
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 08 December 2021
Sec. Computational Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.767694
This article is part of the Research TopicNetwork Based Multi-Omics Analyses to Reveal Mechanisms and Biomarkers of Complex DiseasesView all 8 articles
 Hang Chen1†
Hang Chen1† Zeyang Hu1†
Zeyang Hu1† Menglu Sang2†
Menglu Sang2† Saiqi Ni3†
Saiqi Ni3† Yao Lin1†
Yao Lin1† Chengfang Wu1†
Chengfang Wu1† Yinyu Mu2
Yinyu Mu2 Kaitai Liu2
Kaitai Liu2 Shibo Wu2
Shibo Wu2 Ni Li2
Ni Li2 Guodong Xu2*
Guodong Xu2*Autophagy is closely associated with the tumor immune microenvironment (TIME) and prognosis of patients with lung adenocarcinoma (LUAD). In the present study, we established a signature on the basis of long noncoding RNAs (lncRNAs) related to autophagy (ARlncRNAs) to investigate the TIME and survival of patients with LUAD. We selected ARlncRNAs associated with prognosis to construct a model and divided each sample into different groups on the basis of risk score. The ARlncRNA signature could be recognized as an independent prognostic factor for patients with LUAD, and patients in the low-risk group had a greater survival advantage. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology enrichment analysis suggested that several immune functions and pathways were enriched in different groups. A high-risk score correlated significantly negatively with high abundance of immune cells and stromal cells around the tumor and high tumor mutational burden. Low-risk patients had a higher PD-1, CTLA-4, and HAVCR2 expression and had a better efficacy of immune checkpoint inhibitors, including PD-1/CTLA-4 inhibitor. A reliable signature on the basis of ARlncRNAs was constructed to explore the TIME and prognosis of patients with LUAD, which could provide valuable information for individualized LUAD treatment.
Lung cancer is one of the malignant tumors with the highest morbidity and mortality in the world (Nasim et al., 2019). The incidence and mortality of lung cancer in the United States in 2021 are estimated to be 235,760 and 131,880, respectively (Siegel et al., 2021). In China, there were estimated 733,000 new lung cancer cases and 610,000 deaths in 2015 (Chen et al., 2016). Non–small cell lung cancer (NSCLC) accounts for about 85% of lung cancers, of which adenocarcinoma accounts for about 50% of NSCLC (Shi et al., 2019). Although the advent of radiotherapy and chemotherapy has revolutionized the NSCLC treatment, the 5-year survival rate of NSCLC with distant metastasis is only 7% (American Cancer Society, 2021). Therefore, it is crucial to screen a reliable biomarker to guide individualized treatment of NSCLC.
As a crucial part of the recycling process in complicated tumor immune microenvironment (TIME), autophagy in tumor immunity has been increasingly appreciated (Li et al., 2015). For example, Jiang et al. illustrated the impact of autophagy on TIME from three perspectives and proposed that autophagy-based therapy combined with immunotherapy may be promising (Jiang et al., 2021). Gerada and Ryan (2020) explained that TIME determines whether autophagy promotes or inhibits tumors. Long noncoding RNAs (LncRNAs) are a class of RNA that do not code proteins with transcripts >200 nucleotides (Cai et al., 2020). They participated in the progression and metastasis of lung adenocarcinoma (LUAD) and were associated with immune pathways and even served as a biomarker for prognosis of LUAD (Wei et al., 2017; Pang et al., 2021). Recently, the prognostic signatures on the basis of coding or noncoding genes to predict the prognosis of patients with LUAD have been a research hot spot (Zhang et al., 2021a; Li et al., 2021a). However, utilizing lncRNAs related to autophagy (ARlncRNAs) to construct models and exploring the tumor immunity and the efficacy of immunotherapy in patients with LUAD were still lacking. In this study, we established a novel ARlncRNA signature to analyze the TIME and prognosis of patients with LUAD, which represented a step toward individualized immunotherapy in LUAD.
A signature on the basis of ARlncRNAs was established by a multi-step approach (Figure 1). We acquired the transcriptome profiles and the corresponding clinical information of patients with LUAD and normal samples from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) (Tomczak et al., 2015) database in December 2020. The gene transfer format (GTF) files were obtained from Ensembl (http://asia.ensembl.org) (Howe et al., 2021) to distinguish lncRNAs and messenger RNAs (mRNAs). Furthermore, a list of autophagy-related genes (ARGs) from the Human Autophagy Database (HADb) (http://www.autophagy.lu/) was acquired. We identified ARlncRNAs by calculating the correlation coefficient between the ARGs and lncRNAs (|cor| > 0.4 and p < 0.001).
Kaplan–Meier survival analysis combined with univariate Cox analysis was performed to screen the ARlncRNAs expression levels that were significantly associated with the overall survival (OS) of the patients with LUAD (log-rank p < 0.05, and p < 0.05). They were chosen for subsequent Cox proportional hazard regression analysis, and a heatmap was plotted for visualization. Subsequently, the multivariate Cox regression analysis was performed to construct the prognostic signature by running the R-x64-4.0.4 survival package, and the risk score of every patient with LUAD was calculated on the basis of the following formula:
Coef(i) (Table 1) and E(i) represent the regression coefficient of the multivariate Cox analysis for each ARlncRNA and each ARlncRNA expression level, respectively. The median value of risk score was considered as the cutoff point for differentiating each patient into different groups.
Several receiver operating characteristic (ROC) curves were generated, and area under the ROC curve (AUC) was calculated by R-x64-4.0.4 survival, survminer, and timeROC packages to validate the predictive value of the prognostic signature. Kaplan–Meier log-rank test was conducted for comparing the OS between different risk groups to assess the predictive value of the prognosis model. A barplot and a boxplot were generated by R-x64-4.0.4 packages plyr, ggplot2, and ggpubr to study whether there are statistical differences in OS between different risk groups. In addition, univariate and multivariate Cox regression analyses were performed to explore whether the prognostic signature was a potential independent prognostic indicator for patients with LUAD, and the results were visualized with two forest maps. To study the correlation between risk score and clinicopathological characteristics, Wilcoxon rank sum tests were performed to plot boxplots. A heatmap and multiple Kaplan–Meier survival analyses were generated to visualize the expression and prognosis of ARlncRNAs included in the process of modeling, respectively.
Differentially expressed genes between different risk groups were identified by performing differential expression analysis. The R-x64-4.0.4 packages limma was utilized, and the significance threshold for determining differentially expressed genes was log fold change > 1 and false discovery rate < 0.05. To filter functional phenotypes in different risk groups, we performed gene set enrichment analysis (GSEA) 4.0.1 (https://www.gsea-msigdb.org/gsea/index.jsp) (Subramanian et al., 2005) by Kyoto Encyclopedia of Genes and Genomes (KEGG) (p < 0.05 and q < 0.25). Gene Ontology (GO) function enrichment analysis was conducted to study the functional phenotypes where differentially expressed ARlncRNAs were enriched (p < 0.05 and q < 0.05).
Spearman analysis was conducted to calculate the correlation coefficient between the tumor mutational burden (TMB) and the constructed model on the basis of R-x64-4.0.4 ggplot2, ggpubr, and ggExtra packages. The differences in TMB between different risk groups were explored by Wilcoxon signed-rank test, and a boxplot was plotted. To study the connection between TMB and the prognostic signature comprehensively, Kaplan–Meier survival analyses were performed for comparing OS between high TMB and low TMB.
