
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 11 August 2020
Sec. Systems Biology Archive
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.00789
This article is part of the Research Topic Molecular Biomarkers for Cancer Control View all 10 articles
 Huan Luo1,2
Huan Luo1,2 Peng Wang1,2
Peng Wang1,2 Hua Ye1,2
Hua Ye1,2 Jianxiang Shi2,3
Jianxiang Shi2,3 Liping Dai2,3
Liping Dai2,3 Xiao Wang2,3
Xiao Wang2,3 Chunhua Song1,2
Chunhua Song1,2 Jianying Zhang1,2,3*
Jianying Zhang1,2,3* Jitian Li4*
Jitian Li4*Recent reports suggest that microRNAs (miRNAs) may serve as prognostic biomarkers in osteosarcoma. Due to osteosarcoma's early metastasis and poor prognosis, it is very important to find novel prognostic biomarkers for improving osteosarcoma's prognosis. Herein we propose a meta-analysis for serum miRNA's prognostic value in osteosarcoma. In this study, the literature available from PubMed, Web of Science, Embase, and Cochrane Library databases was reviewed. The pooled hazard ratios (HRs) with their 95% confidence intervals (CIs) were calculated to evaluate miRNAs prognostic values. A total of 20 studies investigating serum miRNAs were included in this meta-analysis; the initial terminal point of these reports included overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), and recurrence-free survival (RFS). For prognostic meta-analyses, the pooled HR for terminal events of higher expression of miRNAs and lower expression of miRNAs were 5.68 (95% CI 4.73–6.82, P < 0.05) and 3.78 (95% CI 3.27–4.37, P < 0.05), respectively. Additionally, subgroup analyses were conducted based on the analysis methods applied and clinicopathological features reported. In the pooled analyses, the miRNA expression levels are associated with poor prognosis according to both univariate and multivariate analyses. Furthermore, serum miRNAs (miRNA-195, miRNA-27a, miRNA-191, miRNA-300, miRNA-326, miRNA-497, miRNA-95-3p, miRNA-223, miRNA-491-5p, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-194) were associated with poor OS and found to be closely correlated with clinical stage and distant metastasis in osteosarcoma. The results illustrate that low or high expression of these specific miRNAs are both potentially useful as prognostic serum biomarkers in osteosarcoma, and miRNAs (miRNA-195, miRNA-27a, miRNA-191, miRNA-300, miRNA-326, miRNA-497, miRNA-95-3p, miRNA-223, miRNA-491-5p, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-194) may indicate clinical stage and metastasis in this form of cancer.
Patient survival in osteosarcoma has improved in recent decades. Osteosarcoma is the most common malignant bone tumor, with a worldwide incidence of approximately one to three cases annually per million (Kansara et al., 2014). Current therapies include surgical resection and combination neoadjuvant chemotherapy, which is reported to have a curative effect in ~70% of patients (Collins et al., 2013). Metastasis and recurrence are common challenges in refractory osteosarcoma, that worsen patient prognosis (Bielack et al., 2002). The highly malignant nature of osteosarcoma, as well as its high rates of recurrence and lung metastasis represent strong concerns (Jones et al., 2012; Ogawa et al., 2013). Clinically, histological examination of the biopsy specimens is preferred for the diagnosis or prognostic evaluation of osteosarcoma. However, such invasive tests may be burdensome when monitoring the progression of the disease, and the accuracy of diagnosis and prognostic evaluation may vary because of differences in sample collection and personnel. Therefore, it is essential to develop novel approaches for the timely diagnosis of osteosarcoma in order to achieve better prognosis (Gu et al., 2014).
MiRNAs are small (about 21-nucleotide-long) non-coding RNAs which can regulate gene expression (Filipowicz et al., 2008). Elevated or downregulated miRNAs may act as oncogenes or tumor suppressors in various cancers (Hayashita et al., 2005; He et al., 2005; Kent and Mendell, 2006; Tian et al., 2019). Additionally, miRNAs that are stable in serum or plasma, or in other biological samples, may have potential utility as diagnostic or prognostic biomarkers in different cancers (Calin and Croce, 2006; Esquela-Kerscher and Slack, 2006; Mitchell et al., 2008; Zhou et al., 2016). These findings show that miRNAs warrant attention as potential novel biomarkers for diagnosis or prognosis in osteosarcoma.
Although numerous recent studies have reported a correlation between prognosis in osteosarcoma and miRNA expression, none have demonstrated sufficient evidence for clinical translation of their findings. For instance, two previous meta-analyses have concluded the prognostic value of miRNA expression in osteosarcoma (Cheng et al., 2017; Kim et al., 2017); however, in these studies, either tissue or both tissue and blood were used as samples. Tissue samples' obtainment are invasive for patients than serum samples. To optimally obtain samples from patients and increase patients' acceptability, it is important for us to find novel serum biomarker for osteosarcoma. To the best of our knowledge, very few studies have provided robust evidence on the potential prognostic utility of serum miRNAs in osteosarcoma. Therefore, in the present work, we conducted a meta-analysis of studies in which serum samples were analyzed, to explore the prognostic value of miRNAs in osteosarcoma. Following which, subgroup analyses included analysis method and clinicopathological features were also explored to better analyze the prognostic value of various groups.
This study was implemented according to the guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Stroup et al., 2000), and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009). We have completed the prognostic value of serum microRNA. In constructing the prognostic value of serum microRNA, we comply with the population, interventions, comparators, out-comes, and study designs (PICOS) principle to complete the research design.
