- 1Key Laboratory of Biotherapy of Zhejiang Province, Department of Cardiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Cardiology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Although several observational studies have suggested an association of elevated plasma homocysteine (Hcy) levels with increased risk of atrial fibrillation (AF), it remains unclear whether this association reflects causality. In this study, we aimed to investigate the causal association of plasma Hcy levels with AF risk.
Methods: A two-sample Mendelian randomization (MR) study was designed to investigate the causal association of Hcy with AF. Summary data on association of single nucleotide polymorphisms (SNPs) with Hcy were extracted from the hitherto largest genome-wide association study (GWAS) with up to 44,147 individuals, and statistics data on association of SNPs with AF were obtained from another recently published GWAS with up to 1,030,836 individuals. SNPs were selected at a genome-wide significance threshold (p < 5 × 10–8). Fixed-effect inverse variance weighting (IVW) method was used to calculate the causal estimate. Other statistical methods and leave-one-out analysis were applied in the follow-up sensitivity analyses. MR-Egger intercept test was conducted to detect the potential directional pleiotropy.
Results: In total, nine SNPs were identified as valid instrumental variables in our two-sample MR analysis. Fixed-effect IVW analysis indicated no evidence of causal association of genetically predicted Hcy with AF. The odds ratio (OR) and 95% confidence interval (CI) of AF per standard deviation (SD) increase in Hcy were 1.077 (0.993, 1.168), p = 0.075. Similar results were observed in the sensitivity analyses. MR-Egger intercept test suggested no evidence of potential horizonal pleiotropy.
Conclusions: This two-sample MR analysis found no evidence to support causal association of Hcy with AF.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered clinically (Lip et al., 2012; Liu et al., 2018), and millions of individuals are expected to develop AF in the next decades (Violi et al., 2016). Patients with AF are at an increased risk of developing serious complications, including stroke, heart failure, dementia, and an early mortality (Magnani et al., 2011). Early detection and intervention for patients with AF can significantly reduce the risk of complications and improve survival rate (Ponikowski et al., 2016; Marrouche et al., 2018). Several risk factors for AF have been so far identified (Magnani et al., 2011).
As a well-known marker for pro-oxidation and proinflammation, elevated levels of homocysteine (Hcy) are considered as a risk factor/biomarker/predictor for developing cardiovascular disease (CVD) (Ganguly and Alam, 2015). In addition, several randomized controlled trials have suggested protective effect of lowering plasma Hcy levels with vitamin B supplements on overall CVD risk (Qin et al., 2016; Ahmed et al., 2019). However, only several studies have addressed the association between Hcy and AF risk (Schnabel et al., 2010). Although these studies have reported an association of elevated plasma Hcy levels with increased AF risk, it is difficult to distinguish causal association from spurious association in observational studies (Snezhitsky et al., 2016; Wei et al., 2017; Yao et al., 2017a). Further studies are warranted to clarify the causative relationship between Hcy and AF.
Mendelian randomization (MR) is an epidemiological approach for making causal inferences from observational data by using genetic variants as instrumental variables (Davey Smith and Hemani, 2014; Davies et al., 2018). Compared with classical observational studies, MR can effectively avoid biased associations because of confounding or reverse causality (Sekula et al., 2016). In this study, we aimed to investigate the causality between plasma Hcy levels and AF risk.
Materials and Methods
Study Design
A two-sample MR study was designed to explore the causal association of Hcy with AF. The single nucleotide polymorphisms (SNPs) that were selected as instrumental variables (IVs) were expected to fulfill three core assumptions (Figure 1; Sekula et al., 2016). First, the identified SNPs must be strongly associated with Hcy. Second, SNPs should not be related to confounders. Third, SNPs were only associated with AF through Hcy; in other words, SNPs should not be directly associated with AF.
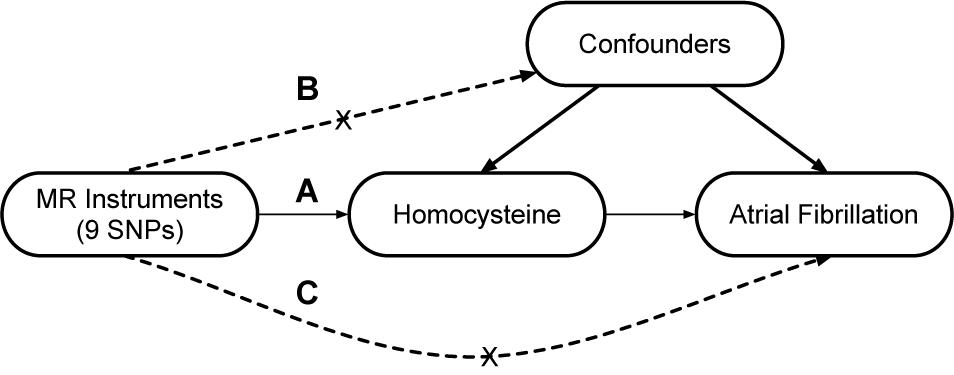
Figure 1. Design of the two-sample Mendelian randomization study. Three core assumptions were as follows: (A) the SNPs should be strongly associated with homocysteine; (B) the SNPs should not be related to confounders; (C) the SNPs should not be directly associated with atrial fibrillation.
