- 1Department of Pediatrics, Ruijin Hospital Affiliated to Shanghai Jiao Tong University, Shanghai, China
- 2State Key Laboratory of Molecular Biology, Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China
- 3Shanghai QingCongquan Training Center for Children With Special Needs, Shanghai, China
- 4Cancer Center, School of Medicine, Shanghai Tenth People's Hospital, Tongji University, Shanghai, China
Background: Central precocious puberty (CPP) is one of the most common and complex problems in clinical pediatric endocrinology practice. Mutation of the MKRN3 gene can cause familial CPP.
Methods and Results: Here we reported a Chinese patient bearing a novel MKRN3 mutation (c.G277A/p.Gly93Ser) and showing the CPP phenotype. Functional studies found that this mutation of MKRN3 attenuated its autoubiquitination, degradation, and inhibition on the transcriptional activity of GNRH1, KISS1, and TAC3 promoters.
Conclusion: MKRN3 (Gly93Ser) is a loss-of-function mutation, which attenuates the inhibition on GnRH1-related signaling, suggesting that this mutant can lead to central precocious puberty.
Introduction
Normal puberty initiation is a process of hypothalamic pituitary gonadal axis activation by pulse secreting of hypothalamic gonadotropin releasing hormone (GnRH) (Yang et al., 2019). The timing of initiation of puberty is determined by the co-regulation of some unknown activation or inhibitory factors. Studies have found that many genes regulate puberty initiation, such as KISS1, GPR54, LIN28B, and MKRN3 (He et al., 2018; Pagani et al., 2020). Mutations or single nucleotide polymorphisms are associated with precocious puberty. Central precocious puberty (CPP) is one of the most common and complex problems in clinical pediatric endocrinology practice. Most CPP patients are sporadic, and almost 30% of them are familial (Li C. Y. et al., 2020). Familial precocious puberty caused by MRKN3 mutations was first reported by Abreu et al. (2013). So far, more and more MKRN3 mutation-related familial CPP has been reported (Aycan et al., 2018; Lu et al., 2018; Fanis et al., 2019; Filibeli et al., 2019). The MKRN3 gene is a maternal imprinted gene, located on chromosome 15q11-q13, which contains only one exon, without introns (Li C. Y. et al., 2020). The gene is highly conserved among different species, and encodes E3 ubiquitin ligases, which participate in the process of selective degradation of proteins in organisms (Abreu et al., 2015; Filibeli et al., 2019).
Our previous study demonstrated that genetic ablation of Mkrn3 did accelerate mouse puberty onset with increased production of hypothalamic GnRH1. MKRN3 interacts with and ubiquitinates MBD3, which epigenetically silences GNRH1 through disrupting MBD3 binding to the GNRH1 promoter and recruitment of DNA demethylase TET2 (Li C. Y. et al., 2020). In this study, a novel MKRN3 variant (c.G277A/p.Gly93Ser) was found in a Chinese patient with familial precocious puberty, and functional tests indicated it as a loss-of-function mutation.
Materials and Methods
Editorial Policies and Ethical
A Chinese patient with a novel MKRN3 gene mutation was recruited. This study was approved by the Institutional Review Board of Ruijin Hospital. Informed consent was obtained from the participant.
Molecular Investigations
DNA was extracted from peripheral blood leukocytes using a DNA extraction kit (Qiagen, Hilden, Germany). A custom gene panel was designed and used to capture the targeted sequence, covering all exons and flanking sequences (including the 10 bp of introns) of 187 genes which are associated with the growth and development of children. The procedure for preparation of libraries was consistent with standard operating protocols previously described (Dai et al., 2019). The average mean depth for the targeted regions was 370, and 84.4% of the covered exons had ≥10 reads. Available reads data were 35.1M. The candidate mutation was confirmed with Sanger sequencing using the following primers for MKRN3:
Forward primers: 5′-AGCAAGGGAGGGTGTGTCTG-3′;
Reverse primers: 5′-GAGCCAATCACAGGCAAGGAAAG-3′.