The literature available in PubMed, Web of Science, Embase, and Cochrane Library databases, up to June 20, 2020, was investigated. The combination of search terms used was (osteosarcoma OR osteogenic sarcoma) AND (microRNA OR miRNA OR miR) AND (prognosis OR survival OR prognostic OR outcome). Only studies of the Chinese population published in English were included, and studies analyzing samples other than serum were excluded.
Inclusion criteria for studies in this review were as follows: (1) studies investigating the utility of miRNAs for evaluating prognosis in osteosarcoma, (2) serum miRNAs' assay method based on quantitative real-time polymerase chain reaction, (3) studies presenting sufficient data to allow calculation of HR and 95% CI, and (4) studies in which a cut-off value was defined. Studies were subject to the following exclusion criteria: (1) studies reporting duplicate data; studies in non-Chinese populations, (2) non-English publications, review articles, or meta-analysis, (3) studies reporting insufficient data for pooled analysis, and (4) studies of tissue, cell lines, or animal experiments.
For prognostic meta-analyses, the quality of included studies was assessed using the Newcastle–Ottawa Scale (NOS), based on the following categories: selection, comparability, and outcome; the highest score was 9, with scores ≥6 indicating studies of high quality (Stang, 2010). The extracted data and information included were as follows: the first author, the year of publication, the country of origin, osteosarcoma sample size, sample type, cut off value, miRNAs characteristics, analysis methods, clinical outcomes, and detection methods. Two investigators retrieved and assessed the literature, respectively, and disagreements were resolved by extensive discussion.
All analyses were performed using STATA 12.0 software. Based on the information provided in the included studies, the pooled HRs with 95% CIs were calculated using this meta-analysis model. Forest plots were used to estimate the effect of miRNA expression on overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), and recurrence free-survival (RFS) (Hong et al., 2014; Zhang et al., 2014a,b; Cai et al., 2015; Tang et al., 2015; Wang N. G. et al., 2015; Wang T. et al., 2015; Yang et al., 2015; Cao et al., 2016; Dong et al., 2016; Liu et al., 2016; Niu et al., 2016; Pang et al., 2016; Wang S. N. et al., 2017; Wang Z. et al., 2017; Cong et al., 2018; Li et al., 2018; Yao et al., 2018; Zhou et al., 2018; Shi et al., 2020). I2 index was used to assess the between-study heterogeneity, with I2 > 50% indicating a large degree of heterogeneity; in this case, a random effect model was applied. I2 ≤ 50% implied that there was no significant heterogeneity, and the fixed effect model was used. Next, subgroup analyses were conducted to identify potential sources of heterogeneity and assess the prognostic value of different subgroups; the level of significance was set at P < 0.05. In addition, Begg's test (Begg and Mazumdar, 1994) and Egger's test (Egger et al., 1997) were performed to assess the publication bias; values of P < 0.05 indicated significant publication bias.
The screening process for the studies is shown in detail in Figure 1. A primary search of the PubMed, Web of Science, Embase, and Cochrane Library databases, using the search strategy described, identified 2,596 articles. The innovative contribution of this work is focused on studies which using serum sample, herein studies using plasma or tissue were excluded, as such, all included studies examined serum samples. The data extracted from the included studies, the quality of the reports and heterogeneity are shown in Tables 1, 2. The osteosarcoma sample size ranged from 60 to 185 subjects. The assay method was based on qRT-PCR. Cut off values were defined in the included studies to differentiate between high-expression miRNAs and low-expression miRNAs, and multivariate or univariate analyses were performed. The quality of each study was high according to the NOS (Stang, 2010). A total of 20 studies and 2,242 osteosarcoma patients were included in this prognostic meta-analysis.
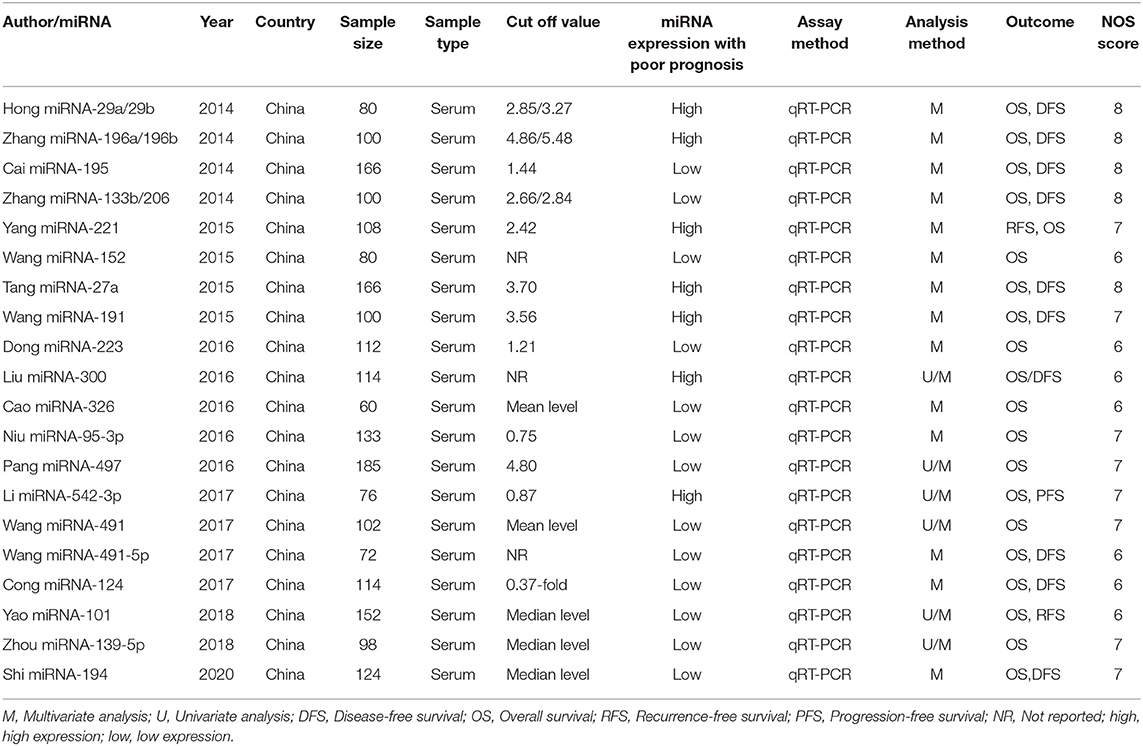
Table 1. The extracted data and quality assessment of literature on the prognostic utility of miRNAs in osteosarcoma.
