- 1Department of Pharmacology, Faculty of Medicine, Hacettepe University, Ankara, Turkey
- 2Department of Chest Diseases, Faculty of Medicine, Hacettepe University, Ankara, Turkey
- 3Department of Biostatistics, Faculty of Medicine, Hacettepe University, Ankara, Turkey
Objectives: To determine the effects of genetic polymorphisms of ABCB1 (MDR1), CYP2A6, CYP2B6 on smoking status, and clinical outcomes of smoking cessation therapies in a Turkish population.
Methods: 130 smokers and 130 non-smokers were recruited. Individuals who never smoked were described as non-smokers. 130 smokers were treated with nicotine replacement therapy (NRT) (n = 40), bupropion (n = 47), bupropion + NRT (n = 15), and varenicline (n = 28). Smokers were checked by phone after 12 weeks of treatment whether they were able to quit smoking or not. Genotyping and phenotyping were performed.
Results: Cessation rates were as follows; 20.0% for NRT, 29.8% for bupropion, 40.0% for bupropion + NRT, 57.1% for varenicline (p = 0.013). The frequency of ABCB1 1236TT-2677TT-3435TT haplotype was significantly higher in non-smokers as compared to smokers (21.5% vs. 10.8, respectively; p = 0.018). Neither smoking status nor smoking cessation rates were associated with genetic variants of CYP2A6 (p = 0.652, p = 0.328, respectively), or variants of CYP2B6 (p = 0.514, p = 0.779, respectively).
Conclusion: Genetic variants of the drug transporter ABCB1 and the 1236TT-2677TT-3435TT haplotype was significantly associated with non-smoking status. Neither ABCB1 nor CYP2A6, CYP2B6 genetic variants were associated with smoking cessation rates at the 12th week of drug treatment.
Introduction
Tobacco smoking is a major health problem globally. Over one billion people around the world consume tobacco products and an estimated six million people, including children are clinically affected. This number is expected to rise to eight million by the year 2030 (WHO, 2017). Among many chemicals in cigarettes, nicotine is the main ingredient responsible for dependence (Miwa et al., 2011). Nicotine addiction has been classified as the tobacco use disorder according to DSM V criteria (American Psychiatric Association, 2013).
Among current first-line pharmacotherapies in smoking cessation are nicotine replacement therapy (NRT), bupropion, and varenicline. The success of smoking cessation treatments may be altered by some pharmacogenetic factors, including genetic variants of drug metabolizing enzymes or drug transporters (Lerman et al., 2006; Benowitz, 2008; Bergen et al., 2013; Chenoweth and Tyndale, 2017).
ABCB1 (MDR1, P-glycoprotein) is an efflux protein expressed in many tissues including the blood-brain barrier. Its activity is involved in the determination of tissue levels of many endogenous substances and xenobiotics (Choi, 2005; Bruhn and Cascorbi, 2014). Genetic polymorphisms of ABCB1 have been shown to affect clinical responses to drugs (Zhou, 2008; Zoto et al., 2015). Three commonly investigated single nucleotide polymorphisms (SNPs) in the protein coding region C1236T (rs1128503), G2677T/A (rs2032582), and C3435T (rs1045642) have been associated with changes in ABCB1 activity (Bruhn and Cascorbi, 2014; Wolking et al., 2015; Kim et al., 2018). Hoffmeyer et al. (2000) found one of the first evidence on pharmacogenetic influences of ABCB1 activity in humans. They determined that in Caucasians, homozygous mutant carriers for the C3435T variant were shown to have a 38% increase in peak steady-state concentrations of digoxin, which is a P-gp substrate, compared to wild-type genotype carriers (Hoffmeyer et al., 2000). This result suggested a reduced activity of ABCB1 related to this variant leading to a decreased intestinal secretion of digoxin. Of these three SNPs, C1236T and C3435T are silent polymorphisms. While C1236T does not affect protein expression or mRNA stability, altered mRNA, and protein expression levels in the duodenum and kidney were reported for C3435T polymorphism (Siegsmund et al., 2002; Wang D. et al., 2005). On the other hand, G2677T/A genetic polymorphism is a non-synonymous mutation with substitution of alanine with serine or threonine (Hodges et al., 2011). It has been associated with altered transporter activity (Kim et al., 2001). Investigations on the effects of these three genotypic variants on the pharmacokinetics of P-gp substrates have suggested functionally important yet inconclusive results (Gerloff et al., 2002; Hsin et al., 2020).
Nicotine was reported to alter the expression level or function of ABCB1 (Wang J. S. et al., 2005; Pan et al., 2009; Pal et al., 2011; Takano et al., 2016), which is responsible for the transportation of many endogenous substances, such as opioid peptides, steroid hormones, and endorphin (Oude Elferink and Zadina, 2001). These endogenous or some exogenous ABCB1 substrates may contribute in the formation of substance abuse in the central nervous system (Oude Elferink and Zadina, 2001; Choi, 2005). For example, Levran et al. (2008) showed that ABCB1 function and genetic polymorphisms are involved in heroin abuse. It is conceivable that ABCB1 genetic variants may play a role in nicotine addiction and may be affecting the status of tobacco consumption or outcomes of cessation therapies. Such an association has not been shown in the literature.
Therefore, we aimed to investigate the effects of genetic polymorphisms of ABCB1 on the severity of nicotine dependence and clinical outcomes of smoking cessation therapies in a cohort of Turkish population. Also, genetic variants of metabolizing enzymes CYP2A6 and CYP2B6 which are involved in nicotine metabolism were examined.
Materials and Methods
Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Hacettepe University Ethics Committee (Protocol number: GO-16/416-03) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
A total of 260 subjects were recruited for the study. Among these subjects, 130 (60 females, 70 males) were smokers who applied to the Smoking Cessation Unit and 130 (73 females, 57 males) were non-smokers as the control group. Individuals who never smoked were described as non-smokers. The age range of subjects was 18–71 years old. Subjects with severe heart, liver, kidney diseases, anxiety disorder according to DSM V, pregnant women, and subjects using a nicotine product other than cigarettes or those having an addiction other than nicotine were excluded from the study. Subjects using drugs that would change CYP2A6 or CYP2B6 activities were also excluded. Smoking status was determined by the Fagerström Test for Nicotine Dependence (FTND) and verified by exhaled CO measurements by using piCO Smokerlyzer (Bedfont Scientific Ltd., Kent, United Kingdom). Subjects with exhaled CO values higher than 4 ppm were evaluated as active smokers. All smokers were treated with the appropriate choice of drug therapies according to the European Network for Smoking and Tobacco Prevention (ENSP) Guidelines for Treating Tobacco Dependence (2016) (ENSP, 2016).
