
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 10 December 2020
Sec. RNA
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.579215
This article is part of the Research TopicRNA Biology in Cardiovascular DiseaseView all 11 articles
Approximately 13,000 people die of an abdominal aortic aneurysm (AAA) every year. This study aimed to identify the immune response-related genes that play important roles in AAA using bioinformatics approaches. We downloaded the GSE57691 and GSE98278 datasets related to AAA from the Gene Expression Omnibus database, which included 80 AAA and 10 normal vascular samples. CIBERSORT was used to analyze the samples and detect the infiltration of 22 types of immune cells and their differences and correlations. The principal component analysis showed significant differences in the infiltration of immune cells between normal vascular and AAA samples. High proportions of CD4+ T cells, activated mast cells, resting natural killer cells, and 12 other types of immune cells were found in normal vascular tissues, whereas high proportions of macrophages, CD8+ T cells, resting mast cells, and six other types of immune cells were found in AAA tissues. In the selected samples, we identified 39 upregulated (involved in growth factor activity, hormone receptor binding, and cytokine receptor activity) and 133 downregulated genes (involved in T cell activation, cell chemotaxis, and regulation of immune response mediators). The key differentially expressed immune response-related genes were screened using the STRING database and Cytoscape software. Two downregulated genes, PI3 and MAP2K1, and three upregulated genes, SSTR1, GPER1, and CCR10, were identified by constructing a protein–protein interaction network. Functional enrichment of the differentially expressed genes was analyzed, and the expression of the five key genes in AAA samples was verified using quantitative polymerase chain reaction, which revealed that MAP2K1 was downregulated in AAA, whereas SSTR1, GEPR1, and CCR10 were upregulated; there was no significant difference in PI3 expression. Our study shows that normal vascular and AAA samples can be distinguished via the infiltration of immune cells. Five genes, PI3, MAP2K1, SSTR1, GPER1, and CCR10, may play important roles in the development, diagnosis, and treatment of AAA.
With changes in lifestyle, the incidence rates of cardiovascular and cerebrovascular diseases have increased every year, making them a serious public health problem. According to World Health Organization statistics, cardiovascular disease is the leading cause of death worldwide. In 2011, coronary heart disease and cerebrovascular disease caused 13.2 million deaths, accounting for 24% of the total global deaths. It is estimated that by 2030, the number of deaths due to cardiovascular diseases will increase to 23.3 million, and cardiovascular diseases will continue to be the leading cause of death (World Health Organization, 2013). Reportedly, approximately 13,000 people die of abdominal aortic aneurysm (AAA) every year (Rooke et al., 2012).
Current studies suggest that age (more than 65 years), family history, sex (male), and smoking are important risk factors for AAA (Sakalihasan et al., 2018). AAA has become an important cause of death in the elderly who are more than 65 years old and is a serious aortic disease involving irreversible radial dilatation of the abdominal aorta more than 3 cm or 1.5 times the normal diameter due to various reasons (Umebayashi et al., 2018). Most AAA patients are asymptomatic and cannot be treated before the tumor ruptures, or the patient dies (Golledge and Norman, 2011). Aneurysm rupture is an important cause of mortality in patients with AAA (Golledge et al., 2006), which is reportedly as high as 80%. Open surgery and interventional surgery are the main methods to treat AAA. However, they have limited use because the prevalence of AAA is positively correlated with age, and patients with AAA often suffer from other cardio-cerebrovascular diseases, such as heart failure, atherosclerosis, and ischemic cardiomyopathy (Arya et al., 2015).
Studies have shown that if the increase in the aortic diameter can be slowed down by 50%, the annual rates of aortic reconstruction surgery for AAA can be halved. In clinical practice, only patients whose AAAs are greater than 5.5 cm and who are at risk of rupture are treated with open surgery (Golledge et al., 2006), and there is no drug to slow down the development of AAA. The occurrence and development of AAA is a complex process involving multiple factors. It is generally believed that AAA is directly related to atherosclerosis, hypertension, chronic obstructive pulmonary disease, and a variety of proteases, but there is no clear evidence to explain the roles of these factors in the pathogenesis of AAA. The pathophysiological processes of AAA include infiltration of inflammatory cells (Moxon et al., 2010; Gordon and Toursarkissian, 2014), degradation of elastic and collagen fibers, death of smooth muscle cells, defects of the arterial wall, and increased oxidative stress (Newman et al., 1994).
Vascular inflammation is the first event in the development of AAA. In the early stages of the disease, immune cells such as lymphocytes, macrophages, mast cells, neutrophils, and natural killer (NK) cells infiltrate and accumulate in the blood vessels and surrounding tissues, causing a series of inflammatory reactions in the vascular wall (Eliason et al., 2005; Forester et al., 2006; Rateri et al., 2011; Wang et al., 2014a; Yan et al., 2016). The invasion of inflammatory cells often stimulates smooth muscle cells to secrete matrix metalloproteinases, which degrade elastin and collagen, thus reducing the stability of the arterial wall and inducing the apoptosis of vascular smooth muscle cells (Rizzo et al., 1989; Sakalihasan et al., 1996; Culav et al., 1999; Longo et al., 2002, 2005; Keeling et al., 2005). Inflammatory response plays an important role in the immune system and is involved in the occurrence and development of AAA (Yamaguchi et al., 2000; Liu et al., 2015). Indeed, lowering interleukin (IL)-17 levels in animal models slows down the increase in aortic diameter in aneurysms (Chang et al., 2015). Therefore, this study aimed to analyze the infiltration of immune cells in AAA and identify genes related to the immune response that play a role in the development of AAA.
We searched the Gene Expression Omnibus database1 and obtained two gene expression datasets, GSE57691 (including 49 AAA samples and 10 normal aortic vessels samples) (Biros et al., 2015) and GSE98278 (Gäbel et al., 2017) (including 31 AAA samples), which included 80 samples from AAAs and 10 samples from normal aortic vessels. We used Limma (Ritchie et al., 2015) and SVA (Leek et al., 2019) packages in R (3.61) to correct the sample data.
To investigate the infiltration of immune cells in AAAs and normal aortic vessels and evaluate and predict the enrichment of immune cells in the samples, we used CIBERSORT (Newman et al., 2015). CIBERSORT is a tool used to deconvolute the expression matrix of immune cell subtypes based on the principle of linear support vector regression. RNA-Seq data were used to estimate the infiltration of immune cells. CIBERSORT analyzed the relative abundance of 22 types of immune infiltrating cells in each sample, including NK cells, T cells, B cells, and macrophages. The 69 samples were screened according to the P-value predicted by CIBERSORT (P < 0.05).
We reduced the dimensions of the samples and performed principal component analysis (PCA) of the infiltration of 22 types of immune cells in the samples.
From ImmPort2, we downloaded 2,498 immune response-related genes, including those related to antigen-presenting cells, chemokines and their receptors, cytokines and their receptors, interferons, and ILs. We used the Limma package (Ritchie et al., 2015) in R (3.61) to compare gene expression data of immune response-related genes in AAA and normal aortic blood samples downloaded from the Gene Expression Omnibus database and extracted information on the expression levels of immune response-related genes in the samples.
After the data were standardized using the Limma software package (Ritchie et al., 2015) in R, we identified immune response-related, differentially expressed genes by comparing normal aortic blood vessel samples with AAA samples. If the change factor was greater than onefold (|fold change| ≥ 1) and the corrected P-value (false discovery rate) ≤ 0.05, the gene was considered to be differentially expressed.
We used the STRING3 database to construct a protein–protein interaction (PPI) network of the differentially expressed gene products. The PPI file was imported to Cytoscape 3.6.04, and the MCODE plug-in was used to map the PPIs. The degree, closeness, intermediate degree of each node in the network, and the average value of each protein’s nodal degree were defined as the threshold of the PPI network nodes, and the proteins whose degrees were greater than the threshold value were selected. The key nodes of the PPI network were identified, and the correlation scores of the nodes and their interacting proteins were calculated.
Gene ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses were performed using clusterProfiler (Yu et al., 2012) package in R (3.6.1). The hypergeometric distribution was used to analyze and calculate the significance levels of these differentially expressed genes in each signaling pathway to identify the signaling pathways that were significantly affected (P < 0.05).
From June 2019 to February 2020, we recruited eight patients with AAA who underwent surgical resection in the Second Affiliated Hospital of Nanchang University and Sun Yat-sen University. All tissue samples were frozen in liquid nitrogen during the surgery. Two experienced pathologists confirmed AAA. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University and Sun Yat-sen University. Written informed consent was obtained from each patient before surgery. The samples were pretreated, and RNA was extracted with TRIzol reagent (German DBI) for real-time quantitative polymerase chain reaction (qPCR). Reverse transcription of RNA into complementary DNA was performed using the Bestar qPCR RT Kit (DBI, Germany) following the manufacturer’s instructions. qPCR was performed to determine the expression levels of PI3, MAP2K1, SSTR1, GPER1, CCR10, IL-6, IL-17, tumor necrosis factor (TNF)-α, and ACTB (internal reference gene) in the samples. Primer sequences were obtained from PrimerBank5 (Table 1).
