- 1École de santé publique, Université de Montréal, Montréal, QC, Canada
- 2Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CRCHUM), Montréal, QC, Canada
The strong correlation between adiposity and blood pressure (BP) might be explained in part by shared genetic risk factors. A recent study identified three nucleotide variants [rs16933812 (PAX5), rs7638110 (MRPS22), and rs9930333 (FTO)] associated with both body mass index (BMI) and systolic blood pressure (SBP) in adolescents age 12–18years. We attempted to replicate these findings in a sample of adolescents of similar age. A total of 713 adolescents were genotyped and had anthropometric indicators and blood pressure measured at age 13, 15, 17, and 24years. Using linear mixed models, we assessed associations of these variants with BMI and SBP. In our data, rs9930333 (FTO) was associated with body mass index, but not systolic blood pressure. Neither rs16933812 (PAX5) nor rs7638110 (MRPS22) were associated with body mass index or systolic blood pressure. Although, differences in phenotypic definitions and in genetic architecture across populations may explain some of the discrepancy across studies, nucleotide variant selection in the initial study may have led to false-positive results that could not be replicated.
Introduction
In 2015, high blood pressure (BP) and high body mass index (BMI) were the first and fourth risk factors, respectively, for disability-adjusted life-years (Forouzanfar et al., 2016). BP and adiposity are strongly correlated (Man et al., 2020), and higher adiposity is an important risk factor for elevated BP, both in adults (Jayedi et al., 2018) and adolescents (Chorin et al., 2015). The common variants/multiple disease hypothesis first introduced by Becker (2004) posits that correlation between related traits such as adiposity and BP, may be explained at least in part by shared genetic risk factors. The estimated genetic correlation between 24h ambulatory systolic BP (SBP) and BMI (rg: 0.33; Man et al., 2020) suggests the presence of common genes affecting both traits, but further investigation is needed to identify pleiotropic genes at play.
Melka et al. (2012) identified three single nucleotide polymorphisms (SNPs; rs16933812 on PAX5, rs7638110 on MRPS22 and rs9930333 on FTO) associated with both BMI and SBP in a population-based sample of 598 adolescents age 12–18years in Saguenay-Lac-St-Jean, Quebec, Canada as part of the Saguenay Youth Study (SYS). However, these results have not yet been replicated in an independent sample. Variants rs16933812 (PAX5) and rs7638110 (MRPS22) were newly discovered variants in the SYS but they were not associated with either BP or BMI in recent meta-analyses for these traits (Evangelou et al., 2018; Yengo et al., 2018). FTO is a gene well-known for its association with obesity phenotypes and rs9930333 (FTO) has been associated with BMI in recent adult (Yengo et al., 2018) and child (Vogelezang et al., 2020) meta-analyses, but not with BP (Evangelou et al., 2018).
There is evidence that some BP-associated variants are expressed differently in youth than in adults, highlighting the need for replication of newly discovered findings, specifically in adolescents. A large genome-wide association study (GWAS) reported 31 genetic variants that had age-specific effects on adult BP, one of which had opposite effects in younger vs. older persons (Simino et al., 2014). Some suggest the emergence of novel genetic effects during adolescence, the activation of which is probably related to pubertal changes (Kupper et al., 2006). Others have noted that not all genetic variants identified in adult samples are associated with BP in children (Wang et al., 2015), suggesting that genetic variants have an age-dependent effect on BP. However, genetic association studies for BMI and BP in youth compared to adults are scarce (Albuquerque et al., 2015; Ahn and Gupta, 2018) and rarely replicated.
In the current study, we sought to replicate main findings of Melka et al. (2012) that SNPs on three genes (rs16933812 on PAX5, rs7638110 on MRPS22, and rs9930333 on FTO) are associated with both BMI and SBP. We used data from the Nicotine Dependence In Teens (NDIT) study (O’Loughlin et al., 2014), a longitudinal investigation of adolescents in Montreal, Quebec, Canada followed from age 12 to 24 (i.e., from 1999 to 2012). The NDIT sample was similar in age and geographic location to SYS.
Materials and Methods
Data were drawn from the NDIT study, a longitudinal investigation of the natural course of nicotine dependence across adolescence (O’Loughlin et al., 2014). A total of 1,294 grade 7 students age 12–13years were recruited in 1999–2000 in 10 Montreal-area (Canada) high schools purposively selected to include French and English schools, schools located in rural, urban, and suburban areas, and schools serving socioeconomically diverse neighborhoods. Data was collected in self-administered questionnaires across 23 data collection cycles. Data in cycles 1–20 were collected in grade 7–11 (i.e., the last year of high school in the province of Quebec) every 3months during the 10-month school year over 5years. Post high school data were collected in cycles 21, 22, and 23 when participants were age 20, 24, and 31years on average. A detailed description of study variables collected in self-administered questionnaires including the cycles in which the variable was available, the questionnaire item(s), response choices, whether the questionnaire items used to measure specific variables changed over time and recoding for analysis is available in Supplementary Table 1.
DNA samples were collected from 943 of 1,294 NDIT participants (73%). The 713 participants who were unrelated, of European descent, had at least one BMI or SBP measure available, and whose DNA sample passed genetic quality control comprised the analytical sample. Anthropometric and BP measurements were obtained at cycles 1, 12, 19, 22, and 23 when participants were age 13, 15, 17, 24, and 31years on average, respectively. Analyses excluded cycle 23 to focus on participants with an age range closer to that observed in SYS.
Informed consent was obtained from parents at inception and from participants post-high school when they had attained legal age. Separate consent was obtained for collection of blood and saliva samples. This study was approved by the ethics review committees of the Montréal Department of Public Health, the McGill University Faculty of Medicine, and the Centre de Recherche du Centre Hospitalier de l’Université de Montréal.
