- Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby, BC, Canada
We investigate the impact of confounding on the results of a genome-wide association analysis by Beaty et al., which identified multiple single nucleotide polymorphisms that appeared to modify the effect of maternal smoking, alcohol consumption, or multivitamin supplementation on risk of cleft palate. The study sample of case-parent trios was primarily of European and East Asian ancestry, and the distribution of all three exposures differed by ancestral group. Such differences raise the possibility that confounders, rather than the exposures, are the risk modifiers and hence that the inference of gene-environment (G×E) interaction may be spurious. Our analyses generally confirmed the result of Beaty et al. and suggest the interaction G×E is driven by the European trios, whereas the East Asian trios were less informative.
1. Introduction
In a case-parent trio study we collect genotypes, G, on affected children and their parents. We may also collect environmental exposures or non-genetic attributes, E, on the children. Gene-environment interaction (G × E) is a statistical interaction term in the standard log-linear model of association between covariates and disease status. G × E interaction can be interpreted as genes that modify the effect of an exposure, or as an exposure that modifies the effect of genes.
Beaty et al. (2011) conducted an analysis of the GENEVA Oral Cleft Study data, a genome-wide association study identifying genetic and environmental factors associated with cleft palate (CP). They found multiple single nucleotide polymorphisms (SNPs) appeared to modify the effect of maternal smoking, maternal alcohol consumption, or maternal multivitamin supplementation on the risk of CP. The study sample of case-parent trios was primarily of European and East Asian ancestry, and the distribution of all three exposures differ by ancestral group, which raises the possibility of spurious G × E.
When the test locus, G′, is not causal, disease risk at G′ can appear to be modified by E without G × E interaction when there is exposure-related population structure (Shi et al., 2011; Weinberg et al., 2011). Exposure-related population structure may be thought of as a form of confounding that occurs when both GG′ haplotype frequencies and E distributions differ by ancestral group. Differences in GG′ haplotypes can lead to differences in G′ risk that may be tagged by E, suggesting G′ × E even in the absence of true G × E interaction.
One of the requirements for exposure-related population structure is different E distributions in the different ancestral groups. Such is the case in the GENEVA Oral Cleft Study where maternal smoking, maternal alcohol consumption and maternal multivitamin supplementation were all more common in self-reported Europeans than in self-reported East Asian populations (see Table 2). If, in addition, haplotype frequencies for markers in the vicinity of a causal SNP also vary by ancestral groups, any inferences of G × E interaction could be spurious. Shin et al. (2012) proposed bias-reduced methods for inference of G × E interaction from case-parent trio data in the presence of exposure-related population structure. In this article we use these methods to adjust the analyses of Beaty et al. (2011) for potential confounding effects of population stratification.
2. The GENEVA Oral Cleft Study
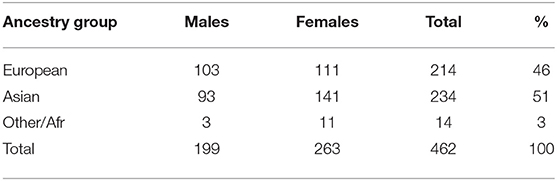
The GENEVA Oral Cleft study (gen, 2010) was comprised of 550 case-parent trios from 13 different sites across the United States, Europe, Southeast Asia, and East Asia. For our analyses, data were obtained through dbGAP at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000094.v1.p1 with accession number phs000094.v1.p1. Of the 550 trios included in the GENEVA Oral Cleft study, 462 were available for our analyses. Summaries of the trios by ancestry and gender of the affected child are shown in Table 1.
The objective of the GENEVA Oral Clefts study was to discover genetic contributions to orofacial clefts, the most common type of craniofacial birth defect in humans, and to assess whether these genes modify the effect of exposures known to be associated with cleft palate. Maternal exposure to multivitamins, alcohol and smoking were assessed through maternal interviews focused on the peri-conceptual period (3 months prior to conception through the first trimester), which includes the first 8–9 weeks of gestation when palatal development is completed. Exposure status is summarized in Table 2. From this table we see the ancestry of the sample is predominantly European (46%) and East Asian (51%), and these three exposures are all more common in Europeans.
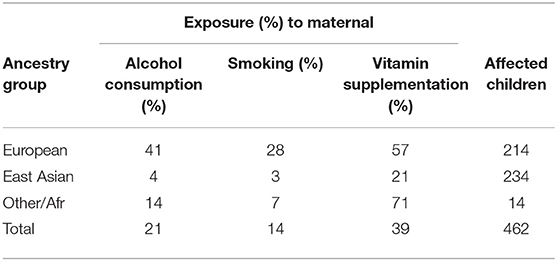
Table 2. Exposure rates for maternal alcohol consumption, maternal smoking, and maternal vitamin supplementation for the total CP group and for three ancestral groups.