Plasmids Construction
The plasmids pCDNA3.0-MKRN3-3xFlag, pRK5-HA-UB, pRL-TK, pGL3-basic, pGL3-miniCMV, and pGL3-GNRH1-p were kindly provided by Professor Ronggui Hu (Chinese Academy of Sciences, Shanghai, China). Mutation of MKRN3 (c.G277A/p.Gly93Ser) was introduced by site-directed mutagenesis as previously reported (Xu et al., 2018). The promoter regions of the KISS1 and TAC3 genes were amplified from human HEK293T genomic DNA (gDNA) and inserted into the pGL3-basic plasmid, generating pGL3-KISS1-p and pGL3-TAC3-p plasmids.
Cell Culture and Transfection
Human HEK293T and mouse GT1-7 cell lines were kindly provided by Professor Ronggui Hu (Chinese Academy of Sciences, Shanghai, China) and cultured in Dulbecco's modified Eagle medium (DMEM, Life Technologies, USA) or DMEM/F12 (1:1) medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 mg/ml of streptomycin (all from Gibco, Layola, USA) in a 37°C humidified atmosphere of 5% CO2. Plasmids were transfected into HEK293T cells using Lipofectamine 2000 (Life Technologies, Carlsbad, USA) according to the manufacturer's instructions.
Immunoblotting
Immunoblotting was done as previously described (Li C. et al., 2020). Briefly, the lysates of HEK293T cells transfected with plasmids were lysed in RIPA buffer (50 mM of Tris–HCl (PH 7.6), 150 mM of NaCl, 5 mM of EDTA, 0.1% sodium dodecyl sulfate (SDS), and 1% NP-40) supplemented with protease inhibitor cocktails (Roche, Germany). The cleared supernatant lysates were incubated with specific antibodies and protein G agarose beads or incubated with Anti-Flag affinity gels. The immunoprecipitants were denatured at 100°C for 10 min in 2 × SDS-PAGE sampling buffer. The inputs, immunoprecipitants, and other cell lysates were then subjected to SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, USA). The membranes were incubated with the appropriate antibodies against GAPDH (1:5000, 60004-1-Ig, Proteintech, China), Flag (1:1000, 20543-1-AP, Proteintech), or HA (1:2000, 51064-2-AP, Proteintech). Secondary antibodies were labeled with HRP, and the signals were visualized using the Tanon 5200 Imaging System (Tanon, China).
Luciferase Reporter Assays
HEK293T cells were seeded at 0.5 × 105 cells/well in 24-well plates. After overnight culture, cells were transiently transfected with pGL3-GNRH1-p, pGL3-KISS1-p, or pGL3-TAC3-p together with other vectors (pRL-TK, wild-type MKRN3, or its G73S mutant). A total of 48 h after transfection, the cells were harvested, lysed with 5X passive buffer, and subjected to a Dual-Luciferase Reporter assay according to the manufacturer's instructions (Promega, USA). Data are expressed as mean ± SD and analyzed using one-way ANOVA with Bonferroni post-hoc test. *P < 0.05 denotes significant difference and **P < 0.01 denotes very significant difference over three independent experiments.
Enzyme-Linked Immunosorbent Assay
A total of 48 h after the GT1–7 cells were transfected, the complete medium was replaced with serum-free DMEM for 24 h to synchronize the cell cycles. Then, 24 h after co-incubation, the supernatants were harvested and subjected to GnRH1 (Phoenix pharmaceuticals, RK-040-02) concentration analysis according to the manufacturer's recommendations (Li C. Y. et al., 2020).
In silico Analysis of the Variant
The computational algorithms Polyphen2, SIFT, and Mutation Taster were used to predict the pathogenicity of the missense variant.
Statistics
Data were analyzed by two tailed unpaired t-test or one-way ANOVA with Bonferroni post-hoc test using GraphPad Prism 7. *p < 0.05 was considered to be significant, **p < 0.01 was considered to be very significant.