Outcome Variables
Trained technicians measured height and weight (Seca Portable Stadiometer – Model 214 and Seca Scale – Model 761, Seca Corporation, Columbia, MD, United States) according to a standardized protocol (Evers and Hooper, 1995). BMI was calculated as weight (kg) divided by height squared (m2). BP was assessed three times at 1-min intervals with an oscillometer (Dinamap XL, CR9340, Critikon Co, Tampa, Fla) calibrated to a mercury sphygmomanometer. SBP was computed as the average of the second and third measurements. Additional information on BMI and SBP measurements, such as how measures were taken, was reported previously (O’Loughlin et al., 2014) and can also be found in Supplementary Text 1.
SNP Genotyping
In addition to the three SNPs associated with both adiposity and SBP in SYS [i.e., rs16933812 (PAX5), rs7638110 (MRPS22), and rs9930333 (FTO)], we also investigated two additional SNPs that were associated with adiposity but not SBP in SYS [i.e., rs7120548 (MTCH2), rs17773430 (MC4R)]. This was performed to further validate that associations with both phenotypes would not be specific to the NDIT sample. Genotyping was performed by Genome Québec using the Illumina Global Screening Array-24 v1.0 (GSA) and imputation was done with Minimac3 software (Das et al., 2016) according to haplotypes from the 1,000 Genomes Phase 3 reference panel (Auton et al., 2015). The SNP rs17773430 (MC4R) was not available within the genotyping array used and was therefore replaced with a proxy, rs111638368 (MC4R), in strong linkage disequilibrium (LD; r2=0.976) in a comparable population to NDIT [i.e., Utah residents with Northern and Western European ancestry (CEU)] in the 1,000 Genomes Project Phase 3 (Machiela and Chanock, 2015). Additional details on the genotyping process and genetic quality control are available in Supplementary Text 2.
In SYS, SNPs rs9930333 (FTO), rs7120548 (MTCH2), and rs111638368 (MC4R) were selected to be tested for their association with SBP based on their strong association with BMI (p < 5×10−4) and because they were in high LD (D′ > 0.90) with at least one of 33 SNPs previously identified in published GWAS for BMI [respectively rs9939609 (FTO), rs3817334 (MTCH2), rs17782313 (MC4R)]. LD measures were calculated in NDIT to examine potential discrepancies with the values observed in SYS (Table 1). Consistent with SYS, high LD was observed in NDIT for the FTO (D′=0.96 for rs9930333 and rs9939609) and MTCH2 SNPs (D′=1.00 for rs7120548 and rs3817334). In contrast, rs111638368 (MC4R) was in low LD with rs17782313 (MC4R) in NDIT (D′=0.33). To assess whether the difference in LD between SYS and NDIT could lead to differences in the strength of associations with BMI and SBP, we included both rs111638368 (MC4R) and rs17782313 (MC4R) in the main analysis. Additionally, LD patterns at both rs16933812 (PAX5) and rs7638110 (MRPS22) locus observed in NDIT were comparable to those observed in SYS (Supplementary Figures 1, 2).
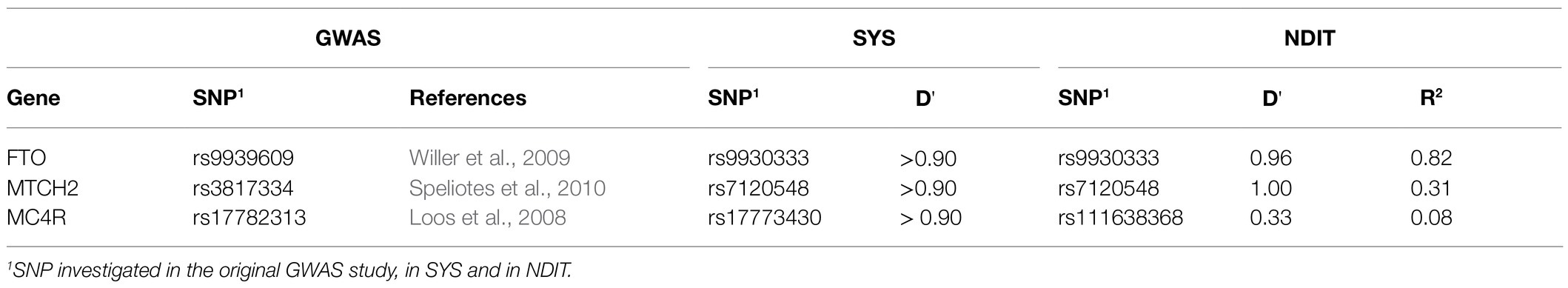
Table 1. Measure of linkage disequilibrium (LD; D') between single nucleotide polymorphisms (SNPs) previously reported in genome-wide association study (GWAS), Melka et al. (2012) and Nicotine Dependence In Teens (NDIT) studies.
Data Analysis
Characteristics of the 713 participants retained in the NDIT sample were described at each cycle. Normally distributed variables were expressed as mean (SD), non-normally distributed variables as medians (IQR), and categorical variables as frequencies.