To analyze the prognostic value of miRNA expression in osteosarcoma, forest plots of data from the 19 studies, in accordance with HRs and their 95% CIs, are shown in Figure 2. The HRs were calculated on the basis of low-expression or high-expression miRNAs, respectively. HR >1 or <1 implied poor or good prognosis for patients with osteosarcoma, respectively. The pooled HRs for low- and high-expression miRNAs were 3.78 (95% CI 3.27–4.37, P < 0.05) and 5.68 (95% CI 4.73–6.82, P < 0.05), respectively, and both tended to be associated with a poorer outcome. Additionally, low-expression miRNAs were stratified by outcomes, including OS (pooled HR = 3.59, 95% CI 3.02–4.26, P < 0.05), DFS (pooled HR = 4.25, 95% CI 3.14–5.76, P < 0.05), and RFS (pooled HR = 4.34, 95% CI 2.48–7.60, P < 0.05). Furthermore, high-expression miRNAs were classified by outcomes, including OS (pooled HR = 5.98, 95% CI 4.58–7.80, P < 0.05), DFS (pooled HR = 4.80, 95% CI 3.53–6.53, P < 0.05), RFS (pooled HR = 6.82, 95% CI 2.13–21.88, P < 0.05), and PFS (pooled HR = 6.95, 95% CI 4.34–11.12, P < 0.05), and these miRNAs were also associated with poor prognosis in osteosarcoma.
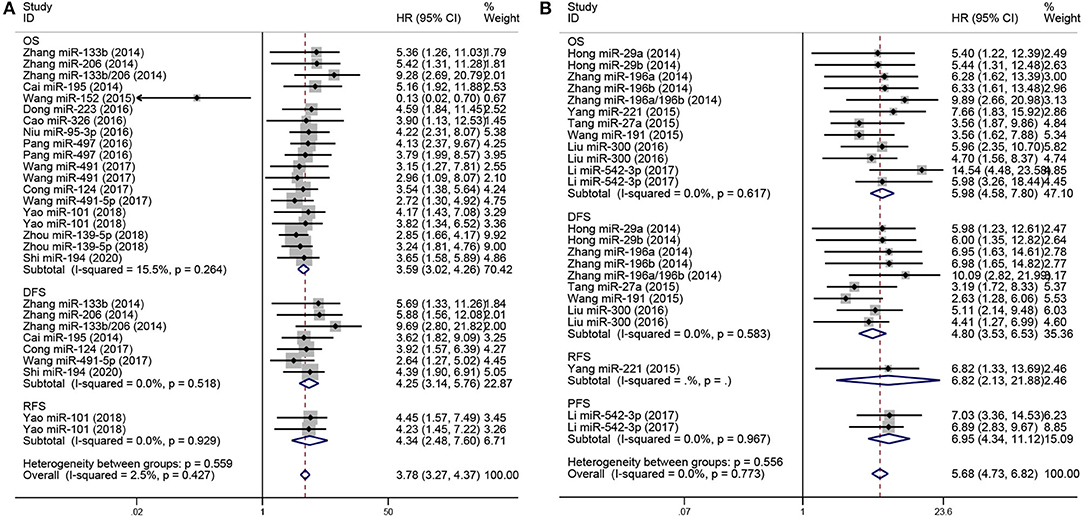
Figure 2. Forest plot for miRNA expression and prognosis in osteosarcoma, stratified by outcomes included (OS, DFS, RFS, PFS). (A) Low-expression miRNAs correlated with poor prognosis in osteosarcoma, stratified by outcomes (OS, DFS, RFS). (B) High-expression miRNAs correlated with poor prognosis in osteosarcoma, stratified by event times (OS, DFS, RFS, PFS).
Subgroup analyses were performed according to analysis method and clinicopathological features in order to explore the correlation of miRNA expression on prognosis in osteosarcoma, as shown in Figures 3, 4, respectively. As shown in Figure 3A, subgroup analyses reveal that low expression levels of miRNA were significantly correlated with poor prognosis in osteosarcoma according to both multivariate (pooled HR = 3.88, 95% CI 3.29–4.58, P < 0.05) and univariate analyses (pooled HR = 3.47, 95% CI 2.57–4.68, P < 0.05). Similar results are shown in Figure 3B: multivariate (pooled HR = 5.28, 95% CI 4.29–6.51, P < 0.05) and univariate analyses (pooled HR = 7.23, 95% CI 4.93–10.59, P < 0.05) both indicate that high expression of miRNA are correlated with poor prognosis in osteosarcoma. Additionally, as shown in Figure 4, the level of expression of serum miRNAs is closely correlated with distant metastasis (pooled HR = 3.30, 95% CI 2.77–3.94, P < 0.05), and clinical stage (pooled HR = 3.48, 95% CI 2.91–4.15, P < 0.05) in osteosarcoma.