Data Sources
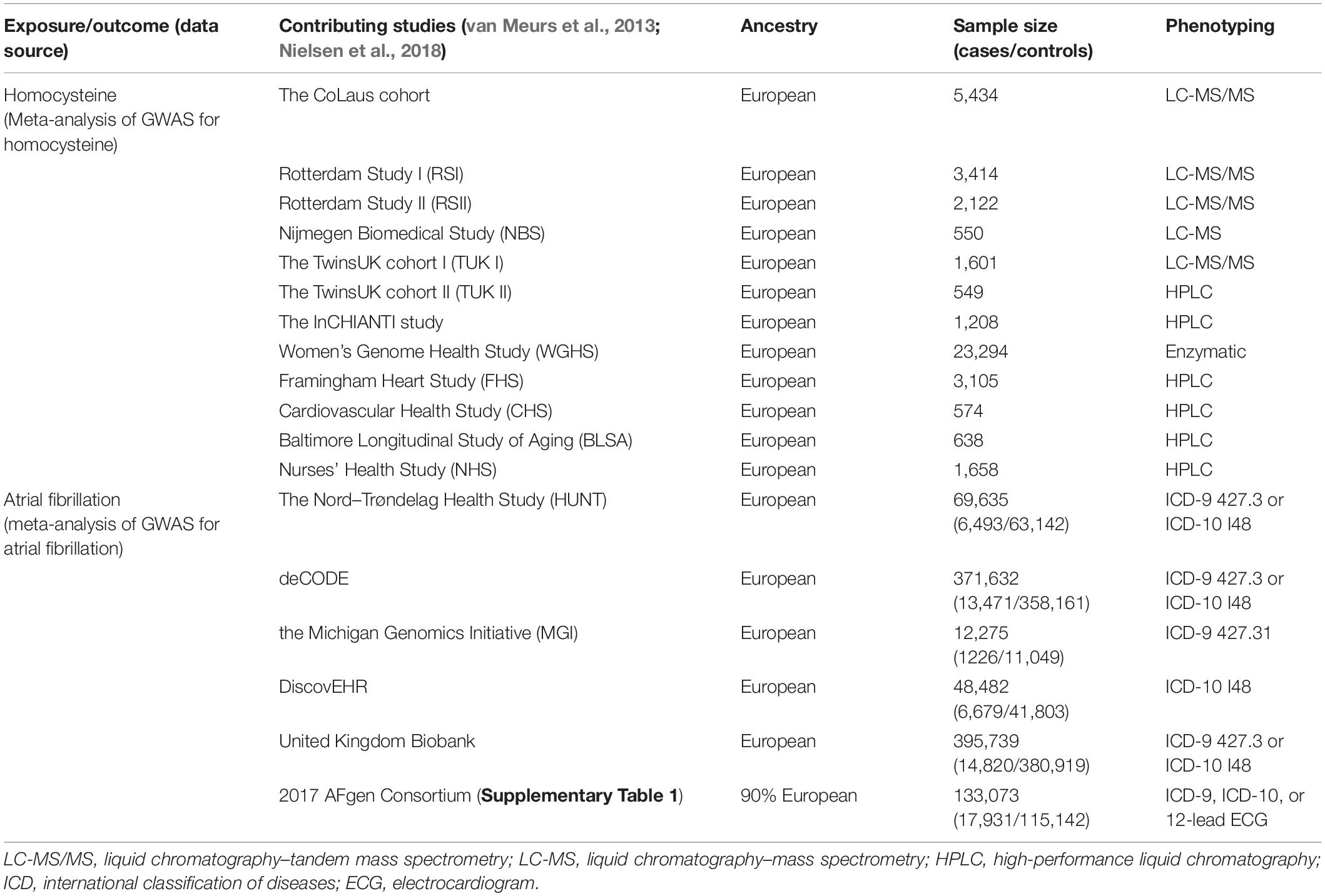
The exposure in this study was genetically predicted plasma Hcy levels. The genetic associations with Hcy were extracted from the hitherto largest genome-wide association study (GWAS) meta-analysis on Hcy. This meta-analysis included data from a total of 10 independent cohorts of European ancestry, with up to 44,147 individuals (Table 1; van Meurs et al., 2013).