Results
Clinical Characteristics and Identification of MKRN3 Gene Mutation
The proband is a 9-year-old girl and her breasts enlarged at the age of 7.5 with knots and tenderness. Her growth velocity (GV) had no significant acceleration (height 128 cm) and the age of bone was not advanced. Her breasts had enlarged significantly with vaginal discharge in the last 9 months, with a GV increase of 11 cm/year. She had not yet started menstruating. She denied a history of supplements and special drugs. Her height was 143.4 cm (1.15SD) and her weight was 46.8 kg. Her BMI was 22.8kg/m2. She was well-balanced and had no facial irregularities. No milk or coffee spots were observed, and no deformity of limbs and spine was found. Anthropometric and laboratory parameters for the proband are shown in Table 1.
Her father was 169 cm tall and his voice changed at 14 years old. Her mother was 163 cm tall and menarched at 12 years old. The patient's target height was 159.5 cm. Her grandfather was 167 cm tall, and her grandmother, who had an early menarche at 9 years old, was 147 cm tall.
Genes panel analysis indicated that the proband had a novel MKRN3 gene mutation (Figure 1A). Her mutation was a point mutation (c.G277A) in exon1 of the MKRN3 gene which leads to glycine (G) being substituted with serine (S) at condon 93 (p.Gly93Ser) (Figures 1B, 2A). Further Sanger sequencing exhibited that her father had the same mutation, and no MKRN3 gene mutation was found in her mother (Figure 1B).
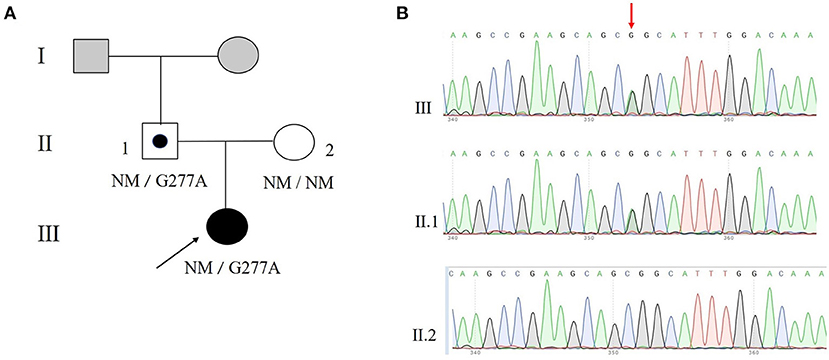
Figure 1. A novel mutation of MKRN3 in a central precocious puberty (CPP) patient. (A) Pedigree of a family with a novel c.227G>A mutation in the human MKRN3 gene. (B) Partial sequencing chromatographs of the MKRN3 gene of the CPP patient and her family members. Squares indicate male family members; circles, female family members; black, individuals with CPP; gray, individuals CPP status unknown; symbol with black dot inside, asymptomatic carriers; NM, no mutation allele. The arrow indicates the proband.