In order to assess the effects of genetic polymorphisms on the severity of nicotine dependence, 260 participants were separated into the following 2 groups as smokers and non-smokers. Smokers were treated with NRT, bupropion, bupropion + NRT, and varenicline, as indicated by the ENSP Guideline. We included both nicotine gum, and nicotine patch users in the NRT group because it was shown that form or dosage of nicotine did not affect smoking cessation rates (Stead et al., 2012).
The success of the smoking cessation was checked by re-calling patients after 12 weeks after the beginning of drug treatment.
Genotyping
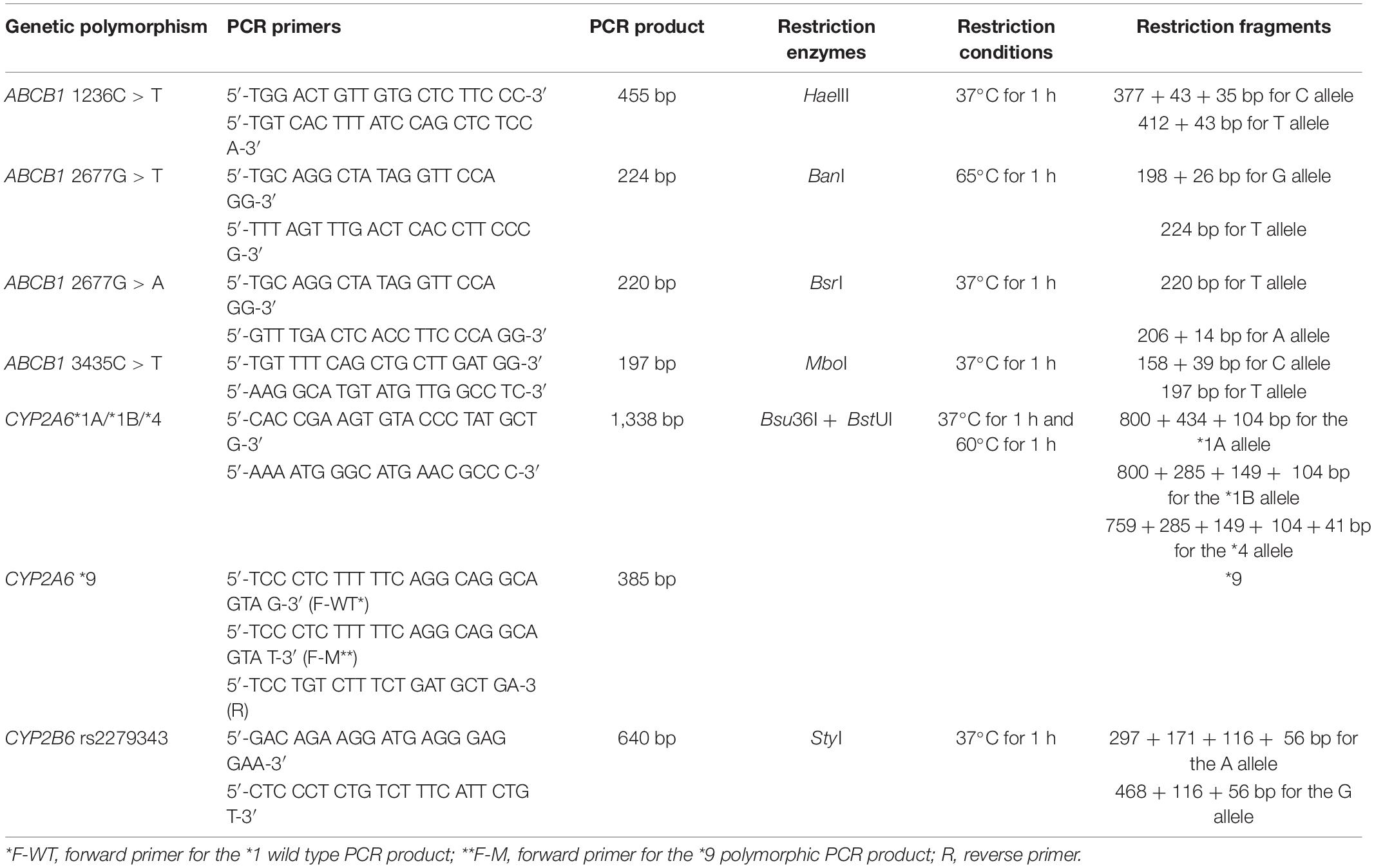
Genomic DNA was extracted using Whole Blood DNA Purification Kit (Thermo Fischer Scientific, Waltham, MA, United States) from venous blood. Genotyping was performed by a Hot Start PCR-RFLP method for the ABCB1 2677G > T/A-3435C > T, CYP2A6∗1, ∗4, and CYP2B6 rs2279343 genetic polymorphisms as described previously (Cascorbi et al., 2001; Nakajima et al., 2006; Tomaz et al., 2015). An allele-specific PCR method was used to genotype CYP2A6∗9 polymorphism (Nakajima et al., 2006). For the ABCB1 1236C > T genetic polymorphism, new PCR primers were designed. A total volume of 25 μl containing 200 μM of each dATP, dCTP, dGTP, dTTP, 2.5 mM MgCl2, BSA, and 12.5 pmol of each primer, 1 unit of Taq DNA Polymerase, and 100 ng of genomic DNA used as PCR mixture (Solis BioDyne, Tartu, Estonia). Hot start PCR conditions were; 95°C for 15 min following 30 cycles of 95°C for 20 s, 60°C for 30 s, 72°C for 1 min, and 72°C for 10 min. PCR cycles were performed by a thermal cycler (Bio-Rad T100 Thermal Cycler, Bio-Rad Laboratories, Taipei, Taiwan). PCR products were restricted by restriction enzymes (New England Biolabs, Ipswich, MA, United States). Restriction fragments were separated by 3% agarose gel electrophoresis and visualized under UV light (Kodak, Rochester, NY, United States). Table 1 shows the PCR primers, restriction enzymes, and restriction conditions (Tufan et al., 2007; Zoto et al., 2015; Karaca et al., 2017).