The outcome of this study was the lifetime risk of AF. The summary statistics data for AF were obtained from the recently published largest GWAS. This GWAS tested the associations between up to 34,740,186 genotyped SNPs and AF, including a total of 1,030,836 individuals (60,620 cases and 970,216 controls) of European ancestry from six resources [The Nord-Trøndelag Health Study (HUNT), deCODE, the Michigan Genomics Initiative (MGI), DiscovEHR, United Kingdom Biobank, and the AFGen Consortium] (Table 1 and Supplementary Table 1; Nielsen et al., 2018).
Studies contributing data to these GWAS meta-analyses had received ethical approval from relevant institutional review boards. In the present study, we only made use of the summarized data from these studies; hence, no additional ethics approval was required.
SNPs Selection
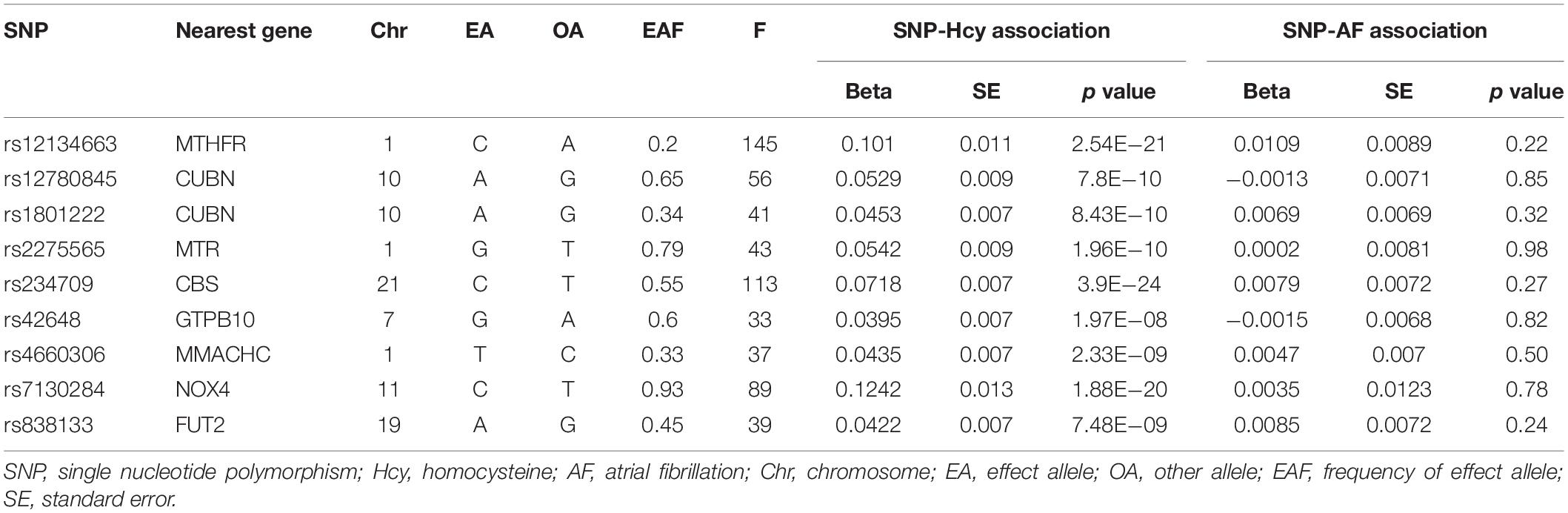
To ensure a strong association between IVs and plasma Hcy levels, we selected SNPs associated with Hcy at a genome-wide significance threshold (p < 5 × 10–8) from the corresponding dataset. Then, we used LD-Link based on European to check for the independence of selected SNPs by calculating the pairwise-linkage disequilibrium (LD) (Machiela and Chanock, 2015; Myers et al., 2020). When r2 > 0.001, the SNP correlated with more SNPs or with higher p value dropped. In addition, we looked up the selected SNPs on PhenoScanner to evaluate whether these SNPs were associated with other traits (Staley et al., 2016). We performed an additional exclusion of SNPs associated with confounding traits at genome-wide significance level, which may affect the results. Subsequently, we extracted the genetic associations with the remaining SNPs and AF from the GWAS dataset on AF. When the specified SNP was not available in the AF dataset, a highly correlated SNP (r2 > 0.8) was selected for proxy. Any SNP directly associated with AF at genome-wide significance level was excluded. Finally, estimated variance in Hcy explained by each SNP and corresponding F statistics were calculated to evaluate the strength of IVs.
