
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 20 July 2021
Sec. Genetics of Common and Rare Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.703911
This article is part of the Research Topic Genetic Studies on Spondyloarthritis: from Disease Predictors to Therapeutic Targets View all 10 articles
Objective: Spondyloarthritis (SpA) are a group of diseases with a high heritability, whose pathogenesis is strongly determined by an interplay between genetic and environmental factor. Therefore, the aim of our study was to determine whether genetic variants could also influence response to therapy in SpA.
Methods: A systematic literature review (SLR) was conducted in PubMed and Web of Science core collection, without publication-year restrictions (Last search 8th April 2021). The search strategy was formulated according to the PEO format (Population, Exposure, Outcome) for observational studies. The population was adult (≥18 years) patients with SpA. The exposure was inheritable genetic variations of any gene involved in the disease pathogenesis/drug metabolism. The outcome was response to the drug, both as dichotomous (response yes/no) and as continuous outcomes. Exclusion criteria were: (1) languages other than English, (2) case series, case reports, editorials, and reviews, (3) studies reporting genetic contribution to drug response only limited to extra-musculoskeletal features of SpA, (4) epigenetic modifications. Quality of the included study was independently assessed by two authors.
Results: After deduplication, 393 references were screened by two authors, which led to the final inclusion of 26 articles, pertinent with the research question, that were considered for qualitative synthesis. Among these, 10 cohort, one cross-sectional, and five case-control studies were considered of at least good quality according to Newcastle-Ottawa Scale (NOS). In studies about TNF-blockers therapy: (1) polymorphisms of the TNF receptor superfamily 1A/1B (TNFRSF1A/1B) genes were most frequently able to predict response, (2) −238 and −308 polymorphisms of TNFα gene were studied with conflicting results, (3) TNFα polymorphism rs1799724, rs1799964, −857, −1,013, +489 predicted drug response in non-adjusted analysis, (4) PDE3A rs3794271 had a linear relationship with DAS28 reduction after anti-TNFα therapy. DHFR polymorphism +35,289 was able to predict response to methotrexate.
Conclusions: Our SLR highlighted the existence of a genetic component in determining drug response. However, further studies are warranted to better define quantify it.
Spondyloarthritis (SpA) is a group of systemic inflammatory diseases with common clinical characteristics and a shared genetic background (Costantino et al., 2018). The typical clinical features include (1) musculo-skeletal manifestations, with axial skeleton (spine and sacroiliac joints) involvement, peripheral arthritis, enthesitis, dactylitis, and (2) extra-musculoskeletal manifestations (EMMs) such as inflammatory bowel disease (IBD), psoriasis, and anterior uveitis. Depending on the main clinical and radiological presentation, the following disease subset have been identified and included under the umbrella term of SpA: ankylosing spondylitis (AS), psoriatic arthritis (PsA), arthritis associated with IBD, reactive arthritis, and undifferentiated SpA (Costantino et al., 2018). Spondyloarthritis have a high heritability, with a complex genetic background that has only been partially elucidated, but which is surely dominated by the Human Leukocyte Antigen (HLA-B27) allele: positive individuals have a relative risk of SpA onset of about 40 compared to those who are HLA-B27 negative. HLA-B27 is part of the Major Histocompatibility Complex class I and it accounts for 20% of the SpA heritability (Costantino et al., 2018). Thus, as strong as its association with the disease might be, HLA-B27 is not the only responsible for SpA genetic susceptibility, as genome wide studies have highlighted in 2007 (Wellcome Trust Case Control Consortium et al., 2007). In particular, among the non-MHC loci, endoplasmic reticulum amino peptidase (ERAP)1 and Interleukin-23 receptor (IL23R) genes were found to be strongly associated with SpA (Wellcome Trust Case Control Consortium et al., 2007). This discovery even led to new pathogenetic hypothesis, with important therapeutic implications (Gaffen et al., 2014).
The importance of genetic factors in the disease susceptibility, prompted researchers to investigate the role of genes in response to therapy as well (Song et al., 2015; Costantino et al., 2018). In fact, heterogeneity in drug response, even with the most effective drugs, has been observed in different disease phenotypes or -in general- in different patients (Ferraccioli et al., 2007). As an example, IL-23 inhibitors are effective in peripheral but not axial manifestations of SpA (Deodhar et al., 2019). Moreover, many patients do not experience adequate disease control with first-line therapy, such as non-steroidal anti-inflammatory drugs or conventional synthetic Disease Modifying Rheumatic Drugs (csDMARDs) and there are no clear indicators to predict this (van der Heijde et al., 2017; Gossec et al., 2020). Furthermore, a consistent proportion of patients (up to one-third) does not even respond to the first biotechnological drug (representing second-line therapy), whichever this might be (Merola et al., 2017). Thus, genetic variants of genes involved in both SpA pathogenesis and phenotypic expression, as well as in the drug metabolism, could play a role in determining drug response (Ferraccioli et al., 2007).
Therefore, the aim of the present study was to collect existing evidence supporting the role of genetics in predicting response to therapy in SpA.
A systematic literature review (SLR) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) was conducted (Moher et al., 2009). PubMed and Web of Science core collection were searched, without publication year restrictions. Last search was on 8th April 2021.
The research question was formulated according to the PEO format (Population, Exposure, Outcome) for observational studies. The population (P) of interest was considered to be adult (≥18 years) patients with SpA. Studies including patients with other rheumatic diagnoses were considered eligible only if the results for SpA were presented separately. The exposure (E) was represented by genetic predisposition, meaning specific inheritable genetic variations of any gene that could be involved in the disease pathogenesis, or in drug metabolism. The outcome of interest was drug response, both as dichotomous outcome (response yes/no according to various disease activity status criteria or response criteria) and as continuous outcomes. Examples of dichotomous outcomes were: the Assessment of SpondyloArthritis international Society (ASAS)-based indices ASAS 20, ASAS 40, Bath Ankylosing Spondylitits Disease Activity Index (BASDAI) 50, BASDAI ≥ 4 (Anderson et al., 2001; Rudwaleit et al., 2004). Among continuous outcomes the following were considered: tender/swollen joint count, BASDAI, percentage of patients, Disease Activity Score on 28-joints count (DAS28) (van der Heijde et al., 1990; Garrett et al., 1994).
Inclusion criteria regarding population were: (1) adult axSpA patients as defined by: clinical diagnosis, ASAS criteria for axSpA or modified NY criteria for AS (van der Linden et al., 1984; Rudwaleit et al., 2009); (2) PsA patients as defined by rheumatologist diagnosis or ClAssification criteria for Psoriatic ARthritis (CASPAR) criteria (Taylor et al., 2006); (3) SpA associated to IBD, reactive arthritis or undifferentiated arthritis (if included).
