
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 07 January 2022
Sec. Applied Genetic Epidemiology
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.791368
Background: Several studies have examined the association between vitamin D receptor (VDR) polymorphisms and osteoporotic fracture risk; however, the results are not uniform. Furthermore, many new articles have been published, and therefore, an updated meta-analysis was performed to further explore these issues.
Objectives: The aim of the study was to investigate the association between VDR, BsmI, ApaI, TaqI, FokI, and Cdx2 polymorphisms and osteoporotic fracture risk.
Methods: The odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the association between VDR BsmI, ApaI, TaqI, FokI, and Cdx2 polymorphisms and the risk of osteoporotic fracture. We also used the false-positive reporting probability (FPRP) test and the Venice criteria to evaluate the credibility of the statistically significant associations.
Results: Overall, this study found that the VDR ApaI and BsmI polymorphisms significantly increased the risk of osteoporotic fracture in European countries and America, respectively. However, when sensitivity analysis was performed after excluding low-quality and Hardy–Weinberg disequilibrium (HWD) studies, it was found that only individuals with the double-mutated genotype have an increased risk of osteoporotic fracture in European countries. In addition, when the credibility of the positive results was assessed, it was found that the positive results were not credible.
Conclusion: This meta-analysis indicates that there may be no significant association among the polymorphisms of VDR BsmI, ApaI, TaqI, FokI, and Cdx2 and the risk of osteoporotic fracture. The increased risk of osteoporotic fracture is most likely due to false-positive results.
Osteoporosis is characterized by reduced bone density and increased bone fragility, leading to an increased risk of fracture (Recker, 2005). Its clinical significance lies in the triggering of osteoporotic fractures (e.g., fractures of the forearm, vertebrae, and hip) (Cummings and Melton, 2002). The World Health Organization estimates that 200 million people worldwide suffer from osteoporosis (Uzzan et al., 2007), placing a huge burden on families and society, and that osteoporosis has become a major public health problem. Therefore, it is important to explore the underlying pathogenic factors.
The main factors in the development of osteoporosis encompass both environmental and genetic factors. The environmental factors include smoking, exercise, and alcohol consumption (Ng et al., 2006; Kaufman et al., 2008; Binici and Gunes, 2010). Many studies have found that genetic factors play an important role in the pathogenesis of osteoporosis (Jin and Ralston, 2001; Recker and Deng, 2002). It has been estimated that the heritability of osteoporosis-related traits (e.g., bone mineral density) can be as high as 60–80% (Uitterlinden et al., 2004). To date, dozens of risk genes for osteoporosis have been identified, of which ESR1, LRP4, ITGA1, LRP5, SOST, SPP1, TNFRSF11A, TNFRSF11B, and TNFSF11 are thought to be involved in bone mineral density (BMD) homeostasis, bone remodeling, and bone matrix composition, and thus influence BMD and osteoporotic fractures. In addition, a number of candidate genes have been investigated (COL1A1, TGFB1, TGFB3, and VDR), but no clear genome-wide significant association with osteoporosis has been demonstrated (Saccone et al., 2015).
The vitamin D receptor (VDR) gene is located on chromosome 12q13 (Seuter et al., 2016) and exerts various biological effects by mediating the downstream signaling 1,25-dihydroxycholecalciferol (1,25(OH)2D3) (Fang et al., 2003). In human monocytes, 1,25(OH)2D3 regulates chromatin susceptibility at 8979 loci (Ling et al., 2016), and as such, VDR single-nucleotide polymorphisms (SNPs) have been associated with various diseases, including reduced bone mineral density and osteoporosis (Gómez et al., 1999; Garnero et al., 2005). In recent years, numerous studies have reported the association of VDR polymorphisms (e.g., BsmI, ApaI, TaqI, FokI, and Cdx2) with osteoporotic fractures. However, these results were inconsistent and even conflicting. For example, Garnero et al. found that the VDR BsmI B allele was associated with lower BMD and an increased risk of fracture (Alvarez-Hernández et al., 2003). In contrast, other studies found no association between the B allele and the risk of osteoporotic fractures (Uitterlinden et al., 2001; Horst-Sikorska et al., 2005; Iván et al., 2008; Karpiński et al., 2017). Similarly, there were conflicting associations between the ApaI, TaqI, FokI, and Cdx2 polymorphisms and osteoporotic fractures in different studies (Gennari et al., 1999; Gómez et al., 1999; Garnero et al., 2005; Nguyen et al., 2005; Fang et al., 2006; Ji et al., 2010; Horst-Sikorska et al., 2013; Jawiarczyk-Przybyłowska et al., 2019; Iveta et al., 2020). These different results may be owing to differences in sample size, ethnicity, and sampling methods used. Although correlations between the VDR BsmI, ApaI, TaqI, and FokI polymorphisms and the risk of osteoporotic fracture development have been reported in several meta-analyses (Aerssens et al., 2000; Moher et al., 2009; Shen et al., 2014; Gao et al., 2015), there are some limitations in these studies. First, their findings are inconsistent; in the study of Ji et al., the bb genotype in the BsmI gene significantly reduced the risk of fracture (odds ratio (OR) 0.87, 95% confidence interval (CI): 0.76–0.98); in the grouped study, they found that the frequency of the bb genotype was significantly decreased in patients with hip fracture, and the frequency of the Tt genotype was also decreased in patients with hip fracture (Gao et al., 2015), while the frequency of the tt genotype was increased in patients with hip fracture. In addition, they observed an increase in the frequency of the Aa genotype in patients with vertebral fractures. Similarly, in a subgroup analysis, Gao et al. found that the BsmI gene was associated with osteoporotic fractures when the control group was population-derived (OR BB vs. bb 1.22, 95% CI 1.01–1.48; OR B vs. b 1.10, 95% CI 1.00–1.20) (Aerssens et al., 2000). No significant association was found between the BmsI and TaqI by Fang et al. and the BmsI by Shen et al. (BsmI OR 0.98, 95% CI 0.86–1.12; BsmI [b vs. B] OR 1.07, 95% CI 0.90–1.29; TaqI [T vs. t] OR 0.89, 95% CI 0.68–1.15; ApaI [A vs. a] OR 0.91, 95% CI 0.76–1.08; FokI [F vs. f] OR 1.20, 95% CI 0.76–1.90) (Moher et al., 2009; Shen et al., 2014). Second, a literature quality assessment was not performed in some of the meta-analyses (Shen et al., 2014; Gao et al., 2015). Finally, the Hardy–Weinberg equilibrium (HWE) test was not performed in the three studies (Moher et al., 2009; Shen et al., 2014; Gao et al., 2015), and not all studies on the VDR polymorphisms with osteoporosis fracture risk adjusted the P-value (Aerssens et al., 2000; Moher et al., 2009; Shen et al., 2014; Gao et al., 2015). Therefore, an updated meta-analysis was conducted to provide results that were more reliable regarding these issues.
The present meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. The databases searched included PubMed, EMBASE, China Knowledge Network, and China Wanfang Data Knowledge Service Platform to analyze the relationship between VDR polymorphisms and osteoporotic fracture risk. The search strategy was (“vitamin D receptor” or “VDR”) and (“polymorphism” or “variant” or “variation” or “mutation” or “SNP” or “genome-wide association study” or “genetic association study” or “genotype” or “allele”) and (“Fractures, Bone” or “Broken Bones” or “Fractures” or “Fracture” or “Broken Bone” or “Bone Fractures” or “Bone Fracture”). The search deadline was March 2021.
The inclusion criteria were as follows: 1) case–control or cohort studies; 2) investigation of the association between VDR BsmI, ApaI, TaqI, FokI, and Cdx2 polymorphisms and osteoporosis risk; and 3) detailed control and case group genotype data or their OR with 95% CI. The exclusion criteria were as follows: 1) overlapping studies; 2) articles without detailed genotype data; and 3) abstracts, case reports, editorials, reviews, letters, and meta-analyses.
