
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 26 August 2021
Sec. Livestock Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.671235
 Muhammad Saif-ur Rehman1,2†
Muhammad Saif-ur Rehman1,2† Saif ur Rehman1†
Saif ur Rehman1† Wasim Yousaf2
Wasim Yousaf2 Faiz-ul Hassan2
Faiz-ul Hassan2 Waqas Ahmad3
Waqas Ahmad3 Qingyou Liu1*
Qingyou Liu1* Hongping Pan1*
Hongping Pan1*Toll-like receptors (TLRs) are pathogen recognition receptors, and primitive sources of innate immune response that also play key roles in the defense mechanism against infectious diseases. About 10 different TLRs have been discovered in chicken that recognize ligands and participate in TLR signaling pathways. Research findings related to TLRs revealed new approaches to understand the fundamental mechanisms of the immune system, patterns of resistance against diseases, and the role of TLR-specific pathways in nutrient metabolism in chicken. In particular, the uses of specific feed ingredients encourage molecular biologists to exploit the relationship between nutrients (including different phytochemicals) and TLRs to modulate immunity in chicken. Phytonutrients and prebiotics are noteworthy dietary components to promote immunity and the production of disease-resistant chicken. Supplementations of yeast-derived products have also been extensively studied to enhance innate immunity during the last decade. Such interventions pave the way to explore nutrigenomic approaches for healthy and profitable chicken production. Additionally, single-nucleotide polymorphisms in TLRs have shown potential association with few disease outbreaks in chickens. This review aimed to provide insights into the key roles of TLRs in the immune response and discuss the potential applications of these TLRs for genomic and nutritional interventions to improve health, and resistance against different fatal diseases in chicken.
Innate and adaptive immunity have been considered as largely separate through complementary mechanisms of defense against microbes (Tipping, 2006). Both immune systems distinguish foreign organisms as non-self and trigger the corresponding defence action. Specifically, antigen receptors on lymphocytes are key feature of adaptive immunity, while innate immunity relies on antigen presenting cells (APCs) and phagocytic cells including dendritic cells (DCs), granulocytes, and macrophages (Tate et al., 2010). Macrophages and DCs belong to the innate immune cells that are activated by microbial components, such as lipopolysaccharide (LPS) of Gram-negative bacteria (Tate et al., 2010). Macrophages perform functions, such as phagocytosis, production of chemokines and cytokine, secretion of antimicrobial peptides, and antigen presentation (Parihar et al., 2010).
Toll-like receptors (TLRs) are a group of pattern recognition receptors (PRRs) and are also the main components of innate immunity (Kawai and Akira, 2010). These receptors provide protection against a wide range of pathogens (Zhang and Liang, 2016). TLRs modulate signaling pathways in the host defence system to control the infection and repair damaged cells (Wang et al., 2016). Agonists/ligands are specialized structural motifs present on microbes that activate the macrophages upon binding with the corresponding TLRs. The binding of specific ligands to TLRs activates various adaptor proteins, transcriptional factors, and stimulates cytokine genes (Kawasaki and Kawai, 2014). These cytokines increase inflammatory responses and protect against various diseases (Bresnahan and Tanumihardjo, 2014). There are multiple factors that can regulate the functions of TLRs including genetic polymorphism and nutrients (Vidya et al., 2017; El-Zayat et al., 2019a).
Genetic associations among different TLRs can help scientists to study the genetic potential and prevention of infectious diseases in birds. To date, 10 TLRs genes have been characterized in chicken (Roach et al., 2005; Yilmaz et al., 2005; Iqbal et al., 2005b; Higgs et al., 2006). Variations in the sequence of TLRs change the recognition patterns of PAMPs and modify the host resistance against pathogenic infections (Ruan and Zheng, 2011; Ruan et al., 2015). TLRs polymorphism can play a crucial role as a genetic marker for the selective breed improvement programs in chicken. This literature review highlights the key roles of TLRs in the immune mechanisms, and further describes potential applications of these TLRs for genomic and nutritional interventions to improve health and resistance against different infectious diseases in chicken.
Phagocytic recognition of invading microbes activates inflammatory reactions against these pathogens via a set of PRRs including the scavenger receptors, TLRs, complement receptors, integrins, and members of the C-type lectin receptor family. These germline receptors are specialized structures that change over time to identify specific motifs on pathogens that are absent in higher-order eukaryotes, and these motifs control the invading pathogens (Aderem and Smith, 2004). TLRs are conserved transmembrane proteins of the PRRs family and categorized by the presence of an extracellular domain, which further contains LRR and a cytoplasmic domain (TIR domain; Akira et al., 2006).
PAMPs have usually been found invariant which also help pathogens in survival and support to maintain divergent feature from “self” (Haunshi and Cheng, 2014). Recognition of “alert signals” in the host during aberrant localization or presence of inflammatory molecules or different cellular stress is mostly assisted by PRRs (Beg, 2002). Upon recognition by PAMPs, PRRs indicate the presence of infectious agents either by presentation at the cell surface or activate antimicrobial and proinflammatory responses through a signal to the host immune system (Figure 1). This mechanism further elicits several intracellular signaling pathways, such as kinases, transcription factors, and adaptor molecules (Akira and Takeda, 2004). The chicken TLRs (chTLRs) also recognize specific components of pathogens (agonist) as shown in Table 1.
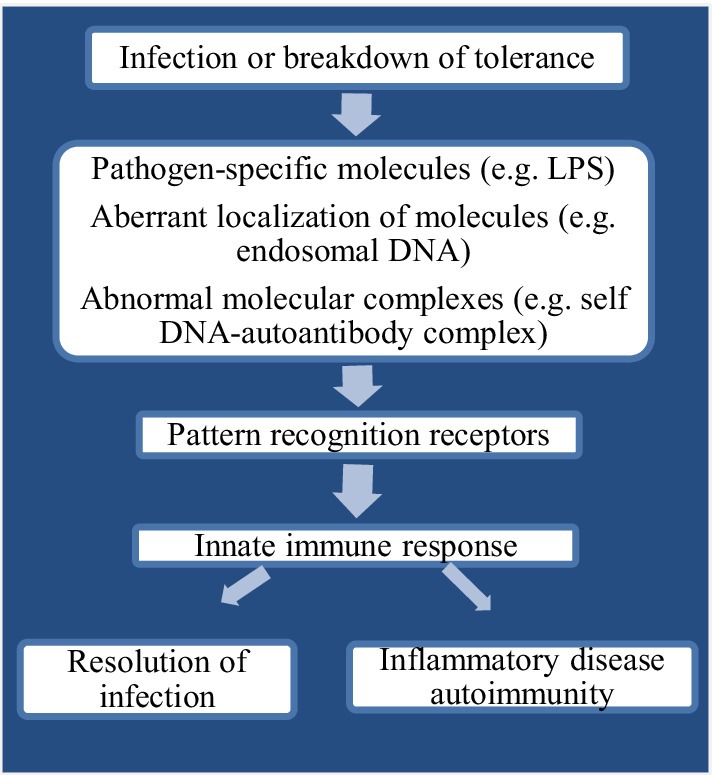
Figure 1. Flow chart diagram showing innate immune recognition through pattern recognition receptors (PRRs).
First TLR was identified in Drosophila (Chu and Mazmanian, 2013). Later on, the regulation of innate immunity was also observed in fungal infections (Plato et al., 2015). TLR is a primitive pathogen scrutiny system and widespread in animals and plants. At present, 13 receptors have been recognized in mammals, but TLR 10, 11, and 13 have exhibited specie specific gene expression (Leulier and Lemaitre, 2008). Many vertebrate contains 10 to 13 TLRs (Roach et al., 2005), e.g., humans carry TLR1-10 while TLR1-13 are present in mice. The chTLRs gene expression has demonstrated their conserved nature during evolution. At least 10 diverse TLRs have been recognized in chicken so far, but they express differently in contrast to mammals as shown in Table 1 (Brownlie and Allan, 2011). These chTLRs are originated from gene duplication, a phenomenon known as paralogs (Roach et al., 2005), and these paralogs are further classified into six major groups through phylogenetic analysis; TLR2 cluster (include TLRs 1, 2, 6, 10, and 14), TLR3, TLRs4, TLRs5, TLRs7/8/9, and lastly, TLR11 cluster that contains TLRs 11, 12, 13, 21, 22, and 23, respectively.
ChTLRs are slightly different from other vertebrates due to the presence of the pseudogene TLR8, chTLR1LA, chTLR1LB, chTLR15 and chTLR2, and the absence of TLR9 (Brownlie and Allan, 2011). Explicitly, orthologs to mammals have been characterized in chicken, such as for mTLR3, mTLR4, mTLR5, and mTLR7, whereas chicken lacks TLR8/9. However, mTLR1, mTLR6, and mTLR10 are exchanged with TLR1A and B, while TLR2A and TLR2B are replaced with TLR2 because of gene doubling. TLR15 and TLR21 are the two additional chTLRs. Avian species have distinct TLR15, which is stimulated after the invasion of bacterial proteases and virulent fungi (Iqbal et al., 2005a; Yilmaz et al., 2005; Temperley et al., 2008; de Zoete et al., 2010), while TLR21 is discrete from mTLR9 which has chief orthologs in amphibians and fish (Brownlie et al., 2009).
The primary function of TLRs during innate immune actions is similar in both birds and mammalian species, and APCs surface assists TLRs for their expression. TLRs identify PAMPs and establish signaling to the innate immune response by stimulating the reactive oxygen and nitrogen intermediates. APCs stimulate the upregulation of co-stimulatory molecules and pro-inflammatory cytokines (Takeda and Akira, 2005). APCs also play a role of junction between innate and adaptive immunity in pathway stimulated by TLRs. Although, peripheral tissues have immature DCs that are considered as affinity sites for microbial invasion (Mbongue et al., 2014), stimulation of TLRs results in the maturation of immature DCs that not only regulate major histocompatibility complex molecules and co-stimulatory molecules, but also boost lymphoid organs to stimulate T cells for antigen activation. Cellular activation is controlled by special receptors “chemokine” which are activated by DCs and upregulate TLRs (Cutler et al., 2001).
The chTLRs stimulate multiple pro-inflammatory cytokines through monocytes like interleukin macrophage inflammatory protein-1β, IL-6, IL-1β, and IL-8 and these cytokines have innate immune and inflammatory responses (He et al., 2011). The first step in TLR signaling is the myeloid differentiation protein 88 (MyD88)-dependent pathway which in return activates mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), following the production of inflammatory chemokines and cytokines. The second pathway is MyD88-independent that stimulates various factors including interferon-inducible genes, type-1 interferons (IFN) as shown in Figure 2 (Takeda and Akira, 2005; Kawai and Akira, 2006). The expression of antimicrobial peptides is due to the TLR signaling pathway and these are the major molecules of innate immune systems. Taken together, these observations explain the potential role of TLRs to modulate innate and adaptive immunity, and this intrinsic property of TLRs can be used as adjuvants in vaccines (Birchler et al., 2001).
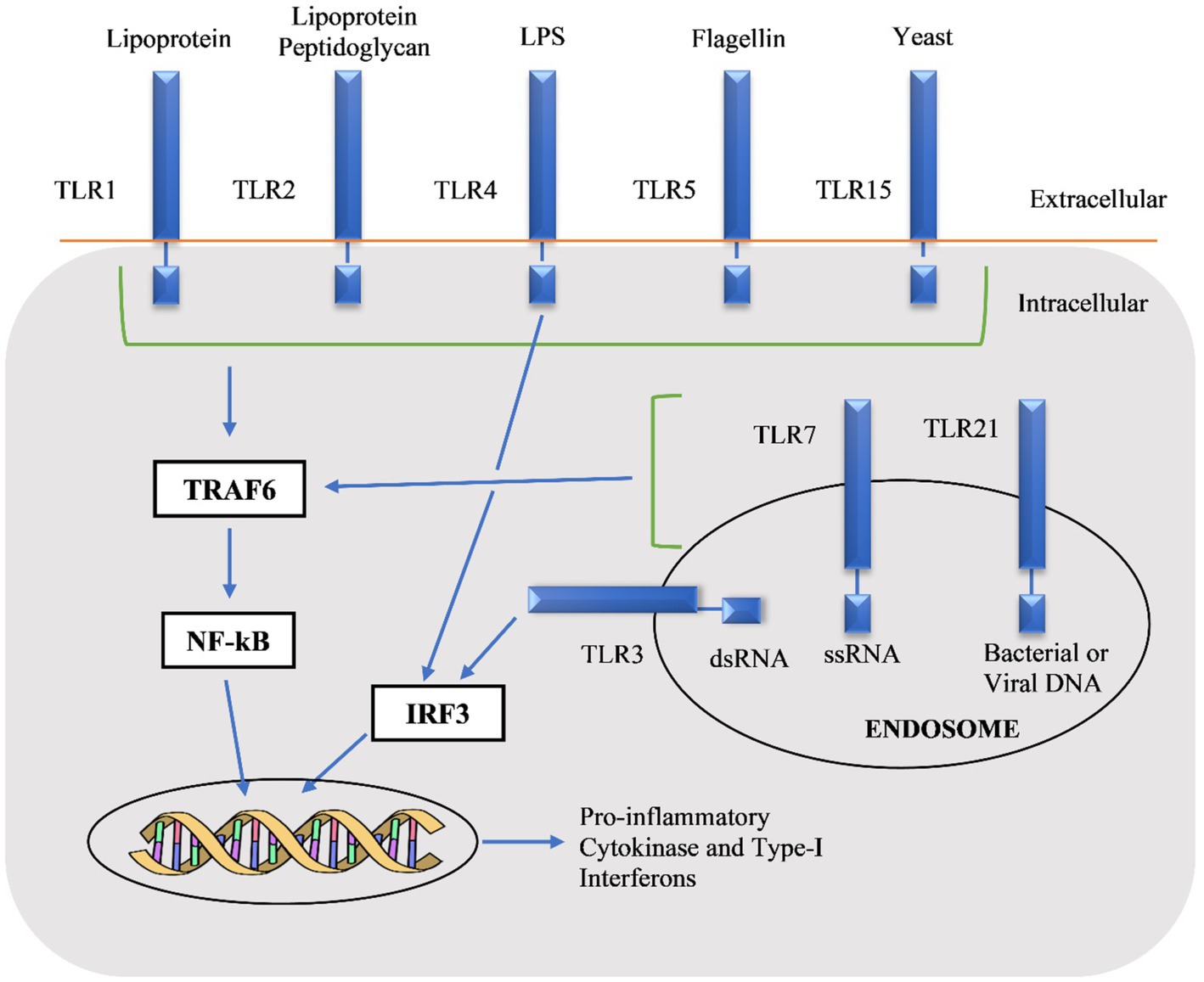
Figure 2. Pathways of chicken toll-like receptors (chTLRs) by recognition of their agonists (Gupta et al., 2014).