Data were analyzed using GraphPad Prism 7 (GraphPad Software Inc.) and IBM SPSS 17.00 (IBM Analytics, United States). Chi-square and Fisher’s exact tests were used for the qualitative analysis of the data. Data were expressed as the mean ± standard deviation (x ± s) and compared using Student’s t-test. PCR data were analyzed using GraphPad Prism 8. P < 0.05 was considered as statistically significant and P < 0.01 as very significant.
We used CIBERSORT to analyze immune cell infiltration in the samples and selected 69 AAA samples and 10 normal aortic samples that met the standard according to the analysis (P < 0.05). We found a significant difference in the abundance of 22 types of immune cells in the 10 normal aortic samples and 69 AAA samples (Figure 1A). There were also significant differences in the infiltration ratio of plasma cells, T cells, naïve CD4+ T cells, resting memory CD4+ T cells, and macrophages M0 in normal aortic and AAA samples (Figure 1B). Correlation analysis of the 22 types of immune cells revealed that the negative correlation between resting mast cells and activated mast cells was the strongest and that between macrophages M0 and plasma cells, macrophages M0 and resting memory CD4+ T cells, and monocytes and plasma cells was strong. A negative correlation was observed between resting CD4+ memory T cells and naïve CD4+ T cells and between plasma cells and CD4+ T cells; a strong positive correlation was observed between naïve CD4+ T cells (Figure 2A). Figure 2B clearly shows that there are 18 types of immune cells with different proportions in normal and AAA samples, such as resting dendritic cells (Figure 3A), eosinophils (Figure 3B), macrophages M0 (Figure 3C), macrophages M1 (Figure 3D), macrophages M2 (Figure 3E), resting mast cells (Figure 3F), monocytes (Figure 3G), activated NK cells (Figure 3H), activated CD4+ memory T cells (Figure 3I), CD8+ T cells (Figure 3J), and volatile T helper cells (Figure 3K). The proportion of regulatory T cells (Figure 3L) was increased in AAA samples, whereas the proportions of activated mast cells (Figure 4A), neutrophils (Figure 4B), resting NK cells (Figure 4C), plasma cells (Figure 4D), resting memory CD4+ T cells (Figure 4E), and naïve CD4+ T cells (Figure 4F) were increased in normal aortic samples.
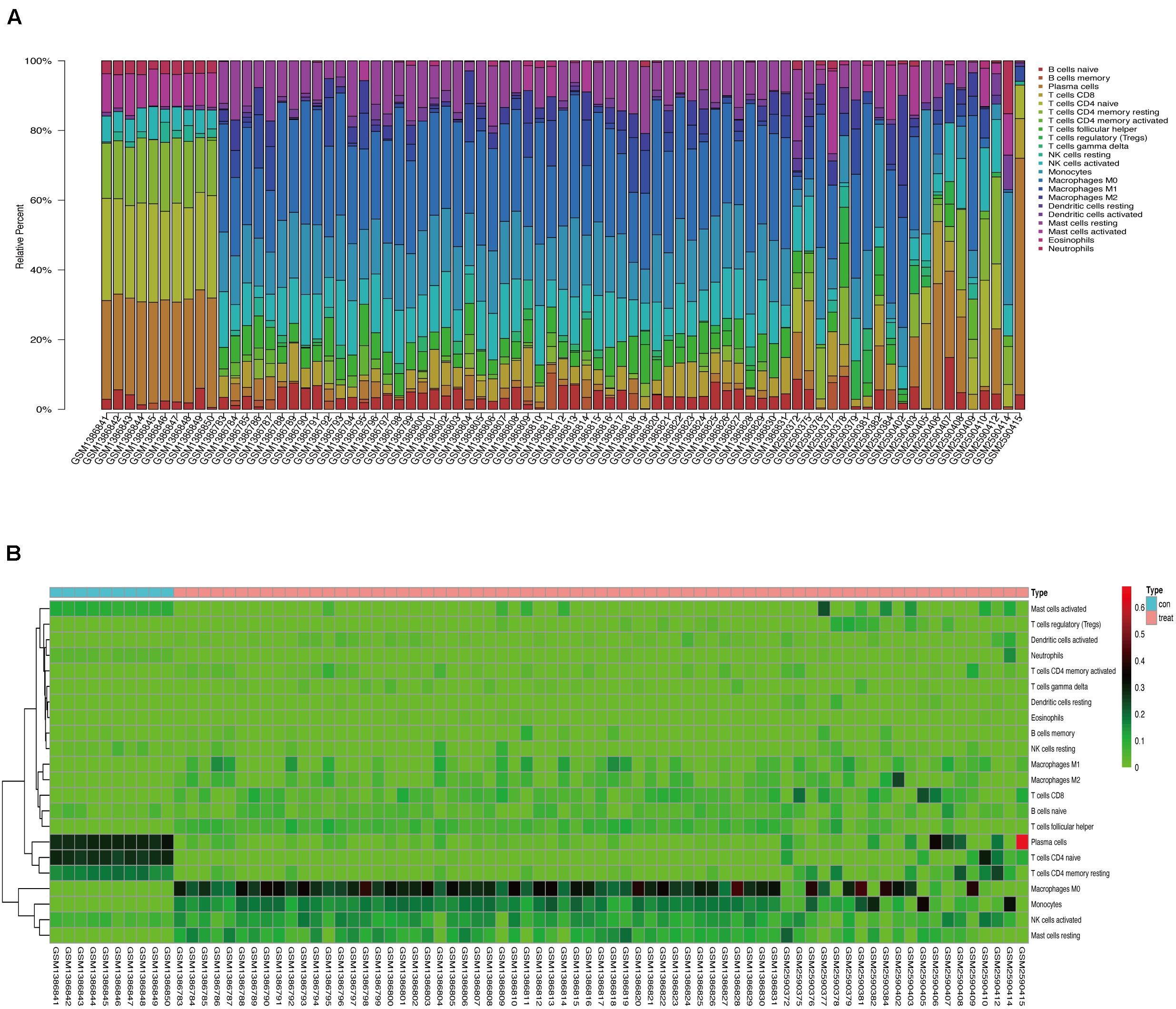
Figure 1. (A) Proportion of 22 kinds of immune cells in the chip. (B) Expression heat map of differential immune cells in the sample.
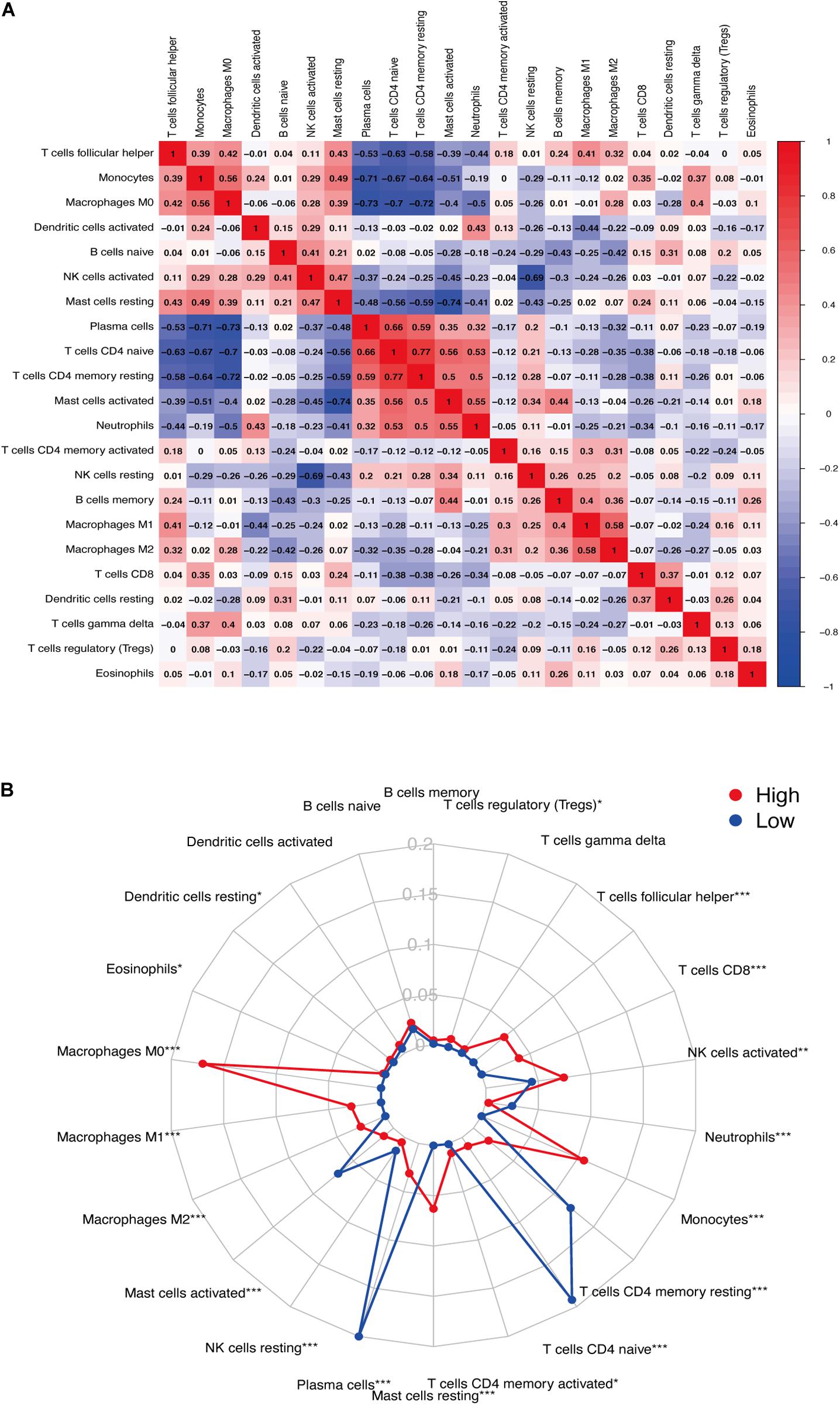
Figure 2. (A) Correlation between immune cells. (B) Infiltration of immune cells (*P < 0.05; **P < 0.01; ***P < 0.001).