Statistical Analysis
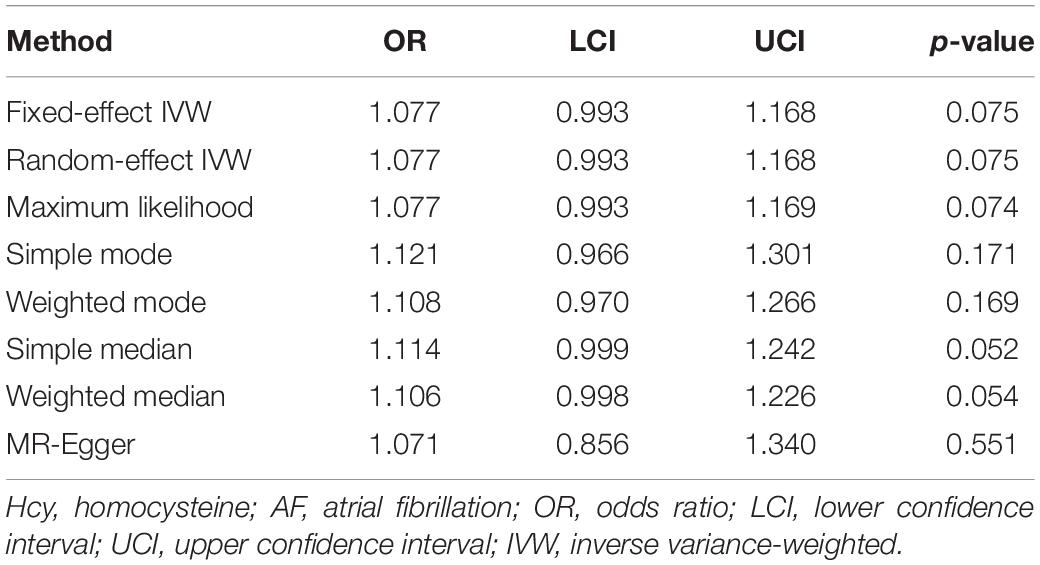
Fixed-effect inverse variance weighting (IVW) method was employed to estimate the causal effect of Hcy on AF. Specifically, Wald ratio was estimated for each SNP, and then, IVW mean of these ratio estimates was calculated as the effect estimate (Burgess et al., 2013). In the sensitivity analyses, the random-effect IVW, maximum likelihood, simple mode, weighted mode, simple median, weighted median, and MR-Egger methods were performed to test the robustness of the effect estimate. In addition, a leave-one-out sensitivity analysis was conducted to determine whether the result was affected by a single SNP (Burgess and Thompson, 2017). Subsequently, MR-Egger intercept test was applied to evaluate the potential horizontal pleiotropy, and funnel plot was generated to provide a visual inspection (Sterne et al., 2011; Bowden et al., 2015). Finally, mRnd was used to calculate the statistical power of the two-sample MR analysis, which required at least 80% (Freeman et al., 2013). The two-sided p < 0.05 was considered statistically significant. All the analyses were conducted using R packages (“MendelianRandomization” and “TwoSampleMR”) with R version 3.6.2 (Yavorska and Burgess, 2017; Hemani et al., 2018).
Results
In total, 18 SNPs were extracted at a genome-wide significance threshold from the Hcy dataset. Among them, four SNPs (rs12921383, rs1801133, rs2851391, and rs957140) were dropped due to the linkage disequilibrium (Supplementary Table 2). Additional five SNPs (rs154657, rs2251468, rs548987, rs7422339, and rs9369898) were removed because of their associations with confounding traits. Specifically, rs154657 was associated with hypertension; rs2251468 was associated with cholesterol, C-reactive protein, and coronary artery disease; rs548987 was associated with body mass index; rs7422339 was associated with mass, cholesterol, and blood pressure; and rs9369898 was associated with cholesterol (Supplementary Table 3). After exclusion of these nine SNPs, the remaining nine SNPs were identified as IVs in our two-sample analysis. All the nine SNPs were valid (F > 10), and their characteristics are shown in Table 2.