Beaty et al. found evidence for G × E interaction for maternal alcohol consumption and SNPs in the genes MLLT3 and SMC2, for maternal smoking and SNPs in the genes TBK1 and ZNF236, and for maternal multivitamin use and SNPs in the BAALC gene.
3. Models and Methods
G × E interaction is defined as the statistical interaction term βGE in a log-linear model for the probability of disease:
where D = 1 indicates the child is affected with cleft palate, G is the child's genotype, coded as 0, 1, or 2 copies of the minor allele, E is an exposure variable and βE is the environmental main effect, which cannot be directly estimated in the case-parent trio design. To simplify the presentation, we assume throughout E is a binary variable with value 1 indicating exposure and 0 indicating no exposure. A non-zero interaction effect, βGE, suggests G modifies the effect of E on disease risk.
Assuming G and E are independent given parental genotypes Gp, and the risk model in Equation (1), one can derive the conditional distribution of G given D = 1, E and Gp, which can be stated in terms of the genotypic odds
for a constant kp that depends on gp (Appendix A.1). The environmental main effect does not appear in this genotypic odds and cannot be estimated from case-parent trio data. In general, any regression effect that does not involve G cannot be estimated from case-parent trio data.
To reduce bias from population stratification, robust methods are required. Robust methods may be classified as design- or data-based. For a binary environmental exposure, the design-based approach of Shi et al. (2011) augments the basic case-parent trio with exposure information on an unaffected sibling. Shi et al. showed that by including the sibship-averaged exposure over two sibs (affected/unaffected) in the linear model controls this potential bias. Weinberg et al. (2011) showed all information about interaction in the tetrad design of Shi et al. (2011) comes from the siblings, not the parents, which lead them to propose a sibling-augmented case-only design and analysis. Shin et al. (2012) took a data-based approach and replaced the sibship-averaged exposure of Shi et al. (2011) with the predicted exposure given ancestry, as reflected by principal components (PCs) computed from independent genetic markers. Their data-based approach is applicable for arbitrary exposures, including continuous measures, and does not require additional siblings. In this report we consider this data-based approach of Shin et al. (2012) to explore whether the G × E interaction effects reported by Beaty et al. (2011) could be spurious.
Let XE be a categorical variable indicating ancestral groups with different E distributions. Shin et al. introduce a separate genetic effect for each level of XE into the risk model Equation (1). When XE is binary, this modified risk model becomes:
In the above model, βGXE controls bias by allowing different genetic effects in the two XE-groups. The term βGEXE allows for different G × E effects in the two groups, which can improve power to detect G × E interaction (Shin et al., 2012).
As XE is not known, it must be replaced by some surrogate, . We consider two surrogates, (i) the expectation of E given genetic markers (EEGM) and (ii) self-reported ancestry (SRA). The idea behind the EEGM approach is to distinguish exposure distributions by their mean, which may vary across ancestry groups, S.Though S is not known, it is reflected in the principal components, M, computed from genetic marker data available on all ancestry groups in these data. The expectation of E given M can be estimated by linear regression of E on M when E is continuous, or by logistic regression when E is binary, as in the current study. Thus, for EEGM adjustment, we estimate the expected exposure within ancestral groups with in the risk model shown in Equation (2) below. We consider EEGM adjustment to be the gold-standard, because, for the case of two ancestral groups, Shin et al. (2012) showed the resulting tests of G × E interaction achieve the nominal type I error rates. To align this EEGM adjustment results with those based on SRA adjustment, we transform EEGM to the unit interval by subtracting the minimum value and dividing by the range. The use of SRA as a surrogate for XE is straightforward and leads to more interpretable models (see below), but is subject to some bias, because self-reported ancestry may not accurately reflect genetic ancestry (Wang et al., 2010). To simplify notation, we assume only two self-reported ancestry groups, encoded in as zero or one.
Distributions of EEGM by SRA for maternal exposure to alcohol, smoking, and vitamin supplements, are shown in Figure 1. As expected, there is a clear separation of EEGM between self-reported Europeans and East Asians for all three exposures considered here. In our analyses we would therefore expect similar results with adjustment by either EEGM or SRA.