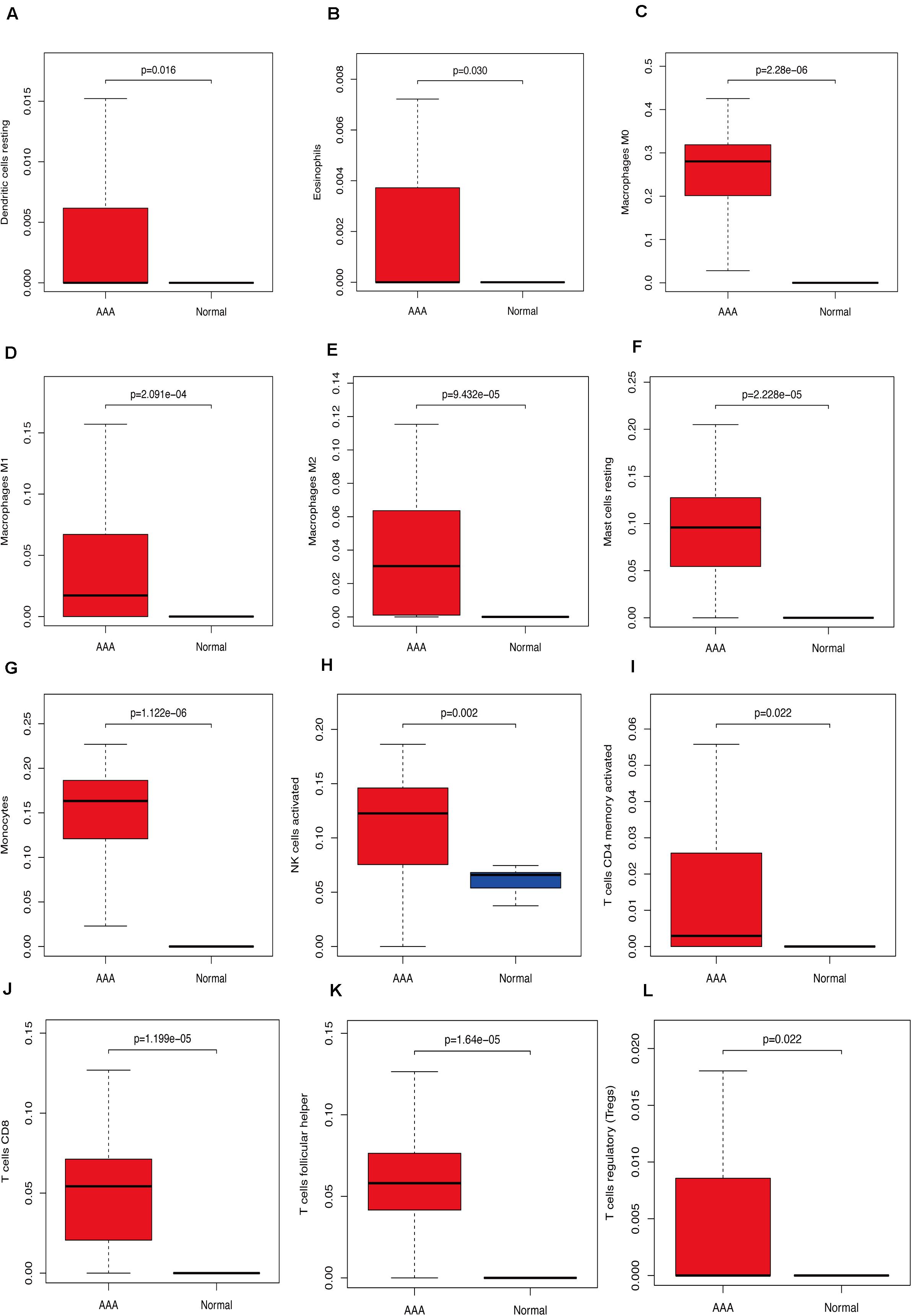
Figure 3. Immunocyte expression ratio, red represents the expression of immune cells in abdominal aortic aneurysm, and blue represents the expression of immune cells in normal vascular tissues. (A) The expression of resting dendritic cells. (B) The expression of eosinophils. (C) The expression of macrophages M0. (D) The expression of macrophages M1. (E) The expression of macrophages M2. (F) The expression of resting mast cells. (G) The expression of monocytes, (H) Activated NK cell expression, (I) activated CD4 memory cell expression, (J) CD8 cell expression, (K) follicular helper cell expression, (L) T regulatory cell (Tregs) expression.
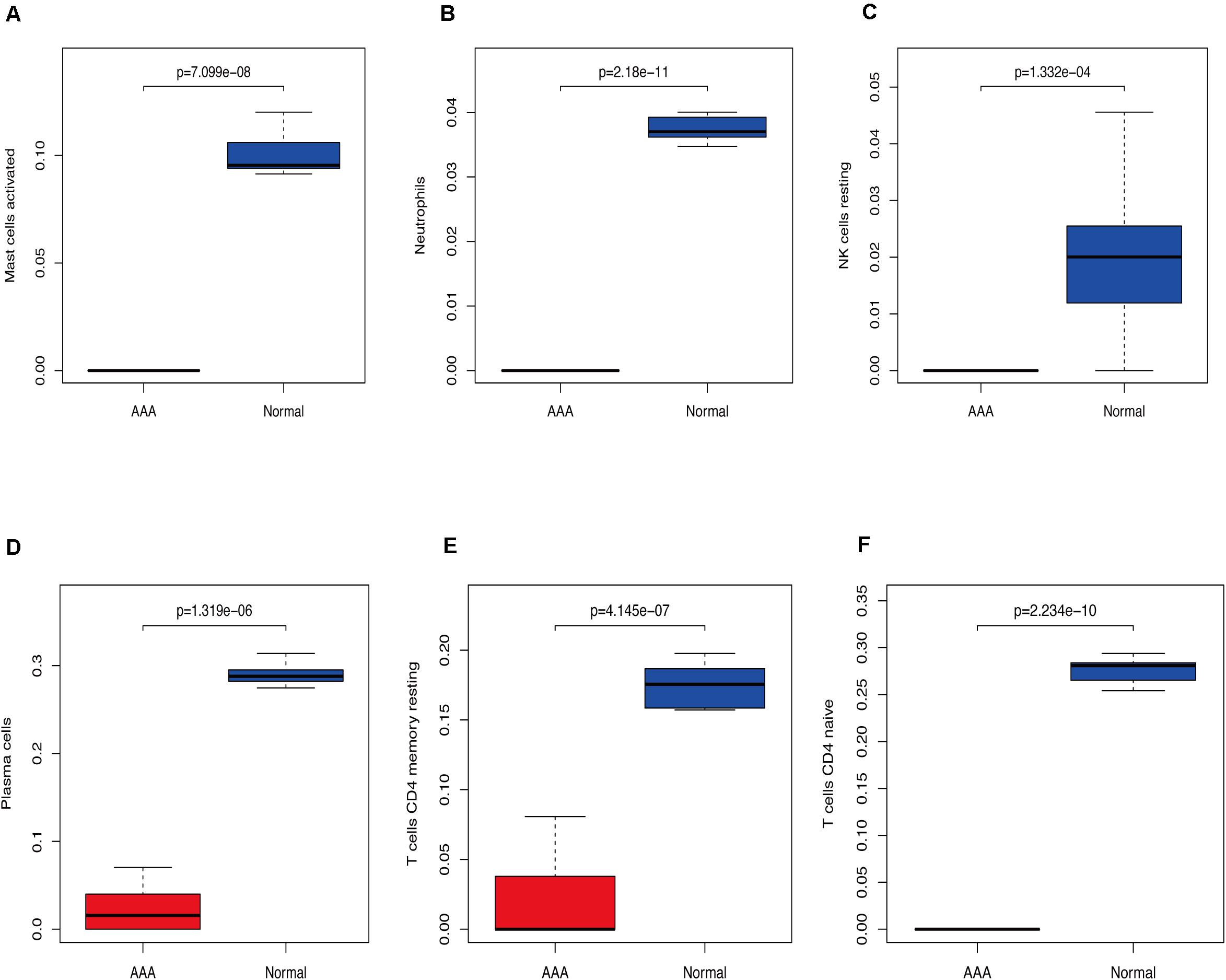
Figure 4. Immunocyte expression ratio, red represents the expression of immune cells in abdominal aortic aneurysm, and blue represents the expression of immune cells in normal vascular tissues (A) Expression of activated mast cells. (B) Expression of neutrophils. (C) The expression of resting NK cells. (D) Expression of plasma cells. (E) Expression of resting CD4 memory cells. (F) Expression of CD4 naïve cells.
