- 1Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 3Institute of Clinical Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 4Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 5Department of Obstetrics and Gynecology and Women’s Health, Taichung Veterans General Hospital, Taichung, Taiwan
- 6Institute of Biomedical Science and Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 7School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 8Department of Public Health, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
- 9Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung, Taiwan
- 10Department of Mathematics and Information Education, National Taipei University of Education, Taipei, Taiwan
- 11Department of Health Care Management, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
- 12Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung, Taiwan
- 13Institute of Public Health and Community Medicine Research Center, National Yang Ming Chiao Tung University, Taipei, Taiwan
Objectives: Genetic variants and obesity are risk factors for hyperuricemia (HUA). Recent genome-wide association studies have identified ABCG2 rs2231142 as one of the most prominent genetic variants for HUA in an East Asian population. Nevertheless, no large-scale studies have demonstrated any interactive effects between this variant and obesity on serum urate level in Asians. This study aimed to determine the interaction of ABCG2 rs2231142 variant and body mass index (BMI) and its effect on risk of HUA in an East Asian population.
Methods: The study was conducted using the Taiwan Biobank database, a population-based biomedical research database of patients with Taiwanese Han Chinese ancestry aged 30–70years between September 2014 and May 2017. Detailed physical information on participants were collected by questionnaires and genotyping using Affymetrix TWB 650K SNP chip. The primary outcome was HUA, defined as a serum uric acid level>7.0mg/dl. Odds ratio (OR) of HUA was analyzed using logistic regression models and the effects of interaction between ABCG2 rs2231142 variants and BMI on serum uric acid level were explored.
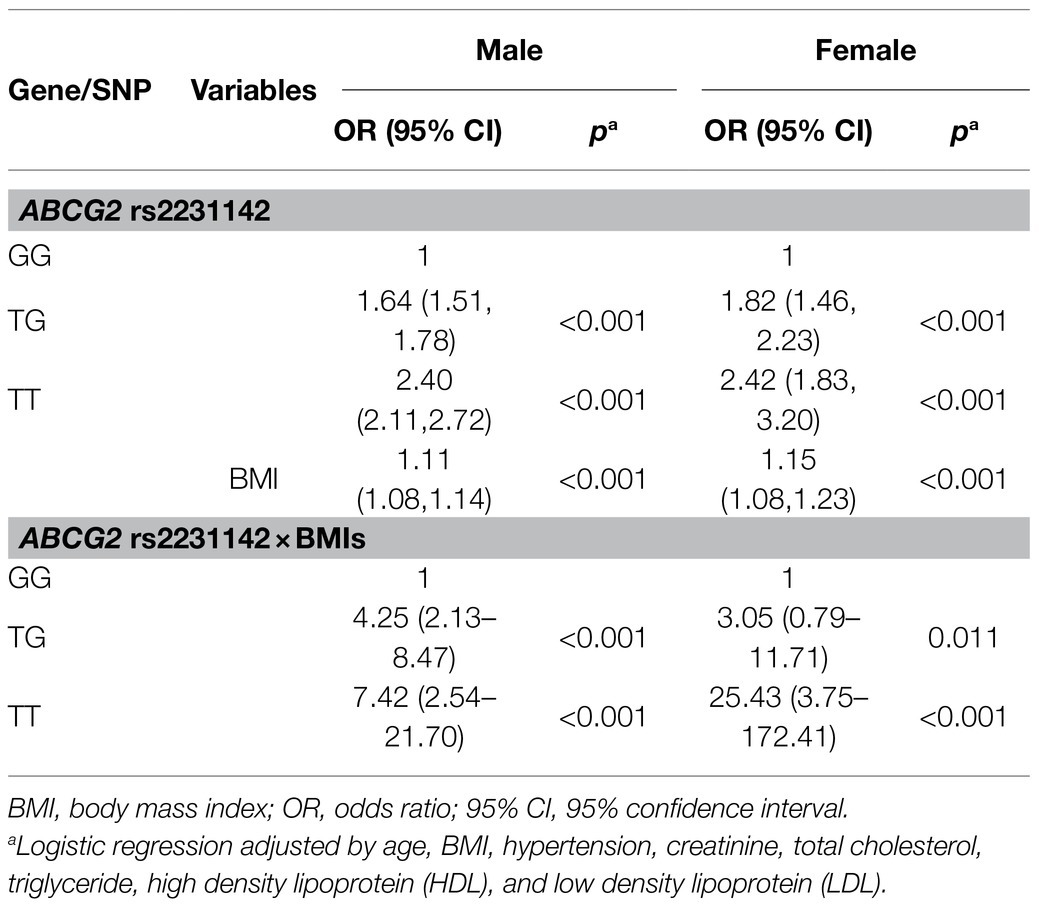
Results: We identified 25,245 subjects, 4,228 (16.75%) of whom had HUA. The prevalence of HUA was 30% in men and 3.8% in women. The risk of HUA was significantly associated with ABCG2 rs2231142 risk T allele, with more HUA in TT genotype (OR: 2.40, 95% CI: 2.11–2.72, p<0.001) and TG genotype (OR: 1.64, 95% CI: 1.51–1.78, p<0.001) in men, and TT genotype (OR: 2.42, 95% CI: 1.83–3.20, p<0.001) and TG genotype (OR: 1.82, 95% CI: 1.46–2.23, p<0.001) in women, compared with their counterparts. Moreover, we found a strong genetic-environmental interaction associated with the risk of HUA. There was increased risk of HUA by the interaction of ABCG2 rs2231142 variant and BMI for TT genotype (OR: 7.42, 95% CI: 2.54–21.7, p<0.001) and TG genotype (OR: 4.25, 95% CI: 2.13–8.47, p<0.001) in men compared with the GG genotype in men, and for TT genotype (OR: 25.43, 95% CI: 3.75–172.41, p<0.001) and TG genotype (OR: 3.05, 95% CI: 0.79–11.71, p=0.011) in women compared with the GG genotype in women.
