0 ± 3.0 mmol/L 15 out of 229 participants were found to suffer from genetically confirmed FH. Carriers of causal genetic variants for FH had higher on-treatment LDL-c compared to those without causal variants (5.7 ± 1.5 vs 4.7 ± 1.0; p = 3.7E-04). The groups did not differ regarding high-density lipoprotein cholesterol, triglycerides, body mass index, blood pressure, glucose, and glycated haemoglobin.
Conclusion: This study reveals a low 7% prevalence of genetic FH in Emiratis with marked hypercholesterolemia as determined by correcting LDL-c for the use of lipid-lowering treatment. The portfolio of mutations identified is, to a large extent, unique and includes gene duplications. Our findings warrant further studies into origins of hypercholesterolemia in these patients. This is further supported by the fact that these patients are also characterized by high prevalence of type 2 diabetes (42% in the current study cohort) which already puts them at an increased risk of atherosclerotic cardiovascular disease. These results may also be useful in public health initiatives for FH cascade screening programs in the UAE and maybe the Gulf region.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in the Middle East (Bamimore et al., 2015). Hypercholesterolemia, characterised by increased levels of low-density lipoprotein cholesterol (LDL-c), is a major established risk factor for ASCVD (Nordestgaard et al., 2013; Ference et al., 2017). It is well-established that functional mutations in LDLR (Brown and Goldstein, 1974), APOB (Innerarity et al., 1987) and PCSK9 (Abifadel et al., 2003) can cause autosomal dominant hypercholesterolemia and that mutations in LDLRAP1 (Ceska et al., 2019) can cause autosomal recessive hypercholesterolemia. Combined, this set of genetic disorders is generally referred to as familial hypercholesterolemia (FH). Since patients with FH are characterized by a 22-fold increased risk for ASCVD, compared to non-carriers with comparable LDL-c concentrations (Abul-Husn et al., 2016; Khera et al., 2016), knowing the aetiology of severe hypercholesterolemia is of major importance for an early diagnosis and pro-active cardiovascular healthcare.
Programs to identify patients with FH are conducted worldwide to improve management and enable early prevention through cascade screening (Vallejo-Vaz et al., 2018; Ceska et al., 2019). These initiatives (reviewed in (Knowles et al., 2017)) have been shown to reduce the average age at which individuals with genetic FH are diagnosed, to improve treatment initiation/adherence (Perez de Isla et al., 2016), and reduce LDL-c and ASCVD in a cost-effective way (Wonderling et al., 2004; Nherera et al., 2011).
The prevalence of clinically defined FH is currently estimated to be 1:217–250 across different Caucasian populations (Benn et al., 2016; de Ferranti et al., 2016). In patients who are referred to specialized lipid clinics, the proportion of genetically confirmed cases can be 50% or more depending on the inclusion criteria (Wang et al., 2016).
A reportedly high prevalence of clinical FH in the Arabian Gulf region (Al-Rasadi et al., 2018a) recently increased awareness of severe dyslipidaemias and a need for cascade screening. Several promising large-scale initiatives are currently under way, but to date publicly available data on a genetic diagnosis of FH in this part of the world are very limited (Bamimore et al., 2015; Al-Rasadi et al., 2018a).
In this study, we screened patients that were referred to the Imperial College London Diabetes Centre in Abu Dhabi (ICLDC), for LDL-C above the 95% percentile. Ninety-six percent of the patients were using lipid-lowering medication while 42% were suffering from type 2 diabetes. The mean LDL-C value corrected for lipid-lowering medication was 10 mmol/L but we could only identify a genetic origin for FH in 7% of the patients.
229 unrelated Emiratis were recruited using the LDL-C cut-off levels used in the Gulf FH Registry final protocol conditions (https://gulfheart.org) [(Al-Rasadi et al., 2018a; Al-Rasadi et al., 2018b) and Supplemental methods]. In brief, adult patients were recruited by Imperial College London Diabetes Centre in Abu Dhabi (ICLDC), presenting with plasma LDL-c concentrations higher than 4.9 mmol/L (with or without lipid lowering treatment) and plasma triglycerides <5 mmol/L. Patients suffering from history of untreated hypothyroidism; history of proteinuria ≥1g/L; history of obstructive liver disease; history of chronic renal failure; human immunodeficiency virus infection or on immunosuppressant or steroid or psychiatric medications, were excluded. Data on family history and physical examination were not available. Ethical approval was obtained from ICLDC Research Ethics Committee. Participants were consented for research by a research patient recruitment officer and were asked for additional blood samples during their regular clinical visit to ICLDC.