Measuring Plasma Cotinine and Trans-3′-Hydroxycotinine Levels
Plasma cotinine and Trans-3′-hydroxycotinine (3-HC) levels were measured by high performance liquid chromatography (HPLC) as described by a previous study (Malaiyandi et al., 2006). Agilent Systems 1200 Series (Agilent Technologies, Santa Clara, CA, United States) liquid chromatography system was used. Chemicals were acquired from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). Samples were measured at the 260 nm wavelength. 0–100–250–500–1,000 ng/ml cotinine and 3-HC solutions were used for method validation. The correlation of the calibration curve fit was calculated larger than 0.9999 for both metabolites. The detection limits of the HPLC-UV assay for cotinine and 3-HC were found to be 50 ng/ml and 25 ng/ml, respectively. Inter- and intraday accuracies were calculated to be between 97.9–102.4% for 3-HC, and 93.4–102.2% for cotinine (n = 6). Autosampler stability was at least 24 h at 4°C.
Nicotine metabolite ratio (NMR = plasma 3-HC/cotinine ratios) was used to determine CYP2A6 enzyme activity.
Statistical Analyses
Allele, genotype, and haplotype frequencies among groups were compared by using Chi-Square and Fischer’s exact tests. Comparisons of the distributions of the variables were performed by using Kruskal–Wallis and Mann–Whitney U tests, where applicable. p values under 0.05 were accepted as statistically significant post hoc analyses were performed and adjusted p values by using Dunn’s test. Statistical analyses were performed by using GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla, CA, United States).
For estimation of ABCB1 haplotype frequencies and their effects on smoking status, the internet resource SNPStats1 was used as an in silico statistical tool.
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Demographics and Treatment Results
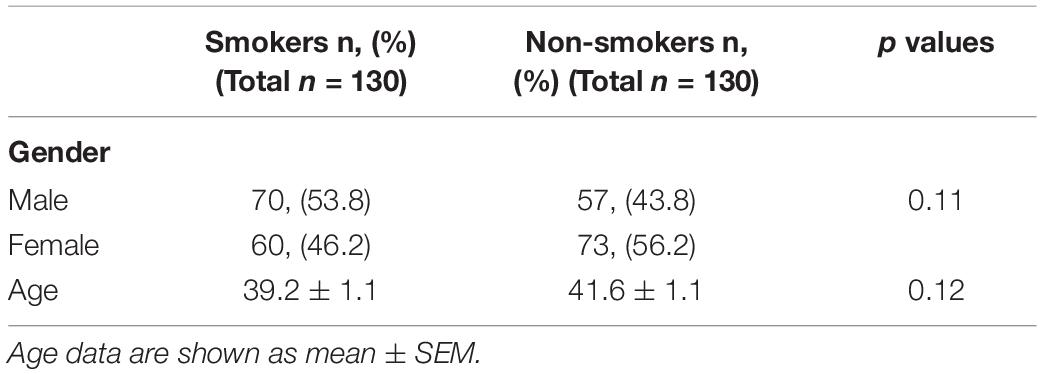
The demographics and characteristics of subject groups are given in Table 2. There were no statistically significant differences between smokers and non-smokers among genders and ages. Distribution of the FTND results among the patients treated for nicotine dependence were shown in Supplementary Figure 1. Cigarettes per day and exhaled CO levels (mean ± standard error of means) were 23.7 ± 0.9 and 14.2 ± 0.8 ppm for smokers, respectively. The distribution of all genetic variants examined were in agreement with the Hardy–Weinberg equilibrium (p > 0.05).
Among 130 smokers, 40 were treated with NRT, 47 with bupropion, 15 with bupropion + NRT, and 28 with varenicline. 12 weeks after the beginning of the treatment, a total of 44 (33.8%) smokers were able to quit smoking. Smoking cessation rates for subgroups are summarized in Table 3.
Effect of ABCB1 Genetic Polymorphisms
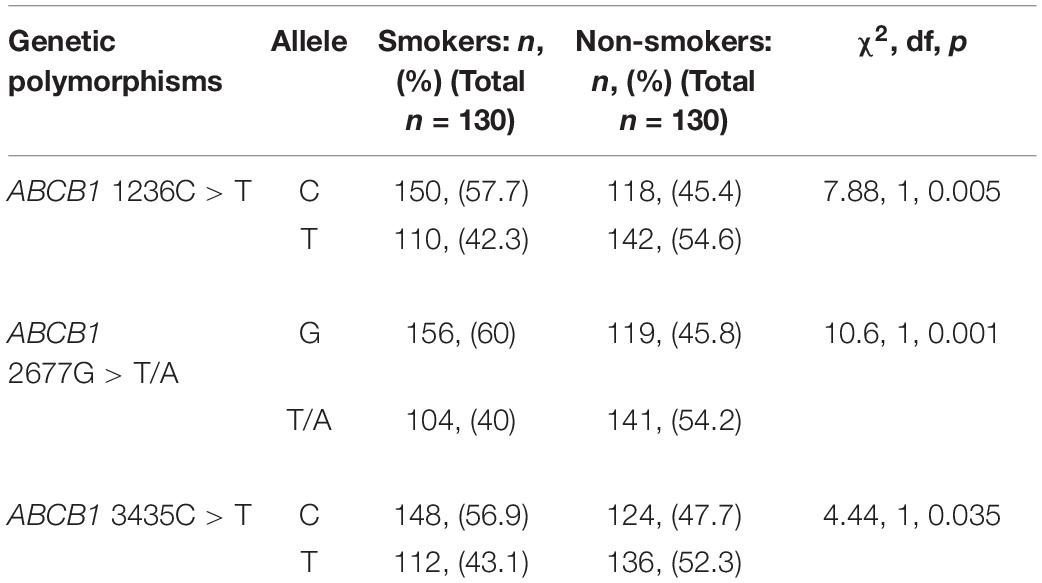
The frequencies of the variant alleles are shown in Table 4. The distributions of alleles for the ABCB1 C1236T, G2677T/A, and C3435CT polymorphisms were significantly different between smokers and non-smokers (p = 0.005, 0.001, and 0.035 respectively).
As shown in Figure 1, the frequency of all-variant haplotype for ABCB1 (1236TT-2677TT-3435TT) was significantly higher in non-smokers as compared to that in smoker subjects (21.5% vs. 10.8, respectively; χ2 = 5.57, df = 1, p = 0.018).