Conclusion: The risk of HUA was markedly increased by the interaction of ABCG2 rs2231142 variant and BMI, both in men and in women. Body weight control and reduction in BMI are recommended in high-risk patients with the ABCG2 rs2231142 risk T allele.
Introduction
Hyperuricemia (HUA; serum uric acid levels>7.0mg/dl) is a major risk factor for gout, which is known as “the disease of kings and the king of diseases.” Acute gouty attacks cause severe pain, difficulty in walking, functional impairments, and major disability, as well as decreased productivity if left untreated. Moreover, HUA is associated with multiple comorbidities, including obesity, hypertension, type 2 diabetes, hyperlipidemia, cardiovascular diseases, chronic kidney diseases, stroke, osteoporosis, erectile dysfunction, obstructive sleep apnea, and is an independent predictor of premature mortality (Vincent et al., 2016; Singh and Cleveland, 2019; Huang et al., 2020). The treatment target of gout is to reduce the levels of serum uric acid, and then to minimize medical comorbidities.
A number of genes associated with gout and HUA were identified by genome-wide association studies (Köttgen et al., 2013; Liu et al., 2020), and most were related to the urate-transport system, such as ATP-binding cassette subfamily G member 2 (ABCG2), glucose transporter type 9 (GLUT9, also known as SLC2A9), and urate anion transporter 1 (URAT1, also known as SLC22A12). ABCG2 encodes a high-capacity transporter for urate efflux and the ABCG2 rs2231142 single nucleotide polymorphism (SNP) is one of the most prominent genetic variants associated with HUA and gout (Dehghan et al., 2008; Woodward et al., 2009). Moreover, the association between ABCG2 rs2231142, HUA and gout risk varies with ethnicity (Dong et al., 2015), with an almost 3-fold increase of T-risk allele frequency in the East Asian population compared to the European population (Okada et al., 2012).
Obesity is one of the major risk factors for HUA, and body mass index (BMI) was positively correlated with serum uric acid levels in previous studies (R=0.96; p<0.00001; Shirasawa et al., 2020; Yu et al., 2021). Tai et al. (2020) has reported increased risk of gout when urate genetic risk scores are higher in low/normal-weight (OR: 2.89, 95% CI: 2.42–3.47), overweight (OR 3.09, 95% CI: 2.84–3.36), and obese individuals (OR 2.65, 95% CI: 2.46–2.86) based on BMI. Cheng et al. (2017) have reported that interaction effects between ABCG2 rs2231142 and obesity on serum uric acid levels in women in a Han Taiwanese population (p for interaction=0.0189). Other studies have also demonstrated that interactions between BMI and genetic variants affect serum urate concentrations and gout risk, but with conflicting results (Brandstätter et al., 2008; Li et al., 2013). However, it may not be possible to use the previously developed urate genetic risk score for non-European ancestry. Also, the score includes some SNPs with a very low minor allele frequency, which might reduce the power (Tai et al., 2020). Furthermore, previous studies using single SNP analysis revealed conflicting results, which were likely due to the fact that a single ethnicity was studied and the sample sizes were relatively small. In addition to the sample size (n=459), the definition of obesity was obscure in a study of ABCG2 rs2231142 in East Asians (Cheng et al., 2017). Therefore, it is currently unclear whether ABCG2 rs2231142 contributes to serum uric acid level according to BMI in East Asians. This study aimed to determine the impact of the interaction between ABCG2 rs2231142 and BMI on serum uric acid level, according to gender, in an East Asian population using a large sample size.
Materials and Methods
Participants
This study was conducted using the Taiwan Biobank (TWB), a population-based biomedical research database of patients with Taiwanese Han Chinese ancestry aged 30–70years, without a history of cancer. The patients were enrolled from 29 recruitment centers throughout Taiwan and all of the participants provided informed consent. Our study cohort was composed of 25,245 individuals with genotyping information, serum uric acid reports and the demographics, medical history, lifestyle modality, body fat evaluation including BMI, waist circumference, and body fat percentage, as well as biochemical reports including serum creatinine, cholesterol and fasting glucose level, were all identified from the database. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Taichung Veterans General Hospital’s Institutional Review Board (IRB no. CE16270B-2). All available data were achieved from TWB, which collected specimens and information in a complete and standardized procedure to fit researchers’ needs in different fields (Pan et al., 2006; Chen et al., 2016; Lin et al., 2019).
Genotyping and Quality Controls
The individual genotyping information was obtained from the TWB participants. A qualified and trained researcher with a medical background assisted in collecting 30-ml blood and extracting DNA from blood sample by employing QIAamp DNA blood kits following the manufacturer’s instructions (Qiagen, Valencia, CA, United States). After evaluating the quality of the isolated genomic DNA (Pan et al., 2006; Chen et al., 2016), it was genotyped by using custom Taiwan BioBank 2.0 SNP chip and an Axiom Genome-Wide Array Plate System (Affymetrix, Santa Clara, CA, USA) at the National Center for Genome Medicine in Academia Sinica, Taiwan. Affymetrix TWB 2.0 SNP chip contained 653, 291 SNPs and was designed specifically for Taiwan’s Han Chinese population. The genotype information and linkage disequilibrium of participants were released by the Ethics and Governance Committee (EGC) of Taiwan Biobank (TaiwanView: https://taiwanview.twbiobank.org.tw/index; Fan et al., 2008). PLINK was used for analysis and a quality control procedure was performed to exclude markers that failed Hardy–Weinberg equilibrium tests with value of p<1×10−6, minor allele frequency<0.01, and a genotyping call rate less than 90% (Purcell et al., 2007).