To explore the abundance of immune cells and stromal cells between different groups, StromalScore, ImmuneScore, and ESTIMATEScore (StromalScore + ImmuneScore) of each patient were calculated by R-x64-4.0.4 estimate and limma packages. Then, Wilcoxon rank sum tests were utilized to explore the differences in StromalScore, ImmuneScore, and ESTIMATEScore between different groups, and three column diagrams were plotted for visualization. Single-sample GSEA (ssGSEA) was conducted for scoring LUAD-infiltrating immune cells to quantify their relative content. The scores of immune cells and pathways in different groups were shown on multi-boxplots, respectively.
The emergence of immunotherapy has revolutionized the treatment of NSCLC. National Comprehensive Cancer Network (NCCN) guidelines (National Comprehensive Cancer Network, 2021) recommend a series of immunotherapy drugs, including CTLA-4 and PD-1 blocking antibodies for NSCLC treatment. Expression analyses of common immune checkpoint inhibitor (ICI)–related immunosuppressive molecules (e.g., PD-1, CTLA-4, and HAVCR-2) were conducted by R-x64-4.0.3, ggpubr, and limma packages to detect any statistical differences in expression of common ICI-related immunosuppressive molecules between different risk groups, and several violin plots were generated for visualization. Immunophenoscore (IPS) representing the immunotherapy effect of patients with LUAD was extracted and obtained from the The Cancer Immunome Atlas (https://tcia.at/) database (Hugo et al., 2017). The difference in immunotherapy efficacy for patients with LUAD between different groups was calculated by Wilcoxon rank sum tests, and boxplots were labeled for visualization as follows: ***<0.001, **<0.01, and *<0.05.
Figure 1 shows a multi-step approach that was conducted to identify ARlncRNAs. A total of 551 LUAD transcriptome data, corresponding clinical data from TCGA-LUAD cohort (54 normal samples and 497 LUAD samples), and 232 ARGs from HADb were downloaded. Subsequently, we distinguished the mRNAs and lncRNAs by GTF files and identified 1,071 ARlncRNAs on the basis of Spearman correlation analysis.
Using univariate Cox analysis combined with Kaplan–Meier survival analysis, we screened out 57 ARlncRNAs associated with survival of patients with LUAD (Figure 2A). Multivariate Cox regression analysis yielded 14 ARlncRNAs among ARlncRNAs related to prognosis for subsequent modeling. The risk score of every patient was calculated on the basis of correlation coefficients calculated by multivariate Cox regression analysis, and each sample was differentiated into different groups by median value of risk score.
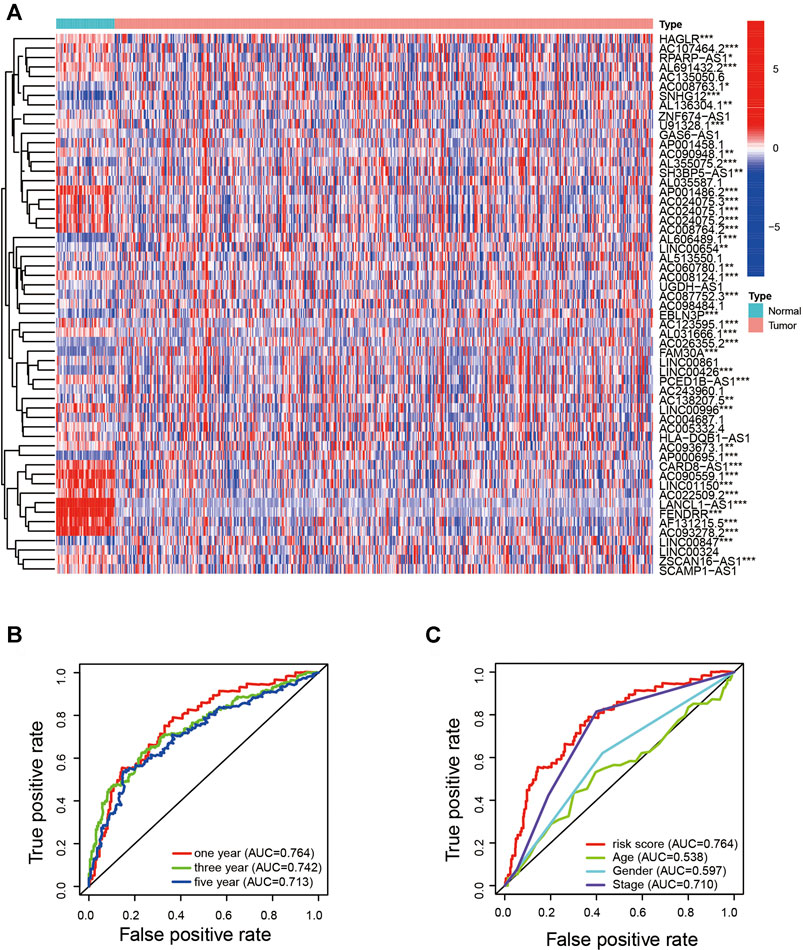
FIGURE 2. Identification of the ARlncRNA signature. (A) Heatmap shows the expression of ARlncRNAs related to survival in LUAD and normal samples. (B) The 1-, 3-, and 5-year AUC values were >0.7. (C) Compared with AUC of age, sex, and stage, the 1-year AUC value was the maximum.
To quantify the predictive ability of the ARlncRNA signature, we generated several ROC for validation. The curves demonstrated that 1-, 3-, and 5-year AUC values were 0.764, 0.742, and 0.713, respectively (Figure 2B). Furthermore, compared with other clinicopathological characteristics (e.g., age, gender, and stage), the 1-year AUC value was the maximum (Figure 2C). The survival curve, boxplot, and barplot indicated that patients with low risk had a better prognosis and were statistically significant (Figures 3A–C). To explore the relationship between the ARlncRNA signature with clinicopathological characteristics, we performed univariate and multivariate Cox regression analysis, which suggested that two factors, i.e., the risk score [hazard ratio (HR) = 1.589, confidence interval (CI) = 1.406–1.797, p < 0.001] and stage (HR = 1.470, CI = 1.265–1.710, p < 0.001), correlated with the survival (Figures 3D,E). The 14 ARlncRNAs included in the modeling process were closely associated with survival outcomes of patients with LUAD (Figures 4A–N). The results above suggested that the ARlncRNA signature could act as a potential independent prognostic indicator for patients with LUAD. The heatmap (Figure 5A) suggested that stage (p < 0.001) and ImmuneScore (p < 0.001), N (p < 0.01) were statistically different in different groups. In addition, most ARlncRNAs included in the process of modeling were enriched in the low-risk group, suggesting that autophagy was more active in the low-risk group. The boxplots indicated that patients with LUAD with advanced T (Figure 5B, T3-4, p < 0.01), advanced stage (Figure 5C, Stage III-IV, p < 0.001), or advanced N (Figure 5D, N1-3, p < 0.001) tended to have higher-risk score and statistically significant.
