
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 27 November 2020
Sec. Epigenomics and Epigenetics
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.596947
This article is part of the Research Topic The Genetic and Epigenetic Bases of Cellular Response to Ionizing Radiation View all 9 articles
The study of the genetic basis of the manifestation of radiation-induced effects and their transgenerational inheritance makes it possible to identify the mechanisms of adaptation and possible effective strategies for the survival of organisms in response to chronic radioactive stress. One persistent hypothesis is that the activation of certain genes involved in cellular defense is a specific response of the cell to irradiation. There is also data indicating the important role of transposable elements in the formation of radiosensitivity/radioresistance of biological systems. In this work, we studied the interaction of the systems of hobo transposon activity and DNA repair in the cell under conditions of chronic low-dose irradiation and its participation in the inheritance of radiation-induced transgenerational instability in Drosophila. Our results showed a significant increase of sterility and locus-specific mutability, a decrease of survival, fertility and genome stability (an increase the frequency of dominant lethal mutations and DNA damage) in non-irradiated F1/F2 offspring of irradiated parents with dysfunction of the mus304 gene which is responsible for excision and post-replicative recombination repair and repair of double-stranded DNA breaks. The combined action of dysfunction of the mus309 gene and transpositional activity of hobo elements also led to the transgenerational effects of irradiation but only in the F1 offspring. Dysfunction of the genes of other DNA repair systems (mus101 and mus210) showed no visible effects inherited from irradiated parents subjected to hobo transpositions. The mei-41 gene showed specificity in this type of interaction, which consists in its higher efficiency in sensing events induced by transpositional activity rather than irradiation.
Ionizing radiation induces biological and genetic effects that have been the subject of detailed study for many years. One of the most important cellular systems responding to irradiation is the DNA repair system which is actively involved in the elimination of DNA damages even before they turn into mutational events (Bregliano et al., 1995). When the cell is irradiated, an increased activity of transposable elements (TEs) associated with stress resistance genes, in particular, DNA repair genes, is observed. TEs are a movable part of the genome and DNA fragments that can move around the genome and destabilize it (Finnegan, 1989; Kaufman and Rio, 1992; Jurka et al., 2007; McCullers and Steiniger, 2017). To date, a connection has been established between P elements and repair processes which is described details in Banga et al. (1991) and Yushkova and Zainullin (2016). The P transposon acting as a “model” intracellular factor is capable of destabilizing the genome only in a certain genetic P-M system of hybrid dysgenesis (HD) (Kidwell et al., 1977). HD is a syndrome that manifests in offspring in the form of an increased level of gene mutations, chromosomal aberrations, chromosome non-disjunction during meiosis, and sterility. HD occurs with certain crossing combinations. As a result of such crosses, inactive transposons (P and hobo) of males of strong P and H strains become active after crossing with females of M and E strains which lack the repressor protein of transposition (Kidwell et al., 1977; Bazin and Higuet, 1996). Entering the cytoplasm lacking the repressor protein of transposition, P and hobo elements encode the enzyme (transposase) of their own transposition which lead to DNA damage and genetic breakdowns (Bazin et al., 1999). As a result, dysgenic events occur in the offspring in early ontogeny, causing atrophy of the reproductive organs, a decrease of fertility and survival at the later stages of their development (Niki and Chigusa, 1986). The presence of certain properties inherited through the maternal line is a key characteristic of the “cytotype” and determines the level of functional activity of transposons responsible for the manifestation of HD (Engels, 1981). A feature of P and hobo elements is their wide distribution and a high level of diversity in various organisms, including humans (Menzel et al., 2014). Unlike other TEs (retrotransposons that move by the “copy-paste” mechanism and do not break the integrity of the DNA structure), transposons are able to move through the genome using the “cut-paste” mechanism and lead to the formation of double-stranded DNA breaks (DSBs) (Sobels and Eeken, 1981; Finnegan, 1989; Kaufman and Rio, 1992).
Recently, there has been a growing interest in studies of the behavior of hobo elements which not only can be massively activated under conditions of classical dysgenic crossing [H-males (with hobo transposons) × E-females (without hobo)] (Blackman et al., 1987) but they can also be active in non-genetic systems, i.e., under conditions of interlinear (between H-males and H-females) and reciprocal (between H-females and E-males) crosses (Bazin and Higuet, 1996; O’Hare et al., 1998; Zakharenko et al., 2006; Yushkova, 2019). The hobo transposon of D. melanogaster belongs to the Ac elements of the hAT superfamily (Loreto et al., 2018). It is active in the germ line and somatic tissue cells (Calvi and Gelbart, 1994; O’Hare et al., 1998; Zakharenko et al., 2006) and also is sensitive to radiation exposure (Ivashenko and Grishaeva, 2002; Yushkova and Zainullin, 2014). The full-length hobo element (∼ 3 kb) has an open reading frame (ORF) encoding transposase and short terminal inverted repeats (TIRs) 12 bp in size, forms duplications at the insertion site of 8 bp (Loreto et al., 2018). Many studies have shown that Drosophila genomes are loaded the defect or truncated hobo copies (up to 1.5 kb) which have a high similarity to a transcriptionally active canonical hobo transposons (Torres et al., 2006; Ortiz and Loreto, 2008; Deprá et al., 2009). Such copies move with high frequency and may play a key role in the formation of dysgenic status in animals (Bazin and Higuet, 1996). Canonical hobo elements have ORF or only part of it (due to internal deletions of hobo copies) and TIRs (Loreto et al., 2018). Transposons of the hAT superfamily have some structural and functional homology with full-length human transposons (Charlie 1-8, Cheshire, Zaphod, and MER69) which account for 1.55% (195 Mbp) of the total genome (Lander et al., 2001). In general, transposons are not active in the human genome because they are not autonomous (McCullers and Steiniger, 2017). However, there is information confirming the presence of active THAP9 transposons in the human genome capable to transpositions, like the P elements of D. melanogaster (Majumdar et al., 2013; Majumdar and Rio, 2015).
This article is a continuation of the study of the mechanisms of interaction between the systems of transposition of TEs as components of epigenetic regulation and DNA repair in the cell under conditions of chronic radiation exposure (Yushkova and Zainullin, 2016). We have established that there is a specificity of turning-on of certain repair processes and their genes as a result of a particular effect (transposition of P elements or irradiation). The “universality” of some repair genes functioning upon the simultaneous action of external (irradiation) and intracellular (P elements) factors has also been discovered. The question remains to what extent this cell response depends on the “transposon-specificity” in this type of interaction. One of the possible approaches to solving this problem is to study the involvement of DNA repair genes in the processes of DNA damage repair induced by the transpositions of hobo elements under conditions of chronic irradiation.
The ability of transposons to cause such serious damage as (DSBs) attracts a significant interest in the problem of radiation-induced activation of transposition systems under conditions of impaired DNA repair. Given the fact that dysfunction of certain repair genes can lead to the inheritance of genetic effects by the offspring of irradiated parents (Yushkova, 2020), it would be interesting to consider how the interaction of the repair genes and transpositional activity of TEs affect the transgenerational transmission of radiation-induced genome instability. Moreover, the aforementioned studies on the transgenerational effects of irradiation have been mainly carried out on animals using high doses of irradiation (Wiley et al., 1997; Barber et al., 2002; Sarapultseva and Malina, 2009; Sarapultseva and Bychkovskaya, 2010; Sarapultseva and Gorski, 2013; Gomes et al., 2015; Sarapultseva and Dubrova, 2016; Lecomte-Pradines et al., 2017; Fuller et al., 2019; Hancock et al., 2019). Therefore, from this point of view, it is also important to consider whether parental radiation exposure affect cytogenetic and epigenetic traits inherited by the offspring of irradiated parents.
The strains were obtained from the Bloomington Drosophila Stock Center and characterized using molecular (PCR analysis) and genetic (instability of the mini-white locus) methods, which made it possible to determine the content of full-length hobo copies in their genomes (Galindo et al., 1995; Bazin and Higuet, 1996; Zakharenko et al., 2006; Yushkova and Zainullin, 2014).
ORS (genotype: C1DX, y1f1;OR) is a subline of the H-cytotype with attached X-chromosomes. It is derived from the wild-type Oregon-R (OR) strain containing full functional copies of the hobo element in the genotype (Galindo et al., 1995). It was created by crossing three females of the C1DX, y1f1/Y strain with three OR males followed by backcrossing their offspring (females) with OR males, for 8 generations (Margulies, 1990). It is capable of high induction of sterility and is subject to instability of the mini-white locus at a frequency of ∼9%. PCR analysis revealed the presence of full-length hobo elements in its genotype. We used this strain as an inducer strain of the H-cytotype.
HiRS (genotype: C1DX, y1f1; HiR) is a subline of the E-cytotype that does not have hobo transposons. It was obtained according to the same crossing scheme as the ORS subline (from the wild-type Hikone-R strain which does not contain hobo elements (Galindo et al., 1995). We used it in crosses as a reactive (sensitive to hobo transposition) E-cytotype strain that is not subject to mutability in the mini-white locus.
mei-41 (genotype: mei-41RT1 f1/FM7a) is a strain of the E-cytotype with a mutation in the mei-41 gene involved in the DNA damage sensing, post-replicative DNA repair, meiotic recombination and the G2/M checkpoint (Hari et al., 1995; Laurencon et al., 2003). The mei-41 gene is also involved in maintaining chromosome stability upon transposon activity (Koromyslov et al., 2003). We used this strain as a conditional positive control. It is not subject to mutability in the mini-white locus.
mus210G1 (genotype: mus210G1/CyO) are strain of the E-cytotype with a mutation in the mus210 gene, which participates in excision repair (Sekelsky et al., 1998). The presence of mutations in genes responsible for the excision repair in the genome does not impair the stability of chromosomes experiencing transposon activity (Chmuzh et al., 2007). We used this strain as a conditional negative control. When it was crossed with the haw strain, no mutability in the mini-white locus was found.
mus101D1 (genotype: w1mus101D1) is a strain of the E-cytotype with a mutation in the mus101 gene involved in the control of the cell cycle and non-recombination post-replicative DNA repair (de Buendßa et al., 2003; Choi et al., 2017). When it was crossed with the haw strain, no mutability in the mini-white locus was found.
mus304D1 (genotype: st1mus304D1/TM3,Sb1Ser1) is a strain of the E-cytotype with a mutation in the mus304 gene involved in the cell cycle control, in post-replicative DNA repair and recombination, DSB repair and excision repair of pyrimidine dimers (Harris and Boyd, 1993; Brodsky et al., 2000). It is not subject to mutability in the mini-white locus.
mus309D3 (genotype: mus309D3,ry/CyO) is an E-strain with a mutation in the mus309 gene involved in the DSB repair system (Portin, 2010). When it was crossed with the haw strain, no mutability in the mini-white locus was found.
haw (genotype: ywHw+,haw1) is an E-strain carrying two genetically engineered marker elements hobo(w+) on the X chromosome with an inserted mini-white reporter gene which is responsible for the orange color of the eyes. A change in the eye color in males (less brightly colored eyes) indicates the excision of one of the hobo(w+) elements (Calvi and Gelbart, 1994). This strain was kindly provided by B. R. Calvi (University of Philadelphia, Philadelphia, PA, United States). We used this strain as a way to quantify the activity of transposase of hobo elements.
ywf/mei-41 (genotype: mei-41/y&C1DX;y1w1f1/y) has attached X chromosomes and traits y (yellow – yellow body), f (forked – underdeveloped bristle), and w (white – white eyes). We used this strain in individual crosses to determine mutability in the mini-white locus.
