- 1Tianjin Key Laboratory of Cerebrovascular and of Neurodegenerative Diseases, Department of Neurology, Tianjin Huanhu Hospital, Tianjin, China
- 2Tianjin Key Laboratory of Cerebrovascular and of Neurodegenerative Diseases, Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, China
The susceptibility of the GAK rs1564282 variant in Parkinson’s disease (PD) in Europeans was identified using a series of published genome-wide association studies. Recently, some studies focused on the association between rs1564282 and PD risk in Chinese populations but with inconsistent results. Thus, we conducted an updated meta-analysis with a total of 7,881 samples (4,055 PD cases and 3,826 controls) from eligible studies. After excluding significant heterogeneity, we showed that the rs1564282 variant was significantly associated with PD in Chinese populations (p = 1.00E-04, odds ratio = 1.28 and 95% confidence interval = 1.16–1.42). The sensitivity analysis showed that the association between rs1564282 and PD was not greatly influenced, and there was no significant publication bias among the included studies. Consequently, this meta-analysis indicates that the GAK rs1564282 variant is significantly associated with susceptibility to PD in Chinese populations.
Introduction
Parkinson’s disease (PD) is the second-most common neurodegenerative disease after Alzheimer’s disease (Ascherio and Schwarzschild, 2016). With the widespread use of genome-wide association studies (GWAS), more genetic components of PD have been identified, and potential mechanisms of PD have been uncovered (Nalls et al., 2014; Nalls et al., 2019). In 2009, Pankratz et al. designated GAK/DGKQ as a new PD risk region in a Caucasian population (Pankratz et al., 2009). The following GWAS showed that the GAK rs1564282 variant was associated with the increasing risk of PD (Spencer et al., 2011). Subsequently, the underlying associations between rs1564282 and PD were investigated in Chinese populations.
Li et al. selected 812 PD patients and 763 control individuals from west China and first corroborated that rs1564282 was associated with PD in a Chinese population (p = 0.017) (Li et al., 2012). Then a meta-analysis using a European population reached a similar conclusion (Li et al., 2012).
In 2013, Chen et al. recruited 376 PD patients and 277 healthy controls from west China and identified an association between rs1564282 and PD (Chen et al., 2013). The presence of rs1564282 was reported to significantly increase the risk of PD progression (Chen et al., 2013). However, Lin team and Tseng team evaluated Chinese populations from Taiwan and Singapore respectively and demonstrated no association between rs1564282 and PD (Lin et al., 2013; Tseng et al., 2013).
In 2015, Yu et al. analyzed 534 PD patients and 435 neurologically healthy controls from west China and found that rs156428 was significantly associated with PD (Yu et al., 2015).
The inconsistent association results from the previous studies may be due to at least two reasons: genetic heterogeneity and small sample sizes. Firstly, genetic heterogeneity among the previous studies may lead to the inconsistency. Although all the previous studies included Chinese populations, their population compositions (or structures) and geographical environment at largely varied. In other words, the associations between the risk variants and PD may be different among different populations. Secondly, the smaller sample sizes of the previous studies may also contribute to the inconsistency. In these previous studies, Tseng et al. recruited 978 and 777 samples from Taiwan and Singapore population respectively while the Tian et al. used 2049 individuals for analysis. The results of these studies showed that larger sample sizes provided greater power in discovering significant genetic associations. Because of the inconsistent results, the association between rs1564282 and PD in Chinese populations needs further research. Thus, we conducted a new meta-analysis to investigate the association between rs1564282 and PD via combining previous case–control cohort data.
Materials and Methods
Systemic Literature Search
A systemic literature search was performed in four databases: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (https://scholar.google.com/), China National Knowledge Infrastructure (CNKI, http://www.cnki.net/) and Wanfang Medicine database (http://www.wanfangdata.com.cn/). We screened all the relevant studies using the following terms: “Parkinson’s disease”, “GAK” and “Chinese or China”. Literature published before July 31, 2021 was selected. The detailed content of the inclusion and exclusion criteria is given in the Study Selection section.
Study Selection
The eligible studies satisfied the inclusion criteria: 1) case–control designed studies in humans, 2) studies calculating the association between rs1564282 variant and PD and 3) studies providing the number of rs1564282 genotypes or adequate data for the calculation of the odds radio (OR) and a 95% confidence interval (CI). Studies that did not satisfy the inclusion criteria were excluded.