Associations of each SNP investigated with BMI and SBP were estimated using linear mixed models to account for repeated measures. Models were adjusted for age at baseline, sex, time elapsed since baseline (0, 36, 57, and 144months), and height (SBP only) and a compound symmetry correlation structure was assumed for the error terms. The fit of the models under alternative correlation structures were compared in sensitivity analyses, but the results did not warrant we re-examine our initial selection (Supplementary Table 2; Supplementary Text 3). Genotype was coded according to a genotypic model (i.e., minor allele homozygous, heterozygous, or major allele homozygous) to compare CIs to those estimated in SYS. Because an additive model has more power to detect genetic associations, additive coding was assessed in sensitivity analyses for all SNPs for both BMI and SBP. The analysis of residuals (Supplementary Figures 3–8) did not suggest any important violations of the assumptions of normality, linearity, or homogeneity of variance required for valid inference in the linear mixed models (Jacqmin-Gadda et al., 2007). All statistical analysis were conducted with R statistical software version 3.6.3 (R Core Team, 2019) and all LD calculations and mapping in NDIT were conducted using Haploview v4.2 (Barrett et al., 2004).
Results
Table 2 describes participant characteristics in cycles 1, 12, 19, and 22. There were more females than males across all cycles (54–56%). Mean BMI increased over time from 20.2kg/m2 at cycle 1 to 24.6kg/m2 at cycle 22. Mean BP increased from cycle 1 to 12 but plateaued from cycle 19 to 22. The sample size decreased due to attrition, from 713 in cycle 1 to 601 (84% of 713) in cycle 22. The number of missing values in cycles 1, 12, 19, and 22 are shown in Supplementary Table 3. Compared to participants lost-to-follow-up before cycle 22, those retained were younger, relatively more were female (56 vs. 42%), fewer had ever smoked (29 vs. 39%) and they had a lower BMI (1.2kg/m2) on average at baseline (Supplementary Table 4).
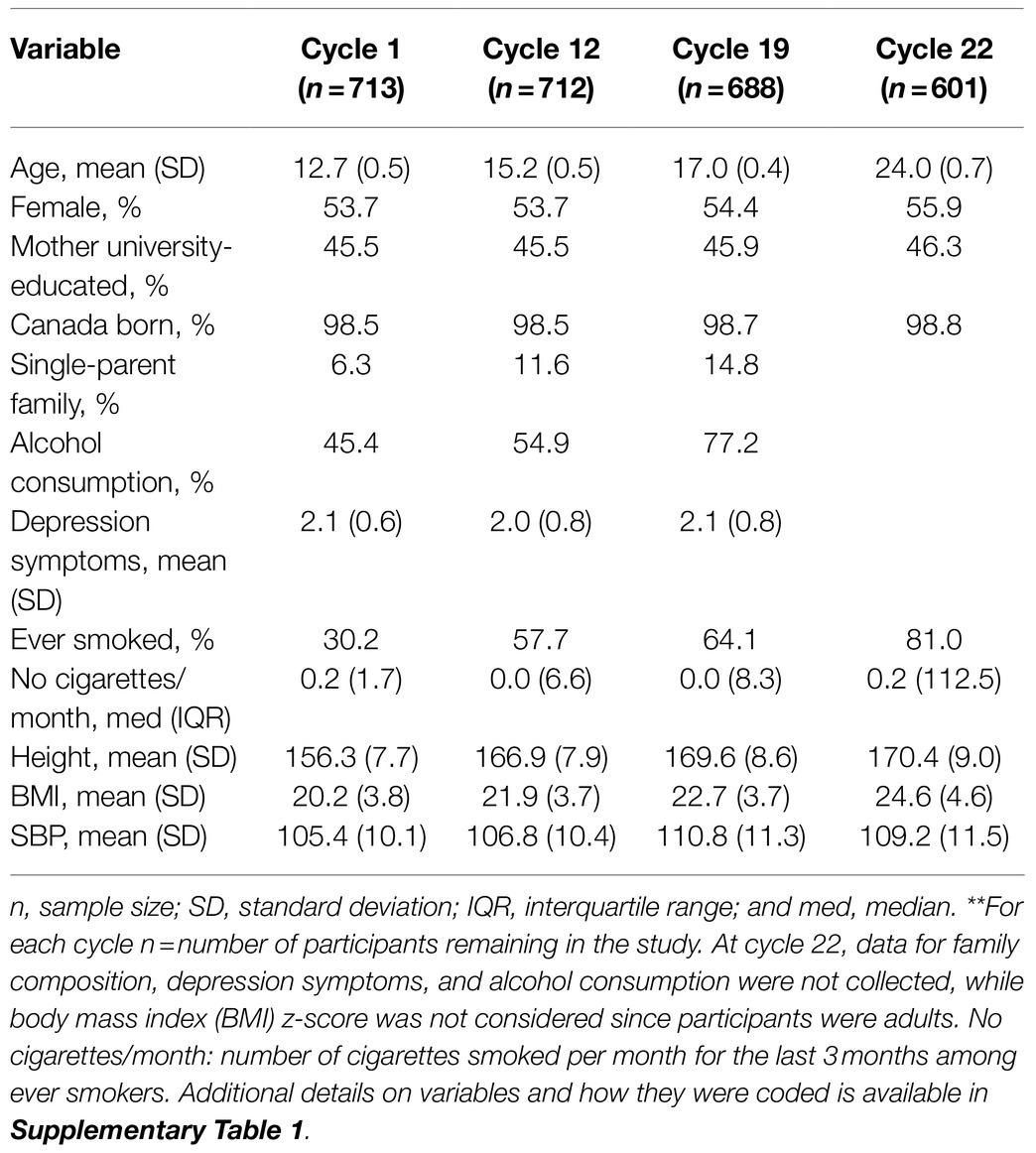
Table 2. Characteristics of analytical sample at cycles 1, 12, 19, and 22, NDIT 1999–2012**.