The analysis of the transpositional activity of hobo elements under conditions of impaired protective intracellular processes was carried out by inducing crosses. To obtain F1 offspring, mass crosses of males of the ORS strain carrying full-length copies of hobo elements with females of the E-strains HiRS, mei-41, and mus-groups were carried out. To obtain F2 offspring, F1 specimens were individually crossed with the studied E-strains. The offspring from these crosses were conventionally designated as variants HiR[hobo+], mus[hobo+], and mei-41[hobo+]. As a control, we used specimens obtained by crossing HiRS males without hobo sequences with females of HiRS, mus101D1, mus210G1, mus304D1, mus309D3, and mei-41 strains. The control variants received the following designations: HiR[hobo-], mus[hobo-] and mei-41[hobo-]. A total of 24 crossing variants were analyzed, including irradiated variants.
Chronic irradiation of parents was performed from a 226Ra source (56 mGy/h) at a dose rate of 0.42 mGy/h. The cumulative dose was 120.9 mGy (from the egg stage to the imago, 12 days). Thermoluminescent dosimeters “DTU-1” with detectors “DTG-4” (“Dosa,” Russia) were used to measure the irradiation doses. Background specimens underwent the same procedure without irradiation. The parental generation of all variants was maintained under strictly controlled laboratory conditions with a photoperiod of 12 h light/12 h darkness at 25 ± 0.1°C and 60% humidity on a standard sugar-yeast medium (Ashburner, 1989).
The analysis of dominant lethal mutations (DLMs) was carried out according to the generally accepted method (Watti and Tikhomirova, 1976; Aslanian et al., 1994). The F1/F2 offspring were crossed with virgin females of the corresponding strain. The final progeny at the egg stage was evaluated for the presence of colored (brown) embryos. These changes called DLMs are caused by genetic abnormalities, which are large chromosomal rearrangements (Watti and Tikhomirova, 1976). To increase the visibility of the laid eggs, we used a sugar-yeast nutrient medium supplemented with activated carbon (Richardson and Kojima, 1965). The DLM frequency was calculated as a ratio of the number of later lethals to the total number of laid embryos. Each variant of the experiment was conducted in three replicates.
From each variant, we selected F1/F2 males (100 specimens) within 24 h after hatching of the imago. The studied variants were maintained under constant conditions on a depleted medium (sugar-agar) at 25 ± 0.1°C and 60% humidity with a 12 h light/12 h darkness cycle (Ashburner, 1989). Specimens were transferred to a fresh medium twice a week. The survival rate of specimens was assessed daily. Each variant of the experiment was conducted in three replicates.
From each F1/F2 variant, we selected 50 control and experimental males of the same age and crossed them with virgin females of the corresponding genotypes at a 1:3 ratio. For 10 days, the number of embryos laid by females per day was counted. Tubes with counted eggs were stored at 25 ± 0.1°C and 60% humidity with a 12 h light/12 h darkness photoperiod until the pupation stage, after which the pupae were counted. For a more accurate visual registration of the laid eggs, a nutrient sugar-yeast medium supplemented with activated carbon was used (Richardson and Kojima, 1965). Fertility was calculated as the average number of pupae per average number of embryos per female. Each variant of the experiment was conducted in three replicates.
From each F1/F2 variant, males of the same age (100 specimens) were selected. They were crossed with wild-type E-females (♂HiR[hobo-] or ♂HiR[hobo+] × 3♀♀HiRS) and mutant genotype females (♂mus(mei-41)[hobo-] or mus(mei-41)[hobo+] × 3♀♀mus(mei-41)). Sterility (GD) was assessed using the method of gonadal atrophy. The presence [unilateral (GD1) and bilateral (GD0)] of hybrid females of each F1/F2 variant was determined by dissecting the abdomen. After emergence from puparia, females were maintained on a nutrient medium for 3 days until the full maturation of the gonads. The GD sterility was calculated using the formula: GD (%) = GD0 (%) + 1/2 GD1 (%) (Marin et al., 2000). Each variant of the experiment was conducted in three replicates.
The parameter “mutability of the mini-white locus” makes it possible to assess the activity of transposase, an enzyme responsible for the transpositions of hobo transposon, in somatic cells (Bazin and Higuet, 1996). For this, a series of successive crosses of experimental F1/F2 males with haw and ywf/mei-41 test strains were carried out. The offspring were screened for specific specimens with a yellow (yellow body), forked (underdeveloped bristle) phenotype and mosaic eye pigmentation that occurs during somatic transposition or excision of hobo(w+) during development. This test allows the assessment of excision (Me) and transposition (Mt) of two hobo(w+) reporter hobo elements and tracking the activity of hobo elements in the tested males through the transposition of hobo(w+) from the X chromosome to autosomes (Calvi et al., 1991; Bazin and Higuet, 1996). The appearance in the offspring of males with lightened eyes (less-colored-eyes) indicates hobo(w+) excision. No excision was found in our study. While the appearance of females with pigmented eyes indicates an autosomal transposition of hobo(w+) (Mt). The level of transpositions (%Mt) was assessed by the number of hybrid males that had at least one transposition event in their offspring. Each variant of the experiment was conducted in three replicates.
DNA damage in neuroblasts and midgut cells of F1/F2 third instar larvae was assessed using the Comet assay (Yushkova and Zainullin, 2016). The cell suspension was obtained by treating somatic tissues to a collagenase solution (type IV, 0.5 mg/ml PBS) at 37°C. The obtained cells of the nerve ganglia and midgut (10 μl) were mixed with 0.5% low melting point agarose (100 μl) and applied on glass slides coated with a layer of 1% normal melting point agarose. The slides were kept in a cold environment (4°C) for 20 min and subjected to further lysis (2.5 Ì NaCl, 10 ìÌ Na2EDTA, 20 ìÌ Tris–HCl, 1% Triton X-100, pH 10.0) at 4°C. Electrophoresis was performed (at 30 mA, 0.7 V/cm, 4°C, 20 min) in a cooled (4°C) neutral buffer (0.9 M Trizma-Base, 0.9 M Boric acid, 20 mM Na2EDTA, pH 8.2). The neutral version of the method assesses predominantly DSBs (Olive and Banath, 2006). The preparations were fixed in 70% ethanol for 15 min, dried and stained with the SYBR Green I solution (0.2 μL/ml in TE buffer, pH 7.5) (DNA synthesis, Russia). The images of “comets” were analyzed using an Infiniti XS-148 FS fluorescence microscope and processed using the CometScoreTM software (version 1.5, TriTek Corp., United States)1. The DSBs were assessed by the Olive tail moment (OTM). OTM is the product of the distance from the center of the head to the density center of the comet’s tail multiplied by tDNK[%] (concentration (%) of DNA in the tail of the “comet”) (Olive, 1999). For each experimental point (10 specimens), 3 slides were taken. On each slide, 50 cells were counted. Given into account three replicates of each variant of the experiment, 450 cells were analyzed per variant, per generation.
We used the following reagents: sodium chloride (NaCl), agarose, triton-X, boric acid, tris hydrochloride (Tris–HCl) (PanReac, Spain), collagenase type IV, phosphate buffered saline (PBS), tris(hydroxymethyl)aminomethane (Trizma Base) (Sigma, CIIIA), disodium EDTA (Na2EDTA) (AppliChem, Germany).
PCR analysis was performed as described in Yushkova and Zainullin (2014). DNA was isolated from the imago using the DiatomTM DNA Prep 200 kit (IzoGen, Russia). The primers for the open reading frame of the full-length hobo element were 5′-GCGCCATACATAATGATTG-3′ and 5′-CTATTGCGAGTTGTTTAG-3′ (Zakharenko et al., 2006). The DNA concentration was measured using a Qubit® fluorometer (Invitrogen, Waltham, MA, United States). Amplification of the isolated DNA was carried out with the addition of GenPak® PCR MasterMix Core reagents using an ESCO Swift Mini Pro thermal cycler (ESCO, Singapore). PCR reaction parameters: initial denaturation at 94°C for 3 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 54°C for 30 s, synthesis at 72°C for 100 s; final elongation at 72°C for 5 min. As a control, deionized water was used. PCR products were stained with the SYBR Green I dye (DNA synthesis, Russia) in 1.3% agarose gel and separated by horizontal electrophoresis. Electrophoretograms were visualized using an ECX-15.M transilluminator (Vilber Lourmat, France) and a Gel Imager video system (DNA-Technology, Russia).
Three independent experiments were carried out, the results of which were summarized and presented as a mean value and the corresponding standard error of the mean (SEM). Statistical analysis of differences between the mean values of the parameters (the frequency of DNA damage and DLMs, the level of sterility and Mt) of the experimental variants were determined by the Student’s t-test. The obtained data were processed using the Statistica program (version 7.0.61.0, StatSoft, Inc., United States). The significance of differences of the results of the fertility analysis was assessed by the Chi-square test in the R program. To compare the statistical differences in survival functions and median lifespan, as well as maximum lifespan between the control and experimental groups, the Kolmogorov-Smirnov, log-rank and Wang-Allison tests were used, respectively (Mantel, 1966; Breslow, 1970; Fleming et al., 1980; Han et al., 2016). Data were processed using the Online Application for Survival Analysis 2 (OASIS 2)2.
GD-sterility is one of the important traits of the manifestation of the H-E system of the HD which is based on the high transposition activity of hobo elements (Bazin and Higuet, 1996). The results obtained showed that increased GD-sterility is characteristic for all offspring obtained by crosses with ORS males (Table 1). On the contrary, we did not observe offspring with gonadal atrophy when crossed with male HiRS. It follows from this that the observed sterility, albeit indirectly, testifies to the transpositional activity of hobo elements. The strains HiRS, mei-41, and mus304D1 were the most sensitive to hobo transpositions. The offspring with dysfunction of the mei-41 and mus304 genes exhibited high sterility (p < 0.05) independent of parental exposure. For this parameter, transgenerational effects of irradiation (p < 0.05) were observed in F1/F2 offspring with normal functioning of repair genes (HiRS).