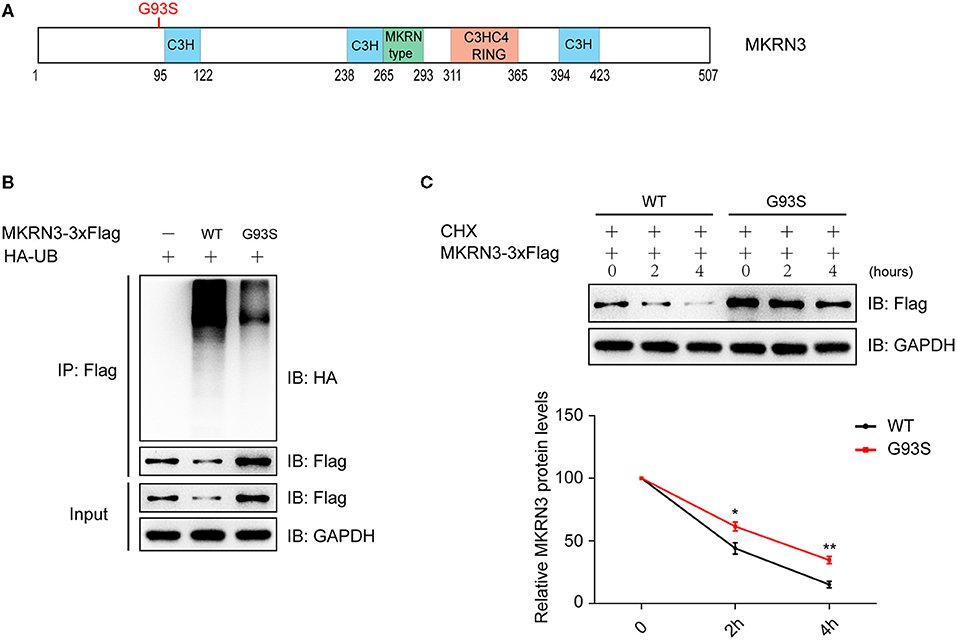
Figure 2. Mutation of MKRN3 attenuated its autoubiquitination and degradation. (A) Schematic view of human MKRN3 protein mutation involved in this study. H, histidine; C, cysteine. (B) Ubiquitination of the wild-type MKRN3 protein was more significant than that of the disease-associated mutant (G93S) in HEK293T cells. Cells were transformed with plasmids encoding HA-Ub and Flag-tagged wild-type MKRN3, or MKRN3 (G93S) mutant. MKRN3 proteins were immunoprecipitated using anti-Flag beads followed by immunoblotting with anti-HA to detect ubiquitination signals. WT, wild-type. (C) The wild-type MKRN3 protein was less stable than the G93S mutant. Flag-tagged wild-type MKRN3 or G93S mutant were expressed in HEK293T cells. Cells were treated with CHX (100 ug/ml) at different time durations (0, 2, or 4 h) before harvest for immunoblotting analysis. Data are presented as mean ± SD, one-way ANOVA was used with the Bonferroni post-hoc test over three independent experiments. *P < 0.05, significant difference; **P < 0.01, very significant difference, over three independent experiments.
In silico Analysis of the Variant
We used several in silico computational algorithms (Polyphen2, SIFT, and Mutation Taster) to predict the protein function. PolyPhen-2 classified the variant as “probably damaging” with a score of 1, while SIFT predicted that the mutation was neutral, and Mutation Taster thought it was a polymorphism.
Mutation of MKRN3 Attenuated Its Autoubiquitination and Degradation
The point mutation (p.G93S) is near the C3H domain of the MKRN3 protein (Figure 2A). As revealed by a previous study, CPP-associated mutations compromise the auto-ubiquitination of MKRN3 (Abreu et al., 2020; Li C. Y. et al., 2020). Our study found that the ubiquitination of wild-type MKRN3 protein was more significant than that of the disease-associated mutant (G93S) in HEK293T cells (Figure 2B), and protein stability detected by immunoblotting analysis indicated that wild-type MKRN3 was less stable than the G93S mutant (Figure 2C).
Mutation of MKRN3 Attenuated Its Inhibition on GnRH1-Related Signaling
To study the function of wild-type and mutant MKRN3, luciferase reporter vectors for human GNRH1, KISS1, and TAC3 gene promoters were constructed, and the miniCMV promoter acted as a negative control (Figure 3A). As showed in Figure 3B, MKRN3 showed little activity on the miniCMV promoter. Compared to wild-type MKRN3, the G93S mutant led to weaker suppression of the transcriptional activity of the GNRH1, KISS1, and TAC3 promoters, as revealed in Figures 3C–E. In GT1-7 cells that were derived from mouse hypothalamic GnRH-positive neurons, wild-type MKRN3 significantly repressed the protein level of GnRH1, while the G93S mutant lost this effect (Figure 3F).