We reduced the dimensions of the samples and performed PCA based on the infiltration of 22 types of immune cells in the samples. The results showed that normal aortic and AAA samples could be clearly distinguished based on the infiltration of immune cells (Figure 5A).
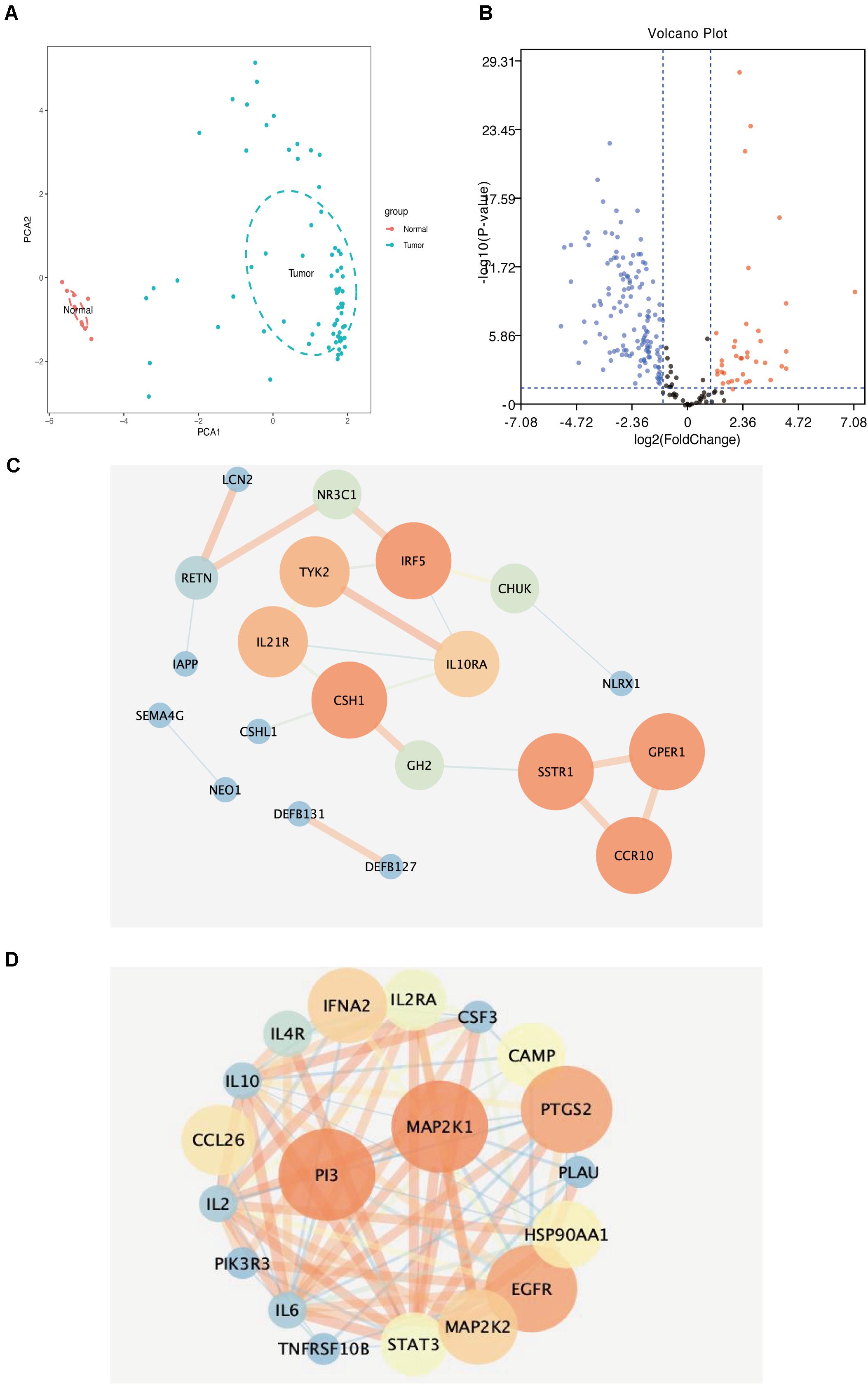
Figure 5. (A) Principal component analysis distribution of normal aortic samples and abdominal aortic aneurysm samples. (B) Volcanic map of differential immune-related genes in samples (blue dots represent downregulated genes, red represents core genes in immune-related genes downregulated by upregulated genes). (C) Downregulated immune-related genes (the darker the color, the larger the circle, the higher the score of immune-related genes). (D) Upregulated immune-related genes (the darker the color, the larger the circle, the higher the score of immune-related genes).
We extracted and analyzed differences in the expression of immune response-related genes in 69 AAA and 10 normal aortic samples. The results showed 39 upregulated and 133 downregulated immune response-related genes (Figure 5B).
We uploaded 39 upregulated and 133 downregulated genes into the STRING database (Supplementary Figures 1A,B) to construct the PPI network. Then, we imported the PPI network into Cytoscape and used MCODE to identify the related nodes. As shown in Figures 5C,D, the larger the degree of the node in the graph, the darker the color and the larger the diameter of the node. Among the upregulated genes, SSTR1, GPER1, and CCR10 had the largest nodes (highest degrees), whereas, among the downregulated genes, PI3 and MAP2K1 had the largest nodes (highest degrees) (Table 2).
Figures 6A,B show that the biological processes regulated by the upregulated immune-response-related genes include response to growth hormone, peptide hormone response, growth factor activity, and cytokine receptor activity. These processes were closely related to the growth hormone response, positive regulation of the JAK-STAT cascade, growth hormone receptor signaling, regulation of tyrosine phosphorylation of STAT proteins (Figures 6C,D), and hormone-mediated signaling. Figures 7A,B show that the downregulated genes were mainly involved in T cell activation, peptide tyrosine phosphorylation, peptidyl tyrosine modification, negative regulation of external stimulus-response, response to TNF, cell chemotaxis, as well as regulation of immune response molecular mediators, platelet α granules, and protein tyrosine kinase activity. This was related to T cell activation, TNF-mediated positive regulation of STAT signaling cascade, negative regulation of external stimulation response, positive regulation of protein kinase B signal transduction, leukocyte migration, leukocyte proliferation, regulation of cell morphogenesis during differentiation, regulation of STAT cascade, leukocyte chemotaxis, and lymphocyte proliferation (Figures 7C,D).