All samples were analysed for autosomal dominant or autosomal recessive hypercholesterolemia (LDLR, APOB, PCSK9, LDLRAP1) using a custom targeted next generation sequencing gene panel. The analysis pipeline focused on: 1- rare genetic variants in the general population [including a control population from ME countries (Scott et al., 2016)]; 2- genetic variants affecting coding and splicing regions of targeted genes or located in promoter regions of LDLR. Pathogenicity of identified variants was determined using clinical genetic databases and in silico prediction algorithms (see Supplemental Methods). The targeted genes were tested for copy number variations (CNV) using the same analysis pipeline (Johansson et al., 2016; Balder et al., 2018) and validated using the Infinium Global Screening Array (Illumina®). Full details of the technical approach are provided in the supplementary methods.
T-test was used to compare the lipid parameters between carriers of causal variants in LDLR, APOB and PCSK9 and non-carriers and Chi2 test was used to compare the proportion of participants between subgroups.
Baseline characteristics of the 229 participants (118 women and 111 men) are shown in Table 1. Mean age of the participants at consent was 46 years (±10). Patients presented with average body mass index of 31 (±6). On treatment plasma lipids and lipoproteins were as follows: Total cholesterol 6.3 mmol/L (±1.1), LDL-c: 4.7 mmol/L (±1.1), High-density lipoprotein cholesterol (HDL-c): 1.3 mmol/L (±0.3); Triglycerides: 2.0 mmol/L (±0.9). Ninety-six percent of the patients were using lipid-lowering drugs (Rosuvastatin (n = 106), Atorvastatin (n = 71), Pitavastatin (n = 6); Simvastatin (n = 2), Rosuvastatin+Ezetimibe (n = 22), Atorvastatin+Ezetimibe (n = 10), Simvastatin+Ezetimibe (n = 2); and Ezetimibe (n = 1). When corrected for the reported use of lipid-lowering drugs (conversion factors are given in Supplementary Table S1), the corrected LDL-c was on average 10 mmol/L (±3.0).
Targeted sequencing rendered a mean coverage depth of 678X (±263) for each base with 98% (±0.01) of the targeted regions covered at least 30 times. This result allowed for an efficient and robust detection of heterozygous variants as well as copy number variations calling.
Fifteen out of 229 participants (7%) were diagnosed with genetically defined FH (FH+). Thirteen variants were located in coding (missense or frameshift mutations) or promoter regions of LDLR gene; one in APOB and one in PCSK9 (Table 2). Two rare causal variants were identified only twice which does not support a hypothesis of large impact of founder mutations in Emiratis, as is the case in the Lebanese population (Abifadel et al., 2009). We did not observe known causal rare variants in the APOB gene which in Western countries accounts for 13% of the FH cases (Abul-Husn et al., 2016; Khera et al., 2016) but we did identify 28 rare APOB variants of unknown clinical significance (Supplementary Table S2). Follow-up studies are needed to better understand and determine the potential causality of these variants.
Of the mutations identified, none were previously reported in the Middle East so far (Bamimore et al., 2015). Among the fifteen identified causative FH mutations, LDLR p. Asn169fs, p. Cys243Tyr and p. Gly537fs are reported for the first time worldwide according to current publicly available information.
Two participants were identified as compound heterozygotes. The first case (Table 2; ID:#53), a 41 years old woman with LDL-c of 9.4 mmol/L on lipid-lowering treatment (Simvastatin 20 mg + Ezetimibe 10 mg) (corrected LDL-c = 18.8 mmol/L), carried two mutations in LDLR (p.Asp178Asn and p. Cys243Tyr). The genetic analysis performed does not allow for determining if these mutations are located on the same allele. The second case (Table 2; ID:#423), a 45 years old man with LDL-c of 6.8 mmol/L on lipid-lowering treatment (Rosuvastatin 20 mg + Ezetimibe 10 mg) (corrected LDL-c = 18.2 mmol/L), carried a variant in LDLR (p.Asp477Asn) and one in PCSK9 (p.Leu108Arg), both previously reported as FH causal mutations.