A total of 221 articles were retrieved from all databases. In all, 194 articles were subsequently excluded because they were abstracts, case reports, editorials, reviews, letters, or meta-analyses. When the remaining 27 articles were read, two articles were excluded because patients with both osteoporosis and osteoporotic fractures were considered in the same group. In addition, two articles were found to be repetitive, and one article had missing genotype data, and attempts to contact the corresponding author have not been answered. In the end, 23 relevant studies were included. In the process of article screening, the retrieval work and the screening process were performed by Yi-yang Mu and Biao-Liu independently and then summarized, and the author Bin-Chen made the final decision when there was any disagreement.
We predesigned the data extraction form. The data from the selected articles were extracted and cross-checked according to the defined inclusion and exclusion criteria. When different results were obtained, and no consensus could be reached after the discussion, a third author was invited to repeat the data extraction and check for confirmation. If the data were unclear or questionable in the article, the author was contacted to obtain the original data. The following information was extracted: first author of the article, year of publication, country of study, corresponding continent, origin of cases and controls, type of osteoporotic fracture, sex of study subjects, number of cases and controls, number of genotypes distributed among cases and controls, diagnostic criteria for osteoporotic fractures, and conclusion of the investigators.
The quality of all articles was independently assessed by two authors. We adopted and refined the quality assessment criteria from two previous meta-analyses (Aerssens et al., 2000; Moher et al., 2009). Supplementary Table S1 lists the quality assessment scales for studies on the factors associated with osteoporotic fracture risk. A total of 20 points were awarded, with articles scoring above 12 rated as excellent, those lying between 9 and 12 labeled as moderate, and studies scoring below 9 rated as poor.
The strength of association was evaluated using ORs with their 95% CIs and was considered statistically significant when the P-value was <0.05. Comparisons were performed using the following five genetic models: 1) allelic model, 2) additive model, 3) dominant model, 4) recessive model, and 5) over-dominant model. The chi-square–based Q test and I2 values were used to assess heterogeneity. P > 0.10 and/or I2 < 50% indicated no significant heterogeneity among the included studies, and a fixed-effects model was used. Otherwise, a random-effects model was applied. Publication bias was detected using Begg’s funnel plot and Egger’s test. Sensitivity analyses were assessed using three methods: 1) exclusion of one included study; ,2) exclusion of included HWD studies and low-quality studies, and 3) only including high-quality studies, the Hardy–Weinberg equilibrium (HWE), and matched studies. A chi-square goodness-of-fit test was applied to assess the HWE, and controls were identified as the HWE when p > 0.05. In addition, the false-positive reporting probability (FPRP) test and Venice criteria were used to assess the credibility of statistically significant associations. The abovementioned statistical analyses were made possible using Stata 12.0 software.
A total of 221 relevant studies were retrieved, and 23 articles met our criteria (5,844 osteoporotic fracture cases and 19,339 controls), of which 18 articles examined VDR BsmI (involving 2,429 osteoporotic fracture cases and 5,187 controls), eight studies discussed VDR ApaI (involving 875 osteoporotic fracture cases and 2,120 controls), nine studies reported VDR TaqI (involving 860 osteoporotic fracture cases and 2,538 controls), seven studies documented VDR FokI (involving 579 osteoporotic fracture cases and 1635 controls), and three studies investigated VDR Cdx2 (involving 1101 osteoporotic fracture cases and 7859 controls), and how each of these polymorphisms correlates with osteoporotic fracture risk. In addition, 18, 5, and 1 case–control studies have been conducted in European, American, and Asian populations, respectively. Among them, four studies discussed these associations in men, and 22 studies analyzed these relationships in women. Finally, there were six high-quality studies and 12 medium-quality studies discussing VDR BsmI; two high-quality studies and seven medium-quality studies discussing VDR ApaI; two high-quality studies and six medium-quality studies on VDR TaqI; one high-quality, five medium-quality, and one low-quality studies on VDR FokI; and one medium-quality and two low-quality studies on VDR Cdx2. Table 1 shows the detailed characteristics and scores of each study. The literature selection and inclusion processes are illustrated in Figure 1. Tables 2–6 show the genotype frequencies of the VDR BsmI, ApaI, TaqI, FokI, and Cdx2 polymorphisms, and the impact of each on the risk of osteoporotic fracture.
We did not observe a significant association between the VDR BsmI polymorphism and the risk of osteoporotic fractures (p > 0.05) in all genetic models. However, subgroup analysis by race showed that the VDR BsmI B allele increased the risk of osteoporotic fracture (OR 1.17, 95% CI 1.03–1.34), and the BB genotype (additive model: OR 0.74, 95% CI 0.58–0.94; recessive model: OR 0.81, 95% CI 0.66–0.99) reduced the risk of osteoporotic fractures in Americans. We believe that articles with HWD control data should be excluded because the inclusion of HWD articles may interfere with the real results. When HWD-related article data were excluded, the positive results of the subgroup analysis corresponding to race changed. Table 7 summarizes the evaluation of the association between VDR BsmI polymorphism and the risk of osteoporotic fractures. Overall, the VDR BsmI polymorphism did not significantly increase the risk of osteoporotic fractures, as shown in Figure 2.
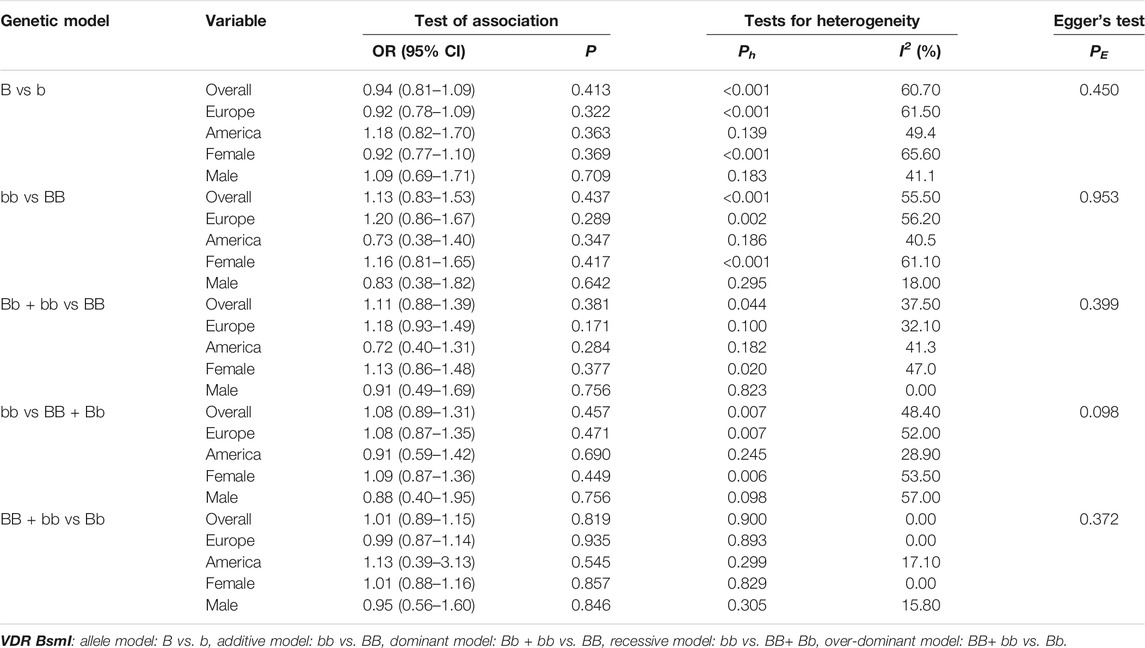
TABLE 7. Pooled estimates of association of VDR BsmI polymorphism and the risk of osteoporotic fracture.