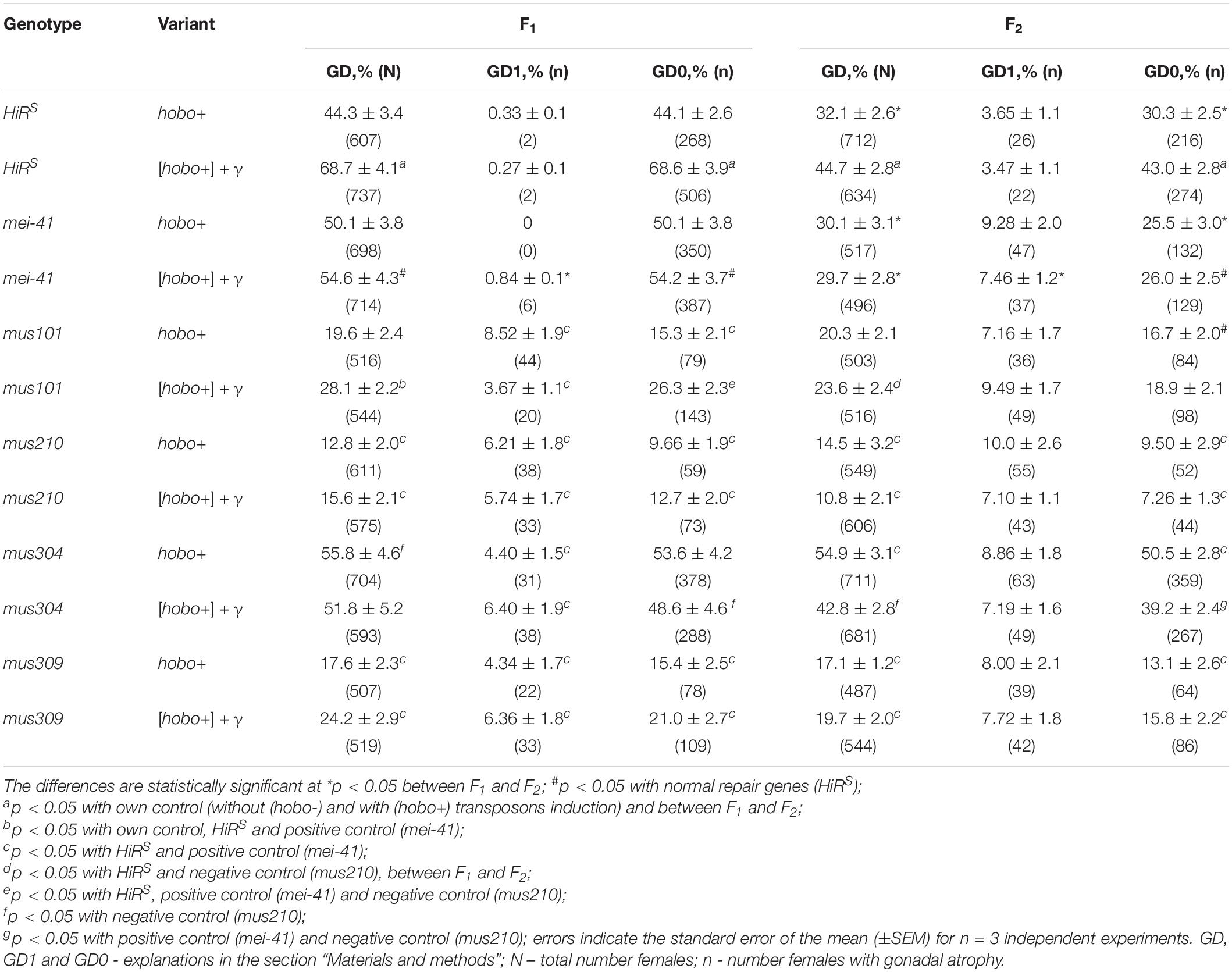
Table 1. Effect of a chronic irradiation in low doses and induction of the hobo elements on the sterility of D. melanogaster females F1/F2 with dysfunction of the repair genes.
To confirm the hobo activity, we also used a specific test for this based on the detection of autosomal excisional and transpositional activity of the constructed marker hobo(w+) elements in the offspring from crosses with males of the studied genotypes with haw females (Calvi et al., 1991; Calvi and Gelbart, 1994). Their activity depends on the transposase encoded by full-length hobo elements presumably present in the paternal genotypes. In the presence of transposase, hobo(w+) elements manifest their activity in the form of excisions and transpositions. In our study, excisions of hobo(w+) elements were not revealed, but were found their transpositions (Mt). The F1/F2 offspring selected as a result of crossing with ORS are characterized by high activity of hobo elements (p < 0.05–0.01). The levels of their GD sterility and Mt varied within 12.8–55.8 and 6.3–30.4% (in the offspring of non-irradiated parents) and 10.8–68.9 and 8.1–39.8% (in the offspring of irradiated parents), respectively. This proves the presence of statistically significant transposition activity of hobo elements in the studied genotypes. Transgenerational inheritance of different levels hobo activity (from low to high) is retained in all offspring of all genotypes independent of parental exposure. Statistically significant (p < 0.01) transgenerational effects of irradiation were found in F1/F2 offspring with normal functioning of repair genes (HiRS) and F1/F2 offspring with dysfunction of the mus304 gene undergoing hobo transposition (Figure 1).
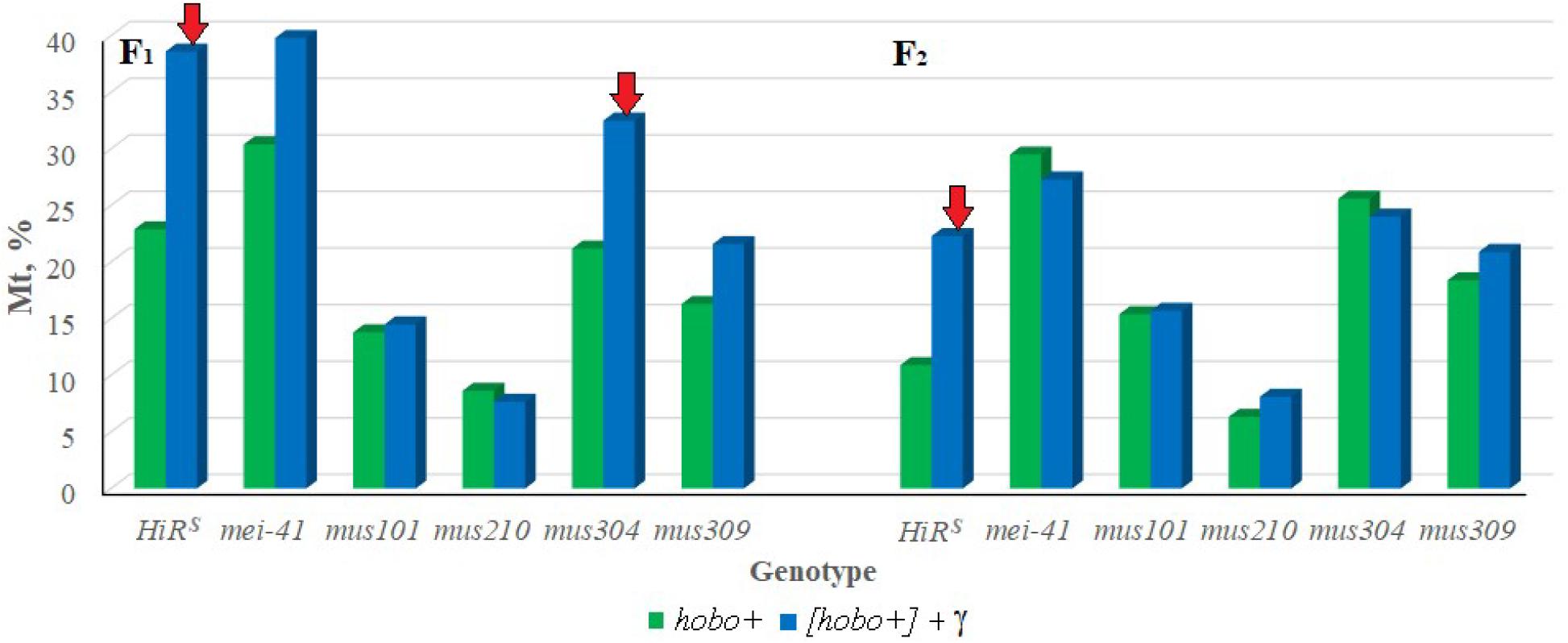
Figure 1. Effect of a chronic irradiation in low doses and induction of the hobo elements on the autosomal transposition hobo(w+) of D. melanogaster females F1/F2 with normal repair genes (HiRS) and dysfunction of the repair genes (mei-41, mus101, mus210, mus304 and mus309). Red arrow – statistically significant (p < 0.05–0.01) transgenerational effects of irradiation in individuals with dysfunction of the repair genes in the presence transposition of hobo elements.
All experimental variants were tested for the content of full-length hobo copies in their genotypes. PCR analysis showed the presence of full-length hobo transposons only in the offspring with hobo activity.
It was shown that disturbances in post-replicative (gene mei-41), excisional (gene mus210) repair, and DNA DSB repair (gene mus309) significantly affect (p < 0.05–0.01) the transmission of DLM frequency to non-dysgenic F1 offspring after irradiation of their parents (Figure 2). In the presence of hobo elements, the frequency of radiation-induced DLM increases (p < 0.01) in offspring with dysfunction of the mei-41, mus304, and mus309 genes. The inheritance of an increased level of DLM by subsequent generations depends on the dysgenic status of animals and the functional state of the mus304 gene in their genome. Dysfunction of the mus304 gene leads to significant (p < 0.01) transgenerational effects of parental irradiation in F2 offspring undergoing transposition of hobo elements.
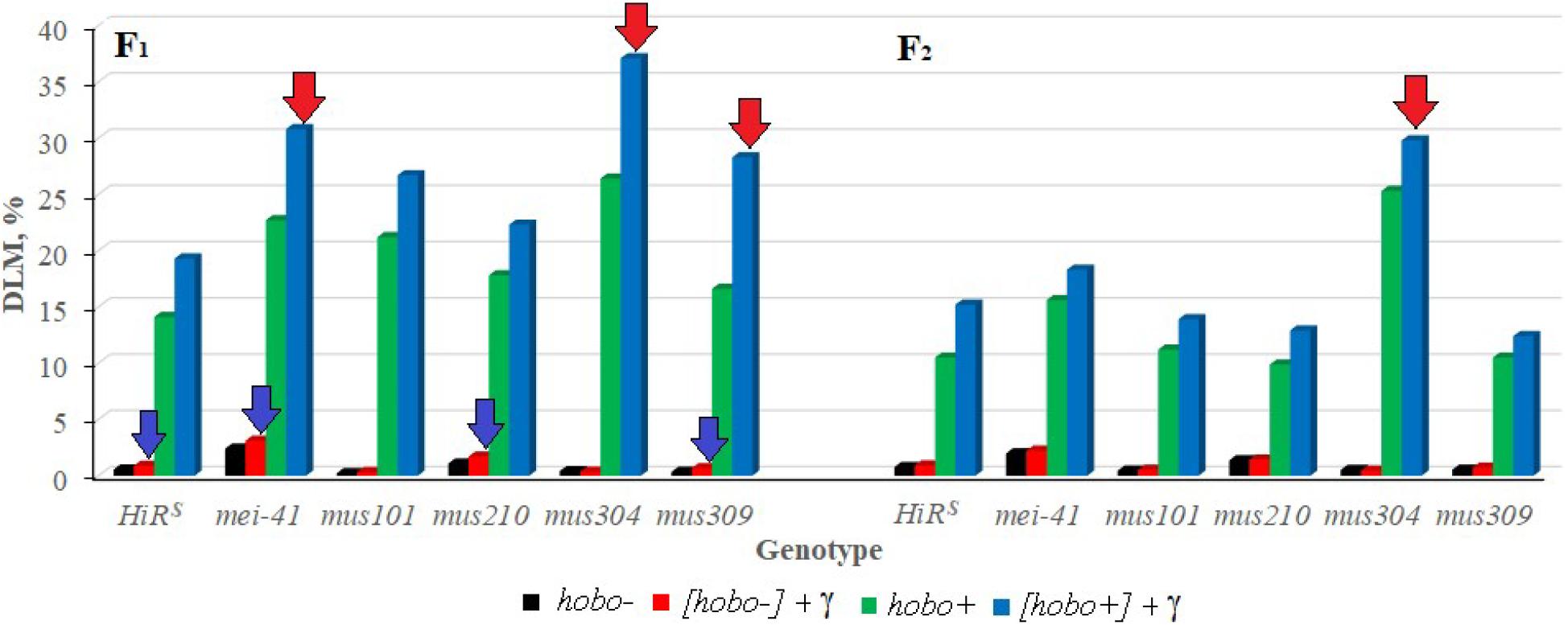
Figure 2. Effect of a chronic irradiation in low doses and induction of the hobo elements on the DLM frequency of D. melanogaster individuals F1/F2 with normal repair genes (HiRS) and dysfunction of the repair genes (mei-41, mus101, mus210, mus304, and mus309). Blue arrow – statistically significant (p < 0.05–0.01) transgenerational effects of irradiation in individuals with dysfunction of the repair genes and without induction of hobo elements; red arrow – statistically significant (p < 0.05–0.01) transgenerational effects of irradiation in individuals with dysfunction of the repair genes in the presence transposition of hobo elements.
