- Health Management Institute, The Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, China
Objectives: To investigate the associations among the methylene tetrahydrofolate reductase rs1801133 C677T gene variant, food groups, and the risk of non-alcoholic fatty liver disease in the Chinese population.
Methods: A study of gene polymorphism was conducted using the polymerase chain reaction method. A total of 4,049 adults participated in the study, and all underwent physical examination and genotyping. Participants filled out a dietary questionnaire to enable us to assess the frequency and quantity of food consumption.
Results: The important variables identified as risk factors of non-alcoholic fatty liver disease were age, smoking, sex, body mass index, hyperlipidemia, diabetes, and methylene tetrahydrofolate reductase genotype (T – allele carriers). The homocysteine content was higher in the non-alcoholic fatty liver disease group than in the control group, and was higher in the T- allele than C- allele carriers. The homocysteine content was the highest in the T- allele carriers. Additionally, certain food groups such as milk and beans were associated with a lower risk of non-alcoholic fatty liver disease. Food groups such as meat, were associated with a higher risk of non-alcoholic fatty liver disease. Fresh fruit and vegetables, salted and smoked foods, desserts, cereals, fish, and eggs were not associated with the risk of non-alcoholic fatty liver disease. However, the influence of salted and smoked foods on non-alcoholic fatty liver disease was different in the C-allele and T-allele carriers of methylene tetrahydrofolate reductase (CT + TT vs. CC, OR = 1.196, P = 0.041 for 1–4 times food per week, OR = 1.580, P = 0.004 for 5–7 times per week). Similarly, salted and smoked foods were also a risk factor for the development of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease.
Conclusion: This study found that the T-allele of the C677T variant of methylene tetrahydrofolate reductase was a risk factor for non-alcoholic fatty liver disease among Chinese people. These results can likely aid the development of novel approaches for managing non-alcoholic fatty liver disease risk.
Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide (Younossi et al., 2016; Subada et al., 2018). NAFLD is a disease caused by excessive fat in the liver (Yki-Järvinen, 2014). According to this report, NAFLD is now an epidemic in China, and the prevalence of NAFLD in South China is 45.4% (Yi et al., 2017). Therefore, identification of modifiable risk factors for the prevention of NAFLD is urgently needed for Chinese patients. NAFLD is a multiple factor disease that is influenced by genetic variation, diet, age, sex, ethnicity, and lifestyle, along with conditions including obesity, insulin resistance, dyslipidemia, hypertension, diabetes mellitus, and metabolic syndrome (Hamabe et al., 2011; Satapathy and Sanyal, 2015; Kalia and Gaglio, 2016; Tong and Guo, 2019; Zhang et al., 2019). Several studies have been conducted on the association between gene polymorphism and NAFLD (Kong et al., 2017; Liu et al., 2019; Xu et al., 2019). Specifically, some studies have indicated that susceptibility genes may play an important role in the pathogenesis of NAFLD (Sookoian and Pirola, 2011; Yang et al., 2011). Methylene tetrahydrofolate reductase (MTHFR) converts 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (Froese et al., 2016). The human MTHFR gene is mapped to chromosome 1p36.3 (Rosenberg et al., 2002). Moreover, the MTHFR rs1801133 C677T gene variant is associated with enzyme activity (Wan et al., 2018). It is known that MTHFR variation will result in a reduction of MTHFR enzyme activity. Specifically, the substitution at 677 bp of the MTHFR gene is a common mutation that leads to hyperhomocysteinemia (Liew and Gupta, 2015). MTHFR C677T has also been associated with NAFLD (Adinolfi et al., 2005). Furthermore, the lack of choline can lead to hyperhomocysteinemia and NAFLD (Leclercq et al., 2000; Liu et al., 2014), and the MTHFR rs1801133 C677T gene variant has been associated with choline status (Abratte et al., 2008). Therefore, homocysteine content can indirectly reflect the choline content. However, choline status is also related to daily diet, and different food groups have varying choline content. Foods that contain choline include liver, beef, fish, pork, fruits, and vegetables, milk, beans, desserts, and salted and smoked foods (Wiedeman et al., 2018; Probst et al., 2019; Ullah et al., 2019). Therefore, the influencing factors between different groups may be different, depending on the interaction of genetic variation and lifestyles (Ma et al., 2017). Although there is growing evidence of a strong relationship between MTHFR variation and NAFLD (Sazci et al., 2008; Franco Brochado et al., 2013; Catalano et al., 2014), the relationship between food groups and NAFLD in different groups has rarely been studied. Here, we systematically analyzed the associations between MTHFR rs1801133 C677T gene variant and food groups in relation to the risk of NAFLD in the Chinese population. We hope that these results will provide novel ideas for the management of NAFLD risk.
Materials and Methods
Subjects
The data were collected from July 2018 to July 2019 at the Health Management Institute of Chinese PLA General Hospital. The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Chinese PLA General Hospital. All the methods were performed in accordance with relevant guidelines and regulations. The study was conducted in accordance with the World Medical Association’s Declaration of Helsinki, and all participants provided written informed consent prior to the study. According to the standardized criteria, fatty liver was diagnosed by abdominal ultrasonography (Saadeh et al., 2002; Hashimoto et al., 2013). All subjects underwent ultrasonography by the same experienced radiologist and using the same equipment. NAFLD was diagnosed according to relevant guidelines and regulations (Fan et al., 2019). The normal control individuals were selected based on abdominal ultrasonography, but those with liver disease were excluded. Finally, a total of 4,091 adults participated in this study, and all underwent both physical examination and genotyping. Among these, 42 patients were excluded for the following reasons: 10 did not sign an informed consent form, 20 did not provide a completed questionnaire, and 12 had inadequate blood samples. Therefore, 4,049 participants were included in this study. Liver fibrosis is the main cause of mortality in patients with non-alcoholic steatohepatitis (NASH), and the BARD score was used to recognize patients that are at high risk of developing advanced fibrosis (Harrison et al., 2008). On the basis of this score, we further screened patients with liver fibrosis from among those with NAFLD. Importantly, patients with a positive BARD score (>2 points) were considered to have liver fibrosis.