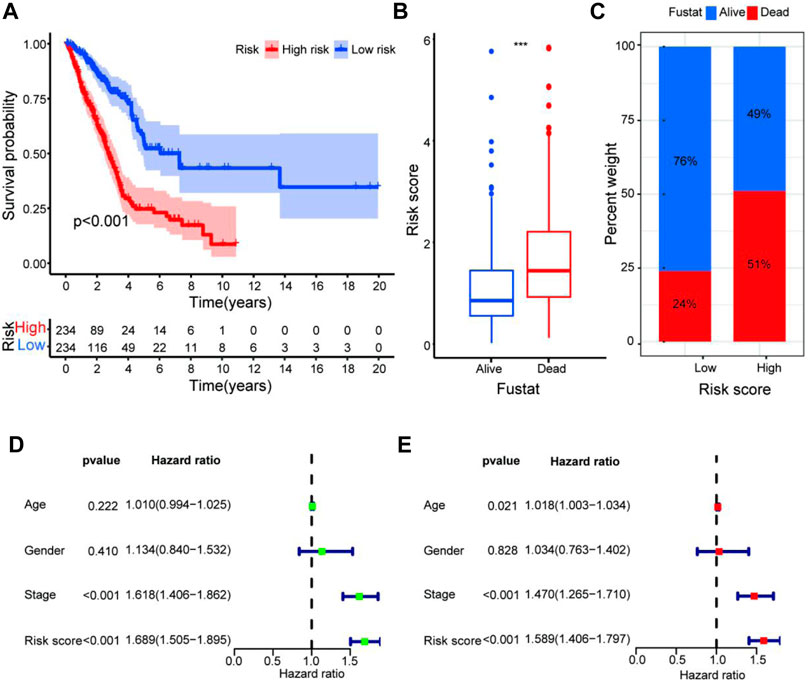
FIGURE 3. Validation of the predictive value of the prognostic signature. (A–C) The survival curve (A), boxplot (B), and barplot (C) indicate that the ARlncRNA signature was associated with survival outcomes of patients with LUAD, and patients with low risk have a significant survival advantage. (D,E) Univariate (D) and multivariate (E) Cox regression analyses including age, gender, stage, and risk score suggest that risk score (HR = 1.589, CI = 1.406–1.797, p < 0.001) and stage (HR = 1.470, CI = 1.265–1.710, p < 0.001) could act as an independent prognostic factor for LUAD.
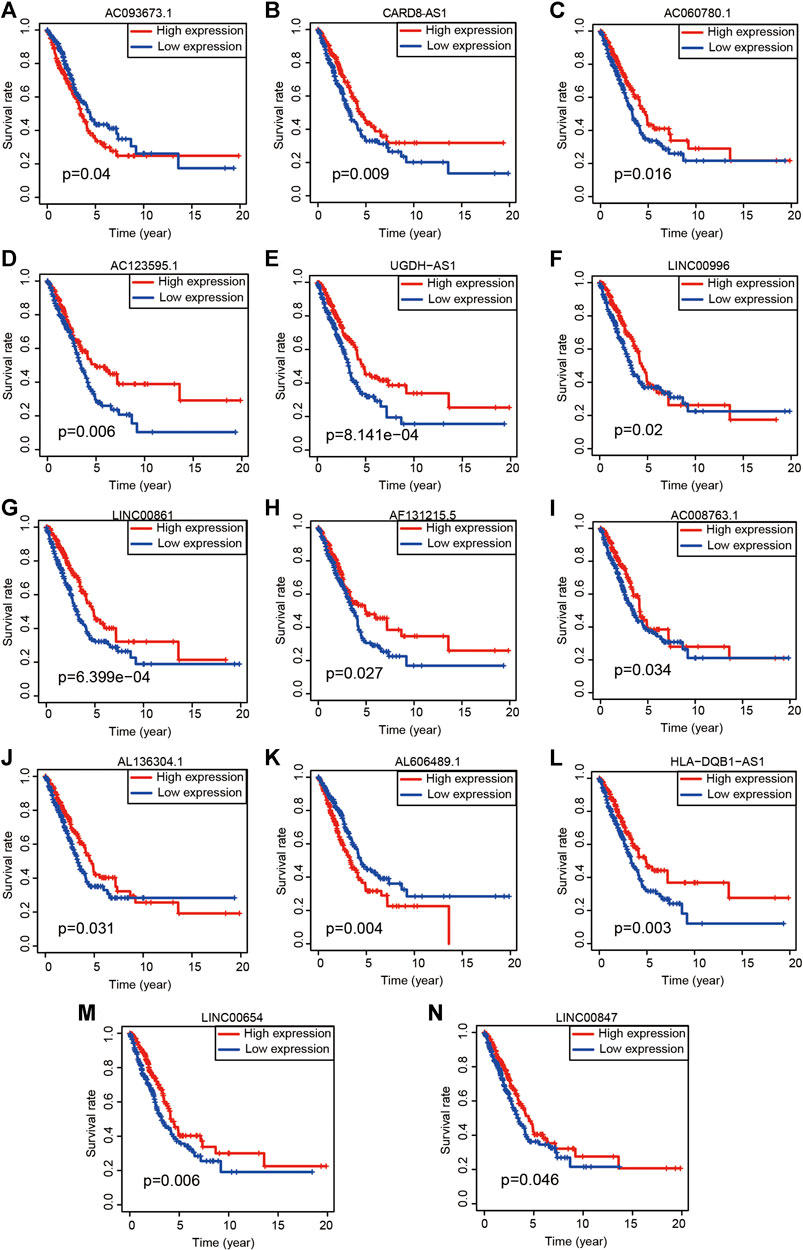
FIGURE 4. Kaplan-Meier survival analyses of 14 ARlncRNAs included in the modeling process. (A–N) The survival curves of 14 ARlncRNAs included by the multivariate Cox regression.
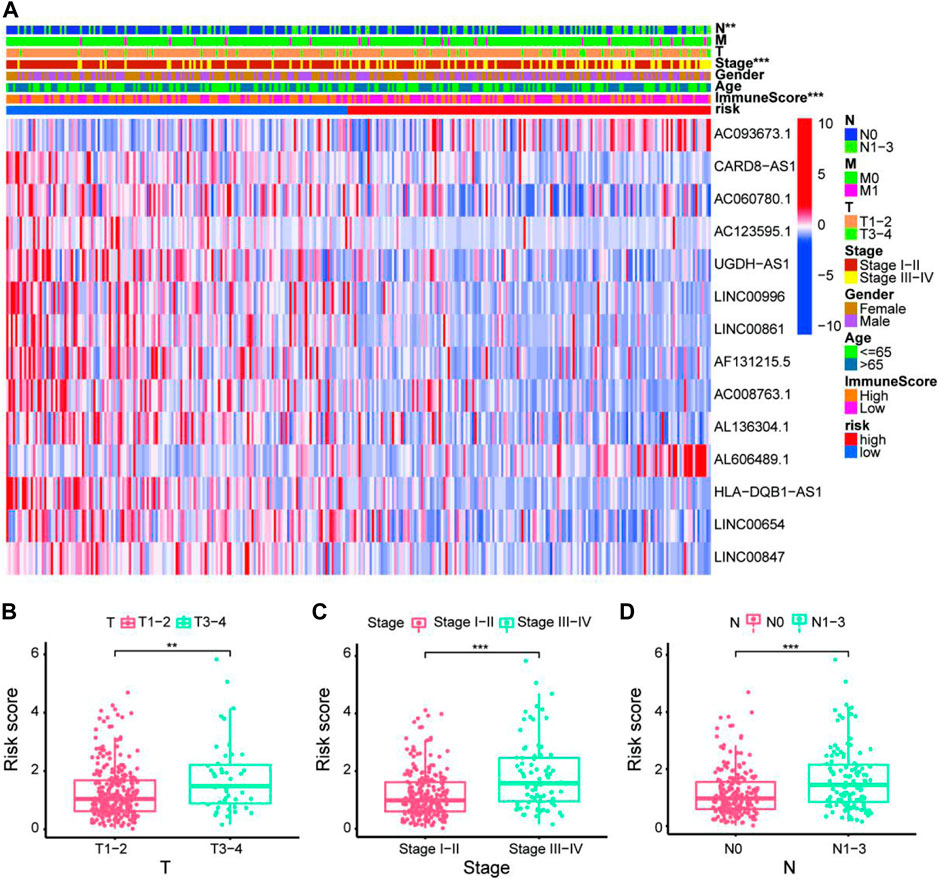
FIGURE 5. Exploration of clinicopathological characteristics of the 14-ARlncRNA signature. (A) The heatmap suggested that stage (p < 0.001), ImmuneScore (p < 0.001), and N (p < 0.01) were statistically different in different groups. In addition, most ARlncRNAs included in the modeling process were enriched in the low-risk group, suggesting that autophagy was more active in the low-risk group. (B–D) The scatter diagrams indicated that patients with LUAD with advanced T, advanced stage, or advanced N tended to have higher-risk score and statistically significant.