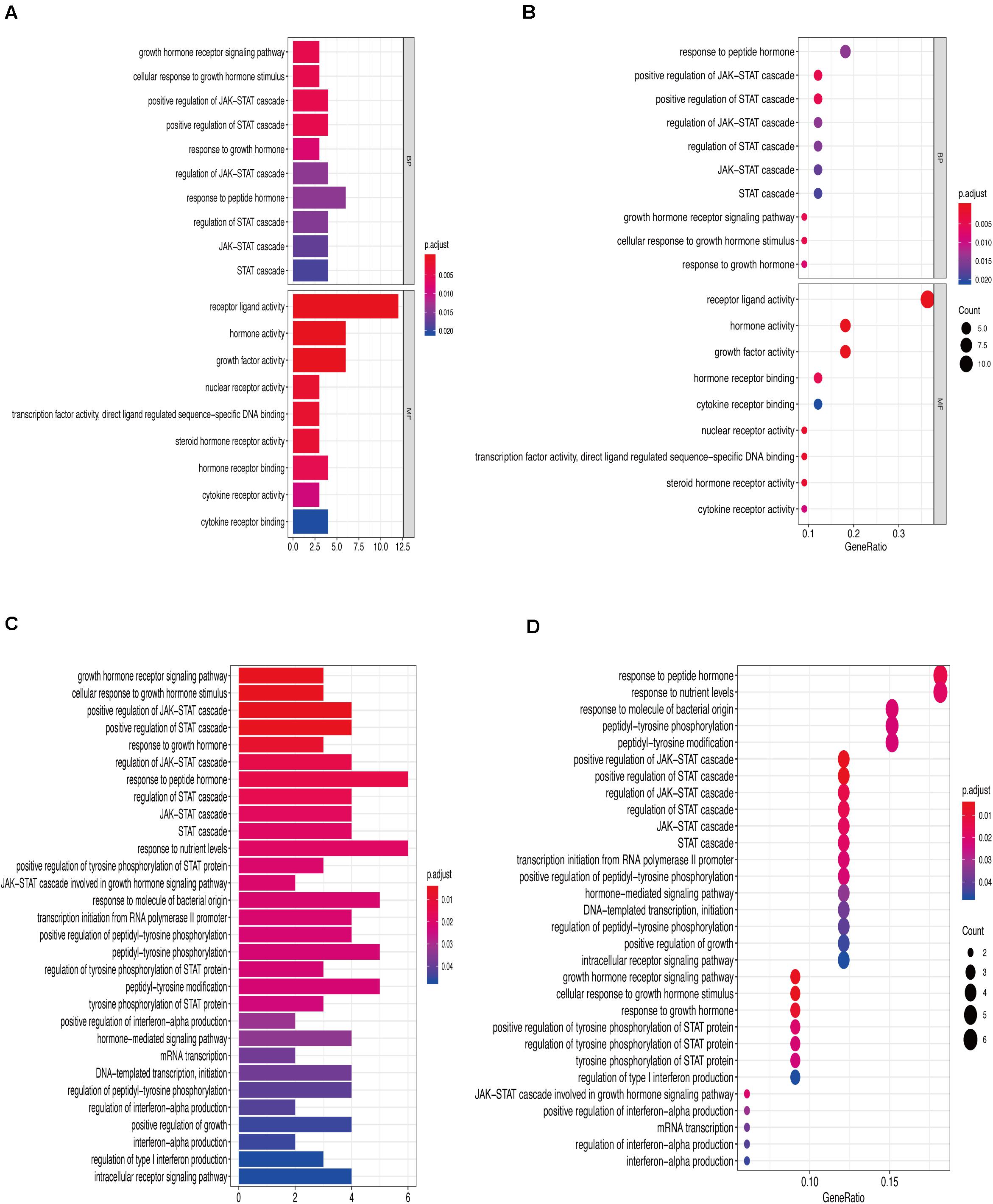
Figure 6. (A) Gene Ontology enrichment analysis histogram of upregulated immune-related genes. (B) Upregulation of immune-related genes, Gene Ontology enrichment analysis histogram. (C) Upregulation of immune-related genes, Kyoto Encyclopedia of Genes and Genomes enrichment analysis histogram. (D) Upregulation of immune-related genes, Kyoto Encyclopedia of Genes and Genomes enrichment analysis histogram.
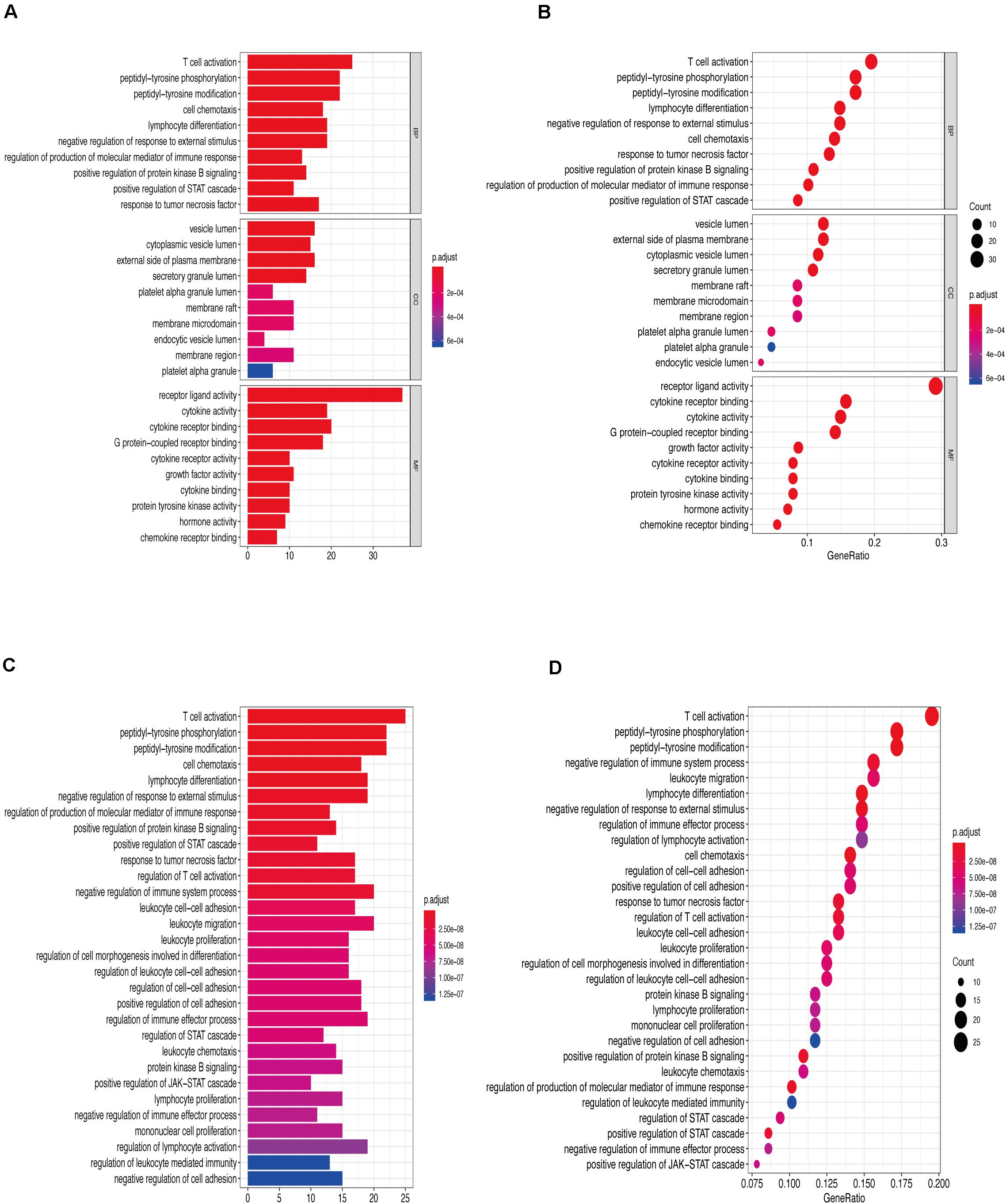
Figure 7. (A) Downregulation of immune-related genes, Gene Ontology enrichment analysis histogram. (B) Downregulation of immune-related genes, Gene Ontology enrichment analysis histogram. (C) Downregulated immune-related genes Kyoto Encyclopedia of Genes and Genomes enrichment analysis histogram. (D) Downregulated immune-related genes Kyoto Encyclopedia of Genes and Genomes enrichment analysis dot map.
We collected eight AAA samples and six samples from the adjacent aortic aneurysm vessels at the Second Affiliated Hospital of Nanchang University. The expression levels of SSTR1, GPER1, CCR10, PI3, and MAP2K1 were measured using qPCR. The relative expression of each target gene in Figure 5 was calculated as follows: relative expression = 2 – Δ CT, where Δ CT = CT value of target gene – CT value of the internal reference gene (actin). Compared with normal samples, the expression levels of SSTR1, GPER1, and CCR10 were increased, whereas those of MAP2K1 were decreased in AAA samples; no significant difference was observed in the expression of PI3 (Figure 8). We found that the expression of vascular inflammatory factors IL-6, IL-17, and TNF-α increased significantly in AAA samples (Supplementary Figure 2).