Data Collection and Outcome Identification
Body fat evaluation including BMI, waist circumference, waist-hip ratio, and body fat percentage were obtained. BMI was weight in kilograms divided by height in meters squared (kg/m2) and overweight was defined as BMI≥24kg/m2 (for the East Asian population). Body fat percentage was the percentage of fat by weight (Kelishadi et al., 2015). Moreover, baseline characteristics of social history (habits of alcohol drinking, smoking, and betel nut chewing) and physical activity were collected by a questionnaire through a face-to-face interview with one of the Taiwan Biobank researchers. Alcohol use was defined as a weekly intake of more than 150ml alcohol for at least 6months and smoking was defined as daily use of tobacco for an uninterrupted period of at least 6months. The social history status was dichotomized as a current- or ever-user vs. a non-user. The extent of physical activity was dichotomized as non-regular vs. regular exercise, which was defined as exercise for more than 30min at least three times a week. Biochemical data including serum creatinine, lipid profile, and fasting glucose level were also collected and provided by the TWB database. Hyperlipidemia was defined as a level of total cholesterol ≥200mg/dl, low density lipoprotein (LDL)≥100mg/dl, triglyceride (TG)≥150mg/dl, or high density lipoprotein (HDL)<40mg/dl in men or <50mg/dl in women. Hyperglycemia was defined as fasting glucose level≥100mg/dl.
The primary outcome was hyperuricemia, which was defined as a level of serum uric acid>7.0mg/dl. The level of serum uric acid was measured using an Architect i2000SR Analyzer (Abbott Diagnostics, Abbott Park, Chicago, IL, United States) by the uricase method (Liao et al., 2006).
Statistical Analysis
The demographic information is shown as mean±standard deviation for continuous variables and number (percent) for categorical variables. Student’s t-test for continuous variables and Chi-square test for categorical variables were used to compare data between HUA and non-HUA controls. Odds ratios (OR) and 95% confidence interval (95% CI) of variables and ABCG2 rs2231142 on HUA were calculated by logistic regression. Multiple logistic regression models were also used for evaluating the effect of interaction of ABCG2 rs2231142 and BMI on HUA. All data were analyzed using R, version 4.0.0 software. Statistically significance was set at p values less than 0.05.