Here we report a duplication of the entire gene APOB in a 29 year old man (Table 2; ID:#662) with LDL-c of 6.5 mmol/L under lipid-lowering treatment (Rosuvastatin 20 mg; corrected LDL-c = 17.5 mmol/L). This ∼1.2 Mb long duplication [chr2: 21,130,084–22,324,616 (Hg19)] has been validated using Global Screening Array (Supplemental Methods and Supplementary Figure S1). Whether 3 APOB gene copies can cause FH is topic of on-going studies, but this specific case illustrates the potential of next generation sequencing to improve our understanding of previously unexplained FH.
Compared to FH-, the FH+ group had significantly higher total cholesterol (7.2 vs. 6.2 mmol/L; p = 2.1e-03), on treatment LDL-c (5.7 vs 4.7 mmol/L; p = 3.7e-04), and corrected LDL-c (9.8 mmol/L (±2.7) vs. 13.9 mmol/L (±4.5); p = 2.1E-07). The FH− and FH+ group were similar with regard to gender distribution, age, BMI, blood pressure, plasma glucose concentration, HbA1c, HDL-c, and triglycerides plasma levels (Table 3).
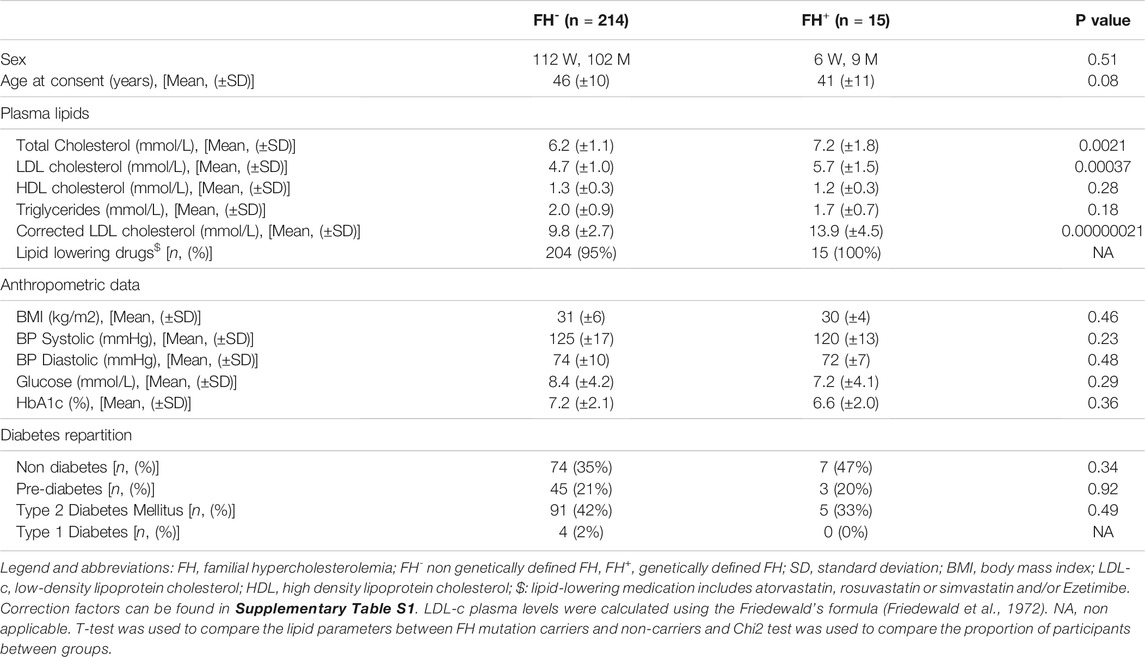
TABLE 3. Differences between participants with (FH+) and without (FH-) rare causal variants in LDLR, APOB, and PCSK9.
We further evaluated the proportion of patients with FH within different LDL-c ranges using on-treatment or corrected LDL-c values. We observed that the chance of identifying monogenic origins of hypercholesterolemia only tends to increase with on treatment LDL-c levels (Figure 1A). In a second step, we used LDL-c values, corrected for lipid-lowering medication with the following cut-offs: <8 mmol/L, >8 to 12 mmol/L, >12 mmol/L (Figure 1B). Here, we show that the chances of finding patients with genetic FH increase significantly over the three groups (p = 3.6E-3). In the group with corrected LDL-c above 12 mmol/L, 21% was found to be mutation positive.