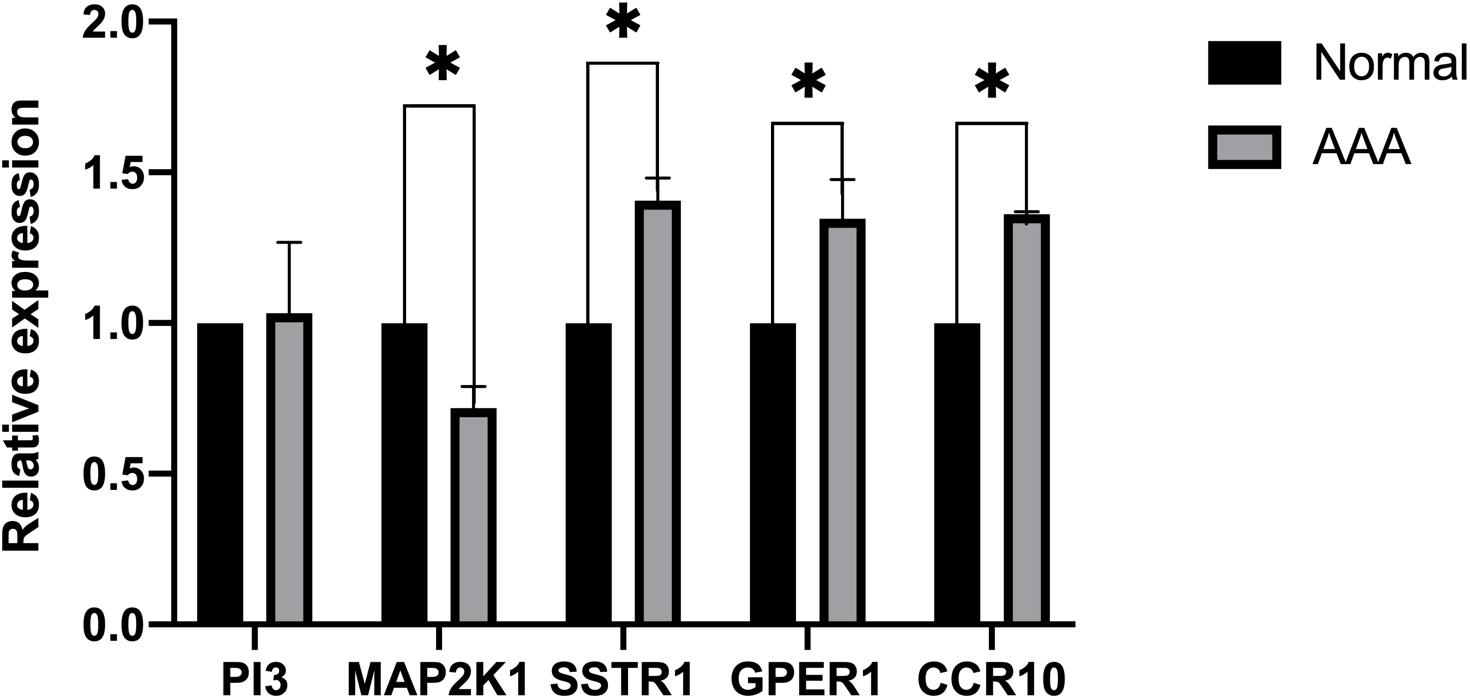
Figure 8. Expression of five immune-related key genes in the samples was analyzed using PCR (∗P < 0.05).
Abdominal aortic aneurysm has always been the focus of vascular surgery research. Due to second-generation sequencing development, more and more researchers began to use bioinformatics technology to study AAA. Wang et al. thought that UBB, NFIA, sparcl1, and other genes play an important role in AAA by comparing gene expression levels and simply constructing a regulatory network (Wang et al., 2018). Gan et al. found that hsa-mir-30a-gng2 and hsa-mir-15b-acss2 may play a role in the development of AAA by screening differentially expressed miRNAs and mRNA and predicting the regulatory relationship through a database (Gan et al., 2019). Gäbel et al. (2017) showed that ccl4l1 and ANGPTL4 were closely related to AAA rupture. In addition, according to Biros et al. (2015), small AAA and large AAA have some unique immune characteristics. They found that cytotoxic T lymphocyte-associated protein 4 (CTLA4) was upregulated in small AAA, and CD8a was upregulated in large AAA. Subsequent studies found that the downregulation of CTLA4 could promote the immune response induced by T cells, leading to the occurrence of AAA. However, few studies have used bioinformatics technology to comprehensively analyze the infiltration of immune cells in AAA. Only one article analyzes the infiltration of immune cells in a ruptured abdominal aortic aneurysm and stable abdominal aortic aneurysm. The sample size is only 48 cases, and there is no laboratory data validation (Lei et al., 2020).
In this study, we analyzed the GSE57691 and GSE98278 datasets related to AAAs. A total of 90 samples were included to comprehensively analyze the status of immune cell infiltration in AAA and the correlation between immune cells. Furthermore, we screened the genes that play a key role in AAA by constructing a network and verified the results by collecting clinical samples for PCR analysis, which made our research results more reliable. We found a significant difference in the infiltration ratio of 22 types of immune cells between normal aortic samples and AAA samples (Figure 1A). Furthermore, we found that the proportions of cells related to M1 macrophages, M2 macrophages, mast cell quiescence, monocytes, NK cell activation, and activated memory CD4+ T cells increased in AAAs, whereas those related to mast cell activation, NK cell quiescence (Figure 4C), quiescent memory CD4+ T cells (Figure 4E), and naïve CD4+ T cells (Figure 4F) increased in normal aortic samples. Although the role of mast cells in AAAs is not clear (Sillesen et al., 2015), the recruitment of macrophages in aortic tissue marks the beginning of infiltration into the adventitia, which promotes the secretion of matrix degradants to contribute to the formation of AAAs (Blomkalns et al., 2013). Activated memory CD4+ T cells can play a pro-inflammatory role by differentiating into Th2 cells, and studies have shown that the proportion of Th2 cells in AAAs is increased (Wang et al., 2014b). Furthermore, NK cells can produce pro-inflammatory factors, such as IL-2 and interferon-γ, which can lead to increased cytotoxic activity and promote AAA formation (Chan et al., 2005a, b; Forester et al., 2006). In addition, there is evidence that monocyte depletion can inhibit the formation of AAAs (Wang et al., 2010). Moreover, the analysis of these same datasets also showed that the invasion of monocytes and CD4T cells into the vascular wall and expression of cytotoxic mediators might be the cause of AAA (Ritchie et al., 2015; Gäbel et al., 2017). Thus, our results are highly consistent with these findings. The results of the PCA analysis showed that normal aortic and AAA samples could be clearly distinguished by the infiltration of immune cells. Also, our study confirmed that the expression of inflammatory factors IL-6, IL-17, and TNF was significantly increased in abdominal aortic aneurysm samples compared with adjacent aorta samples. Therefore, searching for the key immune-related genes in AAA is a new direction to study the occurrence and development of AAA.
We screened 172 immune-response-related genes that were differentially expressed in normal aortic vascular samples and AAA samples, including 39 upregulated genes and 133 downregulated genes. These genes were used to construct a PPI network. SSTR1, GPER1, and CCR10 were found to be important genes in the upregulated gene network, whereas PI3 and MAP2K1 were important genes in the downregulated gene network. SSTR1, one of the five somatostatin receptors (SSTRs), plays an important role in neuroendocrine tumors, such as lung carcinoid (Vesterinen et al., 2019), and the overexpression of SSTR1 can inhibit cell proliferation (Zou et al., 2015). Compared with normal uterine tissues, the expression of GPER1 in uterine leiomyoma is higher and increases cell migration (Kim et al., 2020), which may be one reason for its high expression in AAAs. CCR10 is an important receptor-mediating chemokine, which participates in angiogenesis by endothelial cells. It can promote angiogenesis and improve wound healing by inhibiting the reaction between CCL28 and CCR10 (Chen et al., 2020). CCR10 is expressed in the skin of most patients with psoriasis and atopic or allergic contact dermatitis and plays a key role in T-cell-mediated skin inflammation (Homey et al., 2002). Studies on the same datasets also showed that chemokines and their ligands were upregulated in AAA and could interact with other factors to play a role in AAA. For example, CCL4L1, CCL3L3, CXCL1, CXCL2, CXCL13, and CCR7 were all highly expressed in AAA (Gäbel et al., 2017; Gan et al., 2019). In one study, GNG2, CXCL1, and CCR7 were considered as central genes in the AAA network, and GNG2 interacted with CXCL1 and CCR7 to participate in the chemokine signaling road (Gan et al., 2019). PI3 is an important mediator in the occurrence and development of inflammation and synthesized and secreted by infiltrating neutrophils. Lipopolysaccharide, elastase, and TNF-α can also promote its production, and although the expression of PI3 is increased in the airway and mucosa where inflammatory stimulation persists (Sallenave et al., 1994; Pfundt et al., 1996, 2000; Reid et al., 1999), this does not mean that PI3 is a pro-inflammatory factor. On the contrary, PI3 seems to play a protective role in inflammation; for example, it is downregulated in the acute phase of acute respiratory distress syndrome, and in an experimental study, the plasma PI3 levels of control subjects were much higher than those of patients with respiratory distress syndrome. Therefore, a decrease in PI3 expression may lead to a decrease in the body’s tolerance for inflammation (Tejera et al., 2009). The mutation of MAP2K1 is considered the most common cause of extracranial arteriovenous malformations. MAP2K1 encodes MAP-extracellular signal-regulated kinase 1 (MEK1) and affects cell development through the RAS/MAPK signaling pathway. Extracranial arteriovenous malformations are characterized by vascular endothelial cell dysfunction and the promotion of arteriovenous malformations (Couto et al., 2017; Konczyk et al., 2020). These findings are mostly consistent with our results.