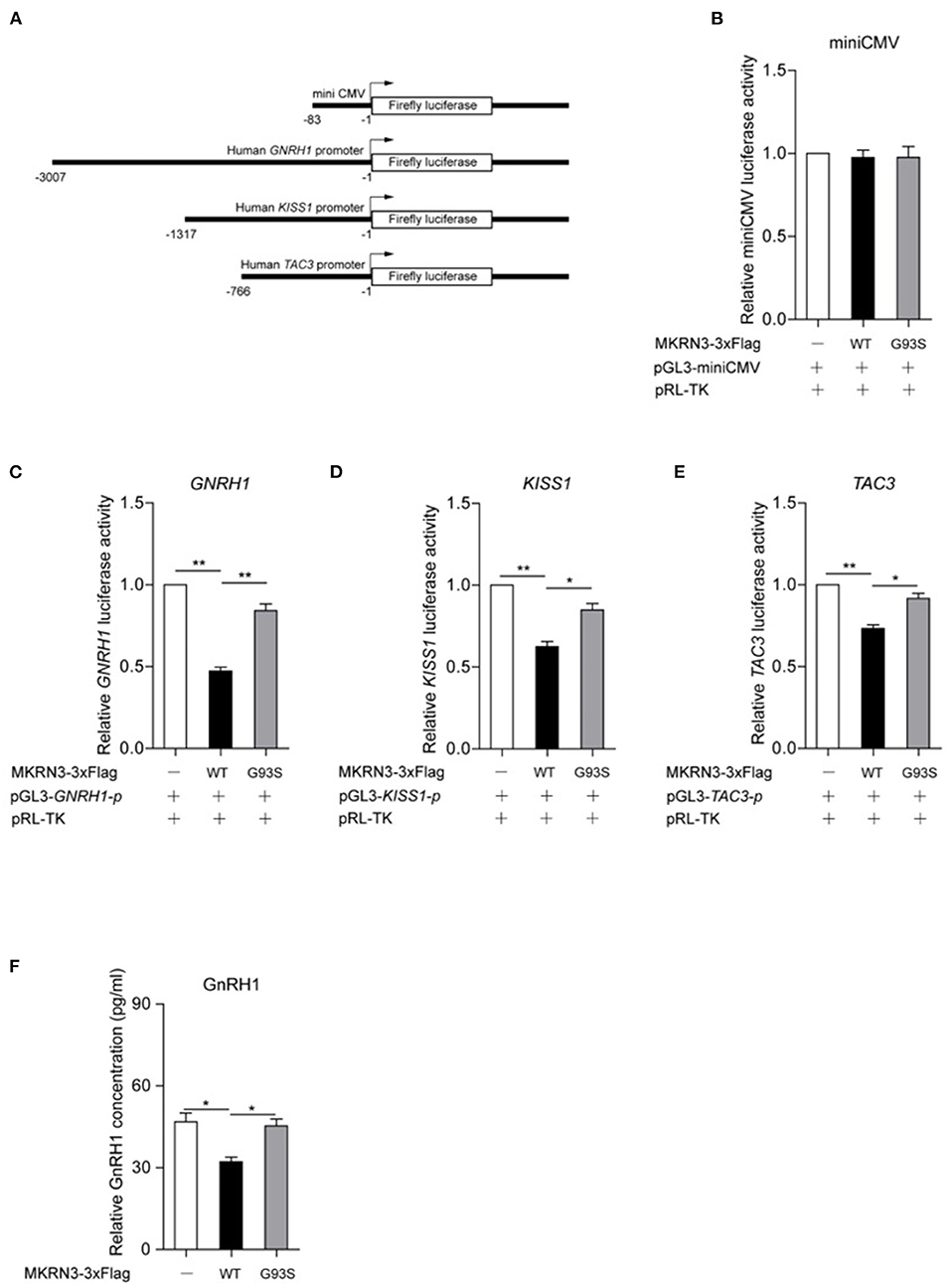
Figure 3. Mutation of MKRN3 attenuated its inhibition on GnRH1-related signaling. (A) Schematic diagrams for the construction of luciferase reporter vectors for the human GNRH1, KISS1, and TAC3 gene promoters. The indicated region of promoters was amplified and inserted into the pGL3-basic vector. The miniCMV promoter was inserted and acted as a negative control. (B–D) A luciferase reporter assay was used to detect the relative activities of (B) miniCMV, (C) GNRH1, (D) KISS1, and (E) TAC3 promoters in HEK293T cells. HEK293T cells were transfected with the indicated plasmids and luciferase activities were detected. Data are presented as mean ± SD, one-way ANOVA was used with the Bonferroni post-hoc test over three independent experiments. (F) Mutation of MKRN3 attenuated its inhibition on GnRH1 levels in GT1-7 cells. GT1-7 cells were transfected with empty vector, wild-type MKRN3, or the G93S mutant. A total of 48 h later, the supernatant was collected and detected by ELISA. Data are presented as mean ± SD, one-way ANOVA was used with the Bonferroni post-hoc test over three independent experiments. *P < 0.05, significant difference; **P < 0.01, very significant difference, over three independent experiments.