Results
Baseline Characteristics of the Participants
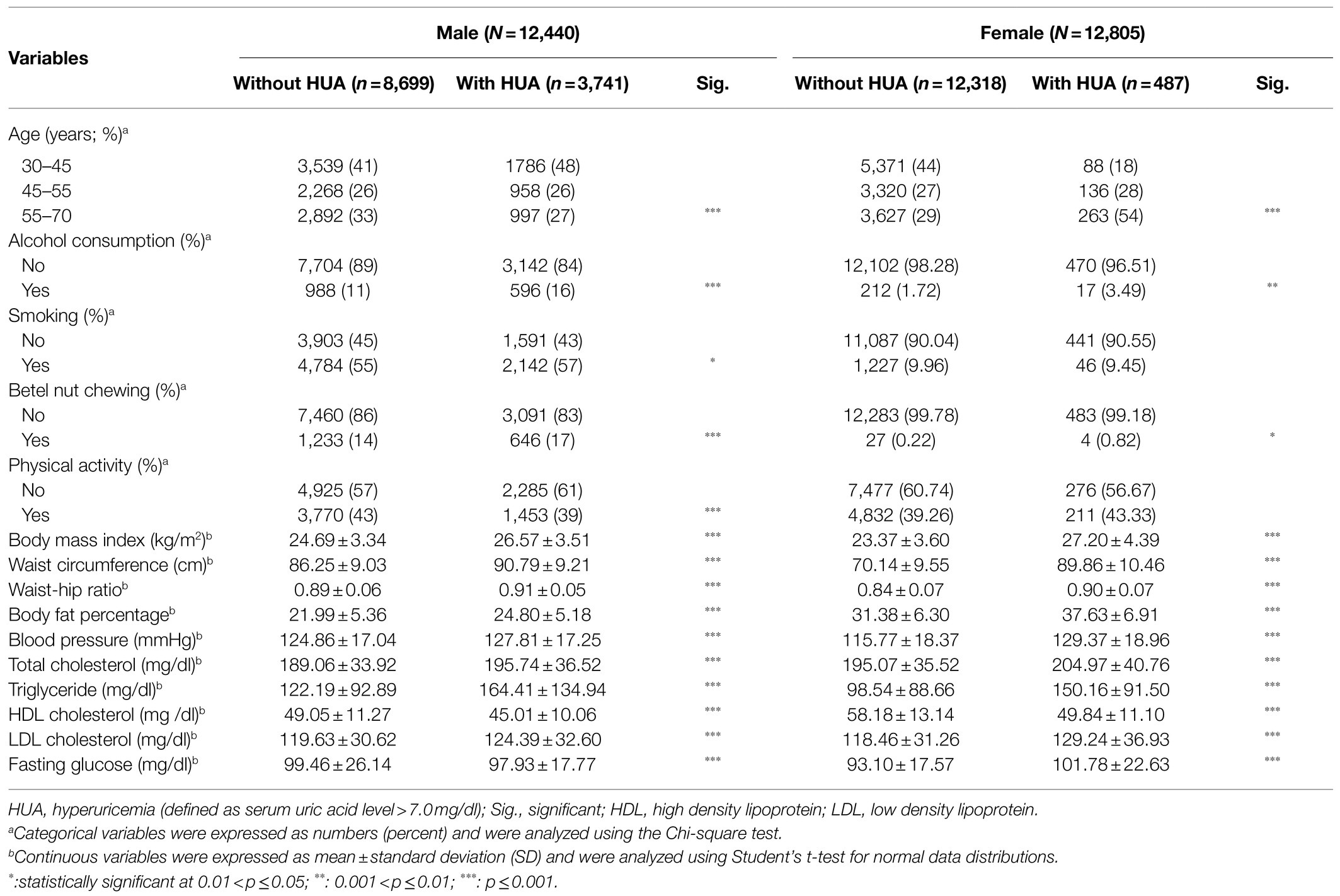
Between September 2014 and May 2017, we identified 25,245 subjects in the final analysis, including 4,228 (16.75%) with hyperuricemia and 21,017 (83.25%) in the control group (Table 1). The prevalence of HUA was 30% in men and 3.8% in women. More postmenopausal women aged 55–70years had HUA compared to the control group (54 vs. 29%, p<0.001) but surprisingly, more men aged 30–45years had HUA (48%). Higher BMI (men: 26.57±3.51 vs. 24.69±3.34kg/m2, p<0.001; women: 27.20±4.39 vs. 23.37±3.60kg/m2, p<0.001), waist circumference (men: 90.79±9.21 vs. 86.25±9.03cm, p<0.001; women: 89.86±10.46 vs. 70.14±9.55cm, p<0.001), waist-hip ratio (men: 0.91±0.05 vs. 0.89±0.06, p<0.001; women: 0.90±0.07 vs. 0.84±0.07, p<0.001), and body fat percentage (men: 24.80±5.18 vs. 21.99±5.36, p<0.001; women: 37.63±6.91 vs. 31.38±6.30, p<0.001) were observed in both men and women with HUA, compared with their counterparts. Furthermore, those with HUA were more likely to be users of alcohol or betel nut, and exhibited higher systolic blood pressure, higher blood total cholesterol level, higher LDL level, higher triglyceride level, but lower HDL level, compared with those without HUA.
Genotype Frequencies of ABCG2 rs2231142 and Association With HUA
The genotype frequency of ABCG2 rs2231142 is shown in Table 2. More TT and TG genotypes of ABCG2 rs2231142 were found in the HUA group than in the control group, with a similar distribution in men (TT: 14.5 vs. 8.3%, p<0.001; TG: 48.8 vs. 41.2%, p<0.001) and women (TT: 15.9 vs. 9.8%, p<0.001; TG: 52.2 vs. 42.7%, p<0.001). The minor T allele of ABCG2 rs2231142 was 38.9% in the HUA population, compared with 28.9% in the control group in men, and 42.0 and 31.1% in females, respectively. Furthermore, the risk of HUA was significantly associated with ABCG2 rs2231142 risk T allele, and both TT and TG genotypes contributed to increased risk of HUA in men (TT: OR: 2.40, 95% CI: 2.11–2.72, p<0.001; TG: OR: 1.64, 95% CI: 1.51–1.78, p<0.001) and women (TT: OR: 2.42, 95% CI: 1.83–3.20, p<0.001; TG: OR: 1.82, 95% CI: 1.46–2.23, p<0.001) compared with their counterparts.
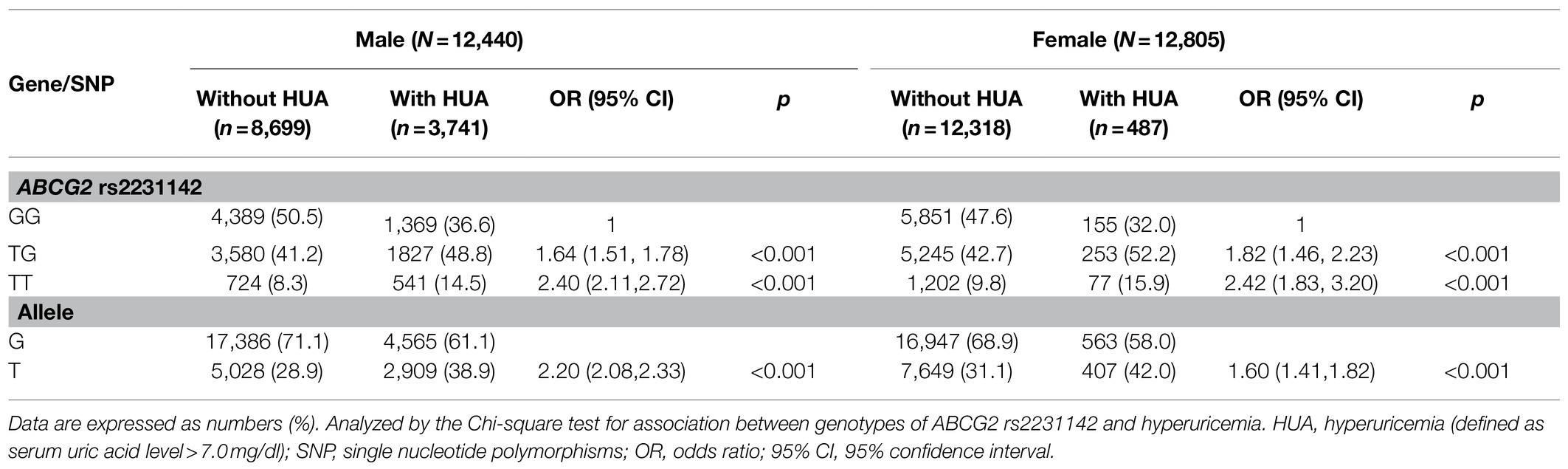
Table 2. Genotype and allele frequencies of ABCG2 rs2231142 and risk of hyperuricemia in the participants.