Our results indicate a significant increase in the frequency of DSBs in somatic cells of substantially all dysgenic F1 offspring (except for specimens with dysfunction of the mus101 and mus210 genes) (Table 2). Transgenerational transmission of DSBs formed as a result of hobo activity was observed in the F1/F2 offspring of the HiRS, mei-41, and mus304D1 genotypes (p < 0.01). Parental radiation exposure enhances the hobo activity, leading to the formation of DNA damage in the cells of the F1 offspring of the HiRS strain and strains with disfunction of the mei-41 (only in midgut cells), mus304, and mus309 (in all tissues) genes (p < 0.05–0.01). The transgenerational effect of irradiation is retained in all studied somatic cells in the F2 offspring with disfunction of the mus304 (p < 0.01), mei-41 (only in brain cells, p < 0.05) and mus309 (only in midgut cells, p < 0.05) genes. Disgenic offspring with disfunction of the mus304 gene after parental radiation exposure (as well as the positive control – mei-41) had higher OTM values.
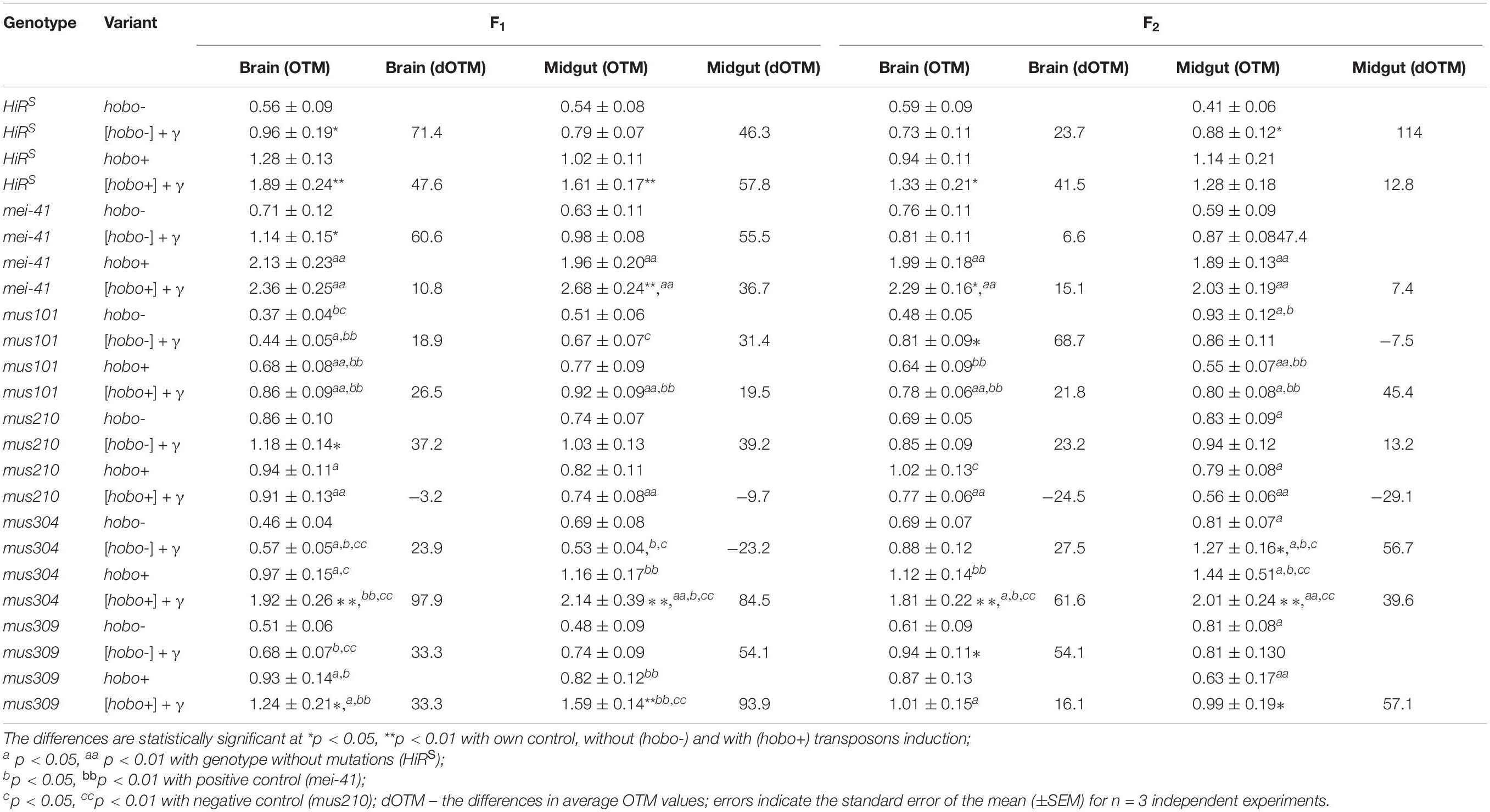
Table 2. Radiation- and hobo-induced DSB DNA (OTM, arb. units) in midgut and brain cells of D. melanogaster larvae.
The results show (Figure 3) that, as well as HiRS, the F1 offspring with disfunction of the mei-41, and mus309 genes and with high hobo activity have low (p < 0.05–0.01) fertility. This tendency is observed in the F2 offspring with disfunction of the mei-41 gene irrespective of parental radiation exposure. Despite the fact that the fertility of the offspring with disfunction of the mus304 gene is higher than the fertility of the above variants, they experience the effect of parental radiation exposure (p < 0.01) which persists in subsequent generations. Along with the negative effects of radiation, the stimulating effect of chronic exposure to low doses of radiation on the reproductive rate was observed. This is typical of the F1 offspring with disfunction of the mei-41 and mus101 genes, the parental exposure of which led to an increase in fertility.
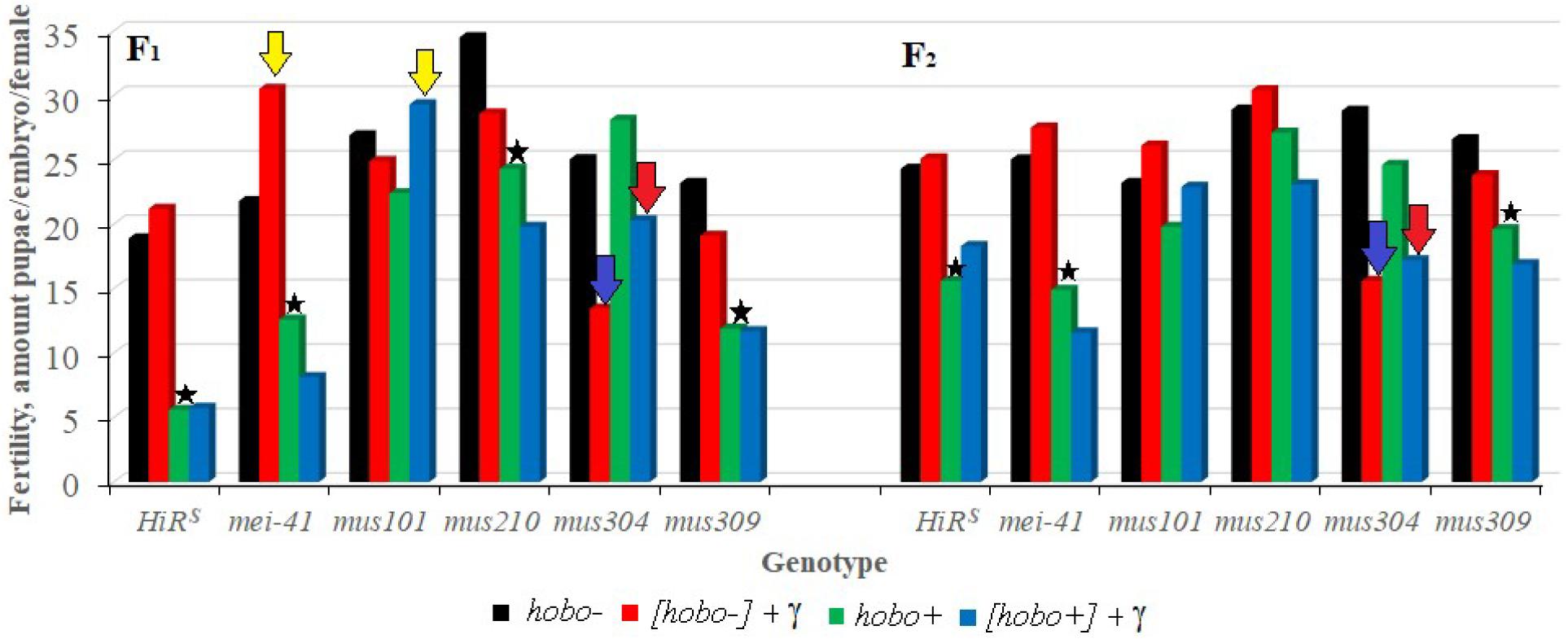
Figure 3. Effect of a chronic irradiation in low doses and induction of the hobo elements on the fertility of D. melanogaster females F1/F2 with normal repair genes (HiRS) and dysfunction of the repair genes (mei-41, mus101, mus210, mus304, and mus309). Blue arrow – statistically significant (p < 0.01) transgenerational effects of irradiation in individuals with dysfunction of the repair genes and without induction of hobo elements; red arrow – statistically significant (p < 0.01) transgenerational effects of irradiation in individuals with dysfunction of the repair genes in the presence transposition of hobo elements; yellow arrow – stimulating effect (p < 0.01) of parental radiation exposure on fertility in females with dysfunction of the repair genes and in the presence of transpositions of hobo elements; asterisk – negative effect (p < 0.01) of induction of hobo elements on fertility of females.
The influence of parental irradiation either did not have a statistically significant effect or led to a decrease in the median survival by 7.7–29.6% (p < 0.01–0.001) in all F1 offspring (except for offspring with disfunction of the mus304 gene) and the F2 offspring of HiRS strain without hobo elements (Table 3 and Figure 4). The maximum lifespan was reduced only in specimens with dysfunction of the mei-41 (in F1/F2 offspring), mus210 (in F1 offspring), mus304 (in F2 offspring), and mus309 (in F1 offspring) genes by 8.8–26.5% (p < 0.05–0.01). However, in the presence of hobo elements in the genomes of D. melanogaster strains, not only the absence of changes in the survival rate and its decrease but also an increase in this parameter were found. Parental radiation exposure had an adverse effect (p < 0.01–0.001) on the median lifespan of F1 specimens with dysfunction of the mei-41, mus101, and mus304 genes. At the same time, a decrease in the maximum lifespan (by 17.6%, p < 0.05) occurred only in offspring with dysfunction of the mus101 gene. We observed an increase in survival rate in the next generation. Thus, in F2 offspring HiRS with normal functioning of the repair genes, the median survival rate increased by 25.6% (p < 0.01). A significant increase in maximum lifespan (by 5%, p < 0.01) was also recorded in F2 offspring with dysfunction of the mei-41 gene.