In our study, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses of differentially expressed genes showed that the upregulated genes were mainly involved in growth hormone response, peptide hormone response, growth factor activity, cytokine receptor activity, positive regulation of JAK-STAT cascade, and hormone-mediated signaling. Downregulated genes were mainly involved in T cell activation, negative regulation of external stimulus–response, response to TNF, cell chemotaxis, regulation of immune response molecular mediators, T cell activation, and leukocyte migration and proliferation. These biological processes and pathways are closely related to inflammation and immunity. Other studies on AAA have also confirmed that the interaction between cytokines and cytokine receptors, chemokine signaling pathway and T cell receptor signaling pathway play an important role in the occurrence and development of AAA (Biros et al., 2015). Therefore, we believe that the five immune response-related genes, SSTR1, GPER1, CCR10, PI3, and MAP2K1, may play important roles in the formation of AAA.
This study has the limitation of a small sample size for the measurement of selected gene expression; indeed, this may be the reason why we found no significant difference in PI3 expression. Hence, we plan to perform more in-depth studies on the selected genes to elucidate the mechanisms of action of these genes in the development of AAA.
In this study, by analyzing an AAA microarray, we found that normal aorta and AAA samples could be clearly distinguished via the infiltration of immune cells and identified the key differentially expressed immune response-related genes, SSTR1, GPER1, CCR10, PI3, and MAP2K1.
Based on our results and the literature, we hypothesize that these five genes participate in the immune response and play important roles in the development of AAA. Thus, these genes may be key targets for the diagnosis and treatment of AAA.
Publicly available datasets were analyzed in this study. This data can be found here: GSE57691 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57691) and GSE98278 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE98278).
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
HN: designing research direction and writing the manuscript. JQ: searching for references and writing the manuscript. SW: helping to revise manuscript. WZ: reviewing and revising the manuscript and guidance for manuscript preparation. All authors contributed to the article and approved the submitted version.
The authors’ research was supported by the research fund of The National Natural Science Foundation of China (13008426) and the Key R&D Projects of Jiangxi Province (20171ACG70008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.579215/full#supplementary-material
Supplementary Figure 1 | (A) Upregulation gene network map downloaded from STRING. (B) Downregulation gene network map downloaded from STRING.
Supplementary Figure 2 | The expression of IL-6, IL-17 and TNF-α in the samples was analyzed using PCR (∗ represents P < 0.05).
Arya, S., Kim, S. I., Duwayri, Y., Brewster, L. P., Veeraswamy, R., Salam, A., et al. (2015). Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J. Vasc. Surg. 61, 324–331. doi: 10.1016/j.jvs.2014.08.115
Biros, E., Gäbel, G., Moran, C. S., Schreurs, C., Lindeman, J. H., Walker, P. J., et al. (2015). Differential gene expression in human abdominal aortic aneurysm and aortic occlusive disease. Oncotarget 6, 12984–12996. doi: 10.18632/oncotarget.3848
Blomkalns, A. L., Gavrila, D., Thomas, M., Neltner, B. S., Blanco, V. M., Benjamin, S. B., et al. (2013). CD14 directs adventitial macrophage precursor recruitment: role in early abdominal aortic aneurysm formation. J. Am. Heart Assoc. 2:e000065. doi: 10.1161/JAHA.112.000065
Chan, W. L., Pejnovic, N., Hamilton, H., Liew, T. V., Popadic, D., Poggi, A., et al. (2005a). Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ. Res. 96, 675–683. doi: 10.1161/01.RES.0000160543.84254.f1
Chan, W. L., Pejnovic, N., Liew, T. V., and Hamilton, H. (2005b). Predominance of Th2 response in human abdominal aortic aneurysm: mistaken identity for IL-4-producing NK and NKT cells? Cell. Immunol. 233, 109–114. doi: 10.1016/j.cellimm.2005.04.020
Chang, T. W., Gracon, A. S., Murphy, M. P., and Wilkes, D. S. (2015). Exploring autoimmunity in the pathogenesis of abdominal aortic aneurysms. Am. J. Physiol. Heart Circ. Physiol. 309, H719–H727. doi: 10.1152/ajpheart.00273.2015
Chen, Z., Haus, J. M., Chen, L., Wu, S. C., Urao, N., Koh, T. J., et al. (2020). CCL28-induced CCR10/eNOS interaction in angiogenesis and skin wound healing. FASEB J. 34, 5838–5850. doi: 10.1096/fj.201902060R
Couto, J. A., Huang, A. Y., Konczyk, D. J., et al. (2017). Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am. J. Hum. Genet. 100, 546–554. doi: 10.1016/j.ajhg.2017.01.018
Culav, E. M., Clark, C. H., and Merrilees, M. J. (1999). Connective tissues: matrix composition and its relevance to physical therapy. Phy. Ther. 79, 308–319. doi: 10.1093/ptj/79.3.308
Eliason, J. L., Hannawa, K. K., Ailawadi, G., Sinha, I., Ford, J. W., and Deogracias, M. P. (2005). Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 112, 232–240. doi: 10.1161/CIRCULATIONAHA.104.517391
Forester, N. D., Cruickshank, S. M., Scott, D. J. A., and Carding, S. R. (2006). Increased natural killer cell activity in patients with an abdominal aortic aneurysm. Br. J. Surg. 93, 46–54. doi: 10.1002/bjs.5215
Gäbel, G., Northoff, B. H., Weinzierl, I., Ludwig, S., Hinterseher, I., Wilfert, W., et al. (2017). Molecular fingerprint for terminal abdominal aortic aneurysm disease. J. Am. Heart Assoc. 6:e006798. doi: 10.1161/JAHA.117.006798
Gan, S., Pan, Y., and Mao, J. (2019). miR-30a-GNG2 and miR-15b-ACSS2 interaction pairs may be potentially crucial for development of abdominal aortic aneurysm by influencing inflammation. DNA Cell Biol. 38, 1540–1556. doi: 10.1089/dna.2019.4994
Golledge, J., and Norman, P. E. (2011). Current status of medical management for abdominal aortic aneurysm. Atherosclerosis 217, 57–63. doi: 10.1016/j.atherosclerosis.2011.03.006
Golledge, J., Muller, J., Daugherty, A., and Norman, P. (2006). Abdominal aortic aneurysm: pathogenesis and implications for management. Arteriosclerosis Thrombosis Vascul. Biol. 26, 2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb
Gordon, P. A., and Toursarkissian, B. (2014). Treatment of abdominal aortic aneurysms: the role of endovascular repair. AORN 100, 241–259. doi: 10.1016/j.aorn.2014.01.025
Homey, B., Alenius, H., Müller, A., Soto, H., Bowman, E. P., Yuan, W., et al. (2002). CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157–165. doi: 10.1038/nm0202-157
Keeling, W. B., Armstrong, P. A., Stone, P. A., Bandyk, D. F., and Shames, M. L. (2005). An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc. Endovasc. Surg. 39, 457–464. doi: 10.1177/153857440503900601
Kim, M., Kim, Y. S., Choi, J. I., Kim, J. M., Lee, H. H., and Kim, T. H. (2020). G protein-coupled estrogen receptor 1 expression in normal myometrium, leiomyoma, and adenomyosis tissues of premenopausal women. Gynecol. Endocrinol. 36, 599–604. doi: 10.1080/09513590.2020.1751108
Konczyk, D. J., Goss, J. A., Smits, P. J., Sudduth, C. L., Al-Ibraheemi, A., and Greene, A. K. (2020). Arteriovenous malformation MAP2K1 mutation causes local cartilage overgrowth by a cell-non autonomous mechanism. Sci. Rep. 10:4428. doi: 10.1038/s41598-020-61444-x
Leek, J. T., Johnson, W. E., Parker, H. S., Fertig, E. J., Jaffe, A. E., Storey, J. D., et al. (2019). sva: Surrogate Variable.Analysis. R package version 3.34.0.