Data Extraction
Two investigators independently extracted the following available data from studies: 1) name of the first author; 2) year of publication; 3) population of study; 4) numbers of PD cases and controls; 5) genotype distribution of rs1564282 in cases and controls; and 6) OR with 95% CI or data for calculating OR and 95% CI.
Genetic Model
The additive genetic model was used to estimate the association between rs1564282 and PD: the T allele versus the C allele.
Statistical Analysis
The Hardy–Weinberg equilibrium (HWE) of rs1564282 in the control for each study was calculated respectively with a chi-squared test at p < 0.001. We conducted the heterogeneity test using Cochran’s Q test and I2 statistic (Liu et al., 2013). The Q statistic follows a χ2 distribution with k−1 degrees of freedom (k means the number of researches selected in calculation). The p-value of Cochran’s Q test <0.1 means a significant heterogeneity exists among the studies. The statistic I2 (
Results
Systematic Literature Search
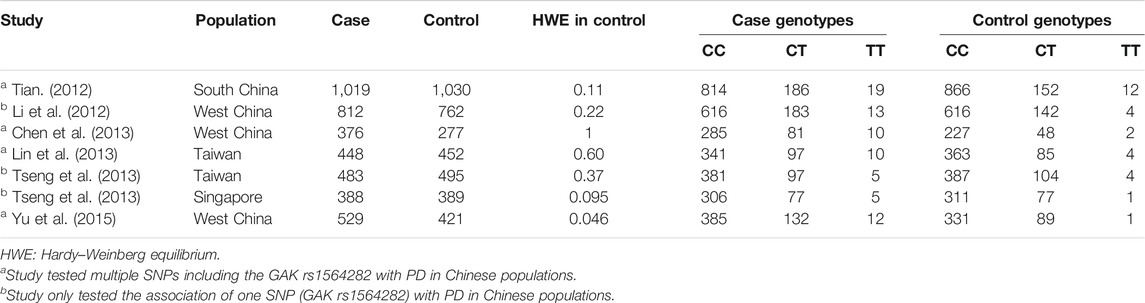
Utilizing our literature search methods, we obtained 20 articles from four databases (Figure 1). Firstly, three articles were removed due to duplication or being a review. Subsequently, 11 articles were excluded because they did not estimate the association between rs1564282 variant and PD or have sufficient data to compute OR. Finally, six articles that included seven studies, with a total of 4,055 PD patients and 3,826 controls, were selected for the meta-analysis. Detailed characteristics of the eligible studies are listed in Table 1.
HWE and Heterogeneity Test
We evaluated the HWE of rs1564282 in controls for each study respectively. We did not find significant deviation from HWE at p < 0.001. Neither Cochran’s Q test nor I2 statistic identified significant heterogeneity of rs1564282 polymorphism among the seven studies in Chinese populations (p = 0.44 and I2 = 0%).
Meta-Analysis
Because there was no significant heterogeneity of rs1564282 polymorphism, we computed the general OR with 95% CI using a fixed-effect model. The meta-analysis results demonstrated significant association between GAK rs1564282 and PD with p = 1.00E-04, OR = 1.28 and 95% CI = 1.16–1.42. More information on the meta-analysis results are shown in Figure 2.
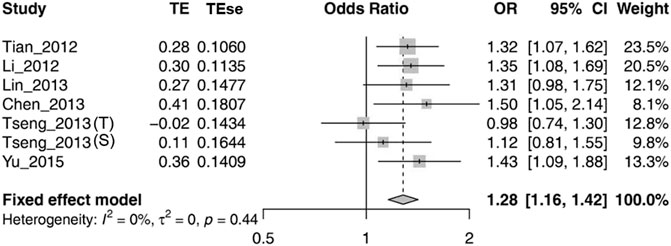
FIGURE 2. Forest plot for meta-analysis of the association between rs1564282 polymorphism and the risk of PD. Tseng_2013 (T) stands for the Taiwan population in the Tseng_2013 study. Tseng_2013 (S) represents the Singapore population in the Tseng_2013 study. TE = Treatment Effect; TEse = Treatment Effect standard error; CI = Confidence Interval.
Sensitivity Analysis and Publication Bias Analysis
The sensitivity analysis was conducted by removing each study at a time. We found that omitting any eligible study did not substantially influence the overall association between rs1564282 and PD (Figure 3).