Table 3 compares SYS results to those obtained in NDIT for BMI and SBP. Minor allele frequencies (MAFs) were similar across studies except for rs16933812 (PAX5), which was higher in NDIT than in SYS (37 vs. 22%). Of the three SNPs associated with both adiposity and SBP in SYS, only rs9930333 (FTO) was significantly associated with BMI in NDIT. The mean BMI difference between minor allele homozygotes and major allele homozygotes for rs9930333 (FTO) was 1.18kg/m2 (95% CI 0.42; 1.94). The mean difference in SBP between minor allele homozygotes and major allele homozygotes for this SNP was not significant [i.e., 0.64mmHg (95% CI −1.08; 2.35)]. Neither rs16933812 (PAX5) nor rs7638110 (MRPS22) were associated with BMI or SBP in NDIT. The estimated difference in BMI for the PAX5 SNP was close to zero with a narrow CI and was in the opposite direction compared to SYS for the MRPS22 SNP. The CIs for the estimated association between both the PAX5 and MRPS22 SNPs with SBP included 0 and both were in the opposite direction in NDIT compared to SYS.
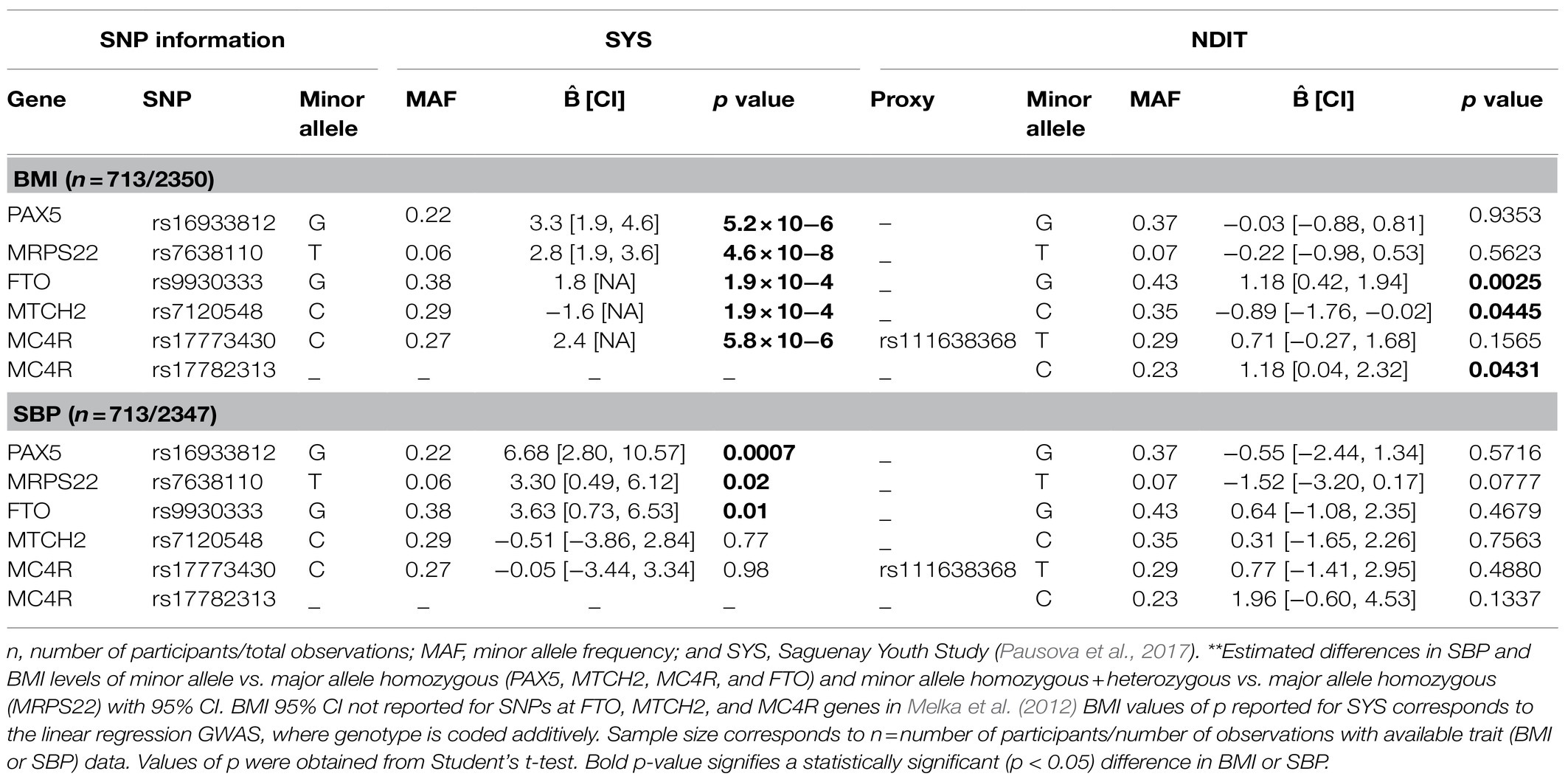
Table 3. Estimated coefficients of the investigated SNPs in their association with BMI and SBP, NDIT 1999–2012 and Melka et al. (2012) 2003–2009**.
Consistent with SYS, SNP rs7120548 (MTCH2) was associated with BMI, but not SBP in NDIT. The mean BMI difference between minor allele homozygotes and major allele homozygotes was −0.89kg/m2 (95% CI −1.76; −0.02) for rs7120548 (MTCH2). The MC4R SNP rs111638368 investigated in SYS was not significantly associated with BMI, while the previously identified MC4R SNP rs17782313 showed a greater difference in BMI than rs111638368 and was statistically significant. The mean BMI difference between minor allele homozygotes and major allele homozygotes was 0.71kg/m2 (95% CI −0.27; 1.68) for rs111638368 (MC4R) and 1.18kg/m2 (95% CI 0.04; 2.32) for rs17782313 (MC4R).