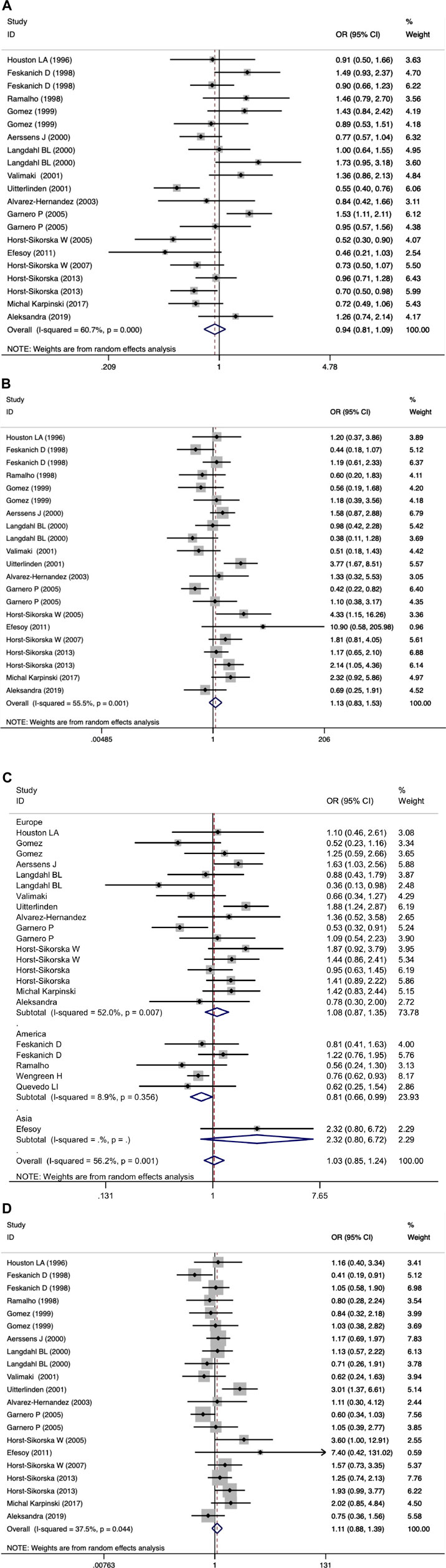
FIGURE 2. Forest plots of all selected studies on the association between VDR Bsml polymorphism and the risk of osteoporotic fracture in different races [(A) allele model, (B) additive model, (C) dominant model, and (D) recessive model].
In the overall analysis, it was not found whether VDR ApaI polymorphism could significantly increase the risk of osteoporotic fracture (p > 0.05 in all genetic models). When stratified by race, the results showed that in the European population, the aa genotype increased the risk of osteoporotic fracture compared with the AA genotype (allelic model: OR 0.83, 95% CI 0.71–0.97; additive model: OR 1.50, 95% CI 1.09–2.07; dominant model: OR 1.26, 95% CI 1.02–1.56; recessive model: OR 1.40, 95% CI 1.07–1.83). All data are shown in Table 8 and Figure 3.
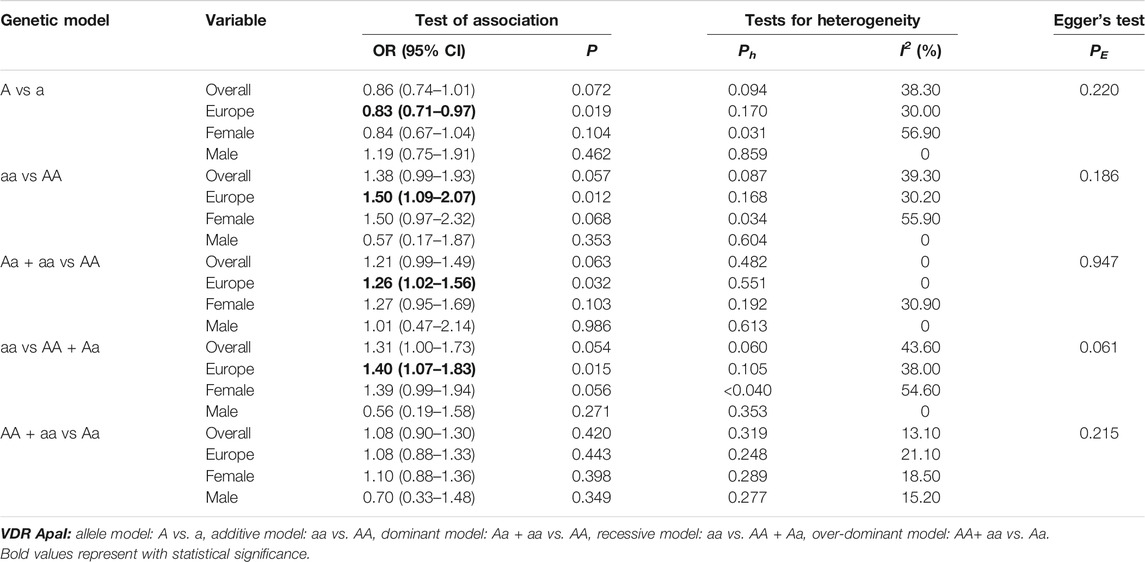
TABLE 8. Pooled estimates of association of VDR ApaI polymorphism and the risk of osteoporotic fracture.
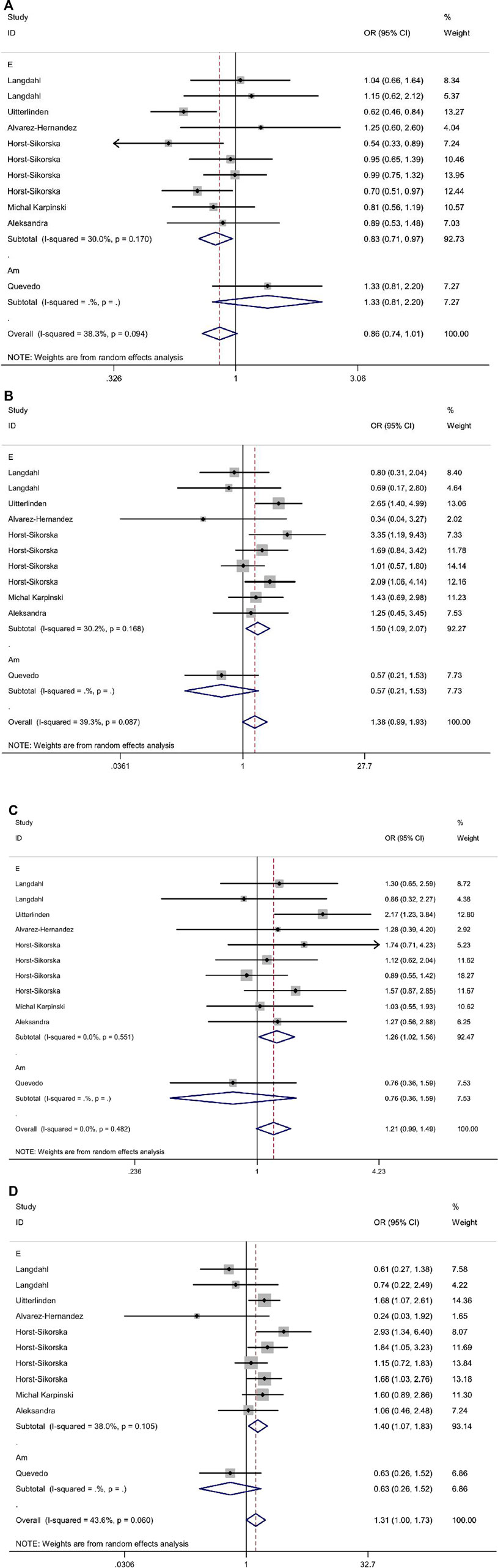
FIGURE 3. Forest plots of all selected studies on the association between VDR Apal polymorphism and the risk of osteoporotic fracture in different races [(A) allele model, (B) additive model, (C) dominant model, and (D) recessive model].
As shown in Tables 9–11 and Figures 4–6, there were no significant associations between the VDR TaqI, FokI, and Cdx2 polymorphisms and the risk of osteoporotic fractures.
