
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 14 October 2021
Sec. RNA
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.756784
This article is part of the Research Topic Machine Learning-Based Methods for RNA Data Analysis, Volume II View all 15 articles
 Chenxi Xiang1†
Chenxi Xiang1† Huimin Ni2†
Huimin Ni2† Zhina Wang3†
Zhina Wang3† Binbin Ji4,5
Binbin Ji4,5 Bo Wang4,5
Bo Wang4,5 Xiaoli Shi4,5
Xiaoli Shi4,5 Wanna Wu2
Wanna Wu2 Nian Liu2
Nian Liu2 Ying Gu2
Ying Gu2 Dongshen Ma1
Dongshen Ma1 Hui Liu2*
Hui Liu2*Over 50% of diffuse large B-cell lymphoma (DLBCL) patients are diagnosed at an advanced stage. Although there are a few therapeutic strategies for DLBCL, most of them are more effective in limited-stage cancer patients. The prognosis of patients with advanced-stage DLBCL is usually poor with frequent recurrence and metastasis. In this study, we aimed to identify gene expression and network differences between limited- and advanced-stage DLBCL patients, with the goal of identifying potential agents that could be used to relieve the severity of DLBCL. Specifically, RNA sequencing data of DLBCL patients at different clinical stages were collected from the cancer genome atlas (TCGA). Differentially expressed genes were identified using DESeq2, and then, weighted gene correlation network analysis (WGCNA) and differential module analysis were performed to find variations between different stages. In addition, important genes were extracted by key driver analysis, and potential agents for DLBCL were identified according to gene-expression perturbations and the Crowd Extracted Expression of Differential Signatures (CREEDS) drug signature database. As a result, 20 up-regulated and 73 down-regulated genes were identified and 79 gene co-expression modules were found using WGCNA, among which, the thistle1 module was highly related to the clinical stage of DLBCL. KEGG pathway and GO enrichment analyses of genes in the thistle1 module indicated that DLBCL progression was mainly related to the NOD-like receptor signaling pathway, neutrophil activation, secretory granule membrane, and carboxylic acid binding. A total of 47 key drivers were identified through key driver analysis with 11 up-regulated key driver genes and 36 down-regulated key diver genes in advanced-stage DLBCL patients. Five genes (MMP1, RAB6C, ACCSL, RGS21 and MOCOS) appeared as hub genes, being closely related to the occurrence and development of DLBCL. Finally, both differentially expressed genes and key driver genes were subjected to CREEDS analysis, and 10 potential agents were predicted to have the potential for application in advanced-stage DLBCL patients. In conclusion, we propose a novel pipeline to utilize perturbed gene-expression signatures during DLBCL progression for identifying agents, and we successfully utilized this approach to generate a list of promising compounds.
Diffuse large B-cell lymphoma (DLBCL) is the most commonly diagnosed non-Hodgkin lymphoma (NHL), representing approximately 25% of new NHL cases each year in the United States (Liu and Barta, 2019). In practice, about one half of DLBCL patients presented with advanced-stage disease (Prakash et al., 2012), featuring bulky tumor burden and poor patient response to treatment. According to published data, advanced-stage DLBCL (stage I/II and stage III/IV) may be both biologically and clinically different from limited-stage DLBCL cases (stage I and II). For example, advanced-stage DLBCL patients were more likely to express higher levels of CD30 (Rodrigues-Fernandes et al., 2021) and CD25 (Oka et al., 2020), both of which are biomarkers of B-cell activation. In addition, advanced-stage DLBCL was also shown to be associated with a higher immune-inflammation index (Wang et al., 2021) and an increased level of lymphopenia at diagnosis (Shin et al., 2020), highlighting its deteriorating immune regulation. Green and Johnson et al. reported there were a few biological factors known to adversely impact the prognosis of DLBCL patients, including the cell-of-origin, co-expression of MYC/BCL2 and co-occurrence of MYC and BCL2/BCL6 rearrangements failed to predict poorer prognosis in limited stage DLBCL(Green et al., 2012; Johnson et al., 2012). Ajay, Major et al reported that stage I and II DLBCL cases had a slightly increased risk of secondary primary malignancies after DLBCL treatment in long-term follow-up (>20 years) (Major et al., 2020). Comparing with limited stage DLBCL, advanced-stage DLBCL patients were more likely to benefit from intensified radiotherapy (Hoiland et al., 2020; Freeman et al., 2021). Also, the pattern of late disease relapses observed in advanced stage DLBCL cases was different from that of limited-stage cases, further corroborating that limited and advanced stage DLBCL were biologically heterogeneous (Hoiland et al., 2020). All of these observations prompted us to treat advanced- and limited-stage DLBCL with different strategies, better tailoring for their specific biological and clinical characteristics.
However, there is limited knowledge regarding the genomic and transcriptomic differences between limited- and advanced-stage DLBCL. Two previous large analyses exploring the genetic landscape of DLBCL were not intended to compare the limited and advanced stages of the disease (Reddy et al., 2017; Schmitz et al., 2018). Moreover, at the single gene or single locus level, advanced- and limited-stage DLBCL may also be different in terms of their altered gene regulation and regulatory/co-expression networks, which was confirmed in other clinical comparisons such as cancer vs normal tissue (Zhang et al., 2018; Xu et al., 2019) and young vs old (Yang et al., 2015; Yang et al., 2016b).