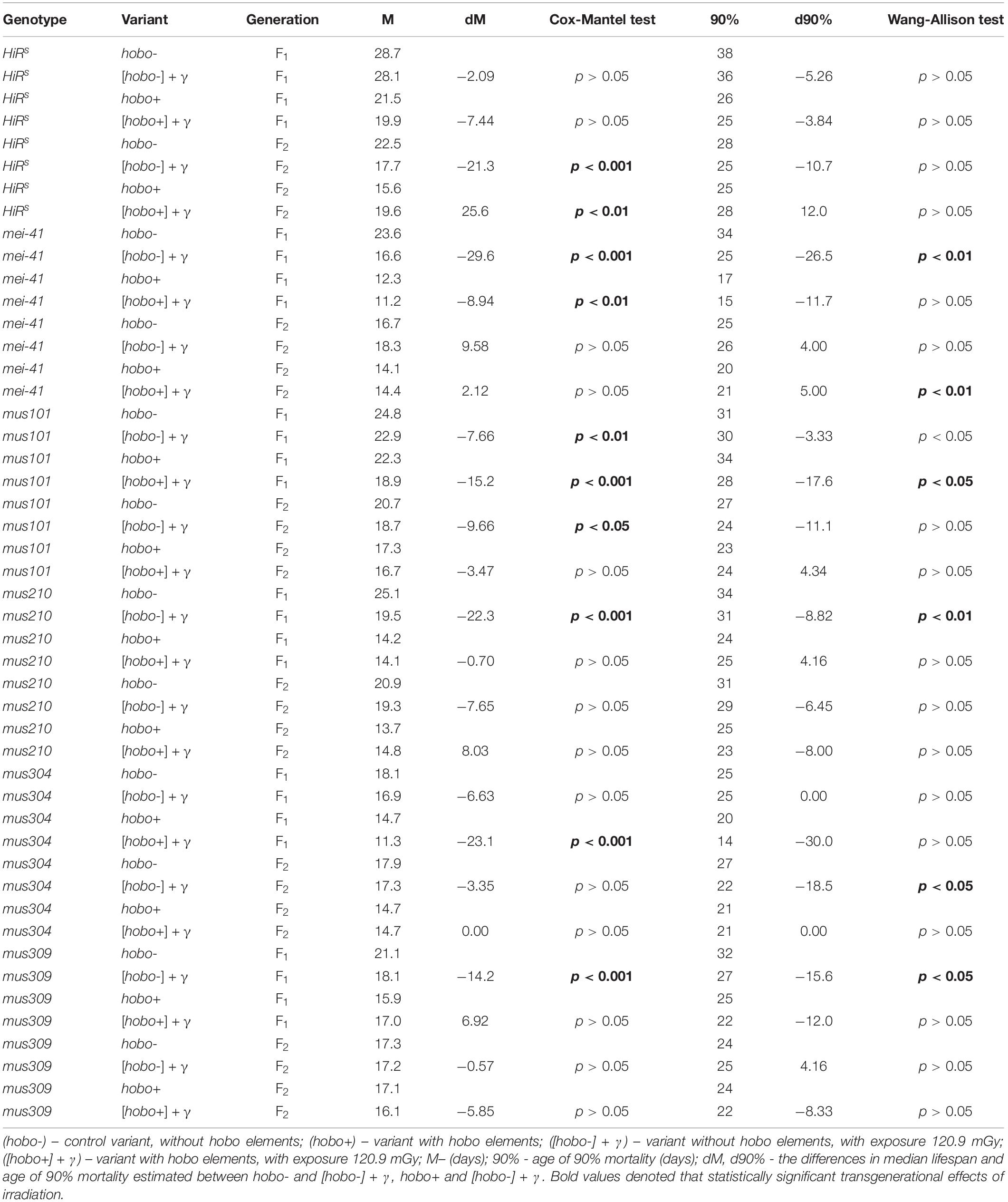
Table 3. Effects of DNA repair genes and system of radiation-induced activation of hobo elements on the lifespan of D. melanogaster male imago.
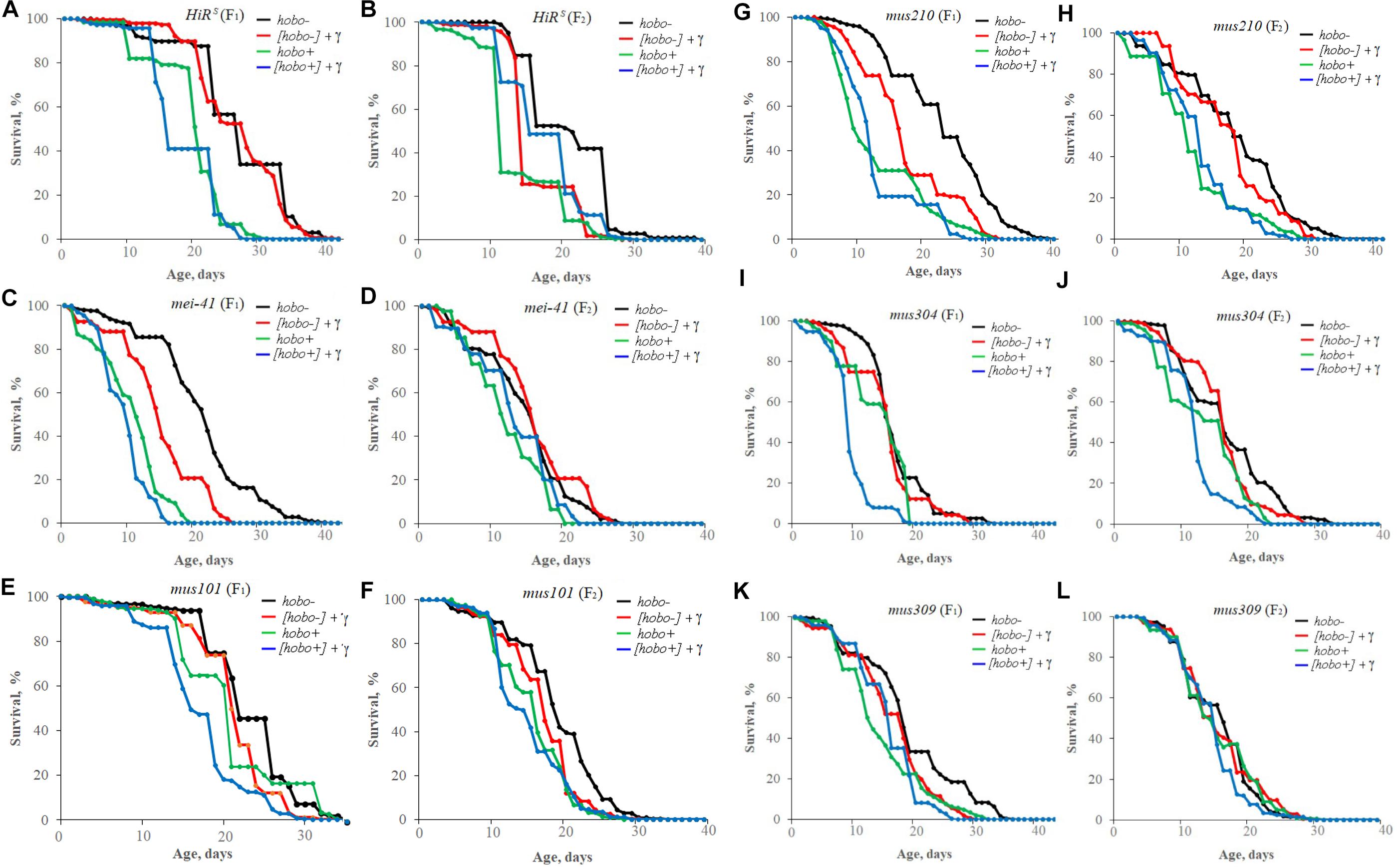
Figure 4. Effect of a chronic irradiation in low doses and induction of the hobo elements on the survival of D. melanogaster male imago F1 (A,C,E,G,I,K)/F2 (B,D,F,H,J,L) with normal repair genes [HiRS (A,B)] and dysfunction of the repair genes: mei-41 (C,D), mus101 (E,F), mus210 (G,H), mus304 (I,J), and mus309 (K,L).
The increased level of transpositional activity of functional transposons in genetic dysgenic systems suggests a high sensitivity of genotypes to the action of DNA-damaging factors and disruptions in the processes of cellular defense. This is confirmed not only by numerous studies of other authors, but by our own experimental facts. Both irradiation and mutations in stress-response genes have been shown to affect the effects of TE transpositions under dysgenic conditions (Yushkova and Zainullin, 2016). This is reflected in an increase of sterility, the frequency of locus-specific mutability, recessive and dominant lethal mutations (Margulies et al., 1986, 1987; Yushkova and Zainullin, 2014). The effects of irradiation and transposon activity are associated with the induction of DSBs (Sobels and Eeken, 1981; Langen et al., 2020). As shown in previous studies, certain stress-response genes are involved in sensing these types of DNA damage. Among them, there are genes that are turned on in response to DNA damage induced by either P-transpositions or irradiation (Yushkova and Zainullin, 2016).
In this work, if we consider these factors separately, we can see that the manifestation of transgenerational instability in D. melanogaster as a result of induction of hobo elements is stronger than as a result of low-dose irradiation. Since in this experiment we used strains of the E-cytotype, the observed effects are mainly due to the influence of dysgenesis. Given this fact, it can be assumed that defects in the DNA repair processes can enhance those dysgenic changes that are accompanied by cell death and dominant lethality (Slatko et al., 1984). The data obtained indicate that defects in excision repair, post-replicative DNA repair and recombination (mus304 and mei-41 genes), as well as DSB repair (mus304 and mus309 genes) affect the frequencies of DNA damage and DLMs during hobo element transpositions. At the same time, against the background of relative sensitivity to hobo factors (in parameters of GD sterility, Mt and DLM), a positive effect of disfunction of the mus101 gene was noted, which was manifested in the form of a decrease in DNA damage and an increase in offspring fertility in response to hobo transposition, both with and without irradiation. The mus101 gene is known to be involved in post-replicative DNA repair and meiosis it is also involved in repair of DSBs induced by low-dose radiation in germline cells (Gatti, 1979; Yushkova, 2020). In mitotically active somatic tissues, the mechanisms associated with the mus101 gene (control of the cell cycle, non-recombination post-replicative DNA repair) are obviously less active than the mechanisms of post-replicative and excision repair, recombination repair (mus304 and mei-41 genes) and DSB repair (mus309 gene).
Note that “DLM” is one of the reliable parameters that has been used in studies of the transgenerational effect irradiation in different animal organisms (from Drosophila to humans) for more than 40 years (Luning et al., 1976; Zainullin et al., 1992; O’Reilly et al., 1994; O’Reilly and Mothersill, 1997; Creane et al., 1999; Mothersill et al., 2000; Shi et al., 2018; Vo et al., 2019). Chromosomal rearrangements that underlie the formation of DLMs in germ cells indicate the manifestation of genome instability (Mazurik and Mikhailov, 2001). Increased genetic instability is a main factor influencing the inheritance of transgenerational effects (Mothersill et al., 2017). In mammals, transgenerational inheritance of an increased level of DLM can be observed oftener in F1/F2 offspring of irradiated parents (Dubrova and Sarapultseva, 2020). In D. melanogaster, the inherited effects observed in the “DLM” parameter can be detected not only in the first generations but also 160 generations after the initial irradiation (E. Yushkova, L. Bashlykova, unpublished data). In our study, using this parameter we confirmed the transmission of DLMs to dysgenic and non-dysgenic F1 offspring of irradiated parents and showed that the formation of transgenerational effects of irradiation depends on the functional level of post-replicative and excision repair and DNA DSB repair. Inheritance of the increased frequency of radiation-induced DLM by subsequent generations is mainly due to the transposition activity of hobo elements and the level of the mus304 gene activity which is responsible for the complex of repair systems. It is known that the increased frequency of hobo transpositions can persist for many tens of generations after inducing crosses (Yurchenko et al., 2011).