Additional sensitivity analyses were done to verify robustness to the various model specifications used and results are available in Supplementary Text 3, Supplementary Tables 5–9, and Supplementary Figure 9. Overall, these analyses revealed that our results are robust to the genotype coding model, the use of BMI compared to BMI z-score as the adiposity measure, the use of young adults in our analytical sample, and to potential confounding by population stratification. Because BMI is a strong predictor of SBP in youth (Chorin et al., 2015), we re-estimated the SBP models, this time adjusting for BMI. Among the three SNPs associated with SBP in SYS (i.e., FTO, PAX5, and MRPS22), adjusting for BMI did not change the estimated coefficient of the PAX5 SNP, while the estimated coefficients for FTO and MRPS22 decreased toward the null, suggesting that BMI might be an intermediary variable between these two SNPs and SBP.
Discussion
Investigating the genetic underpinnings of adiposity and BP in youth is important because it cannot be assumed that the genetic architecture in adults is applicable in children and adolescents (Wang et al., 2015). Further, investigating genetic susceptibility in youth, who are rarely treated with antihypertensive medication and who are less affected than adults by the cumulative impact of exposure to the environment, might help identify genetic factors that influence both adiposity and BP (Bradfield et al., 2012; Parmar Priyakumari et al., 2016). Consistent with SYS, we replicated the association of rs9930333 on FTO with BMI. This observation aligns with several other studies in children and adolescents that observed an association between SNPs on FTO and adiposity traits, such as BMI (den Hoed et al., 2010; Felix et al., 2016), BMI z-score (Namjou et al., 2013), and obesity (Zhao et al., 2011; Bradfield et al., 2012; da Silva et al., 2018). We did not replicate the associations of SNPs rs16933812 and rs7638110 on PAX5 and MRPS22 with BMI. Additionally, none of the three associations with SBP were replicated. Overall, our results do not support the hypothesis that the variants investigated in Melka et al. (2012) contribute to the correlation between BMI and SBP levels in youth. Potential explanations for the lack of replication are reviewed, including statistical considerations and genetic and phenotypic differences across studies.
Statistical Considerations
First, several genetic associations reported in Melka et al. (2012), could represent spurious findings that cannot be replicated in independent datasets. Two SNPs associated with BMI and SBP in Melka et al. (2012) [i.e., rs16933812 (PAX5), rs7638110 (MRPS22)] were novel discoveries that were not associated with either adiposity or BP in the most recent GWAS for each trait (Evangelou et al., 2018; Yengo et al., 2018). Melka et al. (2012) used the same dataset for discovery and effect estimation, which may lead to overestimation of genetic effects (Göring et al., 2001). Further, Melka et al. (2012) used a two-stage procedure in which several SNPs were first selected conditional on the value of p of their association with BMI, before their association with SBP was assessed. This could also lead to an overestimation of associations because estimated effects are strongly correlated with p-values (Faye et al., 2011). Supporting this, coefficients estimated in SYS for the association of novel SNPs rs16933812 (PAX5) and rs7638110 (MRPS22) on BMI were higher compared to those for known BMI SNPs rs9930333 (FTO), rs7120548 (MTCH2), or rs17773430 (MC4R) and thus, would be expected to have been discovered in previous studies with much larger sample sizes.
Although, some GWAS have detected associations between rs9930333 (FTO) and BP traits (Feitosa et al., 2018; Sung et al., 2018; Takeuchi et al., 2018), the most recent consortiums have not identified such an association (Hoffmann et al., 2017; Evangelou et al., 2018). Inconsistencies between SYS and NDIT for the estimated associations between SNP rs9930333 (FTO) and SBP may be due to the potential role of BMI as an intermediate variable. BMI is a known predictor of SBP in adolescents (Chorin et al., 2015) and thus, in the absence of adjustment for BMI, the association between FTO and SBP could solely capture the indirect effect of FTO on SBP through BMI. In NDIT, the coefficient of association between rs9930333 (FTO) and SBP, although not significant, was reduced to zero after adjusting for BMI (Supplementary Table 6), suggesting that BMI could be an intermediate variable responsible for the association between FTO and SBP in SYS. In another study using SYS participants (Pausova et al., 2009), adjusting for BMI reduced the effect of rs9939609 (FTO) on SBP, although it remained significant. The association between these SNPs on FTO and SBP warrants additional investigation, especially considering the potential role of BMI in the association.
Genetic Differences Across Studies
Second, differences in LD pattern between populations can result in inconsistencies in genetic effects, since most SNPs detected in genetic association studies are not causal for the trait investigated but are in high LD with a causal SNP. Although both samples were of European ancestry, SYS participants comprised adolescents from a founder population in Saguenay-Lac-St-Jean (Pausova et al., 2017), in contrast to the admixed NDIT sample in Montréal, Canada. Founder populations usually show a higher degree of LD than admixed populations (Service et al., 2006). In SYS, rs17773430 (MC4R) was in high LD (D′ ˃ 0.9) with rs17782313 (MC4R), a SNP previously identified for its association with BMI in a GWAS (Loos et al., 2008). However, LD measures between these two SNPs are much lower in the 1,000 Genomes Project Phase 3 [D′=0.31 using the Utah residents with Northern and Western European ancestry (CEU); Machiela and Chanock, 2015], which closely resembled the LD between rs17782313 (MC4R) and a proxy of rs17773430 (MC4R) in NDIT. This suggests that the difference in LD observed between SYS and NDIT likely reflects a population-specific LD pattern in the Saguenay-Lac-St-Jean founder population. This difference in LD likely explains the non-replication of the association between the proxy of rs17773430 (MC4R) and BMI in NDIT, as rs17782313 (MC4R) was associated with BMI in NDIT.