Detection of the MTHFR Genotype and Biochemical Indicators
MTHFR polymorphisms were detected by using a gene chip hybrid analysis. Venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes. DNA was extracted from whole blood using the QIAampR DNA Mini Kit (CAT No. 51304, Germany). The MTHFR genotype was determined by PCR-genotyping microarray analysis of three gene types (MTHFR Genotyping kit, BaiO, Shanghai, China). The detection of MTHFR mutation was performed in accordance with the manuals of the Gene Array Kit (BaiO Genotype Detection Gene Array Kit, Cat. No. 51304, Germany) and equipment (BaiO Technology Corp., Shanghai, China). Homocysteine (Hcy) levels were determined by fluorescence detection (F-1080, Hitachi Ltd., Tokyo, Japan) on high-performance liquid chromatography (HPLC; LC-9A, Shimadzu Corp., Kyoto, Japan). Fasting plasma glucose (Glu) was detected by using the hexokinase method. Last, the total cholesterol (TC), high-density lipoprotein (HDL), triglyceride (TG), hemoglobin (Hb), alanine aminotransferase (ALT), and aspartate transaminase (AST) were measured by an autoanalyzer (Cobas c 501 autoanalyzer, Roche Diagnostics, Germany).
Evaluation of Food Groups
As recommended by the China Health and Nutrition Survey (CHNS) (Ge, 1995) and our slightly changed forms (Ma et al., 2017; Zeng et al., 2017) based on the current Chinese lifestyle, all subjects were asked to provide food-related information through a self-questionnaire at their first visit to enable us to assess their diet. The questionnaire included items on socio demographic characteristics, smoking, eating habits, drinking, family history, and medical history, including drug use, allergies, and surgeries. The food groups included cereals, fish, eggs, fruits, and vegetables, soy products, salted, and smoked foods, desserts, milk, and meat. The food frequencies were ranked from 1 to 3 (1: less than 1 day per week. 2: 1–4 days/week. 3: 5–7 days/week). The daily quantity of food groups consumed was estimated as follows: cereal (100–500 g/day), meat (100–200 g/day), fruits and vegetables (200–500 g/day), sugar (30–50 g/day), and salt (6–8 g/day). The dietary intake collection method that was used has been formally validated in the Chinese population (Ma et al., 2017; Zeng et al., 2017), and other questionnaires used the estimated diet record, which is reliable and widely accepted (Bonifacj et al., 1997).