In the offspring of unirradiated parents, the influence of the mei-41, mus304, and mus309 (only in F2 offspring) genes on the level of hobo transpositions was shown.
This indicates the existence of interaction between the processes of TE transpositions and the mechanisms of cell defense during transgenerational inheritance. The importance of the existence of various systems of DNA repair in the cell, in particular, post-replicative and excision repair, is confirmed by studies of the viability in the mei- and mus-strains undergoing P-element transpositions. It turned out that specimens with both disfunctions in the DNA repair system and active P-elements in the genome have high sterility, low fertility, and premature aging of male germline cells (Margulies, 1990; Yushkova and Zainullin, 2016). Analyzing our own results and literature data, we can confidently speak about the differences in the responses of mutant genotypes to the action of one or another TE. The exception is specimens with a mutation in the mus304 gene which equally respond to the transposition of the P and hobo elements. Other strains with disfunction of the genes (mus101 and mus309), despite their sensitivity to both transposons, showed conflicting effects on the levels of DNA damage, survival and fertility of the offspring. Only in terms of DLM and sterility parameters, both transposons were equally effective, which confirms the existence of a common mechanism for the formation of P-M and H-E genetic systems in early ontogenesis. In the early stages of development, transposons are highly active and cause serious genetic damage. Such genetic abnormalities lead to the death of germline cells, resulting in sterility of the offspring (Niki and Chigusa, 1986).
Sterility characterizes the changes that take place in the germline cells. In Drosophila females, DNA damage in germline cells causes cell cycle arrest and apoptosis, which explains the absence of developing egg chambers under the mass transpositions (Hassel et al., 2014; Shim et al., 2014). The p53 transcription factor and the Chk2 protein (Checkpoint kinase 2) are of great importance for protecting germline cells from DNA damage. Studies of their role in DNA damage repair after transpositions have shown that mutations in Chk2 suppress transposon-induced ovarian atrophy, while mutations in p53, on the contrary, aggravate female sterility. The authors concluded that the p53 gene is required to make female germline cells tolerant to TE activity (Tasnim and Kelleher, 2018). It is possible that the mei-41 and mus304 genes can also suppress sterility during hobo transpositions. On the other hand, the mus101, mus210, and mus309 genes can aggravate the sterilizing effect in the presence of hobo elements. The role of mei-41 and mus304 genes in maintaining the integrity of germline cells under conditions of intracellular stress is obvious. To test this assumption, further studies of the effect of mus genes on the resistance of germline cells to transcriptionally active TEs are required.
The diverse effects observed in specimens with the induced activity of P and hobo elements are associated with the functional features of each TE, as well as with the specificity of the regulation of their transpositions. For example, the P element is active only in dysgenic conditions; the hobo transposon can be active without dysgenesis (Calvi and Gelbart, 1994; Zakharenko et al., 2006). The P element activity is limited only to germline cells due to regulated mRNA splicing (Laski et al., 1986). The activity of hobo elements is observed both in germline cells and in somatic tissues (Calvi and Gelbart, 1994; Handler and Gomez, 1995). At the same time, the hobo transcriptional activity in the somatic genome is low and is associated with a weak, although statistically significant, activity of the promoter of hobo (Blackman et al., 1987; Palazzo et al., 2019). The promoters of hobo element are more constructed, can contain several core-promoter motifs, and have AT and GC sequences in equal proportions. This structural organization of the promoters of hobo differs from the structure of highly active promoters of Tc1/mariner transposons that are AT-rich in sequences, have divergent or absent core-promoter motifs, and may be unevenly distributed. As a result, the promoters of Tc1/mariner elements are called “blurry” promoters which are capable of inducing gene transcription not only within one genome of one species, but also in distant genomes (Palazzo et al., 2019). We assume that despite a weak hobo promoter activity in the somatic genome, under dysgenic conditions, the hobo promoter activity increases. This leads to a weakening of the transcriptional regulation of transposase expression and a subsequent increase in the transposition activity of hobo elements.
Unlike the P element, the tissue-specific hobo transposition is regulated by the expression of transposase at the transcriptional level (Calvi and Gelbart, 1994). The frequency of transpositions of such TEs in dysgenic (inducing) crosses is regulated by the level of reactivity of females that is inherited mainly through the maternal line (Udomkit et al., 1996). In this case, the level of reactivity is closely related to the DNA repair and recombination processes and is enhanced by the action of DNA damaging factors (Bregliano et al., 1995). Such interaction is believed to be one of the manifestations of the unified inducible repair-recombination system VAMOS (Variability Modulation System) which, like the SOS response system in bacteria, is involved in modifying the level of variability in response to unfavorable environmental conditions (Laurençon et al., 1997). The molecular mechanisms of the formation of this system have not yet been clarified. To date, there have been several studies supporting the existence of this system. These are the works of Bregliano et al. (1995) and Margulies et al. (1986, 1987); Margulies (1990) describing the participation of mei-9 and mei-41 genes both in control of recombination repair processes and in determination of the level of reactivity (Margulies et al., 1986, 1987; Margulies, 1990; Laurençon and Bregliano, 1995). Our research was also aimed at the development of this hypothesis. The mus genes we are studying belong to the mei group, the mutations of which are known to affect meiosis. The use of chronic low-intensity irradiation (which is a factor that organisms in nature constantly encounter) and genetic systems with mutations of the mus genes can significantly clarify the work of molecular mechanisms of adaptation.
The reaction of offspring with disfunction in the mei-41 and mus genes undergoing transposition of hobo elements after chronic radiation exposure depends on the studied parameter. The most pronounced effect of parental radiation exposure was observed in dysgenic offspring with disfunction of the mus304 gene – in all parameters and in two generations (considering the conditionally positive (mei-41) and negative (mus210) controls). Dysgenic F1/F2 offspring of mus309 strain derived from irradiated parents showed visible effects only at the level of GD sterility, DLMs and DSBs. Specimens with disfunction of the mus101 and mus210 genes reacted only at the level of DLMs and survival, and only in the first generation. The transgenerational effects of irradiation were observed also in the offspring of mei-41 strain in presence of hobo elements, but only in the first generation. It follows from this that the studied interactions of TE transpositions and mutations in DNA repair genes have a general long-term effect on survival. Like mei-41, the mus304 gene is a with a conserved function in the evolution gene capable of participating in the DNA damage repair after TE transpositions or irradiation. The effect of the mei-41 mutation on the recombination frequency which increases as a result of transpositions of P elements is well known (Chmuzh et al., 2006). In this case, the recombinations frequency increases upon irradiation (Zakharenko et al., 2006). Since the mus304 gene is responsible for recombination repair, a mutation in this gene is likely to also affect the recombination processes against the background of increased activity of hAT-transposase (an enzyme involved in the hobo transpositions). Transposase is structurally similar to the V(D)J-recombinase (recombination enzyme) which can cause DNA break during V(D)J-recombination (Zhou et al., 2004). This relationship between the systems of V(D)J-recombination and transpositional activity may significantly contribute to the frequency of DSBs, increasing their level in response to irradiation. Moreover, this type of recombination leads to extensive deletions and duplications, the accumulation of which can lead to cell death and, as a result, to the death of the organism (Preston et al., 1996). In mammals, the ATRIP gene (a homolog of the mus304 gene) plays a dominant role in recombination. Like the ATM gene (the homolog of the Drosophila mei-41 gene), it is involved in the DSB repair along the homologous recombination pathway (Koromyslov et al., 2003; Chmuzh et al., 2006). Since both P and hobo transpositions and irradiation cause the formation of DSBs, it is possible that the mus304 gene has an additional function of repairing such types of DNA damage, reducing the level of transgenerational instability.
The offspring with dysfunction in the mus309 gene turned out to be sensitive, although to a lesser extent, to transpositions and parental radiation exposure. The mus309 gene, a human BLM homolog, is involved in DSB repair and in NHEJ (non-homologous end joining)-mediated DSB repair; it also has an ATP-dependent helicase activity, and is involved in NHEJ (non-homologous end joining)-mediated DSB repair (Adams et al., 2003; Portin, 2010). Recently, a new function of this gene has been identified, namely, participation in the control of the cell cycle in the organism under impact (Kondo and Perrimon, 2011). In the cell exposed to DNA damaging agents, miRNAs have been found to interact with their target genes. The level of genotoxicity strongly depends on changes in miRNA expression, in particular, dme-miR-314-3p which affects the DSB repair by targeting the mus309 gene. Thus activated, the mus309 gene triggers the cell cycle arrest, during which the DNA breaks are repaired (Chandraa et al., 2016).
Fertility, as one of the phenotypic traits epigenetically passed on from parents to their offspring, allows to assess the reproduction potential of the organism and reflects the number of the offspring generated over a certain period of time (Dubrova and Sarapultseva, 2020). In this work, when assessing fertility, we showed the adaptive effect of parental radiation exposure on the fertility of the F1 offspring with impaired function of the mei-41 and mus101 genes. Moreover, this property of the genes depends on the dysgenic status of the animals. The transgenerational inheritance of increased fertility by the offspring of mei-41 (in combination with other parameters) obtained from irradiated parents in non-dysgenic conditions (without hobo transpositions) suggests that the mei-41 gene manifests its activity mainly in sensing transposon-induced and non-radiation-induced events, and that the transmission of genome instability occurs solely due to hobo transpositions. On the other hand, disfunction of the mus101 gene does not affect the inheritance by the offspring of genetic damage caused in the parental genome by radiation-induced hobo element transpositions. Our results on the diverse effects of parental radiation exposure and the inheritance of transpositional activity on the fertility of the offspring with disfunction of the mus304 gene suggest that the mechanisms associated with the mus304 gene under such radiation exposure conditions are able to maintain the integrity of germline cells until the formation of ovarioles. Maintaining the ovarian reserve at an optimal level is one of the possible adaptive strategies for dysgenic females developing under stressful environmental conditions (Yushkova, 2017).
In this work, we for the first time assessed the involvement of DNA repair genes and transpositional activity of hobo elements in the inheritance of radiation-induced transgenerational instability. The specificity of the participation of the mei-41 gene in this type of interaction was established; we found that it plays a predominant role in sensing transposon-induced rather than radiation-induced changes. At the same time, in dysgenic conditions the mus304 gene which differs from genes of other DNA repair systems plays a decisive role in the transgenerational transmission of low-dose radiation effects. Taking into account the literature data and our own results regarding the mus304 gene, no fact responsible for “transposon-specificity” was found. The combined action of the mus309 gene and hobo transpositions on the transgenerational transmission of radiation-induced events had a long-term effect on all studied parameters only in the F1 offspring. Genes (mus101 and mus210) that are not involved in the post-replicative recombination repair and DSB repair were found to be not involved in the repair of genetic material in the offspring of irradiated parents undergoing hobo transpositions.
Requests to access data analyzed in this study should be directed to dXNoa292YUBpYi5rb21pc2MucnU=.
The author conceived and designed the experiments, analyzed and interpreted the results, carried out the statistical analysis, prepared figures and tables as well as wrote the final manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work was carried out on the theme of research “Molecular genetic mechanisms of aging, lifespan and resistance to stress in Drosophila melanogaster” (No. AAAA-A18-118011120004-5).
Adams, M. D., McVey, M., and Sekelsky, J. J. (2003). Drosophila BLM in double-strand breakrepair by synthesis-dependent strand annealing. Science 299, 265–267. doi: 10.1126/science.1077198
Ashburner, M. (1989). Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Aslanian, M. M., Kim, A. I., Magomedova, M. A., and Fatkulbaianova, N. L. (1994). Analysis of dominant and recessive sex-linked lethal mutations induced by low radiation doses in genetically different strains of Drosophila melanogaster w and MS. Rus. J. Genet. 30, 1220–1223.