GSEA (Figure 6A) indicated that several immune pathways, such as B cell receptor signaling, natural killer cell–mediated cytotoxicity, T cell receptor signaling pathway, and vascular endothelial growth factor (VEGF) signaling pathway, were enriched in patients with low risk. Cell cycle, P53 signaling pathway, pathways in cancer, small cell lung cancer, and thyroid cancer were relatively more active in the high-risk group. GO function enrichment analysis indicated that several functions related to tumor immunity, such as humoral immune response, antimicrobial humoral response, antigen processing and presentation, and T cell–mediated cytotoxicity, were enriched in different groups (Figure 6B).
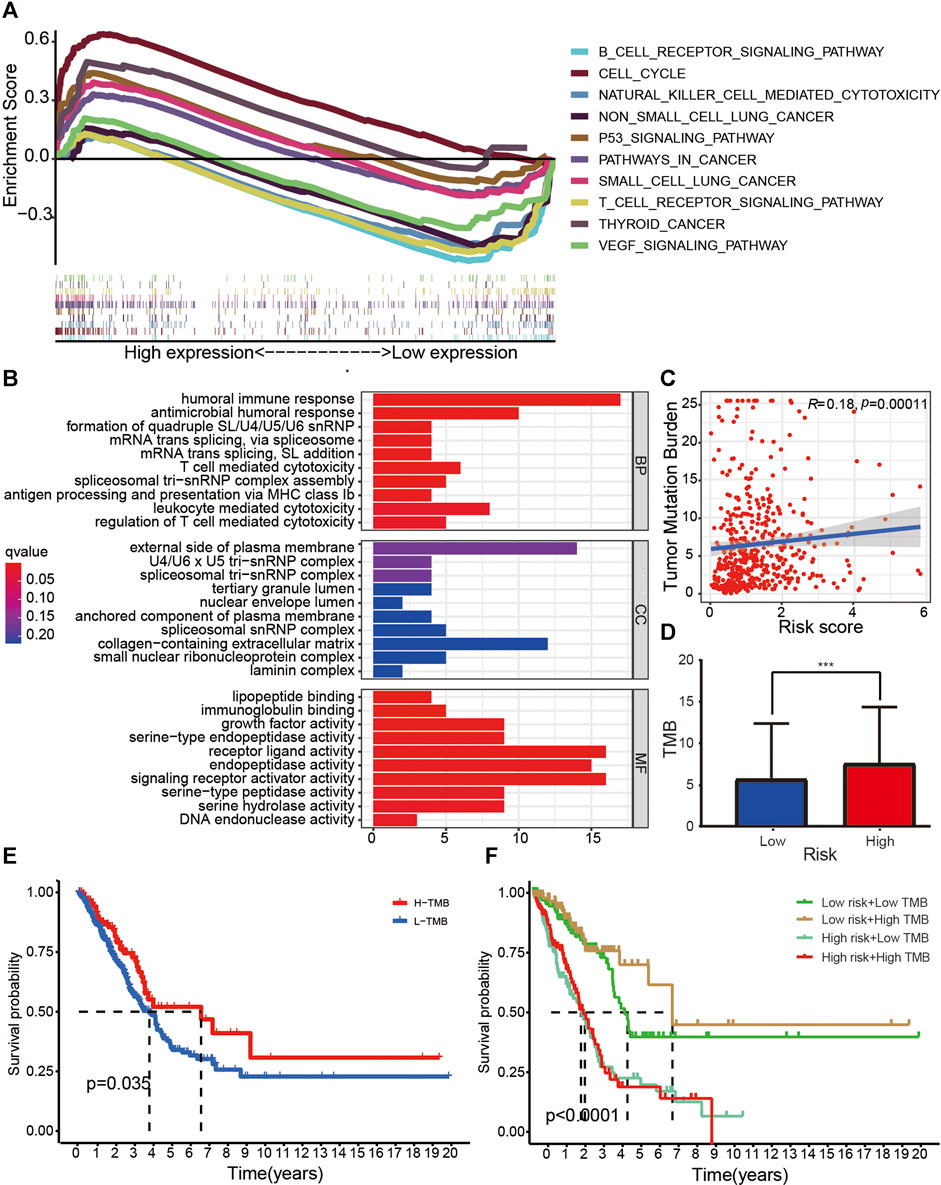
FIGURE 6. Research on tumor immunity of the 14-ARlncRNA signature. (A,B) KEGG (A) and GO (B) function enrichment analyses reveal that several immune pathways and immune function were enriched in different groups, suggesting the ARlncRNA signature may be associated with tumor immunity. (C,D) The scatter diagram (C) and column diagram (D) show that high-risk patients exhibit significantly higher TMB. (E,F) The survival curves indicate that patients with a combination of low-risk and high TMB showed a greater prognosis.
The correlation curve (Figure 6C) and boxplots (Figure 6D) indicated that TMB was significantly negatively correlated with risk score, and the TMB of patients with high risk was significantly higher than those with low risk (p < 0.001). According to survival curves (Figures 6E,F), patients with high TMB had a better survival advantage (p = 0.035), and patients with a combination of low risk and high TMB showed a great prognosis.
We found that StromalScore (Figure 7A, p < 0.001), ImmuneScore (Figure 7B, p < 0.001), and ESTIMATEScore (Figure 7C, p < 0.001) in low-risk patients were significantly higher than that of high-risk patients. The multi-boxplots (Figures 7D,E) indicated that the abundance of activated dendritic cells (aDCs), B cells, dendritic cells (DCs), immature dendritic cells (iDCs), mast cells, neutrophils, plasmacytoid dendritic cells (pDCs), T helper cells, tumor infiltrating lymphocytes (TILs), and regulatory T cells (Tregs) associated significantly negatively with the risk score. Compared with the high-risk group, several immune pathways, e.g., clinical complete response (CCR), checkpoint, cytolytic activity, human leukocyte antigen (HLA), T cell co-inhibition, T cell co-stimulation, and type II interferon (type II IFN) response were more active in the low-risk group.