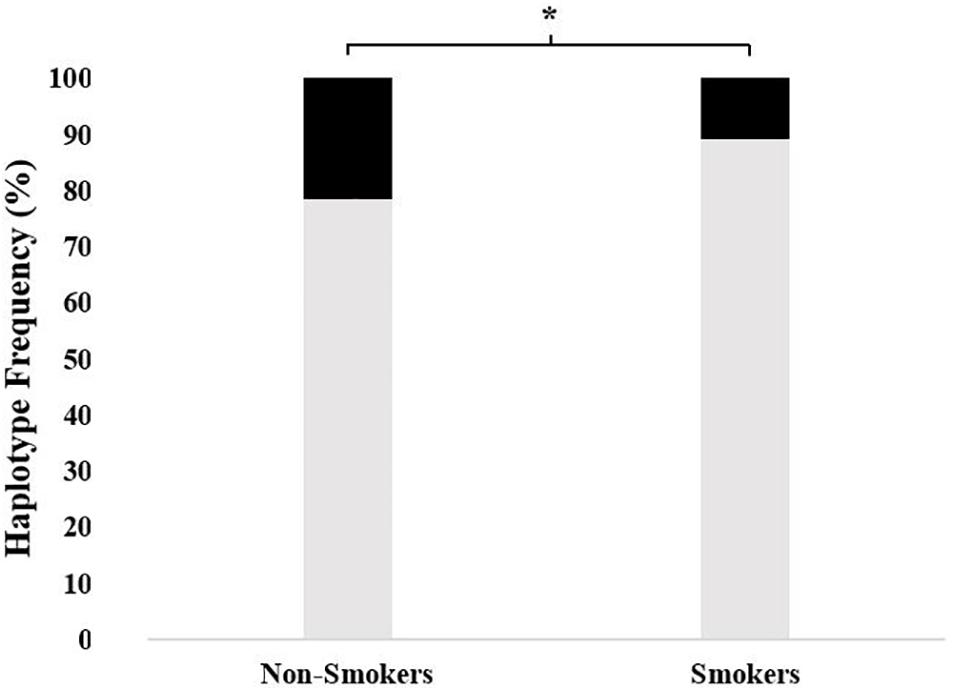
Figure 1. The frequency of 1236TT-2677TT-3435TT haplotype in non-smokers as compared to smokers. The frequencies were 21.5% (95% CI = 14.5–30.3) vs. 10.8% (5.4–16.7), respectively; χ2 = 5.57, df = 1, and *p = 0.018) (■: 1236TT-2677TT-3435TT,  : Other haplotypes).
: Other haplotypes).
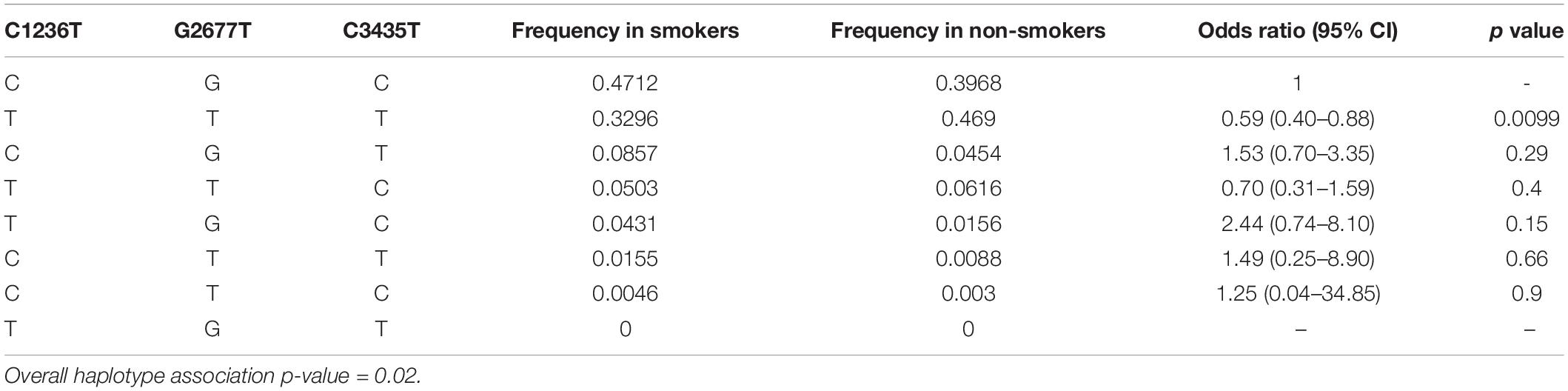
Estimated haplotype distributions and their association with smoking status obtained from in silico analysis by using the web tool SNPStats are provided in Table 5. In accordance with the findings of Figure 1, the subjects who were carriers of the all-variant haplotype (TTT) of ABCB1 were significantly higher in the non-smoker group and this haplotype was significantly associated with smoking status (Table 5, odds ratio = 0.59, confidence interval 95% CI: 0.40–0.88, and p = 0.0099).
There was no association between genetic polymorphisms of ABCB1 and smoking cessation rates (p > 0.05). The haplotypes of ABCB1 and the corresponding smoking cessation rates are presented in Supplementary Table 1.
Effect of CYP2A6 or CYP2B6 Genetic Polymorphisms
Allele frequencies for the CYP2A6∗1, ∗4, and ∗9 variants were 81.9, 3.0, and 0.5% in all participants, respectively. The variant allele for the CYP2B6 rs2279343 polymorphism was determined to be 32.9%. The subjects were grouped according to assumed enzyme activities for CYP2A6. CYP2A6∗1/∗1 genotype carriers were classified as fast metabolizers, while ∗1/∗9 genotype carriers as intermediate metabolizers, and ∗1/∗4, ∗4/∗4, ∗4/∗9, and ∗9/∗9 carriers as slow metabolizers. There was no statistically significant difference between smokers and non-smokers among groups of CYP2A6 activity (p > 0.05). There was also no association between CYP2B6 rs2279343 polymorphism and smoking status (p > 0.05).
Our findings yielded no association between smoking cessation rates and CYP2A6∗1/∗4/∗9 or CYP2B6 rs2279343 genetic polymorphisms (p > 0.05). Data are supplied in Supplementary Table 2.
Plasma Cotinine, Trans-3′-Hydroxycotinine Levels and Nicotine Metabolite Ratios
For CYP2A6 genetic polymorphisms, there was a significant difference among the fast metabolizers, intermediate metabolizers, and slow metabolizers in measured cotinine levels and NMR (Figures 2A,B; p = 0.004 and 0.04, respectively). In addition, the polymorphic GG genotype for the CYP2B6 rs2279343 polymorphism was associated with a higher cotinine level (p = 0.033, data not shown). Cotinine levels were 373 ng/ml (95% CI = 323–423) in AA + AG genotype carriers, 451 ng/ml (95% CI = 336–565) in GG genotype carriers.