The chTLRs expression has been studied against several pathogens and their association was found with disease resistance. The most important chTLRs are discussed below:
The chTLR2 was reported for the first time in 2001 and grouped into two types, TLR2 type 1 and type 2 located on chromosome 4; TLR1 is also classified in a similar manner (Fukui et al., 2001; Yilmaz et al., 2005), and chTLR2 type 1 and 2 are analogous to hTLR2, perhaps due to gene duplication (Plato et al., 2015). The expression pattern of chTLR2 revealed that it is only expressed in caecal tonsils, spleen, liver, bursa, B cells, CD8+ cells, and heterophils (Iqbal et al., 2005a; Kogut et al., 2008). A number of ligands bind to chTLR2 because of heterodimerization of chTLR2 homologs with TLR1 isoforms, and these isoformic properties of TLRs initiate activity to other agonists. Therefore, chTLR2/TLR1 heterodimerization upregulates the activity against synthetic tri-acylated lipopeptide in chicken, whereas chTLR2-1/TLR1-2 heterodimerization activates upon binding with peptidoglycans (Keestra et al., 2007; Higuchi et al., 2008). The TLR2 upregulates initial response of cytokine in host cells upon recognition of M. pulmonis (Love et al., 2010). ChTLR2 response was significantly highest in Hela 57A against Campylobacter spp. (p<0.05) when treated with lysed Campylobacter (de Zoete et al., 2010). In addition, TLR2 members were also associated with Clostridium perfringens and their expression was upregulated in the spleen. Moreover, the combined gene expression levels of TLR2.2 and TLR1.1 were also higher which also suggested the role of TLR1 in the regulation of innate host response (Lu et al., 2009).
The chTLR3 is located on chromosome 4 (Yilmaz et al., 2005) and expressed in the thymus, duodenum, ileum, jejunum, colon, caecum, B cells, T cells, and heterophils (Iqbal et al., 2005a). In chicken, it binds with polyinosinic polycytidylic [Poly (I:C)] acid (He et al., 2007, 2012; Schwarz et al., 2007). The said acid is a double-stranded RNA (dsRNA) homolog that induces stimulation of IL-10, IFN-α, IFN-γ, and IFN-β similar to mammals. However, it produces a scanty amount of nitric oxide in monocytes and also decreases the activation of pro-inflammatory cytokines (He et al., 2012).
ChTLR4 recognizes a major structural component of the cell wall of Gram-negative bacteria which is known as LPS and stimulates the immune system. Upon interaction with the extracellular proteins, LPS makes a complex of myeloid differentiation protein 2 (MD2), cluster of differentiation 14 (CD14), and TLR4 (Oshiumi et al., 2003). TLR4 and MD2 have 43 and 31% similarity with protein orthologs present in humans, respectively, and both were cloned in HeLa cells, and their expression profiles are noteworthy (Keestra et al., 2007). LPS binds with TLR4 to induce a cascade of signaling pathways following activation of NF-κB that results in pro-inflammatory chemokines and cytokines production (Fukui et al., 2001). TLR4 signaling also produces many chemokines, cytokines, IL-6, IL-8, and IL-1β upon invasion of viral and bacterial pathogens (Heggen et al., 2000; Kaiser et al., 2000; Laurent et al., 2001). ChTLR4 has a range of expression pattern, but its genetic expression level is higher in heterophils and macrophages (Leveque et al., 2003; Kogut et al., 2005; Iqbal et al., 2005a).
ChTLR5 is activated by bacterial flagellin and plays a key function in host resistance to pathogenic bacteria (Hayashi et al., 2001; Wlasiuk et al., 2009). It is located on chromosome 3 and possesses almost 50% homology with hTLR5 at amino acid level (Iqbal et al., 2005a,b). Upregulated gene expression of chTLR5 has been observed in a few tissues that induces pro-inflammatory responses. Flagellin-based stimulation of Stromal and HD11 cells results in the production of IL1-β (Iqbal et al., 2005b). In heterophils, the expression levels of inflammatory chemokines and IL6 are increased upon binding with LPS/flagellin (Kogut et al., 2008). TLR5 expresses in HeLa cells and activates NF-κB when treated with purified flagellin or Salmonella enterica serovar Enteritidis (Keestra et al., 2008). ChTLR5-mediated molecular mechanisms may contribute to lung infection and cause acute respiratory syndrome (Zhang et al., 2012). However, few bacteria like Campylobacter Jejuni do not carry TLR5-binding sites that make chTLR5 sense them because of variation in flagellin (Paul et al., 2013).
ChTLR7 is alternatively spliced that owes 63% similarity with chTLR7 at amino acid level and expressed in the forms of three different transcripts to produce two types of proteins (Philbin et al., 2005). ChTLR7 is expressable in a number of cells/tissues likewise other TLRs (Iqbal et al., 2005a; Philbin et al., 2005). Single-stranded RNA has been identified as a ligand for TLR7 which is controlled by a gene similar to TLR8 (Kawai and Akira, 2010). RNA ligands stimulate splenocytes and HD11 cells that cause activation of IL-1β, IL-6, IL-8, and cytokines. This stimulation of cytokines is important for endosomal acidification due to chloroquine sensitivity (Kogut et al., 2005; He et al., 2006). Another research found contradictory results for TLR7 agonist loxoribine that downregulated pro-inflammatory cytokines (Kogut et al., 2005) and it was a contradictory observation reported for neutrophils in humans (Hayashi et al., 2003). A research report described that infection of avian influenza in chicken macrophages exhibited significant upregulation of TLR7 gene expression initially (Kawai and Akira, 2010), but the gene expression decreased later on.
The expression of TLRs mRNA is not limited to the tissues involved in the immune system including spleen, thymus, tonsils, lymphatic vessels, and lymph nodes, but it is also found in the peripheral blood leukocytes, vital organs, pancreas, colon, small intestine, ovary, placenta, testis, prostate, and skeletal muscles (Zarember and Godowski, 2002). Moreover, the gene expression of TLRs has also been observed in various cells of the immune system including macrophages, NK cells, DCs, circulating leukocytes, adaptive immune cells, and non-immune cells like epithelial and endothelial cells and fibroblasts (Delneste et al., 2007). All the 10 TLRs are found on chicken heterophils and can be functionally activated in vitro with either TLR agonists or intact bacterial cells (Kogut et al., 2005, 2006, 2008). A broader and enriched expression pattern of TLRs has been observed in heterophils which suggests that heterophils might play a major role as first-line effector cells through TLR-induced signaling pathway (Kogut et al., 2005, 2006). In addition, TLRs are bridging molecules since they are also expressed on DCs that join innate and adaptive immunity (Gao et al., 2017). This is the reason that the induction of innate immunity by TLRs further stimulates adaptive immunity (Akira and Takeda, 2004). TLRs recognize the pathogen and convey the signal to APCs which kill the microbes by phagocytosis (Moresco et al., 2011; Sharma et al., 2013). Moreover, the presence of a total group of TLRs on immature DCs assists in their maturation process (Re and Strominger, 2011). The DCs initiate the immune response in the chicken gut by the production of cytokines and stimulate other immune competent cells. Different feed additives like phytochemicals have shown the potential to mediate the functions of DCs through both direct and indirect ways to effectively modulate the immune response (Teng and Kim, 2018).
The dynamic expression of TLRs may indicate the chances of genetic modulation and production of enhanced immunological response due to higher expression levels of TLRs in chicken. The TLR2 and TLR4 are only expressed on the surface of DCs, natural killer (NK) cells, and monocytes, but intracellular expression has also been noticed in the endothelial cells (Tamiru et al., 2019). Similarly, NK cells use a variety of cellular receptors, such as Ly49 and CD94 to induce immunological responses (Boudreau and Hsu, 2018). The DCs are grouped into CD11c positive myeloid and CD11c negative plasmacytoid (PDCs). These PDCs secrete IL-6, IL-10, IL-12, IP10, and tumor necrosis factor (TNF)-α as soon as they are exposed to pathogens and also regulate the TLR7 and TL9 dependent immune pathways (Katherine et al., 2020). The expression profiles of TLR3 and TLR7 have been found higher in lungs of the post-hatch chicks, while the expression of TLR7 increases with the age of the birds (Karpala et al., 2012). Different expression patterns of TLRs generate responses against pathogens of various poultry diseases; however, the individual role of TLRs in causing disease resistance is limited. The expression patterns of TLRs in a few infectious disease of poultry have been discussed below.
A comparative study between resistant and susceptible chicken embryo fibroblasts elucidated that resistant chicken shows higher TLR3, IL8, and TLR7 gene expression when treated with Marek’s disease and TLR3 ligand Poly(I:C; Haunshi and Cheng, 2014). The synthetic analogy to dsRNA like Poly (I:C) stimulates chTLR3 in DF-1 cells and spleen (Karpala et al., 2008) that acts as an adjuvant against Marek’s disease (Parvizi et al., 2012). The findings of Haunshi and Cheng (2014) are similar to the fact that the expression of chTLR3 increases in the bursa (Jie et al., 2013) and in lungs (Abdul-Careem et al., 2009) upon infection with Marek’s disease. The higher expression level of chTLR3 highlights the role in resistant birds in comparison with susceptible ones (Haunshi and Cheng, 2014). When CEFs were treated with TLR3 ligand, Poly (I:C) increased the expression level of IL-6, IL-1β, and IL-18 that subsequently resulted in 81% reduction in Marek’s disease (Zou et al., 2017; Bavananthasivam et al., 2018). The expression pattern of TLR in CEFs is different Bavananthasivam et al. (2018) as compared to Haunshi and Cheng (2014) study. This difference in expression confers that the genetic makeup of chicken may influence the TLR expression and develops resistance against diseases.
In Newcastle disease, a similar expression level of TLR3 and TLR7 is reported in chicken embryo fibroblasts and duck embryonic fibroblasts that activate host innate immune responses upon signaling cues received by pro-inflammatory cytokines and IFNs. The gene expression levels of TLR3 and TLR7 were higher in chicken embryo fibroblast due to the species differences in chicken and ducks (Kang et al., 2016). Additionally, the antiviral effect of TLR7 against Newcastle disease infection was also characterized in layers and reported presence of different haplotypes that responded to viral attacks as a front-line immune response (Rowland et al., 2018). In chicken bone marrow macrophage cell line HD11, TLR7 inhibited the replication of Newcastle disease (Zhang et al., 2018).
The chTLR7 agonist Poly-C has shown to inhibit influenza virus replication in the chicken macrophage efficiently than TLR3 ligand Poly (I:C), but TLR3 also exhibited significant effect (Karpala et al., 2008; Stewart et al., 2012). The TLR3 agonist enhances the stimulation of IL-6, IL-12, and IFN-γ when these are used as an adjuvant with avian influenza virus (Liang et al., 2013). Moreover, the significant antiviral response of ligands of TLR2, 3, 4, 7, and 21, i.e., Poly (I:C), Pam3CSK4, LPS, and CpG, has been observed against H4N6 avian influenza virus infection. Ligands Pam3CSK4, CpG, and LPS reduce the growth of avian influenza virus in macrophages of chicken and increase expression profile of interferon regulatory factor-7, IL-1b, IFN-c, and IFN-b (Barjesteh et al., 2014). All TLR ligands reduce the shedding of virus with the greatest avian influenza virus immunity when treated with Poly (I:C; Paul et al., 2012). On the basis of these findings, it could be concluded that TLRs might have substantial ability to serve as an antiviral agent in chicken to control viral infections.