Although frontline chemoimmunotherapies have been shown to cure up to 60% of patients with advanced-stage disease, with a clear plateau in progression-free survival (PFS) and rare relapses beyond 5 years (Coiffier et al., 2010), there still is a fraction of patients who are subject to relapse and have tumors that are refractory to treatment (Coiffier et al., 2010), highlighting the heterogeneity of advanced DLBCL. Thus, it is critical to develop new drugs for improving the treatment of advanced-stage DLBCL, so that it might be effectively treated by using existing treatment strategies as limited-stage DLBCL patients are. However, the development of a novel drug is usually costly and time-consuming (Liu et al., 2020; Yang et al., 2020) and highlights the need for effective drug repositioning strategies. There are many computer-based drug repositioning methods that have been used for cancers (Xu et al., 2019; Liu et al., 2020) and other diseases, such as Coronavirus disease 2019 (COVID-19) (Tang et al., 2020; Li et al., 2021).
In this study, we propose a new strategy for identifying new agents that have the potential to specifically target advanced-stage DLBCL. In general, we retrieved advanced-stage DLBCL-specific expressed genes by comparing the transcriptome of advanced-stage disease with that of limited-stage DLBCL. These differentially expressed genes (DEGs) were then subjected to weighted gene correlation network analysis (WGCNA) to discover the co-expression modules that may contribute to the progression of this disease. Finally, potential personal agents were obtained from the Crowd Extracted Expression of Differential Signatures (CREEDS) based on the down-regulation and up-regulation of genes (see Materials and methods for details). We aimed to specifically reveal the transcriptomic scenario occurring in advanced-stage DLBCL and to elucidate the genes that were most likely contributing to disease progression. Based on this knowledge, we then identified some potential agents for the treatment of advanced-stage DLBCL in future clinical practice.
RNA sequencing data from patients with DLBCL cancer were collected from the cancer genome atlas (TCGA). Based on the imaging results, including computed tomography (CT) scans, magnetic resonance imaging (MRI) or positron emission tomography (PET) scanning, patients were divided into four stages (I–IV) according to the Ann Arbor system (Heidelberg, 2020).
An expression matrix of 42 patients and their group information (stage I/II or III/IV) were used as the input for DEG discovery. DEGs between samples at stage I/II and stage III/IV were obtained using DESeq2 (Love et al., 2014) using log2 |fold change| ≧ 1 and a p value ≦ 0.05.
After identifying DEGs, we performed survival analysis on these genes for all of the patients. Next, Kaplan-Meier (Bland and Altman, 1998) survival estimation was used for all differentially expressed genes to identify genes correlated with overall survival. Kaplan- Meier arranged the survival time in descending order, at each death node, it estimated the proportion of the observed values that survived for a certain period of time under the same circumstances, which could intuitively show the survival and mortality rates of two or more groups. The R packages survival and survminer were used for survival analysis and curve plotting, respectively.
The WGCNA package in R (Peter and Horvath, 2008) was used to construct a co-expression network. For this step, we randomly picked 400 genes from the stage III/IV patients to generate a topological overlap matrix since the gene number was too large to perform this analysis using all of the genes. For the constructed gene network to conform to a scale-free distribution, a soft threshold was used to select the appropriate
KEGG pathway (Ogata et al., 1999) analysis and Gene Ontology (GO) analysis (Botstein et al., 2000), including biological process (BP), cellular composition (CC) and molecular function (MF), were performed on the genes in the module identified by WGCNA to understand the biological significance of the progression of DLBCL. The R package clusterProfiler (Yu et al., 2012) was used in the process of enrichment analysis to analyze the functions of the genes from these modules.
For key driver analysis, we used up- or down-regulated genes separately as inputs to identify key drivers. Key driver analysis (Yang et al., 2016a) (KDA) was used to identify hub genes, and protein actions v11.0 was used as a reference protein–protein interaction network (Szklarczyk et al., 2021). Parameters were set as follows: nlayerExpansion was set to 1, nlayerSearch was set to 6 and enrichedNodesPercentCut was set to −1. A p value_whole ≦ 0.05 was used to filter out key drivers. The hub genes were of great significance in terms of the occurrence and development of DLBCL.
CREEDS includes single gene perturbation signatures, as well as disease and drug perturbation signatures, and it can be used to identify the relationship between gene, disease and drug (Gillies et al., 2016). CREEDS is composed of single-drug perturbation-induced gene expression signatures. Utilizing this database, agents that can reverse the behavior of up/down-regulated genes can be discovered, and the best matched agents are reported. We used this tool for drug discovery for advanced-stage DLBCL. In this work, we combined differentially expressed genes and key driver genes as a new gene set to discover new agents related to advanced-stage DLBCL.
For the purpose of specifically developing new agents that could be utilized in combination with R- CHOP backbones to treat advanced stage DLBCL patients, we proposed a new method of drug repurposing based on gene expression and network perturbation (Figure 1). In order to identify key factors for DLBCL progression, WGCNA and DEG, differential module (DM) and key driver (KD) analyses were performed. Then, the key factors of DLBCL progression and drug perturbation signature were used to predict potential agents for the treatment of advanced stage DLBCL. Finally, some previous studies were reviewed to demonstrate the effectiveness of the newly identified agents.
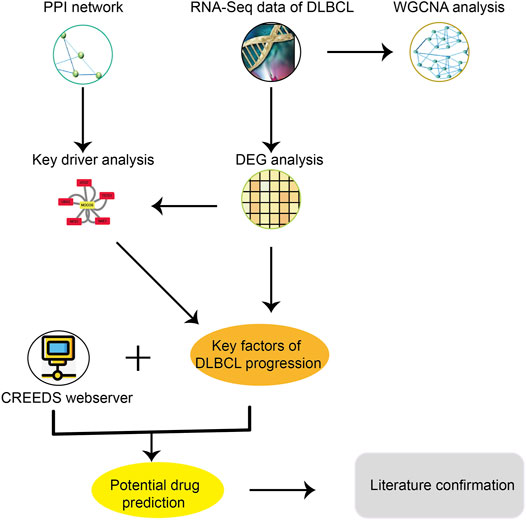
FIGURE 1. A brief study design for drug repurposing, including these major steps: 1) Download and organize the RNA-seq data and clinical information of DLBCL from TCGA; 2) Got key factors of DLBCL progression through DEG analysis, key driver analysis and WGCNA analysis; 3) Potential drug prediction through CREEDs; 4) Literature confirmation.