Discussion
Puberty is a transition period from childhood to adulthood with a gradual maturation of the sexual and reproductive systems. Children with central precocious puberty (CPP) show an advanced initiation of hypothalamus-pituitary-gonadal (HPG) axis function, which leads to a rapid development of internal and external reproductive organs and secondary sexual sign before the age of 8 in girls and the age of 9 in boys (Leger, 2002; Kirkgoz et al., 2020). The incidence of CPP is about 1/5,000–10,000, accounting for more than 30% of the total number of pediatric endocrine outpatients (Abreu et al., 2015; Li C. Y. et al., 2020). Children with CPP will suffer from short stature in adulthood as a result of a premature and massive secretion of sex hormones that accelerate bone maturation and premature epiphyseal fusion (Bodicoat et al., 2014). Pre-mature breast development and early onset in girls increases the risk of breast cancer in adulthood and may increase the risk of obesity, diabetes, and cardiovascular disease in the future (Elks et al., 2013; Prentice and Viner, 2013; Bodicoat et al., 2014). The premature development of children's sexual characteristics alongside their immature intelligence and sexual psychology can lead to children's psychological disorders or cause various social problems, so early diagnosis and intervention are needed.
The timing of puberty initiation is thought to be determined by the co-regulation of unknown activation or inhibitory factors. Our recent study demonstrated that MKRN3 acts as an important mammalian puberty initiation regulator (Abreu et al., 2020). A previous study proposed that MKRN3 does not directly alter GNRH1 expression (Yellapragada et al., 2019). However, there are many environmental and metabolic signals postnatally, that may regulate further maturation and function of GnRH neurons. In our study, MKRN3 interacts with and ubiquitinates MBD3, which epigenetically silences GNRH1 through disrupting MBD3 binding to the GNRH1 promoter and recruitment of DNA demethylase TET2 (Li C. Y. et al., 2020). As revealed in this study, which was similar to our previous study, MKRN3 represses the transcriptional activity of human GNRH1 promoter activity, but mutation of MKRN3 attenuates this affect (Li C. et al., 2020). We also found that MKRN3 represses the transcriptional activity of human KISS1 and TAC3 promoter activity, which was consistent with the study conducted by Abreu et al. (2020).