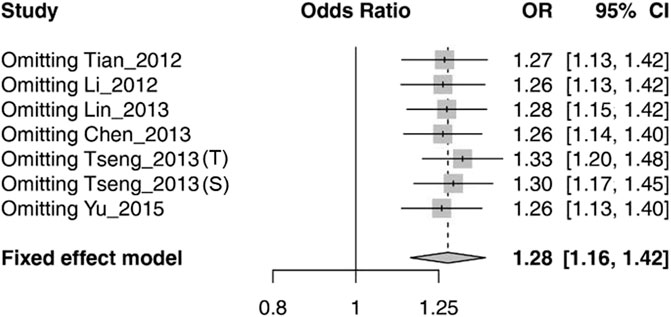
FIGURE 3. Sensitivity analysis of meta-analysis by omitting every study in turn. Tseng_2013 (T) stands for the Taiwan population in the Tseng_2013 study. Tseng_2013 (S) represents the Singapore population in the Tseng_2013 study. CI = Confidence Interval.
The funnel plot was used to estimate the publication bias of the included studies. The shape of the funnel plot was symmetrical and inverted (Figure 4). The regression test showed no significant publication bias among the seven included studies in this meta-analysis (p = 0.77).
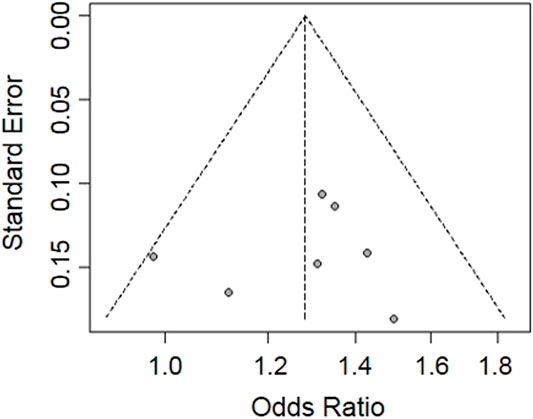
FIGURE 4. Funnel plot for publication bias analysis of the eligible studies evaluating the relationship between rs1564282 polymorphism and the risk of PD. The x-axis and y-axis represent the ORs and standard errors for every eligible study, respectively.
Discussion
The genetic association between the GAK rs1564282 and PD was first reported in a familial PD GWAS and replicated by following studies in European populations (Pankratz et al., 2009; Hamza et al., 2010; Lill et al., 2012; Nalls et al., 2014). Furthermore, the underlying mechanism of GAK in PD has been explored. GAK is ubiquitously expressed and participates in various biological processes such as clathrin-mediated membrane traffic and hepatitis C virus entry (Olszewski et al., 2014; Neveu et al., 2015).
In the pathogenesis of PD, rs1564282 was significantly associated with a higher expression level of α-synuclein expression (encoded by SNCA gene) in the cortex of PD cases than controls using microarray data (Dumitriu et al., 2011). Dumitriu et al. further investigated the interaction between GAK expression and SNCA (Dumitriu et al., 2011). They executed small interfering RNA knockdown of GAK in HEK293 cells that overexpressed the SNCA protein and reported that lack of GAK expression increased the cytotoxicity based on the overexpression of a-synuclein (Dumitriu et al., 2011).
In addition to the synergistic action with SNCA, evidence also showed that GAK impacted the leucine-rich repeat kinase 2 (LRRK2) by forming a complex (Beilina et al., 2014). The gene for LRRK2 has been identified as risk both for monogenic and sporadic PD (Gasser, 2009; Sharma et al., 2012). Beilina et al. utilized the protein–protein arrays to explore the potential interaction mechanisms of LRRK2 in PD pathogenesis (Beilina et al., 2014). The results indicated that GAK was a part of a LRRK2-related complex that helped the autophagy–lysosome system to clean vesicles from the Golgi (Beilina et al., 2014).
Nagle’s team conducted deep RNA sequencing in human brain tissue from dead PD patients (Nagle et al., 2016). Compared with controls, GAK was a unique gene in the 4p16.3 region which had significantly increased expression in PD after adjustment (q value = 4.80E-09) (Nagle et al., 2016). Song et al. studied the function of auxilin, the Drosophila GAK homolog, via an in vivo model (Song et al., 2017). Through systematic experimentation, auxilin was identified as playing a vital role in PD pathogenesis (Song et al., 2017). Researchers proved that reduced auxilin expression resulted in the progressive loss of dopaminergic neurons (Song et al., 2017). Furthermore, the concurrence of reduced auxilin expression and increased SNCA expression accelerated the early death of dopaminergic neurons (Song et al., 2017). Recent evidence showed that GAK was one candidate PD gene that had association with N6-methyladenosine modification (Qiu et al., 2020).