The TLR4, TLR15, TLR21, MD-2, ILs, IFNs, and iNOS have been reported as resistant genes against salmonella infections (Tohidi et al., 2012; Gupta et al., 2014). ChTLR21 acts as a receptor for microbial genetic material (Tamiru et al., 2019). MD2 is a specialized molecule needed by TLR4 to recognize LPS ligands (Shimazu et al., 1999). Upon salmonella infection, LPS serves as an inflammatory agent that is stimulated by TLR4. After inflammation, LPS binds to CD14 present on macrophage and these macrophages send signals to the TLR4/MD2 complex (Akashi et al., 2001). The said pathway takes part in the transcription of genes related to immunity during salmonella infection. MD2 is required for the LPS recognition of TLR4, intracellular distribution, and cell surface expression (Nagai et al., 2002). A number of TLR4 receptors vary among different chicken species, therefore, the expression levels of LPS-binding receptors vary between them (Dil and Qureshi, 2002). Two research studies proved that the susceptibility of salmonellosis is associated with chTLR4 (Beaumont et al., 2003; Leveque et al., 2003).
The relationship between the chTLR4 gene and susceptibility/resistance to salmonella infection has been studied (Leveque et al., 2003). Maximum resistant level of 93% has been observed in chicken due to TLR4 expression profiles and W1 (resistant) gene at NRAMP1 in comparison with those that have TLR4 and C alleles at locus NRAMP1 (58%). Furthermore, gene expression of TLR4, TLR5, and TLR21 increases substantially in Salmonella enterica serovar Typhimurium infected chicken, as soon as they bind with their respective ligands. Genetic expression of these TLRs suggests a positive role in resistance or susceptibility against Salmonella serovars (Shaughnessy et al., 2009).
Gene expression of different TLRs in birds challenged with C. perfringens has been studied. It was observed that the infection upregulated the genetic expression of TLRs (TLR2 family, TLR15, and TLR21) and related genes that induced the TLR signaling in ilea and spleens (Lu et al., 2009). TLRs are the key activator of TNF-α production and also modulate the gene expression of TNF-α inducing factor homolog to activate NF-κB that turns out to be a stimulant for inflammation. In chicken exposed to Eimeria and Salmonella species, the assimilation of TNF-α modulation increases with the corresponding increase in the inflammatory cytokines (Higuchi et al., 2008). Inflammation and stimulation of innate response have been studied in necrotic enteritis (Lu et al., 2009), whereas the homolog expression of TNF-α inducing factor may have the ability to initiate the TNF-α response against C. perfringens in chicken. These findings highlight the importance of specific TLRs in birds’ disease resistance and further studies are required to explore the possible mechanism of action and related molecular targets.
Restricted uses of growth-promoting antibiotics motivate the use of alternate natural compounds for profitable chicken production. Natural plant-derived compounds are called phytonutrients that carry multiple healthy effects and also restrict the multiplication of microbial growth. Phytochemicals can promote quality food production in the chicken industry while keeping the taste of the food intact. Phytonutrients include natural plant extracts, essential oils, and phytochemical compounds that modulate innate/non-specific and humoral/specific immune responses in chickens (Karásková et al., 2015; Singh et al., 2016; Catanzaro et al., 2018). TLRs have been identified in B cells, macrophages, and heterophils, where host responses are mediated by enhanced cellular activity and cytokines production. In response to pathogen invasion and infection, TLRs elicit reactive oxygen species, inflammatory cytokines, upregulate inflammatory reaction, and promote host adaptive immune response (Keestra et al., 2013; Huang, 2017). No doubt, TLRs are essentially important in the innate immune system and play crucial roles in the host defence against microbial invasions, but overstimulation of TLRs disturbs the body homeostasis resulting in the production of excessive pro-inflammatory cytokines that subsequently leads to the onset of many autoimmune and inflammatory diseases. Thus, inhibition of TLRs signaling pathways has been considered as a potential therapeutic strategy to mitigate undesirable, disease-related inflammatory cascades (Gao et al., 2017: El-Zayat et al., 2019b).
Target-specific inhibition of TLRs can be sought through two possible ways (1) by blocking the binding of TLRs ligands to the corresponding receptor and (2) by interfering with the intracellular signaling pathways to stop the signal transduction. Based on these facts, it has been suggested that therapeutic interventions among TLRs pathways offer potential remedies to reverse chronic liver diseases (Catanzaro et al., 2018; Katherine et al., 2020). Many inhibitory agents for TLRs signaling have been developed to control excessive inflammation, such as small inhibitory molecules (synthetic or naturally derived chemical weak bases, e.g., antimalarial drugs), antibodies, oligonucleotides, lipid-A analogs, microRNAs, and new emerging nano-inhibitors (Gao et al., 2017). In addition, phytochemicals like curcumin and its analogs have shown to modulate JK/KB signaling pathway that results in NF-κB activation by inhibiting IκB phosphorylation and degradation (Ullah et al., 2020).
Moreover, 6-shogaol is a compound present in ginger which can inhibit the TRIF (Toll/interleukin-1 (IL-1) receptor domain-containing adapter inducing interferon)-dependent signaling pathway of TLRs by targeting TANK-binding kinase1, and, hence effectively modulate TLR-derived immune/inflammatory target gene expression induced by microbial infection (Park et al., 2009b).
Phytonutrients restrict the proliferation of T cells induced by PHA and also reduce the production of IL-2, nitric oxide (NO), LPS-dependent NF-κB-mediated inflammatory pathway, and augments NK cell cytotoxicity. Besides, these compounds also inhibit NF-κB, and MAPK signaling pathways to inhibit stimulatory signals necessary for T cell activation. Phytonutrients also impair the growth of pro-inflammatory (IL-12) cytokines. In several in vivo and in vitro studies, curcumin produces the preceding advantages of phytonutrients (Catanzaro et al., 2018). It is imperative to modulate the gene expression of TLR and/or LPS oligomerization of TLRs to prevent excessive energy loss and host cell damage. Several phytochemicals modulate the gene expression of TLRs or prevent oligomerization of TLRs by pathogen LPS. This ability of phytonutrients mediates the expression patterns of TLRs and opens the horizon for potential nutrigenomic interventions to modulate the immunogenic response in chicken. Effects of potent phytochemicals with superior ability to affect the regulation of TLRs are described as under:
Inflammatory responses stimulated by TLRs via MyD88 pathways produce pro-inflammatory cytokines (Netea et al., 2004). Mammalian TLR4 can identify LPS which is a unique feature of Gram-negative bacteria, while TLR2 recognizes peptidoglycans of Gram-positive bacteria (Pasare and Medzhitov, 2005). During C. perfringens infections in broilers, mRNA expression of TLR2 was increased. On the other hand, supplementation of essential oils (EO) containing thymol and carvacrol decreased the mRNA expression of the same protein and improved resistance against the pathogens (Du et al., 2016). Thymol and carvacrol improve not only cellular immunity, but also increase the function of humoral system, whereas these chemicals also increase the expression of genes involved in chicken’s immune response (Awaad et al., 2014). Thymol and carvacrol inhibited inflammatory cell recruitment, pro-inflammatory cytokines, and oxidative impairment (Riella et al., 2012). However, pro-inflammatory response via cytokine production might damage the gut health and increase energy consumption (Lee et al., 2013a). Therefore, EO supplementation decreases TLR2 and pro-inflammatory cytokine that improves the health status of the gut (Du et al., 2016). When broiler birds were supplemented with carvacrol, TLR4 expression decreased considerably and inhibited the secretion of inflammatory cytokines (Liu et al., 2019). The blends of EO (25% thymol and 25% carvacrol) against necrotic enteritis have been well studied in broilers that were treated with C. perfringens. The supplementation of EO downregulated the TLR2 expression in challenged birds (Yin et al., 2017). This suggests that thymol and carvacrol regulate gene expression of TLR2 and TLR4 in diseased birds. Phytogenic feed additives including thymol and carvacrol have shown to downregulate the gene expression of TLR2 in chicken (Paraskeuas and Mountzouris, 2019).
Andrographolide is a constituent of Andrographis paniculata, a medicinal plant that has been traditionally used to treat infectious diseases, inflammation, fever, and cold (Chandrasekaran et al., 2011). At present, andrographolide serves as a modulator of adaptive immune response and also regulates the TLRs activities (Thakur et al., 2014). Morinda citrifolia is commonly known as Noni which is a popular medicinal plant that contains multiple phytochemicals, such as gums and mucilages, carbohydrates, proteins, fats, amino acids, anthraquinone glycosides, flavonoids, coumarin glycosides, alkaloids, phenolic compounds, citric acid, and tannins (Nayak et al., 2011).
Kalmegh and Noni supplementation influenced the gene expression profile of TLR2, 3, 4, 5, 15, and 21 (Sunder et al., 2016). Phytochemicals and polysaccharides present in Noni fruit also modulated the NF-B signal transduction pathways in a dose-dependent manner (Desai et al., 2009). The upregulation of genetic expression of TLR3, 4, and 5 might be attributed to enhanced TLR signaling mediated by quercetin treatment which is a phytochemical found abundantly in Noni and accelerates IFN-γ production (Park et al., 2009a). These antiviral products upregulate TLR3, TLR4, and TLR5 genes (Tanabe et al., 2003). The higher gene expression of TLR3, TLR4, and TLR5 and lower gene expression of TLR7 indicate the antiviral and antibacterial activities of IFN-γ. Andrographolide induces the APK and PI3K signaling pathways that activate macrophages (Wang et al., 2010). Moreover, andrographolide is more effective in downregulate gene expression of TLR7 and TLR8 which suggests that the modulation of cytokine might be due to the inhibition of TLR7 and TLR8 gene expression (Thakur et al., 2014). TLR7 and TLR8 are similarly expressed in HL-60 cell lines treated with andrographolide and revealed the anti-inflammatory effect of andrographolide (Thakur et al., 2015). It also increases cellular apoptosis and downregulates NF-κB protein and TLR4 expression to control the inflammatory response (Gao and Wang, 2016). The significant effect of andrographolide to downregulate expression profiles of TLR3, TLR7, and TLR8 in the intestine of Monopterus albus suggested its key role as health promotor in aquaculture (Shi et al., 2020). However, more detailed dose–response studies are necessary to reaffirm this assertion (Thakur et al., 2014).
Allium sativum L. is commonly known as garlic and it has been extensively used in various medical treatments for a long time. It has an immunomodulatory effect that increases T-lymphocyte blasto-genesis, phagocytosis, and cytokine (Hodge et al., 2002). S-Allylcysteine is a key compound in garlic extract that mediates repressive effects on NF-κB as it also serves as a transcriptional modulator during an adaptive immune response and stands as a sole regulator of proinflammatory gene expression (Ho et al., 2001). Allicin is also an antibiotic constituent of garlic that suppresses the bacterial growth in intestines and also inhibits fungal growth that produces aflatoxin (Ho et al., 2001). Holy basil (Ocimum sanctum) has the features of anti-inflammation, wound-healing, and modulates humoral immunity (Cohen, 2014). EO and biologically active compounds (ethanol, methanol, linalool, and eugenol) of O. sanctum have resourceful antibacterial functions especially against Shigella spp., Staphylococcus aureus, Salmonella typhi, Bacillus pumilus, E. coli, and Pseudomonas aeruginosa (Prakash and Gupta, 2005). Monoterpene, a component of O. sanctum, is a phenolic compound in nature and exhibits immunomodulatory effect by increasing IFN-γ, IL-4, T-helper, and NK cells that result in phagocytic activity (Mondal et al., 2011). In a study, Eimeria acervuline challenged chickens were fed garlic metabolites (propyl thiosulfinate and propyl thiosulfinate oxide) and these metabolites significantly decreased mRNA expression of TLR3 and TLR5 and also increased anti-inflammatory response (Kim et al., 2013a). Garlic powder and leaf powder of Holi basil promote mRNA translation of TLR2, TLR4, and TLR7 of broilers (Sheoran et al., 2017). Since TLR2 and TLR4 increase innate immunity against various Gram-positive and Gram-negative bacteria, therefore, changes in their genetic expression through garlic powder and leaf powder of Holi basil suggest a positive effect against bacterial infections. TLR7 recognizes viral nucleic acid and increases the level of TLR7 either by garlic powder alone or in combination with leaf powder. Allicin and Ajoene are present in garlic that enhance the host innate immunity against plasmodium and HIV infection, respectively (Feng et al., 2012).