The ubiquitin-proteasome system (UPS) mediates the degradation of most cell proteins, and the selection of target proteins is mainly controlled by E3 ligases (Hu and Sun, 2016). CPP-associated mutations compromise the auto-ubiquitination and degradation of MKRN3, including the MKRN3 (G93S) mutant involved in our study (Abreu et al., 2020; Li C. Y. et al., 2020). As revealed in our previous study, MKRN3 mediates the ubiquitination of MBD3 which binds and activates the GNRH1 promoter, resulting in a low level of GnRH1. Children with loss-of-function MKRN3 mutations tend to have an advanced initiation of the hypothalamus-pituitary-gonadal (HPG) axis, and a rapid development of internal and external reproductive organs and secondary sexual sign (Abreu et al., 2015). In our study, functional tests indicated that MKRN3 (G93S) is a loss-of-function mutation, which attenuated the inhibition of GnRH1-related signaling, suggesting this mutant can lead to central precocious puberty.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ruijin Hospital Affiliated to Shanghai Jiao Tong University, Shanghai. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WL and CL: conceptualization and writing—original draft preparation. XY, JW, and TH: methodology, validation, and formal analysis. WL: investigation. WL, WW, and ZD: resources. XY: data curation. CL, WL, and YL: writing—review and editing. CL: visualization. ZD: supervision. WL: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Shanghai Science & Technology Committee (14411958600), the Shanghai Talent Development Fund (2017120), and the National Natural Science Foundation of China (31900804).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the patient and her family who participated in this study.
References
Abreu, A. P., Dauber, A., Macedo, D. B., Noel, S. D., Brito, V. N., Gill, J. C., et al. (2013). Central precocious puberty caused by mutations in the imprinted gene MKRN3. New. Engl. J. Med. 368, 2467–2475. doi: 10.1056/NEJMoa1302160
Abreu, A. P., Macedo, D. B., Brito, V. N., Kaiser, U. B., and Latronico, A. C. (2015). A new pathway in the control of the initiation of puberty: the MKRN3 gene. J. Mol. Endocrinol. 54, R131–R139. doi: 10.1530/JME-14-0315
Abreu, A. P., Toro, C. A., Song, Y. B., Navarro, V. M., Bosch, M. A., Eren, A., et al. (2020). MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J. Clin. Invest. 130, 4486–4500. doi: 10.1172/JCI136564
Aycan, Z., Savas-Erdeve, S., Cetinkaya, S., Kurnaz, E., Keskin, M., Sahin, N. M., et al. (2018). Investigation of MKRN3 mutation in patients with familial central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 10, 223–229. doi: 10.4274/jcrpe.5506
Bodicoat, D. H., Schoemaker, M. J., Jones, M. E., McFadden, E., Griffin, J., Ashworth, A., et al. (2014). Timing of pubertal stages and breast cancer risk: the breakthrough generations study. Breast Cancer Res. 22:R18. doi: 10.1186/bcr3613
Dai, Y., Liang, S. R., Dong, X., Zhao, Y. H., Ren, H. T., Guan, Y. Z., et al. (2019). Whole exome sequencing identified a novel DAG1 mutation in a patient with rare, mild and late age of onset muscular dystrophy-dystroglycanopathy. J. Cell. Mol. Med. 23, 811–818. doi: 10.1111/jcmm.13979
Elks, C. E., Ong, K. K., Scott, R. A., van der Schouw, Y. T., Brand, J. S., Wark, P. A., et al. (2013). Age at menarche and type 2 diabetes risk the EPIC-interact study. Diabetes Care 36, 3526–3534. doi: 10.2337/dc13-0446
Fanis, P., Skordis, N., Toumba, M., Papaioannou, N., Makris, A., Kyriakou, A., et al. (2019). Central precocious puberty caused by novel mutations in the promoter and 5 '-utr region of the imprinted MKRN3 gene. Front. Endocrinol. 10:677. doi: 10.3389/fendo.2019.00677
Filibeli, B. E., Ayranci, I., Manyas, H., Kirbiyik, O., Dundar, B., and Catli, G. (2019). MKRN3 gene mutation in a case of familial central precocious puberty. Hormone Res. Paediatr. 91:595.