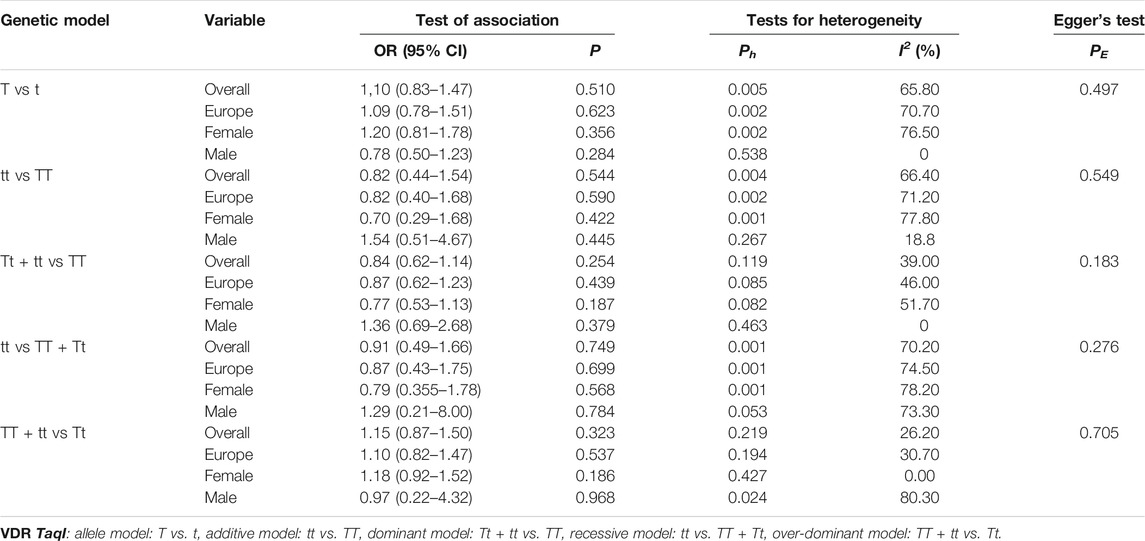
TABLE 9. Pooled estimates of association of VDR TaqI polymorphism and the risk of osteoporotic fracture.
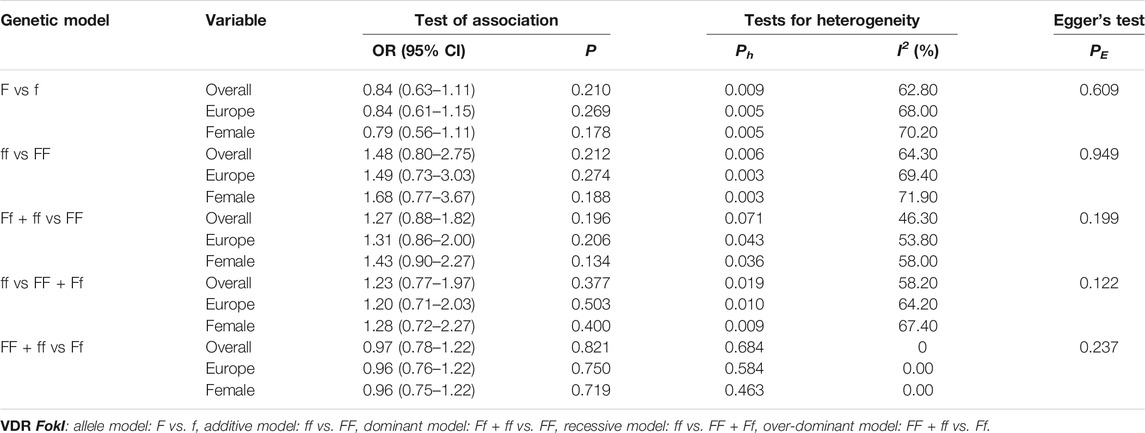
TABLE 10. Pooled estimates of association of VDR FokI polymorphism and the risk of osteoporotic fracture.
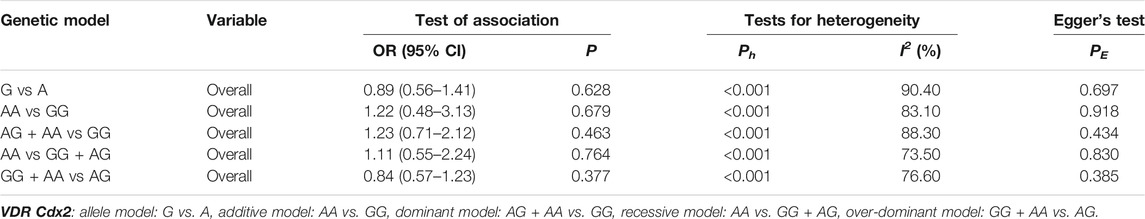
TABLE 11. Pooled estimates of association of VDR Cdx2 polymorphism and the risk of osteoporotic fracture.
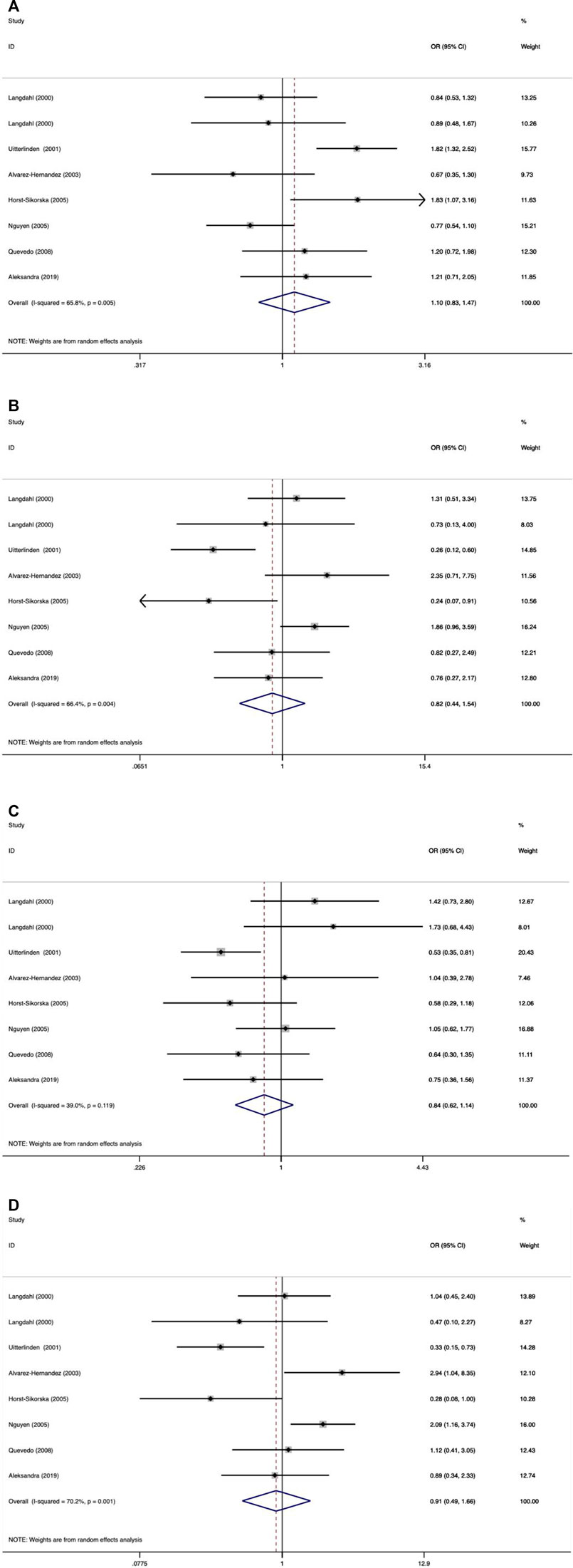
FIGURE 4. Forest plots of all selected studies on the association between VDR Taql polymorphism and the risk of osteoporotic fracture in different races [(A) allele model, (B) additive model, (C) dominant model, and (D) recessive model].