The clinical characteristics of DLBCL cancer patients collected from TCGA are presented in Table 1, including 25 patients at clinical stage I/II and 17 patients at clinical stage III/IV. It was more likely to occur in elder patients and involve extranodal sites or organs. Patients of advanced stage disease also tended to have B symptoms. No gender preference was observed in this group of patients and all patients received no treatment before resection of tumors.
After collecting data from TCGA, DEGs were obtained using DESeq2, by comparing the transcriptome of advanced stage DLBCL with limited stage DLBCL. Of the 93 DEGs that were identified with a log2 |fold change| ≧ 1 and a p value ≦ 0.05, 20 genes were up-regulated and 73 genes were down-regulated in advanced DLBCL. The top 10 genes that were differently expressed between advanced and limited stage DLBCL are shown in Figure 2A.

FIGURE 2. Analysis of differentially expressed genes. (A) Heat map of the top 10 differentially expressed genes. The x-axis represents different samples from TCGA, blue indicates samples at limited stage (stage I/II) and red indicates samples at advanced stage (stage III/IV). The y-axis represents differentially expressed genes. (B) Survival curve of the association between the expression levels of DAB1 and survival time after diagnosis with DLBCL.
We aimed to evaluate whether this set of differentially expressed genes could define a group of patients with poorer prognosis. We dichotomized 42 DLBCL cases into either the high expression group or the low expression group as per the mean expression level of each DEG. In addition, the Kaplan-Meier survival estimation method was used to evaluate all DEGs to study the relationship between gene expression and overall survival. Through this Kaplan-Meier survival estimation analysis, we found that DAB1 was negatively correlated with overall survival, while other DEGs were not correlated with overall survival.
WGCNA, based on a scale-free network to analyze genes according to their expression patterns, was used to cluster highly related genes into one module. As can be seen from Figure 3A, the soft threshold value was set at 10 to build this scale-free network. Next, 79 gene modules were identified by hierarchical clustering and dynamic branch cutting, and each module was assigned a unique color identifier (Supplementary Figure S4). We then selected a portion of these genes to construct a topological overlapping heat map, shown in Figure 3B. Through differential module analysis, we found that the thistle1 module was most relevant to advance stage of DLBCL in this dataset (Figure 3C).
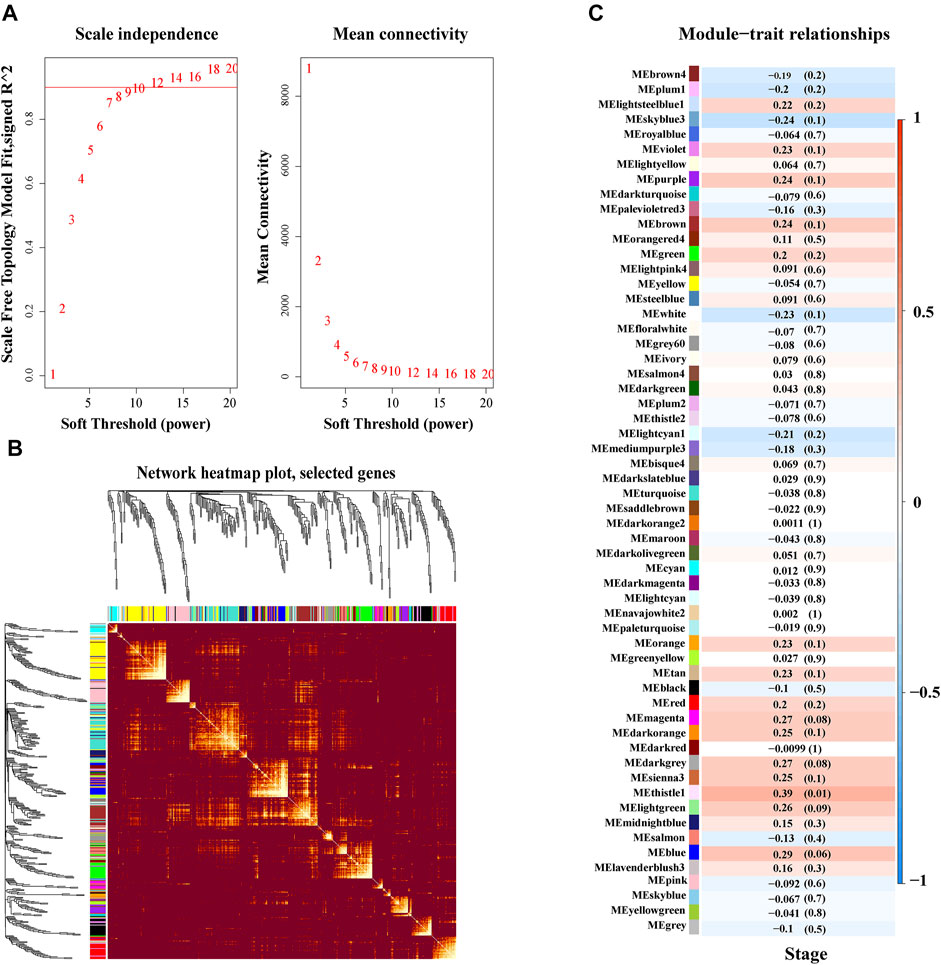
FIGURE 3. Weighted co-expression and key module identification associated with clinical DLBCL stage. (A) Determination of soft threshold in WGCNA. (B) Topological overlapping WGCNA heat map. (C) The relationship between modules and clinical traits. Pearson correlation coefficient was used to calculate the correlation degree between each module and trait.