Exclusion criteria were: (1) studies in languages other than English, (2) case series, case reports, editorials, and reviews, (3) studies reporting genetic contribution to drug response only limited to EMMs, such as IBD or psoriasis, and not presenting data for patients with SpA separately, (4) epigenetic modifications (e.g., DNA methylation and miRNA).
We checked MeSH terms for SpA, genetics, drug response to identify search terms in an attempt to capture all possible synonyms. In the final search, however, MeSH terms were not used to avoid excluding more recent works. The detailed search strategy is indicated in the Supplementary File.
Two reviewers (AO, GC) assessed titles and abstracts on suitability for inclusion, according to the inclusion/exclusion criteria, followed by a full-text review if necessary. Discrepancies were resolved by consensus. The following information was extracted from the study: author, year, study design, number of included patients, characteristics of the study population (disease classification, gender, age, disease duration), of the exposure (gene where a variation was detected, and type of variation), and outcome measures. The quality of the extracted studies was then evaluated by Newcastle-Ottawa Scale (NOS) for cross-sectional, cohort, and case-control studies (Wells et al., 2021). Newcastle-Ottawa Scale study quality was then graded according to the total score. Cross-sectional studies were graded as: very good = 9–10; good = 7–8; satisfactory = 5–6; unsatisfactory = 0–4 (Modesti et al., 2016). Cohort and case–control studies were graded as: very good = 8–9; good = 7; satisfactory = 5–6; unsatisfactory = 0–4.
A PRISMA flowchart was generated for the final selection of the studies to be included (see Results section for details).
Exposure was expressed as presence or absence of a specific genetic variation. Outcome was expressed according to the analysis presented in the study. If analysis were adjusted, odds ratio (95% Confidence Interval-CI), hazard rate (95%CI), or beta (95%CI) were reported for logistic regression, Cox regression or linear regression, respectively. Otherwise, only p-value was reported for descriptive statistics. Due to heterogeneity of the included population, exposure, and outcomes a meta-analysis could not be performed.
A total of 524 references were retrieved by the databases search. After removing duplicates, titles, and abstracts of the remaining 393 references were screened for eligibility, which led to the elimination of 330 articles. This was mainly due to wrong target population (e.g., rheumatoid arthritis, psoriasis, gout), wrong exposure (e.g., monocytes expression profile, long non-coding mRNA as inflammatory modulators), or wrong outcome (e.g., disease onset or severity instead of response to therapy); two papers were not in English. The full-text of 61 articles was examined, resulting in the exclusion of 35 further articles that did not fulfill inclusion/exclusion criteria: 29 were reviews or book chapters, one did not present data for SpA separately, one did not specify treatment, four were congress abstracts with insufficient information to extract. The remaining 26 articles were considered for qualitative evaluation.
The PRISMA flowchart is displayed in Figure 1.
The 26 studies that were included in the qualitative assessment were thoroughly examined to identify: author, year, study design, number of participants, definition of population, exposure, outcome. The main characteristics of the studies are displayed in Table 1. There were 15 cohort studies (Tutuncu et al., 2005; Seitz et al., 2007; Chandran et al., 2010; Eder et al., 2010; Morales-Lara et al., 2010, 2012; Ramírez et al., 2012; Julià et al., 2014; Schiotis et al., 2014; Fabris et al., 2016; Chen, 2017; Yan et al., 2017; Liu et al., 2019; Ovejero-Benito et al., 2019; Polo Y La Borda et al., 2019), eight case-control studies (Manolova et al., 2014; Murdaca et al., 2014; Ma et al., 2017; Wang et al., 2017; Zhao et al., 2017; Aita et al., 2018; Xing-Rong et al., 2018; Xu et al., 2020; Sokolik et al., 2021) and one cross sectional study (Nossent et al., 2014). The definition of the populations was heterogeneous, with studies conducted in Europe, USA, and China, and mainly including AS and PsA patients (Table 1). Exposure was also heterogeneous, as several genetic polymorphisms were evaluated, with target genes implicated in the pathogenesis (e.g., C Reactive Protein—CRP, Tumor Necrosis Factor α–TNFα), drug metabolism (e.g., Cytocrome P450), drug immunogenicity (e.g., Fc receptor). The response to therapy was variably evaluated by validated outcomes of the following types: (1) dichotomous: ASAS 20, ASAS 40, BASDAI 50, American College of Rheumatology (ACR) 20, Psoriatic Arthritis Response Criteria (PsARC) (2) categorical: EULAR response criteria; (3) continuous: tender or swollen joint count, DAS28, BASDAI change score, morning stiffness. Some studies used non-validated but clinically significant outcomes, among which (1) a ≥70% improvement in physician global assessment (PhGA) and SJC/TJC plus a ≥50% improvement in two of: erythrocyte sedimentation rate, CRP, patient global assessment (PGA) (Tutuncu et al., 2005) (2) BASDAI ≤ 4 (Aita et al., 2018) (3) a ≥50% in a Numerical Rating Scale (NRS) for pain (Ovejero-Benito et al., 2019), (4) necessity of therapeutic switch yes/no (Fabris et al., 2016), (5) actively inflamed joint count (meaning tender and/or swollen joints; Chandran et al., 2010).