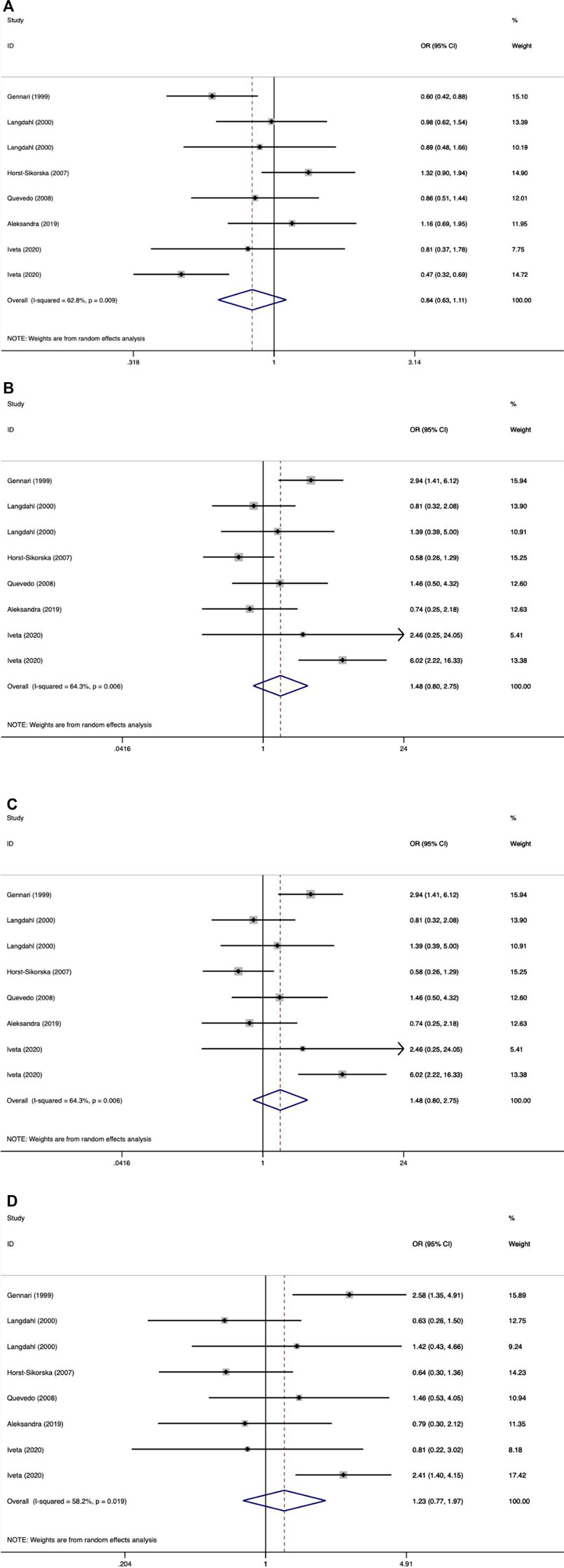
FIGURE 5. Forest plots of all selected studies on the association between VDR Fokl polymorphism and the risk of osteoporotic fracture in different races [(A) allele model, (B) additive model, (C) dominant model, and (D) recessive model].
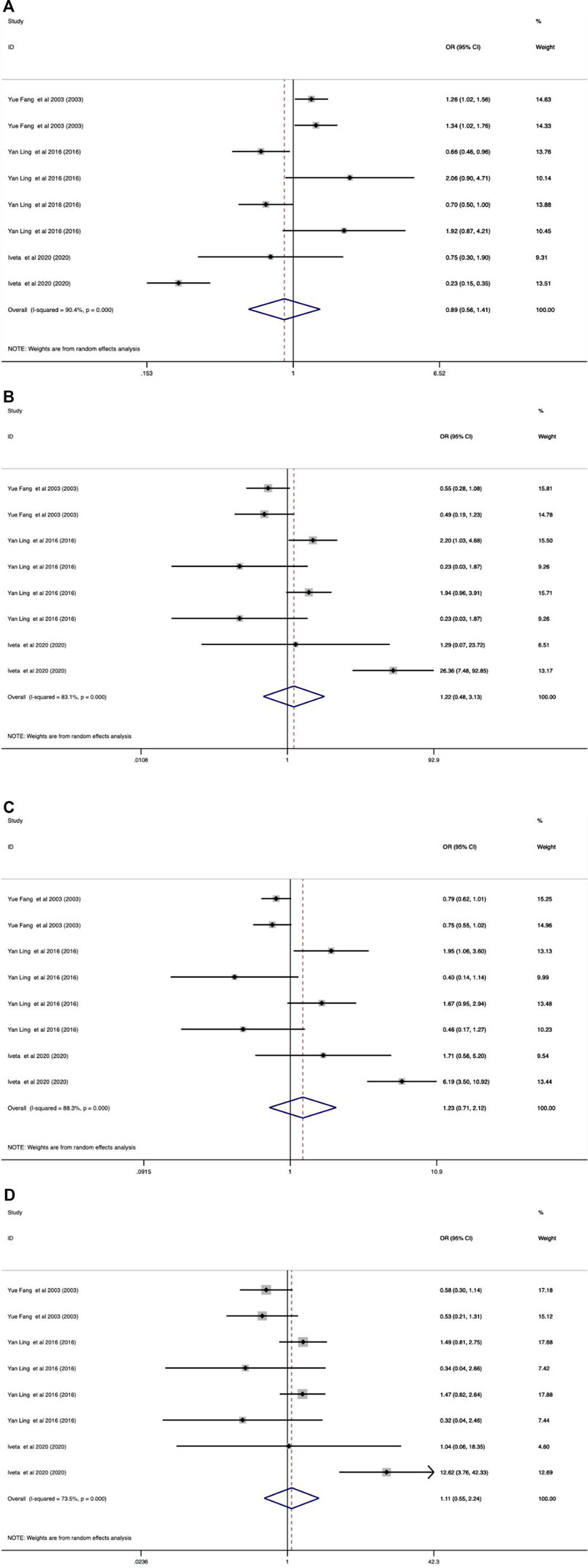
FIGURE 6. Forest plots of all selected studies on the association between VDR Cdx-2 polymorphism and the risk of osteoporotic fracture in different races [(A) allele model, (B) additive model, (C) dominant model, and (D) recessive model].
Table 12 shows the results of articles that did not exclude HWD.
We observed heterogeneity in the overall and several subgroup analyses. Heterogeneity may be attributed to factors such as race, sex, and HWE. To explore the source of heterogeneity, a regression meta-analysis was used. However, no obvious source of heterogeneity was found by the results of regression meta-analyses. However, if it was taken into consideration that the previous exclusion of HWD-related articles leads to significant results in subgroup analysis, then it can be said that the source of heterogeneity might be HWD-related. Sensitivity analysis was estimated using three methods. First, a study was deleted every time to evaluate its robustness, and no change was observed in the research results. However, a significant change was observed in the obtained results once when low-quality and HWD studies were excluded. In previous studies, the VDR BsmI B allele increased the risk of osteoporotic fracture (OR 1.17, 95% CI 1.03–1.34), and the bb genotype reduced the risk of osteoporotic fracture in the United States (additive model: OR 0.74, 95% CI 0.88–0.94; allelic model: OR 0.81, 95% CI 0.66–0.99), but after excluding low-quality and HWD studies, the results showed no significant association between VDR BsmI gene polymorphism and fracture risk in the American population (allelic model: OR 1.18, 95% CI 0.82–1.70; additive model: OR 0.73, 95% CI 0.38–1.40; recessive model: OR 0.91, 95% CI 0.59–1.42). In addition, an increased risk of osteoporosis fracture was found in individuals with the AA genotype only in the European population (allele model: OR 0.83, 95% CI 0.70–0.98; additive model: OR 1.52, 95% CI 1.07–2.16; dominant model: OR 1.26, 95% CI 1.01–1.57; recessive model: OR 1.42, 95% CI 1.06–1.90), which was also different from previous studies (allelic model: OR 0.83, 95% CI 0.71–0.97; additive model: OR 1.50, 95% CI 1.09–2.07; dominant model: OR 1.26, 95% CI 1.02–1.56; recessive model: OR 1.40, 95% CI 1.07–1.83). In addition, when the studies were limited to only high quality, HWE, and matching, the corresponding total OR value was not significantly changed. The sensitivity analysis results are presented in Table 13.