Statistical Analysis
Student’s t-test, one way-ANOVA, chi-squared tests, Fisher’s exact test, and binary logistic regression were performed using SPSS version 24.0. A Hardy–Weinberg equilibrium analysis was performed by using the chi-square test. All analyses were performed with 95% confidence intervals, and p-values < 0.05 were considered to indicate statistical significance.
Results
Hardy-Weinberg Equilibrium
The results showed that the genotypes of the NAFLD patients and controls conformed to the Hardy-Weinberg equilibrium. Table 1 shows the genotype and allele frequencies in NAFLD and controls.

Table 1. Hardy–Weinberg equilibrium testing for MTHFR C677T genotypes in the NAFLD and control groups.
Clinical Characteristics
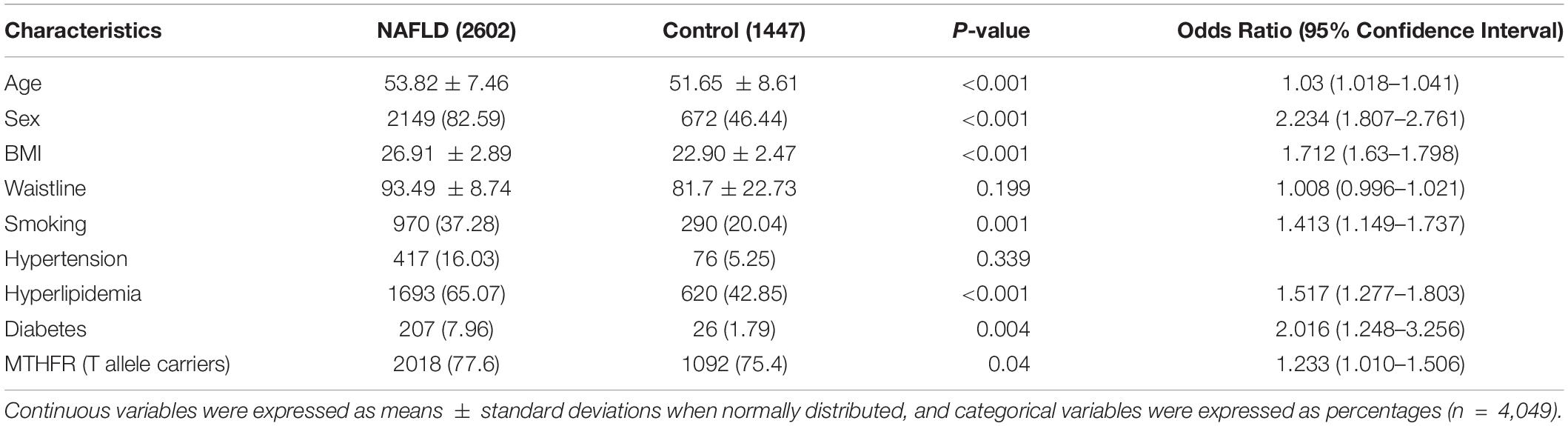
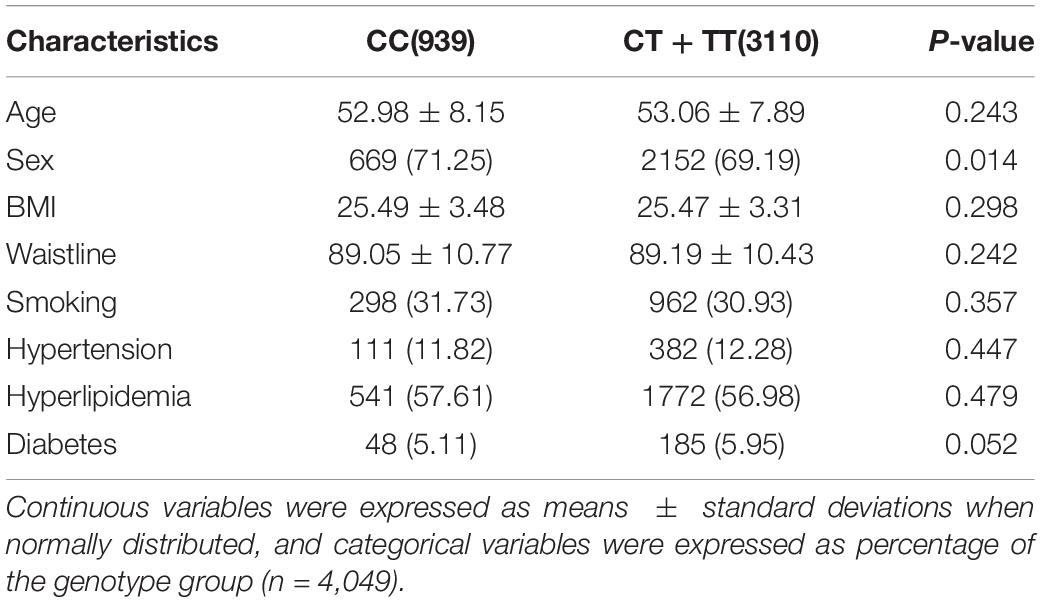
In total, 4,049 patients were included in the study. The frequencies of MTHFR alleles were 23.19% (CC), 47.67% (CT), and 29.14% (TT). The average age of patients with NAFLD was 53.82 ± 7.46 years. The important variables identified as risk factors of NAFLD were age, sex, BMI, smoking, hyperlipidemia, diabetes, and MTHFR (T allele carriers) (Table 2). Importantly, subjects carrying CT or TT were at a higher risk of developing NAFLD than those carrying CC (OR = 1.233, 95% CI: 1.010–1.506, P = 0.04). Waistline and hypertension were not risk factors for NAFLD (P > 0.05). However, factors such as age, waistline, BMI, smoking, hyperlipidemia, hypertension, and diabetes were not significantly different between the C- allele and T- allele carriers (Table 3).
The Relevant Lifestyle and Baseline Characteristics of NAFLD
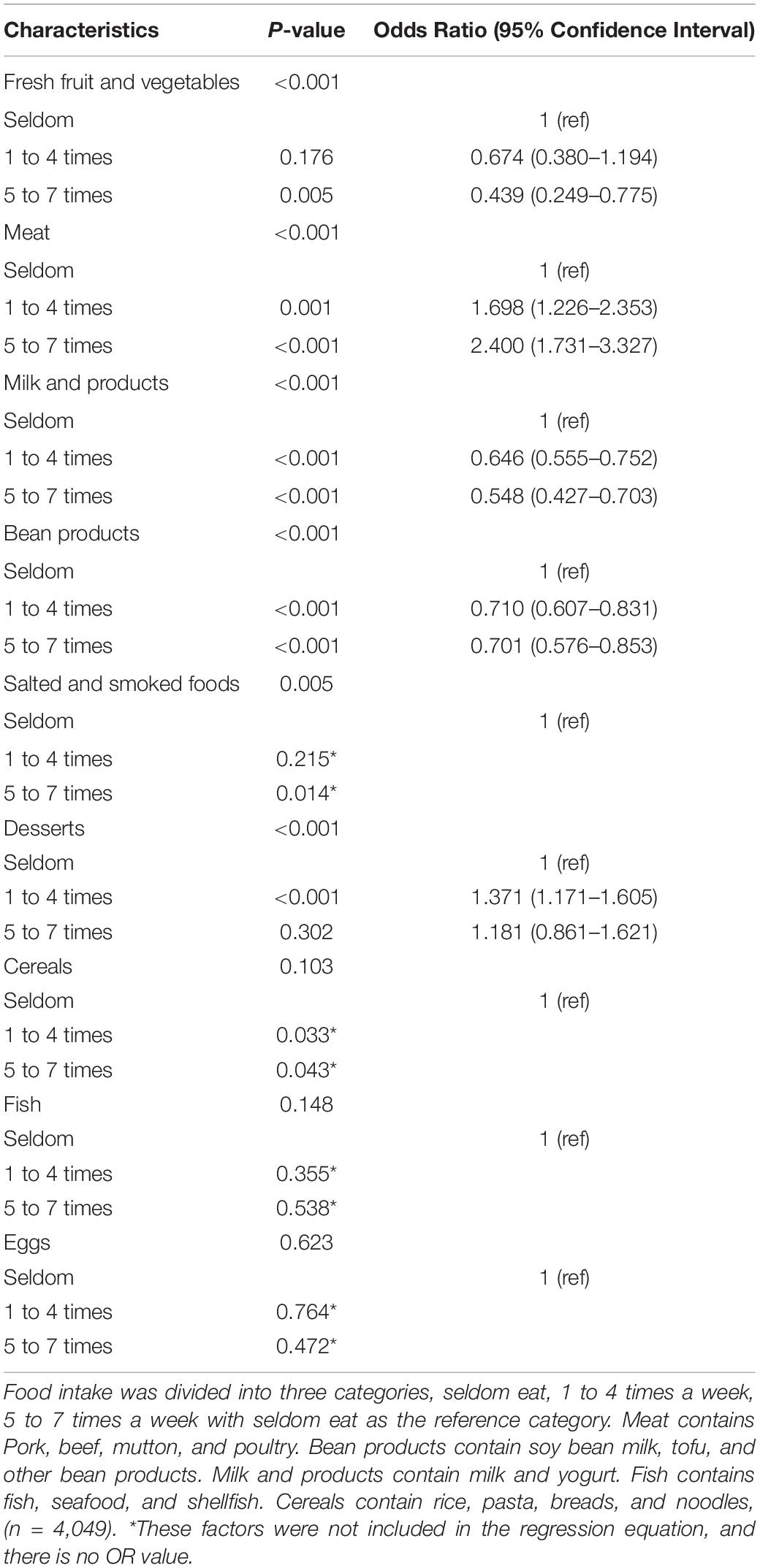
According to the results shown in Table 4, certain food groups such as milk and beans were associated with a lower risk of NAFLD. Meat, on the other hand, was associated with a higher risk of NAFLD. Fresh fruit and vegetables, salted, and smoked foods, desserts, cereals, fish, and eggs were not associated with the risk of NAFLD. After adjustment for age, this trend remained significant.