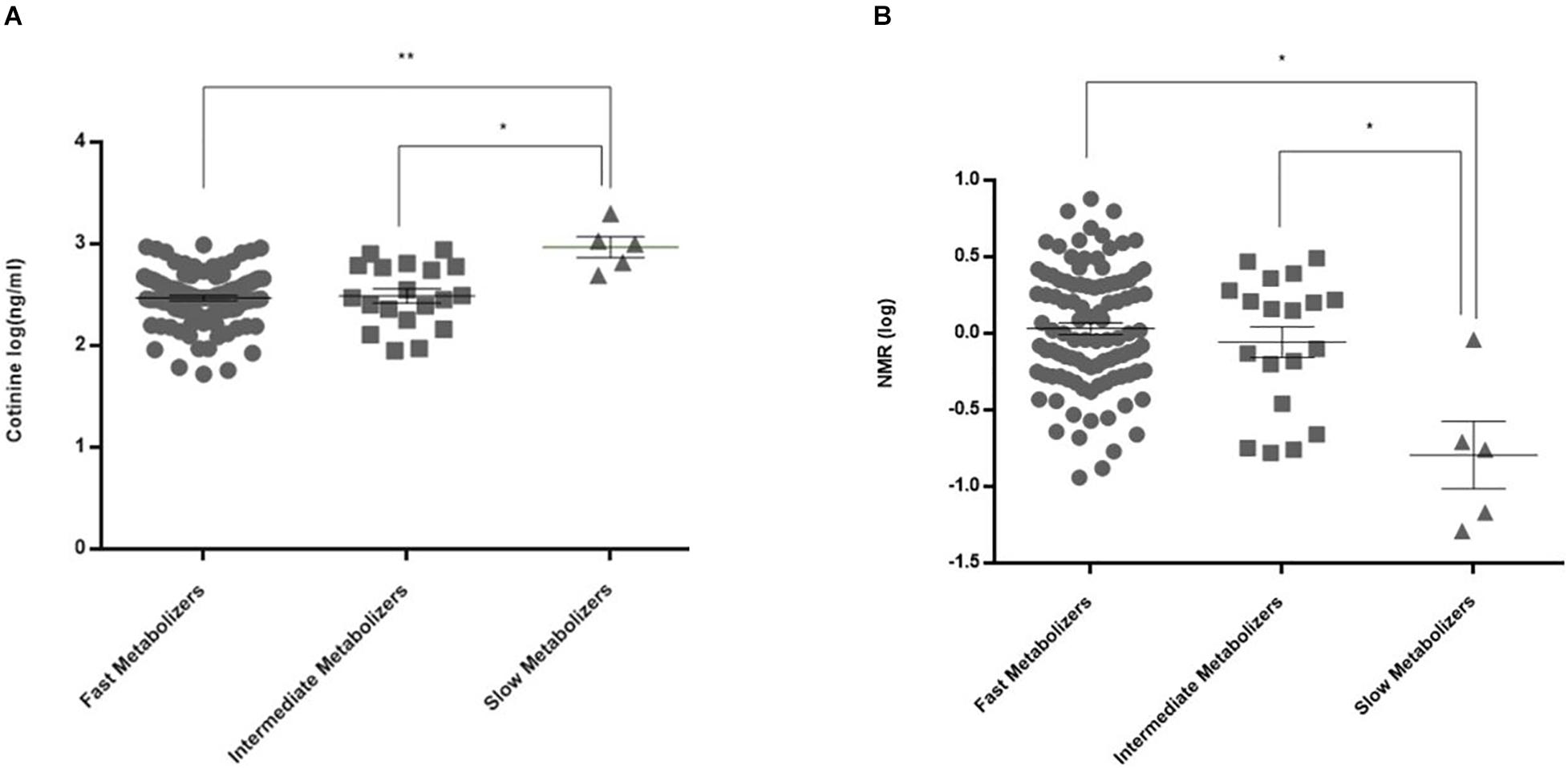
Figure 2. Plasma cotinine levels and NMR values in CYP2A6 activity subgroups. (A) Cotinine levels were 348 ± 20 (95% CI = 308–387) ng/ml in fast metabolizers (n = 104), 383 ± 56 (266–501) ng/ml in intermediate metabolizers (n = 19), and 1,046 ± 216 (490–1,601) ng/ml in slow metabolizers (n = 5) (**adjusted p = 0.0024, *adjusted p = 0.0105). (B) NMRs in CYP2A6 activity subgroups were 1.57 ± 0.14 (95% CI = 1.29–1.85) in fast metabolizers (n = 102), 1.28 ± 0.22 (0.82–1.74) in intermediate metabolizers (n = 19), and 0.34 ± 0.20 (–0.28–0.96) in slow metabolizers (n = 4) (*p = 0.04).
We found no statistically significant differences in cotinine, 3-HC levels, and NMR between quitters and non-quitters (p > 0.05). We also found no statistically significant differences in cotinine, 3-HC levels, and NMR between subjects with the all-variant haplotype 1236TT-2677TT-3435TT as compared with other haplotypes. Data are presented in Supplementary Table 3.
Discussion
Our results showed that the genetic polymorphisms of the drug transporter ABCB1 (MDR1) were significantly associated with non-smoking status in a cohort of Turkish subjects. We also found that an all-variant haplotype 1236TT-2677TT-3435TT was about two times more common in non-smokers as compared to smokers (Figure 1). To our knowledge, this is the first study to report an association between ABCB1 genetic polymorphisms and smoking status.