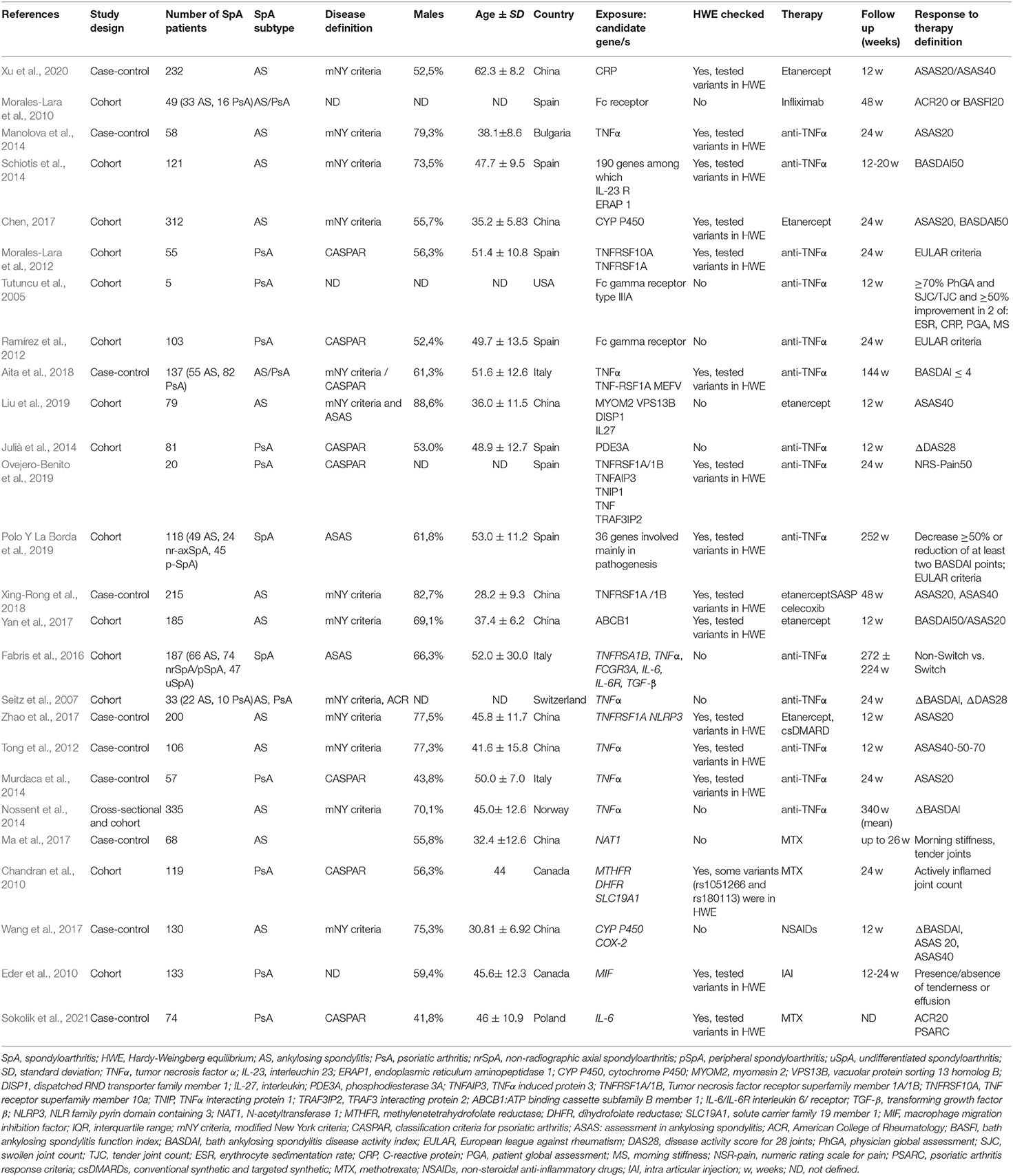
Table 1. Characteristics of the studies satisfying inclusion and exclusion criteria for the SLR, with particular reference to study design and characteristics of population, exposure, outcome.
According to the NOS for cohort studies, 11 studies were graded as very good or good (Chandran et al., 2010; Eder et al., 2010; Morales-Lara et al., 2012; Ramírez et al., 2012; Julià et al., 2014; Schiotis et al., 2014; Fabris et al., 2016; Chen, 2017; Yan et al., 2017; Liu et al., 2019; Polo Y La Borda et al., 2019), and were therefore included in the qualitative synthesis. One study was deemed unsatisfactory (Morales-Lara et al., 2010) and three were only satisfactory (Tutuncu et al., 2005; Seitz et al., 2007; Ovejero-Benito et al., 2019), thus their results are not discussed in detailed. The lone cross-sectional study was considered of good quality according to NOS (Nossent et al., 2014). Among the case-control studies, four were only satisfactory (Manolova et al., 2014; Wang et al., 2017; Xu et al., 2020; Sokolik et al., 2021), one was unsatisfactory (Ma et al., 2017), and five good (Tong et al., 2012; Zhao et al., 2017; Aita et al., 2018) or very good (Murdaca et al., 2014; Xing-Rong et al., 2018). The latter were the ones that were taken into consideration for the qualitative synthesis. A common reason for higher grades in the cohort studies was the fact that the exposure (genetic polymorphism) was surely present at the start of the study and likely unbiased, resulting from the same laboratory test applied for the whole sample. In general, across all study designs, comparability grading was not always optimal as a minority of studies applied proper correction for several covariates, while the majority only corrected for one important factor or reported unadjusted analysis. Table 2 reports the detailed grading of each study.
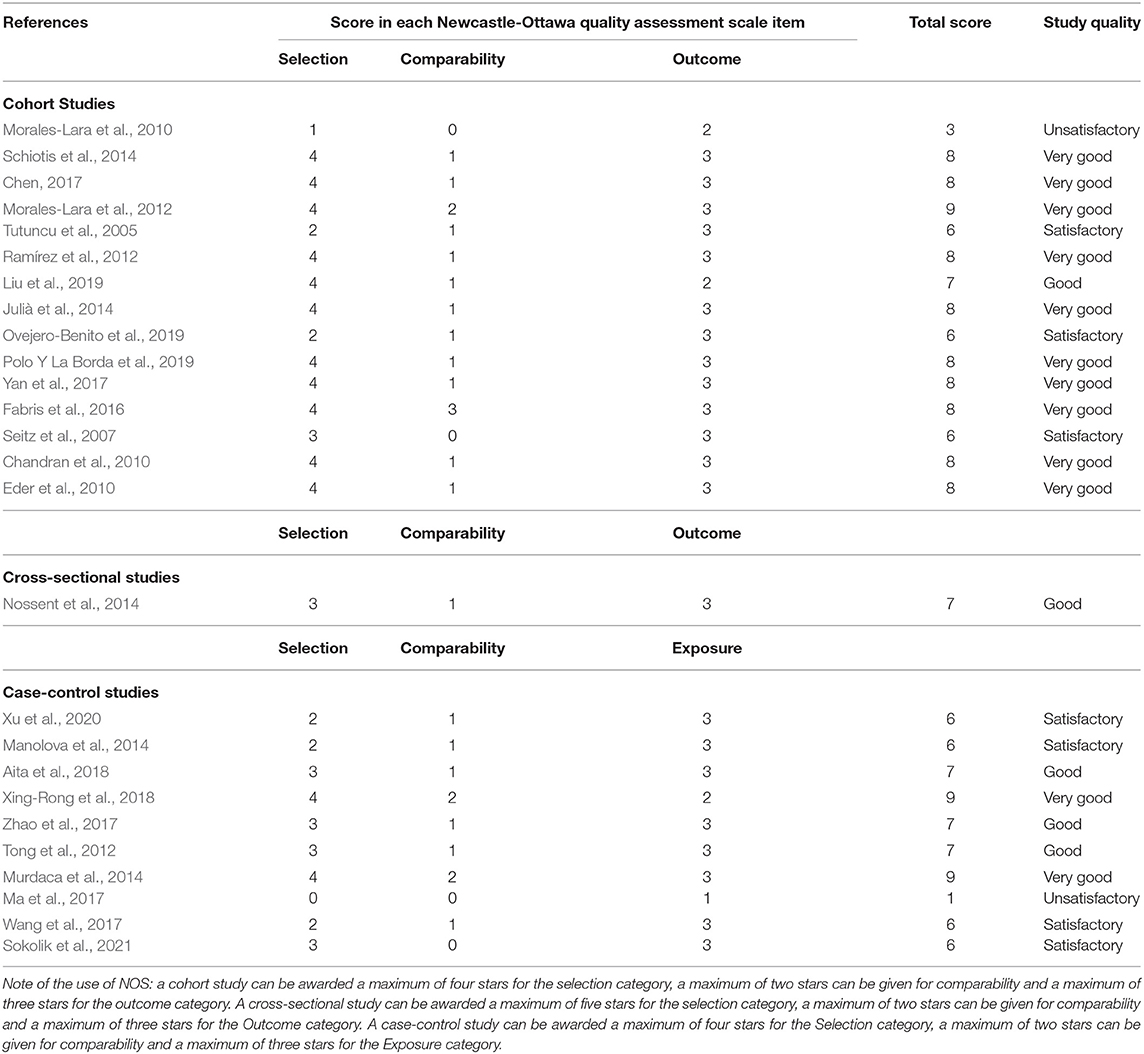
Table 2. Application of Newcastle-Ottawa quality assessment scale (NOS) for cohort, cross-sectional and case control studies.