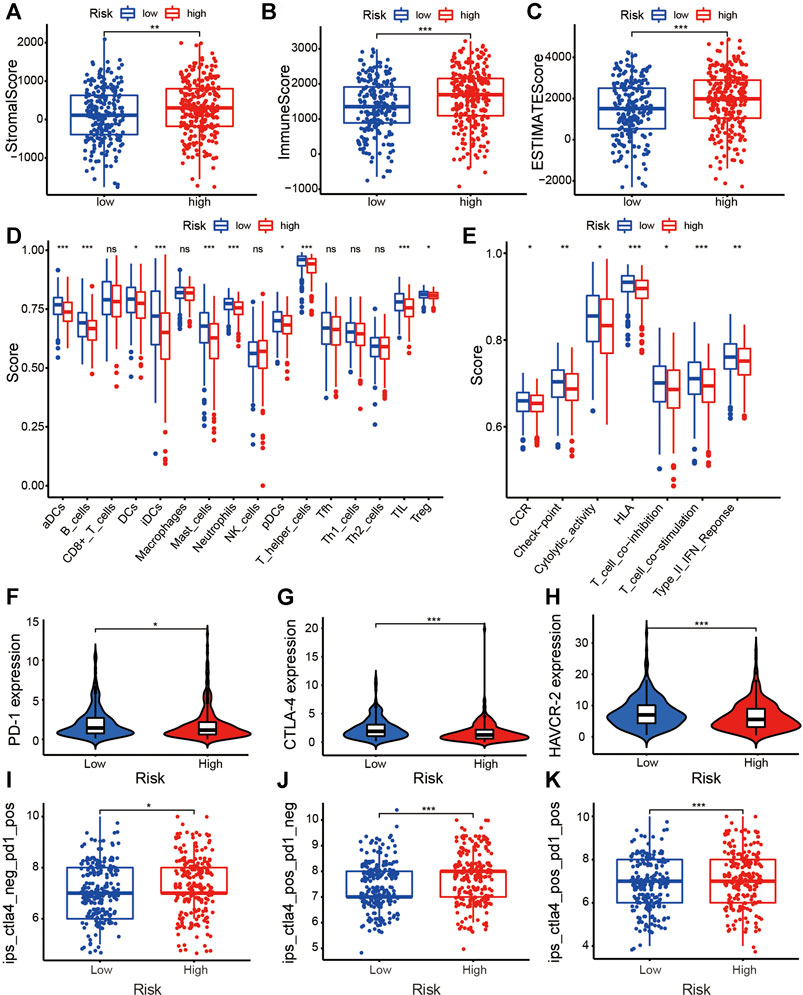
FIGURE 7. Exploration of the tumor immunity and ICI efficacy of the ARlncRNA signature. (A–C) The column diagrams reveal that low-risk group patients have a higher StromalScore (A), ImmuneScore (B), and ESTIMATEScore (C). (D,E) The multi-boxplots show that patients in low-risk group have a higher abundance of aDCs, B cells, DCs, iDCs, mast cells, neutrophils, pDCs, T helper cells, TILs, and Tregs (D). Several immune pathways, including CCR, checkpoint, cytolytic activity, HLA, T cell co-inhibition, T cell co-stimulation, and type II IFN response were enriched in low-risk patients (E). (F–H) The violin plots indicate that high-risk score correlated significantly negatively with high PD-1 (F), CTLA-4 (G), and HAVCR-2 (H) expression, revealing low-risk patients are more applicable for ICIs treatment. (I–K) The violin plots validate the results above that regardless of whether it is a PD-1 blocker alone (I), a CTLA-4 blocker alone (J), or a combination of the two (K), the efficacy of patients with low-risk is better than that of high-risk patients.
The violin plots showed that the expression of common ICI-related immunosuppressive molecules, e.g., PD-1 (Figure 7F, p < 0.05), CTLA-4 (Figure 7G, p < 0.001), and HAVCR-2 (Figure 7H, p < 0.001), in low-risk group was significantly higher than those in high-risk group, which indicated that the efficacy of the abovementioned ICIs seem to be better for low-risk patients. The boxplots on the basis of IPS validated the above results, suggesting that, whether it is PD-1 inhibitor alone (Figure 7I, p < 0.05), CTLA-4 inhibitor alone (Figure 7J, p < 0.001), or a combination of the two (Figure 7K, p < 0.001), the efficacy of low-risk patients is better than that of high-risk patients.
Recently, the prognostic signatures on the basis of bioinformatics analyses for forecasting the survival outcome of cancer patients have become more and more popular (Liu et al., 2021a; Zhang et al., 2021b). Although there are already lots of signatures utilizing ARlncRNAs (Wu et al., 2021a) or ARGs (Duan et al., 2021) to predict the survival outcomes of patients with LUAS, we are the first to explore the tumor immunity of the ARlncRNA model in detail. We conducted a detailed study on the tumor immunity of the ARlncRNA signature, which renders the constructed signature applicable for guiding the clinical personalized treatment of patients with LUAD.
First, the lncRNA and ARG transcriptome profiles were obtained, and ARlncRNAs related to prognosis of patients with LUAD on the basis of co-expression analysis and univariate Cox analysis were identified. Next, we calculated the 1-, 3-, and 5-year AUC values to obtain an ideal signature and differentiated each patient into different groups on the basis of median value. Then, the survival and clinicopathological characteristics were analyzed to assess the predictive value of the ARlncRNA signature. Subsequently, we conducted a comprehensive assessment of the tumor immunity of the ARlncRNA signature, including GO and KEGG function enrichment analyses, TMB, TME, infiltration of immune cells, expression of common ICI-related immunosuppressive molecules, and efficacy of ICIs.
Previous studies on LUAD have mostly focused on single genes or noncoding genes, which are unable to illustrate the complex tumorigenesis and development process (Fan et al., 2018; Wang et al., 2020). In recent years, a combination of several genes to improve the predictive value of OS in patients with LUAD was gradually identified. For example, Duan et al. (2021) constructed a prognostic signature on the basis of ARGs, which served as a novel biomarker in LUAD. Meanwhile, Zhu et al. (2021) identified a ferroptosis-related gene signature and explored the immune cells infiltration. In this study, several ARlncRNAs included in the modeling process have been already reported in various malignant tumors, such as CARD8-AS1 (Lin et al., 2018; Li and Zhan, 2019), AC060780.1 (Curty et al., 2020), AC123595.1 (Li et al., 2020; Zheng et al., 2020), UGDH-AS1 (Liu et al., 2020), LINC00996 (Ge et al., 2018), LINC00861 (Qian et al., 2018; Liu et al., 2021b; Hu et al., 2021), AL606489.1 (Wu et al., 2021a), HLA-DQB1-AS1 (Singh and Gasman, 2020), LINC00654 (Li et al., 2017), and LINC00847 (Tu et al., 2020; Li et al., 2021b). Whereas, others have not been discovered yet and may be potential novel biomarkers for further study.
Researchers found that autophagy played a vital role in tumorigenesis and development. Gu et al. confirmed that bupivacaine-induced autophagy through Akt/mTOR signaling, inhibiting the progression of NSCLC (Gu et al., 2021). Lin et al. proposed that high expression of miR-30a improved the prognosis of NSCLC after neoadjuvant chemotherapy by reducing autophagy caused by chemotherapy drugs (Lin et al., 2021). In addition, Li et al. found that the dysfunction of autophagy mediated by c-myc/miR-150/EPG5 had a great impact on the progression of NSCLC (Li et al., 2019). Collectively, autophagy was probably involved in the occurrence and development of LUAD through a certain signaling pathway, having a significant impact on the prognosis of patients with LUAD.
Then, GSEA enrichment analysis suggested that patients with low risk had more autophagy and were enriched in B cell receptor signaling, natural killer cell–mediated cytotoxicity, T cell receptor signaling pathway, and the VEGF signaling pathway. Recent searches confirmed the strong correlation between autophagy and VEGF. For example, Chen et al. found that VEGF promoted the occurrence of autophagy and VEGF knockdown decreased the autophagy level (Chen et al., 2020). In addition, Spengler et al. (2020) discovered that VEGF signaling pathway regulated autophagy in endothelial cells. Together, we speculated that autophagy probably contributed to the occurrence and development of LUAD through VEGF signaling pathway and that the ARlncRNA signature was closely associated with tumor immunity.