Lei, C., Yang, D., Chen, S., Chen, W., Sun, X., Wu, X., et al. (2020). Patterns of immune infiltration in stable and raptured abdominal aortic aneurysms: A gene-expression-based retrospective study. Gene 762:145056. doi: 10.1016/j.gene.2020.145056
Liu, Y., Liao, J., Zhao, M., Wu, H., Yung, S., Chan, T. M., et al. (2015). Increased expression of TLR2 in CD4(+) T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur. J. Immunol. 45, 2683–2693. doi: 10.1002/eji.201445219
Longo, G. M., Buda, S. J., Fiotta, N., Xiong, W., Griener, T., Shapiro, S., et al. (2005). MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery 137, 457–462. doi: 10.1016/j.surg.2004.12.004
Longo, G. M., Xiong, W., Greiner, T. C., Zhao, Y., Fiotti, N., and Baxter, B. T. (2002). Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110, 625–632. doi: 10.1172/JCI0215334
Moxon, J. V., Parr, A., Emeto, T. I., Walker, P., Norman, P. E., and Golledge, J. (2010). Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr. Probl. Cardiol. 35, 512–548. doi: 10.1016/j.cpcardiol.2010.08.004
Newman, A. M., Liu, C. L., Green, M. R., Gentles, A. J., Feng, W., and Xu, Y. (2015). Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457. doi: 10.1038/nmeth.3337
Newman, K. M., Jean-Claude, J., Li, H., Ramey, W. G., and Tilson, M. D. (1994). Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 90, II224–II227.
Pfundt, R., van Ruissen, F., van Vlijmen-Willems, I. M., Alkemade, H. A., Zeeuwen, P. L., Jap, P. H., et al. (1996). Constitutive and inducible expression of SKALP/elafin provides anti-elastase defense in human epithelia. J. Clin. Invest. 98, 1389–1399. doi: 10.1172/jci118926
Pfundt, R., Wingens, M., Bergers, M., Zweers, M., Frenken, M., and Schalkwijk, J. T. N. F. - (2000). Alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch. Dermatol. Res. 292, 180–187. doi: 10.1007/s004030050475
Rateri, D. L., Howatt, D. A., Moorleghen, J. J., Charnigo, R., Cassis, L. A., and Daugherty, A. (2011). Prolonged infusion of angiotensin II in apoE–/– mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am. J. Pathol. 179, 1542–1548. doi: 10.1016/j.ajpath.2011.05.049
Reid, P. T., Marsden, M. E., Cunningham, G. A., Haslett, C., and Sallenave, J. M. (1999). Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett. 457, 33–37. doi: 10.1016/s0014-5793(99)01004-2
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. doi: 10.1093/nar/gkv007
Rizzo, R. J., McCarthy, W. J., Dixit, S. N., Lilly, M. P., Shively, V. P., Flinn, W. R., et al. (1989). Collagen types and matrix protein content in human abdominal aortic aneurysms. J. Vasc. Surg. 10, 365–373. doi: 10.1067/mva.1989.13151
Rooke, T. W., Hirsch, A. T., Misra, S., Sidawy, A. N., Beckman, J. A., Findeiss, L. K., et al. (2012). American college of cardiology foundation., american heart association., society for cardiovascular angiography and interventions., society of interventional radiology., society for vascular medicine., society for vascular surgery.(2012). 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the american college of cardiology foundation/american heart association task force on practice guidelines: developed in collaboration with the society for cardiovascular angiography and interventions, society of interventional radiology, society for vascular medicine, and society for vascular surgery. Catheter Cardiovasc. Interv. 79, 501–531. doi: 10.1002/ccd.23373
Sakalihasan, N., Delvenne, P., Nusgens, B. V., Limet, R., Lapière, C. M., et al. (1996). Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J. Vasc. Surg. 24, 127–133. doi: 10.1016/s0741-5214(96)70153-2
Sakalihasan, N., Michel, J. B., Katsargyris, A., Kuivaniemi, H., Defraigne, J. O., Nchimi, A., et al. (2018). Abdominal aortic aneurysms. Nat. Rev. Dis. Primers 4:34. doi: 10.1038/s41572-018-0030-7
Sallenave, J. M., Shulmann, J., Crossley, J., Jordana, M., and Gauldie, J. (1994). Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am. J. Respir. Cell Mol. Biol. 11, 733–741. doi: 10.1165/ajrcmb.11.6.7946401
Sillesen, H., Eldrup, N., Hultgren, R., Lindeman, J., Bredahl, K., and Thompson, M. (2015). AORTA trial investigators. Randomized clinical trial of mast cell inhibition in patients with a medium-sized abdominal aortic aneurysm. Br. J. Surg. 102, 894–901. doi: 10.1002/bjs.9824
Tejera, P., Wang, Z., Zhai, R., Su, L., Sheu, C. C., Taylor, D. M., et al. (2009). Genetic polymorphisms of peptidase inhibitor 3 (elafin) are associated with acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 41, 696–704. doi: 10.1165/rcmb.2008-0410OC
Umebayashi, R., Uchida, H. A., and Wada, J. (2018). Abdominal aortic aneurysm in aged population. Aging 10, 3650–3651. doi: 10.18632/aging.101702
Vesterinen, T., Leijon, H., Mustonen, H., Remes, S., Knuuttila, A., Salmenkivi, K., et al. (2019). Somatostatin receptor expression is associated with metastasis and patient outcome in pulmonary carcinoid tumors. J. Clin. Endocrinol. Metab. 104, 2083–2093. doi: 10.1210/jc.2018-01931
Wang, G., Bi, L., Wang, G., Huang, F., Lu, M., and Zhu, K. (2018). Microarray analysis to identify the similarities and differences of pathogenesis between aortic occlusive disease and abdominal aortic aneurysm. Vascular 26, 301–314. doi: 10.1177/1708538117736695
Wang, J., Lindholt, J. S., Sukhova, G. K., Shi, M. A., Xia, M., Chen, H., et al. (2014a). IgE actions on CD4+ T cells, mast cells, and macrophages participate in the pathogenesis of experimental abdominal aortic aneurysms. EMBO Mol. Med. 6, 952–969. doi: 10.15252/emmm.201303811
Wang, L., Gao, S., Xu, W., Zhao, S., Zhou, J., Wang, N., et al. (2014b). Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice. J. Mol. Cell Cardiol. 72, 20–27. doi: 10.1016/j.yjmcc.2014.02.005
Wang, Y., Ait-Oufella, H., Herbin, O., Bonnin, P., Ramkhelawon, B., Taleb, S., et al. (2010). TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 120, 422–432. doi: 10.1172/JCI38136
World Health Organization (2013). Media centre, Cardiovascular diseases (CVDs). Available Online at: http://www.who.int/mediacentre/factsheets/fs317/zh/index.html. Accessed Jan 29, 2015
Yamaguchi, T., Yokokawa, M., Suzuki, M., Higashide, S., Katoh, Y., Sugiyama, S., et al. (2000). The effect of immunosuppression on aortic dilatation in a rat aneurysm model. Surg. Today 30, 1093–1099. doi: 10.1007/s005950070007
Yan, Y. W., Fan, J., Bai, S. L., Hou, W. J., Li, X., and Tong, H. (2016). Zinc prevents abdominal aortic aneurysm formation by induction of A20-mediated suppression of NF-κB pathway. PLoS One 11:e0148536. doi: 10.1371/journal.pone.0148536
Yu, G., Wang, L.-G., Han, Y., and He, Q.-Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287. doi: 10.1089/omi.2011.0118
Keywords: immune related genes, abdominal aortic aneurysm, bioinformatics, vascular, surgery
Citation: Nie H, Qiu J, Wen S and Zhou W (2020) Combining Bioinformatics Techniques to Study the Key Immune-Related Genes in Abdominal Aortic Aneurysm. Front. Genet. 11:579215. doi: 10.3389/fgene.2020.579215
Received: 02 July 2020; Accepted: 10 November 2020;
Published: 10 December 2020.
Edited by:
Maarten M. G. van den Hoogenhof, Heidelberg University Hospital, GermanyReviewed by:
Tobias Jakobi, Heidelberg University Hospital, GermanyCopyright © 2020 Nie, Qiu, Wen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weimin Zhou, d2VpbWluemhvdW5jdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.