In order to understand the causes of DLBCL deterioration from the biological level, we analysed the genes in the thistle1 module using KEGG pathway and GO enrichment analysis. KEGG pathway analysis results indicated that the development of DLBCL was very strongly correlated to the NOD-like receptor signalling pathway, osteoclast differentiation, leishmaniasis, Staphylococcus aureus infection and viral protein interaction with cytokine and cytokine receptor (Figure 4A). Furthermore, GO enrichment was performed based on three aspects: BP, CC and MF. In the BP analysis, we found that the genes in the thistle1 module were mainly related to neutrophil activation, positive regulation of response to external stimulus and response to interferon−gamma (Figure 4B). In addition, in the CC analysis, the genes in the thistle1 module were related to secretory granule membrane, endocytic vesicle and apical part of cell (Figure 4C). Moreover, the genes in the thistle1 module were mainly enriched in 7 MFs, including carboxylic acid binding, organic acid binding, cysteine−type endopeptidase activity, manganese ion binding, ligand−gated cation channel activity, immunoglobulin G (IgG) binding and immunoglobulin binding (Figure 4D).
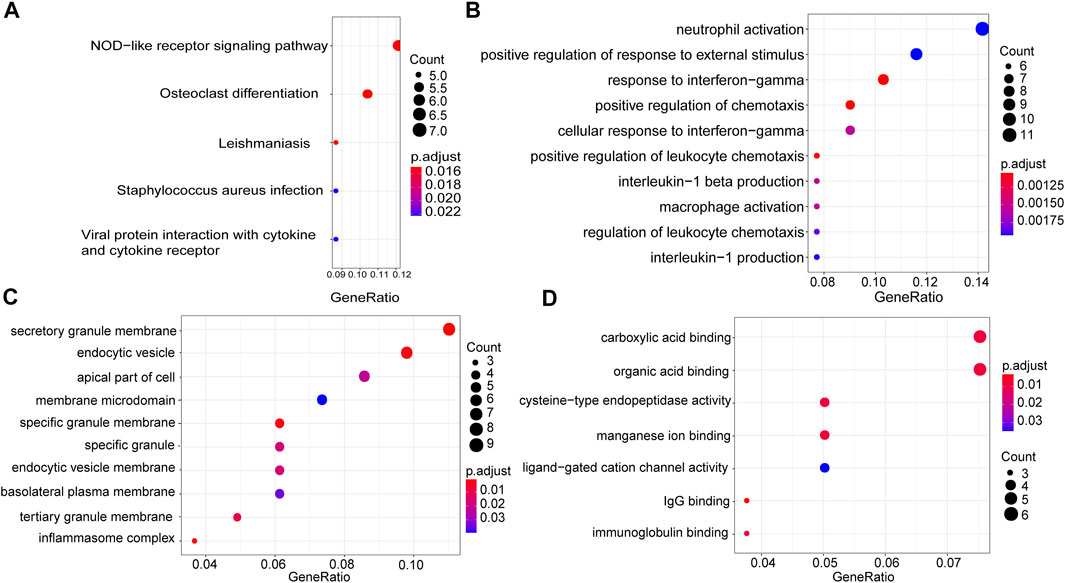
FIGURE 4. Pathway and functional enrichment analysis of genes in the thistle1 module. (A) KEGG pathway analysis. (B) GO enrichment for biological process. (C) GO enrichment for cellular composition. (D) GO enrichment for molecular function. The x-axes are the ratio of genes, and the y-axes are the GO terms.
A total of 47 key drivers were identified through key driver analysis, with 11 up-regulated key driver genes and 36 down-regulated key diver genes being diagnostic of advanced-stage DLBCL relative to limited-stage DLBCL. Then, five hub genes were identified from key drivers as shown in Figure 5, which were most related to the occurrence and development of DLBCL. MMP1 (Rosas et al., 2008), also known as matrix metalloproteinase-1, encodes a protein of 469 amino acid residues and is a kind of photolytic enzyme closely related to tumor genesis, invasion and metastasis. Rab6c (Young et al., 2010) is a member of the RAS family. Its mutation can affect the balance of Ras-GTP and cause malignant transformation of cells. Gene ontology annotations for 1-Aminocyclopropane-1-Carboxylate Synthase Homolog (Inactive) Like (ACCSL) (Chen and Karampinos, 2020) include pyridoxal phosphate binding. Dysregulation of gene levels of molybdenum cofactor sulfurase (MOCOS) (Kurzawski et al., 2012) can lead to cell disorders. Studies have demonstrated that this gene can be used as a key detection gene for kidney genetic diseases. RGS21 (Von Buchholtz et al., 2004), a new member of the regulator of G protein signaling (RGS) protein family. It can inhibit signal transduction by increasing GTPase activity.