Recently, the interaction between autophagy and tumor immunity was investigated comprehensively. For example, TMB and TIME were identified as important determinants of the efficacy of ICIs and in the prognosis of cancer patients (Wu et al., 2021b). Moreover, Jena et al. (2021) have demonstrated that autophagy was closely related to TIME and participated in tumor progression. To explore the relationship between the ARlncRNA signature and tumor immunity thoroughly, we conducted ssGSEA to investigate the immune status in different groups. The patients in low-risk group had a higher abundance of immune cells and were more active in immune pathways, most of which were validated closely associated with autophagy. For example, Di Rita and Strappazzon (2021) found that CALCOCO2, an autophagy receptor, was mainly expressed in B cells, which mediated autophagy. In addition, the function of DCs to secrete cytokines has been shown to be inhibited by autophagy (Liang et al., 2021). Turan et al. (2019) proved through experiments that iDCs infected with TSV-1 could induce autophagy. Li et al. (2021c) demonstrated that autophagy of mast cells could serve as a therapeutic target for allergic reactions. Ding et al. (2021) revealed that neutrophils were associated with autophagy. Autophagy has been validated to fuel pDCs (Basit et al., 2018). Schmid et al. (2006) proposed that CD4(+) T helper cells could recognize MHCII molecules presented after autophagy. Lu et al. (2021) demonstrated that autophagy could mediate the function of Tregs. Samuel et al. (2013) proved that cellular metabolism facilitated autophagy to mediate the cytolytic effect. Zhang et al. (2020) confirmed the firm correlation between autophagy and HLA. These studies above suggest that autophagy is closely linked to tumor immunity, and autophagy probably participates in LUAD progression by regulating tumor immunity.
At present, PD-1 (Fan et al., 2021) and CTLA-4 (Sun et al., 2020) inhibitors have been validated to benefit patients with advanced NSCLC in clinical trials. Furthermore, the research indicated that the autophagy of tumor cells increased the expression of ICI-related immunosuppressive molecules (e.g., PD-1 and CTLA-4) and affected anti-tumor immune responses directly. In this study, expression analyses were conducted to study the correlation between the ARlncRNA signature and the expression of common ICI-related immunosuppressive molecules, which revealed that low-risk patients always had a higher expression of them and a better efficacy of ICIs. Subsequently, we analyzed the efficacy of ICIs and verified the results above, demonstrating that regardless of whether it is a PD-1 inhibitor alone, a CTLA-4 inhibitor alone, or a combination of the two, the efficacy of patients in the low-risk group is better than that of the high-risk patients. Overall, the ARlncRNA signature could serve as a novel indicator for screening patients applicable for ICIs.
According to our data, we speculated that compared with high-risk patients, low-risk patients have more active autophagy, stronger tumor immunity, and greater survival advantage and are more applicable for ICIs treatment. Autophagy could play a crucial part in the progression of LUAD by regulating the tumor immunity through VEGF signaling pathway, which had a great impact on prognosis of patients with LUAD.
However, there are several limitations in our research. First, it is the bias of the information in the analysis process, because the profiles were obtained from public database. Second, it was difficult to find an ideal Gene Expression Omnibus set including both 14 lncRNAs newly identified and detailed clinical information to validate the constructed ARlncRNA signature. Last, external validation, such as quantitative real-time PCR and microarrays are necessary to increase credibility.
In conclusion, we constructed a novel ARlncRNA signature and predicted the survival of patients with LUAD, the state of TIME, and even the efficacy of ICIs accurately on the basis of the expression of the 14 ARlncRNAs included in the modeling process, which may benefit patients with advanced NSCLC. Immunotherapy combined with TIME-targeted therapy may improve individualized treatment of LUAD in the future.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
HC and GX contributed to the conception of the study; ZH, MS, and SN performed the R language; YL, CW, and YM contributed significantly to analysis and manuscript preparation; HC performed the data analyses and wrote the manuscript; KL, SW, and NL helped perform the analysis with constructive discussions.
The study was funded by Basic public welfare project of Ningbo (grant no. 2019C50041), Zhejiang Province Medical and Health Project (grant nos. 2019ZD019 and 2019KY606), the Natural Science Foundation of Ningbo (grant no. 202003N4273), and Nature Science Foundation of Ningbo city (grant no. 2019A610230).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the TCGA database for generously sharing a large amount of data.
American Cancer Society 2021. Non-small Cell Lung Cancer Survival Rates by Stage[OL]. Available at: www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html (Accessed April 20, 2021).
Basit, F., Mathan, T., Sancho, D., and de Vries, I. J. M. (2018). Human Dendritic Cell Subsets Undergo Distinct Metabolic Reprogramming for Immune Response. Front. Immunol. 9, 2489. doi:10.3389/fimmu.2018.02489
Cai, P., Otten, A. B. C., Cheng, B., Ishii, M. A., Zhang, W., Huang, B., et al. (2020). A Genome-wide Long Noncoding RNA CRISPRi Screen Identifies PRANCR as a Novel Regulator of Epidermal Homeostasis. Genome Res. 30 (1), 22–34. doi:10.1101/gr.251561.119
Chen, L., Lin, G., Chen, K., Wan, F., Liang, R., Sun, Y., et al. (2020). VEGF Knockdown Enhances Radiosensitivity of Nasopharyngeal Carcinoma by Inhibiting Autophagy through the Activation of mTOR Pathway. Sci. Rep. 10 (1), 16328. doi:10.1038/s41598-020-73310-x
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA: A Cancer J. Clinicians 66 (2), 115–132. doi:10.3322/caac.21338
Curty, G., Beckerle, G. A., Iñiguez, L. P., Furler, R. L., de Carvalho, P. S., Marston, J. L., et al. (2020). Human Endogenous Retrovirus Expression Is Upregulated in the Breast Cancer Microenvironment of HIV Infected Women: A Pilot Study. Front. Oncol. 10, 553983. doi:10.3389/fonc.2020.553983
Di Rita, A., and Strappazzon, F. (2021). A Protective Variant of the Autophagy Receptor CALCOCO2/NDP52 in Multiple Sclerosis (MS). Autophagy 17 (6), 1565–1567. doi:10.1080/15548627.2021.1924969