Studies have suggested that physical activity (Kilpeläinen et al., 2011; Young et al., 2016; Graff et al., 2017), alcohol consumption (Young et al., 2016), diet (Young et al., 2016), and sleep duration (Young et al., 2016) modify the associations between variants on FTO and BMI. Also, stronger associations between variants on FTO and SBP has been observed in alcohol users compared to non-users (Feitosa et al., 2018). The strength of the associations estimated in different samples can vary because of gene–environment interactions when the environmental factors vary in their distributions between samples (Schielzeth et al., 2018). Although such differences between SYS and NDIT could not be assessed, the age range in the two samples was similar and further, participants lived in the same geographic location, which limits the likelihood that gene–environment interactions explain the lack of replication.
Differences in Phenotypes Across Studies
Third, differences in BP measurement techniques may contribute to failed replication of the associations between rs16933812 (PAX5), rs7638110 (MRPS22), and rs9930333 (FTO) and SBP. Average BP in SYS was measured in a 52-min protocol including measures at different postures (supine, standing, and sitting) and different levels of stress (during mental stress and during mental stress recovery; Pausova et al., 2017). In NDIT, BP was measured in sitting position only according to a standard protocol (O'Loughlin et al., 2015). Short-term variation in BP in response to position change or mental effort is regulated by multiple factors including sympathetic outflow, cardiopulmonary reflexes, blood vessel elasticity, blood viscosity and hormones, and the genetic architecture of this variation remains largely unknown (Parati et al., 2013). Inconsistencies in associations with SBP across studies may reflect heterogeneity of BP sub-phenotypes related to differences in measurement.
Limitations of this study include lack of power to detect the effect of low MAF SNPs and the potential for selection bias. Specifically, NDIT had less power to detect SNP rs7638110 (MRPS22), which has the MAF of only 7% compared to the other SNPs investigated, which have MAFs over 20%. Although retention in NDIT was good (i.e., 84% of participants at inception were available in cycle 22), loss-to-follow-up could still induce selection bias. Although debated, selection bias can occur in longitudinal genetic association studies when the genetic variants studied are associated with a secondary phenotype which affects retention in the study (Munafò et al., 2018).
In conclusion, we replicated two associations observed in an earlier study of Quebec adolescents of European ancestry between SNPs in the well-established adiposity genes FTO and MTCH2 and BMI. However, we could not replicate SNPs at genes PAX5, MRPS22, or MC4R. None of the SNPs showed an association with SBP. Our findings underscore again that initial discovery of genetic associations should be interpreted cautiously. Since lack of genetic data on youth limits the possibility of large sample size genetic association studies, replication of smaller analyses in comparable study population remains crucial to inform the genetic architecture of youth adiposity and BP.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: NDIT data. The datasets generated and/or analyzed during the current study are not publicly available due to participant confidentiality issues but are available from the Principal Investigator (
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics review committees of the Montréal Department of Public Health, the McGill University Faculty of Medicine, and the Centre de Recherche du Centre Hospitalier de l’Université de Montréal. Informed consent was provided at inception by participants’ legal guardians. Informed consent was provided by participants (who had attained legal age) post high school.
Author Contributions
DG wrote the initial draft, and was responsible for conceptualization, formal analysis, and methodology. JO’L did review and editing. M-PS did review and editing and was responsible for conceptualization, methodology, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Canadian Cancer Research Society (grant numbers 010271 and 017435), the Cancer Research Society (grant number 21098), and the Canadian Institutes for Health Research (364471). M-PS is supported by a Chercheur-Boursier career award from the Fonds de Recherche du Quebec-Santé (FRQS). JO’L holds a Canada Research Chair in the Early Determinants of Adult Chronic Disease. The funders were not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This article is also available in the institutional repository of Université de Montréal as part of a thesis authored by DG for an M.Sc. in Epidemiology degree (Goulet, 2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.690335/full#supplementary-material
Abbreviations
BP, Blood pressure; BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; SNP, Single nucleotide polymorphism; NDIT study, Nicotine dependence in teens study; SYS, Saguenay Youth Study; LD, Linkage disequilibrium; MAF, Minor allele frequency.