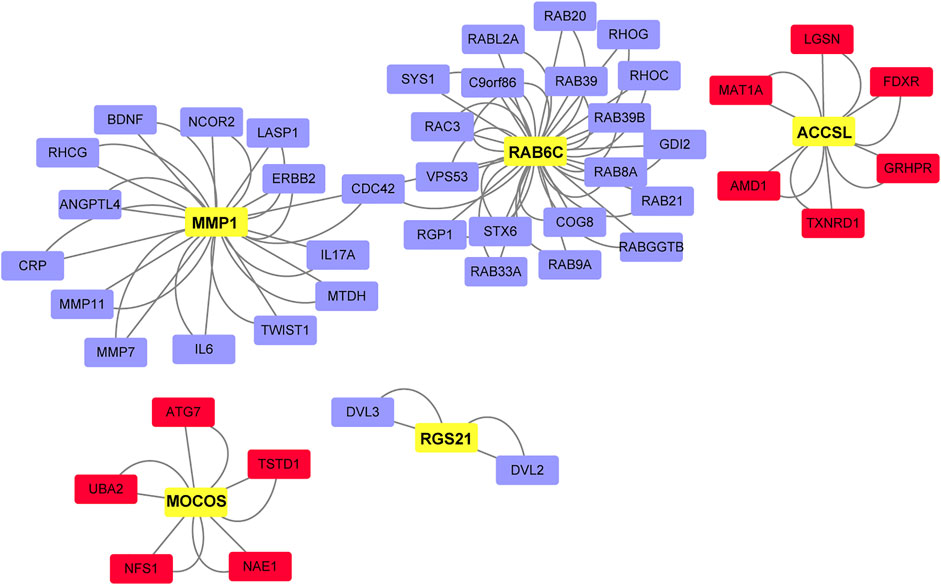
FIGURE 5. Network of key DLBCL drivers and hub genes. Red, key drivers from up-regulated gene set in advanced-stage samples. Blue, key drivers from down-regulated gene set in advanced-stage samples. Yellow, hub genes.
Potential personal agents associated with DLBCL were identified according to the differences between differential genes and drug signaling. Approximately 10 potential agents were selected according to their drug perturbation-induced gene expression signatures, and detailed information on these agents is presented in Table 2, including the type, drug/small molecule, possible effect and evidence for activity. The top five agents could reverse the expression of down-regulated genes, and the remaining agents could reverse the expression of up-regulated genes. In other words, after treatment with these drugs, gene expression levels may return to normal. The top five agents that may reverse down-regulated gene expression are formaldehyde, ethanol, dibutyl phthalate, paclitaxel, and prednisolone. Ethanol (EtOH) is similar to pharmacological mTOR inhibitors and has been shown to inhibit the mTOR signaling pathway. Mazan et al. studied the influence of EtOH on the mTOR signaling pathway and explored the translational group analysis of downstream effects of EtOH in DLBCL, and the results showed that EtOH partially inhibited mTOR signaling and protein translation (Mazan-Mamczarz et al., 2015). In a previous study, newly diagnosed DLBCL patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) were evaluated for their clinical characteristics, therapeutic efficacy and patient survival, and DLBCL patients treated with R-CHOP had better survival than other patients (Hong et al., 2011). Ohe et al. also reported a case of DLBCL successfully treated with prednisolone (Ohe et al., 2012). The top five agents that may reverse up-regulated gene expression are oxaliplatin, eribulin, NC1153, EPZ-6438 and R547. Oxaliplatin selectively inhibits the synthesis of deoxyribonucleic acid (DNA). Shen et al. studied the efficacy, safety and feasibility of the combination of rituximab, gemcitabine, and oxaliplatin (R-GemOx) as a first-line treatment in elderly patients with DLBCL. They found that R-GemOx might be a therapeutic option for the management of DLBCL (Shen et al., 2018).
DLBCL remains a highly heterogenous disease, with the frontline R-CHOP modality achieving only a 40–60% complete response (CR) rate in unselected patients. The prognosis of patients with DLBCL with refractory tumors or relapse remains dismal. As a result, designing more sophisticated personal treatment modalities has the potential to improve the outcomes in high-risk DLBCL patients. Although a wealth of studies has focused on targeted therapies based on the molecular classification of DLBCL, the clinical stage of DLBCL remains an important factor for choosing an appropriate treatment regime. DLBCL patients with advanced- and limited-stage disease have different responses to standard chemoimmunotherapies, due to the different genomic profiles of advanced-stage disease relative to limited-stage disease (Miao et al., 2019). In this study, we propose a new approach to gain insights into the intrinsic heterogeneity of DLBCL, which focused on comparing the transcriptomic profile of advanced- and limited-stage DLBCL and distilling the disease to a few distinctly expressed genes and hub genes that might contribute to disease progression. In general, 20 genes were up-regulated and 73 genes were down-regulated in advanced-stage samples compared to limited-stage samples. We also found that DAB1 was negatively correlated with overall survival through survival analysis of all identified DEGs (Figure 2B, p = 0.045). Due to the limitations of differential expression analysis, it is impossible to group genes with the same function together. Therefore, we carried out weighted gene co-expression network analysis and analysis on different modules. During these analyses, 79 similar gene expression modules were found using WGCNA, among which, the thistle1 module was highly related to disease stage. KEGG pathway and GO enrichment analyses of the genes in the thistle1 module indicated that DLBCL progression was mainly related to the NOD-like receptor signaling pathway, neutrophil activation, secretory granule membrane and carboxylic acid binding. There is evidence that tumors and their mesenchymal cells produce many cytokines and chemokines to stimulate the differentiation of N2 neutrophils (Valerius et al., 1993; Souto et al., 2014). However, neutrophils can cause DNA damage through reactive oxygen species and related products of myeloperoxidase (MPO), and N2 cells secrete VEGF, TNF and other cytokines to promote tumor angiogenesis and, at the same time, synthesize and secrete MMP and NE to the tumor stroma to participate in the tumor reconstruction of the extracellular matrix to promote tumor growth and metastasis (Zvi et al., 2009; Mishalian et al., 2013; Zhou et al., 2016). During key driver analysis, 47 key drivers were identified and five hub genes were extracted from these key drivers, including MMP1. MMP1 (Rosas et al., 2008) can alter the microenvironment of cells. When MMP1 is out of balance, it accelerates the degradation of the matrix barrier and promotes the formation and growth of tumors by releasing matrix-related growth factors. Studies have shown that MMP1 is associated with lung squamous cell carcinoma, colon cancer and adenocarcinoma.