Turmeric is the most extensively studied medicinal plant among the Curcuma genus and possesses many phytochemicals to trigger immunity (Li et al., 2011). Curcumin (diferuloylmethane) has properties of antioxidant, anti-inflammation, lipid peroxidase inhibitor, antiviral, free radical scavenger, antimicrobial, antitumor, antiprotozoal, and immunity enhancer (Pulido-Moran et al., 2016; Amalraj et al., 2017). Curcuminoids (a mixture of curcumin, dimethoxy curcumin, and bisdemethoxycurcumin) are effective anti-inflammatory agents by acting through multiple mechanisms, such as suppression of NF-κB, inhibition of cyclooxygenase-2, downregulation of the metastatic gene products, cell proliferation, and anti-apoptotic (Aggarwal et al., 2006). Curcuminoids also modulate the proliferation and cellular response of macrophages, B cells, neutrophils, T cells, DCs, and natural killer (NK) cells (Chandrasekaran et al., 2013). LPS activates TLR4 that stimulates MAPK and NF-κB pathways to produce cytokines (Kagan and Medzhitov, 2006). For that reason, reduction of the inflammatory response is needed via downregulation of NF-κB pathway. Supplementation of curcumin downregulates the gene expression of TLR4, and associated downstream molecules (NF-κB, MyD88, TNF-α, 1l-1β, and IL-6) in laying hens (Nawab et al., 2019). TLR4 activates MyD88 and regulates NF-κB activation (Karnati et al., 2015). Moreover, IL1β, IL6, IL12, IL18, and TNF15 increase in chicken macrophages treated in vitro with organic extract of turmeric (Lee et al., 2010). Turmeric also downregulates CD28, myeloperoxidase, and lactotransferrin in broiler birds that are associated with inflammatory response (Kim et al., 2013b).
A study reported the effect of 10 ethanolic extracts including Castanea sativa leaves, barks of Cinchona pubescens, Cinnamomum verum, Salix alba, Rheum palmatum root extract, Alchemilla vulgaris plant, Humulus lupulus cones, Vaccinium myrtillus berries, Curcuma longa root, and Arctostaphylos uvaursi leaves which worked as an anti-inflammatory drug. These extracts significantly mitigated TLR4 and TLR2 signaling pathways (Schink et al., 2018). Resveratrol acts as an anti-inflammatory agent that is an important phytoalexin found in various fruits like berries, peanuts, and grape skins (Saleh et al., 2021). It inhibits TLR4 expression in macrophages and heart tissues and reduces inflammation (Li et al., 2015; Tong et al., 2020).
A group of flavonoids including flavones, flavonols, isoflavones, flavanones, anthocyanidin, and flavanols demonstrated anti-inflammatory properties. Flavonoids are polyphenols that serve as antioxidant and anti-inflammatory agents in diet and regulate TLR gene expression (Pérez-Cano et al., 2014). Significant downregulation of TLR4 has been noticed after the treatment with flavonoids (Pérez-Cano et al., 2014). Flavonoids have shown to mediate the molecular targets and TLR-mediated signaling pathways. The potential effects of flavonoids on immunoregulatory response in the body are mediated through three primary levels (1) by modulating the composition of the microbiota (2) by mediating the expression and activation of TLRs, and (3) through modulating the downstream signaling pathways involved. The combined action of all these pathways might explain the crucial utility of flavonoids in preventing various diseases and immune-related disorders in different avian species (Pérez-Cano et al., 2014). The suppression of TLRs activation by flavonoids offers the opportunity to develop new and alternative therapeutic interventions for the treatment of inflammatory diseases.
Yeast (Saccharomyces cerevisiae)-based probiotic has been a promising substitute for antibiotics, as it is capable to modulate bacterial population (Trckova et al., 2014). Yeast can stimulate an immune response by providing binding sites to pathogens (Gao et al., 2008).
The immune modulation mechanism in yeast has not been fully understood. Polysaccharide is the main constituent of the yeast cell wall (YCW) that partially consists of β1, 3–1, 6-glucans, and mannan which modulate PRRs expression to enhance the innate immunity (Mogensen, 2009). Recognition of YCW by PRRs may activate macrophages and DCs of innate immunity following the modulation of cytokines (IL-4 and IL-10) that facilitate antibiotic production (Haghighi et al., 2006).
Gene expression of chTLR2b and chTLR4 supplemented with yeast-derived macromolecules improves the spleen and bursa of Fabricius (Yitbarek et al., 2013). Dietary nucleotides increase cell-mediated immunity, improve host resistance, and humoral immunity against invaded bacteria (Hess and Greenberg, 2012). Higher TLR2b level enhances barriers of the gastro-intestinal epithelium to block the invading pathogens (Chen et al., 2007).
Mannan oligosaccharides (MOS) are yeast-derived carbohydrates that have great potential to control necrotic enteritis in chicken, while simultaneously control pathogenic invasion (Hajati and Rezaei, 2010). ChTLR2 acts as a receptor for lipoproteins and LPS (Fukui et al., 2001). Therefore, broiler chicken fed on MOS challenged with C. perfringens show upregulated TLR2b gene expression (Yitbarek et al., 2012). However, zymosan derived from Saccharomyces cerevisiae is recognized through TLR2 which initiates the cascade of pro-inflammatory stimulation (Sato et al., 2003). Thus, the inclusion of MOS in broiler chickens might benefit to perform the proper functions of innate immunity in the ileum. Upregulated TLR4 gene expression in caecal tonsil and ileum in MOSC-treated birds prove that TLR4 is a key receptor to identify the LPS (Yitbarek et al., 2012). Studies implicate that upregulated gene expression of TLR4 is associated with resistance to Salmonella Saccharomyces cerevisiae, glucuronoxylomannan, and Candida albicans derivatives in chicken (Chaussé et al., 2011).
Various components of YCW produce immunomodulatory functions, and supplementation of even 0.05% YCW enhances cell-mediated and humoral immune reactions in chicken (Gómez-Verduzco et al., 2009). Particularly, β-glucans supplementation stimulates innate immunity and increases resistance to S. enterica (Lowry et al., 2005). Nucleotides of yeast and YCW have also immunomodulatory properties (Hess and Greenberg, 2012). Genetic expression of TLR4 gene was upregulated in chicken supplemented with nucleotide-rich diet (Alizadeh et al., 2016) and YCW supplementation also upregulated TLR2b gene expression (Alizadeh et al., 2016). This may be due to a noteworthy level of mannan and β1,3–1,6-glucan (Sato et al., 2003). Mannan oligosaccharides improve chicken production and increase innate immunity. Its use in the diet may also exert beneficial effects. This discussion highlights the importance of yeast and yeast-derived products during gene regulation of TLRs.
A study was conducted to evaluate the effect of probiotics (Lactobacillus acidophilus) on different genes expression levels of TLRs in chicken’s cecal tonsil and the TLR2, TLR4, and TLR5 gene expression were different in chickens fed with probiotic mixed diet in comparison with the birds in the control group (Asgari et al., 2018). Same probiotic supplementation increased the TLR2 regulation in cecal tonsils of S. enteritidis-infected chickens that helped in lowering the infection level. The previous studies showed that Lactobacillus-based probiotics could reduce the level of pro-inflammatory cytokines in the intestine of S. Enteritidis-infected chickens and increased the gene expression of TLR2 in their cecal tonsils (Penha Filho et al., 2015). The effects of probiotics on genetic expression of TLRs in dairy cow were also evaluated, and gene expression of TLR2, TLR6, TLR7, and TLR8 was downregulated. Studies in cattle TLRs suggested that probiotic behaved like anti-inflammatory agents and control TLRs genetic expression and innate immune response (Adjei-Fremah et al., 2018).
Inflammatory responses of TLR2 and TLR4 have also been studied against high-fat diet in rats (Wan et al., 2014; Lee et al., 2015). These treatments downregulated the gene expression of TLR2 and TLR4 in CD14 monocytes and impaired their functions (Wan et al., 2014). This diet activated the macrophages that significantly increased stimulation of NF-κB and IL-6 (Lee et al., 2015). TLR-dependent vitamin D mediated innate immunity has also been studied (Arababadi et al., 2018). Innate immunity regulation by vitamin D was also associated with TLRs regulation (Sadeghi et al., 2006). It was confirmed that TLR4 caused the activation of APCs that reduced the inflammatory response of innate immunity (Gambhir et al., 2012; Calton et al., 2015).
Polymorphic variants in TLRs may control the reaction of hosts against different pathogenic microbes, and these phenomena control the susceptibility and resistance against diseases (O’Dwyer et al., 2013). These variations may also affect the recognition patterns of ligands by TLRs to differentiate host resistance to pathogenic infections. The single-nucleotide polymorphisms (SNPs) found within the PRRs change the structural orientation of the receptors and associated interactive features between the ligands and their corresponding receptors (Skevaki and Pararas, 2015). These topological variations influence the signaling pathways and also enable to recognize different pathogens.
A study reported 27 polymorphic sites in the amino acid sequence of chTLR1; 14 in type 1, while 13 in type 2 (Ruan and Zheng, 2011). Scientists declared that variations in the sequence of amino acids G560S and T130A present in LRR site of type 1 and C228S, F129L, G285S, and L275P in type 2 LRR site might change PAMP recognition by these TLRs. LRRs are the extracellular domains of TLRs to facilitate functional domains for the recognition of ligands (Jin and Lee, 2008). Moreover, two distinctive polymorphic sites have been found; first (A645T) in chTLR1 type 1 and second (L275P) in chTLR1 type 2, and both occur in LRR domains in White-Leghorn chicken (Table 2). These variants characterize resistance against salmonellosis (Wigley, 2004).
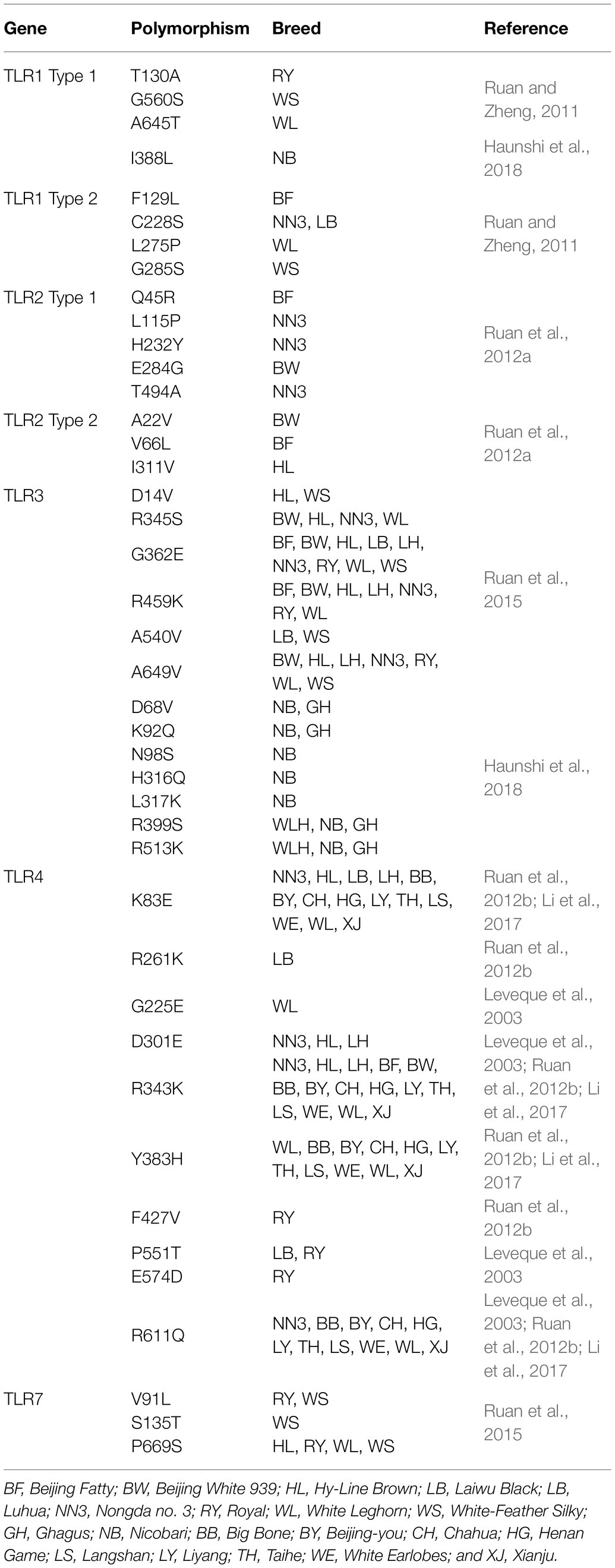
Table 2. Allelic variation and major single-nucleotide polymorphisms in the different TLRs in chicken.