ABCB1 C1236T and C3435T polymorphisms are silent mutations, albeit with functional consequences. For example, C1236T has been associated with methadone doses required for effective treatment of heroin dependence (Levran et al., 2008). Decreased protein and mRNA expression levels of P-gp in the duodenum and kidney were found in carriers of the variant allele for the C3435T polymorphism (Siegsmund et al., 2002; Wang D. et al., 2005). G2677T/A polymorphism was associated with substitution of alanine with serine or threonine and altered transporter activity (Kim et al., 2001). An interesting and recent population pharmacokinetics study in humans by Hsin et al. (2020) investigated in vivo functional effects of common SNP combinations and haplotypes of the ABCB1 gene by measuring the apparent bioavailability of digoxin, which is an ABCB1 substrate. They reported a 35% higher bioavailability of this substrate in individuals carrying the 1236TT-2677TT-3435TT haplotype as compared to those carrying the wild type haplotype (Hsin et al., 2020). They suggested a reduced ABCB1 transporter activity in the gastrointestinal system associated with increased bioavailability of digoxin. Their results may conform with our findings since it provides biological plausibility for our finding of about two-times higher frequency of the all-variant TT-TT-TT haplotype in non-smokers since ABCB1 has been implicated in the transportation of endogenous compounds such as opioid peptides, steroids, glutamate, and endorphin, which act as neuronal modulators in the central nervous system, and which may play roles in mechanisms of substance dependence (Oude Elferink and Zadina, 2001; Choi, 2005; Zhou, 2008). Indeed, the effects of ABCB1 polymorphisms on substance abuse has been reported previously. For example, genetic polymorphisms of ABCB1 affected the dosage of methadone in the treatment of opioid or heroin addiction (Levran et al., 2008; Yuferov et al., 2010). Also, nicotine was shown to alter ABCB1 expression (Pal et al., 2011; Takano et al., 2016) and 4-methylnitrosamino-1–3-pyridyl-1-butanone, which is an ingredient in tobacco smoke, to induce ABCB1 mRNA expression (Nieh et al., 2015). Therefore, it may be conceivable that ABCB1 genetic polymorphisms may interact with mechanisms related to substance dependence, including nicotine addiction. However, our results do not allow us to draw a direct conclusion since molecular mechanisms related to biological mechanisms have not been investigated.
Our findings also showed that there was no association found between ABCB1 genetic variants and the success of drug treatments for smoking cessation. The finding of a higher level of cotinine in the 2677TT sub-group may be a coincidental finding since existing literature do not largely support the view that ABCB1 variants largely affect plasma concentrations of drugs (Bruhn and Cascorbi, 2014).
Earlier studies reported that CYP2A6 enzyme activity affected nicotine dependence and cigarette consumption (Lerman et al., 2006; Benowitz, 2008; Chenoweth and Tyndale, 2017). Its activity was also found to alter cessation rates during NRT and drugless interventions (Lerman et al., 2006; Schnoll et al., 2009; Chenoweth and Tyndale, 2017; Tanner and Tyndale, 2017). Twin studies indicated that 60–80% of the variation in CYP2A6 enzyme activity can be linked to genetic factors (Swan et al., 2009; Loukola et al., 2015). Asian populations tend to have a higher frequency of polymorphic alleles which cause loss of function (Benowitz et al., 2002; Park et al., 2016). Similarly, African-Americans have lower enzyme activity as compared to Caucasians (Nakajima et al., 2006). We found the frequencies of ∗4 and ∗9 alleles, which result in activity loss, were lower in our patient population as compared to Asian populations (0.5 and 3%, respectively). Previous studies found that smokers who have slower enzyme activity benefited more from NRT when compared to smokers with high enzyme activity (Lerman et al., 2006; Schnoll et al., 2009). Thus, it was suggested that individuals with high enzyme activity should be treated with varenicline (Lerman et al., 2015). Unlike previous studies (Chenoweth and Tyndale, 2017; Tanner and Tyndale, 2017), our study found no association between high or low enzyme activity groups for CYP2A6 activity and the outcome of cessation. This finding may be due to the population difference in these studies. Our study was limited for not being able to examine ∗2 and ∗12 polymorphisms of CYP2A6 (Benowitz et al., 2006). Our finding of an association between plasma cotinine levels, or NMRs and assumed CYP2A6 enzyme activities are in agreement with previous findings (Tanner and Tyndale, 2017; Figures 2A,B).
CYP2B6 is the main metabolizing enzyme for bupropion and has a minor role in nicotine metabolism (Thorn et al., 2010). The expression of CYP2B6 in the brain might be related to a role in nicotine dependence (Garcia et al., 2015). It was reported that there was a reduced enzyme activity in carriers of the single nucleotide variant rs2279343 of CYP2B6 (Al Koudsi and Tyndale, 2010). A study conducted on African-American smokers demonstrated that CYP2B6∗6 haplotype, which included rs2279343 and rs3745274 variants was associated with poorer clinical outcomes with bupropion treatment (Zhu et al., 2012). The same study suggested that higher bupropion doses should be preferred in carriers for the CYP2B6∗6 (516G > T and 785A > G) haplotype. In addition, the same haplotype was associated with increased risk of relapse after cessation in Caucasians (Lee et al., 2007). We found the frequency of variant allele CYP2B6 rs2279343 was 32.9% in our study population, supporting a previous study conducted on an another Turkish population (Yuce-Artun et al., 2014). Our study showed no association of CYP2B6 rs2279343 polymorphism with smoking status or cessation rates. Our study was limited for not being able to examine ∗9 polymorphism of CYP2B6. It is another limitation of our study that it was conducted on a relatively small sample size of subjects. Therefore, our findings need to be confirmed by other studies in various populations.
In conclusion, we found for the first time in the literature that genetic variants of the drug transporter ABCB1 (MDR1) and an all-variant haplotype (1236TT-2677TT-3435TT) were significantly associated with non-smoking status. This haplotype and the related allelic variants may serve as a biomarker for protection against nicotine addiction if confirmed by other studies in different populations.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: Mendeley Data; doi: 10.17632/f8xhczy3f9.1.
Ethics Statement
The studies involving human participants were reviewed and approved by Hacettepe University Ethics Committee (Protocol number: GO-16/416-03). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AM, EB, and MB contributed to the conception and design of the study. EB, AM, SE, and ETK recruited the subjects. AM, EB, MB, MO, and ETK contributed to the acquisition of the data. AM, EB, MB, SE, AI, and EK contributed to the interpretation of data. All authors contributed to the interpretation of the results and manuscript preparation.
Funding
This study was supported by Hacettepe University Research Fund (TSA-2017-12810).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.571997/full#supplementary-material
Footnotes
References
Al Koudsi, N., and Tyndale, R. F. (2010). Hepatic CYP2B6 is altered by genetic, physiologic, and environmental factors but plays little role in nicotine metabolism. Xenobiotica 40, 381–392. doi: 10.3109/00498251003713958
American Psychiatric Association, (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Arlington, VA: American Psychiatric Association.