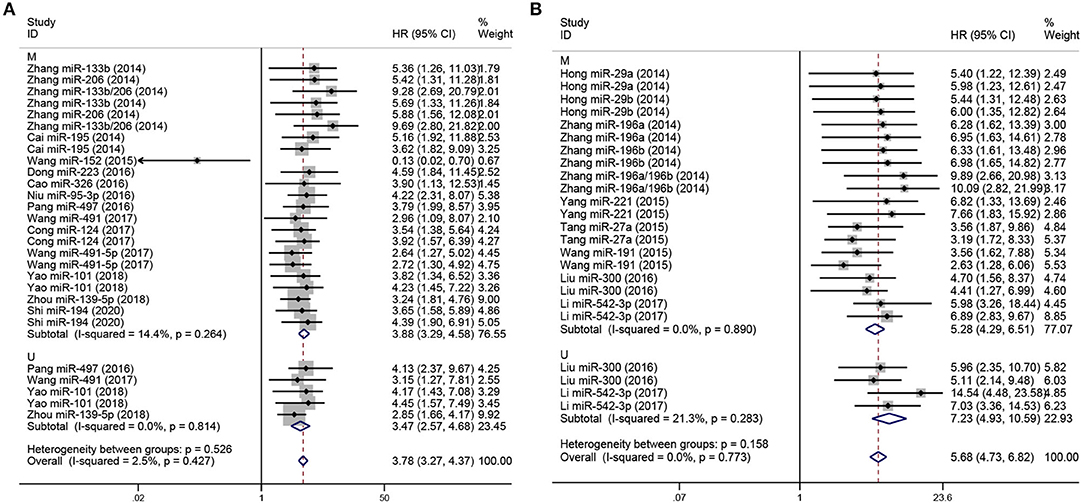
Figure 3. Forest plot for miRNA expression and prognosis of osteosarcoma, stratified by analysis method. (A) Low-expression miRNAs correlated with poor prognosis in osteosarcoma, stratified by analysis method (M and U). (B) High-expression miRNAs with poor prognosis in osteosarcoma, stratified by analysis method (M and U). The p-values for heterogeneity of HR by subgroup, and overall, are shown. M, Multivariate analysis; U, Univariate analysis.
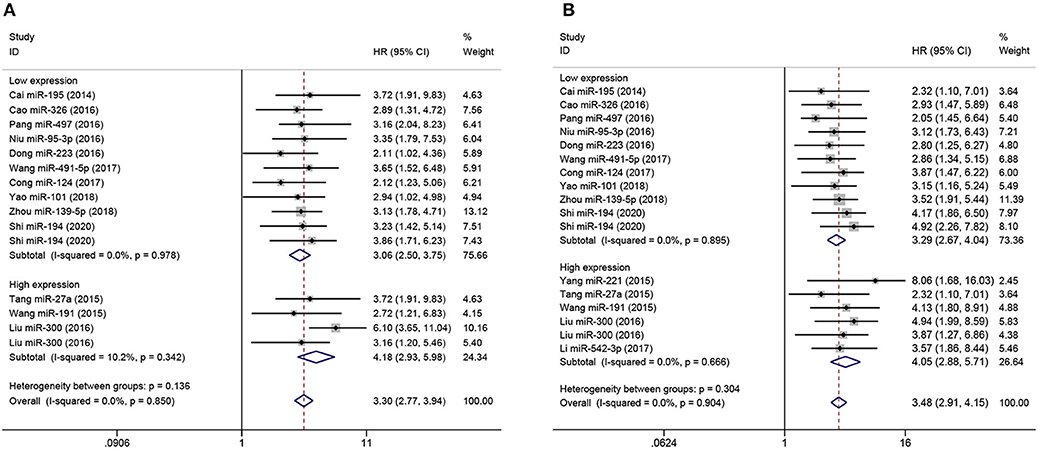
Figure 4. Forest plot of the correlation between metastasis, clinical stage, and miRNA expression in studies with poor overall survival. (A) Correlation between miRNA level and tumor metastasis in osteosarcoma with poor overall survival, stratified by miRNAs expression (high-expression miRNA or low-expression miRNA). (B) Correlation between miRNA level and clinical stage in osteosarcoma with poor overall survival, stratified by miRNA expression (high-expression miRNA or low-expression miRNA).
The P-values for Begg's tests of low-expression miRNAs and high-expression miRNAs were 0.028 and 0.602, respectively, and the corresponding P-values for Egger's tests were 0.544 and 0.283. Furthermore, the funnel plots of Begg's and Egger's are all symmetrical demonstrating that there is no significant publication bias in this research (Figure 5). Sensitivity analyses revealed that none of the studies were outliers, suggesting that the pooled results of this research are credible.