The Association of BMI With HUA
After adjusting for potential confounders, BMI was associated with a higher risk of HUA (Table 3). The BMI was analyzed as a continuous variable in the regression analysis, and for each one-unit increase in the BMI, the risk for HUA increased by 11% in men (OR: 1.11, 95% CI: 1.08–1.14, p<0.001) and by 15% in women (OR: 1.15, 95% CI: 1.08–1.23, p<0.001). In this study, we build a multiple logistic regression model for predicting serum uric acid levels based on ABCG2 rs2231142 genotypes. As Figure 1 shown, the mean uric acid of people with genotype GG is the lowest, followed by TG, and the highest is of people with TT genotype. As the BMI increased, the serum uric acid level rose in all genotypes, the correlation between BMI and uric acid is positive as a whole. The level of serum uric acid was higher in men than in women, not only at baseline but also at the peak level, and reached a plateau in women with the TT genotype of ABCG2 rs2231142. In addition, we further characterized the relationship between BMI and serum uric acid levels when BMI exceeds 24 with different genotypes. Based on 3 BMI categories, the distributions of genotypes are almost the same for different gender (Supplementary Figure 1). In female, the mean serum uric acid levels were increased by ABCG2 rs2231142 risk T allele. Similar results were observed for male where uric acid levels were elevated by TT and TG genotypes increase in BMI, there is an interaction between BMI and ABCG2 rs2231142 on the effect of uric acid.
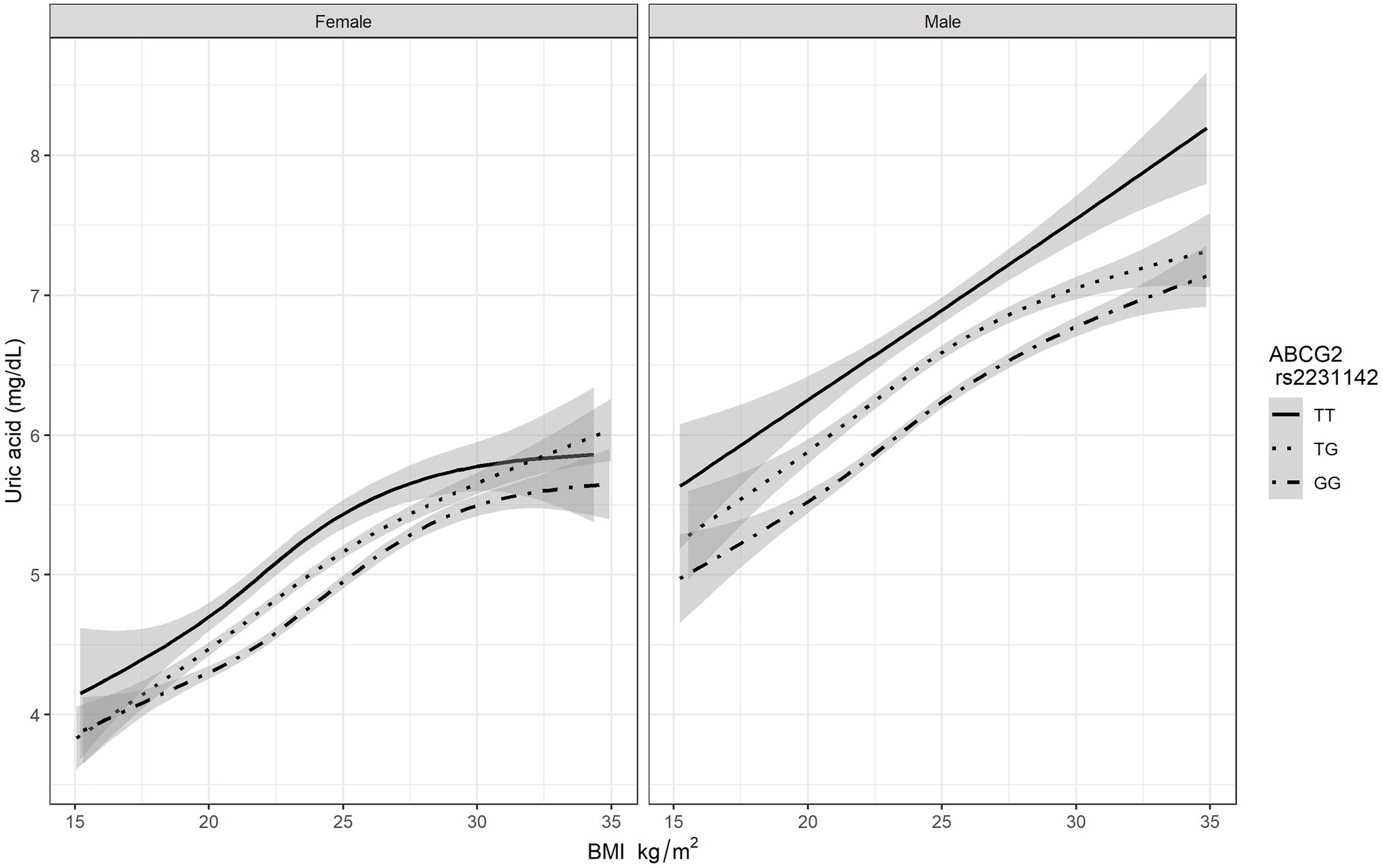
Figure 1. The association BMI and serum uric acid level based on different genotypes of ABCG2 rs2231142.