Similarly, 10 polymorphic sites in the amino acid sequences of TLR2 type1 and type 2 have been reported among seven chicken breeds, i.e., six and four in type 1 and type 2, respectively (Ruan et al., 2012a). Five SNPs have been found in LRR of TLR2 type 1 (Q45R, L115P, H232Y, E284G, and T494A) while three were LRR of type 2 (V66L, I311V, and A22V). However, TIR domain is similar to the IL-1 receptor that is highly conserved and interacts with adapter proteins, such as MyD88 (Verstak et al., 2009). Various polymorphic sites also report in the TIR domain (Table 2) that cause loss of MyD88 binding and reduce TLR2/TLR4 signaling, because TIR is the functional domain of subsequent recruitment of intracellular adapter proteins (Nagpal et al., 2009; Werling et al., 2009).
A study reported nine polymorphisms in the amino acid chain of chTLR4 including eight in extracellular sites, while only one in cytoplasmic sites (Ruan et al., 2012b). Out of these, nine reported SNPs, five were like the SNPs in the LRR region as shown in Table 2 (Leveque et al., 2003). Similarly, nineteen amino acid variants represented 10 novel mutations, while nine were reported previously. Seven new and four old mutations (E83K, R343K, H383Y, and Q611R) have been featured in LRR (Li et al., 2017). Moreover, E83K of TLR4 has more resistance against SE, and E83K mutation has been documented as AA/AG genotypes because of G to A substitution at nucleotide 247, and GG is known as a wild genotype. However, this SNP shows significant resistance against salmonella with prominent results in the GG genotype (Li et al., 2017).
Similarly, genotype GG has been associated in Sentul chicken against salmonella and Newcastle disease (Mamutse et al., 2018). However, all genotypes (AA, AG, and GG) were also found with a similar resistance pattern against salmonella in Kampung chicken (Ulupi et al., 2013). A group finds polymorphism in TLR4 gene’s exon 2 in 14 Chinese chicken including Red Jungle fowl and Tibetan chicken. Particularly, these chickens are more resistant to disease, and they are only BB at exon 2 of TLR4, while the rest of all breeds is AA, BB, and AB. Here, BB has been reported as a wild type at TLR4 exon 2, and AA genotype is because of G/A mutations at nucleotide 114 and 142 of exon 2, respectively, as reported as G114A and G142A. The genotype BB may be beneficial for immunity in chickens, and the B allele might have a significant association with resistance (Liu et al., 2011). They also found two mutations in the amino acid sequence of TLR4, G114A, and G142A. Further investigation to find the relationship between these SNPs and resistibility to disease will reveal selective breeding programs in the future.
ChTLR3 and chTLR7 have the potential to increase innate immunity against viral attacks as discussed in this review. Therefore, various polymorphic regions might play frequent roles in resistance or susceptibility of disease in chickens. A study has published six SNPs (R345S, D14V, G362E, A540V, A649V, and R459K) in the LRR region of TLR3, while a single one (T713S) has been found in TIR domain. Three polymorphic sites have been found in the LRR region of TLR7 (V91L, S135T, and P669S), while one (V876M) has been located in the TIR domain as shown in Table 2 (Ruan et al., 2015). Ten SNPs in the LRR region of TLR3 have been observed in Indian local chicken breeds and eight in the LRR domain as shown in Table 2 (Haunshi et al., 2018). Polymorphic regions in TLR3 and TLR7 might exert potential effects on host responses against viruses to establish patterns of variable disease resistance or susceptibility (Al Qahtani et al., 2012; O’Dwyer et al., 2013). A number of reports have presented polymorphic regions in TLR3 gene that are associated with resistance against viral load (Kindberg et al., 2011; Sironi et al., 2012; Svensson et al., 2012; Lee et al., 2013b; Studzińska et al., 2017). A parallel study also reported significant relationship of different TLR3 variants in the susceptibility or resistance against various chicken diseases. These are non-synonymous changes in SNPs due to change in amino acid sequence. These can be beneficial, harmful, or neutral against proteins functions, while it may be neutral if SNPs have synonymous substitution (Downing et al., 2010).
The available variations in chTLRs suggest a positive selection of resistance or susceptibility against diseases. A sufficient research gap is yet to be exploited to identify the association of SNPs with resistance against microbial infections in chicken. Identification of associated polymorphic regions may contribute to the genomic selection of chicken and help to design future breeding programs. There is also a dire need to conduct some association studies among these variants and resistance in chicken for the improvement of commercial chicken.
Ten different TLRs activate TLR signaling pathways including ChTLR1, 2, 3, 4, and 7 which elicit essential immunogenic and inflammatory responses during different viral and bacterial diseases. Several nutrients and phytochemicals have proven as an excellent source to trigger innate immunity via stimulation of TLRs in chicken suggesting that some TLRs are nutrient specific. Yeast derivatives (as probiotic and prebiotic) have also been revealed as potential modulators of TLRs to enhance the immune response in chicken under health and disease conditions. Such interventions suggest the nutrigenomic potential of TLRs to improve the health status and production through dietary supplementation in chicken feed with specific nutrients particularly phytonutrients. Moreover, variations in TLRs have been identified that link potential association with disease susceptibility and resistance in chicken. Such genetic variations including SNPs are particularly useful for the genomic selection of chicken to produce birds with better genetic resistance and resilience against different diseases. However, a lot of work is needed to explain the role of nutrients and phytochemicals that modulate the genetic expression of TLRs.
QL and HP: conceptualization and project administration. F-uH, SR, HP, and QL: resources. MR, SR, WY, WA, and F-uH: data searching. MR and SR: writing and original draft preparation. F-uH, SR, WY, WA, QL, and HP: review and editing. QL: funding acquisition. All authors read and approved the final version of the manuscript.
The present study was supported by the National Natural Science Fund (U20A2051, 31760648, and 31860638), the Guangxi Natural Science Foundation (AB18221120), and the Guangxi Distinguished scholars Program (201835).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul-Careem, M. F., Haq, K., Shanmuganathan, S., Read, L. R., Schat, K. A., Heidari, M., et al. (2009). Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus. Virology 393, 250–257. doi: 10.1016/j.virol.2009.08.001
Aderem, A., and Smith, K. D. (2004). A systems approach to dissecting immunity and inflammation. Sem. Immunol. 6, 55–67. doi: 10.1016/j.smim.2003.10.002
Adjei-Fremah, S., Ekwemalor, K., Asiamah, E. K., Ismail, H., Ibrahim, S., and Worku, M. (2018). Effect of probiotic supplementation on growth and global gene expression in dairy cows. J. Appl. Anim. Res. 46, 257–263. doi: 10.1080/09712119.2017.1292913
Aggarwal, S., Ichikawa, H., Takada, Y., Sandur, S. K., Shishodia, S., and Aggarwal, B. B. (2006). Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Mol. Pharmacol. 69, 195–206. doi: 10.1124/mol.105.017400
Akashi, S., Nagai, Y., Ogata, H., Oikawa, M., Fukase, K., Kusumoto, S., et al. (2001). Human MD-2 confers on mouse toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 13, 1595–1599. doi: 10.1093/intimm/13.12.1595
Akira, S., and Takeda, K. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511. doi: 10.1038/nri1391
Akira, S., Uematsu, S., and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. doi: 10.1016/j.cell.2006.02.015
Al Qahtani, A., Al Ahdal, M., Abdo, A., Sanai, F., AlAnazi, M., Khalaf, N., et al. (2012). Toll like receptor 3 polymorphism and its association with hepatitis B virus infection in Saudi Arabian patients. J. Med. Virol. 84, 1353–1359. doi: 10.1002/jmv.23271
Alizadeh, M., Rodriguez-Lecompte, J., Echeverry, H., Crow, G., and Slominski, B. (2016). Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on antibody-mediated immune response and gene expression of pattern recognition receptors and cytokines in broiler chickens immunized with T-cell dependent antigens. Poult. Sci. 95, 823–833. doi: 10.3382/ps/pev449
Amalraj, A., Pius, A., Gopi, S., and Gopi, S. (2017). Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 7, 205–233. doi: 10.1016/j.jtcme.2016.05.005
Arababadi, M. K., Nosratabadi, R., and Asadikaram, G. (2018). Vitamin D and toll like receptors. Life Sci. 203, 105–111. doi: 10.1016/j.lfs.2018.03.040
Asgari, F., Falak, R., Teimourian, S., Pourakbari, B., Ebrahimnezhad, S., and Shekarabi, M. (2018). Effects of oral probiotic feeding on toll-like receptor gene expression of the chicken’s cecal tonsil. Rep. Biochem. Mol. Biol 6, 151–157.
Awaad, M., Elmenawey, M., and Ahmed, K. A. (2014). Effect of a specific combination of carvacrol, cinnamaldehyde, and on the growth performance, carcass quality and gut integrity of broiler chickens. Vet. World 7, 284–290. doi: 10.14202/vetworld.2014.284-290
Barjesteh, N., Behboudi, S., Brisbin, J. T., Villanueva, A. I., Nagy, É., and Sharif, S. (2014). TLR ligands induce antiviral responses in chicken macrophages. PLoS One 9:e105713. doi: 10.1371/journal.pone.0105713
Bavananthasivam, J., Kulkarni, R. R., Read, L., and Sharif, S. (2018). Reduction of Marek's disease virus infection by toll-like receptor ligands in chicken embryo fibroblast cells. Viral Immunol. 31, 389–396. doi: 10.1089/vim.2017.0195
Beaumont, C., Protais, J., Pitel, F., Leveque, G., Malo, D., Lantier, F., et al. (2003). Effect of two candidate genes on the salmonella carrier state in fowl. Poult. Sci. 82, 721–726. doi: 10.1093/ps/82.5.721
Beg, A. A. (2002). Endogenous ligands of toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509–512. doi: 10.1016/S1471-4906(02)02317-7
Birchler, T., Seibl, R., Büchner, K., Loeliger, S., Seger, R., Hossle, J. P., et al. (2001). Human toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur. J. Immunol. 31, 3131–3137. doi: 10.1002/1521-4141(200111)31:11<3131::AID-IMMU3131>3.0.CO;2-G
Boudreau, J. E., and Hsu, K. C. (2018). Natural killer cell education in human health and disease. Curr. Opin. Immunol. 50, 102–111. doi: 10.1016/j.coi.2017.11.003
Bresnahan, K. A., and Tanumihardjo, S. A. (2014). Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Advances in nutrition 5, 702–711. doi: 10.3945/an.114.006361
Brownlie, R., and Allan, B. (2011). Avian toll-like receptors. Cell Tissue Res. 343, 121–130. doi: 10.1007/s00441-010-1026-0
Brownlie, R., Zhu, J., Allan, B., Mutwiri, G. K., Babiuk, L. A., Potter, A., et al. (2009). Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 46, 3163–3170. doi: 10.1016/j.molimm.2009.06.002
Calton, E. K., Keane, K. N., Newsholme, P., and Soares, M. J. (2015). The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One 10:e0141770. doi: 10.1371/journal.pone.0141770
Catanzaro, M., Corsini, E., Rosini, M., Racchi, M., and Lanni, C. (2018). Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules 23:2778. doi: 10.3390/molecules23112778
Chandrasekaran, C. V., Kannan Sundarajan, J. R. E., Gururaja, G. M., Mundkinajeddu, D., and Agarwal, A. (2013). Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharm. Res. 5, 71–79. doi: 10.4103/0974-8490.110527
Chandrasekaran, C., Thiyagarajan, P., Deepak, H., and Agarwal, A. (2011). In vitro modulation of LPS/calcimycin induced inflammatory and allergic mediators by pure compounds of Andrographis paniculata (king of bitters) extract. Int. Immunopharmacol. 11, 79–84. doi: 10.1016/j.intimp.2010.10.009
Chaussé, A.-M., Grépinet, O., Bottreau, E., Le Vern, Y., Menanteau, P., Trotereau, J., et al. (2011). Expression of toll-like receptor 4 and downstream effectors in selected cecal cell subpopulations of chicks resistant or susceptible to salmonella carrier state. Infect. Immun. 79, 3445–3454. doi: 10.1128/IAI.00025-11
Chen, J., Rao, J. N., Zou, T., Liu, L., Marasa, B. S., Xiao, L., et al. (2007). Polyamines are required for expression of toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointestinal Liver Physiol. 293, G568–G576. doi: 10.1152/ajpgi.00201.2007
Chu, H., and Mazmanian, S. K. (2013). Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 14, 668–675. doi: 10.1038/ni.2635
Cohen, M. M. (2014). Tulsi-Ocimum sanctum: A herb for all reasons. J. Ayurveda Int. Med. 5, 251–259. doi: 10.4103/0975-9476.146554
Cutler, C. W., Jotwani, R., and Pulendran, B. (2001). Dendritic cells: immune saviors or Achilles' heel? Infect. Immun. 69, 4703–4708. doi: 10.1128/IAI.69.8.4703-4708.2001
de Zoete, M. R., Keestra, A. M., Roszczenko, P., and van Putten, J. P. (2010). Activation of human and chicken toll-like receptors by campylobacter spp. Infect. Immun. 78, 1229–1238. doi: 10.1128/IAI.00897-09
Delneste, Y., Beauvillain, C., and Jeannin, P. (2007). Innate immunity: structure and function of TLRs. Med. Sci. 23, 67–73. doi: 10.1051/medsci/200723167
Desai, F., Ramanathan, M., Fink, C. S., Wilding, G. E., Weinstock-Guttman, B., and Awad, A. B. (2009). Comparison of the immunomodulatory effects of the plant sterol β-sitosterol to simvastatin in peripheral blood cells from multiple sclerosis patients. Int. Immunopharmacol. 9, 153–157. doi: 10.1016/j.intimp.2008.10.019
Dil, N., and Qureshi, M. (2002). Differential expression of inducible nitric oxide synthase is associated with differential toll-like receptor-4 expression in chicken macrophages from different genetic backgrounds. Vet. Immunol. Immunopathol. 84, 191–207. doi: 10.1016/S0165-2427(01)00402-0
Downing, T., Lloyd, A. T., O’Farrelly, C., and Bradley, D. G. (2010). The differential evolutionary dynamics of avian cytokine and TLR gene classes. J. Immunol. 184, 6993–7000. doi: 10.4049/jimmunol.0903092
Du, E., Wang, W., Gan, L., Li, Z., Guo, S., and Guo, Y. (2016). Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotech. 7:19. doi: 10.1186/s40104-016-0079-7
El-Zayat, S. R., Sibaii, H., and Mannaa, F. A. (2019a). Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl. Res. Cent. 43, 187. doi: 10.1186/s42269-019-0227-2
El-Zayat, S. R., Sibaii, H., and Mannaa, F. A. (2019b). Micronutrients and many important factors that affect the physiological functions of toll-like receptors. Bull. National Res. Cent. 43, 123.