Comparison of the Effects of Food Groups Between the C- and the T-Allele Carriers
Table 5 shows comparison of risk factors of NAFLD between the C- and T-allele carriers. The influence of most food groups, such as desserts, fresh fruit, and vegetables, meat, cereals, fish, and eggs was not significantly different between CC and TT + CT genotype groups. However, it was marginally different for other food groups, namely milk and beans. In addition, the influence of salted and smoked foods on NAFLD was obviously different between the two groups. The intake of salted and smoked foods was found to be a risk factor for NAFLD in the TT and CT groups (OR = 1.196, 95% CI: 1.007–1.420, P = 0.041 for 1–4 times food per week intake. OR = 1.580, 95% CI: 1.156–2.159, P = 0.004 for 5–7 times per week intake), but it had no effect on the CC genotype group. According to the results shown in Table 5, ORs and 95% CIs had the same sense of base characteristics in relation to the risk of NAFLD. In addition, regular consumption (i.e., several times/week) of salted and smoked foods was associated with a higher risk of developing NAFLD than occasional consumption. After adjustment for age, this trend with salted and smoked foods intake remained significant.
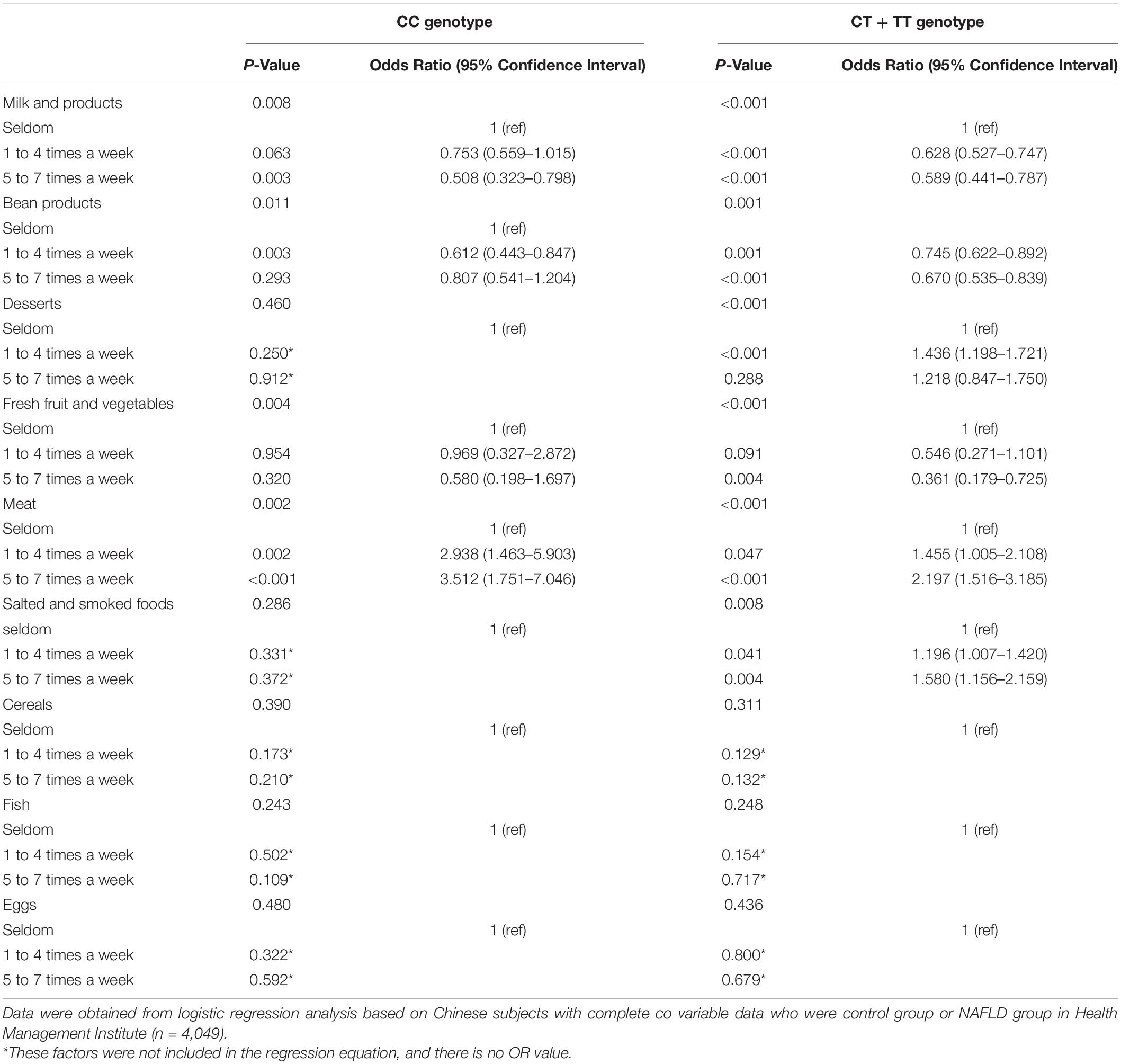
Table 5. Comparison of the relationship between food groups and NAFLD in the C- and T- allele carriers.
Association Between MTHFR Genotype and Blood Biochemical Indicators
Table 6 shows the results of blood lipids, blood glucose, hepatic function, hemoglobin, and homocysteine in the NAFLD and control groups between the C- and the T- allele carriers. Levels of ALT, AST, TC, TG, Hb, Glu, and Hcy of the CC genotype groups were significantly different between the NAFLD and control groups. On the contrary, no significant association was found in HDL-C in CC genotype between the NAFLD and control groups. Levels of ALT, AST, TG, Hb, and Hcy were significantly differences between the CT and TT genotype groups. However, no significant differences were observed for TC, HDL-C, and Glu between the NAFLD and control groups. Homocysteine content was higher in the NAFLD group than in the control group, and also higher in the CT + TT genotype group than in the CC group. The homocysteine content was the highest in the CT + TT genotype group (Table 6).