Based on gene expression and network perturbations, 10 potential agents for the treatment of DLBCL were obtained. For instance, NC1153 can inhibit JAK3 specifically and induce the apoptosis of certain leukemia/lymphoma cell lines. Using Affymetrix microarray profiling following NC1153 treatment, Nagy et al. reported that JAK3-dependent survival modulating pathways (p53, TGF-beta, TNFR and ER stress) were altered in Kit225 cells (Nagy et al., 2010). EPZ-6438 selectively inhibited intracellular H3K27 methylation in a concentration- and time-dependent manner in both EZH2 wild-type and mutant lymphoma cells. Inhibition of H3K27 trimethylation (H3K27Me3) leads to selective cell killing of human lymphoma cell lines bearing EZH2 catalytic domain point mutations (Knutson et al., 2014).
In summary, we proposed a novel pipeline to utilize perturbed gene-expression signatures during DLBCL progression for identifying agents, and we successfully utilized this approach to generate a list of promising compounds. Whether this can be used clinically needs further research. We will continue to follow the latest developments of these agents in the treatment of DLBCL and explore its pharmaco-mechanisms under the aid of stage-of-art technologies in the future.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
HL and CX designed the project, CX, HN, and ZW wrote the manuscript, BJ and XS collected data, BW carried out data analysis, NL and WW analyzed experimental results. YG and DM researched literatures. All authors read and gave their approval for the final version of the manuscript.
Authors BJ, BW and XS were employed by Geneis Beijing Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
The handling Editor declared a past co-authorship/collaboration with one of the authors BW.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.756784/full#supplementary-material
Bland, J. M., and Altman, D. G. (1998). Statistics Notes: Survival Probabilities (The Kaplan-Meier Method). Bmj 317, 1572–1580. doi:10.1136/bmj.317.7172.1572
Botstein, D., Cherry, J. M., Ashburner, M., Ball, C., Blake, J., Butler, H., et al. (2000). Gene Ontology: tool unification Biol. 25, 25–29. doi:10.1038/75556
Chen, W., and Karampinos, D. C. (2020). Chemical‐Shift Encoding-Based Water-Fat Separation With Multifrequency Fat Spectrum Modeling in Spin‐Lock MRI. Magn. Reson. Med. 83, 1608–1624. doi:10.1002/mrm.28026
Coiffier, B., Thieblemont, C., Van Den Neste, E., Lepeu, G., Plantier, I., Castaigne, S., et al. (2010). Long-Term Outcome of Patients in the LNH-98.5 Trial, the First Randomized Study Comparing Rituximab-CHOP to Standard CHOP Chemotherapy in DLBCL Patients: a Study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 116, 2040–2045. doi:10.1182/blood-2010-03-276246
Freeman, C. L., Savage, K. J., Villa, D. R., Scott, D. W., Srour, L., Gerrie, A. S., et al. (2021). Long-Term Results of PET-Guided Radiation in Patients With Advanced-Stage Diffuse Large B-Cell Lymphoma Treated With R-CHOP. Blood. 137, 929–938. doi:10.1182/blood.2020005846
Gillies, R. J., Kinahan, P. E., and Hricak, H. (2016). Radiomics: Images Are More Than Pictures, They Are Data. Radiology. 278, 563–577. doi:10.1148/radiol.2015151169
Green, T. M., Young, K. H., Visco, C., Xu-Monette, Z. Y., Orazi, A., Go, R. S., et al. (2012). Immunohistochemical Double-Hit Score Is a Strong Predictor of Outcome in Patients With Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J. Clin. Oncol. 30, 3460–3467. doi:10.1200/jco.2011.41.4342
Heidelberg, S. B. (2020). Ann Arbor Staging System. Springer Berlin Heidelberg. doi:10.1007/978-3-540-47648-1_287
Hoiland, R. L., Fergusson, N. A., Mitra, A. R., Griesdale, D. E. G., Devine, D. V., Stukas, S., et al. (2020). The Association of ABO Blood Group With Indices of Disease Severity and Multiorgan Dysfunction in COVID-19. Blood Adv. 4, 4981–4989. doi:10.1182/bloodadvances.2020002623
Hong, J., Park, S., Park, J., Kim, H. S., Kim, K.-H., Ahn, J. Y., et al. (2011). Evaluation of Prognostic Values of Clinical and Histopathologic Characteristics in Diffuse Large B-Cell Lymphoma Treated with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone Therapy. Leuk. Lymphoma. 52, 1904–1912. doi:10.3109/10428194.2011.588761
Johnson, N. A., Slack, G. W., Savage, K. J., Connors, J. M., Ben-Neriah, S., Rogic, S., et al. (2012). Concurrent Expression of MYC and BCL2 in Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J. Clin. Oncol. 30, 3452–3459. doi:10.1200/jco.2011.41.0985