Banga, S. S., Velazquez, A., and Boyd, J. B. (1991). P transposition in Drosophila provides a new tool for analyzing postreplication repair and double-strand break repair. Mutat. Res. 255, 79–88. doi: 10.1016/0921-8777(91)90020-p
Barber, R., Plumb, M. A., Boulton, E., Roux, I., and Dubrova, Y. E. (2002). Elevated mutation rates in the germ line of first-and second-generation offspring of irradiated male mice. Proc. Natl. Acad. Sci. U. S. A. 99, 6877–6882. doi: 10.1073/pnas.102015399
Bazin, C., Denis, B., Capy, P., Bonnivard, E., and Higuet, D. (1999). Characterization of permissivity for hobo-mediated gonadal dysgenesis in Drosophila melanogaster. Mol. Gen. Genet. 261, 480–486. doi: 10.1007/s004380050991
Bazin, C., and Higuet, D. (1996). Lack of correlation between dysgenic traits in the hobo system of hybrid dysgenesis in Drosophila melanogaster. Genet. Res. 67, 219–226. doi: 10.1017/s001667230003370x
Blackman, R. K., Grimaila, R., Macy, M., Koehler, D., and Gelbart, W. M. (1987). Mobilization of hobo elements residing within the Decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell 49, 497–505. doi: 10.1016/0092-8674(87)90452-1
Bregliano, J. C., Laurençon, A., and Degroote, F. (1995). Evidence for an inducible repair-recombination system in the female germ line of Drosophila melanogaster. I. Induction by inhibitors of nucleotide synthesis and by gamma rays. Genetics 141, 571–578.
Breslow, N. A. (1970). Generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 57, 579–594. doi: 10.1093/biomet/57.3.579
Brodsky, M. H., Sekelsky, J. J., Tsang, G., Hawley, R. S., and Rubin, G. M. (2000). Mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14, 666–678. doi: 10.1101/gad.14.6.666
Calvi, B. R., Hong, T. J., Findley, S. D., and Gelbart, W. M. (1991). Evidence for a common evolutionary origin of inverted repeat transposons in Drosophila and plants: hobo, activator, and Tam3. Cell. 66, 465–471. doi: 10.1016/0092-8674(81)90010-6
Calvi, B. R., and Gelbart, W. M. (1994). The basis for germline specificity of the hobo transposable element in Drosophila melanogaster. EMBO J. 13, 1636–1644. doi: 10.1002/j.1460-2075.1994.tb06427.x
Chandraa, S., Khatoona, R., Pandeya, A., Sainia, S., Vimala, D., Singha, P., et al. (2016). Dme-miR-314-3p modulation in Cr(VI) exposed Drosophila affects DNA damage repair by targeting mus309. J. Hazard Mater. 304, 360–369. doi: 10.1016/j.jhazmat.2015.10.075
Chmuzh, E. V., Shestakova, L. A., Volkova, V. S., and Zakharov, I. K. (2006). Diversity of mechanisms and functions of enzyme systems of DNA repair in Drosophila melanogaster. Rus. J. Genet. 42, 363–375. doi: 10.1134/S1022795406040028
Chmuzh, E. V., Shestakova, L. A., Volkova, V. S., and Zakharov, I. K. (2007). Highly sensitive systems for experimental insertional mutagenesis in repair-deficient genetic environment in Drosophila melanogaster: new opportunities for studying postreplication repair of double-stranded DNA breaks and mechanisms of transposable element migration. Rus. J. Genet. 43, 41–47. doi: 10.1134/S1022795407010073
Choi, S. H., Park, J.-H., Nguyen, T. T. N., Shim, H. J., and Song, Y.-H. (2017). Initiation of Drosophila chorion gene amplification requires Claspin and mus101, whereas Claspin, but not mus101, plays a major role during elongation. Dev. Dyn. 246, 466–474. doi: 10.1002/dvdy.24499
Creane, M., Seymour, C. B., and Mothersill, C. (1999). Effect of docetaxel (Taxotere) on expression of radiation-induced lethal mutations in human cell lines. Int. J. Radiat. Biol. 75, 725–730. doi: 10.1080/095530099140069
de Buendßa, P. G., Herrera, O. L., and Arteaga, C. E. (2003). Different contributions of pre- and post-replication DNA repairs for information maintenance. Environ. Mol. Mutagen 41:169. doi: 10.1002/em.10145
Deprá, M., Valente, V. L. S., Margis, R., and Loreto, E. (2009). The hobo transposon and hobo-related elements are expressed as developmental genes in Drosophila. Gene 448, 57–63. doi: 10.1016/j.gene.2009.08.012
Dubrova, Y. E., and Sarapultseva, E. I. (2020). Radiation-induced transgenerational effects in animals. Int. J. Radiat. Biol. 3, 1–7. doi: 10.1080/09553002.2020.1793027
Engels, W. R. (1981). Germline hypermutability in Drosophila and its relation to hybrid dysgenesis and cytotype. Genetics 98, 565–587.
Finnegan, D. J. (1989). Eukaryotic transposable elements and genome evolution. Trends Genet. 5, 103–107. doi: 10.1016/0168-9525(89)90039-5
Fleming, T. R., O’Fallon, J. R., O’Brien, P. C., and Harrington, D. P. (1980). Modified Kolmogorov-Smirnov test procedures with application to arbitrarily right-censored data. Biometrics 36, 607–625. doi: 10.2307/2556114
Fuller, N., Ford, A. T., Lerebours, A., Gudkov, D. I., Nagorskaya, L. L., and Smith, J. T. (2019). Chronic radiation exposure at Chernobyl shows no effect on genetic diversity in the freshwater crustacean, Asellus aquaticus thirty years on. Ecol. Evol. 9, 10135–10144. doi: 10.1002/ece3.5478
Galindo, M. I., Ladevéze, V., Lemeonier, F., Kalmes, R., Periquet, G., and Pascual, L. (1995). Spread of the autonomous transposable element hobo in the genome of Drosophila melanogaster. Mol. Biol. Evol. 5, 723–734. doi: 10.1093/oxfordjournals.molbev.a040251
Gatti, M. (1979). Genetic control of chromosome breakage and rejoining in Drosophila melanogaster: spontaneous chromosome aberrations in X-linked mutants defective in DNA metabolism (recombination-defective meiotic mutants/mutagen-sensitive mutants/chromosome aberrations in neuroblast cells). Genetics 76, 1377–1381. doi: 10.1073/pnas.76.3.1377
Gomes, A. M. G. F., Barber, R. C., and Dubrova, Y. E. (2015). Paternal irradiation perturbs the expression of circadian genes in offspring. Mutat. Res. 775, 33–37. doi: 10.1016/j.mrfmmm.2015.03.007
Han, S. K., Lee, D., Lee, H., Kim, D., Son, H. G., Yang, J.-S., et al. (2016). OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7, 56147–56152. doi: 10.18632/oncotarget.11269
Hancock, S., Vo, N. T. K., Omar-Nazir, L., Batlle, J. V. I., Otaki, J. M., Hiyama, A., et al. (2019). Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 168, 230–240. doi: 10.1016/j.envres.2018.09.039
Handler, A. M., and Gomez, S. P. (1995). The hobo transposable element has transposase-dependent and -independent excision activity in drosophilid species. Mol. Gen. Genet. 247, 399–408. doi: 10.1007/BF00293140
Hari, K. L., Santerre, A., Sekelsky, J. J., McKim, K. S., Boyd, J. B., and Hawley, R. S. (1995). The mei-41 gene of Drosophila melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell 82, 815–821. doi: 10.1016/0092-8674(95)90478-6
Harris, P. V., and Boyd, J. B. (1993). Re-evaluation of excision repair in the mus304, mus306 and mus308 mutants of Drosophila. Mutat. Res. 301, 51–55. doi: 10.1016/0165-7992(93)90056-2
Hassel, C., Zhang, B., Dixon, M., and Calvi, B. R. (2014). Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development 141, 112–123. doi: 10.1242/dev.098871
Ivashenko, N. I., and Grishaeva, T. M. (2002). The features of induced mutagenesis in systems of hybrid dysgenesis in Drosophila melanogaster. Rus. J. Genet. 38, 1351–1356. doi: 10.1023/A:1020696603337
Jurka, J., Kapitonov, V. V., Kohany, O., and Jurka, M. V. (2007). Repetitive sequences in complex genomes: structure and evolution. Annu. Rev. Genomics Hum. Genet. 8, 241–259. doi: 10.1146/annurev.genom.8.080706.092416
Kaufman, P. and Rio, D. (1992). P element-mediated transformation of D. melanogaster using purified P element transposase. Drosophila Inform. Serv. 71, 146.
Kidwell, M. G., Kidwell, J. P., and Sved, J. A. (1977). Hybrid dysgenesis in Drosophila melanogaster. A syndrome of aberrant traits including mutation, sterility and male recombination. Genetics 36, 813–833.
Kondo, S., and Perrimon, N. (2011). A genome—wide RNAi screen identifies core components of the G(2)-M DNA damage checkpoint. Sci. Signal. 4:rs1. doi: 10.1126/scisignal.2001350
Koromyslov, Y. A., Chmuzh, E. V., Shestakova, L. A., Tcheressiz, S. V., Yurchenko, N. N., and Zakharov, I. K. (2003). Mutability of unstable sex-linked alleles of Drosophila melanogaster and their interaction with mutations of the genes of the repair system. Drosophila Inform. Serv. 86, 35–38.
Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin, J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. doi: 10.1038/35057062
Langen, B., Helou, K., and Forssell-Aronsson, E. (2020). The IRI-DICE hypothesis: ionizing radiation-induced DSBs may have a functional role for non-deterministic responses at low doses. Radiat. Environ. Bioph. 59, 349–355. doi: 10.1007/s00411-020-00854-x
Laski, F. A., Rio, D. C., and Rubin, G. V. (1986). Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell 44, 7–19. doi: 10.1016/0092-8674(86)90480-0
Laurençon, A., and Bregliano, J. C. (1995). Evidence for an inducible repair-recombination system in the female germ line of Drosophila melanogaster. II. Differential sensitivity to gamma rays. Genetics 141, 579–585.
Laurençon, A., Gay, F., Ducau, J., and Bregliano, J. C. (1997). Evidence for an inducible repair-recombination system in the female germ line of Drosophila melanogaster. III. Correlation between reactivity levels, crossover frequency and repair efficiency. Genetics 146, 1333–1344.
Laurencon, A., Purdy, A., Sekelsky, J., Hawley, R. S., and Su, T. T. (2003). Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164, 589–601.
Lecomte-Pradines, C., Hertel-Aas, T., Coutris, C., Gilbin, R., Oughton, D., and Alonzo, F. (2017). A dynamic energy-based model to analyze sublethal effects of chronic gamma irradiation in the nematode Caenorhabditis elegans. J. Toxicol. Environ. Health A. 80, 830–844. doi: 10.1080/15287394.2017.1352194
Loreto, E. L. S., Deprá, M., Diesel, J. F., Panzera, Y., Lucia, V., and Valente-Gaiesky, S. (2018). Drosophila relics hobo and hobo-MITEs transposons as raw material for new regulatory networks. Genet. Mol. Biol. 41, 198–205. doi: 10.1590/1678-4685-GMB-2017-0068
Luning, K. G., Frolen, H., and Nilsson, A. (1976). Genetic effects of 239Pu salt injections in male mice. Mutat. Res. 34, 539–542. doi: 10.1016/0027-5107(76)90229-3
Majumdar, S., and Rio, D. C. (2015). P transposable elements in Drosophila and other eukaryotic organisms. Microbiol. Spectr. 3, MDNA3–MDNA0004. doi: 10.1128/microbiolspec.MDNA3-0004-2014
Majumdar, S., Singh, A., and Rio, D. C. (2013). The human THAP9 gene encodes an active P-element DNA transposase. Science 339, 446–448. doi: 10.1126/science.1231789
Mantel, N. (1966). Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 50, 163–170.