In order to synthetize results regarding the influence of genetic variants on response to therapy, only data from studies that were deemed of good or very good quality were extracted and are presented in Table 3.
Most of the studies focused on anti-TNFα therapy. Several of them investigated genes involved in SpA pathogenetic mechanisms, in particular TNFα, TNFα receptors, and several interleukins (IL) both with pro inflammatory (e.g., IL-6) and anti-inflammatory (e.g., IL-10) effects.
Polymorphisms of the TNF receptor superfamily 1 A and 1B (TNFRSF1A/1B) genes were those that most frequently were able to predict response (TNFRSF1A rs767455 genotype AA, TNFRSF1A rs1800693 genotype GG, TNFRSF1B rs1061622 genotype TT and GG) according to various criteria such as BASDAI 50, EULAR response, or ASAS 20, ASAS 40 (Morales-Lara et al., 2010; Julià et al., 2014; Ovejero-Benito et al., 2019). Notably, Schiotis et al., who also investigated the TNFRSF1B polymorphism rs1061622, found that the GG genotype was associated with non-response, thus reaching opposite conclusion compared to the previously mentioned studies despite a fair numerosity and correcting for other polymorphisms (Schiotis et al., 2014). Other authors simply could not demonstrate any association to response according to the ASAS 20 for the polymorphisms they investigated in the TNFRSF1A gene (rs2234649, rs4149570, rs4149621, rs4149569; Zhao et al., 2017; Xing-Rong et al., 2018). Notably, among the investigated genetic variations, also TNFRSF1A rs767455 was present, and its association with clinical response was therefore not confirmed by all authors (Zhao et al., 2017; Xing-Rong et al., 2018).
The TNFα gene was also frequently studied in relation to therapy response, with two studies failing to demonstrate an association of the −238G>A (rs361525) and −308G>A (rs1800629) polymorphisms and clinical response according to ACR 20 and BASDAI (Murdaca et al., 2014; Nossent et al., 2014). These studies, however, did not correct for any confounding factor. Conversely, Fabris et al., correcting the association of the same −308 A polymorphism to therapy response (according to ASAS0 20) for age, gender, disease duration, and diagnosis, found a significantly positive association (Fabris et al., 2016). Other TNFα gene polymorphism described to be associated to either ASAS 40, ASAS 50, or ACR 20 response were −857C>T (rs1799724), −1031T>C (rs1799964), while +489G>A (rs80267959) was associated to ACR 20 response. All these findings derived, however, from non-adjusted analysis and were not confirmed by all studies (Tong et al., 2012; Murdaca et al., 2014; Aita et al., 2018).
Furthermore, genes encoding for molecules implicated in the signaling transduction cascade (including inflammatory cascade), such as phosphodiesterase (PDE)3A, were shown to have a linear relationship with DAS28 reduction after anti-TNFα therapy (Julià et al., 2014). Other polymorphisms implicated in SpA pathogenesis that were found to be independently associated to non-response were: rs755622 in macrophage migration inhibition factor (MIF), rs917997 in IL18-receptor accessory protein (IL18-RAP), rs3740691 in ADP Ribosylation Factor GTPase Activating Protein 2 (ARFGAP2), rs1800896 in IL-10, 2rs4240847 in Mitogen-Activated Protein Kinase-Activated Protein Kinase (MAPKAPK2), rs11096957 in Toll like receptor-10 (TLR-10), rs11541076 in Interleukin 1 Receptor Associated Kinase 3 (IRAK-3) (Schiotis et al., 2014; Polo Y La Borda et al., 2019).
Finally, one study, among those of good quality investigating pathogenetic genes, explored the role of MIF polymorphism rs755622 in predicting clinical response to intra-articular steroid injections in PsA; the analysis failed to show any association when correcting for age, sex, disease duration, and activity (Eder et al., 2010).
Fewer authors took into consideration genes that might be involved in drug metabolism or immunogenicity. Amid these, genes encoding for enzymes that are part the cytochrome (CYP) P450 superfamily have been tested: the allele variants CYP2D6*10 and CYP3A*3 were more frequently found in BASDAI50 responders than non-responders to etanercept (Chen, 2017). Other works examined genes encoding for the Fc fragment receptor 2A and 3A, under the hypothesis that polymorphisms resulting in a higher/lower affinity to the Fc region of TNFα blockers may modulate both their half-life and cellular effects, and may therefore produce differential therapeutic effects in individuals (Ramírez et al., 2012). Ramirez et al. found that FCGR3A was indeed associated to EULAR response, although in a non-adjusted analysis (Ramírez et al., 2012). Fabris et al. were not able to confirm this finding after adjusting for age, gender, disease duration, and diagnosis (AS/PsA) (Fabris et al., 2016).
One study investigated response to methotrexate in terms of reduction of at least 50% of “actively inflamed joints,” meaning tender and/or swollen joints, highlighting that DHFR polymorphism +35289A>G (rs1232027) was able to predict response to methotrexate (even when correcting for concomitant medications; Chandran et al., 2010).
A synthesis of the genes that have been found to be associated to drug response is represented in Table 4.