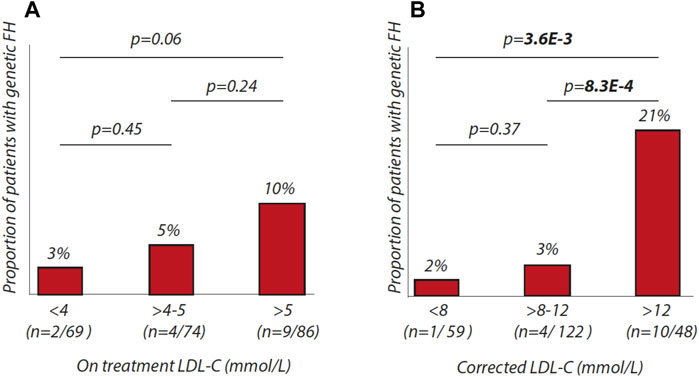
FIGURE 1. Percentages of patients with genetic FH in different LDL-C ranges. (A) proportion of patients with genetic FH according to on-treatment LDL-C ranges (<4 mmol/L, > 4 to 5 mmol/L, > 5 mmol/L). (B) proportion of patients with genetic FH according to corrected-LDL-C ranges (<8 mmol/L, > 8 to 12 mmol/L, > 12 mmol/L).
The current study demonstrates that in the studied cohort of Emirati participants, we have been able to identify a genetic origin of FH in 1 out of 15 individuals with an LDL-c>95th percentile.
It is tempting to speculate that the low prevalence of FH in our study is related to the fact that these patients are characterized by not only hypercholesterolemia but also increased triglycerides and low HDL-c and recruited at the Imperial College London Diabetes Centre in Abu Dhabi, a specialised diabetes centre. In our study, participants have an average BMI of 31, fasting glucose of 8.3 mmol while 57 % were on anti-diabetic medication. Similar characteristics were seen in the Gulf FH registry (Al-Rasadi et al., 2018b). We believe, however, that it is unlikely that inclusion of patients with pre-diabetes or type 2 diabetes in our study can explain the low prevalence of genetic FH compared to studies in Western countries as insulin-resistance is generally not characterised by high LDL-c concentrations but increased levels of triglycerides and decreased levels of HDL-c (Feingold et al., 1992). On the other hand, carriers of FH causal variants are typically characterized by an isolated high LDL-c. It appears that FH causing variants in our study confer classic hypercholesterolemia on top of an unfavourable metabolic plasma lipid phenotype. This is illustrated by the fact that FH+ and FH- patients are similar when it comes to age, BMI, blood pressure, high sensitivity CRP, plasma glucose concentration, HbA1c, HDL-c, and triglycerides plasma levels. This observation may warrant attention as patients referred to a diabetes centre can also suffer from hypercholesterolemia which likely puts them at an even higher risk of ASCVD which is especially true for identified with causal mutations in LDL genes.
Taken the lifelong genetic burden of increased LDL-c in the 15 identified FH+ patients combined with their detrimental metabolic phenotype; the cardiovascular consequences are anticipated to be more serious than observed in classical FH. The relatively young age of our study cohort (45 yrs), however, makes it difficult to evaluate their atherosclerotic burden and cardiac complications but it is noteworthy that ASCVD-associated mortality rate in the Middle East is one of the highest worldwide while the mean age of patients suffering from myocardial infarction in this part of the world is 10–12 years younger when compared to Western countries (Ramahi, 2010; Gehani et al., 2014).
This brings us to the FH- patients for which there is no explanation for their hypercholesterolemia. Since in this study FH+ and FH- patients have similar metabolic phenotypes, it appears unlikely that this trait would drive hypercholesterolemia in FH- patients. Having excluded causal canonical gene defects, one can imagine that a better understanding of factors driving hypercholesterolemia in our FH- Emirati cohort may provide novel insights into lipid metabolism. Additional studies are in this regard needed to address the origin of hypercholesterolemia in the FH- patients to search for novel genes or other factors that can explain their phenotype.