References
Ahn, S.-Y., and Gupta, C. (2018). Genetic programming of hypertension. Front. Pediatr. 5:285. doi: 10.3389/fped.2017.00285
Albuquerque, D., Stice, E., Rodríguez-López, R., Manco, L., and Nóbrega, C. (2015). Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol. Gen. Genomics. 290, 1191–1221. doi: 10.1007/s00438-015-1015-9
Auton, A., Abecasis, G. R., Altshuler, D. M., Durbin, R. M., Abecasis, G. R., Bentley, D. R., et al. (2015). A global reference for human genetic variation. Nature 526, 68–74. doi: 10.1038/nature15393
Barrett, J. C., Fry, B., Maller, J., and Daly, M. J. (2004). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265. doi: 10.1093/bioinformatics/bth457
Becker, K. G. (2004). The common variants/multiple disease hypothesis of common complex genetic disorders. Med. Hypotheses 62, 309–317. doi: 10.1016/S0306-9877(03)00332-3
Bradfield, J. P., Taal, H. R., Timpson, N. J., Scherag, A., Lecoeur, C., Warrington, N. M., et al. (2012). A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 44, 526–531. doi: 10.1038/ng.2247
Chorin, E., Hassidim, A., Hartal, M., Havakuk, O., Flint, N., Ziv-Baran, T., et al. (2015). Trends in adolescents obesity and the association between BMI and blood pressure: a cross-sectional study in 714,922 healthy teenagers. Am. J. Hypertens. 28, 1157–1163. doi: 10.1093/ajh/hpv007
da Silva, T. E. R., Andrade, N. L., Cunha, D. D. O., Leão-Cordeiro, J. A. B., Vilanova-Costa, C. A. S. T., and Silva, A. M. T. C. (2018). The FTO rs9939609 polymorphism and obesity risk in teens: evidence-based meta-analysis. Obes. Res. Clin. Pract. 12, 432–437. doi: 10.1016/j.orcp.2018.08.001
Das, S., Forer, L., Schönherr, S., Sidore, C., Locke, A. E., Kwong, A., et al. (2016). Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287. doi: 10.1038/ng.3656
den Hoed, M., Ekelund, U., Brage, S., Grontved, A., Zhao, J. H., Sharp, S. J., et al. (2010). Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 59, 2980–2988. doi: 10.2337/db10-0370
Evangelou, E., Warren, H. R., Mosen-Ansorena, D., Mifsud, B., Pazoki, R., Gao, H., et al. (2018). Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 50, 1412–1425. doi: 10.1038/s41588-018-0205-x
Evers, S. E., and Hooper, M. D. (1995). Dietary intake and anthropometric status of 7 to 9 year old children in economically disadvantaged communities in Ontario. J. Am. Coll. Nutr. 14, 595–603. doi: 10.1080/07315724.1995.10718548
Faye, L. L., Sun, L., Dimitromanolakis, A., and Bull, S. B. (2011). A flexible genome-wide bootstrap method that accounts for rankingand threshold-selection bias in GWAS interpretation and replication study design. Stat. Med. 30, 1898–1912. doi: 10.1002/sim.4228
Feitosa, M. F., Kraja, A. T., Chasman, D. I., Sung, Y. J., Winkler, T. W., Ntalla, I., et al. (2018). Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLoS One 13:e0198166. doi: 10.1371/journal.pone.0198166
Felix, J. F., Bradfield, J. P., Monnereau, C., van der Valk, R. J. P., Stergiakouli, E., Chesi, A., et al. (2016). Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25, 389–403. doi: 10.1093/hmg/ddv472
Forouzanfar, M. H., Afshin, A., Alexander, L. T., Anderson, H. R., Bhutta, Z. A., Biryukov, S., et al. (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388, 1659–1724. doi: 10.1016/S0140-6736(16)31679-8
Göring, H. H., Terwilliger, J. D., and Blangero, J. (2001). Large upward bias in estimation of locus-specific effects from genomewide scans. Am. J. Hum. Genet. 69, 1357–1369. doi: 10.1086/324471
Goulet, D. (2020). Variants génétiques associés à l’adiposité et à la pression artérielle: Une réplication [In french, master's thesis]. Université de Montréal.
Graff, M., Scott, R. A., Justice, A. E., Young, K. L., Feitosa, M. F., Barata, L., et al. (2017). Genome-wide physical activity interactions in adiposity — A meta-analysis of 200,452 adults. PLoS Genet. 13:e1006528. doi: 10.1371/journal.pgen.1006528
Hoffmann, T. J., Ehret, G. B., Nandakumar, P., Ranatunga, D., Schaefer, C., Kwok, P.-Y., et al. (2017). Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat. Genet. 49, 54–64. doi: 10.1038/ng.3715
Jacqmin-Gadda, H., Sibillot, S., Proust, C., Molina, J.-M., and Thiébaut, R. (2007). Robustness of the linear mixed model to misspecified error distribution. Comput. Stat. Data Anal. 51, 5142–5154. doi: 10.1016/j.csda.2006.05.021
Jayedi, A., Rashidy-Pour, A., Khorshidi, M., and Shab-Bidar, S. (2018). Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose–response meta-analysis of more than 2.3 million participants. Obes. Rev. 19, 654–667. doi: 10.1111/obr.12656
Kilpeläinen, T. O., Qi, L., Brage, S., Sharp, S. J., Sonestedt, E., Demerath, E., et al. (2011). Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 8:e1001116. doi: 10.1371/journal.pmed.1001116
Kupper, N., Ge, D., Treiber Frank, A., and Snieder, H. (2006). Emergence of novel genetic effects on blood pressure and hemodynamics in adolescence. Hypertension 47, 948–954. doi: 10.1161/01.HYP.0000217521.79447.9a
Loos, R. J. F., Lindgren, C. M., Li, S., Wheeler, E., Zhao, J. H., Prokopenko, I., et al. (2008). Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40, 768–775. doi: 10.1038/ng.140
Machiela, M. J., and Chanock, S. J. (2015). LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557. doi: 10.1093/bioinformatics/btv402
Man, T., Nolte, I. M., Jaju, D., Al-Anqoudi, Z. A. M., Muñoz, M. L., Hassan, M. O., et al. (2020). Heritability and genetic correlations of obesity indices with ambulatory and office beat-to-beat blood pressure in the Oman Family study. J. Hypertens. 38, 1474–1480. doi: 10.1097/HJH.0000000000002430