The Effect of Interaction of ABCG2 rs2231142 and BMI on HUA
ABCG2 rs2231142 and BMI both had significant impacts on risk of HUA, with genotypes dominant. Table 3 shows the markedly increased risk of HUA due to the interaction of ABCG2 rs2231142 and BMI. Compared with subjects with the GG genotype of ABCG2 rs2231142, the risk for HUA increased by 325% (OR: 4.25, 95% CI: 2.13–8.47, p<0.001) in men and by 205% (OR: 3.05, 95% CI: 0.79–11.71, p=0.011) in women with the TG genotype, and elevated remarkably by 642% (OR: 7.42, 95% CI: 2.54–21.70, p<0.001) in men and by 2,443% (OR: 25.43, 95% CI: 3.75–172.41, p<0.001) in women with the TT genotype when the BMI increased by one unit.
Discussion
This cohort study demonstrated a significantly increased chance of HUA in individuals with either the ABCG2 rs2231142 risk T allele or higher BMI in both men and women. Moreover, we demonstrated a strong genetic-environmental interaction associated with the risk of HUA. Individuals with elevated BMI in combination with the ABCG2 rs2231142 risk T allele had a much higher risk of HUA compared with the other subgroups. Of note, compared with those without risk factors, the ORs for the TT genotype of ABCG2 rs2231142 and higher BMI were 7.42 (95% CI 2.54–21.70, p<0.001) in men and 25.43 (95% CI 3.75–172.41, p<0.001) in women.
We demonstrated that serum uric acid level rose in all genotypes of ABCG2 rs2231142 as BMI increased, especially for the TT genotype. The gene-environmental interaction between ABCG2 rs2231142 and BMI on the serum uric acid level could be explained by some reasons. The level of ABCG2 and URAT1 increased significantly in an obese mouse model, which might intensify reabsorption of urate (Doshi et al., 2011). Besides, higher BMI and obesity might lead to obesity-induced hyperinsulinemia, enhance renal proximal urate reabsorption and further cause HUA (Facchini et al., 1991; Quiñones Galvan et al., 1995). Our result was similar to a previous study that showed the probability of gout increased when urate genetic risk scores were elevated in different BMI categories (Tai et al., 2020). However, this urate genetic risk score was constructed for gout, not for HUA, and it is unclear whether the score could be completely applicable to HUA prediction. Moreover, the urate genetic score was composed of numerous SNPs, some of which had very low minor allele frequency, and the impact of the top ranking urate loci might be diluted. Additionally, the composite genetic risk score for risk prediction is currently not feasible due to the high cost and the lack of access to this technique. Our study highlighted the effect of ABCG2 rs2231142, a single first-place genetic variant that impacts serum uric acid in an East Asian population, in order to explore its effects and to identify possible practical measures that could be implemented in clinical practice for treatment and prevention.
In the present study, we found a significantly increased risk of HUA due to the interaction of ABCG2 rs2231142 and BMI both in men and women. Similarly, Shih-Tsung Cheng and his colleagues determined that the ABCG2 rs2231142 risk allele exerted a greater effect on frequency of hyperuricemia, especially in obese patients in an East Asian cohort, but the difference in serum uric acid level was not significant in nonobese patients with this risk allele (Cheng et al., 2017). However, the sample size was small, resulting in limited power to detect associations among ABCG2 rs2231142, BMI, and serum uric acid level in East Asians. Moreover, conflicting results were reported in non-East Asian populations. A study on participants of European descent which analyzed 22 population cohorts (n=42,741) showed significant associations of the ABCG2 rs2231142 variant and BMI with serum uric acid level in males, but not in females (Huffman et al., 2015). Another study revealed no significant effect of interaction between ABCG2 rs2231142 genotypes and obesity on serum uric acid level in Mexicans (Macias-Kauffer et al., 2019). There were some differences between the results of our study and those reported in previous studies conducted on Western populations, possibly due to ethnic differences, distinct genetic factors, and dietary intake. One meta-analysis demonstrated that the results could be explained by ethnicity, which contributed to 66.67% heterogeneity in the ABCG2 rs2231142 TT vs. the GG genotype model (Dong et al., 2015). Further subgroup analysis revealed different gout risks in different ethnicities and a similar trend was expected in HUA risk. The odds ratios of gout risk were 2.80 (p=0.001), 4.56 (p<0.001), and 7.67 (p=0.002) for Caucasian, Mongoloid, and Polynesian populations carrying the ABCG2 rs2231142 TT genotype, respectively, compared with the GG genotype. Apart from genetic variants, dietary habits differed based on distinct ethnicities, and the effects of different diets on obesity status also varied. This has been confirmed in both animal and human studies. C57BL/6 mice fed with a cafeteria diet or a high-fat diet for 12weeks became obese (weight gain 179% on the cafeteria diet and 194% on the high-fat diet), but obesity was not found in those fed a standard chow diet or normal-fat diet (Lang et al., 2019). Since the degree of obesity varied based on different diet habits among ethnicities, and it is well-known that obesity is associated with serum uric acid level according to previous studies (Shirasawa et al., 2020), we might further infer that different dietary habits among ethnicities could be associated with various serum uric acid levels.