According to the fixed-effect IVW analysis results, the odds ratio (OR) and 95% confidence interval (CI) of AF per standard deviation (SD) increase in Hcy were 1.077 (0.993, 1.168), p = 0.075 (Figure 2, Table 3, and Supplementary Figure 1). These results suggested that genetically predicted plasma Hcy levels were not associated with AF. Similar results were observed using other statistical methods (Table 3). The leave-one-out analysis results indicated that the overall estimate was not driven by any SNP (Figure 3). According to the MR-Egger intercept test results, the overall estimate and 95% CI of the intercept were 0.000 (−0.013, 0.014), p = 0.958 (Table 4). Together with the almost symmetrical funnel plot (Supplementary Figure 2), these results suggested no evidence of horizonal pleiotropy. In addition, no significant association between Hcy and HF was observed in MR analyses that included nine SNPs dropped due to linkage disequilibrium or potential pleiotropy (Supplementary Table 4).
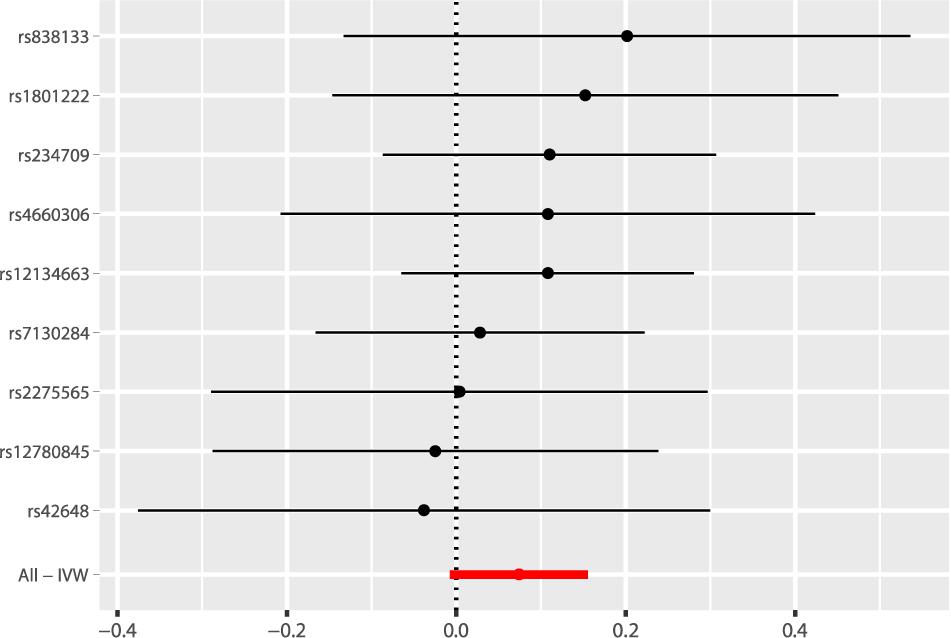
Figure 2. Fixed-effect IVW analysis of the causal association of Hcy with AF. The black dots and bars indicated the causal estimate and 95% CI using each SNP. The red dot and bar indicated the overall estimate and 95% CI meta-analyzed by fixed-effect IVW method. IVW, inverse variance weighted; Hcy, homocysteine; AF, atrial fibrillation; CI, confidence interval; SNP, single nucleotide polymorphism.
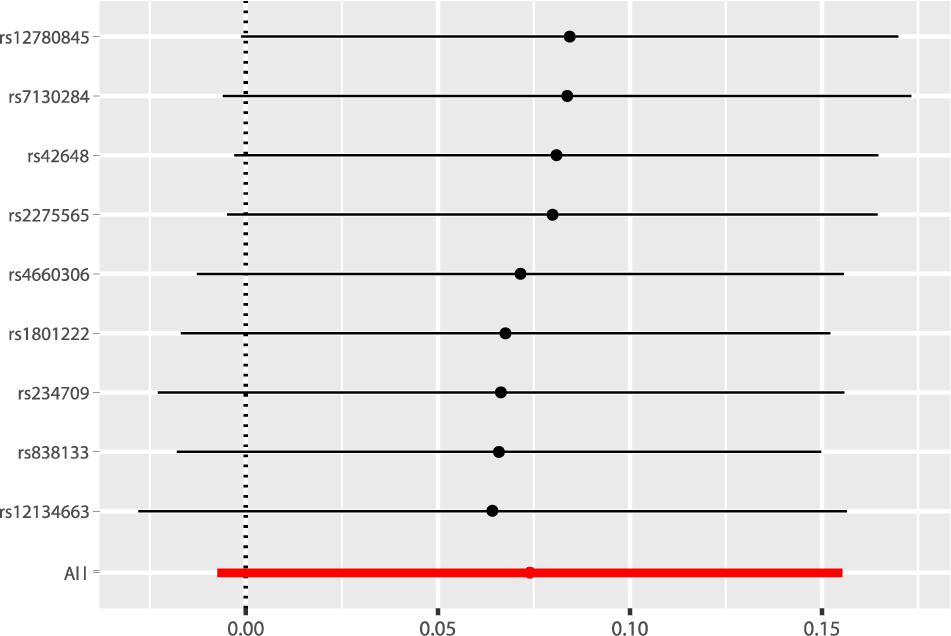
Figure 3. Leave-one-out analysis of the causal association of Hcy with AF. The black dots and bars indicated the causal estimate and 95% CI when a SNP was removed in turn. The red dot and bar indicated the overall estimate and 95% CI using fixed-effect IVW method. Hcy, homocysteine; AF, atrial fibrillation; CI, confidence interval; SNP, single nucleotide polymorphism; IVW, inverse variance weighted.