Benowitz, N. L. (2008). Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 83, 531–541. doi: 10.1038/clpt.2008.3
Benowitz, N. L., Perez-Stable, E. J., Herrera, B., and Jacob, P. III (2002). Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J. Natl. Cancer Inst. 94, 108–115. doi: 10.1093/jnci/94.2.108
Benowitz, N. L., Swan, G. E., Jacob, P. III, Lessov-Schlaggar, C. N., and Tyndale, R. F. (2006). CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80, 457–467. doi: 10.1016/j.clpt.2006.08.011
Bergen, A. W., Javitz, H. S., Krasnow, R., Nishita, D., Michel, M., Conti, D. V., et al. (2013). Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet. Genomics 23, 94–103. doi: 10.1097/FPC.0b013e32835cdabd
Bruhn, O., and Cascorbi, I. (2014). Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin. Drug Metab. Toxicol. 10, 1337–1354. doi: 10.1517/17425255.2014.952630
Cascorbi, I., Gerloff, T., Johne, A., Meisel, C., Hoffmeyer, S., Schwab, M., et al. (2001). Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin. Pharmacol. Ther. 69, 169–174. doi: 10.1067/mcp.2001.114164
Chenoweth, M. J., and Tyndale, R. F. (2017). Pharmacogenetic optimization of smoking cessation treatment. Trends Pharmacol. Sci. 38, 55–66. doi: 10.1016/j.tips.2016.09.006
Choi, C. H. (2005). ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 5:30. doi: 10.1186/1475-2867-5-30
ENSP, (2016). ENSP Guidelines for Treating Tobacco Dependence. Available online at: http://elearning-ensp.eu/assets/English%20version.pdf (accessed June 12, 2020).
Garcia, K. L., Coen, K., Miksys, S., Le, A. D., and Tyndale, R. F. (2015). Effect of brain cyp2b inhibition on brain nicotine levels and nicotine self-administration. Neuropsychopharmacology 40, 1910–1918. doi: 10.1038/npp.2015.40
Gerloff, T., Schaefer, M., Johne, A., Oselin, K., Meisel, C., Cascorbi, I., et al. (2002). MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br. J. Clin. Pharmacol. 54, 610–616. doi: 10.1046/j.1365-2125.2002.01691.x
Hodges, L. M., Markova, S. M., Chinn, L. W., Gow, J. M., Kroetz, D. L., Klein, T. E., et al. (2011). Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet. Genomics 21, 152–161. doi: 10.1097/FPC.0b013e3283385a1c
Hoffmeyer, S., Burk, O., von Richter, O., Arnold, H. P., Brockmoller, J., Johne, A., et al. (2000). Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. U.S.A. 97, 3473–3478. doi: 10.1073/pnas.050585397
Hsin, C. H., Stoffel, M. S., Gazzaz, M., Schaeffeler, E., Schwab, M., Fuhr, U., et al. (2020). Combinations of common SNPs of the transporter gene ABCB1 influence apparent bioavailability, but not renal elimination of oral digoxin. Sci. Rep. 10:12457. doi: 10.1038/s41598-020-69326-y
Karaca, R. O., Kalkisim, S., Altinbas, A., Kilincalp, S., Yuksel, I., Goktas, M. T., et al. (2017). Effects of genetic polymorphisms of cytochrome P450 enzymes and MDR1 transporter on pantoprazole metabolism and Helicobacter pylori eradication. Basic Clin. Pharmacol. Toxicol. 120, 199–206. doi: 10.1111/bcpt.12667
Kim, R. B., Leake, B. F., Choo, E. F., Dresser, G. K., Kubba, S. V., Schwarz, U. I., et al. (2001). Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin. Pharmacol. Ther. 70, 189–199. doi: 10.1067/mcp.2001.117412
Kim, T. E., Shin, K. H., Park, J. E., Kim, M. G., Yun, Y. M., Choi, D. H., et al. (2018). Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des. Devel. Ther. 12, 2139–2147. doi: 10.2147/DDDT.S148257
Lee, A. M., Jepson, C., Hoffmann, E., Epstein, L., Hawk, L. W., Lerman, C., et al. (2007). CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol. Psychiatry. 62, 635–641. doi: 10.1016/j.biopsych.2006.10.005
Lerman, C., Schnoll, R. A., Hawk, L. W. Jr., Cinciripini, P., George, T. P., Wileyto, E. P., et al. (2015). Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 3, 131–138. doi: 10.1016/S2213-2600(14)70294-2
Lerman, C., Tyndale, R., Patterson, F., Wileyto, E. P., Shields, P. G., Pinto, A., et al. (2006). Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin. Pharmacol. Ther. 79, 600–608. doi: 10.1016/j.clpt.2006.02.006
Levran, O., O’Hara, K., Peles, E., Li, D., Barral, S., Ray, B., et al. (2008). ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum. Mol. Genet. 17, 2219–2227. doi: 10.1093/hmg/ddn122
Loukola, A., Buchwald, J., Gupta, R., Palviainen, T., Hallfors, J., Tikkanen, E., et al. (2015). A genome-wide association study of a biomarker of nicotine metabolism. PLoS Genet. 11:e1005498. doi: 10.1371/journal.pgen.1005498
Malaiyandi, V., Goodz, S. D., Sellers, E. M., and Tyndale, R. F. (2006). CYP2A6 genotype, phenotype, and the use of nicotine metabolites as biomarkers during Ad libitum smoking. Cancer Epidemiol. Biomarkers Prev. 15, 1812–1819. doi: 10.1158/1055-9965.EPI-05-0723
Miwa, J. M., Freedman, R., and Lester, H. A. (2011). Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 70, 20–33. doi: 10.1016/j.neuron.2011.03.014
Nakajima, M., Fukami, T., Yamanaka, H., Higashi, E., Sakai, H., Yoshida, R., et al. (2006). Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin. Pharmacol. Ther. 80, 282–297. doi: 10.1016/j.clpt.2006.05.012
Nieh, S., Jao, S. W., Yang, C. Y., Lin, Y. S., Tseng, Y. H., Liu, C. L., et al. (2015). Regulation of tumor progression via the Snail-RKIP signaling pathway by nicotine exposure in head and neck squamous cell carcinoma. Head Neck 37, 1712–1721. doi: 10.1002/hed.23820
Oude Elferink, R. P., and Zadina, J. (2001). MDR1 P-glycoprotein transports endogenous opioid peptides. Peptides 22, 2015–2020. doi: 10.1016/s0196-9781(01)00564-2
Pal, D., Kwatra, D., Minocha, M., Paturi, D. K., Budda, B., and Mitra, A. K. (2011). Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 88, 959–971. doi: 10.1016/j.lfs.2010.09.012