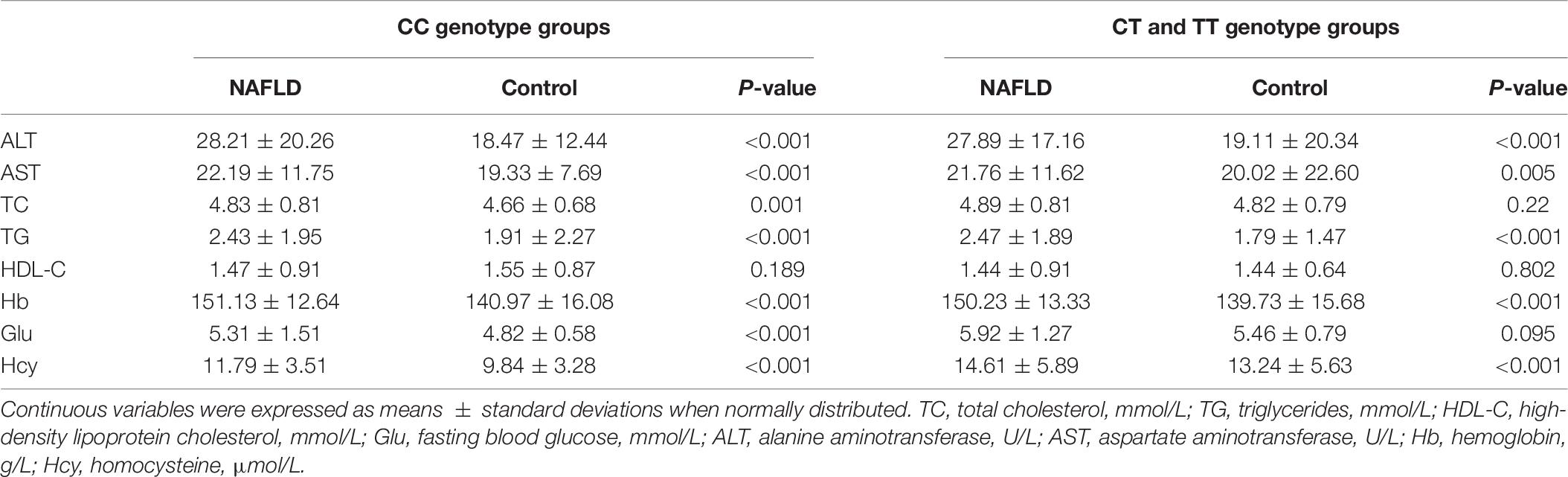
Table 6. Comparison of the levels of blood lipids, blood glucose, hepatic markers, hemoglobin, and homocysteine between the C- and T-allele carriers.
Comparison of the Effects of the Salted and Smoked Foods on Liver Fibrosis
According to the BARD score, there were 528 patients with liver fibrosis in the CC group and 1,838 in the CT + TT group. The influence of salted and smoked foods on liver fibrosis was significantly different between the two groups (Table 7). The intake of salted and smoked foods was found to be a risk factor for the development of liver fibrosis in the TT and CT genotype group (OR = 1.429, 95% CI: 1.040–1.962, P = 0.028 for 1–4 times food per week intake. OR = 2.231, 95% CI: 1.170–4.256, P = 0.015 for 5–7 times food per week intake), but there was no comparable effect in the CC genotype group (Table 7). Additionally, regular consumption (i.e., several times/week) of salted and smoked foods was associated with a higher risk of developing liver fibrosis than occasional consumption.
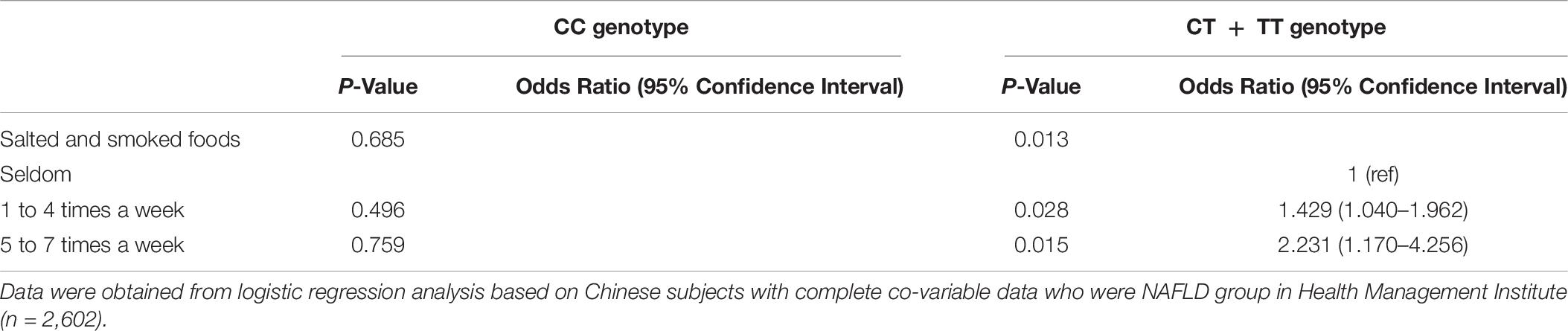
Table 7. Comparison of the relationship between salted and smoked foods and liver fibrosis in the C- and T-allele carriers.