Feng, Y., Zhu, X., Wang, Q., Jiang, Y., Shang, H., Cui, L., et al. (2012). Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 11:268. doi: 10.1186/1475-2875-11-268
Fukui, A., Inoue, N., Matsumoto, M., Nomura, M., Yamada, K., Matsuda, Y., et al. (2001). Molecular cloning and functional characterization of chicken toll-like receptors a single chicken toll covers multiple molecular patterns. J. Biol. Chem. 276, 47143–47149. doi: 10.1074/jbc.M103902200
Gambhir, V., Yildiz, C., Mulder, R., Siddiqui, S., Guzzo, C., Szewczuk, M., et al. (2012). The TLR2 agonists lipoteichoic acid and Pam3CSK4 induce greater pro-inflammatory responses than inactivated mycobacterium butyricum. Cell. Immunol. 280, 101–107. doi: 10.1016/j.cellimm.2012.12.001
Gao, H., and Wang, J. (2016). Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 13, 1827–1832. doi: 10.3892/mmr.2015.4703
Gao, W., Xiong, Y., Li, Q., and Yang, H. (2017). Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 8, 508–517. doi: 10.3389/fphys.2017.00508
Gao, J., Zhang, H., Yu, S., Wu, S., Yoon, I., Quigley, J., et al. (2008). Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 87, 1377–1384. doi: 10.3382/ps.2007-00418
Gómez-Verduzco, G., Cortes-Cuevas, A., López-Coello, C., Ávila-González, E., and Nava, G. M. (2009). Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 51:11. doi: 10.1186/1751-0147-51-11
Gupta, S. K., Deb, R., Dey, S., and Chellappa, M. M. (2014). Toll-like receptor-based adjuvants: enhancing the immune response to vaccines against infectious diseases of chicken. Expert Rev. Vaccines 13, 909–925. doi: 10.1586/14760584.2014.920236
Haghighi, H. R., Gong, J., Gyles, C. L., Hayes, M. A., Zhou, H., Sanei, B., et al. (2006). Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 13, 975–980. doi: 10.1128/CVI.00161-06
Hajati, H., and Rezaei, M. (2010). The application of prebiotics in poultry production. Int. J. Poult. Sci. 9, 298–304. doi: 10.3923/ijps.2010.298.304
Haunshi, S., Burramsetty, A. K., Ramasamy, K., and Chatterjee, R. N. (2018). Polymorphisms in pattern recognition receptor genes of indigenous and white Leghorn breeds of chicken. Arch. Anim. Breed. 61:441. doi: 10.5194/aab-61-441-2018
Haunshi, S., and Cheng, H. H. (2014). Differential expression of toll-like receptor pathway genes in chicken embryo fibroblasts from chickens resistant and susceptible to Marek's disease. Poult. Sci. 93, 550–555. doi: 10.3382/ps.2013-03597
Hayashi, F., Means, T. K., and Luster, A. D. (2003). Toll-like receptors stimulate human neutrophil function. Blood 102, 2660–2669. doi: 10.1182/blood-2003-04-1078
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Eugene, C. Y., Goodlett, D. R., et al. (2001). The innate immune response to bacterial flagellin is mediated by toll-like receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
He, H., Genovese, K. J., Nisbet, D. J., and Kogut, M. H. (2006). Profile of toll-like receptor expressions and induction of nitric oxide synthesis by toll-like receptor agonists in chicken monocytes. Mol. Immunol. 43, 783–789. doi: 10.1016/j.molimm.2005.07.002
He, H., Genovese, K. J., Nisbet, D. J., and Kogut, M. H. (2007). Synergy of CpG oligodeoxynucleotide and double-stranded RNA (poly I: C) on nitric oxide induction in chicken peripheral blood monocytes. Mol. Immunol. 44, 3234–3242. doi: 10.1016/j.molimm.2007.01.034
He, H., Genovese, K. J., Swaggerty, C. L., MacKinnon, K. M., and Kogut, M. H. (2012). Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-γ and regulatory cytokine IL-10 expression in chicken monocytes. Dev. Comp. Immunol. 36, 756–760. doi: 10.1016/j.dci.2011.11.006
He, H., MacKinnon, K. M., Genovese, K. J., and Kogut, M. H. (2011). CpG oligodeoxynucleotide and double-stranded RNA synergize to enhance nitric oxide production and mRNA expression of inducible nitric oxide synthase, pro-inflammatory cytokines and chemokines in chicken monocytes. Innate Immun. 17, 137–144. doi: 10.1177/1753425909356937
Heggen, C., Qureshi, M., Edens, F., and Barnes, H. (2000). Alterations in macrophage-produced cytokines and nitrite associated with poult enteritis and mortality syndrome. Avian Dis. 44, 59–65. doi: 10.2307/1592508
Hess, J. R., and Greenberg, N. A. (2012). The role of nucleotides in the immune and gastrointestinal systems: potential clinical applications. Nutr. Clin. Pract. 27, 281–294. doi: 10.1177/0884533611434933
Higgs, R., Cormican, P., Cahalane, S., Allan, B., Lloyd, A. T., Meade, K., et al. (2006). Induction of a novel chicken toll-like receptor following salmonella enterica serovar Typhimurium infection. Infect. Immun. 74, 1692–1698. doi: 10.1128/IAI.74.3.1692-1698.2006
Higuchi, M., Matsuo, A., Shingai, M., Shida, K., Ishii, A., Funami, K., et al. (2008). Combinational recognition of bacterial lipoproteins and peptidoglycan by chicken toll-like receptor 2 subfamily. Dev. Comp. Immunol. 32, 147–155. doi: 10.1016/j.dci.2007.05.003
Ho, S., Ide, N., and Lau, B. (2001). S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 8, 39–46. doi: 10.1078/0944-7113-00005
Hodge, G., Hodge, S., and Han, P. (2002). Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry J. Int. Society Anal. Cytol. 48, 209–215. doi: 10.1002/cyto.10133
Huang, S. (2017). Upregulation of TLR4 mRNA expression levels in broiler chickens under acute heat stress. Braz. J. Poult. Sci. 19, 87–94. doi: 10.1590/1806-9061-2016-0344
Iqbal, M., Philbin, V. J., and Smith, A. L. (2005a). Expression patterns of chicken toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 104, 117–127. doi: 10.1016/j.vetimm.2004.11.003
Iqbal, M., Philbin, V. J., Withanage, G., Wigley, P., Beal, R. K., Goodchild, M. J., et al. (2005b). Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with salmonella enterica serovar typhimurium. Infect. Immun. 73, 2344–2350. doi: 10.1128/IAI.73.4.2344-2350.2005
Jie, H., Lian, L., Qu, L., Zheng, J., Hou, Z., Xu, G., et al. (2013). Differential expression of toll-like receptor genes in lymphoid tissues between Marek's disease virus-infected and noninfected chickens. Poult. Sci. 92, 645–654. doi: 10.3382/ps.2012-02747
Jin, M. S., and Lee, J.-O. (2008). Structures of the toll-like receptor family and its ligand complexes. Immunity 29, 182–191. doi: 10.1016/j.immuni.2008.07.007
Kagan, J. C., and Medzhitov, R. (2006). Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell 125, 943–955. doi: 10.1016/j.cell.2006.03.047
Kaiser, P., Rothwell, L., Galyov, E. E., Barrow, P. A., Burnside, J., and Wigley, P. (2000). Differential cytokine expression in avian cells in response to invasion by salmonella typhimurium, salmonella enteritidis and salmonella gallinarumThe GenBank accession numbers for the sequences reported in this paper are AI982185 for chicken IL-6 cDNA and AJ250838 for the partial chicken IL-6 genomic sequence, respectively. Microbiology 146, 3217–3226. doi: 10.1099/00221287-146-12-3217
Kang, Y., Feng, M., Zhao, X., Dai, X., Xiang, B., Gao, P., et al. (2016). Newcastle disease virus infection in chicken embryonic fibroblasts but not duck embryonic fibroblasts is associated with elevated host innate immune response. Virol. J. 13:41. doi: 10.1186/s12985-016-0499-1
Karásková, K., Suchý, P., and Straková, E. (2015). Current use of phytogenic feed additives in animal nutrition: a review. Czech J. Anim. Sci. 60, 521–530. doi: 10.17221/8594-CJAS
Karnati, H. K., Pasupuleti, S. R., Kandi, R., Undi, R. B., Sahu, I., Kannaki, T., et al. (2015). TLR-4 signalling pathway: MyD88 independent pathway up-regulation in chicken breeds upon LPS treatment. Vet. Res. Commun. 39, 73–78. doi: 10.1007/s11259-014-9621-2
Karpala, A. J., BagnaudBaule, A., Goossens, K. E., Lowenthal, J. W., and Bean, A. G. D. (2012). Ontogeny of the interferon system in chickens. J. Reprod. Immunol. 94, 169–174. doi: 10.1016/j.jri.2012.02.008
Karpala, A. J., Lowenthal, J. W., and Bean, A. G. (2008). Activation of the TLR3 pathway regulates IFNβ production in chickens. Dev. Comp. Immunol. 32, 435–444. doi: 10.1016/j.dci.2007.08.004
Katherine, A., Fitzgerald,, and Kagan, J. C. (2020). Toll-like receptors and the control of immunity. Cell 180, 1044–1066. doi: 10.1016/j.cell.2020.02.041
Kawai, T., and Akira, S. (2006). TLR signaling. Cell Death Differ. 13, 816–825. doi: 10.1038/sj.cdd.4401850
Kawai, T., and Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 11, 373–384. doi: 10.1038/ni.1863
Kawasaki, T., and Kawai, T. (2014). Toll-like receptor signaling pathways. Front. Immunol. 5:461. doi: 10.3389/fimmu.2014.00461
Keestra, A. M., de Zoete, M. R., Bouwman, L. I., Vaezirad, M. M., and van Putten, J. P. (2013). Unique features of chicken toll-like receptors. Dev. Comp. Immunol. 41, 316–323. doi: 10.1016/j.dci.2013.04.009
Keestra, A. M., de Zoete, M. R., van Aubel, R. A., and van Putten, J. P. (2007). The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. J. Immunol. 178, 7110–7119. doi: 10.4049/jimmunol.178.11.7110
Keestra, A. M., de Zoete, M. R., van Aubel, R. A., and van Putten, J. P. (2008). Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 45, 1298–1307. doi: 10.1016/j.molimm.2007.09.013
Kim, D. K., Lillehoj, H. S., Lee, S. H., Jang, S. I., Lillehoj, E. P., and Bravo, D. (2013a). Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 92, 2635–2643. doi: 10.3382/ps.2013-03095
Kim, D. K., Lillehoj, H. S., Lee, S. H., Lillehoj, E. P., and Bravo, D. (2013b). Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 109, 76–88. doi: 10.1017/S0007114512000530
Kindberg, E., Vene, S., Mickiene, A., Lundkvist, Å., Lindquist, L., and Svensson, L. (2011). A functional toll-like receptor 3 gene (TLR3) may be a risk factor for tick-borne encephalitis virus (TBEV) infection. J. Infect. Dis. 203, 523–528. doi: 10.1093/infdis/jiq082
