- 1Key Laboratory of Animal (Poultry) Genetics Breeding and Reproduction, Ministry of Agriculture and Rural Affairs; Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
- 2College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 3College of Food Science and Engineering, Qingdao Agricultural University, Qingdao, China
Dietary threonine (Thr) deficiency increases hepatic triglyceride accumulation in Pekin ducks, which results in fatty liver disease and impairs hepatic function. However, the underlying molecular mechanisms altered by dietary Thr deficiency are still unknown. To identify the underlying molecular changes, 180 one-day-old ducklings were divided into three groups, including Thr deficiency group (Thr-D), Thr sufficiency group (Thr-S), and pair-fed group (Pair-F) that was fed with a Thr-sufficient diet but with reduced daily feed intake. The results showed that feed intake was similar between Thr-D and Pair-F groups, but weight gain rate and final body weight in the Thr-D group were lower than those in the Pair-F group. Feed intake, weight gain, and body weight in Thr-D and Pair-F groups were lower than those in the Thr-S group. The Thr-D diet reduced abdominal fat percentage but increased hepatic triglyceride content when compared with that of the Thr-S and Pair-F groups. The Pair-F reduced hepatic levels of C15:0, C17:0, C18:0, C20:0, C20:4n6, and C22:0 and also reduced total fatty acid, saturated fatty acid, and unsaturated fatty acid content when compared with those of the Thr-D and Thr-S groups. The Thr-D diet increased hepatic content of C6:0, C17:1, C18:3n6, C20:0, C20:1n9, and C22:2, as well as reduced the content of C18:2n6t and C23:0 when compared with those of the Thr-S group. Transcriptome analysis in the liver indicated that the Thr-D diet upregulated genes related to fatty acid and triglyceride synthesis and downregulated genes related to fatty acid oxidation and triglyceride transport. Gene ontology analysis showed that more genes related to lipid metabolism processes and molecular function were differentially expressed in the Thr-D group relative to Thr-S and Pair-F groups than in the Pair-F group relative to the Thr-S group. KEGG pathway analysis showed that differentially expressed genes were enriched in signal transduction, immune, hormone, lipid, and amino acid metabolism pathways. Our findings indicated that the Thr-D diet increased hepatic triglyceride and fatty acid accumulation via increasing fatty acid and triglyceride synthesis and reducing fatty acid oxidation and triglyceride transport. These findings provide novel insights into our understanding of the molecular mechanisms underlying fat accumulation in the liver caused by dietary threonine deficiency.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an increasingly prevalent disease worldwide that leads to chronic hepatic injury (Loomba and Sanyal, 2013). It is estimated that NAFLD affects approximately 25% of the world population (Lazo and Clark, 2008; Rector et al., 2008). Recently, the occurrence of NAFLD has been increasing in adolescents as the prevalence of childhood obesity rises (Janssen et al., 2005; Park et al., 2005). Lipid accumulation in the liver of people who drink little or no alcohol is the main characteristic of NAFLD. Such a fatty liver is more susceptible to inflammatory cytokines and oxidative stress (Browning and Horton, 2004; Cohen et al., 2011) and may gradually develop into steatohepatitis, liver fibrosis, and hepatocellular carcinoma (Chalasani et al., 2012).
Threonine (Thr) is an essential amino acid for humans and poultry (Kidd and Kerr, 1996) and plays important roles in various metabolic processes, such as hormone secretion and immune defense. Its importance is highlighted by the adverse effects of Thr deficiency in experimental animals, including anorexia, impaired immunity, excessive energy expenditure, and growth retardation (Ross-Inta et al., 2009; Xie et al., 2014; Jiang et al., 2016; Zhang et al., 2016). For example, dietary Thr deficiency reduces the food intake of poultry (Xie et al., 2014; Zhang et al., 2014; Jiang et al., 2016; Zhang et al., 2017), whereas such a reduced feeding leads to low fat deposition in young growing cattle (Basarab et al., 2003). Moreover, a threonine-deficient diet elicits lipid accumulation in the liver of rats (Methfessel et al., 1964). In broilers, dietary Thr deficiency increases fat content in breast muscle and whole body (Rangel-Lugo et al., 1994; Ciftci and Ceylan, 2004). More recently, we demonstrated that dietary Thr deficiency increases hepatic lipid deposition and reduces abdominal fat levels in ducks (Jiang et al., 2017).