Discussion
Risk Factors of NAFLD and MTHFR Genotypes
Our results indicate a relationship between lifestyle and NAFLD in different MTHFR genotype groups. First, the occurrence of NAFLD was significantly higher in participants with the CT or TT genotype than in those with the CC genotype. This indicated that the TT genotype of MTHFR was more likely to be related to susceptibility to NAFLD (Serin et al., 2007; Liew and Gupta, 2015). MTHFR variation was significantly associated with NAFLD risk in a Turkish study (Sazci et al., 2008), and our results were consistent with that study. Second, our results indicated that the risk factors of NAFLD are age, sex, BMI, smoking, hypertension, and diabetes, which was consistent with another review of literature (Benedict and Zhang, 2017). Lonardo et al’s epidemiological review (Non-alcoholic Fatty Liver Disease Study Group, Lonardo et al., 2015) showed NAFLD was more common in men. Similarly, our study found that the prevalence of NAFLD in males was 82.59%. Third, in the CC-genotype group, the levels of ALT, AST, TG, Hb, and Hcy were obviously different in the NAFLD and control groups, and the results were the same in the CT- and TT-genotype groups. We concluded that these changes were related to NAFLD. Kwo et al. (2017) reported that the degree of elevation of ALT and or AST levels could aid in the assessment of NAFLD. Similarly, Cohen et al., reported that patients with NAFLD exhibited an increased plasma concentration of TG and TC (Cohen and Fisher, 2013). Moreover, Hu et al. (2019) suggested that Glu levels are related to NAFLD. Hb has also been found to be associated with NAFLD (Jiang et al., 2014). The association of the C677T gene variant of MTHFR with total cholesterol were consistently more marked in Asian populations (Luo et al., 2018). Luo et al. (2018) found that the C677T gene variant was associated with increased risk of coronary artery disease and elevated levels of total cholesterol. However, we found that total cholesterol levels were different between the C- and T-allele carriers. The mechanisms by which MTHFR variation is associated with TC levels have not been clarified yet. Homocysteine levels were also reported to be associated with NAFLD (Liang et al., 2019).
Influence of Food Groups on MTHFR Genotypes
We found that certain food groups such as milk and beans were associated with a lower risk of NAFLD, whereas meats were associated with a higher risk of NAFLD. According to the results of the logistic regression, the influence of most food groups on NAFLD was not significantly different between the CC and TT + CT groups. However, the influence of salted and smoked foods was obviously different between the two groups. After adjustment for age, this trend of salted and smoked foods intake remained significant. We speculate that this phenomenon is likely related to the choline content in the food. Importantly, choline has been found to be associated with MTHFR variation, and Jian Yan et al.’s findings indicate that the MTHFR C677T genotype favors the use of choline as a methyl donor (Yan et al., 2011). The liver is an important organ for metabolism and storage of choline (Zeisel, 2006). Studies have shown that choline deficiency can cause NAFLD and non-alcoholic steatohepatitis (NASH) (Yao and Vance, 1988; Corbin and Zeisel, 2012; Matsumoto et al., 2013). The MTHFR C677T variation can lead to hyperhomocysteinemia (Liew and Gupta, 2015), and the lack of choline can lead to hyperhomocysteinemia (Liu et al., 2014). Schwahn et al. reported that betaine supplementation of MTHFR-deficient mice reduced lipid deposition in the liver, and increased homocysteine and choline metabolism (Schwahn et al., 2003). We found that the content of homocysteine was higher in the NAFLD group than in the control group, and the homocysteine content was the highest in the T-genotype group of NAFLD. Therefore, we speculate that choline is deficient in the T-genotype group of NAFLD. In addition, foods that contain choline include meat, fruits and vegetables, milk, beans, desserts, and salted and smoked foods, but the choline content is lower in salted and smoked foods. For example, Frankfurter sausages have less choline than unprocessed meat (Probst et al., 2019). Therefore, adults who consumed salted and smoked foods were more susceptible to NAFLD. We also found that salted and smoked foods were a risk factor for NAFLD in the T-genotype group, while this effect was not seen in the C-genotype group. The likely reason may be that choline content is lower in salted and smoked foods, which is more strongly associated with NAFLD. Similarly, salted and smoked foods intake was a risk factor for liver fibrosis in the TT + CT group in patients with NAFLD. Importantly, patients with NAFLD who consumed salted and smoked foods were more susceptible to NASH. These results indicate that food intervention may be more effective for reducing the prevalence of NAFLD in different genotype groups.
Limitations
Our study has some limitations. First, we combined the CT and TT genotypes as one group. The C677T mutation represents the substitution of C (cytosine) for T (thymine) at nucleotide position 677, which results in the reduction of MTHFR enzyme activity. Therefore, including the T genotype as a group may have affected the MTHFR enzyme activity. Second, we only studied choline in certain foods, and intend to study more food groups in the future. Third, although NAFLD is a multifactorial disease, we only studied the relationship between diet and NAFLD. Moreover, we only studied the homocysteine levels and lack nutrient status/biochemical data (e.g., choline plasma levels). We plan to study the other biochemical data in future studies. Finally, we only studied a limited range of consumption quantities of each food, and aim to further evaluate the daily consumption quantities of each food by individuals in future studies.
Conclusion
This study’s findings indicated that MTHFR CT and TT genotypes are risk factors for NAFLD among Chinese people. The results also suggested that the genetic risk factor may interact with dietary factors, particularly salted and smoked foods. The interaction between food groups intake and MTHFR C677T variation may affect NAFLD incidence in the adult Chinese population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XH, CM, TX, LO, and QZ: data curation. XH: investigation. CM and QZ: methodology. TX and LO: software. XH and CM: writing–original draft. XH and QZ: writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81872920).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all study participants for their cooperation.
References
Abratte, C. M., Wang, W., Li, R., Moriarty, D. J., and Caudill, M. A. (2008). Folate intake and the MTHFR C677T genotype influence choline status in young Mexican American women. J. Nutr. Biochem. 19, 158–165. doi: 10.1016/j.jnutbio.2007.02.004
Adinolfi, L. E., Ingrosso, D., Cesaro, G., Cimmino, A., D’Anto, M., Capasso, R., et al. (2005). Hyperhomocysteinemia and the MTHFR C677T polymorphism promote steatosis and fibrosis in chronic hepatitis C patients. Hepatology 41, 995–1003. doi: 10.1002/hep.20664
Benedict, M., and Zhang, X. (2017). Non-alcoholic fatty liver disease: an expanded review. World J. Hepatol. 9, 715–732. doi: 10.4254/wjh.v9.i16.715
Bonifacj, C., Gerber, M., Scali, J., and Daures, J. P. (1997). Comparison of dietary assessment methods in a southern French population: use of weighed records, estimated-diet records and a food-frequency questionnaire. Eur. J. Clin. Nutr. 51, 217–231. doi: 10.1038/sj.ejcn.1600387
Catalano, D., Trovato, G. M., Ragusa, A., Martines, G. F., Tonzuso, A., Pirri, C., et al. (2014). Non-alcoholic fatty liver disease (NAFLD) and MTHFR 1298A > C gene polymorphism. Eur. Rev. Med. Pharmacol. Sci. 18, 151–159.