Ding, L., Yang, J., Zhang, C., Zhang, X., and Gao, P. (2021). Neutrophils Modulate Fibrogenesis in Chronic Pulmonary Diseases. Front. Med. 8, 616200. doi:10.3389/fmed.2021.616200
Duan, J., Lei, Y., Lv, G., Liu, Y., Zhao, W., Yang, Q., et al. (2021). Identification of a Novel Autophagy Signature for Predicting Survival in Patients with Lung Adenocarcinoma. PeerJ 9, e11074. doi:10.7717/peerj.11074
Fan, T., Liu, Y., Liu, H., Wang, L., Tian, H., Zheng, Y., et al. (2021). Comprehensive Analysis of a Chemokine- and Chemokine Receptor Family-Based Signature for Patients with Lung Adenocarcinoma. Cancer Immunol. Immunother. doi:10.1007/s00262-021-02944-1
Fan, X., Weng, Y., Bai, Y., Wang, Z., Wang, S., Zhu, J., et al. (2018). Lipin-1 Determines Lung Cancer Cell Survival and Chemotherapy Sensitivity by Regulation of Endoplasmic Reticulum Homeostasis and Autophagy. Cancer Med. 7 (6), 2541–2554. doi:10.1002/cam4.1483
Ge, H., Yan, Y., Wu, D., Huang, Y., and Tian, F. (2018). Potential Role of LINC00996 in Colorectal Cancer: a Study Based on Data Mining and Bioinformatics. Ott 11, 4845–4855. doi:10.2147/OTT.S173225
Gerada, C., and Ryan, K. M. (2020). Autophagy, the Innate Immune Response and Cancer. Mol. Oncol. 14 (9), 1913–1929. doi:10.1002/1878-0261.12774
Gu, J.-H., Liu, C.-C., Xie, J.-L., Ma, B., Cui, S.-M., Yang, G.-Z., et al. (2021). The Local Anesthetic Bupivacaine Inhibits the Progression of Non-small Cell Lung Cancer by Inducing Autophagy through Akt/mTOR Signaling. Front. Oncol. 11, 616445. doi:10.3389/fonc.2021.616445
Howe, K., Achuthan, P., Allen, J., Alvarez-Jarreta, J., Amode, M. R., Armean, I. M., et al. (2021). Ensembl 2021. Nucleic Acids Res. 49 (D1), D884–D891. doi:10.1093/nar/gkaa942
Hu, W., Wang, Y., Fang, Z., He, W., and Li, S. (2021). Integrated Characterization of lncRNA-Immune Interactions in Prostate Cancer. Front. Cel Dev. Biol. 9, 641891. doi:10.3389/fcell.2021.641891
Hugo, W., Zaretsky, J. M., Sun, L., Song, C., Moreno, B. H., Hu-Lieskovan, S., et al. (2017). Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 168 (3), 542. doi:10.1016/j.cell.2017.01.010
Jena, B. C., Rout, L., Dey, A., and Mandal, M. (2021). Active Autophagy in Cancer‐associated Fibroblasts: Recent Advances in Understanding the Novel Mechanism of Tumor Progression and Therapeutic Response. J. Cel Physiol 236, 7887–7902. doi:10.1002/jcp.30419
Jiang, T., Chen, X., Ren, X., Yang, J.-M., and Cheng, Y. (2021). Emerging Role of Autophagy in Anti-tumor Immunity: Implications for the Modulation of Immunotherapy Resistance. Drug Resist. updates 56, 100752. doi:10.1016/j.drup.2021.100752
Li, H., Chen, Y.-k., Wan, Q., Shi, A.-q., Wang, M., He, P., et al. (2021). Long Non-coding RNA LINC00847 Induced by E2F1 Accelerates Non-small Cell Lung Cancer Progression through Targeting miR-147a/IFITM1 Axis. Front. Med. 8, 663558. doi:10.3389/fmed.2021.663558
Li, H., Liu, J., Cao, W., Xiao, X., Liang, L., Liu-Smith, F., et al. (2019). C-myc/miR-150/EPG5 axis Mediated Dysfunction of Autophagy Promotes Development of Non-small Cell Lung Cancer. Theranostics 9 (18), 5134–5148. doi:10.7150/thno.34887
Li, J.-P., Li, R., Liu, X., Huo, C., Liu, T.-T., Yao, J., et al. (2020). A Seven Immune-Related lncRNAs Model to Increase the Predicted Value of Lung Adenocarcinoma. Front. Oncol. 10, 560779. doi:10.3389/fonc.2020.560779
Li, J., Ullah, M. A., Jin, H., Liang, Y., Lin, L., Wang, J., et al. (2021). ORMDL3 Functions as a Negative Regulator of Antigen-Mediated Mast Cell Activation via an ATF6-UPR-autophagy-dependent Pathway. Front. Immunol. 12, 604974. doi:10.3389/fimmu.2021.604974
Li, L., Gan, Z. H., Qin, L., Jiao, S. H., and Shi, Y. (2017). AIB1 Regulates the Ovarian Cancer Cell Cycle through TUG1. Eur. Rev. Med. Pharmacol. Sci. 21 (24), 5610–5617. doi:10.26355/eurrev_201712_14002
Li, N., and Zhan, X. (2019). Identification of Clinical Trait-Related lncRNA and mRNA Biomarkers with Weighted Gene Co-expression Network Analysis as Useful Tool for Personalized Medicine in Ovarian Cancer. EPMA J. 10 (3), 273–290. doi:10.1007/s13167-019-00175-0
Li, Y.-Y., Lam, S.-K., Zheng, C.-Y., and Ho, J. C.-M. (2015). The Effect of Tumor Microenvironment on Autophagy and Sensitivity to Targeted Therapy in EGFR-Mutated Lung Adenocarcinoma. J. Cancer 6 (64), 382–386. doi:10.7150/jca.11187
Li, Y., Shen, R., Wang, A., Zhao, J., Zhou, J., Zhang, W., et al. (2021). Construction of a Prognostic Immune-Related LncRNA Risk Model for Lung Adenocarcinoma. Front. Cel Dev. Biol. 9, 648806. doi:10.3389/fcell.2021.648806
Liang, L., Zhou, Q., and Feng, L. (2021). Decreased microRNA-155 in Behcet's Disease Leads to Defective Control of Autophagy Thereby Stimulating Excessive Proinflammatory Cytokine Production. Arthritis Res. Ther. 23 (1), 135. doi:10.1186/s13075-021-02517-8
Lin, X., Jiang, T., Bai, J., Li, J., Wang, T., Xiao, J., et al. (2018). Characterization of Transcriptome Transition Associates Long Noncoding RNAs with Glioma Progression. Mol. Ther. - Nucleic Acids 13, 620–632. doi:10.1016/j.omtn.2018.10.009
Lin, X., Lai, X., Feng, W., Yu, X., Gu, Q., and Zheng, X. (2021). MiR-30a Sensitized Lung Cancer against Neoadjuvant Chemotherapy by Depressing Autophagy. Jpn. J. Clin. Oncol. 51 (5), 675–684. doi:10.1093/jjco/hyaa272
Liu, H., Zhang, L., Ding, X., and Sui, X. (2021). LINC00861 Inhibits the Progression of Cervical Cancer cells by Functioning as a ceRNA for miR-513b-5p A-nd regulating the PTEN/AKT/mTOR S-ignaling P-athway. Mol. Med. Rep. 23 (1), 1. doi:10.3892/mmr.2020.11662
Liu, H., Zhang, Q., Lou, Q., Zhang, X., Cui, Y., Wang, P., et al. (2020). Differential Analysis of lncRNA, miRNA and mRNA Expression Profiles and the Prognostic Value of lncRNA in Esophageal Cancer. Pathol. Oncol. Res. 26 (2), 1029–1039. doi:10.1007/s12253-019-00655-8
Liu, W., Jiang, Y., Peng, L., Sun, X., Gan, W., Zhao, Q., et al. (2021). Inferring Gene Regulatory Networks Using the Improved Markov Blanket Discovery Algorithm. Interdiscip. Sci. Comput. Life Sci. doi:10.1007/s12539-021-00478-9
Lu, J., Liu, Y., Wang, W., Li, P., and Qi, F. (2021). Knockdown of miR-146a in Regulatory T Cells Suppresses Heart Transplantation Rejection in Mice by Increasing Autophagy. Transpl. Immunol. 65, 101372. doi:10.1016/j.trim.2021.101372
Nasim, F., Sabath, B. F., and Eapen, G. A. (2019). Lung Cancer. Med. Clin. North America 103 (3), 463–473. doi:10.1016/j.mcna.2018.12.006
National Comprehensive Cancer Network (2021). NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer. V.1 2021. Available at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed April 5, 2021).