There were some strengths in our study. First of all, this study was a population-based study and explored the effects of interaction between ABCG2 rs2231142 risk allele and BMI on hyperuricemia in a population with a large sample size, which provided enough power for multiple logistic regression analysis and interaction analysis. In addition, the exploration of a single gene effect may be more feasible in clinical practice in the near future. Moreover, the Axiom Genome-Wide TWB Array Plate is a validated chip that is subject to rigorous quality control and is better suited to genotyping of East Asians.
However, this study has some limitations. First, the participants were all East Asians aged 30–70years and thus the results may not be generalizable to Western populations or other age groups (younger than 30years or older than 70years). Further genetics research based on different ethnicities and subgroups are warranted. Second, the majority of subjects recruited from the Taiwan Biobank were healthy, and therefore the results may not be generalizable to hospital-based populations. Third, the BMI of participants was checked at a single time point and may not reflect the trends of BMI change related to the serum level of uric acid. This issue remains to be explored in future studies.
This study sheds new light on the joint effect of ABCG2 rs2231142 risk allele and BMI on HUA. Based on the results of this study, the authors recommend the following. First, for high-risk patients with the ABCG2 rs2231142 risk T allele, body weight control and reduction in BMI are recommended to prevent hyperuricemia and possibly avoid development of gouty arthritis as well as associated comorbidities. Second, for high BMI subjects, the ABCG2 rs2231142 genotype should be checked and the serum uric acid level should also be assessed in those with the ABCG2 rs2231142 risk T allele. Third, for an individual with elevated BMI, the trend of serum uric acid should be monitored frequently with early implementation of a primary prevention strategy, such as dietary control, weight reduction, and lifestyle modification, in order to prevent development of hyperuricemia.
Conclusion
We conducted a case–control study using a population-based biomedical research database of subjects with Taiwanese Han Chinese ancestry. The risk of HUA was markedly increased by the interaction of ABCG2 rs2231142 variant and BMI, both in men and in women. Therefore, body weight control and reduction in BMI are strongly recommended in high-risk patients with the ABCG2 rs2231142 risk T allele. Prospective studies are needed to evaluate the effects of BMI change on serum uric acid level in individuals with the ABCG2 rs2231142 risk T allele, which is a high-risk gene.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Taichung Veterans General Hospital’s Institutional Review Board (IRB no. CE16270B-1). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
C-HL, Y-MC, and Y-JC conceived and designed the study. C-HL, Y-MC, P-CC, Y-JC, Y-CL, and I-CC performed the literature search and interpretation of data. Y-JC drafted the manuscript. P-CC conducted data extraction and methodological quality assessments, and performed the analysis. C-HL, P-CC, Y-MC, T-HH, Y-CL, J-CC, I-CC, and H-JL performed the critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from Taichung Veterans General Hospital, Taiwan (TCVGH-1087312C).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.709887/full#supplementary-material
Supplementary Figure 1 | Box plots for the relationship between BMI and serum uric acid levels when BMI exceeds 24 with different genotypes.
References
Brandstätter, A., Kiechl, S., Kollerits, B., Hunt, S. C., Heid, I. M., Coassin, S., et al. (2008). Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care 31, 1662–1667. doi: 10.2337/dc08-0349
Chen, C. H., Yang, J. H., Chiang, C. W. K., Hsiung, C. N., Wu, P. E., Chang, L. C., et al. (2016). Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 25, 5321–5331. doi: 10.1093/hmg/ddw346
Cheng, S. T., Wu, S., Su, C. W., Teng, M. S., Hsu, L. A., and Ko, Y. L. (2017). Association of ABCG2 rs2231142-A allele and serum uric acid levels in male and obese individuals in a Han Taiwanese population. J. Formos. Med. Assoc. 116, 18–23. doi: 10.1016/j.jfma.2015.12.002
Dehghan, A., Köttgen, A., Yang, Q., Hwang, S. J., Kao, W. L., Rivadeneira, F., et al. (2008). Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 372, 1953–1961. doi: 10.1016/S0140-6736(08)61343-4
Dong, Z., Guo, S., Yang, Y., Wu, J., Guan, M., Zou, H., et al. (2015). Association between ABCG2 Q141K polymorphism and gout risk affected by ethnicity and gender: a systematic review and meta-analysis. Int. J. Rheum. Dis. 18, 382–391. doi: 10.1111/1756-185X.12519
Doshi, M., Takiue, Y., Saito, H., and Hosoyamada, M. (2011). The increased protein level of URAT1 was observed in obesity/metabolic syndrome model mice. Nucleos. Nucleot. Nucl. Acids 30, 1290–1294. doi: 10.1080/15257770.2011.603711
Facchini, F., Chen, Y. D., Hollenbeck, C. B., and Reaven, G. M. (1991). Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266, 3008–3011. doi: 10.1001/jama.1991.03470210076036
Fan, C. T., Lin, J. C., and Lee, C. H. (2008). Taiwan Biobank: a project aiming to aid Taiwan's transition into a biomedical island. Pharmacogenomics 9, 235–246. doi: 10.2217/14622416.9.2.235
Huang, W. S., Lin, C. L., Tsai, C. H., and Chang, K. H. (2020). Association of gout with CAD and effect of antigout therapy on CVD risk among gout patients. J. Investig. Med. 68, 972–979. doi: 10.1136/jim-2019-001140
Huffman, J. E., Albrecht, E., Teumer, A., Mangino, M., Kapur, K., Johnson, T., et al. (2015). Modulation of genetic associations with serum urate levels by body-mass-index in humans. PLoS One 10:e0119752. doi: 10.1371/journal.pone.0119752
Kelishadi, R., Mirmoghtadaee, P., Najafi, H., and Keikha, M. (2015). Systematic review on the association of abdominal obesity in children and adolescents with cardio-metabolic risk factors. J. Res. Med. Sci. 20, 294–307.