Our study presents limitations. First of all, we had to account for the absence of pre-treatment LDL-c levels while 96% of our study participants were using lipid-lowering medication. Using widely used adjustment criteria for various lipid-lowering drugs (Wensel et al., 2010; Haralambos et al., 2015), we calculated pre-treatment LDL-c levels to be on average 10 mmol/l in our study cohort which we consider a solid basis to start screening for mutations in genes involved in LDL metabolism. To illustrate this point, the DLCN indicates that only LDL-c values above 8.5 mmol/l can be categorized as probable FH. This suggests that even in the case that adjustments would overestimate pre-treatment LDL-c levels, it is likely that true pre-treatment LDL-c levels are still well above 8 mmol/l. Our study also provides support that use of the corrections for LDL-c values are valid as we show that the chance of finding a mutation in LDL candidate genes increases significantly over three strata of estimated pre-treatment LDL-c (Figure 1). We were furthermore unable to retrieve information on family history and physical characteristics to complete the DLCN score. These parameters would, however, only have increased the DCLN scores which would further underline the need for molecular diagnostics in our cohort.
In conclusion, this study into the genetics of hypercholesterolemia in the selected Emirati population shows that chances of finding FH causing mutations are low (7%) when standard criteria are used, despite marked hypercholesterolemia. Taking into consideration the limitations of the study, further investigations are needed to explain the discrepancy with similar studies in Western societies. The study, however, warrants further consideration in regard to FH screening initiatives in the Gulf region and further highlights the health risks in both FH- and FH+ individuals with hypercholesterolemia, obesity and type 2 diabetes.
All datasets presented in this study are included in the article/Supplementary Material.
This study was reviewed and approved by ICLDC Research Ethics Committee. The participants provided their written informed consent to participate in this study.
HD and AA collected data; AR, MV, RK, and LJ were involved in the analysis; AR, RD, AB, HD, PL, RS, and JK were involved in design of the study, data interpretation and drafting paper.
This study is supported by Netherlands CardioVascular Research Initiative: “the Dutch Heart Foundation, Dutch Federation of University Medical Centers, Netherlands Organization for Health Research and Development and the Royal Netherlands Academy of Sciences” (CVON 2017–2020; Acronym Genius2 to Kuivenhoven) and by a personal grant of Prof. Kuivenhoven (Established Investigator of the Netherlands Heart Foundation; 2015T068). The study was also funded by ICLDC, covering all sample-related costs (including DNA extraction and analysis).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Bindu Shobi, Dalia Haj, Tariq Ghulam, Suma Antony and Alshafi Mohammed for their contribution to this project (recruitment, sample processing, data collection, manuscript revision).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.809256/full#supplementary-material
Abifadel, M., Rabès, J.-P., Jambart, S. l., Halaby, G., Gannagé-Yared, M.-H. l. n., Sarkis, A., et al. (2009). The Molecular Basis of Familial Hypercholesterolemia in Lebanon: Spectrum ofLDLRmutations and Role ofPCSK9as a Modifier Gene. Hum. Mutat. 30 (7), E682–E691. doi:10.1002/humu.21002
Abifadel, M., Varret, M., Rabès, J.-P., Allard, D., Ouguerram, K., Devillers, M., et al. (2003). Mutations in PCSK9 Cause Autosomal Dominant Hypercholesterolemia. Nat. Genet. 34 (2), 154–156. doi:10.1038/ng1161
Abul-Husn, N. S., Manickam, K., Jones, L. K., Wright, E. A., Hartzel, D. N., Gonzaga-Jauregui, C., et al. (2016). Genetic Identification of Familial Hypercholesterolemia within a Single U.S. Health Care System. Science 354 (6319), aaf7000. doi:10.1126/science.aaf7000
Al-Rasadi, K., Alhabib, K. F., Al-Allaf, F., Al-Waili, K., Al-Zakwani, I., AlSarraf, A., et al. (2018). The Gulf Familial Hypercholesterolemia Registry (Gulf FH): Design, Rationale and Preliminary Results. Curr. Vasc. Pharmacol.