Kogut, M. H., Genovese, K. J., He, H., and Kaiser, P. (2008). Flagellin and lipopolysaccharide up-regulation of IL-6 and CXCLi2 gene expression in chicken heterophils is mediated by ERK1/2-dependent activation of AP-1 and NF-κB signaling pathways. Innate Immun. 14, 213–222. doi: 10.1177/1753425908094416
Kogut, M. H., Iqbal, M., He, H., Philbin, V., Kaiser, P., and Smith, A. (2005). Expression and function of toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 29, 791–807. doi: 10.1016/j.dci.2005.02.002
Kogut, M. H., Swaggerty, C. L., He, H., Pevzner, I., and Kaiser, P. (2006). Toll-like receptor agonists stimulate differential functional activation and cytokine and chemokine gene expression in heterophils isolated from chickens with differential innate responses. Microbes Infect. 8, 1866–1874. doi: 10.1016/j.micinf.2006.02.026
Laurent, F., Mancassola, R., Lacroix, S., Menezes, R., and Naciri, M. (2001). Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 69, 2527–2534. doi: 10.1128/IAI.69.4.2527-2534.2001
Lee, S. O., Brown, R., and Razonable, R. R. (2013b). Association between a functional polymorphism in toll-like receptor 3 and chronic hepatitis C in liver transplant recipients. Transpl. Infect. Dis. 15, 111–119. doi: 10.1111/tid.12033
Lee, S., Lillehoj, H., Hong, Y., Jang, S., Lillehoj, E., Ionescu, C., et al. (2010). In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br. Poult. Sci. 51, 213–221. doi: 10.1080/00071661003745844
Lee, S. H., Lillehoj, H. S., Jang, S. I., Lillehoj, E. P., Min, W., and Bravo, D. M. (2013a). Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 110, 840–847. doi: 10.1017/S0007114512006083
Lee, J. J., Wang, P. W., Yang, I. H., Huang, H. M., Chang, C. S., Wu, C. L., et al. (2015). High-fat diet induces toll-like receptor 4-dependent macrophage/microglial cell activation and retinal impairment. Invs. ophthalmol. Visual Sci. 56, 3041–3050. doi: 10.1167/iovs.15-16504
Leulier, F., and Lemaitre, B. (2008). Toll-like receptors—taking an evolutionary approach. Nat. Rev. Genet. 9, 165–178. doi: 10.1038/nrg2303
Leveque, G., Forgetta, V., Morroll, S., Smith, A. L., Bumstead, N., Barrow, P., et al. (2003). Allelic variation in TLR4 is linked to susceptibility to salmonella enterica serovar Typhimurium infection in chickens. Infect. Immun. 71, 1116–1124. doi: 10.1128/IAI.71.3.1116-1124.2003
Li, P., Wang, H., Zhao, X., Gou, Z., Liu, R., Song, Y., et al. (2017). Allelic variation in TLR4 is linked to resistance to salmonella Enteritidis infection in chickens. Poult. Sci. 96, 2040–2048. doi: 10.3382/ps/pex010
Li, J., Xie, C., Zhuang, J., Li, H., Yao, Y., Shao, C., et al. (2015). Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 11, 1120–1126. doi: 10.3892/mmr.2014.2955
Li, S., Yuan, W., Deng, G., Wang, P., Yang, P., and Aggarwal, B. (2011). Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops. 2, 28–54. doi: 10.2174/2210290601102010028
Liang, J., Fu, J., Kang, H., Lin, J., Yu, Q., and Yang, Q. (2013). Comparison of 3 kinds of toll-like receptor ligands for inactivated avian H5N1 influenza virus intranasal immunization in chicken. Poult. Sci. 92, 2651–2660. doi: 10.3382/ps.2013-03193
Liu, Y., Chang, G. B., Hu, G. S., Li, Q., Xu, Q., and Chen, G. H. (2011). Genetic variation at Exon2 of TLR4 gene and its association with resistant traits in chicken. Afr. J. Biotechnol. 10, 8260–8266. doi: 10.5897/AJB10.2620
Liu, S., Song, M., Yun, W., Lee, J., Kim, H., and Cho, J. (2019). Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 98, 2026–2033. doi: 10.3382/ps/pey575
Love, W., Dobbs, N., Tabor, L., and Simecka, J. W. (2010). Toll-like receptor 2 (TLR2) plays a major role in innate resistance in the lung against murine mycoplasma. PLoS One 5:e10739. doi: 10.1371/journal.pone.0010739
Lowry, V., Farnell, M., Ferro, P., Swaggerty, C., Bahl, A., and Kogut, M. (2005). Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 98, 309–318. doi: 10.1016/j.ijfoodmicro.2004.06.008
Lu, Y., Sarson, A. J., Gong, J., Zhou, H., Zhu, W., Kang, Z., et al. (2009). Expression profiles of genes in toll-like receptor-mediated signaling of broilers infected with Clostridium perfringens. Clin. Vaccine Immunol. 16, 1639–1647. doi: 10.1128/CVI.00254-09
Mamutse, J., Gunawan, A., Sumantri, C., Murtini, S., and Sartika, T. (2018). Association of the Toll-Like Receptor 4 (TLR4) and Myxovirus (MX) genes with resistance to salmonella and Newcastle disease in selected sentul chickens. Int. J. Poult. Sci. 17, 591–599. doi: 10.3923/ijps.2018.591.599
Mbongue, J., Nicholas, D., Firek, A., and Langridge, W. (2014). The role of dendritic cells in tissue-specific autoimmunity. J. Immunol. Res. 2014:857143. doi: 10.1155/2014/857143
Mogensen, T. H. (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273. doi: 10.1128/CMR.00046-08
Mondal, S., Varma, S., Bamola, V. D., Naik, S. N., Mirdha, B. R., Padhi, M. M., et al. (2011). Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J. Ethnopharmacol. 136, 452–456. doi: 10.1016/j.jep.2011.05.012
Moresco, E. M., LaVine, D., and Beutler, B. (2011). Toll-like receptors. Curr. Biol. 21, R488–R493. doi: 10.1016/j.cub.2011.05.039
Nagai, Y., Akashi, S., Nagafuku, M., Ogata, M., Iwakura, Y., Akira, S., et al. (2002). Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672. doi: 10.1038/ni809
Nagpal, K., Plantinga, T. S., Wong, J., Monks, B. G., Gay, N. J., Netea, M. G., et al. (2009). A TIR domain variant of MyD88 adapter-like (mal)/TIRAP results in loss of MyD88 binding and reduced TLR2/TLR4 signaling. J. Biol. Chem. 284, 25742–25748. doi: 10.1074/jbc.M109.014886
Nawab, A., Li, G., An, L., Chao, L., Xiao, M., Zhao, Y., et al. (2019). Effect of curcumin supplementation on TLR4 mediated non-specific immune responses in liver of laying hens under high-temperature conditions. J. Therm. Biol. 84, 384–397. doi: 10.1016/j.jtherbio.2019.07.003
Nayak, S., Chintamaneni, M., and Mengi, S. (2011). Anti-infective potential of Morinda citrifolia fruit extract. Int. J. Pharm. Pharm. Sci. 3, 81–83.
Netea, M. G., Van der Graaf, C., Van der Meer, J. W., and Kullberg, B. J. (2004). Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75, 749–755. doi: 10.1189/jlb.1103543
O’Dwyer, D. N., Armstrong, M. E., Trujillo, G., Cooke, G., Keane, M. P., Fallon, P. G., et al. (2013). The toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 188, 1442–1450. doi: 10.1164/rccm.201304-0760OC
Oshiumi, H., Tsujita, T., Shida, K., Matsumoto, M., Ikeo, K., and Seya, T. (2003). Prediction of the prototype of the human toll-like receptor gene family from the pufferfish, Fugu rubripes, genome. Immunogenetics 54, 791–800. doi: 10.1007/s00251-002-0519-8
Paraskeuas, V. V., and Mountzouris, K. C. (2019). Modulation of broiler gut microbiota and gene expression of toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult. Sci. 98, 2220–2230. doi: 10.3382/ps/pey588
Parihar, A., Eubank, T. D., and Doseff, A. I. (2010). Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2, 204–215. doi: 10.1159/000296507
Park, H.-J., Lee, C.-M., Jung, I. D., Lee, J. S., Jeong, Y.-I., Chang, J. H., et al. (2009a). Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 9, 261–267. doi: 10.1016/j.intimp.2008.10.021
Park, S. J., Lee, M. Y., Son, B. S., and Youn, H. S. (2009b). TBK1-targeted suppression of TRIF-dependent signaling pathway of toll-like receptors by 6-shogaol, an active component of ginger. Biosci. Biotechnol. Biochem. 73, 1474–1478. doi: 10.1271/bbb.80738
Parvizi, P., Mallick, A. I., Haq, K., Haghighi, H. R., Orouji, S., Thanthrige-Don, N., et al. (2012). A toll-like receptor 3 ligand enhances protective effects of vaccination against Marek's disease virus and hinders tumor development in chickens. Viral Immunol. 25, 394–401. doi: 10.1089/vim.2012.0033
Pasare, C., and Medzhitov, R. (2005). “Toll-like receptors: linking innate and adaptive immunity” in Mechanisms of Lymphocyte Activation and Immune Regulation X. eds. S. Gupta, W. E. Paul, and R. Steinman (Boston, MA: Springer), 11–18. doi: 10.1007/0-387-24180-9_2
Paul, M. S., Brisbin, J. T., Abdul-Careem, M. F., and Sharif, S. (2013). Immunostimulatory properties of toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 152, 191–199. doi: 10.1016/j.vetimm.2012.10.013
Paul, M. S., Mallick, A. I., Read, L. R., Villanueva, A. I., Parvizi, P., Abdul-Careem, M. F., et al. (2012). Prophylactic treatment with toll-like receptor ligands enhances host immunity to avian influenza virus in chickens. Vaccine 30, 4524–4531. doi: 10.1016/j.vaccine.2012.04.033
Penha Filho, R. A. C., Díaz, S. J. A., Fernando, F. S., Chang, Y. F., Andreatti Filho, R. L., and Junior, A. B. (2015). Immunomodulatory activity and control of salmonella Enteritidis colonization in the intestinal tract of chickens by lactobacillus based probiotic. Vet. Immunol. Immunopathol. 167, 64–69. doi: 10.1016/j.vetimm.2015.06.006
Pérez-Cano, F. J., Massot-Cladera, M., Rodríguez-Lagunas, M. J., and Castell, M. (2014). Flavonoids affect host-microbiota crosstalk through TLR modulation. Antioxidants 3, 649–670. doi: 10.3390/antiox3040649
Philbin, V. J., Iqbal, M., Boyd, Y., Goodchild, M. J., Beal, R. K., Bumstead, N., et al. (2005). Identification and characterization of a functional, alternatively spliced Toll‐like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 114, 507–521. doi: 10.1111/j.1365-2567.2005.02125.x
Plato, A., Hardison, S. E., and Brown, G. D. (2015). Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 37, 97–106. doi: 10.1007/s00281-014-0462-4
Prakash, P., and Gupta, N. (2005). Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J. Physiol. Pharmacol. 49, 125–131.