Köttgen, A., Albrecht, E., Teumer, A., Vitart, V., Krumsiek, J., Hundertmark, C., et al. (2013). Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154. doi: 10.1038/ng.2500
Lang, P., Hasselwander, S., Li, H., and Xia, N. (2019). Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep. 9:19556. doi: 10.1038/s41598-019-55987-x
Li, W. D., Jiao, H., Wang, K., Zhang, C. K., Glessner, J. T., Grant, S. F., et al. (2013). A genome wide association study of plasma uric acid levels in obese cases and never-overweight controls. Obesity (Silver Spring) 21, E490–E494. doi: 10.1002/oby.20303
Liao, F., Zhao, Y. S., Zhao, L. N., Tao, J., Zhu, X. Y., and Liu, L. (2006). Evaluation of a kinetic uricase method for serum uric acid assay by predicting background absorbance of uricase reaction solution with an integrated method. J. Zhejiang Univ. Sci. B 7, 497–502. doi: 10.1631/jzus.2006.B0497
Lin, J. C., Chen, L. K., Hsiao, W. W., Fan, C. T., and Ko, M. L. (2019). Next chapter of the Taiwan Biobank: sustainability and perspectives. Biopreserv. Biobank. 17, 189–197. doi: 10.1089/bio.2018.0119
Liu, J., Yang, W., Li, Y., Wei, Z., and Dan, X. (2020). ABCG2 rs2231142 variant in hyperuricemia is modified by SLC2A9 and SLC22A12 polymorphisms and cardiovascular risk factors in an elderly community-dwelling population. BMC Med. Genet. 21:54. doi: 10.1186/s12881-020-0987-4
Macias-Kauffer, L. R., Villamil-Ramírez, H., León-Mimila, P., Jacobo-Albavera, L., Posadas-Romero, C., Posadas-Sánchez, R., et al. (2019). Genetic contributors to serum uric acid levels in Mexicans and their effect on premature coronary artery disease. Int. J. Cardiol. 279, 168–173. doi: 10.1016/j.ijcard.2018.09.107
Okada, Y., Sim, X., Go, M. J., Wu, J. Y., Gu, D., Takeuchi, F., et al. (2012). Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 44, 904–909. doi: 10.1038/ng.2352
Pan, W. H., Fann, C. S., Wu, J. Y., Hung, Y. T., Ho, M. S., Tai, T. H., et al. (2006). Han Chinese cell and genome bank in Taiwan: purpose, design and ethical considerations. Hum. Hered. 61, 27–30. doi: 10.1159/000091834
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Quiñones Galvan, A., Natali, A., Baldi, S., Frascerra, S., Sanna, G., Ciociaro, D., et al. (1995). Effect of insulin on uric acid excretion in humans. Am. J. Phys. 268, E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1
Shirasawa, T., Ochiai, H., Yoshimoto, T., Nagahama, S., Watanabe, A., Yoshida, R., et al. (2020). Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr. Disord. 20:2. doi: 10.1186/s12902-019-0481-1
Singh, J. A., and Cleveland, J. D. (2019). Gout is associated with a higher risk of chronic renal disease in older adults: a retrospective cohort study of U.S. Medicare population. BMC Nephrol. 20:93. doi: 10.1186/s12882-019-1274-5
Tai, V., Narang, R. K., Gamble, G., Cadzow, M., Stamp, L. K., Merriman, T. R., et al. (2020). Do serum urate-associated genetic variants differentially contribute to gout risk according to body mass index? Analysis of the UK Biobank. Arthritis Rheum. 72, 1184–1191. doi: 10.1002/art.41219
Vincent, Z. L., Gamble, G., House, M., Knight, J., Horne, A., Taylor, W. J., et al. (2016). Predictors of mortality in people with recent-onset gout: a prospective observational study. J. Rheumatol. 44, 368–373. doi: 10.3899/jrheum.160596
Woodward, O. M., Köttgen, A., Coresh, J., Boerwinkle, E., Guggino, W. B., and Köttgen, M. (2009). Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U. S. A. 106, 10338–10342. doi: 10.1073/pnas.0901249106
Keywords: uric acid, hyperuricemia, gout, ABCG2 rs2231142, BMI, obesity
Citation: Chen Y-J, Chen I-C, Lin H-J, Lin Y-C, Chang J-C, Chen Y-M, Hsiao T-H, Chen P-C and Lin C-H (2021) Association of ABCG2 rs2231142 Allele and BMI With Hyperuricemia in an East Asian Population. Front. Genet. 12:709887. doi: 10.3389/fgene.2021.709887
Edited by:
Taesung Park, Seoul National University, South KoreaReviewed by:
Jinchen Li, Central South University, ChinaZhaoxia Yu, University of California, Irvine, United States
Copyright © 2021 Chen, Chen, Lin, Lin, Chang, Chen, Hsiao, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Heng Lin, ZXBpZEB2Z2h0Yy5nb3YudHc=; Pei-Chun Chen, cGVpY2h1bmNjQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yen-Ju Chen
Yen-Ju Chen I-Chieh Chen
I-Chieh Chen Hsueh-Ju Lin
Hsueh-Ju Lin Ying-Cheng Lin
Ying-Cheng Lin Jui-Chun Chang
Jui-Chun Chang Yi-Ming Chen
Yi-Ming Chen Tzu-Hung Hsiao
Tzu-Hung Hsiao Pei-Chun Chen10*†
Pei-Chun Chen10*† Ching-Heng Lin
Ching-Heng Lin