Al-Rasadi, K., Al-Zakwani, I., Alsheikh-Ali, A. A., Almahmeed, W., Rashed, W., Ridha, M., et al. (2018). Prevalence, Management, and Outcomes of Familial Hypercholesterolemia in Patients with Acute Coronary Syndromes in the Arabian Gulf. J. Clin. Lipidol. 12 (3), 685–692. doi:10.1016/j.jacl.2018.02.003
American Diabetes Association (2018). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 41 (Suppl. 1), S13–S27. doi:10.2337/dc18-S002
Balder, J.-W., Rimbert, A., Zhang, X., Viel, M., Kanninga, R., van Dijk, F., et al. (2018). Genetics, Lifestyle, and Low-Density Lipoprotein Cholesterol in Young and Apparently Healthy Women. Circulation 137 (8), 820–831. doi:10.1161/circulationaha.117.032479
Bamimore, M. A., Zaid, A., Banerjee, Y., Al-Sarraf, A., Abifadel, M., Seidah, N. G., et al. (2015). Familial Hypercholesterolemia Mutations in the Middle Eastern and North African Region: a Need for a National Registry. J. Clin. Lipidol. 9 (2), 187–194. doi:10.1016/j.jacl.2014.11.008
Benn, M., Watts, G. F., Tybjærg-Hansen, A., and Nordestgaard, B. G. (2016). Mutations Causative of Familial Hypercholesterolaemia: Screening of 98 098 Individuals from the Copenhagen General Population Study Estimated a Prevalence of 1 in 217. Eur. Heart J. 37 (17), 1384–1394. doi:10.1093/eurheartj/ehw028
Brown, M. S., and Goldstein, J. L. (1974). Expression of the Familial Hypercholesterolemia Gene in Heterozygotes: Mechanism for a Dominant Disorder in Man. Science 185 (4145), 61–63. doi:10.1126/science.185.4145.61
Ceska, R., Latkovskis, G., Ezhov, M. V., Freiberger, T., Lalic, K., Mitchenko, O., et al. (2019). The Impact of the International Cooperation on Familial Hypercholesterolemia Screening and Treatment: Results from the ScreenPro FH Project. Curr. Atheroscler. Rep. 21 (9), 36. doi:10.1007/s11883-019-0797-3
de Ferranti, S. D., Rodday, A. M., Mendelson, M. M., Wong, J. B., Leslie, L. K., and Sheldrick, R. C. (2016). Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 133 (11), 1067–1072. doi:10.1161/circulationaha.115.018791
Feingold, K. R., Grunfeld, C., Pang, M., Doerrler, W., and Krauss, R. M. (1992). LDL Subclass Phenotypes and Triglyceride Metabolism in Non-insulin-dependent Diabetes. Arterioscler Thromb. 12 (12), 1496–1502. doi:10.1161/01.atv.12.12.1496
Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., et al. (2017). Low-density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38 (32), 2459–2472. doi:10.1093/eurheartj/ehx144
Friedewald, W. T., Levy, R. I., and Fredrickson, D. S. (1972). Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 18 (6), 499–502. doi:10.1093/clinchem/18.6.499
Gehani, A. A., Al-Hinai, A. T., Zubaid, M., Almahmeed, W., Hasani, M. R. M., Yusufali, A. H., et al. (2014). Association of Risk Factors with Acute Myocardial Infarction in Middle Eastern Countries: the INTERHEART Middle East Study. Eur. J. Prev. Cardiolog 21 (4), 400–410. doi:10.1177/2047487312465525
Haralambos, K., Whatley, S. D., Edwards, R., Gingell, R., Townsend, D., Ashfield-Watt, P., et al. (2015). Clinical Experience of Scoring Criteria for Familial Hypercholesterolaemia (FH) Genetic Testing in Wales. Atherosclerosis 240 (1), 190–196. doi:10.1016/j.atherosclerosis.2015.03.003
Innerarity, T. L., Weisgraber, K. H., Arnold, K. S., Mahley, R. W., Krauss, R. M., Vega, G. L., et al. (1987). Familial Defective Apolipoprotein B-100: Low Density Lipoproteins with Abnormal Receptor Binding. Proc. Natl. Acad. Sci. 84 (19), 6919–6923. doi:10.1073/pnas.84.19.6919
Johansson, L. F., van Dijk, F., de Boer, E. N., van Dijk-Bos, K. K., Jongbloed, J. D. H., van der Hout, A. H., et al. (2016). CoNVaDING: Single Exon Variation Detection in Targeted NGS Data. Hum. Mutat. 37 (5), 457–464. doi:10.1002/humu.22969