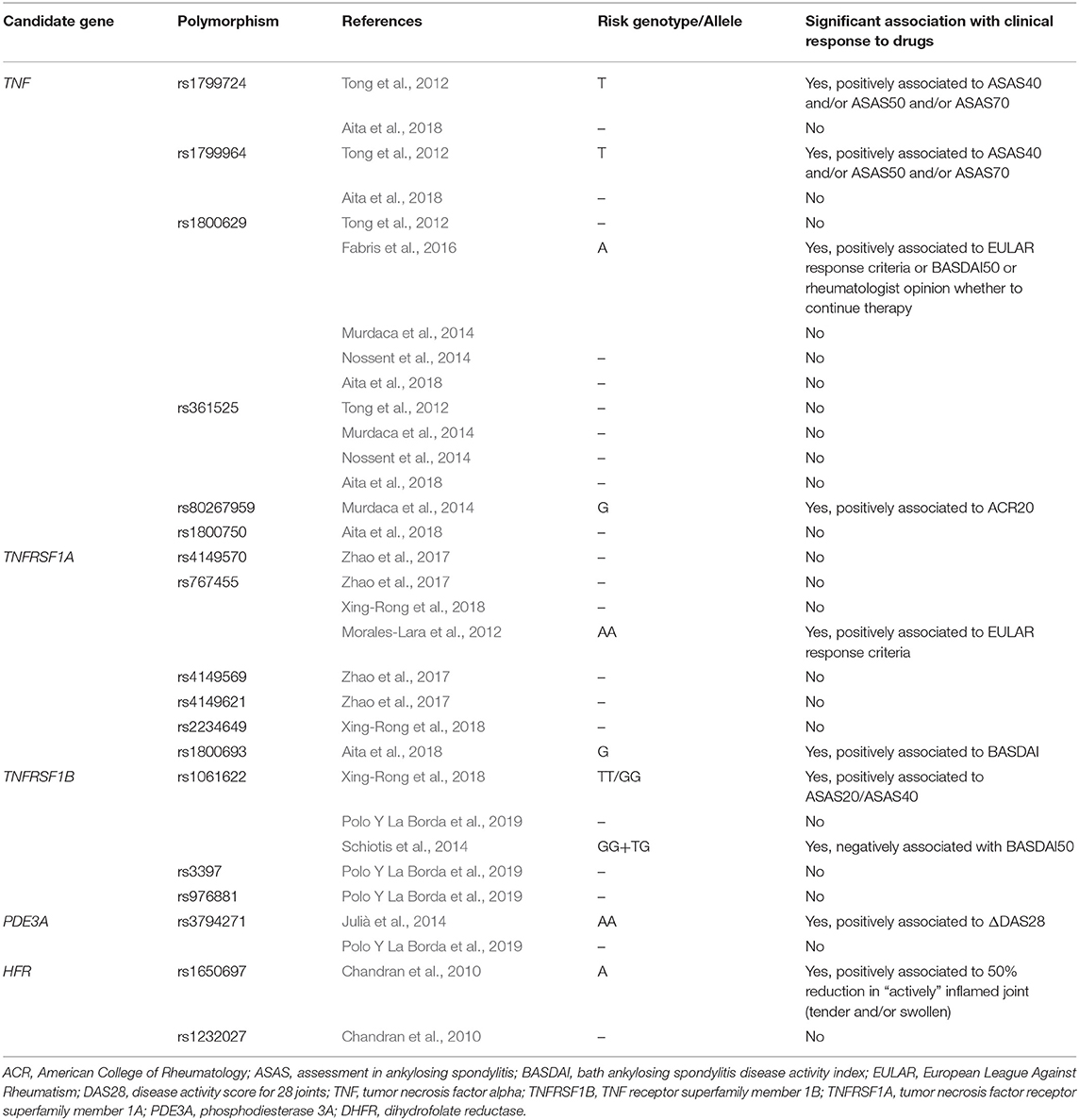
Table 4. Synthesis of genes that have been studied in relation to treatment response in spondyloarthritis, and summary of results.
The results of our SLR highlighted that the genetic component is surely one of the determinants of drug response in SpA. However, the heterogeneity existing in present literature prevented us to quantify the genetic contribution to therapy response, particularly regarding anti-TNFα biological drugs, which were the most studied.
Admittedly, there are several challenges in conducting predictions studies about genetic variants in drug response in SpA. Firstly, given that most studies focused on genes involved in the disease pathogenesis, it must be remembered that several pathways have been implied in this process. Dysregulation of the IL-17/23 axis and the activation of innate immunity, with effectors like gamma-delta T cells, type 3 innate lymphoid cells (ILCs), neutrophils, macrophages, and lately also cytotoxic B lymphocytes have been described in SpA (Tang and Inman, 2021). In addition, interaction with environmental triggers is fundamental for disease onset and perpetuation. As an example, polymorphisms of TLR-2 and−4, key receptors in pathogen recognition expressed by macrophages or dendritic cells, have been associated to SpA onset at an early age (Perica et al., 2015). When certain genetic variants are associated to disease onset or severity, it is logical to suspect they might be involved in drug response as well. However, since pathogenesis is not solely driven by one of these mechanisms, it is unlikely that a single gene, or a narrow spectrum of gene within a particular pathway, might significantly explain the tendency to respond to a certain targeted therapy. Furthermore, several aspects of SpA pathogenesis are still unknown: one above all, it is not clear how HLA-B27 exerts its pathogenetic effect. For this reason, comprehensive genetic approaches, such as genome-wide association studies (GWAS) have been undertaken in order to uncover unknown factors of susceptibility (Jung et al., 2014; Robinson et al., 2016). This kind of studies might also have therapeutic implications, and has the advantage, compared to the classic candidate-gene(s) design, of being hypothesis-free. Both candidate and whole genome strategies have limitations, however, candidate gene approach lacks the objectivity of genome-wide screening in the process of choosing specific candidates from numbers of potential possibilities; the choice of genes depends on the prior knowledge of the illness, which often remains partly unknown (Sabourin et al., 2019). In addition, in order to be clinically useful, a quite strong relation between a certain genetic variant and clinical outcomes has to be highlighted. Not to mention the candidate gene should also have a demonstrated added value, compared to clinical predictors of response (e.g., male sex), to be of interest (Ni et al., 2013; Ramonda et al., 2021). In practice, it is often the case that certain polymorphisms are only weakly associated to drug response. This can clearly be seen from the adjusted OR, along with their wide 95% CI, represented in Table 3 (Schiotis et al., 2014; Fabris et al., 2016; Zhao et al., 2017; Polo Y La Borda et al., 2019).