Discussion
This is a two-sample MR study to investigate the association between plasma Hcy levels and the risk of AF. In the present study, there is no evidence to support causal association of Hcy with AF. It should be cautious, since the observed p value of 0.075 in the primary analysis might be suggestive of a causal relationship and all of the secondary analyses point to a consistent effect direction. However, the MR analyses that used all extracted SNPs also suggested insignificant association between Hcy and AF.
Homocysteine is an intermediate product of methionine metabolism through a series of transmethylation reactions. Hcy-induced oxidative stress and impairments in methylation activity have been thought to play an important role in the pathogenesis of CVD (Ullegaddi et al., 2004; Shen et al., 2010). Previous studies have suggested that elevated plasma Hcy levels (known as hyperhomocysteinemia) were associated with an increased risk of CVD including hypertension (Kim et al., 2018) and coronary artery disease (Jakubowski, 2019), which were potential risks for AF. Increased Hcy confers an independent risk for the thromboembolic and cardiovascular events in AF patients (Yao et al., 2017b). Cross-sectional clinical studies indicated that AF patients had elevated Hcy levels, especially in elderly patients (Marcucci et al., 2004; Shimano et al., 2008). Several observational clinical studies have reported the positive association of Hcy with recurrent AF risk after ablation (Yao et al., 2017b). However, the results of this study about the effects of Hcy on AF were different from those of previous studies. One of the possible reasons is that the sample size might not be large enough in traditional observational studies to detect the exact association. Moreover, confounding factors are inevitable in observational studies, which can cause bias. Hcy may be just a sign of other CVDs, which in turn increased AF risk. Additionally, high plasma Hcy levels observed in traditional studies might also be a consequence of the AF incident.
Observational studies often suffer from confounding factors that can lead to spurious non-replicable findings (Lawlor et al., 2008). Randomized controlled trials (RCTs) are the gold standard to establish causal relationships in the medical research (Evans and Davey Smith, 2015). However, RCTs cannot always be conducted because they can be excessively costly, impractical, even unethical, or difficult to collect a large enough sample (Lawlor et al., 2008; Evans and Davey Smith, 2015). One of the alternative approaches is conducting MR experiments that are based on Mendel’s law of independent assortment, i.e., each trait inherits independently from other traits to the next generation. In MR, the random segregation of alleles (genes) helps in dividing them into exposed and control groups independently, and unmeasured confounders are also equally distributed between two groups (Gupta et al., 2017). The application of MR can ingeniously remedy the shortcomings of traditional epidemiological research, such as confounding factors and reverse causation, providing method for epidemiological research with regard to etiology (Badsha and Fu, 2019). MR studies select suitable genetic instrumental variables from the widely available GWAS, which make it a time- and cost-efficient approach (Holmes et al., 2017). These advantages contribute to its increasing popularity for assessing and screening for potentially causal associations. In this study, we analyzed the association between plasma Hcy levels and AF with the aid of a large-scale GWAS. This study suggested that an increase in plasma Hcy levels did not directly lead to the occurrence of AF. As shown in the research, several recent prospective studies have shown that Hcy might be a novel risk marker for AF rather than a causal risk factor (Schnabel et al., 2010; Kubota et al., 2019). These findings suggested that the possibility of Hcy as an independent risk factor in AF patients was small.
Strengths of the present study include the two-sample MR study design and the large sample size. The following potential limitations also require discussion. First, it is difficult to completely rule out the influence of potential directional pleiotropy, which may lead to biased estimates. However, SNPs associated with known confounding traits were excluded in our analyses. In addition, no evidence of pleiotropic effect was observed in MR-Egger intercept test, and similar results were observed in sensitivity analyses using several other models. Second, there is some degree of overlap between the participants included in the GWAS for Hcy and AF, which can cause biased estimate if substantial. Five cohorts included in the Hcy GWAS are also part of the AF GWAS (3.5%), although true proportions might be smaller. Therefore, risk of bias from sample overlap is probably low. Third, we are unable to perform the reverse analysis because the GWAS for Hcy is not publicly available. Finally, we only reveal the relationship between Hcy and AF from a genetic point of view, without involving other environmental factors.