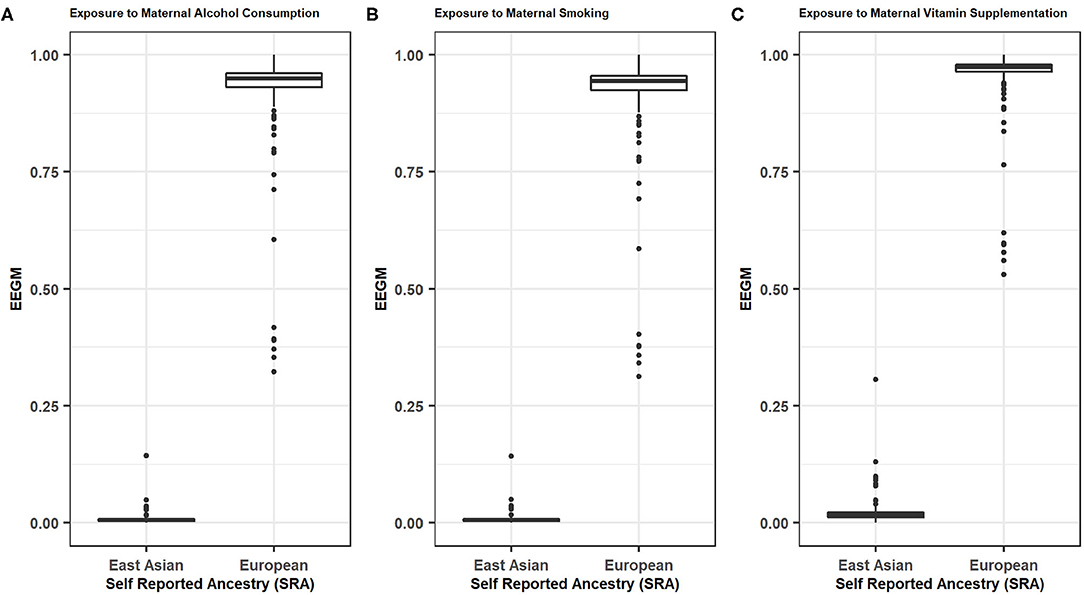
Figure 1. EEGM by SRA for (A) Maternal Alcohol Consumptions, (B) Maternal Smoking, (C) Maternal Vitamin Supplementations.
For either surrogate , let denote its coefficient so we can write the risk model as
leading to genotypic odds
Interpretation of the model in Equation (2) is simplest when is the SRA. If for East Asians and 1 for Europeans, then the relative risk of cleft palate due to exposure in self-reported East Asians with G = g is . Thus, is the multiplicative increase in the relative-risk due to exposure for each additional copy of G in self-reported East Asians. Similarly, the relative risk of cleft palate due to exposure in self-reported Europeans is , so this latter term is the multiplicative increase in the relative-risk due to exposure for each copy of G in self-reported Europeans. To summarize, reflects G × E in self-reported East Asians and reflects G × E in self-reported Europeans.
Inference can be made from the conditional likelihood based on P(G|D = 1, E, Gp), which can be obtained from the genotypic odds shown in Equation (3). Parameter estimates are obtained by maximizing the conditional likelihood, and hypothesis tests are obtained from likelihood ratio statistics. In particular, the hypothesis HO (i.e., not GxE interaction), is tested first by fitting models with and without the interaction terms and comparing the resulting likelihood ratio statistic to the χ2 distribution with 2 degrees of freedom.
4. Results
Inference was obtained under the unadjusted risk model in Equation (1) and the adjusted risk model in Equation (2) with either SRA or EEGM adjustment. The first analysis was restricted to the 6 SNPs in the MLLT3 gene reported by Beaty et al. to have significant G × E with maternal alcohol consumption. P-values for the 1 df (no adjustment) and 2 df tests (with adjustment) are shown in Table 3. We see the results with adjustment roughly agree and are only slightly attenuated compared to the results without adjustment for population stratification of exposures.
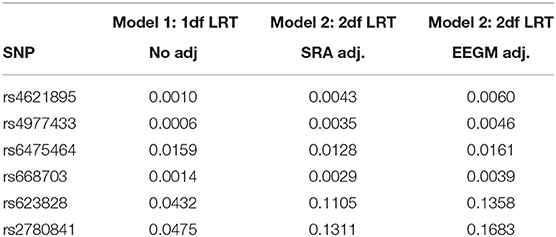
Table 3. P-values of 1 df likelihood ratio test from Model (1), and 2 df likelihood ratio tests from models with SRA and EEGM adjustment for the 6 SNPs in the MLLT3 gene on chromosome 9 show significant evidence of interaction with Maternal Alcohol Consumption.