Cohen, D. E., and Fisher, E. A. (2013). Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin. Liver Dis. 33, 380–388. doi: 10.1055/s-0033-1358519
Corbin, K. D., and Zeisel, S. H. (2012). Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 28, 159–165. doi: 10.1097/MOG.0b013e32834e7b4b
Fan, J. G., Wei, L., Zhuang, H., National Workshop on Fatty Liver, Alcoholic Liver Disease Chinese, Society of Hepatology, et al. (2019). Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J. Dig. Dis. 20, 163–173. doi: 10.1111/1751-2980.12685
Franco Brochado, M. J., Domenici, F. A., Candolo Martinelli Ade, L., Zucoloto, S., de Carvalho da Cunha, S. F., and Vannucchi, H. (2013). Methylenetetrahydrofolate reductase gene polymorphism and serum homocysteine levels in nonalcoholic fatty liver disease. Ann. Nutr. Metab. 63, 193–199. doi: 10.1159/000353139
Froese, D. S., Huemer, M., Suormala, T., Burda, P., Coelho, D., Gueant, J. L., et al. (2016). Mutation update and review of severe methylenetetrahydrofolate reductase deficiency. Hum. Mutat. 37, 427–438. doi: 10.1002/humu.22970
Ge, K. (1995). Dietary pattern and physical development in China– based on the 1992 national nutrition survey. Asia Pac. J. Clin. Nutr. 4(Suppl. 1), 19–23.
Hamabe, A., Uto, H., Imamura, Y., Kusano, K., Mawatari, S., Kumagai, K., et al. (2011). Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J. Gastroenterol. 46, 769–778. doi: 10.1007/s00535-011-0376-z
Harrison, S. A., Oliver, D., Arnold, H. L., Gogia, S., and Neuschwander-Tetri, B. A. (2008). Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57, 1441–1447. doi: 10.1136/gut.2007.146019
Hashimoto, E., Taniai, M., and Tokushige, K. (2013). Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol. 28(Suppl. 4), 64–70. doi: 10.1111/jgh.12271
Hu, D. S., Zhu, S. H., Li, X., Chen, Q. F., Lin, C. J., Fang, D. H., et al. (2019). Association between hemoglobin glycation index and NAFLD in Chinese nondiabetic individuals. Can. J. Gastroenterol. Hepatol. 2019, 8748459. doi: 10.1155/2019/8748459
Jiang, Y., Zeng, J., and Chen, B. (2014). Hemoglobin combined with triglyceride and ferritin in predicting non-alcoholic fatty liver. J. Gastroenterol. Hepatol. 29, 1508–1514. doi: 10.1111/jgh.12580
Kalia, H. S., and Gaglio, P. J. (2016). The prevalence and pathobiology of nonalcoholic fatty liver disease in patients of different races or ethnicities. Clin. Liver Dis. 20, 215–224. doi: 10.1016/j.cld.2015.10.005
Kong, L., Lu, Y., Zhang, S., Nan, Y., and Qiao, L. (2017). Role of nutrition, gene polymorphism, and gut microbiota in non-alcoholic fatty liver disease. Discov. Med. 24, 95–106.
Kwo, P. Y., Cohen, S. M., and Lim, J. K. (2017). ACG clinical guideline: evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 112, 18–35. doi: 10.1038/ajg.2016.517
Leclercq, I. A., Farrell, G. C., Field, J., Bell, D. R., Gonzalez, F. J., and Robertson, G. R. (2000). CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Invest. 105, 1067–1075. doi: 10.1172/JCI8814
Liang, H., Xie, X., Song, X., Huang, M., Su, T., Chang, X., et al. (2019). Orphan nuclear receptor NR4A1 suppresses hyperhomocysteinemia-induced hepatic steatosis in vitro and in vivo. FEBS Lett. 593, 1061–1071. doi: 10.1002/1873-3468.13384
Liew, S. C., and Gupta, E. D. (2015). Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 58, 1–10. doi: 10.1016/j.ejmg.2014.10.004
Liu, M., Liu, S., Shang, M., Liu, X., Wang, Y., Li, Q., Mambiya, M., et al. (2019). Association between ADIPOQ G276T and C11377G polymorphisms and the risk of non-alcoholic fatty liver disease: an updated meta-analysis. Mol. Genet. Genomic Med. 7:e624.