Conclusion
This two-sample MR analysis found no evidence to support causal association of Hcy with AF. It was plausible that simply reducing plasma Hcy levels could not decrease the incidence of AF in clinical practice. However, additional studies are still needed to further confirm our results.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
SC and WZ designed the study. SC, FY, and TX conducted the analysis and drafted the first draft. YW, KZ, and GF reviewed the design and analysis, commented on the text, and provided subject matter expertise. All authors reviewed the final version for intellectual content.
Funding
This study was supported by the National Natural Science Foundation of China (82070408).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.619536/full#supplementary-material
References
Ahmed, S., Bogiatzi, C., and Hackam, D. G. (2019). Vitamin B 12 deficiency and hyperhomocysteinaemia in outpatients with stroke or transient ischaemic attack: a cohort study at an academic medical centre. BMJ Open 9:e026564. doi: 10.1136/bmjopen-2018-026564
Badsha, M. B., and Fu, A. Q. (2019). Learning causal biological networks with the principle of mendelian randomization. Front. Genet. 10:460. doi: 10.3389/fgene.2019.00460
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol 37, 658–665. doi: 10.1002/gepi.21758
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389. doi: 10.1007/s10654-017-0255-x
Davey Smith, G., and Hemani, G. (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98.
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601. doi: 10.1136/bmj.k601
Evans, D. M., and Davey Smith, G. (2015). Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu. Rev. Genomics Hum. Genet. 16, 327–350. doi: 10.1146/annurev-genom-090314-050016
Freeman, G., Cowling, B. J., and Schooling, C. M. (2013). Power and sample size calculations for Mendelian randomization studies using one genetic instrument. Int. J. Epidemiol. 42, 1157–1163. doi: 10.1093/ije/dyt110
Ganguly, P., and Alam, S. F. (2015). Role of homocysteine in the development of cardiovascular disease. Nutr. J. 14:6.
Gupta, V., Walia, G. K., and Sachdeva, M. P. (2017). ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public Health 145, 113–119. doi: 10.1016/j.puhe.2016.12.033
Hemani, G., Zheng, J., and Elsworth, B. (2018). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7:e34408.
Holmes, M. V., Ala-Korpela, M., and Smith, G. D. (2017). Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 14, 577–590. doi: 10.1038/nrcardio.2017.78
Jakubowski, H. (2019). Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 99, 555–604. doi: 10.1152/physrev.00003.2018
Kim, J., Lee, S. K., Yoon, D. W., and Shin, C. (2018). Concurrent presence of obstructive sleep apnea and elevated homocysteine levels exacerbate the development of hypertension: a KoGES Six-year follow-up study. Sci. Rep. 8:2665.
Kubota, Y., Alonso, A., Heckbert, S. R., Norby, F. L., and Folsom, A. R. (2019). Homocysteine and incident atrial fibrillation: the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Heart Lung Circ. 28, 615–622. doi: 10.1016/j.hlc.2018.03.007
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N., and Davey Smith, G. (2008). Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163. doi: 10.1002/sim.3034
Liu, F. D., Zhao, R., Feng, X. Y., Shi, Y. H., Wu, Y. L., Shen, X. L., et al. (2018). Rivaroxaban does not influence hemorrhagic transformation in a diabetes ischemic stroke and endovascular thrombectomy model. Sci. Rep. 8:7408.
Machiela, M. J., and Chanock, S. J. (2015). LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557. doi: 10.1093/bioinformatics/btv402
Magnani, J. W., Rienstra, M., and Lin, H. (2011). Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation 124, 1982–1993. doi: 10.1161/circulationaha.111.039677
Marcucci, R., Betti, I., and Cecchi, E. (2004). Hyperhomocysteinemia and vitamin B6 deficiency: new risk markers for nonvalvular atrial fibrillation? Am. Heart. J. 148, 456–461. doi: 10.1016/j.ahj.2004.03.017
Marrouche, N. F., Brachmann, J., Andresen, D., Siebels, J., Boersma, L., Jordaens, L., et al. (2018). Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 378, 417–427.
Myers, T. A., Chanock, S. J., and Machiela, M. J. (2020). LDlinkR: an R Package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front. Genet. 11:157. doi: 10.3389/fgene.2020.00157
Nielsen, J. B., Thorolfsdottir, R. B., and Fritsche, L. G. (2018). Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 50, 1234–1239.