Figure 2 shows the estimated interaction terms and and their corresponding confidence intervals while Table 4 shows exponentiated parameter estimates and their confidence intervals, as well as p-values from inference based on the risk model adjusted by SRA only. The overlap of the confidence intervals for with zero and the non-overlap of the confidence intervals for suggest no evidence of G × E interaction in self-reported East Asians, but evidence of G × E interaction in self-reported Europeans. The table of exponentiated parameter estimates quantifies this apparent G × E in self-reported Europeans. For example, the estimate suggests that each copy of the minor allele at SNP rs4621895 more than doubles the relative risk of CP due to maternal alcohol exposure.
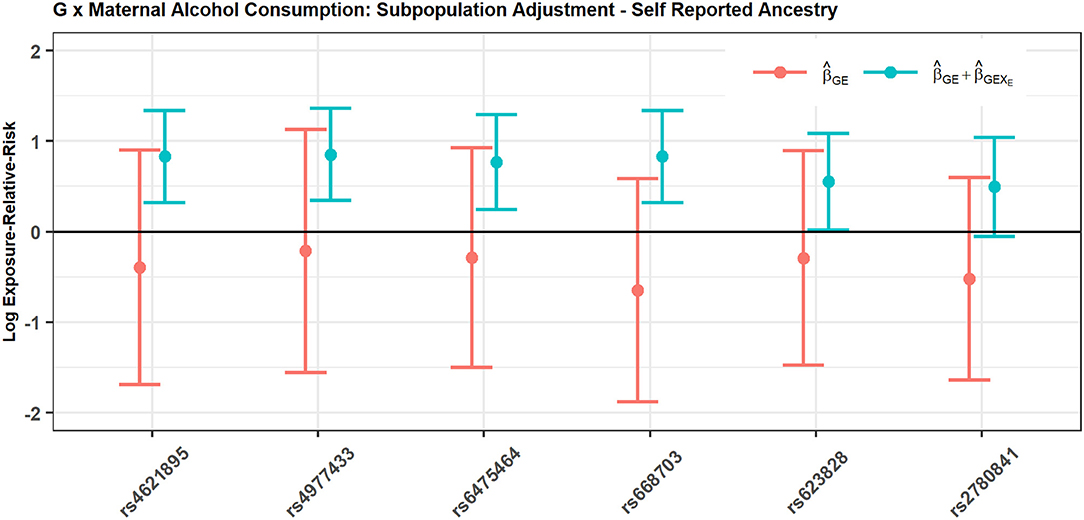
Figure 2. SNPs in MLLT3 gene show significant interaction with Maternal Alcohol Consumption among European CP trios, but not among East Asian CP trios.
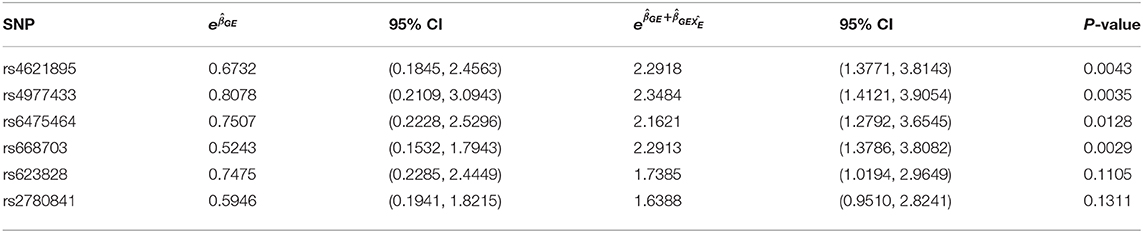
Table 4. Exponentiated G × E parameter estimates with corresponding exponentiated 95% Confidence Intervals with SRA adjustment at 6 the SNPs on MLLT3 (Chr 9) showing significant interaction with Maternal Alcohol Consumption.
Beaty et al. report that CP-affected European trios were gathered from multiple sites. Given that the G × E signal for SNPs in MLLT3 appears to be driven by these trios of European ancestry only, we also investigated whether stratification within Europeans could explain the apparent G × E interaction. To do this, we computed principal components from genetic markers in all self-reported European trios and performed logistic regression of exposure on PCs computed from 6,231,573 SNPs. However, the PCs were not predictive of exposure, and so there was no evidence of ancestral sub-groups with different E distributions within self-reported Europeans.