Hepatic lipid accumulation is a complex process, and the role of Thr in it remains elusive. It is speculated that Thr deficiency increases hepatic lipid deposition by changing lipid catabolic and transport pathways, as well as promoting lipid synthesis (Methfessel et al., 1964). Indeed, we have shown that the expression of several hepatic genes related to lipid uptake, fatty acid synthesis, β-oxidation, ketogenesis, and triglyceride transport is affected by dietary Thr levels in Pekin ducks (Jiang et al., 2019). Nevertheless, the biochemical mechanisms whereby Thr deficiency causes hepatic lipid accumulation in ducks are still unclear.
Therefore, the present study was conducted to investigate the effects of dietary Thr deficiency and feed restriction on the growth performance, plasma parameters, hepatic lipid content, and hepatic fatty acid composition. Moreover, we also assessed the expression of hepatic genes by transcriptome analysis to explore potential pathways whereby Thr deficiency causes triglyceride accumulation in the liver of Pekin ducks. Noteworthy, because the reduced food intake associated with Thr deficiency (Jiang et al., 2017) may affect hepatic lipid levels, it is necessary to compensate for this effect by designing a paired fed group. We used such a group in this study, whose feed consumption was similar between animals fed with Thr-sufficient and Thr-deficient diets.
Materials and Methods
Animals and Experimental Design
A completely randomized design with single factorial arrangement of treatments was used in this experiment. Dietary treatments included a Thr deficiency diet (Thr-D), a Thr sufficiency diet (Thr-S), supplemented with 0.21% (w/w) crystal Thr to basal diet as fed, and a paired fed group (Pair-F), which was fed with Thr sufficiency diet maintaining a similar daily feed intake to that of the Thr-D group by feed restriction. Thus, there were a total of three different treatments (Thr-D, Thr-S, and Pair-F).
One-day-old male Pekin ducks (Pekin Duck Breeding Centre of the Institute of Animal Science), 180 individuals, were randomly assigned to one of the three treatments composed of six replicate cages with 10 ducklings per cage according to average body weight (57.1 ± 3.01 g). All ducks were handled in accordance with the Pekin duck management guidelines. The birds were housed in raised wire floor pens (200 × 100 × 40 cm) with nipple drinkers and tubular feeders and maintained under constant light. The temperature was kept at 30°C from 1 to 3 days of age and then gradually reduced to 25°C until 21 days of age. Feed pellets and water were offered ad libitum.
The basal corn–wheat–peanut meal diet (Supplementary Table S1) was formulated to meet or exceed the current National Research Council recommendation (NRC, 1994), except for Thr concentration. First, a single batch of basal diet was mixed and then divided into two aliquots according to the experimental treatments. According to previous study (Zhang et al., 2014), each sublot was mixed with 0.21% crystalline Thr or cornstarch, which was used to replace crystalline Thr and maintain dietary characteristics. Dietary Thr concentrations in Thr-D and Thr-S diets were 0.53% (w/w) and 0.73% (w/w), respectively. Weight and feed intake per cage were measured weekly to calculate the daily body weight gain, daily feed intake, and gain:feed ratio from day 1 to day 21. All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS20160322, IAS-CAAS, Beijing, China) and performed in accordance with the guidelines. Ethical approval on animal survival was given by the animal ethics committee of IAS-CAAS.
Sample Collection and Preparation
At the 21st day of age, ducks were fasted for 12 h and weighed individually to calculate the average body weight of animals in each cage for each treatment. Then, three birds whose weights were close to the average body weight of animals in each cage were selected. Blood samples were collected by jugular vein puncture with heparinized syringes equipped with stainless steel needles. The plasma was separated by centrifugation and stored at -20°C until analysis. Then, the selected birds were killed by cervical dislocation, and left liver samples were collected. A liver sample from the same location was frozen in liquid nitrogen for analysis of gene expression, and the left sample was frozen at -20°C for analyses of total lipids, cholesterol, total triglycerides, and fatty acid compositions. The three duck samples were pooled into one sample with same volume or weight in individual cage, and then, pooled samples were used for liver lipid analysis, fatty acid analysis, gene expression analysis. Other three birds were selected from each cage to collect breast muscle, thigh muscle, abdominal fat, and liver; these organs were weighed to calculate their weight in relation to the whole body. Finally, other three birds from each group were killed by cervical dislocation, and liver samples were collected and frozen in liquid nitrogen for transcriptome analysis.