Liu, Y. Q., Jia, Z., Han, F., Inakuma, T., Miyashita, T., Sugiyama, K., et al. (2014). Suppression effects of betaine-enriched spinach on hyperhomocysteinemia induced by guanidinoacetic acid and choline deficiency in rats. TheScientificWorldJournal 2014:904501. doi: 10.1155/2014/904501
Luo, Z., Lu, Z., Muhammad, I., Chen, Y., Chen, Q., Zhang, J., et al. (2018). Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 17:191. doi: 10.1186/s12944-018-0837-y
Ma, C., Yu, B., Zhang, W., Wang, W., Zhang, L., and Zeng, Q. (2017). Associations between aldehyde dehydrogenase 2 (ALDH2) rs671 genetic polymorphisms, lifestyles and hypertension risk in Chinese Han people. Sci. Rep. 7:11136. doi: 10.1038/s41598-017-11071-w
Matsumoto, M., Hada, N., Sakamaki, Y., Uno, A., Shiga, T., Tanaka, C., et al. (2013). An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103. doi: 10.1111/iep.12008
Non-alcoholic Fatty Liver Disease Study Group, Lonardo, A., Bellentani, S., Argo, C. K., Ballestri, S., Byrne, C. D., et al. (2015). Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig. Liver Dis. 47, 997–1006. doi: 10.1016/j.dld.2015.08.004
Probst, Y., Guan, V., and Neale, E. (2019). Development of a choline database to estimate australian population intakes. Nutrients 11:913. doi: 10.3390/nu11040913
Rosenberg, N., Murata, M., Ikeda, Y., Opare-Sem, O., Zivelin, A., Geffen, E., et al. (2002). The frequent 5,10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. Am. J. Hum. Genet. 70, 758–762. doi: 10.1086/338932
Saadeh, S., Younossi, Z. M., Remer, E. M., Gramlich, T., Ong, J. P., Hurley, M., et al. (2002). The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123, 745–750. doi: 10.1053/gast.2002.35354
Satapathy, S. K., and Sanyal, A. J. (2015). Epidemiology and natural history of nonalcoholic fatty liver disease. Semin. Liver Dis. 35, 221–235. doi: 10.1055/s-0035-1562943
Sazci, A., Ergul, E., Aygun, C., Akpinar, G., Senturk, O., and Hulagu, S. (2008). Methylenetetrahydrofolate reductase gene polymorphisms in patients with nonalcoholic steatohepatitis (NASH). Cell Biochem. Function 26, 291–296. doi: 10.1002/cbf.1424
Schwahn, B. C., Chen, Z., Laryea, M. D., Wendel, U., Lussier-Cacan, S., and Genest, J. Jr., et al. (2003). Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 17, 512–514. doi: 10.1096/fj.02-0456fje
Serin, E., Guclu, M., Atac, F. B., Verdi, H., Kayaselcuk, F., Ozer, B., et al. (2007). Methylenetetrahydrofolate reductase C677T mutation and nonalcoholic fatty liver disease. Dig. Dis. Sci. 52, 1183–1186. doi: 10.1007/s10620-006-9565-7
Sookoian, S., and Pirola, C. J. (2011). Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 53, 1883–1894. doi: 10.1002/hep.24283
Subada, S., Corey, K. E., Lake, J. E., and Erlandson, K. M. (2018). NAFLD and HIV: do sex, race, and ethnicity explain HIV-related risk? Curr. HIV/AIDS Rep. 15, 212–222.
Tong, J., and Guo, J. J. (2019). Key molecular pathways in the progression of non-alcoholic steatohepatitis. Eur. Rev. Med. Pharmacol. Sci. 23, 8515–8522. doi: 10.26355/eurrev_201910_19165
Ullah, R., Rauf, N., Nabi, G., Ullah, H., Shen, Y., Zhou, Y. D., et al. (2019). Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int. J. Biol. Sci. 15, 265–276. doi: 10.7150/ijbs.30121
Wan, L., Li, Y., Zhang, Z., Sun, Z., He, Y., and Li, R. (2018). Methylenetetrahydrofolate reductase and psychiatric diseases. Translat. Psychiatry 8:242. doi: 10.1038/s41398-018-0276-6
Wiedeman, A. M., Barr, S. I., Green, T. J., Xu, Z., Innis, S. M., and Kitts, D. D. (2018). Dietary choline intake: current state of knowledge across the life cycle. Nutrients 10:1513. doi: 10.3390/nu10101513
Xu, Y., Zhao, Z., Liu, S., Xiao, Y., Miao, M., Dong, Q., and Xin, Y. (2019). Association of nonalcoholic fatty liver disease and coronary artery disease with FADS2 rs3834458 gene polymorphism in the chinese han population. Gastroenterol. Res. Pract. 2019:6069870.
Yan, J., Wang, W., Gregory, J. F. III, Malysheva, O., Brenna, J. T., Stabler, S. P., et al. (2011). MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am. J. Clin. Nutr. 93, 348–355. doi: 10.3945/ajcn.110.005975
Yang, Z., Wen, J., Tao, X., Lu, B., Du, Y., Wang, M., et al. (2011). Genetic variation in the GCKR gene is associated with non-alcoholic fatty liver disease in Chinese people. Mol. Biol. Rep. 38, 1145–1150. doi: 10.1007/s11033-010-0212-1
Yao, Z. M., and Vance, D. E. (1988). The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 263, 2998–3004.
Yi, M., Chen, R. P., Yang, R., and Chen, H. (2017). Increased prevalence and risk of non-alcoholic fatty liver disease in overweight and obese patients with Type 2 diabetes in South China. Diabetic Med. 34, 505–513. doi: 10.1111/dme.13174
Yki-Järvinen, H. (2014). Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910. doi: 10.1016/S2213-8587(14)70032-4
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84.
Zeisel, S. H. (2006). Choline: critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 26, 229–250. doi: 10.1146/annurev.nutr.26.061505.111156
Zeng, Q., Li, F., Xiang, T., Wang, W., Ma, C., Yang, C., et al. (2017). Influence of food groups on plasma total homocysteine for specific MTHFR C677T genotypes in Chinese population. Mol. Nutr. Food Res. 61:1600351. doi: 10.1002/mnfr.201600351
Keywords: food groups, non-alcoholic fatty liver disease, methylene tetrahydrofolate reductase, risk factors, lifestyle, homocysteine
Citation: Hao X, Ma C, Xiang T, Ou L and Zeng Q (2021) Associations Among Methylene Tetrahydrofolate Reductase rs1801133 C677T Gene Variant, Food Groups, and Non-alcoholic Fatty Liver Disease Risk in the Chinese Population. Front. Genet. 12:568398. doi: 10.3389/fgene.2021.568398
Received: 09 June 2020; Accepted: 22 January 2021;
Published: 18 February 2021.
Edited by:
Suresh T. Mathews, Samford University, United StatesReviewed by:
Bibiana Garcia-Bailo, University of Toronto, CanadaPradeep Kumar, Veer Bahadur Singh Purvanchal University, India
Copyright © 2021 Hao, Ma, Xiang, Ou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zeng, WlEzMDFAMTI2LmNvbQ==
 Xiaoyan Hao
Xiaoyan Hao Qiang Zeng
Qiang Zeng