A second, but not less important, issue is represented by the reproducibility of results. Even studies investigating the same polymorphism, such as TNFα −308, which has been associated to anti-TNF response both in adult and juvenile SpA (Scardapane et al., 2012), often have contrasting results (Murdaca et al., 2014; Nossent et al., 2014; Fabris et al., 2016). In part, this could be due to the small sample size of some of these studies or to the diversities in the included ethnicities (e.g., Asian vs. Caucasian). On the other hand, the outcomes of drug response are also not standardized across studies. Moreover, analysis are carried out very differently, adjusting for different sets of factors, or without any/with very little adjustment. All these factors add up to the challenge of detecting significant and reproducible genetic markers of drug response.
Thirdly, it has been highlighted how genetic research is particularly prone to type I error (i.e., the risk of falsely rejecting a true null hypothesis or, in other words, to identify a significant association when indeed no association exists; Sabourin et al., 2019). This might happen because of the highly non-independent nature of the variants in a genome, which implies that the assumptions underlying the commonly used statistical methods are often not met (Sabourin et al., 2019). Furthermore, more commonly type I error may stem from multiple testing (comparison of several variants), genotyping errors, and population stratification, that can result in spurious associations (Jorgensen et al., 2009). One of the most obvious, yet important, remedies for this, would be to correct for multiple testing, especially in the candidate-gene approach studies where several variants are tested. Unfortunately, only a slight minority of the retrieved studies applied this correction (Table 3), although this might be less impactful in those studies which tested a limited number of variants (e.g., 3–4). Another way it has been found to limit this problems is replication or cross-validation within the same sample (Liu et al., 2019).
Certainly, however, the fact that several polymorphisms, mainly implicated in the disease pathogenesis, were able to predict to some extent the treatment response, even in adjusted analysis and with a fair numerosity in study populations, points toward the real existence of a genetic determination of drug response (Julià et al., 2014; Schiotis et al., 2014; Fabris et al., 2016; Zhao et al., 2017). This was especially seen with TNFα-blockers therapy, which is also the most frequently used effective therapy for SpA (van der Heijde et al., 2017). Studies investigating polymorphisms involved in drug metabolism in anti-TNFα were less consistent. Interestingly, also response to methotrexate seemed to be predicted by a polymorphism of a gene involved in drug metabolism (DHFR +35289), which is somehow more expected than for anti-TNFα as methotrexate is a traditional csDMARD, with a prevalent liver metabolism.
Our study had the methodological strength of being a SLR, and therefore we were able to capture all relevant literature pertaining our research questions, as well as providing a quality assessment of each study. The potential limitations are linked to the design of included studies, which all used a candidate-gene approach: this kind of research is more prone to type I error and to publication bias (i.e. the presentation of mostly positive results, neglecting studies with negative findings). To this regard, GWAS studies could be at a lower risk of bias. Moreover, no RCT taking genetic variants into consideration was retrieved, but only observational studies. Other issues were heterogeneity in the description of population, exposure and outcome. The latter prevented us to perform a meta-analysis to quantify the genetic contribution to drug response in SpA.
In conclusion, we were able to identify a genetic component in drug response across all the included study. Incorporating genetic analysis into clinical studies could help to predict responses to different treatment options, aiming toward personalized medicine. However, further studies are warranted to better define the genotypes that are most involved in contributing to response to therapy and to describe the magnitude of this phenomenon, especially in comparison with the most commonly used clinical predictors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
AO and GC participated in study design, data extraction, analysis and synthesis, and drafted the manuscript. ML and PG helped in data collection, critical interpretation of data, and revised the manuscript for important intellectual content. AD and RR conceived the study, analyzed the results critically, and revised the manuscript for important intellectual content. All authors approved the final version to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.703911/full#supplementary-material
Aita, A., Basso, D., Ramonda, R., Moz, S., Lorenzin, M., Navaglia, F., et al. (2018). Genetics in TNF-TNFR pathway: a complex network causing spondyloarthritis and conditioning response to anti-TNFα therapy. PLoS ONE 13:e0194693. doi: 10.1371/journal.pone.0194693
Anderson, J. J., Baron, G., van der Heijde, D., Felson, D. T., and Dougados, M. (2001). Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 44, 1876–1886. doi: 10.1002/1529-0131(200108)44:8<1876::AID-ART326>3.0.CO;2-F
Chandran, V., Siannis, F., Rahman, P., Pellett, F. J., Farewell, V. T., and Gladman, D. D. (2010). Folate pathway enzyme gene polymorphisms and the efficacy and toxicity of methotrexate in psoriatic arthritis. J. Rheumatol. 37, 1508–1512. doi: 10.3899/jrheum.091311
Chen, Y.-Y. (2017). Correlations of CYP2C9*3/CYP2D6*10/CYP3A5*3 gene polymorphisms with efficacy of etanercept treatment for patients with ankylosing spondylitis: a case-control study. Medicine (Baltimore) 96:e5993. doi: 10.1097/MD.0000000000005993
Costantino, F., Breban, M., and Garchon, H.-J. (2018). Genetics and functional genomics of spondyloarthritis. Front. Immunol. 9:2933. doi: 10.3389/fimmu.2018.02933
Deodhar, A., Gensler, L. S., Sieper, J., Clark, M., Calderon, C., Wang, Y., et al. (2019). Three Multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. Hoboken NJ. 71, 258–270. doi: 10.1002/art.40728
Eder, L., Chandran, V., Ueng, J., Bhella, S., Lee, K.-A., Rahman, P., et al. (2010). Predictors of response to intra-articular steroid injection in psoriatic arthritis. Rheumatol. Oxf. Engl. 49, 1367–1373. doi: 10.1093/rheumatology/keq102
Fabris, M., Quartuccio, L., Fabro, C., Sacco, S., Lombardi, S., Ramonda, R., et al. (2016). The −308 TNFα and the−174 IL-6 promoter polymorphisms associate with effective anti-TNFα treatment in seronegative spondyloarthritis. Pharmacogenomics J. 16, 238–242. doi: 10.1038/tpj.2015.49
Ferraccioli, G., Tolusso, B., and De Santis, M. (2007). Pharmacogenetic of antirheumatic treatments: clinical implications. Pharmacogenomics J. 7, 2–9. doi: 10.1038/sj.tpj.6500396
Gaffen, S. L., Jain, R., Garg, A. V., and Cua, D. J. (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600. doi: 10.1038/nri3707
Garrett, S., Jenkinson, T., Kennedy, L. G., Whitelock, H., Gaisford, P., and Calin, A. (1994). A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 21, 2286–2291.