So far, PD GWASs and relevant large-scale meta-analyses have identified tens of risk loci in European population (Hamza et al., 2010; Nalls et al., 2011; Nalls et al., 2014; Nalls et al., 2019). As a vital part of the world population, Chinese population accounts for a certain proportion of global PD patients. Strong evidence provided by Foo team identified that SNCA, LRRK2 and MCCC1 genes had genome-wide significant associations with PD susceptibility in both Chinese and European population (Foo et al., 2017). In the analysis of risk loci, they inferred that MAPT and GBA genes might be “European-specific variant loci” (Foo et al., 2017). In subsequent studies, some PD risk loci with genome-wide significance identified in European population had been confirmed to have association in Chinese population, for example GALC, IL1R2, SATB1, BIN3 and COQ7 genes (Li et al., 2018; Chen et al., 2019; Hu et al., 2020).
Several studies estimated the underlying association between rs1564282 and PD risk in Chinese populations in China and Singapore (Tseng et al., 2013; Yu et al., 2015). However, the results of these studies were not consistent. We integrated the pooled data of previous studies and conducted a new meta-analysis with 4,055 PD patients and 3,826 controls in all. Firstly, we identified that there was no significant genetic heterogeneity of rs1564282 in the included Chinese populations. Subsequently, the meta-analysis using a fixed-effect model showed a significant association between rs1564282 and PD in Chinese populations. Finally, we performed sensitivity and publication bias analysis. Results showed that the association between rs1564282 and PD was not greatly influenced substantially and that there was no significant publication bias among the eligible studies. In conclusion, our meta-analysis provides good evidence on the risk of the GAK rs1564282 variant on PD in Chinese populations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
HL and YJ conceived and designed the study. HL analyzed data and wrote the manuscript. YJ was responsible for research supervision and manuscript revision. CZ provided technical support. All listed authors approved the final version for submission.
Funding
This work was supported by the National Natural Science Foundation (Grant Number 82172282), Science and Technology Project of Tianjin Municipal Health and Health Committee (Grant Nos. ZC20121 and KJ20048).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, confidence interval; GWAS, genome-wide association studies; HWE, Hardy–Weinberg equilibrium; LRRK2, leucine-rich repeat kinase 2; OR, odds radio; PD, Parkinson’s disease.
References
Ascherio, A., and Schwarzschild, M. A. (2016). The Epidemiology of Parkinson's Disease: Risk Factors and Prevention. Lancet Neurol. 15 (12), 1257–1272. doi:10.1016/s1474-4422(16)30230-7
Beilina, A., Rudenko, I. N., Kaganovich, A., Civiero, L., Chau, H., Kalia, S. K., et al. (2014). Unbiased Screen for Interactors of Leucine-Rich Repeat Kinase 2 Supports a Common Pathway for Sporadic and Familial Parkinson Disease. Proc. Natl. Acad. Sci. U S A. 111 (7), 2626–2631. doi:10.1073/pnas.1318306111
Chen, X., Xiao, Y., Guo, W., Zhou, M., Huang, S., Mo, M., et al. (2019). Relationship between Variants of 17 Newly Loci and Parkinson's Disease in a Chinese Population. Neurobiol. Aging 73, e1–e231230. doi:10.1016/j.neurobiolaging.2018.08.017