Pulido-Moran, M., Moreno-Fernandez, J., Ramirez-Tortosa, C., and Ramirez-Tortosa, M. (2016). Curcumin and health. Molecules 21:264. doi: 10.3390/molecules21030264
Re, F., and Strominger, J. L. (2011). Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276, 37692–37699. doi: 10.1074/jbc.M105927200
Riella, K., Marinho, R., Santos, J., Pereira-Filho, R., Cardoso, J., Albuquerque-Junior, R., et al. (2012). Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 143, 656–663. doi: 10.1016/j.jep.2012.07.028
Roach, J. C., Glusman, G., Rowen, L., Kaur, A., Purcell, M. K., Smith, K. D., et al. (2005). The evolution of vertebrate toll-like receptors. Proc. Natl. Acad. Sci. 102, 9577–9582. doi: 10.1073/pnas.0502272102
Rowland, K., Saelao, P., Wang, Y., Fulton, J. E., Liebe, G. N., McCarron, A. M., et al. (2018). Association of candidate genes with response to heat and Newcastle disease virus. Genes. 9:560. doi: 10.3390/genes9110560
Ruan, W., An, J., and Wu, Y. (2015). Polymorphisms of chicken TLR3 and 7 in different breeds. PLoS One 10:e0119967. doi: 10.1371/journal.pone.0119967
Ruan, W., Wu, Y., An, J., Cui, D., Li, H., and Zheng, S. (2012a). Toll-like receptor 2 type 1 and type 2 polymorphisms in different chicken breeds. Poult. Sci. 91, 101–106. doi: 10.3382/ps.2011-01808
Ruan, W., Wu, Y., An, J., and Zheng, S. (2012b). Polymorphisms of chicken toll-like receptors 4, 15, and 21 in different breeds. Poult. Sci. 91, 2512–2516. doi: 10.3382/ps.2012-02319
Ruan, W., and Zheng, S. (2011). Polymorphisms of chicken toll-like receptor 1 type 1 and type 2 in different breeds. Poult. Sci. 90, 1941–1947. doi: 10.3382/ps.2011-01489
Sadeghi, K., Wessner, B., Laggner, U., Ploder, M., Tamandl, D., Friedl, J., et al. (2006). Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 36, 361–370. doi: 10.1002/eji.200425995
Saleh, H. A., Yousef, M. H., and Abdelnaser, A. (2021). The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 12:606069. doi: 10.3389/fimmu.2021.606069
Sato, M., Sano, H., Iwaki, D., Kudo, K., Konishi, M., Takahashi, H., et al. (2003). Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 171, 417–425. doi: 10.4049/jimmunol.171.1.417
Schink, A., Neumann, J., Leifke, A. L., Ziegler, K., Fröhlich-Nowoisky, J., Cremer, C., et al. (2018). Screening of herbal extracts for TLR2-and TLR4-dependent anti-inflammatory effects. PLoS One 13:e0203907. doi: 10.1371/journal.pone.0203907
Schwarz, H., Schneider, K., Ohnemus, A., Lavric, M., Kothlow, S., Bauer, S., et al. (2007). Chicken toll-like receptor 3 recognizes its cognate ligand when ectopically expressed in human cells. J. Interf. Cytokine Res. 27, 97–102. doi: 10.1089/jir.2006.0098
Sharma, N., Akhade, A. S., and Qadri, A. (2013). Sphingosine-1-phosphate suppresses TLR-induced CXCL8 secretion from human T cells. J. Leukoc. Biol. 93, 521–528. doi: 10.1189/jlb.0712328
Shaughnessy, R. G., Meade, K. G., Cahalane, S., Allan, B., Reiman, C., Callanan, J. J., et al. (2009). Innate immune gene expression differentiates the early avian intestinal response between salmonella and campylobacter. Vet. Immunol. Immunopathol. 132, 191–198. doi: 10.1016/j.vetimm.2009.06.007
Sheoran, N., Kumar, R., Kumar, A., Batra, K., Sihag, S., Maan, S., et al. (2017). Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in broilers. Vet. World 10, 121–129. doi: 10.14202/vetworld.2017
Shi, Y., Zhong, L., Liu, Y., Zhang, J., Lv, Z., Li, Y., et al. (2020). Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (monopterus albus). Animals 10:1744. doi: 10.3390/ani10101744
Shimazu, R., Akashi, S., Ogata, H., Nagai, Y., Fukudome, K., Miyake, K., et al. (1999). MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J. Exp. Med. 189, 1777–1782. doi: 10.1084/jem.189.11.1777
Singh, N., Tailang, M., and Mehta, S. (2016). A review on herbal plants as immunomodulators. Int. J. Pharm. Sci. Res. 7, 882–889. doi: 10.13040/IJPSR.0975-8232
Sironi, M., Biasin, M., Cagliani, R., Forni, D., De Luca, M., Saulle, I., et al. (2012). A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J. Immunol. 188, 818–823. doi: 10.4049/jimmunol.1102179
Skevaki, C., and Pararas, M. (2015). Single nucleotide polymorphisms of toll-like receptors and susceptibility to infectious diseases. Clin. Exp. Immunol. 180, 165–177. doi: 10.1111/cei.12578
Stewart, C. R., Bagnaud-Baule, A., Karpala, A. J., Lowther, S., Mohr, P. G., Wise, T. G., et al. (2012). Toll-like receptor 7 ligands inhibit influenza A infection in chickens. J. Interf. Cytokine Res. 32, 46–51. doi: 10.1089/jir.2011.0036
Studzińska, M., Jabłońska, A., Wiśniewska-Ligier, M., Nowakowska, D., Gaj, Z., Leśnikowski, Z. J., et al. (2017). Association of TLR3 L412F polymorphism with cytomegalovirus infection in children. PLoS One 12:e0169420. doi: 10.1371/journal.pone.0169420
Sunder, J., Sujatha, T., Rajas, A., and Kundu, A. (2016). Immunomodulatory effect of Morinda citrifolia and Andrographis paniculata on expression of toll-like receptors in Nicobari fowl. Indian J. Anim. Sci. 86, 1006–1008.
Svensson, A., Tunbäck, P., Nordström, I., Padyukov, L., and Eriksson, K. (2012). Polymorphisms in toll-like receptor 3 confer natural resistance to human herpes simplex virus type 2 infection. J. Gen. Virol. 93, 1717–1724. doi: 10.1099/vir.0.042572-0
Takeda, K., and Akira, S. (2005). Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14. doi: 10.1093/intimm/dxh186
Tamiru, N., Alkie, A. Y., Hodgins, D. C., Kulkarni, R. R., Taha-Abdelaziz, K., and Sharif, S. (2019). Development of innate immunity in chicken embryos and newly hatched chicks: a disease control perspective. Avian Pathol. 48, 288–310. doi: 10.1080/03079457.2019.1607966
Tanabe, M., Kurita-Taniguchi, M., Takeuchi, K., Takeda, M., Ayata, M., Ogura, H., et al. (2003). Mechanism of up-regulation of human toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem. Biophys. Res. Commun. 311, 39–48. doi: 10.1016/j.bbrc.2003.09.159
Tate, M. D., Pickett, D. L., van Rooijen, N., Brooks, A. G., and Reading, P. C. (2010). Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J. Virol. 84, 7569–7580. doi: 10.1128/JVI.00291-10
Temperley, N. D., Berlin, S., Paton, I. R., Griffin, D. K., and Burt, D. W. (2008). Evolution of the chicken toll-like receptor gene family: a story of gene gain and gene loss. BMC Genomics 9:62. doi: 10.1186/1471-2164-9-62
Teng, P. Y., and Kim, W. K. (2018). Review: roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 5:245. doi: 10.3389/fvets.2018.00245
Thakur, A. K., Soni, U. K., Rai, G., Chatterjee, S. S., and Kumar, V. (2014). Protective effects of Andrographis paniculata extract and pure andrographolide against chronic stress-triggered pathologies in rats. Cell. Mol. Neurobiol. 34, 1111–1121. doi: 10.1007/s10571-014-0086-1
Thakur, A. K., Soni, U. K., Rai, G., Chatterjee, S. S., and Kumar, V. (2015). Andrographolide modulate some toll-like receptors and cytokines expressions in HL-60 cell line. Pharm. Pharm. Int. J. 2, 116–120. doi: 10.15406/ppij.2015.02.00027
Tipping, P. G. (2006). Toll-like receptors: the interface between innate and adaptive immunity. J. Am. Soc. Nephrol. 17, 1769–1771. doi: 10.1681/ASN.2006050489
Tohidi, R., Idris, I. B., Panandam, J. M., and Bejo, M. H. (2012). The effects of polymorphisms in IL-2, IFN-γ, TGF-β2, IgL, TLR-4, MD-2, and iNOS genes on resistance to salmonella Enteritidis in indigenous chickens. Avian Pathol. 41, 605–612. doi: 10.1080/03079457.2012.739680
Tong, W., Chen, X., Song, X., Chen, Y., Jia, R., Zou, Y., et al. (2020). Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4, NFκB/MAPKs/IRF3. Exp. Ther. Med. 19, 1824–1834. doi: 10.3892/etm.2019.8396
Trckova, M., Faldyna, M., Alexa, P., Zajacova, Z. S., Gopfert, E., Kumprechtova, D., et al. (2014). The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 92, 767–774. doi: 10.2527/jas.2013-6793
Ullah, M. A., Johora, F. T., Sarkar, B., Araf, Y., and Rahman, M. D. H. (2020). Curcumin analogs as the inhibitors of TLR4 pathway in inflammation and their drug like potentialities: a computer-based study. J. Recep. Signal Transduction 40, 324–338. doi: 10.1080/10799893.2020.1742741
Ulupi, N., Sumantri, C., and Wibawan, I. (2013). Association of TLR4 gene genotype and resistance against salmonella enteritidis natural infection in kampung chicken. Int. J. Poult. Sci. 12, 445–450. doi: 10.3923/ijps.2013.445.450
Verstak, B., Nagpal, K., Bottomley, S. P., Golenbock, D. T., Hertzog, P. J., and Mansell, A. (2009). MyD88 adapter-like (mal)/TIRAP interaction with TRAF6 is critical for TLR2-and TLR4-mediated NF-κB proinflammatory responses. J. Biol. Chem. 284, 24192–24203. doi: 10.1074/jbc.M109.023044
Vidya, M. K., Kumar, V. G., Sejian, V., Bagath, M., Krishnan, G., and Bhatta, R. (2017). Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 37, 20–36. doi: 10.1080/08830185.2017.1380200
Wan, Z., Durrer, C., Mah, D., Simtchouk, S., and Little, J. P. (2014). One-week high-fat diet leads to reduced toll-like receptor 2 expression and function in young healthy men. Nutr. Res. 34, 1045–1051. doi: 10.1016/j.nutres.2014.08.012
Wang, Y., Song, E., Bai, B., and Vanhoutte, P. M. (2016). Toll-like receptors mediating vascular malfunction: lessons from receptor subtypes. Pharmacol. Ther. 158, 91–100. doi: 10.1016/j.pharmthera.2015.12.005
Wang, W., Wang, J., Dong, S.-F., Liu, C.-H., Italiani, P., Sun, S.-H., et al. (2010). Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 31, 191–201. doi: 10.1038/aps.2009.205
Werling, D., Jann, O. C., Offord, V., Glass, E. J., and Coffey, T. J. (2009). Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 30, 124–130. doi: 10.1016/j.it.2008.12.001
Wigley, P. (2004). Genetic resistance to salmonella infection in domestic animals. Res. Vet. Sci. 76, 165–169. doi: 10.1016/S0034-5288(03)00117-6
Wlasiuk, G., Khan, S., Switzer, W. M., and Nachman, M. W. (2009). A history of recurrent positive selection at the toll-like receptor 5 in primates. Mol. Biol. Evol. 26, 937–949. doi: 10.1093/molbev/msp018
Yilmaz, A., Shen, S., Adelson, D. L., Xavier, S., and Zhu, J. J. (2005). Identification and sequence analysis of chicken toll-like receptors. Immunogenetics 56, 743–753. doi: 10.1007/s00251-004-0740-8
Yin, D., Du, E., Yuan, J., Gao, J., Wang, Y., Aggrey, S. E., et al. (2017). Supplemental thymol and carvacrol increases ileum lactobacillus population and reduces effect of necrotic enteritis caused by clostridium perfringes in chickens. Sci. Rep. 7:7334. doi: 10.1038/s41598-017-07420-4
Yitbarek, A., Echeverry, H., Brady, J., Hernandez-Doria, J., Camelo-Jaimes, G., Sharif, S., et al. (2012). Innate immune response to yeast-derived carbohydrates in broiler chickens fed organic diets and challenged with Clostridium perfringens. Poult. Sci. 91, 1105–1112. doi: 10.3382/ps.2011-02109
Yitbarek, A., Rodriguez-Lecompte, J., Echeverry, H., Munyaka, P., Barjesteh, N., Sharif, S., et al. (2013). Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 92, 2299–2310. doi: 10.3382/ps.2013-03141
Zarember, K. A., and Godowski, P. J. (2002). Tissue expression of human toll-like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168, 554–561. doi: 10.4049/jimmunol.168.2.554
Zhang, P., Ding, Z., Liu, X., Chen, Y., Li, J., Tao, Z., et al. (2018). Enhanced replication of virulent Newcastle disease virus in chicken macrophages is due to polarized activation of cells by inhibition of TLR7. Front. Immunol. 9:366. doi: 10.3389/fimmu.2018.00366
Zhang, Y., and Liang, C. (2016). Innate recognition of microbial-derived signals in immunity and inflammation. Sci. China Life Sci. 59, 1210–1217. doi: 10.1007/s11427-016-0325-6
Zhang, L., Liu, R., Ma, L., Wang, Y., Pan, B., Cai, J., et al. (2012). Eimeria tenella: expression profiling of toll-like receptors and associated cytokines in the cecum of infected day-old and three-week old SPF chickens. Exp. Parasitol. 130, 442–448. doi: 10.1016/j.exppara.2012.01.013
Keywords: chicken, toll-like receptors, gene expression, nutrigenomic, polymorphism, immunity
Citation: Rehman MS-u, Rehman Su, Yousaf W, Hassan F-u, Ahmad W, Liu Q and Pan H (2021) The Potential of Toll-Like Receptors to Modulate Avian Immune System: Exploring the Effects of Genetic Variants and Phytonutrients. Front. Genet. 12:671235. doi: 10.3389/fgene.2021.671235
Received: 13 April 2021; Accepted: 27 July 2021;
Published: 26 August 2021.
Edited by:
Jiuzhou Song, University of Maryland, United StatesReviewed by:
Cintia Hiromi Okino, Brazilian Agricultural Research Corporation (EMBRAPA), BrazilCopyright © 2021 Rehman, Rehman, Yousaf, Hassan, Ahmad, Liu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyou Liu, cXlsaXUtZ2VuZUBneHUuZWR1LmNu; Hongping Pan, cGFuaHA2NUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.