Knutson, S. K., Kawano, S., Minoshima, Y., Warholic, N. M., Huang, K.-C., Xiao, Y., et al. (2014). Selective Inhibition of EZH2 by EPZ-6438 Leads to Potent Antitumor Activity in EZH2-Mutant Non-Hodgkin Lymphoma. Mol. Cancer Ther. 13, 842–854. doi:10.1158/1535-7163.mct-13-0773
Kurzawski, M., Dziewanowski, K., Safranow, K., and Drozdzik, M. (2012). Polymorphism of Genes Involved in Purine Metabolism (XDH, AOX1, MOCOS) in Kidney Transplant Recipients Receiving Azathioprine. Ther. Drug Monit. 34, 266–274. doi:10.1097/ftd.0b013e31824aa681
Li, T., Huang, T., Guo, C., Wang, A., Shi, X., Mo, X., et al. (2021). Genomic Variation, Origin Tracing, and Vaccine Development of SARS-CoV-2: A Systematic Review. The Innovation. 2, 100116. doi:10.1016/j.xinn.2021.100116
Liu, C., Wei, D., Xiang, J., Ren, F., Huang, L., Lang, J., et al. (2020). An Improved Anticancer Drug-Response Prediction Based on an Ensemble Method Integrating Matrix Completion and Ridge Regression. Mol. Ther. - Nucleic Acids. 21, 676–686. doi:10.1016/j.omtn.2020.07.003
Liu, Y., and Barta, S. K. (2019). Diffuse Large B‐cell Lymphoma: 2019 Update on Diagnosis, Risk Stratification, and Treatment. Am. J. Hematol. 94, 604–616. doi:10.1002/ajh.25460
Love, M. I., Huber, W., and Anders, S. (2014). Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data With DESeq2. Genome Biol. 15, 550. doi:10.1186/s13059-014-0550-8
Major, A., Smith, D. E., Ghosh, D., Rabinovitch, R., and Kamdar, M. (2020). Risk and Subtypes of Secondary Primary Malignancies in Diffuse Large B‐cell Lymphoma Survivors Change Over Time Based on Stage at Diagnosis. Cancer. 126, 189–201. doi:10.1002/cncr.32513
Mazan-Mamczarz, K., Peroutka, R. J., Steinhardt, J. J., Gidoni, M., Zhang, Y., Lehrmann, E., et al. (2015). Distinct Inhibitory Effects on mTOR Signaling by Ethanol and INK128 in Diffuse Large B-Cell Lymphoma. Cell Commun Signal. 13, 15. doi:10.1186/s12964-015-0091-0
Miao, Y., Medeiros, L. J., Li, Y., Li, J., and Young, K. H. (2019). Genetic Alterations and Their Clinical Implications in DLBCL. Nat. Rev. Clin. Oncol. 16, 634–652. doi:10.1038/s41571-019-0225-1
Mishalian, I., Bayuh, R., Levy, L., Zolotarov, L., Michaeli, J., and Fridlender, Z. G. (2013). Tumor-Associated Neutrophils (TAN) Develop Pro-Tumorigenic Properties During Tumor Progression. Cancer Immunol. Immunother. 62, 1745–1756. doi:10.1007/s00262-013-1476-9
Nagy, Z. S., Ross, J. A., Rodriguez, G., Bader, J., Dimmock, J., and Kirken, R. A. (2010). Uncoupling JAK3 Activation Induces Apoptosis in Human Lymphoid Cancer Cells via Regulating Critical Survival Pathways. FEBS Lett. 584, 1515–1520. doi:10.1016/j.febslet.2010.02.071
Ogata, H., Goto, S., Sato, K., Fujibuchi, W., Bono, H., and Kanehisa, M. (1999). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 27, 29–34. doi:10.1093/nar/27.1.29
Ohe, M., Hashino, S., and Hattori, A. (2012). Successful Treatment of Diffuse Large B-Cell Lymphoma With Clarithromycin and Prednisolone. Korean J. Hematol. 47, 293–297. doi:10.5045/kjh.2012.47.4.293
Oka, S., Ono, K., and Nohgawa, M. (2020). Clinical Effect of CD25 on the Prognosis of Diffuse Large B Cell Lymphoma With Secondary Central Nervous System Relapse. Pathol. Oncol. Res. 26, 1843–1850. doi:10.1007/s12253-019-00778-y
Peter, L., and Horvath, S. (2008). WGCNA: an R Package for Weighted Correlation Network Analysis. Bmc Bioinformatics. 9, 559. doi:10.1186/1471-2105-9-559
Prakash, G., Sharma, A., Raina, V., Kumar, L., Sharma, M. C., and Mohanti, B. K. (2012). B Cell Non-Hodgkin's Lymphoma: Experience From a Tertiary Care Cancer center. Ann. Hematol. 91, 1603–1611. doi:10.1007/s00277-012-1491-5
Reddy, A., Zhang, J., Davis, N. S., Moffitt, A. B., Love, C. L., Waldrop, A., et al. (2017). Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell. 171, 481–494. doi:10.1016/j.cell.2017.09.027
Rodrigues-Fernandes, C. I., Abreu, L. G., Radhakrishnan, R., Perez, D. E. D. C., Amaral-Silva, G. K., Gondak, R. D. O., et al. (2021). Prognostic Significance of CD30 Expression in Diffuse Large B-Cell Lymphoma: A Systematic Review with Meta-Analysis. J. Oral Pathol. Med. 50, 587. doi:10.1111/jop.13208
Rosas, I. O., Richards, T. J., Konishi, K., Zhang, Y., Gibson, K., Lokshin, A. E., et al. (2008). MMP1 and MMP7 as Potential Peripheral Blood Biomarkers in Idiopathic Pulmonary Fibrosis. Plos Med. 5, e93. doi:10.1371/journal.pmed.0050093
Schmitz, R., Wright, G. W., Huang, D. W., Johnson, C. A., Phelan, J. D., Wang, J. Q., et al. (2018). Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 378, 1396–1407. doi:10.1056/nejmoa1801445
Shen, Q.-D., Zhu, H.-Y., Wang, L., Fan, L., Liang, J.-H., Cao, L., et al. (2018). Gemcitabine-Oxaliplatin Plus Rituximab (R-GemOx) as First-Line Treatment in Elderly Patients With Diffuse Large B-Cell Lymphoma: a Single-Arm, Open-Label, Phase 2 Trial. Lancet Haematol. 5, e261–e269. doi:10.1016/s2352-3026(18)30054-1