TABLE 13. Pooled estimates of association of VDR BsmI, ApaI, TaqI, and FokI polymorphisms and the risk of osteoporotic fracture, excluding low-quality and HWD studies.
Publication bias was evaluated using Begg’s funnel plot and Egger’s test. The shape of the funnel plot shows that there was no obvious funnel asymmetry in the entire population (Figure 7). Egger’s test also showed no evidence of significant publication bias (p > 0.05 in all genetic models), as displayed in Tables 7–11.
We determined that significant associations meeting the following statistical criteria were classified as “positive results” (Montazeri et al., 2019): 1) the P value of the Z-test <0.05 in at least two gene models; 2) at the P value level of 0.05, the FPRP was <0.2; 3) statistical power >0.8; and 4) I2 < 50%. Results were considered as “less credible results” with a lower threshold when the following conditions were met: 1) p < 0.05 in at least one of the genetic models; 2) the statistical power was between 50 and 79%, FPRP >0.2, or I2 > 50%. After confidence evaluation, it was determined that the statistically significant associations in this meta-analysis were “unreliable.” The detailed confidence evaluation results are presented in Table 14.
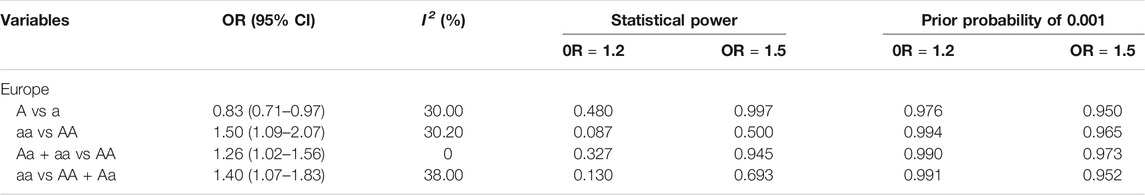
TABLE 14. False-positive report probability values for the statistically significant associations in the current meta-analysis.
Osteoporosis is characterized by decreased bone density and increased bone fragility, which leads to increased fracture risk (Recker, 2005). Genes play an important role in the development of osteoporotic fractures, and the VDR gene has been extensively studied as a candidate gene that plays a key role in regulating bone resorption and metabolism (Jin and Ralston, 2001; Recker and Deng, 2002), and influencing bone mass (Kim et al., 2007). Therefore, it is important to study the relationship between VDR polymorphisms and osteoporotic fracture risk. Many previous studies have attempted to clarify the relationship between the polymorphisms of VDR and the risk of osteoporotic fracture. Unfortunately, there is no reliable evidence to show whether there is a relationship between them, which may be due to different reasons, including small sample size, race, and regional differences. Therefore, a meta-analysis is a valid alternative.
This meta-analysis included 23 studies, among which 18 explored the relationship between the VDR polymorphism BsmI and osteoporosis fracture risk, eight studies reported VDR ApaI polymorphism, nine studies reported VDR TaqI polymorphism, seven studies reported VDR FokI polymorphism, and three studies were related to VDR Cdx2 polymorphism. In addition, five genetic models were compared. Overall, the VDR BsmI polymorphism had no significant effect on the risk of osteoporotic fractures. However, in subgroup analysis, there was a significant correlation between the two. Moreover, the VDR ApaI polymorphism also did not significantly affect the risk of osteoporotic fracture. According to racial stratification, it was found that the genotype aa increased the risk of osteoporotic fracture in European countries compared with the AA genotype. However, no meaningful results were found regarding the relationship between the VDR polymorphisms (TaqI, VDR FokI, and Cdx2) and osteoporotic fracture. Moreover, when the low-quality and HWD research were excluded, and when the combined analysis involved only high-quality, HWE, and matching research, no significant correlation was observed. Furthermore, the current meta-analysis was carried out by applying multiple subgroups and different genetic models at the expense of multiple comparisons; in this case, the aggregated P value must be adjusted (Attia et al., 2003). The Venice standard, statistical ability, and I2 value are important standards (Langdahl et al., 2000). Therefore, the FPRP and Venice standards were used to evaluate positive results. After the credibility evaluation, it was determined that “positive results are not credible,” which are statistically significant in the current meta-analysis. After the regression meta-analysis, no source of obvious heterogeneity was identified. In addition, no obvious asymmetry was found in the study of VDR BsmI, ApaI, TaqI, and FokI polymorphisms using Begg’s funnel plot and Egger’s test. However, owing to the limited number of studies, Begg’s funnel plot was not used to explore publication deviation in VDR Cdx2 research. Finally, Egger’s test showed that there was no clear statistical evidence to show publication bias.
Four meta-analyses analyzed the association between VDR polymorphisms and risk of osteoporotic fracture. Fang et al. (Shen et al., 2014), Shen et al. (Moher et al., 2009), and Gao et al. (Aerssens et al., 2000) discussed the association between the VDR BsmI polymorphism and the risk of osteoporotic fracture, and their results showed that there was no significant association between VDR BsmI polymorphism and the risk of osteoporotic fracture. However, Ji et al. (Gao et al., 2015) examined 17 studies on the relationship of VDR BsmI polymorphism with osteoporotic fracture risk, including 2,112 osteoporotic fracture cases and 4,521 controls, and indicated that there was a statistically significant association between the VDR BsmI polymorphism and osteoporotic fracture risk. In addition, Fang et al. (Shen et al., 2014) and Shen et al. (Moher et al., 2009) examined four and five VDR TaqI studies, respectively, all of which considered that the VDR TaqI polymorphism was not significantly associated with osteoporotic fracture risk. Four studies on VDR ApaI and four studies on VDR FokI analyzed by Shen et al. (Moher et al., 2009) did not find that the VDR ApaI and FokI polymorphisms increased the risk of osteoporotic fracture. In addition, some shortcomings were found when published meta-analyses were carefully checked. First, there was no quality evaluation for the included studies in the two meta-analyses (Shen et al., 2014; Gao et al., 2015), and low-quality studies might have been included, which led to a deviation in the results. Second, the genotype distribution in the control group was not detected by the HWE (Moher et al., 2009; Shen et al., 2014; Gao et al., 2015). The HWE is necessary for a sound genetic association study. If the control group does not meet the requirements of the HWE, there may be selection bias or genotype errors, thus making the results unreliable. Third, the statistical power was not calculated in some previous meta-analyses (Moher et al., 2009; Shen et al., 2014; Gao et al., 2015). At the same time, the statistically significant false-positive report probability was not evaluated in all previously published meta-analyses. Therefore, the meta-analysis results may not be credible. Finally, none of the abovementioned studies discussed the relationship between the VDR Cdx2 polymorphism and osteoporotic fracture.
This meta-analysis had the following advantages: 1) evaluating the quality of the included research; 2) the control group underwent the HWE test; 3) applying the FPRP and Venice criteria to evaluate the correlations that were found to be significant in the current meta-analysis; 4) compared with the previous meta-analysis, the sample size has been significantly expanded; and 5) exploring the sources of heterogeneity based on regression meta-regression analysis. However, there are still some limitations to this study. First, the confounding factors closely related to the outcome were not controlled, such as smoking, drinking, and variable research designs. Second, there are relatively few studies on Americans and Asians in several subgroup analyses, and not enough statistical power to explore the real connection. Moreover, owing to the limited number of studies, a subgroup analysis was not carried out in the summary analysis of the VDR Cdx2 polymorphism and osteoporotic fracture risk. Finally, it was found that the research quality of VDR Cdx2 is low, and hence, the results may not be credible. Future research with large sample sizes and large enough subgroups will help verify our findings.