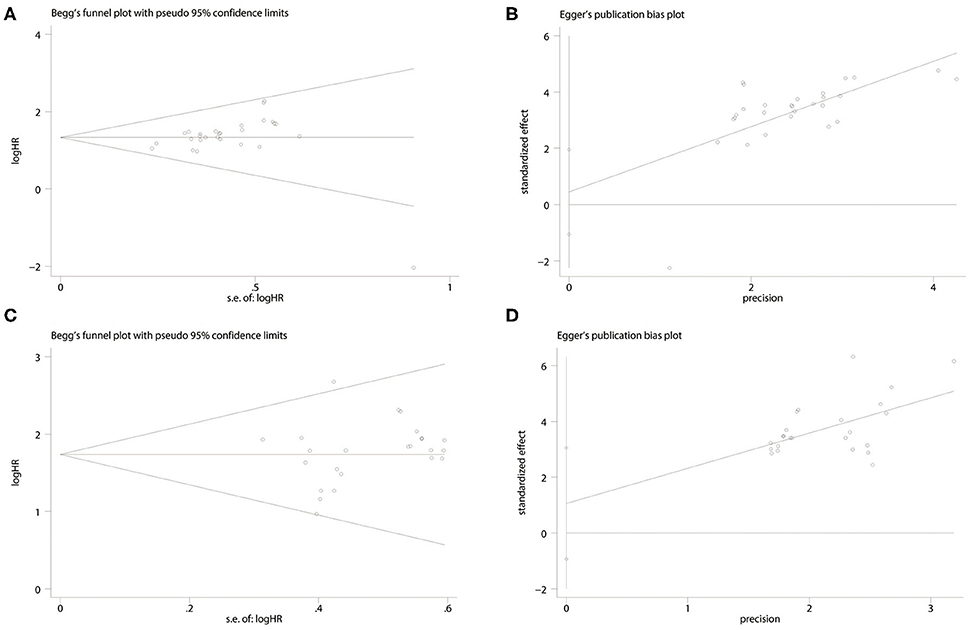
Figure 5. Forest plot of the publication bias. (A) Begg's funnel plot of publication bias for the association between miRNA low expression and poor prognosis. (B) Egger's test of publication bias for the association between miRNA low expression and poor prognosis. (C) Begg's funnel plot of publication bias for the association between miRNA high expression and poor prognosis. (D) Egger's test of publication bias for the association between miRNA high expression and poor prognosis.
Although osteosarcoma is the common malignant bone tumor (Kansara et al., 2014) and extensive progress has been made in the development of effective therapies (Bielack et al., 2002; Collins et al., 2013), patient prognosis remains unsatisfactory. Therefore, for improved treatment, management, and patient prognosis in osteosarcoma, the identification of novel prognostic biomarkers is critical. The potential utility of circulating miRNAs as non-invasive biomarkers has been demonstrated in several types of cancers (Zhou et al., 2016; Tan et al., 2019). Furthermore, blood testing is more easily accepted by patients than other invasive tests. Therefore, we conducted a meta-analysis of studies investigating the prognostic capacity of serum miRNAs in osteosarcoma. A more detailed subgroup analysis was also conducted to further examine the association between analysis methods, clinical stage, metastasis, and miRNA expression level in osteosarcoma.
We investigated 20 studies on 23 different miRNAs in osteosarcoma in this meta-analysis; these included 9 highly expressed miRNAs (miRNA-29a, miRNA-29b, miRNA-196a, miRNA-196b, miRNA-221, miRNA-27a, miRNA-191, miRNA-542-3p, and miRNA-300) (Hong et al., 2014; Zhang et al., 2014a; Tang et al., 2015; Wang T. et al., 2015; Yang et al., 2015; Li et al., 2018), and 14 miRNAs with low expression (miRNA-195, miRNA-223, miRNA-497, miRNA-491, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-326, miRNA-133b, miRNA-206, miRNA-152, miRNA-95-3p, and miRNA-491-5p, miR-194) (Cai et al., 2015; Cao et al., 2016; Dong et al., 2016; Pang et al., 2016; Wang S. N. et al., 2017; Cong et al., 2018; Yao et al., 2018; Zhou et al., 2018; Shi et al., 2020). A previous meta-analysis has reported that aberrant expression of miRNAs, in terms of both elevated and downregulated expression, are associated with poor prognosis in osteosarcoma (Cheng et al., 2017). Similar to the above findings, our data confirm the observation that serum miRNAs with aberrantly elevated or downregulated levels of expression are strongly correlated with poor prognosis for osteosarcoma. Among osteosarcoma patients, high expression of serum miRNAs (miRNA-196a and miRNA-196b) and combined expression of miRNA-196a/miRNA-196b were independent prognostic factors for OS and DFS (Zhang et al., 2014a). Further, Frampton et al. (2014) reported that miRNA-21, miRNA-23a, and miRNA-27a are highly expressed in pancreatic tumor, and their combination could serve as a prognostic biomarker in this tumor. The above results suggest that the development of an optimal panel of miRNA expression would be useful biomarker to improve prognosis in osteosarcoma.