Pan, W. C., Chen, R. M., Shen, Y. C., Chen, C. C., and Ueng, Y. F. (2009). Suppressive effect of tobacco smoke extracts on oral P-glycoprotein function and its impact in smoke-induced insult to oral epidermal cells. Toxicol. Lett. 185, 116–123. doi: 10.1016/j.toxlet.2008.12.007
Park, S. L., Tiirikainen, M. I., Patel, Y. M., Wilkens, L. R., Stram, D. O., Le Marchand, L., et al. (2016). Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risks of lung cancer and effect on their smoking intensity. Carcinogenesis 37, 269–279. doi: 10.1093/carcin/bgw012
Schnoll, R. A., Patterson, F., Wileyto, E. P., Tyndale, R. F., Benowitz, N., and Lerman, C. (2009). Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol. Biochem. Behav. 92, 6–11. doi: 10.1016/j.pbb.2008.10.016
Siegsmund, M., Brinkmann, U., Schaffeler, E., Weirich, G., Schwab, M., Eichelbaum, M., et al. (2002). Association of the P-glycoprotein transporter MDR1(C3435T) polymorphism with the susceptibility to renal epithelial tumors. J. Am. Soc. Nephrol. 13, 1847–1854. doi: 10.1097/01.asn.0000019412.87412.bc
Stead, L. F., Perera, R., Bullen, C., Mant, D., Hartmann-Boyce, J., Cahill, K., et al. (2012). Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 11:CD000146. doi: 10.1002/14651858.CD000146.pub4
Swan, G. E., Lessov-Schlaggar, C. N., Bergen, A. W., He, Y., Tyndale, R. F., and Benowitz, N. L. (2009). Genetic and environmental influences on the ratio of 3’hydroxycotinine to cotinine in plasma and urine. Pharmacogenet. Genomics 19, 388–398. doi: 10.1097/FPC.0b013e32832a404f
Takano, M., Naka, R., Sasaki, Y., Nishimoto, S., and Yumoto, R. (2016). Effect of cigarette smoke extract on P-glycoprotein function in primary cultured and newly developed alveolar epithelial cells. Drug Metab. Pharmacokinet. 31, 417–424. doi: 10.1016/j.dmpk.2016.08.006
Tanner, J. A., and Tyndale, R. F. (2017). Variation in CYP2A6 activity and personalized medicine. J. Pers. Med. 7:18. doi: 10.3390/jpm7040018
Thorn, C. F., Lamba, J. K., Lamba, V., Klein, T. E., and Altman, R. B. (2010). PharmGKB summary: very important pharmacogene information for CYP2B6. Pharmacogenet. Genomics 20, 520–523. doi: 10.1097/fpc.0b013e32833947c2
Tomaz, P. R., Santos, J. R., Issa, J. S., Abe, T. O., Gaya, P. V., Krieger, J. E., et al. (2015). CYP2B6 rs2279343 polymorphism is associated with smoking cessation success in bupropion therapy. Eur. J. Clin. Pharmacol. 71, 1067–1073. doi: 10.1007/s00228-015-1896-x
Tufan, A., Babaoglu, M. O., Akdogan, A., Yasar, U., Calguneri, M., Kalyoncu, U., et al. (2007). Association of drug transporter gene ABCB1 (MDR1) 3435C to T polymorphism with colchicine response in familial Mediterranean fever. J. Rheumatol. 34, 1540–1544.
Wang, D., Johnson, A. D., Papp, A. C., Kroetz, D. L., and Sadee, W. (2005). Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet. Genomics 15, 693–704. doi: 10.1097/01.fpc.0000178311.02878.83
Wang, J. S., Markowitz, J. S., Donovan, J. L., and Devane, C. L. (2005). P-glycoprotein does not actively transport nicotine and cotinine. Addict. Biol. 10, 127–129. doi: 10.1080/13556210500122995
WHO, (2017). WHO Report on Global Tobacco Epidemic. Available online at: https://www.who.int/tobacco/global_report/2017/en/ (accessed June 12, 2020).
Wolking, S., Schaeffeler, E., Lerche, H., Schwab, M., and Nies, A. T. (2015). Impact of genetic polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin. Pharmacokinet. 54, 709–735. doi: 10.1007/s40262-015-0267-1
Yuce-Artun, N., Kose, G., and Suzen, H. S. (2014). Allele and genotype frequencies of CYP2B6 in a Turkish population. Mol. Biol. Rep. 41, 3891–3896. doi: 10.1007/s11033-014-3256-9
Yuferov, V., Levran, O., Proudnikov, D., Nielsen, D. A., and Kreek, M. J. (2010). Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann. N. Y. Acad. Sci. 1187, 184–207. doi: 10.1111/j.1749-6632.2009.05275.x
Zhou, S. F. (2008). Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 38, 802–832. doi: 10.1080/00498250701867889
Zhu, A. Z., Cox, L. S., Nollen, N., Faseru, B., Okuyemi, K. S., Ahluwalia, J. S., et al. (2012). CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin. Pharmacol. Ther. 92, 771–777. doi: 10.1038/clpt.2012.186
Keywords: nicotine, genetic polymorphism, addiction, P-glycoprotein, cytochrome P450 enzymes
Citation: Muderrisoglu A, Babaoglu E, Korkmaz ET, Ongun MC, Karabulut E, Iskit AB, Emri S and Babaoglu MO (2020) Effects of Genetic Polymorphisms of Drug Transporter ABCB1 (MDR1) and Cytochrome P450 Enzymes CYP2A6, CYP2B6 on Nicotine Addiction and Smoking Cessation. Front. Genet. 11:571997. doi: 10.3389/fgene.2020.571997
Received: 12 June 2020; Accepted: 12 November 2020;
Published: 30 November 2020.
Edited by:
Amit V. Pandey, University of Bern, SwitzerlandReviewed by:
Su-Jun Lee, Inje University, South KoreaJulio Benitez, University of Extremadura, Spain
Copyright © 2020 Muderrisoglu, Babaoglu, Korkmaz, Ongun, Karabulut, Iskit, Emri and Babaoglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmet Muderrisoglu, a.muderrisoglu@kku.edu.tr; orcid.org/0000-0003-2954-360X
†These authors have contributed equally to this work and share first authorship
†Present address: Ahmet Muderrisoglu, Department of Pharmacology, Faculty of Medicine, Kırıkkale University, Kırıkkale, Turkey; Salih Emri, Clinic for Chest Diseases, Medicana Kızıltoprak Hospital, Istanbul, Turkey
 Ahmet Muderrisoglu
Ahmet Muderrisoglu Elif Babaoglu
Elif Babaoglu Elif Tugce Korkmaz
Elif Tugce Korkmaz Mert C. Ongun
Mert C. Ongun Erdem Karabulut
Erdem Karabulut Alper B. Iskit1
Alper B. Iskit1 Melih O. Babaoglu
Melih O. Babaoglu