This meta-analysis strongly indicates that there is no significant association between the polymorphisms of VDR BsmI, ApaI, TaqI, FokI, and Cdx2 and the risk of osteoporotic fracture. The increased risk of osteoporotic fracture elucidated in previous studies is most likely due to false-positive results.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Y-yM designed research, performed research, collected data, analyzed data, and wrote the article. BL collected data. BC and W-fZ checked the data. X-HY contributed to methodology. H-zL and X-fH designed research and revised the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the authors of all the original studies included in the meta-analysis. At the same time, we would like to thank X-fH and H-zL for their guidance. In addition, we would like to thank Yu-hui Pan for his help in revising the grammar of this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.791368/full#supplementary-material
BMD, bone mineral density; 95% CI, 95% confidence interval; Fn, femoral neck; FPRP, false-positive report probabilities; HWD, Hardy–Weinberg disequilibrium; HWE, Hardy–Weinberg equilibrium (ideally, the frequency of alleles is constant in heredity; that is, gene balance is maintained); LS, lumbar spine; OR, odds ratio; VDR, vitamin D receptor; PRISMA, preferred reporting items for systematic review and meta-analyses; SNP, single-nucleotide polymorphism.
Aerssens, J., D(equeker, J., Peeters, J., Breemans, S., Broos, P., and Boonen, S. (2000). Polymorphisms of the VDR, ER and COLIA1 Genes and Osteoporotic Hip Fracture in Elderly Postmenopausal Women. Osteoporos. Int. 11, 583–591. doi:10.1007/s001980070079
Alvarez-Hernández, D., Naves, M., Díaz-López, J. B., Gómez, C., Santamaría, I., Cannata-Andía, J. B., et al. (2003). Influence of Polymorphisms in VDR and COLIA1 Genes on the Risk of Osteoporotic Fractures in Aged Men. Kidney International. Suppl. 63 (85), S14–S18. doi:10.1046/j.1523-1755.63.s85.5.x
Attia, J., Thakkinstian, A., and D'Este, C. (2003). Meta-analyses of Molecular Association Studies: Methodologic Lessons for Genetic Epidemiology. J. Clin. Epidemiol. 56 (4), 297–303. doi:10.1016/s0895-4356(03)00011-8
Binici, D. N., and Gunes, N. (2010). Risk Factors Leading to Reduced Bone mineral Density in Hemodialysis Patients with Metabolic Syndrome. Ren. Fail. 32 (4), 469–474. doi:10.3109/08860221003675260
Iveta, B., Jarmila, B., Soňa, M., Ján, K., Zlatica, T., Ivan, B., et al. (2020). Association between Vitamin D Receptor Gene Polymorphisms (Fok I, Cdx-2) and Bone mineral Density in Slovak Postmenopausal Women. Anthropologischer Anzeiger 77, 195–203. doi:10.1127/anthranz/2020/1048
Cummings, S. R., and Melton, L. J. (2002). Epidemiology and Outcomes of Osteoporotic Fractures. The Lancet 359, 1761–1767. doi:10.1016/s0140-6736(02)08657-9
Efesoy, A., Yilmaz, Ö., Erden, G., Güçtekin, A., Bodur, H., Yildirimkaya, M, et al. (2003). Relationship of the Vitamin D Receptor and Collagen Iα1 Gene Polymorphisms with Low Bone Mineral Density and Vertebral Fractures in Postmenopausal Turkish Women. Turk J. Rheumatol. 26, 295–302. doi:10.5606/tjr.2011.047
Fang, Y., Rivadeneira, F., van Meurs, J. B. J., Pols, H. A. P., Ioannidis, J. P. A., and Uitterlinden, A. G. (2006). Vitamin D Receptor Gene BsmI and TaqI Polymorphisms and Fracture Risk: a Meta-Analysis. Bone 39 (4), 938–945. doi:10.1016/j.bone.2006.04.016
Fang, Y., Van Meurs, J. B., Bergink, A. P., Hofman, A., Van Duijn, C. M., Van Leeuwen, J. P., et al. (2003). Cdx-2 Polymorphism in the Promoter Region of the Human Vitamin D Receptor Gene Determines Susceptibility to Fracture in the Elderly. J. Bone Miner. Res. 18 (9), 1632–1641. doi:10.1359/jbmr.2003.18.9.1632
Feskanich, D., Hunter, D. J., Willett, W. C., Hankinson, S. E., Hollis, B. W., Hough, H. L., et al. (1998). Vitamin D Receptor Genotype and the Risk of Bone Fractures in Women. Epidemiology 9, 535–539. doi:10.1097/00001648-199809000-00011
Gao, J., Wang, L., and Zhu, J. (2015). Influence of BsmI Polymorphism in Vitamin D Receptor Gene on the Risk of Fracture in Caucasian Populations: a Meta Analysis. Int. J. Clin. Exp. Med. 8 (1), 589–597.
Garnero, P., Munoz, F., Borel, O., Sornay-Rendu, E., and Delmas, P. D. (2005). Vitamin D Receptor Gene Polymorphisms Are Associated with the Risk of Fractures in Postmenopausal Women, Independently of Bone mineral Density. J. Clin. Endocrinol. Metab. 90 (8), 4829–4835. doi:10.1210/jc.2005-0364
Gennari, L., Becherini, L., Mansani, R., Masi, L., Falchetti, A., Morelli, A., et al. (1999). FokI Polymorphism at Translation Initiation Site of the Vitamin D Receptor Gene Predicts Bone Mineral Density and Vertebral Fractures in Postmenopausal Italian Women. J. Bone Mineral Res. 14, 1379–1386. doi:10.1359/jbmr.1999.14.8.1379
Gómez, C., Naves, M. L., Barrios, Y., Díaz, J. B., Fernández, J. L., Salido, E., et al. (1999). Vitamin D Receptor Gene Polymorphisms, Bone Mass, Bone Loss and Prevalence of Vertebral Fracture: Differences in Postmenopausal Women and Men. Osteoporos. Int. 10 (3), 175–182. doi:10.1007/s001980050213
Horst-Sikorska, W., Wawrzyniak, A., Celczyńska-Bajew, L., Marcinkowska, M., Dabrowski, S., Kalak, R., et al. (2005). Polimorfizm Genu VDR-Efektywny Marker Molekularny Ryzyka Osteoporotycznych Złamań Kości W Grupie Kobiet Po Menopauzie Pochodzących Z Rejonu. Endokrynol Pol. 56, 233–239.
Horst-Sikorska, W., Dytfeld, J., Wawrzyniak, A., Marcinkowska, M, Michalak, M, Franek, E, et al. (2013). Vitamin D Receptor Gene Polymorphisms, Bone mineral Density and Fractures in Postmenopausal Women with Osteoporosis. Mol. Biol. Rep. 40 (1), 383–390. doi:10.1007/s11033-012-2072-3
Horst-Sikorska, W., Kalak, R., Wawrzyniak, A., Marcinkowska, M., Celczynska-Bajew, L., and Slomski, R. (2007). Association Analysis of the Polymorphisms of the VDR Gene with Bone mineral Density and the Occurrence of Fractures. J. Bone Miner. Metab. 25, 310–319. doi:10.1007/s00774-007-0769-5
Houston, L. A., Grant, S. F. A., Reid, D. M., and Ralston, S. H. (1996). Vitamin D Receptor Polymorphism, Bone mineral Density, and Osteoporotic Vertebral Fracture: Studies in a UK Population. Bone 18, 249–252. doi:10.1016/8756-3282(95)00483-1
Iván, Q. L., Milka, M. B., Marcelo, C. N., and Nancy, R. F. (2008). Polimorfismos del gen del receptor de vitamina D y riesgo de fractura de cadera en la mujer adulta mayor de la Región del Bío Bío. Revista Médica De Chile 136 (4), 475–481. doi:10.4067/s0034-98872008000400008
Aleksandra, J. -P., Jowita, H. -Z., Katarzyna, K., Łukasz, G., Agnieszka, Z., and Marek, B. (2019). Association of Vitamin D Receptor Polymorphisms with Activity of Acromegaly, Vitamin D Status and Risk of Osteoporotic Fractures in Acromegaly Patients. Front. Endocrinol. (Lausanne) 10, 643. doi:10.3389/fendo.2019.00643
Ji, G.-R., Yao, M., Sun, C.-Y., Li, Z.-H., Han, Z., and BsmI, Taq. I. (2010). BsmI, TaqI, ApaI and FokI Polymorphisms in the Vitamin D Receptor (VDR) Gene and Risk of Fracture in Caucasians: A Meta-Analysis. Bone 47 (3), 681–686. doi:10.1016/j.bone.2010.06.024
Jin, H., and Ralston, S. H. (2001). Genetics of Osteoporosis. Rev. Endocr. Metab. Disord. 2 (1), 13–21.