The levels of miRNA-27a, miRNA-191, miRNA-195, miRNA-497, miRNA-223, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-326, miRNA-95-3p, miRNA-491-5p, and miRNA-300, miRNA-194 (Cai et al., 2015; Tang et al., 2015; Wang T. et al., 2015; Cao et al., 2016; Dong et al., 2016; Liu et al., 2016; Niu et al., 2016; Pang et al., 2016; Wang Z. et al., 2017; Cong et al., 2018; Yao et al., 2018; Zhou et al., 2018; Shi et al., 2020) are potentially associated with clinical stage as well as the tumor metastasis in osteosarcoma. miRNA-27a, miRNA-191, and miRNA-300 are highly expressed in osteosarcoma, while miRNA-195, miRNA-497, miRNA-223, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-326, miRNA-95-3p, miRNA-194, and miRNA-491-5p are expressed at low levels. In particular, the high expression of miR-191 may affect cancer progression through various pathways, and is associated with therapeutic outcomes or poor prognosis in cancers (Elyakim et al., 2010; Li et al., 2017). As such, miR-27a and miR-191 serve as regulatory factors in osteosarcoma. Low expression of miR-195 may act as a regulator in hepatocellular carcinoma cells, with potential utility in cancer therapy (Xu et al., 2009). In addition, low expression of serum miR-497 may be associated with tumor development (Kong et al., 2015). miR-124 was found to be expressed at low levels and correlated with invasion and metastasis in hepatocellular carcinoma (HCC), which may predict poor prognosis (Zheng et al., 2012). Low expression of miR-101 has been shown to inhibit the progression of bladder transitional cell carcinoma, and may serve as a tumor suppressor gene in this disease (Friedman et al., 2009). miR-139-5p has been shown to be expressed at low levels, and is considered to act as a regulator of cell proliferation, metastasis, apoptosis, and the cell cycle (Zhang et al., 2014c). In glioblastomas, miR-326 shows low expression and acts as a tumor suppressor (Nawaz et al., 2016). These results demonstrate, at a molecular level, that miRNAs have diagnostic or prognostic utility that could be extended to other tumors. miR-195, miR-497, miR-223, miR-124, miR-101, miR-139-5p, and miR-326 act as tumor suppressors in cancers, which is consistent with the results obtained in our study. Therefore, the conclusions that high-expression or low-expression miRNAs are potential novel biomarkers for predicting prognosis in osteosarcoma are reliable.
However, this study has some limitations. All relevant publications may not have been included in the databases, and specific subgroup analyses showed mild heterogeneity. HRs and RRs were merged into HRs in the included literature, potentially leading to slight logical errors, finally the included studies' population limited to Chinese. Despite these limitations, this study suggests that miRNAs have potential utility as novel prognostic markers in osteosarcoma. Further investigation of the dynamic expressional profile of miRNAs during the entire course of the development and treatment of osteosarcoma is needed for clinical implementation of miRNAs as biomarkers in either diagnosis or prognosis.
Despite these limitations, the results of this study suggest that serum miRNAs represent excellent biomarkers of prognosis in osteosarcoma. We conclude that miRNAs in this systematic review with aberrantly low or high expression are indicative of poor prognosis in osteosarcoma. Further, the expression of miRNAs(miRNA-195, miRNA-27a, miRNA-191, miRNA-300, miRNA-326, miRNA-497, miRNA-95-3p, miRNA-223, miRNA-491-5p, miRNA-124, miRNA-101, miRNA-139-5p, miRNA-194) is correlated with clinical stage and tumor metastasis in this disease. However, the implementation of miRNAs as biomarkers for monitoring cancer progression, clinical stage and distant metastasis and guiding therapeutic interventions improving poor prognosis. Future studies should aim to address these critical aspects.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
HL designed the study and collected data. HL and PW drafted the manuscript. PW, HY, JS, LD, XW, and CS contributed to the writing. JZ and JL contributed to the writing and review of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Science and Technology Major Project of China (No. 2018ZX10302205), the Major Project of Science and Technology in Henan Province (No. 161100311400), the Program of Natural Science Foundation of Henan Province (No. 182300410009), and the Major Project of TCM research in Henan Province (No. 2018ZYZD01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors gratefully acknowledge JZ (Henan Academy of Medical and Pharmaceutical Sciences, Zhengzhou University) for providing financial support and language help, and JL (Laboratory of Molecular Biology, Henan Luoyang Orthopedic Hospital) for providing assistance with language and writing of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00789/full#supplementary-material
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi: 10.2307/2533446
Bielack, S. S., Kempf-Bielack, B., Delling, G., Exner, G. U., Flege, S., Helmke, K., et al. (2002). Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 20, 776–790. doi: 10.1200/jco.2002.20.3.776
Cai, H., Zhao, H., Tang, J., and Wu, H. (2015). Serum miR-195 is a diagnostic and prognostic marker for osteosarcoma. J. Surg. Res. 194, 505–510. doi: 10.1016/j.jss.2014.11.025
Calin, G. A., and Croce, C. M. (2006). MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 66, 7390–7394. doi: 10.1158/0008-5472.Can-06-0800