Dietary Protein and Amino Acids
Dietary protein levels were determined according to the Kjeldahl method (Thiex et al., 2002). The amino acid concentrations in diets were measured with an amino acid analyzer (L-800; Hitachi, Tokyo, Japan) after hydrolysis in 6-M HCl for 24 h at 110°C. Dietary tryptophan was determined according to the method of Official Journal of the European Communities (EU2000/45/EC).
Plasma Parameters
The levels of plasma parameters, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), glucose, total triglycerides (TG), total cholesterol (CHO), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC), were measured using an automatic analyzer (Hitachi 7080, Tokyo, Japan) with commercial kit (Maccura, Sichuang, China).
Liver Lipids
Total lipids were extracted by homogenizing minced liver tissue samples in chloroform–methanol (2:1, v/v) as described previously (Folch et al., 1957). The extracts were evaporated under a stream of nitrogen, weighed, and resuspended in chloroform–methanol (2:1) containing 0.01% (w/v) butylated hydroxytoluene. The concentrations of TG and CHO were measured using commercial kits (BioSino Bio-technology and Science Inc, Beijing, China).
Fatty Acids Composition
Liver samples were prepared according to a previously described method (Yang et al., 2010a). Briefly, fatty acids were extracted from total lipids and methylated by adding 1-ml acetyl chloride/methanol (1:10, v/v) and 20 μl of 5 mg/ml nonadecanoic acid (used as internal standard) for 4 h at 80°C. Then 1 ml of hexane and 1.5 ml of 6% (w/v) K2CO3 were added into the tube. After shaking for 10 min, 400 μl of the hexane layer was transferred to a new injection vial after centrifugation for 10 min at 3,000×g for further gas chromatography–mass spectrometry (GC-MS) analysis. The fatty acid methyl esters were separated by GC (Agilent 6890, Agilent Technologies, Santa Clara, CA, USA) using a DB 23 capillary column (60 m × 0.25 mm × 0.25 μm, Agilent Technologies, Santa Clara, USA) with MS detection (5970C, Agilent Technologies, Santa Clara, CA, USA). Samples (1 μl) were injected using an autosampler. The oven was programmed as follows: 50°C for 1 min, ramp to 175°C at 25°C/min holding 3 min, ramp to 200°C at 3.5°C/min holding 3 min, and finally ramp to 230°C at 2°C/min holding 3 min with helium as the carrier gas at a split ratio of 1:50. The GC was operated at constant flow pressure of 33.357 kPa, and the injector temperature, transfer line, and ion source were 250, 250, and 230°C, respectively. Peaks were identified by comparing retention times with those of the corresponding standards (Sigma Aldrich, Saint Louis, MO, USA).
RNA Isolation
Total RNA was extracted from frozen liver samples with RNAiso Plus reagent (code no. 9109, Takara, Dalian, China) according to the manufacturer’s instructions. For quantitative polymerase chain reaction (qPCR) analysis, RNA concentration was measured at 260 nm (NanoDrop™ 2000, Thermo Fisher Scientific, Waltham, MA, USA), and the RNA integrity was evaluated by agarose gel electrophoresis stained with GelRed® (Biotium, Fremont, CA, USA). The mRNA integrity and concentration of samples used in the transcriptome analyses were evaluated with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Transcriptomic Analysis
Whole transcriptomic expression profiles of hepatic RNA samples (n = 3) from each group were analyzed using a HiSeq X Ten system (Illumina, San Diego, CA, USA). STAR and edgeR were used to quantify transcriptomic data as previously described for differential gene expression within RNA samples (Robinson et al., 2010; Dobin et al., 2013). Changes in expression of genes affected by Thr deficiency were further confirmed by qPCR.