He, Y. Y., Han, X. H., Sun, W., Yu, J., and Tamadon, A. (2018). Precocious puberty and the Lin28/Let7 pathway: the therapeutic effect of the nourishing “yin” and purging “fire” traditional chinese medicine mixture in a rat model. Evid. Based Compl. Alternat. Med. 2018:4868045. doi: 10.1155/2018/4868045
Hu, H. B., and Sun, S. C. (2016). Ubiquitin signaling in immune responses. Cell Res. 26, 457–483. doi: 10.1038/cr.2016.40
Kirkgoz, T., Karakoc-Aydiner, E., Bugrul, F., Abali, Z. Y., Helvacioglu, D., Kiykim, A., et al. (2020). Management of systemic hypersensitivity reactions to gonadotropin-releasing hormone analogues during treatment of central precocious puberty. Hormone Res. Paediatr. 93, 66–72. doi: 10.1159/000505329
Leger, J. (2002). Management of central precocious puberty. Arch Pediatr. 9, 1283–1287. doi: 10.1016/S0929-693X(02)00086-6
Li, C., Han, T., Guo, R., Chen, P., Peng, C., Prag, G., et al. (2020). an integrative synthetic biology approach to interrogating cellular ubiquitin and ufm signaling. Int. J. Mol. Sci. 21:4231. doi: 10.3390/ijms21124231
Li, C. Y., Lu, W. L., Yang, L. G., Li, Z. W., Zhou, X. Y., Guo, R., et al. (2020). MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl. Sci. Rev. 7, 671–685. doi: 10.1093/nsr/nwaa023
Lu, W. L., Wang, J. Q., Li, C. Y., Sun, M. Q., Hu, R. G., and Wang, W. (2018). A novel mutation in 5 '-UTR of makorin ring finger 3 gene associated with the familial precocious puberty. Acta Bioch. Bioph. Sin. 50, 1291–1293. doi: 10.1093/abbs/gmy124
Pagani, S., Calcaterra, V., Acquafredda, G., Montalbano, C., Bozzola, E., Ferrara, P., et al. (2020). MKRN3 and KISS1R mutations in precocious and early puberty. Ital. J. Pediatr. 46:39. doi: 10.1186/s13052-020-0808-6
Prentice, P., and Viner, R. M. (2013). Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int. J. Obesity 37, 1036–1043. doi: 10.1038/ijo.2012.177
Xu, X., Li, C., Gao, X., Xia, K., Guo, H., Li, Y., et al. (2018). Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 28, 48–68. doi: 10.1038/cr.2017.132
Yang, D., Zhang, W. J., Zhu, Y. X., Liu, P. N., Tao, B., Fu, Y. C., et al. (2019). Initiation of the hypothalamic-pituitary-gonadal axis in young girls undergoing central precocious puberty exerts remodeling effects on the prefrontal cortex. Front. Psychiatry. 10:332. doi: 10.3389/fpsyt.2019.00332
Keywords: central precious puberty, MKRN3, ubiquitination, GnRH, makorin RING-finger protein 3
Citation: Yin X, Wang J, Han T, Tingting Z, Li Y, Dong Z, Wang W, Li C and Lu W (2021) A Novel Loss-of-Function MKRN3 Variant in a Chinese Patient With Familial Precocious Puberty: A Case Report and Functional Study. Front. Genet. 12:663746. doi: 10.3389/fgene.2021.663746
Received: 03 February 2021; Accepted: 21 June 2021;
Published: 06 August 2021.
Edited by:
William Newman, The University of Manchester, United KingdomReviewed by:
Yuan Gao, Shandong University, ChinaAnna Grandone, University of Campania Luigi Vanvitelli, Italy
Copyright © 2021 Yin, Wang, Han, Tingting, Li, Dong, Wang, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanyin Li, bGljaHVhbnlpbjIwMTNAc2liY2IuYWMuY24=; Wenli Lu, bHdsMjA0MDU5QDEyNi5jb20=
†These authors have contributed equally to this work
 Xueling Yin
Xueling Yin Junqi Wang
Junqi Wang Tianting Han2†
Tianting Han2† Zhang Tingting
Zhang Tingting Zhiya Dong
Zhiya Dong Wei Wang
Wei Wang Chuanyin Li
Chuanyin Li