Khera, A. V., Won, H.-H., Peloso, G. M., Lawson, K. S., Bartz, T. M., Deng, X., et al. (2016). Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients with Severe Hypercholesterolemia. J. Am. Coll. Cardiol. 67 (22), 2578–2589. doi:10.1016/j.jacc.2016.03.520
Knowles, J. W., Rader, D. J., and Khoury, M. J. (2017). Cascade Screening for Familial Hypercholesterolemia and the Use of Genetic Testing. JAMA 318 (4), 381–382. doi:10.1001/jama.2017.8543
Nherera, L., Marks, D., Minhas, R., Thorogood, M., and Humphries, S. E. (2011). Probabilistic Cost-Effectiveness Analysis of cascade Screening for Familial Hypercholesterolaemia Using Alternative Diagnostic and Identification Strategies. Heart 97 (14), 1175–1181. doi:10.1136/hrt.2010.213975
Nordestgaard, B. G., Chapman, M. J., Humphries, S. E., Ginsberg, H. N., Masana, L., Descamps, O. S., et al. (2013). Familial Hypercholesterolaemia Is Underdiagnosed and Undertreated in the General Population: Guidance for Clinicians to Prevent Coronary Heart Disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 34 (45), 3478–3490. doi:10.1093/eurheartj/eht273
Perez de Isla, L., Alonso, R., Watts, G. F., Mata, N., Saltijeral Cerezo, A., Muñiz, O., et al. (2016). Attainment of LDL-Cholesterol Treatment Goals in Patients with Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 67 (11), 1278–1285. doi:10.1016/j.jacc.2016.01.008
Ramahi, T. M. (2010). Cardiovascular Disease in the Asia Middle East Region: Global Trends and Local Implications. Asia Pac. J. Public Health 22 (3 Suppl. l), 83S–89S. doi:10.1177/1010539510373034
Scott, E. M., Halees, A., Halees, A., Itan, Y., Spencer, E. G., He, Y., et al. (2016). Characterization of Greater Middle Eastern Genetic Variation for Enhanced Disease Gene Discovery. Nat. Genet. 48 (9), 1071–1076. doi:10.1038/ng.3592
EAS Familial Hypercholesterolaemia Studies Collaboration Vallejo-Vaz, A. J., De Marco, M., Stevens, C. A. T., Akram, A., Freiberger, T., Hovingh, G. K., et al. (2018). Overview of the Current Status of Familial Hypercholesterolaemia Care in over 60 Countries - the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 277, 234–255. doi:10.1016/j.atherosclerosis.2018.08.051
Wang, J., Dron, J. S., Ban, M. R., Robinson, J. F., McIntyre, A. D., Alazzam, M., et al. (2016). Polygenic versus Monogenic Causes of Hypercholesterolemia Ascertained Clinically. Atvb 36 (12), 2439–2445. doi:10.1161/atvbaha.116.308027
Wensel, T. M., Waldrop, B. A., and Wensel, B. (2010). New Drug Approvals: Pitavastatin: A New HMG-CoA Reductase Inhibitor. Ann. Pharmacother. 44 (3), 507–514. doi:10.1345/aph.1m624
Keywords: familial hypercholestelemia, genetics, screening, prevalence, United Arab Emirates (UAE)
Citation: Rimbert A, Daggag H, Lansberg P, Buckley A, Viel M, Kanninga R, Johansson L, Dullaart RPF, Sinke R, Al Tikriti A, Kuivenhoven JA and Barakat MT (2022) Low Detection Rates of Genetic FH in Cohort of Patients With Severe Hypercholesterolemia in the United Arabic Emirates. Front. Genet. 12:809256. doi: 10.3389/fgene.2021.809256
Received: 04 November 2021; Accepted: 30 November 2021;
Published: 03 January 2022.
Edited by:
Alpo Juhani Vuorio, University of Helsinki, FinlandReviewed by:
Alexandre Pereira, Harvard Medical School, United StatesCopyright © 2022 Rimbert, Daggag, Lansberg, Buckley, Viel, Kanninga, Johansson, Dullaart, Sinke, Al Tikriti, Kuivenhoven and Barakat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Albert Kuivenhoven, ai5hLmt1aXZlbmhvdmVuQHVtY2cubmw=
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.