Gossec, L., Baraliakos, X., Kerschbaumer, A., de Wit, M., McInnes, I., Dougados, M., et al. (2020). EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 79, 700–712. doi: 10.1136/annrheumdis-2020-217159
Jorgensen, T. J., Ruczinski, I., Kessing, B., Smith, M. W., Shugart, Y. Y., and Alberg, A. J. (2009). Hypothesis-driven candidate gene association studies: practical design and analytical considerations. Am. J. Epidemiol. 170, 986–993. doi: 10.1093/aje/kwp242
Julià, A., Rodríguez, J., Fernández-Sueiro, J. L., Gratacós, J., Queir,ó, R., Montilla, C., et al. (2014). PDE3A-SLCO1C1 locus is associated with response to anti-tumor necrosis factor therapy in psoriatic arthritis. Pharmacogenomics 15, 1763–1769. doi: 10.2217/pgs.14.125
Jung, S.-H., Yim, S.-H., Hu, H.-J., Lee, K. H., Lee, J.-H., Sheen, D.-H., et al. (2014). Genome-wide copy number variation analysis identifies deletion variants associated with ankylosing spondylitis. Arthritis Rheumatol. 66, 2103–2112. doi: 10.1002/art.38650
Liu, J., Zhu, Q., Han, J., Zhang, H., Li, Y., Ma, Y., et al. (2019). IgG galactosylation status combined with MYOM2-rs2294066 precisely predicts anti-TNF response in ankylosing spondylitis. Mol. Med. 25:25. doi: 10.1186/s10020-019-0093-2
Ma, X.-F., Wang, X.-D., Liu, R.-R., and Luan, Q.-X. (2017). Efficacy research of salazosulfamide in ankylosing spondylitis and NAT1 gene polymorphism. Exp. Ther. Med. 14, 2999–3003. doi: 10.3892/etm.2017.4844
Manolova, I., Ivanova, M., Stoilov, R., Rashkov, R., and Stanilova, S. (2014). Association of single nucleotide polymorphism at position −308 of the tumor necrosis factor-alpha gene with ankylosing spondylitis and rheumatoid arthritis. Biotechnol. Biotechnol. Equip. 28, 1108–1114. doi: 10.1080/13102818.2014.972147
Merola, J. F., Lockshin, B., and Mody, E. A. (2017). Switching biologics in the treatment of psoriatic arthritis. Semin. Arthritis Rheum. 47, 29–37. doi: 10.1016/j.semarthrit.2017.02.001
Modesti, P. A., Reboldi, G., Cappuccio, F. P., Agyemang, C., Remuzzi, G., Rapi, S., et al. (2016). Panethnic differences in blood pressure in europe: a systematic review and meta-analysis. PLoS ONE 11:e0147601. doi: 10.1371/journal.pone.0147601
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Morales-Lara, M. J., Cañete, J. D., Torres-Moreno, D., Hernández, M. V., Pedrero, F., Celis, R., et al. (2012). Effects of polymorphisms in TRAILR1 and TNFR1A on the response to anti-TNF therapies in patients with rheumatoid and psoriatic arthritis. Joint Bone Spine 79, 591–596. doi: 10.1016/j.jbspin.2012.02.003
Morales-Lara, M. J., Conesa-Zamora, P., García-Simón, M. S., Pedrero, F., Santaclara, V., Perez-Guillermo, M., et al. (2010). Association between the FCGR3A V158F polymorphism and the clinical response to infliximab in rheumatoid arthritis and spondyloarthritis patients. Scand. J. Rheumatol. 39, 518–520. doi: 10.3109/03009741003781969
Murdaca, G., Gulli, R., Spanò, F., Lantieri, F., Burlando, M., Parodi, A., et al. (2014). TNF-α gene polymorphisms: association with disease susceptibility and response to anti-TNF-α treatment in psoriatic arthritis. J. Invest. Dermatol. 134, 2503–2509. doi: 10.1038/jid.2014.123
Ni, X., Zhang, W., and Huang, R. S. (2013). Pharmacogenomics discovery and implementation in genome-wide association studies era. Wiley Interdiscip. Rev. Syst. Biol. Med. 5, 1–9. doi: 10.1002/wsbm.1199
Nossent, J. C., Sagen-Johnsen, S., and Bakland, G. (2014). Tumor necrosis factor-α promoter −308/238 polymorphism association with less severe disease in ankylosing spondylitis is unrelated to serum TNF-α and does not predict TNF inhibitor response. J. Rheumatol. 41, 1675–1682. doi: 10.3899/jrheum.131315
Ovejero-Benito, M. C., Muñoz-Aceituno, E., Reolid, A., Fisas, L. H., Llamas-Velasco, M., Prieto-Pérez, R., et al. (2019). Polymorphisms associated with anti-TNF drugs response in patients with psoriasis and psoriatic arthritis. J. Eur. Acad. Dermatol. Venereol. 33, e175–e177. doi: 10.1111/jdv.15431
Perica, M., Vidović, M., Lamot, L., Bukovac, L. T., Kapitanovi,ć, S., Peri,ć, M., et al. (2015). Single nucleotide polymorphism of toll-like receptor 4 (TLR4) is associated with juvenile spondyloarthritis in Croatian population. Clin. Rheumatol. 34, 2079–2086. doi: 10.1007/s10067-015-2952-8
Polo Y La Borda, J., Campos, J., Sanz, J., Andréu, J. L., Mulero, J., and Sánchez, A. (2019). Predictive clinical-genetic model of long-term non-response to tumor necrosis factor-alpha inhibitor therapy in spondyloarthritis. Int. J. Rheum. Dis. 22, 1529–1537. doi: 10.1111/1756-185X.13607
Ramírez, J., Fernández-Sueiro, J. L., López-Mejías, R., Montilla, C., Arias, M., Moll, C., et al. (2012). FCGR2A/CD32A and FCGR3A/CD16A variants and EULAR response to tumor necrosis factor-α blockers in psoriatic arthritis: a longitudinal study with 6 months of followup. J. Rheumatol. 39, 1035–1041. doi: 10.3899/jrheum.110980
Ramonda, R., Lorenzin, M., Carriero, A., Chimenti, M. S., Scarpa, R., Marchesoni, A., et al. (2021). Effectiveness and safety of secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study. RMD Open 7:e001519. doi: 10.1136/rmdopen-2020-001519
Robinson, P. C., Leo, P. J., Pointon, J. J., Harris, J., Cremin, K., Bradbury, L. A., et al. (2016). Exome-wide study of ankylosing spondylitis demonstrates additional shared genetic background with inflammatory bowel disease. NPJ Genomic Med. 1:16008. doi: 10.1038/npjgenmed.2016.8
Rudwaleit, M., Listing, J., Brandt, J., Braun, J., and Sieper, J. (2004). Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann. Rheum. Dis. 63, 665–670. doi: 10.1136/ard.2003.016386
Rudwaleit, M., van der Heijde, D., Landewé, R., Listing, J., Akkoc, N., Brandt, J., et al. (2009). The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783. doi: 10.1136/ard.2009.108233