Chen, Y. P., Song, W., Huang, R., Chen, K., Zhao, B., Li, J., et al. (2013). GAK Rs1564282 and DGKQ Rs11248060 Increase the Risk for Parkinson's Disease in a Chinese Population. J. Clin. Neurosci. 20 (6), 880–883. doi:10.1016/j.jocn.2012.07.011
Dumitriu, A., Pacheco, C. D., Wilk, J. B., Strathearn, K. E., Latourelle, J. C., Goldwurm, S., et al. (2011). Cyclin-G-associated Kinase Modifies -synuclein Expression Levels and Toxicity in Parkinson's Disease: Results from the GenePD Study. Hum. Mol. Genet. 20 (8), 1478–1487. doi:10.1093/hmg/ddr026
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Foo, J. N., Tan, L. C., Irwan, I. D., Au, W.-L., Low, H. Q., Prakash, K.-M., et al. (2017). Genome-wide Association Study of Parkinson's Disease in East Asians. Hum. Mol. Genet. 26 (1), ddw379–232. doi:10.1093/hmg/ddw379
Gasser, T. (2009). Molecular Pathogenesis of Parkinson Disease: Insights from Genetic Studies. Expert Rev. Mol. Med. 11, e22. doi:10.1017/s1462399409001148
Hamza, T. H., Zabetian, C. P., Tenesa, A., Laederach, A., Montimurro, J., Yearout, D., et al. (2010). Common Genetic Variation in the HLA Region Is Associated with Late-Onset Sporadic Parkinson's Disease. Nat. Genet. 42 (9), 781–785. doi:10.1038/ng.642
Hu, X., Mao, C., Hu, Z., Zhang, Z., Zhang, S., Yang, Z., et al. (2020). Association Analysis of 15 GWAS-Linked Loci with Parkinson's Disease in Chinese Han Population. Neurosci. Lett. 725, 134867. doi:10.1016/j.neulet.2020.134867
Li, G., Cui, S., Du, J., Liu, J., Zhang, P., Fu, Y., et al. (2018). Association of GALC, ZNF184, IL1R2 and ELOVL7 with Parkinson's Disease in Southern Chinese. Front. Aging Neurosci. 10, 402. doi:10.3389/fnagi.2018.00402
Li, N.-N., Chang, X.-L., Mao, X.-Y., Zhang, J.-H., Zhao, D.-M., Tan, E.-K., et al. (2012). GWAS-Linked GAK Locus in Parkinson's Disease in Han Chinese and Meta-Analysis. Hum. Genet. 131 (7), 1089–1093. doi:10.1007/s00439-011-1133-3
Lill, C. M., Roehr, J. T., McQueen, M. B., Kavvoura, F. K., Bagade, S., Schjeide, B.-M. M., et al. (2012). Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database. Plos Genet. 8 (3), e1002548. doi:10.1371/journal.pgen.1002548
Lin, C.-H., Chen, M.-L., Tai, Y.-C., Yu, C.-Y., and Wu, R.-M. (2013). Reaffirmation of GAK, but Not HLA-DRA, as a Parkinson's Disease Susceptibility Gene in a Taiwanese Population. Am. J. Med. Genet. 162 (8), 841–846. doi:10.1002/ajmg.b.32188
Liu, G., Xu, Y., Jiang, Y., Zhang, L., Feng, R., and Jiang, Q. (2017). PICALM Rs3851179 Variant Confers Susceptibility to Alzheimer's Disease in Chinese Population. Mol. Neurobiol. 54 (5), 3131–3136. doi:10.1007/s12035-016-9886-2
Liu, G., Zhang, S., Cai, Z., Ma, G., Zhang, L., Jiang, Y., et al. (2013). PICALM Gene Rs3851179 Polymorphism Contributes to Alzheimer's Disease in an Asian Population. Neuromol Med. 15 (2), 384–388. doi:10.1007/s12017-013-8225-2
Nagle, M. W., Latourelle, J. C., Labadorf, A., Dumitriu, A., Hadzi, T. C., Beach, T. G., et al. (2016). The 4p16.3 Parkinson Disease Risk Locus Is Associated with GAK Expression and Genes Involved with the Synaptic Vesicle Membrane. PLoS One 11 (8), e0160925. doi:10.1371/journal.pone.0160925
Nalls, M. A., Blauwendraat, C., Vallerga, C. L., Heilbron, K., Bandres-Ciga, S., Chang, D., et al. (2019). Identification of Novel Risk Loci, Causal Insights, and Heritable Risk for Parkinson's Disease: a Meta-Analysis of Genome-wide Association Studies. Lancet Neurol. 18 (12), 1091–1102. doi:10.1016/s1474-4422(19)30320-5