Cao, L., Wang, J., and Wang, P. Q. (2016). MiR-326 is a diagnostic biomarker and regulates cell survival and apoptosis by targeting Bcl-2 in osteosarcoma. Biomed. Pharmacother. 84, 828–835. doi: 10.1016/j.biopha.2016.10.008
Cheng, D., Qiu, X., Zhuang, M., Zhu, C., Zou, H., and Liu, Z. (2017). MicroRNAs with prognostic significance in osteosarcoma: a systemic review and meta-analysis. Oncotarget 8, 81062–81074. doi: 10.18632/oncotarget.19009
Collins, M., Wilhelm, M., Conyers, R., Herschtal, A., Whelan, J., Bielack, S., et al. (2013). Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysis. J. Clin. Oncol. 31, 2303–2312. doi: 10.1200/jco.2012.43.8598
Cong, C., Wang, W., Tian, J., Gao, T., Zheng, W., and Zhou, C. (2018). Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark. Sect. A Dis. Mark. 21, 449–454. doi: 10.3233/CBM-170672
Dong, J., Liu, Y., Liao, W., Liu, R., Shi, P., and Wang, L. (2016). miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J. Bone Oncol. 5, 74–79. doi: 10.1016/j.jbo.2016.05.001
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Elyakim, E., Sitbon, E., Faerman, A., Tabak, S., Montia, E., Belanis, L., et al. (2010). hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 70, 8077–8087. doi: 10.1158/0008-5472.Can-10-1313
Esquela-Kerscher, A., and Slack, F. J. (2006). Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269. doi: 10.1038/nrc1840
Filipowicz, W., Bhattacharyya, S. N., and Sonenberg, N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. doi: 10.1038/nrg2290
Frampton, A. E., Castellano, L., Colombo, T., Giovannetti, E., Krell, J., Jacob, J., et al. (2014). MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology 146, 268–277. doi: 10.1053/j.gastro.2013.10.010
Friedman, J. M., Liang, G., Liu, C. C., Wolff, E. M., Tsai, Y. C., Ye, W., et al. (2009). The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 69, 2623–2629. doi: 10.1158/0008-5472.Can-08-3114
Gu, J., Li, J., Huang, M., Zhang, Z., Li, D., Song, G., et al. (2014). Identification of osteosarcoma-related specific proteins in serum samples using surface-enhanced laser desorption/ionization-time-of-flight mass spectrometry. J. Immunol. Res. 2014:649075. doi: 10.1155/2014/649075
Hayashita, Y., Osada, H., Tatematsu, Y., Yamada, H., Yanagisawa, K., Tomida, S., et al. (2005). A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 65, 9628–9632. doi: 10.1158/0008-5472.Can-05-2352
He, L., Thomson, J. M., Hemann, M. T., Hernando-Monge, E., Mu, D., Goodson, S., et al. (2005). A microRNA polycistron as a potential human oncogene. Nature 435, 828–833. doi: 10.1038/nature03552
Hong, Q., Fang, J., Pang, Y., and Zheng, J. (2014). Prognostic value of the microRNA-29 family in patients with primary osteosarcomas. Med. Oncol. 31:37. doi: 10.1007/s12032-014-0037-1
Jones, K. B., Salah, Z., Del Mare, S., Galasso, M., Gaudio, E., Nuovo, G. J., et al. (2012). miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 72, 1865–1877. doi: 10.1158/0008-5472.Can-11-2663
Kansara, M., Teng, M. W., Smyth, M. J., and Thomas, D. M. (2014). Translational biology of osteosarcoma. Nat. Rev. Cancer 14, 722–735. doi: 10.1038/nrc3838
Kent, O. A., and Mendell, J. T. (2006). A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene 25, 6188–6196. doi: 10.1038/sj.onc.1209913
Kim, Y. H., Goh, T. S., Lee, C. S., Oh, S. O., Kim, J. I., Jeung, S. H., et al. (2017). Prognostic value of microRNAs in osteosarcoma: a meta-analysis. Oncotarget 8, 8726–8737. doi: 10.18632/oncotarget.14429
Kong, X. J., Duan, L. J., Qian, X. Q., Xu, D., Liu, H. L., Zhu, Y. J., et al. (2015). Tumor-suppressive microRNA-497 targets IKKbeta to regulate NF-kappaB signaling pathway in human prostate cancer cells. Am. J. Cancer Res. 5, 1795–1804.
Li, H., Zhou, Z. Q., Yang, Z. R., Tong, D. N., Guan, J., Shi, B. J., et al. (2017). MicroRNA-191 acts as a tumor promoter by modulating the TET1-p53 pathway in intrahepatic cholangiocarcinoma. Hepatology 66, 136–151. doi: 10.1002/hep.29116
Li, Q., Song, S., Ni, G., Li, Y., and Wang, X. (2018). Serum miR-542-3p as a prognostic biomarker in osteosarcoma. Cancer Biomark. Sect. A Dis. Mark. 21, 521–526. doi: 10.3233/cbm-170255
Liu, J. D., Xin, Q., Tao, C. S., Sun, P. F., Xu, P., Wu, B., et al. (2016). Serum miR-300 as a diagnostic and prognostic biomarker in osteosarcoma. Oncol. Lett. 12, 3912–3918. doi: 10.3892/ol.2016.5214
Mitchell, P. S., Parkin, R. K., Kroh, E. M., Fritz, B. R., Wyman, S. K., Pogosova-Agadjanyan, E. L., et al. (2008). Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U.S.A. 105, 10513–10518. doi: 10.1073/pnas.0804549105
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Nawaz, Z., Patil, V., Paul, Y., Hegde, A. S., Arivazhagan, A., Santosh, V., et al. (2016). PI3 kinase pathway regulated miRNome in glioblastoma: identification of miR-326 as a tumour suppressor miRNA. Mol. Cancer 15:74. doi: 10.1186/s12943-016-0557-8
Niu, J., Sun, Y., Guo, Q., Niu, D., and Liu, B. (2016). Serum miR-95-3p is a diagnostic and prognostic marker for osteosarcoma. SpringerPlus 5:1947. doi: 10.1186/s40064-016-3640-0
Ogawa, K., Seki, T., Onji, T., Adachi, N., Tanaka, S., Hide, I., et al. (2013). Mutant gammaPKC that causes spinocerebellar ataxia type 14 upregulates Hsp70, which protects cells from the mutant's cytotoxicity. Biochem. Biophys. Res. Commun. 440, 25–30. doi: 10.1016/j.bbrc.2013.09.013
Pang, P. C., Shi, X. Y., Huang, W. L., and Sun, K. (2016). miR-497 as a potential serum biomarker for the diagnosis and prognosis of osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 20, 3765–3769.