Karpiński, M., Galicka, A., Milewski, R., Popko, J., Badmaev, V., and Stohs, S. J. (2017). Association between Vitamin D Receptor Polymorphism and Serum Vitamin D Levels in Children with Low-Energy Fractures. J. Am. Coll. Nutr. 36 (1), 64–71. doi:10.1080/07315724.2016.1218803
Kaufman, J.-M., Ostertag, A., Saint-Pierre, A., Cohen-Solal, M., Boland, A., Van Pottelbergh, I., et al. (2008). Genome-wide Linkage Screen of Bone mineral Density (BMD) in European Pedigrees Ascertained through a Male Relative with Low BMD Values: Evidence for Quantitative Trait Loci on 17q21-23, 11q12-13, 13q12-14, and 22q11. J. Clin. Endocrinol. Metab. 93 (10), 3755–3762. doi:10.1210/jc.2008-0678
Kim, M.-s., Fujiki, R., Murayama, A., Kitagawa, H., Yamaoka, K., Yamamoto, Y., et al. (2007). 1α,25(OH)2D3-Induced Transrepression by Vitamin D Receptor through E-box-type Elements in the Human Parathyroid Hormone Gene Promoter. Mol. Endocrinol. 21, 334–342. doi:10.1210/me.2006-0231
Langdahl, B. L., Gravholt, C. H., Brixen, K., and Eriksen, E. F. (2000). Polymorphisms in the Vitamin D Receptor Gene and Bone Mass, Bone Turnover and Osteoporotic Fractures. Eur. J. Clin. Invest. 30, 608–617. doi:10.1046/j.1365-2362.2000.00686.x
Ling, Y., Lin, H., Aleteng, Q., Ma, H., Pan, B., Gao, J., et al. (2016). Cdx-2 Polymorphism in Vitamin D Receptor Gene Was Associated with Serum 25-hydroxyvitamin D Levels, Bone mineral Density and Fracture in Middle-Aged and Elderly Chinese Women. Mol. Cell. Endocrinol. 427, 155–161. doi:10.1016/j.mce.2016.03.014
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. J. Clin. Epidemiol. 62 (10), 1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Montazeri, Z., Li, X., Nyiraneza, C., Ma, X., Timofeeva, M., Svinti, V., et al. (2019). Systematic Meta-Analyses, Field Synopsis and Global Assessment of the Evidence of Genetic Association Studies in Colorectal Cancer. Gut 69, 1460–1471. doi:10.1136/gutjnl-2019-319313
Ng, M. Y., Sham, P. C., Paterson, A. D., Chan, V., and Kung, A. W. (2006). Effect of Environmental Factors and Gender on the Heritability of Bone mineral Density and Bone Size. Ann. Hum. Genet. 70 (Pt 4), 428–438. doi:10.1111/j.1469-1809.2005.00242.x
Nguyen, T. V., Esteban, L. M., White, C. P., Grant, S. F., Center, J. R., Gardiner, E. M., et al. (2005). Contribution of the Collagen I Alpha1 and Vitamin D Receptor Genes to the Risk of Hip Fracture in Elderly Women. J. Clin. Endocrinol. Metab. 90 (12), 6575–6579. doi:10.1210/jc.2005-1153
Quevedo, L. I., Martínez, B. M., Castillo, N. M., and Rivera, F N. (2008). Polimorfismos del gen del receptor de vitamina D y riesgo de fractura de cadera en la mujer adulta mayor de la Región del Bío Bío (Vitamin D Receptor Gene Polymorphisms and Risk of Hip Fracture in Chilean Elderly Women]. Rev. Med. Chil. 136 (4), 475–81. [Epub ahead of print].
Ramalho, A. C., Lazaretti-Castro, M., Hauache, O., Kasamatsu, T., Brandão, C., Reis, A. F., et al. (1998). Fractures of the Proximal Femur: Correlation with Vitamin D Receptor Gene Polymorphism. Braz. J. Med. Biol. Res. 31, 921–927. doi:10.1590/s0100-879x1998000700006
Recker, R. R. (2005). Summary--Novel Therapies for Osteoporosis. J. Musculoskelet. Neuronal Interact 5, 358–359.
Recker, R. R., and Deng, H.-W. (2002). Role of Genetics in Osteoporosis. Endo. 17, 55–66. doi:10.1385/endo:17:1:55
Saccone, D., Asani, F., and Bornman, L. (2015). Regulation of the Vitamin D Receptor Gene by Environment, Genetics and Epigenetics. Gene 561 (2), 171–180. doi:10.1016/j.gene.2015.02.024
Seuter, S., Neme, A., and Carlberg, C. (2016). Epigenome-wide Effects of Vitamin D and Their Impact on the Transcriptome of Human Monocytes Involve CTCF. Nucleic Acids Res. 44 (9), 4090–4104. doi:10.1093/nar/gkv1519
Shen, H., Xie, J., and Lu, H. (2014). Vitamin D Receptor Gene and Risk of Fracture in Postmenopausal Women: a Meta-Analysis. Climacteric 17 (4), 319–324. doi:10.3109/13697137.2013.856401
Uitterlinden, A. G., Weel, A., Burger, H., Fang, Y., Van Duijn, C. M., Hofman, A., et al. (2001). Interaction between the Vitamin D Receptor Gene and Collagen Type I1 Gene in Susceptibility for Fracture. J. Bone Mineral Res. 16, 379–385. doi:10.1359/jbmr.2001.16.2.379
Uitterlinden, A. G., Fang, Y., van Meurs, J. B. J., Pols, H. A. P., and van Leeuwen, J. P. T. M. (2004). Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 338 (2), 143–156. doi:10.1016/j.gene.2004.05.014
Uzzan, B., Cohen, R., Nicolas, P., Cucherat, M., and Perret, G. Y. (2007). Effects of Statins on Bone mineral Density: a Meta-Analysis of Clinical Studies. Bone 40, 1581–1587. doi:10.1016/j.bone.2007.02.019
Välimäki, S., Tähtelä, R., Kainulainen, K., Laitinen, K., Löyttyniemi, E., Sulkava, R., et al. (2001). Relation of Collagen Type I Alpha 1 (COLIA 1) and Vitamin D Receptor Genotypes to Bone Mass, Turnover, and Fractures in Early Postmenopausal Women and to Hip Fractures in Elderly People. Eur. J. Intern. Med. 12 (1), 48–56. doi:10.1016/s0953-6205(00)00137-0
Keywords: VDR, polymorphism, osteoporosis, risk of fracture, meta-analysis
Citation: Mu Y-y, Liu B, Chen B, Zhu W-f, Ye X-H, Li H-z and He X-f (2022) Evaluation of Association Studies and an Updated Meta-Analysis of VDR Polymorphisms in Osteoporotic Fracture Risk. Front. Genet. 12:791368. doi: 10.3389/fgene.2021.791368
Received: 08 October 2021; Accepted: 03 December 2021;
Published: 07 January 2022.
Edited by:
Melanie Haffner-Luntzer, University of Ulm, GermanyReviewed by:
Fatma Savran Oguz, Istanbul University, TurkeyCopyright © 2022 Mu, Liu, Chen, Zhu, Ye, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-zhuo Li, bGloejA5OTlAc2luYS5jb20=; Xiao-feng He, MzkzMTIwODIzQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.