Nalls, M. A., Nalls, M. A., Plagnol, V., Hernandez, D. G., Sharma, M., Sheerin, U. M., et al. (2011). Imputation of Sequence Variants for Identification of Genetic Risks for Parkinson's Disease: a Meta-Analysis of Genome-wide Association Studies. Lancet 377 (9766), 641–649. doi:10.1016/s0140-6736(10)62345-8
Nalls, M. A., Pankratz, N., Pankratz, N., Lill, C. M., Do, C. B., Hernandez, D. G., et al. (2014). Large-scale Meta-Analysis of Genome-wide Association Data Identifies Six New Risk Loci for Parkinson's Disease. Nat. Genet. 46 (9), 989–993. doi:10.1038/ng.3043
Neveu, G., Ziv-Av, A., Barouch-Bentov, R., Berkerman, E., Mulholland, J., and Einav, S. (2015). AP-2-associated Protein Kinase 1 and Cyclin G-Associated Kinase Regulate Hepatitis C Virus Entry and Are Potential Drug Targets. J. Virol. 89 (8), 4387–4404. doi:10.1128/jvi.02705-14
Olszewski, M. B., Chandris, P., Park, B.-C., Eisenberg, E., and Greene, L. E. (2014). Disruption of Clathrin-Mediated Trafficking Causes Centrosome Overduplication and Senescence. Traffic 15 (1), 60–77. doi:10.1111/tra.12132
Pankratz, N., Wilk, J. B., Latourelle, J. C., DeStefano, A. L., Halter, C., Pugh, E. W., et al. (2009). Genomewide Association Study for Susceptibility Genes Contributing to Familial Parkinson Disease. Hum. Genet. 124 (6), 593–605. doi:10.1007/s00439-008-0582-9
Qiu, X., He, H., Huang, Y., Wang, J., and Xiao, Y. (2020). Genome-wide Identification of m6A-Associated Single-Nucleotide Polymorphisms in Parkinson's Disease. Neurosci. Lett. 737, 135315. doi:10.1016/j.neulet.2020.135315
Sharma, M., Ioannidis, J. P. A., Aasly, J. O., Annesi, G., Brice, A., Van Broeckhoven, C., et al. (2012). Large-scale Replication and Heterogeneity in Parkinson Disease Genetic Loci. Neurology 79 (7), 659–667. doi:10.1212/WNL.0b013e318264e353
Song, L., He, Y., Ou, J., Zhao, Y., Li, R., Cheng, J., et al. (2017). Auxilin Underlies Progressive Locomotor Deficits and Dopaminergic Neuron Loss in a Drosophila Model of Parkinson's Disease. Cel Rep. 18 (5), 1132–1143. doi:10.1016/j.celrep.2017.01.005
Spencer, C. C., Spencer, C. C. A., Plagnol, V., Strange, A., Gardner, M., Paisan-Ruiz, C., et al. (2011). Dissection of the Genetics of Parkinson's Disease Identifies an Additional Association 5' of SNCA and Multiple Associated Haplotypes at 17q21. Hum. Mol. Genet. 20 (2), 345–353. doi:10.1093/hmg/ddq469
Tian, J. (2012). Polymorphism Analysis and Poly Gene Association Analysis of PARK16, PARK17, PARK18 and BST1 Genes in Parkinson's Disease. China National Knowledge Infrastructure Available at: http://cdmd.cnki.com.cn/Article/CDMD-10533-1012475946.htm.
Tseng, W.-E. J., Chen, C.-M., Chen, Y.-C., Yi, Z., Tan, E.-K., and Wu, Y.-R. (2013). Genetic Variations of GAK in Two Chinese Parkinson's Disease Populations: a Case-Control Study. PLoS One 8 (6), e67506. doi:10.1371/journal.pone.0067506
Keywords: Parkinson’s disease, genome-wide association study, GAK, rs1564282, Chinese population
Citation: Li H, Zhang C and Ji Y (2021) Association of GAK rs1564282 With Susceptibility to Parkinson’s Disease in Chinese Populations. Front. Genet. 12:777942. doi: 10.3389/fgene.2021.777942
Received: 16 September 2021; Accepted: 01 November 2021;
Published: 18 November 2021.
Edited by:
Liangcai Zhang, Janssen Research and Development, United StatesReviewed by:
Jinchen Li, Central South University, ChinaJudong Shen, Merck and Co., Inc., United States
Copyright © 2021 Li, Zhang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Ji, aml5b25ndXNhQDEyNi5jb20=
†These authors have contributed equally to this work
 He Li
He Li Chen Zhang
Chen Zhang Yong Ji
Yong Ji