Ponikowski, P., Voors, A. A., and Anker, S. D. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 18, 891–975.
Qin, X., Li, J., Spence, J. D., Zhang, Y., Li, Y., Wang, X., et al. (2016). Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke 47, 2805–2812. doi: 10.1161/strokeaha.116.014578
Schnabel, R. B., Larson, M. G., Yamamoto, J. F., Sullivan, L. M., Pencina, M. J., Meigs, J. B., et al. (2010). Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 121, 200–207. doi: 10.1161/circulationaha.109.882241
Sekula, P., Del Greco, M. F., Pattaro, C., and Kottgen, A. (2016a). Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265. doi: 10.1681/asn.2016010098
Shen, J., Lai, C. Q., Mattei, J., Ordovas, J. M., and Tucker, K. L. (2010). Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 91, 337–342. doi: 10.3945/ajcn.2009.28571
Shimano, M., Inden, Y., Tsuji, Y., Kamiya, H., Uchikawa, T., Shibata, R., et al. (2008). Circulating homocysteine levels in patients with radiofrequency catheter ablation for atrial fibrillation. Europace 10, 961–966. doi: 10.1093/europace/eun140
Snezhitsky, V. A., Yatskevich, E. S., Doroshenko, E. M., Smirnov, V. Y., Dolgoshey, T. S., and Rubinsky, A. Y. (2016). [Homocysteine as a prognostic marker of atrial remodeling and clinical picture in patients with paroxysmal and persistent forms of atrial fibrillation]. Klin. Med. (Mosk). 94, 16–22. doi: 10.18821/0023-2149-2016-94-1-16-22
Staley, J. R., Blackshaw, J., Kamat, M. A., Ellis, S., Surendran, P., Sun, B. B., et al. (2016). PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics 32, 3207–3209. doi: 10.1093/bioinformatics/btw373
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002. doi: 10.1136/bmj.d4002
Ullegaddi, R., Powers, H. J., and Gariballa, S. E. (2004). B-group vitamin supplementation mitigates oxidative damage after acute ischaemic stroke. Clin. Sci. (Lond). 107, 477–484. doi: 10.1042/cs20040134
van Meurs, J. B., Pare, G., Schwartz, S. M., Hazra, A., Tanaka, T., Vermeulen, S. H., et al. (2013). Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am. J. Clin. Nutr. 98, 668–676.
Violi, F., Soliman, E. Z., Pignatelli, P., and Pastori, D. (2016). Atrial fibrillation and myocardial infarction: a systematic review and appraisal of pathophysiologic mechanisms. J. Am. Heart Assoc. 5:e003347.
Wei, Y., Huang, Z., and Li, J. (2017). Research progress on the correlation between hmocysteine and atrial fibrilation. Zhonghua Xin Xue Guan Bing Za Zhi 45, 550–552.
Yao, Y., Shang, M. S., Dong, J. Z., and Ma, C. S. (2017a). Homocysteine in non-valvular atrial fibrillation: role and clinical implications. Clin. Chim. Acta 475, 85–90. doi: 10.1016/j.cca.2017.10.012
Yao, Y., Yao, W., Bai, R., Lu, Z. H., Tang, R. B., Long, D. Y., et al. (2017b). Plasma homocysteine levels predict early recurrence after catheter ablation of persistent atrial fibrillation. Europace 19, 66–71.
Keywords: homocysteine, atrial fibrillation, Mendelian randomization, causal association, genome-wide association study
Citation: Chen S, Yang F, Xu T, Wang Y, Zhang K, Fu G and Zhang W (2021) Appraising the Causal Association of Plasma Homocysteine Levels With Atrial Fibrillation Risk: A Two-Sample Mendelian Randomization Study. Front. Genet. 12:619536. doi: 10.3389/fgene.2021.619536
Received: 21 October 2020; Accepted: 13 April 2021;
Published: 26 May 2021.
Edited by:
David R. Van Wagoner, Case Western Reserve University, United StatesReviewed by:
Shamone Gore Panter, Cleveland State University, United StatesJohn Barnard, Cleveland Clinic, United States
Copyright © 2021 Chen, Yang, Xu, Wang, Zhang, Fu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guosheng Fu, ZnVnc0B6anUuZWR1LmNu; Wenbin Zhang, MzMxMzAxMUB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Songzan Chen
Songzan Chen Fangkun Yang
Fangkun Yang Tian Xu1
Tian Xu1 Guosheng Fu
Guosheng Fu Wenbin Zhang
Wenbin Zhang