Melka, M. G., Bernard, M., Mahboubi, A., Abrahamowicz, M., Paterson, A. D., Syme, C., et al. (2012). Genome-wide scan for loci of adolescent obesity and their relationship with blood pressure. J. Clin. Endocrinol. Metab. 97, E145–E150. doi: 10.1210/jc.2011-1801
Munafò, M. R., Tilling, K., Taylor, A. E., Evans, D. M., and Davey Smith, G. (2018). Collider scope: when selection bias can substantially influence observed associations. Int. J. Epidemiol. 47, 226–235. doi: 10.1093/ije/dyx206
Namjou, B., Keddache, M., Marsolo, K., Wagner, M., Lingren, T., Cobb, B., et al. (2013). EMR-linked GWAS study: investigation of variation landscape of loci for body mass index in children. Front. Genet. 4:268. doi: 10.3389/fgene.2013.00268
O’Loughlin, J., Dugas, E. N., Brunet, J., DiFranza, J., Engert, J. C., Gervais, A., et al. (2014). Cohort profile: The nicotine dependence in teens (NDIT) study. Int. J. Epidemiol. 44, 1537–1546. doi: 10.1093/ije/dyu135
O'Loughlin, J., Dugas, E. N., Brunet, J., DiFranza, J., Engert, J. C., Gervais, A., et al. (2015). Cohort profile: the nicotine dependence in teens (NDIT) study. Int. J. Epidemiol. 44, 1537–1546. doi: 10.1093/ije/dyu135
Parati, G., Ochoa, J. E., Lombardi, C., and Bilo, G. (2013). Assessment and management of blood-pressure variability. Nat. Rev. Cardiol. 10, 143–155. doi: 10.1038/nrcardio.2013.1
Parmar Priyakumari, G., Taal, H. R., Timpson Nicholas, J., Thiering, E., Lehtimäki, T., Marinelli, M., et al. (2016). International genome-wide association study consortium identifies novel loci associated with blood pressure in children and adolescents. Circ. Cardiovasc. Genet. 9, 266–278. doi: 10.1161/CIRCGENETICS.115.001190
Pausova, Z., Paus, T., Abrahamowicz, M., Bernard, M., Gaudet, D., Leonard, G., et al. (2017). Cohort profile: the Saguenay Youth Study (SYS). Int. J. Epidemiol. 46, e19–e19. doi: 10.1093/ije/dyw023
Pausova, Z., Syme, C., Abrahamowicz, M., Xiao, Y., Leonard Gabriel, T., Perron, M., et al. (2009). A common variant of the FTO gene is associated With not only increased adiposity but also elevated blood pressure in French Canadians. Circ. Cardiovasc. Genet. 2, 260–269. doi: 10.1161/CIRCGENETICS.109.857359
R Core Team (2019). “R: A Language and Environment for Statistical Computing”. Vienna, Austria: R Foundation for Statistical Computing.
Schielzeth, H., Rios Villamil, A., and Burri, R. (2018). Success and failure in replication of genotype–phenotype associations: how does replication help in understanding the genetic basis of phenotypic variation in outbred populations? Mol. Ecol. Resour. 18, 739–754. doi: 10.1111/1755-0998.12780
Service, S., DeYoung, J., Karayiorgou, M., Roos, J. L., Pretorious, H., Bedoya, G., et al. (2006). Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat. Genet. 38, 556–560. doi: 10.1038/ng1770
Simino, J., Shi, G., Bis, J. C., Chasman, D. I., Ehret, G. B., Gu, X., et al. (2014). Gene-age interactions in blood pressure regulation: a large-scale investigation with the CHARGE, global BPgen, and ICBP consortia. Am. J. Hum. Genet. 95, 24–38. doi: 10.1016/j.ajhg.2014.05.010
Speliotes, E. K., Willer, C. J., Berndt, S. I., Monda, K. L., Thorleifsson, G., Jackson, A. U., et al. (2010). Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948. doi: 10.1038/ng.686
Sung, Y. J., Winkler, T. W., de las Fuentes, L., Bentley, A. R., Brown, M. R., Kraja, A. T., et al. (2018). A large-scale multi-ancestry genome-wide study accounting for smoking behavior identifies multiple significant loci for blood pressure. Am. J. Hum. Genet. 102, 375–400. doi: 10.1016/j.ajhg.2018.01.015
Takeuchi, F., Akiyama, M., Matoba, N., Katsuya, T., Nakatochi, M., Tabara, Y., et al. (2018). Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat. Commun. 9:5052. doi: 10.1038/s41467-018-07345-0
Vogelezang, S., Bradfield, J. P., Ahluwalia, T. S., Curtin, J. A., Lakka, T. A., Grarup, N., et al. (2020). Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16:e1008718. doi: 10.1371/journal.pgen.1008718
Wang, X., Xu, X., Su, S., and Snieder, H. (2015). Familial aggregation and childhood blood pressure. Curr. Hypertens. Rep. 17, 509–509. doi: 10.1007/s11906-014-0509-x
Willer, C. J., Speliotes, E. K., Loos, R. J. F., Li, S., Lindgren, C. M., Heid, I. M., et al. (2009). Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34. doi: 10.1038/ng.287
Yengo, L., Sidorenko, J., Kemper, K. E., Zheng, Z., Wood, A. R., Weedon, M. N., et al. (2018). Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649. doi: 10.1093/hmg/ddy271
Young, A. I., Wauthier, F., and Donnelly, P. (2016). Multiple novel gene-by-environment interactions modify the effect of FTO variants on body mass index. Nat. Commun. 7, 12724–12724. doi: 10.1038/ncomms12724
Keywords: adiposity, blood pressure, genetic association study, adolescence, replication
Citation: Goulet D, O’Loughlin J and Sylvestre M-P (2021) Association of Genetic Variants With Body-Mass Index and Blood Pressure in Adolescents: A Replication Study. Front. Genet. 12:690335. doi: 10.3389/fgene.2021.690335
Edited by:
Hui-Qi Qu, Children's Hospital of Philadelphia, United StatesReviewed by:
Masayuki Okuda, Yamaguchi University, JapanAsuman Turkmen, The Ohio State University, United States
Copyright © 2021 Goulet, O’Loughlin and Sylvestre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Pierre Sylvestre, bWFyaWUtcGllcnJlLnN5bHZlc3RyZUB1bW9udHJlYWwuY2E=
 Danick Goulet
Danick Goulet Jennifer O’Loughlin
Jennifer O’Loughlin Marie-Pierre Sylvestre
Marie-Pierre Sylvestre