Shin, H. J., Kim, D. Y., Chung, J., Shin, K. H., and Lee, H. (2020). Prognostic Impact of Peripheral Blood T-Cell Subsets at the Time of Diagnosis on Survival in Patients With Diffuse Large B-Cell Lymphoma. Acta Haematol. 144 (4), 427–437. doi:10.1159/000510912
Souto, J. C., Alcolea, S., R, A., Remacha, A., Camacho, M., Soler, M., et al. (2014). Tumour Cell Lines HT-29 and FaDu Produce Proinflammatory Cytokines and Activate Neutrophils In Vitro: Possible Applications for Neutrophil-Based Antitumour Treatment. Mediators Inflamm. 2009, 817498. doi:10.1007/978-3-540-47648-1_287
Szklarczyk, D., Gable, A. L., Nastou, K. C., Lyon, D., Kirsch, R., Pyysalo, S., et al. (2021). The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 49, D605–D612. doi:10.1093/nar/gkaa1074
Tang, X., Cai, L., Meng, Y., Xu, J., Lu, C., and Yang, J. (2020). Indicator Regularized Non-Negative Matrix Factorization Method-Based Drug Repurposing for COVID-19. Front. Immunol. 11, 603615. doi:10.3389/fimmu.2020.603615
Valerius, T., Repp, R., de Wit, T., Berthold, S., Platzer, E., Kalden, J., et al. (1993). Involvement of the High-Affinity Receptor for IgG (Fc Gamma RI; CD64) in Enhanced Tumor Cell Cytotoxicity of Neutrophils during Granulocyte Colony-Stimulating Factor Therapy. Blood. 82, 931–939. doi:10.1182/blood.v82.3.931.931
Von Buchholtz, L., Elischer, A., Tareilus, E., Gouka, R., Kaiser, C., Breer, H., et al. (2004). RGS21 Is a Novel Regulator of G Protein Signalling Selectively Expressed in Subpopulations of Taste Bud Cells. Eur. J. Neurosci. 19, 1535–1544. doi:10.1111/j.1460-9568.2004.03257.x
Wang, Z., Zhang, J., Luo, S., and Zhao, X. (2021). Prognostic Significance of Systemic Immune-Inflammation Index in Patients With Diffuse Large B-Cell Lymphoma. Front. Oncol. 11, 655259. doi:10.3389/fonc.2021.655259
Xu, X., Long, H., Xi, B., Ji, B., Li, Z., Dang, Y., et al. (2019). Molecular Network-Based Drug Prediction in Thyroid Cancer. Int. J. Mol. Sci. 20, 263. doi:10.3390/ijms20020263
Yang, J., Huang, T., Song, W. M., Petralia, F., Mobbs, C. V., Zhang, B., et al. (2016a). Discover the Network Underlying the Connections Between Aging and Age-Related Diseases. Sci. Rep. 6, 32566–32612. doi:10.1038/srep32566
Yang, J., Huang, T., Song, W.-m., Petralia, F., Mobbs, C. V., Zhang, B., et al. (2016b). Discover the Network Mechanisms Underlying the Connections between Aging and Age-Related Diseases. Sci. Rep. 6, 32566. doi:10.1038/srep32566
Yang, J., Huang, T., Huang, T., Petralia, F., Long, Q., Zhang, B., et al. (2015). Synchronized Age-Related Gene Expression Changes Across Multiple Tissues in Human and the Link to Complex Diseases. Sci. Rep. 5, 15145. doi:10.1038/srep15145
Yang, J., Peng, S., Zhang, B., Houten, S., Schadt, E., Zhu, J., et al. (2020). Human Geroprotector Discovery by Targeting the Converging Subnetworks of Aging and Age-Related Diseases. Geroscience. 42, 353–372. doi:10.1007/s11357-019-00106-x
Young, J., Ménétrey, J., and Goud, B. (2010). RAB6C Is a Retrogene that Encodes a Centrosomal Protein Involved in Cell Cycle Progression. J. Mol. Biol. 397, 69–88. doi:10.1016/j.jmb.2010.01.009
Yu, G., Wang, L.-G., Han, Y., and He, Q.-Y. (2012). clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A J. Integr. Biol. 16, 284–287. doi:10.1089/omi.2011.0118
Zhang, W., Long, H., He, B., and Yang, J. (2018). DECtp: Calling Differential Gene Expression Between Cancer and Normal Samples by Integrating Tumor Purity Information. Front. Genet. 9, 321. doi:10.3389/fgene.2018.00321
Zhou, S.-L., Zhou, Z.-J., Hu, Z.-Q., Huang, X.-W., Wang, Z., Chen, E.-B., et al. (2016). Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 150, 1646–1658. e1617. doi:10.1053/j.gastro.2016.02.040
Keywords: diffuse large B-cell lymphoma, drug repurposing, differentially expressed genes, differential module analysis, key driver analysis
Citation: Xiang C, Ni H, Wang Z, Ji B, Wang B, Shi X, Wu W, Liu N, Gu Y, Ma D and Liu H (2021) Agent Repurposing for the Treatment of Advanced Stage Diffuse Large B-Cell Lymphoma Based on Gene Expression and Network Perturbation Analysis. Front. Genet. 12:756784. doi: 10.3389/fgene.2021.756784
Received: 11 August 2021; Accepted: 24 September 2021;
Published: 14 October 2021.
Edited by:
Liqian Zhou, Hunan University of Technology, ChinaReviewed by:
Lina Zhao, Chinese Academy of Medical Sciences, ChinaCopyright © 2021 Xiang, Ni, Wang, Ji, Wang, Shi, Wu, Liu, Gu, Ma and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, aGxpdUB4emhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.