Sabourin, J. A., Cropp, C. D., Sung, H., Brody, L. C., Bailey-Wilson, J. E., and Wilson, A. F. (2019). ComPaSS-GWAS: a method to reduce type I error in genome-wide association studies when replication data are not available. Genet. Epidemiol. 43, 102–111. doi: 10.1002/gepi.22168
Scardapane, A., Breda, L., Lucantoni, M., and Chiarelli, F. (2012). TNF-α polymorphisms in juvenile idiopathic arthritis: which potential clinical implications? Int. J. Rheumatol. 2012, 756291. doi: 10.1155/2012/756291
Schiotis, R., Sánchez, A., Escudero, A., Bartolom,é, N., Szczypiorska, M., Font, P., et al. (2014). Candidate's single-nucleotide polymorphism predictors of treatment nonresponse to the first anti-TNF inhibitor in ankylosing spondylitis. Rheumatol. Int. 34, 793–801. doi: 10.1007/s00296-013-2913-y
Seitz, M., Wirthmüller, U., Möller, B., and Villiger, P. M. (2007). The −308 tumour necrosis factor-alpha gene polymorphism predicts therapeutic response to TNFalpha-blockers in rheumatoid arthritis and spondyloarthritis patients. Rheumatol. Oxf. Engl. 46, 93–96. doi: 10.1093/rheumatology/kel175
Sokolik, R., Iwaszko, M., Swierkot, J., Wysoczańska, B., Korman, L., Wiland, P., et al. (2021). Relationship between interleukin-6−174G/C genetic variant and efficacy of methotrexate treatment in psoriatic arthritis patients. Pharmacogenomics Pers. Med. 14, 157–166. doi: 10.2147/PGPM.S264555
Song, G. G., Seo, Y. H., Kim, J.-H., Choi, S. J., Ji, J. D., and Lee, Y. H. (2015). Association between TNF-α (−308 A/G,−238 A/G,−857 C/T) polymorphisms and responsiveness to TNF-α blockers in spondyloarthropathy, psoriasis and Crohn's disease: a meta-analysis. Pharmacogenomics 16, 1427–1437. doi: 10.2217/pgs.15.90
Tang, M., and Inman, R. D. (2021). Recent advances on the role of cytotoxic T lymphocytes in the pathogenesis of spondyloarthritis. Semin. Immunopathol. 43, 255–264. doi: 10.1007/s00281-021-00846-z
Taylor, W., Gladman, D., Helliwell, P., Marchesoni, A., Mease, P., Mielants, H., et al. (2006). Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 54, 2665–2673. doi: 10.1002/art.21972
Tong, Q., Zhao, D.-B., Bajracharya, P., Xu, X., Kong, R.-N., Zhang, J., et al. (2012). TNF-α−857 and−1031 polymorphisms predict good therapeutic response to TNF-α blockers in Chinese Han patients with ankylosing spondylitis. Pharmacogenomics 13, 1459–1467. doi: 10.2217/pgs.12.133
Tutuncu, Z., Kavanaugh, A., Zvaifler, N., Corr, M., Deutsch, R., and Boyle, D. (2005). Fcgamma receptor type IIIA polymorphisms influence treatment outcomes in patients with inflammatory arthritis treated with tumor necrosis factor alpha-blocking agents. Arthritis Rheum. 52, 2693–2696. doi: 10.1002/art.21266
van der Heijde, D., Ramiro, S., Landewé, R., Baraliakos, X., Van den Bosch, F., Sepriano, A., et al. (2017). 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991. doi: 10.1136/annrheumdis-2016-210770
van der Heijde, D. M., van't Hof, M. A., van Riel, P. L., Theunisse, L. A., Lubberts, E. W., van Leeuwen, M. A., et al. (1990). Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann. Rheum. Dis. 49, 916–920. doi: 10.1136/ard.49.11.916
van der Linden, S., Valkenburg, H. A., and Cats, A. (1984). Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368. doi: 10.1002/art.1780270401
Wang, Y., Yi, X.-D., and Lu, H.-L. (2017). Influence of CYP2C9 and COX-2 genetic polymorphisms on clinical efficacy of non-steroidal anti-inflammatory drugs in treatment of ankylosing spondylitis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 23, 1775–1782. doi: 10.12659/msm.900271
Wellcome Trust Case Control Consortium Australo-Anglo-American Spondylitis Consortium (TASC), Burton, P. R., Clayton, D. G., Cardon, L. R., Craddock, N., et al. (2007). Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337. doi: 10.1038/ng.2007.17
Wells, G. A., Shea, B., O'Connell, D., Peterson, J., Welch, V., and Losos, M. (2021). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http:// www.ohri.ca/program/clinical_epidemiology/oxford.asp (accessed April 8, 2021).
Xing-Rong, W., Sheng-Qian, X., Wen, L., Shan, Q., Fa-Ming, P., and Jian-Hua, X. (2018). Role of TNFRSF1A and TNFRSF1B polymorphisms in susceptibility, severity, and therapeutic efficacy of etanercept in human leukocyte antigen-B27-positive Chinese Han patients with ankylosing spondylitis. Medicine (Baltimore) 97:e11677. doi: 10.1097/MD.0000000000011677
Xu, Y., Jiang, W., and Zhang, H. (2020). Association between C-reactive protein gene variant and treatment efficacy of etanercept in ankylosing spondylitis patients receiving hip arthroplasty. J. Clin. Lab. Anal. 34:e23343. doi: 10.1002/jcla.23343
Yan, R.-J., Lou, T.-T., Wu, Y.-F., and Chen, W.-S. (2017). Single nucleotide polymorphisms of ABCB1 gene and response to etanercept treatment in patients with ankylosing spondylitis in a Chinese Han population. Medicine (Baltimore) 96:e5929. doi: 10.1097/MD.0000000000005929
Keywords: spondyloarthritis, genes, polymorphism, drug, therapy
Citation: Ortolan A, Cozzi G, Lorenzin M, Galozzi P, Doria A and Ramonda R (2021) The Genetic Contribution to Drug Response in Spondyloarthritis: A Systematic Literature Review. Front. Genet. 12:703911. doi: 10.3389/fgene.2021.703911
Received: 01 May 2021; Accepted: 21 June 2021;
Published: 20 July 2021.
Edited by:
Loredana Bury, University of Perugia, ItalyReviewed by:
Henri-Jean Garchon, Université de Versailles Saint-Quentin-en-Yvelines, FranceCopyright © 2021 Ortolan, Cozzi, Lorenzin, Galozzi, Doria and Ramonda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Ramonda, cm9iZXJ0YS5yYW1vbmRhQHVuaXBkLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.