Margulies, L., Briscoe, D. J., and Wallace, S. S. (1986). The relationship between radiation-induced and transposon-induced genetic damage during Drosophila oogenesis. Mutat. Res. 162, 55–68. doi: 10.1016/0027-5107(86)90071-0
Margulies, L., Briscoe, D. J., and Wallace, S. S. (1987). The relationship between radiation-induced and transposon-induced genetic damage during Drosophila spermatogenesis. Mutat. Res. 179, 183–195. doi: 10.1016/0027-5107(87)90309-5
Margulies, L. (1990). A high level of hybrid dysgenesis in Drosophila: high thermosensitivity, dependence on DNA repair, and incomplete cytotype regulation. Mol. Gen. Genet. 220, 448–455. doi: 10.1007/BF00391752
Marin, L., Lehmann, M., Nouaud, D., Izaabel, H., Anxolabehere, D., and Ronsseray, S. (2000). P-element repression in Drosophila melanogaster by a naturally occurring defective telomeric P copy. Genetics 155, 1841–1854.
Mazurik, V. K., and Mikhailov, V. F. (2001). Radiation-induced genome instability: the phenomenon, molecular mechanisms, pathogenetical significance. Radiat. Biol. Radioecol. 41, 272–289.
McCullers, T. J., and Steiniger, M. (2017). Transposable elements in Drosophila. Mob. Genet. Elements 7, 1–18. doi: 10.1080/2159256X.2017.1318201
Menzel, G., Heitkam, T., Seibt, K. M., Nouroz, F., Müller-Stoermer, M., Heslop-Harrison, J. S., et al. (2014). The diversification and activity of hAT transposons in Musa genomes. Chromosome Res. 22, 559–571. doi: 10.1007/s10577-014-9445-5
Mothersill, C., Kadhim, M. A., O’Reilly, S., Papworth, D., Marsden, S. J., Seymour, C. B., et al. (2000). Dose- and time-response relationships for lethal mutations and chromosomal instability induced by ionizing radiation in an immortalized human keratinocyte cell line. Int. J. Radiat. Biol. 76, 799–806. doi: 10.1080/09553000050028959
Mothersill, C., Rusin, A., and Seymour, C. (2017). Low doses and non-targeted effects in environmental radiation protection; where are we now and where should we go? Environ. Res. 159, 484–490. doi: 10.1016/j.envres.2017.08.029
Niki, Y., and Chigusa, S. I. (1986). Developmental analysis of the gonadal sterility of P-M hybrid dysgenesis in Drosophila melanogaster. Jpn. J. Genet. 61, 147–156. doi: 10.1266/jjg.61.147
Olive, P. L. (1999). DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int. J. Radiat. Biol. 75, 395–405. doi: 10.1080/095530099140311
Olive, P. L., and Banath, J. P. (2006). The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 1, 23–29. doi: 10.1038/nprot.2006.5
O’Reilly, J. P., and Mothersill, C. (1997). Comparative effects of UV A and UV B on clonogenic survival and delayed cell death in skin cell lines from humans and fish. Int. J. Radiat. Biol 72, 111–119. doi: 10.1080/095530097143590
O’Reilly, S., Mothersill, C., and Seymour, C. B. (1994). Postirradiation expression of lethal mutations in an immortalized human keratinocyte cell line. Int. J. Radiat. Biol. 66, 77–83. doi: 10.1080/09553009414550961
Ortiz, M. F., and Loreto, E. L. S. (2008). The hobo-related elements in the melanogaster species group. Genet. Res. 90, 243–252.
O’Hare, K., Tam, J. L., Lim, J. K., Yurchenko, N. N., and Zakharov, I. K. (1998). Rearrangements at a hobo element inserted into the first intron of the singed gene in the unstable sn49 system of Drosophila melanogaster. Mol. Gen. Genet. 257, 452–460. doi: 10.1007/s004380050669
Palazzo, A., Lorusso, P., Miskey, C., Walisko, O., Gerbino, A., Marobbio, C. M. T., et al. (2019). Transcriptionally promiscuous “blurry” promoters in Tc1/mariner transposons allow transcription in distantly related genomes. Mobile DNA 10, 1–13. doi: 10.1186/s13100-019-0155-6
Portin, P. (2010). Evidence based on studies of the mus309 mutant, deficient in DNA double-strand break repair, that meiotic crossing over in Drosophila melanogaster is a two-phase process. Genetica 138, 1033–1045. doi: 10.1007/s10709-010-9489-1
Preston, C. R., Sved, J. A., and Engels, W. R. (1996). Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 44, 1623–1638.
Richardson, R. H., and Kojima, K. (1965). The kinds of genetic variability in relation to selection responses in Drosophila fecundity. Genetics 52, 583–598.
Sarapultseva, E., and Gorski, A. (2013). Low-dose γ-irradiation affects the survival of exposed Daphnia and their offspring. Dose Response 11, 460–468. doi: 10.2203/dose-response.12-033.Sarapultseva
Sarapultseva, E. I., and Bychkovskaya, I. B. (2010). Peculiar low radiation effects as a risk factor. Assessment of organism viability in the model experiments with Daphnia magna. Int. J. Low Radiation 7, 1–9. doi: 10.1504/IJLR.2010.032766
Sarapultseva, E. I., and Dubrova, Y. E. (2016). The long-term effects of acute exposure to ionising radiation on survival and fertility in Daphnia magna. Environ. Res. 150, 138–143. doi: 10.1016/j.envres.2016.05.046
Sarapultseva, E. I., and Malina, I. (2009). The change of Daphnia magna viability after low doses of gamma-irradiation. Radiat. Biol. Radioecol. 49, 82–84.
Sekelsky, J. J., Burtis, K. C., and Hawley, R. S. (1998). Damage control: the pleiotropy of DNA repair genes in Drosophila melanogaster. Genetics 148, 1587–1598.
Shi, X., Seymour, C., and Mothersill, C. (2018). Change of cell growth and mitochondrial membrane polarization in the progeny of cells surviving low-dose high-LET irradiation from Ra-226. Environ. Res. 167, 51–65. doi: 10.1016/j.envres.2018.07.002
Shim, H. J., Lee, E.-M., Nguyen, L. D., Shim, J., and Song, Y.-H. (2014). High-dose irradiation induces cell cycle arrest, apoptosis, and developmental defects during Drosophila oogenesis. PLoS One 9:e89009. doi: 10.1371/journal.pone.0089009
Slatko, B. E., Mason, J. M., and Woodruff, R. C. (1984). The DNA transposition system of hybrid dysgenesis in Drosophila melanogaster can function despite defects in host DNA repair. Genet. Res. Camb. 43, 159–171. doi: 10.1017/S0016672300025878
Sobels, F. H., and Eeken, J. C. J. (1981). Influence of the MR (mutator) factor on X-ray-induced genetic damage. Mutat. Res. 83, 201–206. doi: 10.1016/0027-5107(81)90005-1
Tasnim, S., and Kelleher, E. S. (2018). p53 is required for female germline stem cell maintenance in P-element hybrid dysgenesis. Dev. Biol. 434, 215–220. doi: 10.1016/j.ydbio.2017.12.021
Torres, F. P., Fonte, L. F. M., Valente, V. L. S., and Loreto, E. (2006). Mobilization of a hobo-related sequence in the genome of Drosophila simulans. Genetica 126, 101–110. doi: 10.1007/s10709-005-1436-1
Udomkit, A., Forbes, S., McLean, C., Arkhipova, I., and Finnegan, D. J. (1996). Control of expression of the I factor, a LINE-like transposable element in Drosophila melanogaster. EMBO J. 15, 3174–3181. doi: 10.1002/j.1460-2075.1996.tb00680.x
Vo, N. T. K., Seymour, C. B., and Mothersill, C. E. (2019). Radiobiological characteristics of descendant progeny of fish and amphibian cells that survive the initial ionizing radiation dose. Environ. Res. 169, 494–500. doi: 10.1016/j.envres.2018.11.047
Watti, K. V., and Tikhomirova, M. M. (1976). Spontaneous and radiation induced dominant lethal mutations in male and female Drosophila. Res. Genet. 6, 32–43.
Wiley, L. M., Baulch, J. E., Raabe, O. G., and Straume, T. (1997). Impaired cell proliferation in mice that persists across at least two generations after paternal irradiation. Radiat. Res. 148, 145–151.
Yurchenko, N. N., Kovalenko, L. V., and Zakharov, I. K. (2011). Transposable elements: instability of genes and genomes. Vavilov J. Genet. Breeding 15, 263–270.
Yushkova, E. (2019). Effects of ionizing radiation at Drosophila melanogaster with differently active hobo transposons. Int. J. Radiat. Biol. 95, 1564–1572. doi: 10.1080/09553002.2019.1642534
Yushkova, E. A. (2017). Effects of chronic irradiation at low doses on morphological indicators of reproductive system of dysgenic female Drosophila melanogaster. Radiat. Biol. Radioecol. 57, 60–65. doi: 10.7868/S0869803117010167
Yushkova, E. A. (2020). Genetic mechanisms of formation of radiation-induced instability of the genome and its transgenerational effects in the descendants of chronically irradiated individuals of Drosophila melanogaster. Radiat. Environ. Bioph. 59, 221–236. doi: 10.1007/s00411-020-00833-2
Yushkova, E. A., and Zainullin, V. G. (2014). Induction of transpositions of hobo-elements in chronically irradiated cells of dysgenetic and non-dysgenetic individuals of Drosophila melanogaster. Rus. J. Genet. 50, 447–452. doi: 10.1134/S1022795414050123
Yushkova, E. A., and Zainullin, V. G. (2016). Interaction between gene repair and mobile elements induced activity systems after low-dose irradiation. Int. J. Radiat. Biol. 92, 485–492. doi: 10.1080/09553002.2016.1206221
Zainullin, V. G., Shevchenko, V. A., Mjasnjankina, E. N., Generalova, M. V., and Rakin, A. O. (1992). The mutation frequency of Drosophila melanogaster populations living under conditions of increased background radiation due to the Chernobyl accident. Sci. Total. Environ. 112, 37–44. doi: 10.1016/0048-9697(92)90236-L
Zakharenko, L. P., Kovalenko, L. V., Perepelkina, M. P., and Zakharov, I. K. (2006). The effect of γ-radiation on induction of the hobo element transposition in Drosophila melanogaster. Rus. J. Genet. 42, 763–767. doi: 10.1134/S1022795406060056
Keywords: γ-rays, DNA damage, transposons, transgenerational effects, DNA repair genes
Citation: Yushkova E (2020) Involvement of DNA Repair Genes and System of Radiation-Induced Activation of Transposons in Formation of Transgenerational Effects. Front. Genet. 11:596947. doi: 10.3389/fgene.2020.596947
Received: 20 August 2020; Accepted: 04 November 2020;
Published: 27 November 2020.
Edited by:
Ki Moon Seong, Korea Institute of Radiological and Medical Sciences, South KoreaReviewed by:
René Massimiliano Marsano, University of Bari Aldo Moro, ItalyCopyright © 2020 Yushkova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Yushkova, dXNoa292YUBpYi5rb21pc2MucnU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.