Pang, Z., Chen, X., Wang, Y., Wang, Y., Yan, T., Wan, J., et al. (2021). Long Non-coding RNA C5orf64 Is a Potential Indicator for Tumor Microenvironment and Mutation Pattern Remodeling in Lung Adenocarcinoma. Genomics 113, 291–304. doi:10.1016/j.ygeno.2020.12.010
Qian, Y., Mao, Z.-d., Shi, Y.-j., Liu, Z.-g., Cao, Q., and Zhang, Q. (2018). Comprehensive Analysis of miRNA-mRNA-lncRNA Networks in Non-smoking and Smoking Patients with Chronic Obstructive Pulmonary Disease. Cell Physiol Biochem 50 (3), 1140–1153. doi:10.1159/000494541
Samuel, S., Beljanski, V., Van Grevenynghe, J., Richards, S., Ben Yebdri, F., He, Z., et al. (2013). BCL-2 Inhibitors Sensitize Therapy-Resistant Chronic Lymphocytic Leukemia Cells to VSV Oncolysis. Mol. Ther. 21 (7), 1413–1423. doi:10.1038/mt.2013.91
Schmid, D., Dengjel, J., Schoor, O., Stevanovic, S., and Münz, C. (2006). Autophagy in Innate and Adaptive Immunity against Intracellular Pathogens. J. Mol. Med. 84 (3), 194–202. doi:10.1007/s00109-005-0014-4
Shi, J.-F., Wang, L., Wu, N., Li, J.-L., Hui, Z.-G., Liu, S.-M., et al. (2019). Clinical Characteristics and Medical Service Utilization of Lung Cancer in China, 2005-2014: Overall Design and Results from a Multicenter Retrospective Epidemiologic Survey. Lung cancer 128, 91–100. doi:10.1016/j.lungcan.2018.11.031
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer Statistics, 2021. CA A. Cancer J. Clin. 71 (1), 7–33. doi:10.3322/caac.21654
Singh, A. N., and Gasman, B. (2020). Disentangling the Genetics of Sarcopenia: Prioritization of NUDT3 and KLF5 as Genes for Lean Mass & HLA-DQB1-AS1 for Hand Grip Strength with the Associated Enhancing SNPs & a Scoring System. BMC Med. Genet. 21 (1), 40. doi:10.1186/s12881-020-0977-6
Spengler, K., Kryeziu, N., Große, S., Mosig, A. S., and Heller, R. (2020). VEGF Triggers Transient Induction of Autophagy in Endothelial Cells via AMPKα1. Cells 9 (3), 687. doi:10.3390/cells9030687
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., et al. (2005). Gene Set Enrichment Analysis: a Knowledge-Based Approach for Interpreting Genome-wide Expression Profiles. Proc. Natl. Acad. Sci. 102 (43), 15545–15550. doi:10.1073/pnas.0506580102
Sun, T., Zhang, W., Li, Y., Jin, Z., Du, Y., Tian, J., et al. (2020). Combination Immunotherapy with Cytotoxic T-Lymphocyte-Associated Antigen-4 and Programmed Death Protein-1 Inhibitors Prevents Postoperative Breast Tumor Recurrence and Metastasis. Mol. Cancer Ther. 19 (3), 802–811. doi:10.1158/1535-7163.MCT-19-0495
Tomczak, K., Czerwińska, P., and Wiznerowicz, M. (2015). Review the Cancer Genome Atlas (TCGA): an Immeasurable Source of Knowledge. wo 1A, 68–77. doi:10.5114/wo.2014.47136
Tu, L. R., Li, W., Liu, J., Song, X. G., and Xu, H. W. (2020). LncRNA LINC00847 Contributes to Hepatocellular Carcinoma Progression by Acting as a Sponge of miR-99a to Induce E2F2 Expression. J. Biol. Regul. Homeost Agents 34 (6), 2195–2203. doi:10.23812/20-427-L
Turan, A., Grosche, L., Krawczyk, A., Mühl-Zürbes, P., Drassner, C., Düthorn, A., et al. (2019). Autophagic Degradation of Lamins Facilitates the Nuclear Egress of Herpes Simplex Virus Type 1. J. Cel. Biol. 218 (2), 508–523. doi:10.1083/jcb.201801151
Wang, W., Li, A., Han, X., Wang, Q., Guo, J., Wu, Y., et al. (2020). DEPDC1 Up‐regulates RAS Expression to Inhibit Autophagy in Lung Adenocarcinoma Cells. J. Cel. Mol. Med. 24 (22), 13303–13313. doi:10.1111/jcmm.15947
Wei, Y., Yan, Z., Wu, C., Zhang, Q., Zhu, Y., Li, K., et al. (2017). Integrated Analysis of Dosage Effect lncRNAs in Lung Adenocarcinoma Based on Comprehensive Network. Oncotarget 8 (42), 71430–71446. doi:10.18632/oncotarget.19864
Wu, J., Xu, C., Guan, X., Ni, D., Yang, X., Yang, Z., et al. (2021). Comprehensive Analysis of Tumor Microenvironment and Identification of an Immune Signature to Predict the Prognosis and Immunotherapeutic Response in Lung Squamous Cell Carcinoma. Ann. Transl Med. 9 (7), 569. doi:10.21037/atm-21-463
Wu, L., Wen, Z., Song, Y., and Wang, L. (2021). A Novel Autophagy‐related lncRNA Survival Model for Lung Adenocarcinoma. J. Cel Mol Med 25 (12), 5681–5690. doi:10.1111/jcmm.16582
Zhang, B., Wang, R., Li, K., Peng, Z., Liu, D., Zhang, Y., et al. (2021). An Immune-Related lncRNA Expression Profile to Improve Prognosis Prediction for Lung Adenocarcinoma: From Bioinformatics to Clinical Word. Front. Oncol. 11, 671341. doi:10.3389/fonc.2021.671341
Zhang, L., Yang, P., Feng, H., Zhao, Q., and Liu, H. (2021). Using Network Distance Analysis to Predict lncRNA-miRNA Interactions. Interdiscip. Sci. Comput. Life Sci. 13 (3), 535–545. doi:10.1007/s12539-021-00458-z
Zhang, X., Jin, Y., Wang, Q., Jian, F., Li, M., Long, H., et al. (2020). Autophagy-mediated Regulation Patterns Contribute to the Alterations of the Immune Microenvironment in Periodontitis. Aging 13 (1), 555–577. doi:10.18632/aging.202165
Zheng, H., Li, B.-H., Liu, C., Jia, L., and Liu, F.-T. (2020). Comprehensive Analysis of lncRNA-Mediated ceRNA Crosstalk and Identification of Prognostic Biomarkers in Wilms' Tumor. Biomed. Research International 2020, 1–13. doi:10.1155/2020/4951692
Keywords: lung adenocarcinoma, long noncoding RNA, tumor immune microenvironment, prognostic signature, survival
Citation: Chen H, Hu Z, Sang M, Ni S, Lin Y, Wu C, Mu Y, Liu K, Wu S, Li N and Xu G (2021) Identification of an Autophagy-Related lncRNA Prognostic Signature and Related Tumor Immunity Research in Lung Adenocarcinoma. Front. Genet. 12:767694. doi: 10.3389/fgene.2021.767694
Received: 31 August 2021; Accepted: 26 October 2021;
Published: 08 December 2021.
Edited by:
Ben-gong Zhang, Wuhan Textile University, ChinaReviewed by:
Qi Zhao, University of Science and Technology Liaoning, ChinaCopyright © 2021 Chen, Hu, Sang, Ni, Lin, Wu, Mu, Liu, Wu, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guodong Xu, eHVndW9kb25nQG5idS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.