We performed similar analyses to the other four genes implicated in the Beaty study with mixed success. In some instances, the SNPs and exposures were so rare in the self-reported East Asian trios the coefficient in the model (2) could not be estimated. In such situations, we must assume a common G × E effect in both East Asians and Europeans. However, in this extended analysis of the other 4 genes we were still able to find some evidence of G × E interaction, though, as in Table 3, the results were attenuated compared to those when there is no adjustment for confounding. Tables of results are shown in Appendix A.2.
5. Discussion and Conclusions
Case-parent trio studies allow robust inference of genetic main effects, but inference of G × E interaction is susceptible to bias from a particular form of confounding known as exposure-related population structure. In the GENEVA Oral Cleft Study, such confounding would arise from heterogeneity of haplotype and exposure distributions across component sub-populations of the study. This is a concern because of the observed heterogeneity in exposure to maternal tobacco, alcohol, and multivitamin use among European and East Asian populations. We emphasize that differences in exposure need not be genetic in nature; indeed, the differences in exposure distributions in the GENEVA Study are presumably cultural. However, if these non-genetic differences coincide with genetic differences in genes such as MLLT3, SMC2, TBK1, ZNF236, and BAALC, they can result in spurious inference of G × E interaction. We applied the bias-reduced methods of Shin et al. to the GENEVA Oral Cleft Study and generally obtained the same conclusions regarding G × E interaction as an earlier analysis by Beaty et al. However, we found that the data only supported genetic modifiers of the exposure effects in self-reported Europeans. By contrast, all three exposures are too rare in self-reported East Asians to draw any conclusions about the presence of such modifiers in that population.
We further investigated whether confounding within the self-reported Europeans could explain these suggestions of G × E interaction. However, the genetic marker data from the genome-wide marker panel was not predictive of exposure within this population and so the adjustment of Shin et al. was not possible. Along the same lines, we investigated whether confounding in self-reported East Asians could explain the G × E findings of Wu et al., who found evidence of genetic modifiers of the effect of environmental tobacco exposure on CP. The study sample was comprised of trios from Korea, China (Hubei, Sichuan, and Shandong provinces), Taiwan, and Singapore. Differences in exposure rates and haplotype distributions among these populations could lead to spurious evidence of G × E interaction. Again, we found genetic markers were not predictive of exposure, so the methods of Shin et al. could not be applied directly. Both of these scenarios suggest the need for alternative methods for adjusting for exposure-related stratification among sub-populations.
We conclude with a discussion of areas for future work. Exposure-related population structure is illustrated in Figure 3. The link between GG′ haplotype frequencies and E distributions can be broken by adding any of S, XE, or to the risk model. The approach of Shin et al. is to estimate a surrogate for XE. As noted above, this approach may not be sensitive enough to adjust for subtle heterogeneity in genetic and exposure distributions. The diagram suggests alternatives, namely conditioning on S or on . The advantage of conditioning on S, or on principal components that reflect S, is its simplicity and familiarity to researchers who study unrelated subjects. The potential advantage of an approach based on inferring is that it aims to characterize local structure in the genome of study subjects that could be responsible for apparent differences in risk at the test locus caused by differences in Linkage Disequilibrium (LD) with a nearby causal locus. Efforts to develop such methods are ongoing.
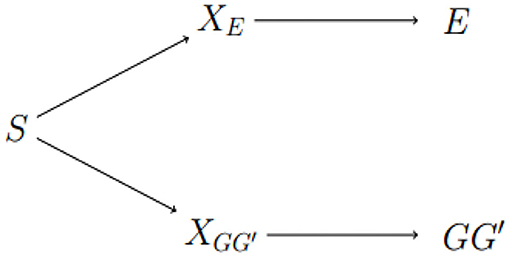
Figure 3. Dependence between E and GG′ is through latent sub-population S. Latent factors XE and indicate different distributions of E and GG′, respectively. E and GG′ are conditionally independent given any of the three variables on the path between them.
Another possible avenue for future research is to develop an approach that is a hybrid of case-parent-trio and case-only designs. The case-only design (Piegorsch et al., 1994) shares key features with the case-parent trio design. Starting with a log-linear model of disease probabilities, and assuming G and E are independent, G × E is inferred from an association between G and E in cases. By conditioning on parental genotypes, inference from case-parent trio data is under the weaker assumption that G and E are independent within families, rather than in the general population, as in the case-only design. However, the number of cases available for analysis may be increased by dropping the requirement of parental genotype data. Thus, robust methods for inference of G × E that make use of cases with or without parental genotypes become of interest.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000094.v1.p1 (dbGaP Study Accession: phs000094.v1.p1).