Shi, L., Xie, C., Zhu, J., and Chen, X. (2020). Downregulation of serum miR-194 predicts poor prognosis in osteosarcoma patients. Ann. Diagn. Pathol. 46:151488. doi: 10.1016/j.anndiagpath.2020.151488
Stang, A. (2010). Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. doi: 10.1001/jama.283.15.2008
Tan, C., Cao, J., Chen, L., Xi, X., Wang, S., Zhu, Y., et al. (2019). Noncoding RNAs Serve as diagnosis and prognosis biomarkers for hepatocellular carcinoma. Clin. Chem. 65, 905–915. doi: 10.1373/clinchem.2018.301150
Tang, J., Zhao, H., Cai, H., and Wu, H. (2015). Diagnostic and prognostic potentials of microRNA-27a in osteosarcoma. Biomed. Pharmacother. 71, 222–226. doi: 10.1016/j.biopha.2015.01.025
Tian, X. P., Huang, W. J., Huang, H. Q., Liu, Y. H., Wang, L., Zhang, X., et al. (2019). Prognostic and predictive value of a microRNA signature in adults with T-cell lymphoblastic lymphoma. Leukemia 33, 2454–2465. doi: 10.1038/s41375-019-0466-0
Wang, N. G., Wang, D. C., Tan, B. Y., Wang, F., and Yuan, Z. N. (2015). Down-regulation of microRNA152 is associated with the diagnosis and prognosis of patients with osteosarcoma. Int. J. Clin. Exp. Pathol. 8, 9314–9319.
Wang, S. N., Luo, S., Liu, C., Piao, Z., Gou, W., Wang, Y., et al. (2017). miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting alphaB-crystallin. Mol. Ther. J. Am. Soc. Gene Ther. 25, 2140–2149. doi: 10.1016/j.ymthe.2017.05.018
Wang, T., Ji, F., Dai, Z., Xie, Y., and Yuan, D. (2015). Increased expression of microRNA-191 as a potential serum biomarker for diagnosis and prognosis in human osteosarcoma. Cancer Biomark. Sect. A Dis. Mark. 15, 543–550. doi: 10.3233/cbm-150493
Wang, Z., Jiang, C., Chang, X., and Dai, Y. (2017). Low miR-491-5p is an unfavorable prognostic marker for osteosarcoma. Int. J. Clin. Exp. Pathol. 10, 3304–3309.
Xu, T., Zhu, Y., Xiong, Y., Ge, Y. Y., Yun, J. P., and Zhuang, S. M. (2009). MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology 50, 113–121. doi: 10.1002/hep.22919
Yang, Z., Zhang, Y., Zhang, X., Zhang, M., Liu, H., Zhang, S., et al. (2015). Serum microRNA-221 functions as a potential diagnostic and prognostic marker for patients with osteosarcoma. Biomed. Pharmacother. 75, 153–158. doi: 10.1016/j.biopha.2015.07.018
Yao, Z. S., Li, C., Liang, D., Jiang, X. B., Tang, J. J., Ye, L. Q., et al. (2018). Diagnostic and prognostic implications of serum miR-101 in osteosarcoma. Cancer Biomark. Sect. A Dis. Mark. 22, 127–133. doi: 10.3233/cbm-171103
Zhang, C., Yao, C., Li, H., Wang, G., and He, X. (2014a). Combined elevation of microRNA-196a and microRNA-196b in sera predicts unfavorable prognosis in patients with osteosarcomas. Int. J. Mol. Sci. 15, 6544–6555. doi: 10.3390/ijms15046544
Zhang, L., Dong, Y., Zhu, N., Tsoi, H., Zhao, Z., Wu, C. W., et al. (2014c). microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol. Cancer 13:124. doi: 10.1186/1476-4598-13-124
Zhang, C., Yao, C., Li, H., Wang, G., and He, X. (2014b). Serum levels of microRNA-133b and microRNA-206 expression predict prognosis in patients with osteosarcoma. Int. J. Clin. Exp. Pathol. 7, 4194–4203.
Zheng, F., Liao, Y. J., Cai, M. Y., Liu, Y. H., Liu, T. H., Chen, S. P., et al. (2012). The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 61, 278–289. doi: 10.1136/gut.2011.239145
Zhou, L., Ma, X., Yue, J., Chen, T., Wang, X. Y., Wang, Z. W., et al. (2018). The diagnostic effect of serum miR-139-5p as an indicator in osteosarcoma. Cancer Biomark. Sect. A Dis. Mark. 23, 561–567. doi: 10.3233/cbm-181744
Keywords: miRNA, osteosarcoma, prognosis, serum, biomarker, meta-analysis
Citation: Luo H, Wang P, Ye H, Shi J, Dai L, Wang X, Song C, Zhang J and Li J (2020) Serum-Derived microRNAs as Prognostic Biomarkers in Osteosarcoma: A Meta-Analysis. Front. Genet. 11:789. doi: 10.3389/fgene.2020.00789
Received: 31 March 2020; Accepted: 02 July 2020;
Published: 11 August 2020.
Edited by:
Dongqing Wei, Shanghai Jiao Tong University, ChinaReviewed by:
Leda Torres, National Institute of Pediatrics, MexicoCopyright © 2020 Luo, Wang, Ye, Shi, Dai, Wang, Song, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianying Zhang, amlhbnlpbmd6aGFuZ0Bob3RtYWlsLmNvbQ==; Jitian Li, aml0aWFubGVlQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.