Author Contributions
PR researched the statistical methods, carried out the analyses, and wrote the manuscript. BM developed the research question, consulted on the analyses, and wrote and proofread the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding support for the study entitled International Consortium to Identify Genes and Interactions Controlling Oral Clefts was provided by several previous grants from the National Institute of Dental and Craniofacial Research (NIDCR). Data and samples were drawn from several studies awarded to members of this consortium. Funding to support original data collection, previous genotyping and analysis came from several sources to individual investigators. Funding for individual investigators include: R21-DE-013707 and R01-DE-014581 (Beaty); R37-DE-08559 and P50-DE-016215 (Murray, Marazita) and the Iowa Comprehensive Program to Investigate Craniofacial and Dental Anomalies (Murray); R01-DE-09886 (Marazita), R01-DE-012472 (Marazita), R01-DE-014677 (Marazita), R01-DE-016148 (Marazita), R21-DE-016930 (Marazita); R01-DE-013939 (Scott). Parts of this research were supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Wilcox, Lie). Additional recruitment was supported by the Smile Train Foundation for recruitment in China (Jabs, Beaty, Shi) and a grant from the Korean government (Jee).
The genome-wide association study, also known the Cleft Consortium, is part of the Gene Environment Association Studies (GENEVA) program of the trans-NIH Genes, Environment and Health Initiative [GEI] supported by U01-DE-018993. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health (NIH) to The Johns Hopkins University, contract number HHSN268200782096C. Funds for genotyping were provided by the NIDCR through CIDR's NIH contract. Assistance with genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01-HG-004446) and by the National Center for Biotechnology Information (NCBI). This work was supported, in part, by the Natural Sciences and Engineering Research Council of Canada.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank all of the patients and families at each recruitment site for participating in this study, and we gratefully acknowledge the invaluable assistance of clinical, field, and laboratory staff who contributed to this effort over the years.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.600232/full#supplementary-material
References
(2010). Geneva Clefts Project Imputation Report - Hapmap III Reference Panel [pdf file]. Available online at: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000094.v1.p1
Beaty, T. H., Ruczinski, I., Murray, J. C., Marazita, M. L., Munger, R. G., Hetmanski, J. B., et al. (2011). Evidence for gene-environment interaction in a genome wide study of nonsyndromic cleft palate. Genet. Epidemiol. 35, 469–478. doi: 10.1002/gepi.20595
Shi, M., Umbach, D. M., and Weinberg, C. R. (2011). Family-based gene-by-environment interaction studies. Epidemiology 22, 400–407. doi: 10.1097/EDE.0b013e318212fec6
Shin, J.-H., Infante-Rivard, C., Graham, J., and McNeney, B. (2012). Adjusting for spurious gene-by-environment interaction using case-parent triads. Stat. Appl. Genet. Mol. Biol. 11. doi: 10.2202/1544-6115.1714
Wang, H., Haiman, C. A., Kolonel, L. N., Henderson, B. E., Wilkens, L. R., Le Marchand, L., et al. (2010). Self-reported ethnicity, genetic structure and the impact of population stratification in a multiethnic study. Hum. Genet. 128, 165–177. doi: 10.1007/s00439-010-0841-4
Weinberg, C. R., Shi, M., and Umbach, D. M. (2011). A sibling-augmented case-only approach for assessing multiplicative gene-environment interactions. Am. J. Epidemiol. 174, 1183–1189. doi: 10.1093/aje/kwr231
Keywords: gene-environment interaction, case-parent trios, population stratification, genome-wide association study, cleft palate
Citation: Ratnasekera P and McNeney B (2021) Re-analysis of a Genome-Wide Gene-By-Environment Interaction Study of Case Parent Trios, Adjusted for Population Stratification. Front. Genet. 11:600232. doi: 10.3389/fgene.2020.600232
Received: 29 August 2020; Accepted: 08 December 2020;
Published: 13 January 2021.
Edited by:
José M. Álvarez-Castro, University of Santiago de Compostela, SpainReviewed by:
Terri Beaty, Johns Hopkins University, United StatesClaire Infante-Rivard, McGill University, Canada
Copyright © 2021 Ratnasekera and McNeney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brad McNeney, bWNuZW5leUBzZnUuY2E=
 Pulindu Ratnasekera
Pulindu Ratnasekera Brad McNeney*
Brad McNeney*