Pathway Analysis With Gene Ontology and Kyoto Encyclopedia Genes and Genomes (KEGG)
Differentially expressed genes (|log2Fold change| > 0.585, P < 0.05) in liver were converted to their FASTA Protein Sequence, and gene enrichment was performed with the FASTA Protein Sequences by “Gene-list Enrichment” in Kobas 3.0 (http://kobas.cbi.pku.edu.cn/) (Wu et al., 2006; Xie et al., 2011). Homo sapiens was selected as the reference species, and hypergeometric test/Fisher’s exact test was applied as statistical method.
qPCR
RNA samples were reverse transcribed to cDNA with the use of PrimerScript™ RT Master Mix (Code No. RR036A, Takara, Dalian, China) following the manufacturer’s instructions. qPCR analysis was performed using real-time PCR quantitative analysis in the fluorescence detection system (ABI Q7, Life Technologies, Shanghai, China) with Power Green Master Mix (Code No.4367659, Life Technologies, NY, USA). Primers used for amplification are listed in Supplementary Table S2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping reference gene to normalize the expression of the targeted genes (Vandesompele et al., 2002). Each specimen was measured independently in triplicate, and PCR amplification efficiency was close to 100%. Relative mRNA expression of target genes was calculated using the 2-∆∆ct method as previously reported (Livak and Schmittgen, 2001).
Statistical Analyses
Data were subjected to one-way ANOVA using general linear model procedure of SAS (version 9.2; SAS Institute Inc). Differences among means were tested by Duncan’s method. Data are expressed as mean and standard error of the mean (SEM). The level of statistical significance was set at P < 0.05.
Results
Growth Performance
Although the feed intake of ducks in the Pair-F group was assured to be similar to that of the Thr-D group by feed restriction, Thr intake, body weight, and weight gain in the Thr-D group were lower (P < 0.0001) than those in the Pair-F group (Table 1). Both Thr-D and Pair-F diets reduced (P < 0.0001) body weight and weight gain of ducks compared with that of the Thr-S group. The gain/feed ratio was lower in the Thr-D group than that in the Pair-F group (P < 0.03).
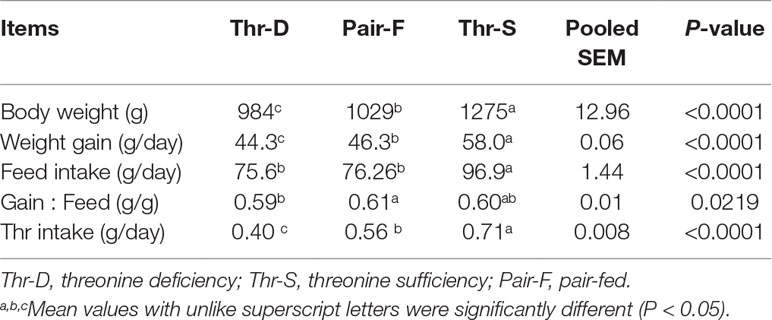
Table 1 Effects of threonine deficiency and feed restriction on growth performance of ducks from 1 to 21 days of age.
Carcass Traits
Both Thr-D and Pair-F diets reduced the relative weights of breast muscle (P < 0.0001) and abdominal fat (P < 0.006) when compared to those of the Thr-S. These treatments had no effect (P > 0.05) on thigh muscle relative weight (Table 2). There were no differences in the relative weights of breast muscle and abdominal fat (P > 0.05) between Thr-D and Pair-F groups.
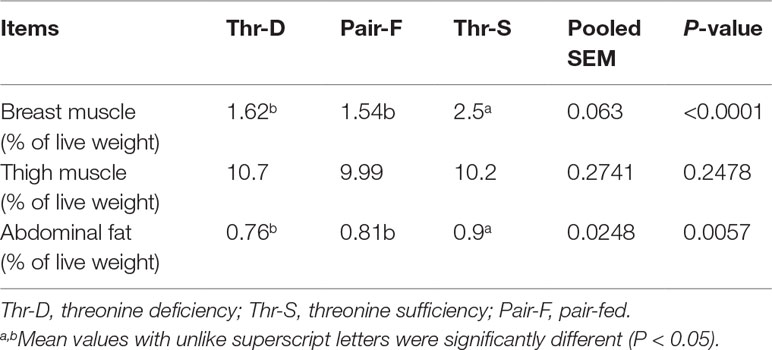
Table 2 Effects of threonine deficiency and feed restriction on carcass traits of ducks at 21 day of age.
Hepatic Lipid Accumulation
The Pair-F diet increased the relative weight of liver tissues compared with those of Thr-D and Thr-S (P < 0.0001), but there was no difference in the relative weight of liver between Thr-D and Thr-S (Table 3, P > 0.05). The Thr-D diet increased hepatic triglyceride concentration in Pekin ducks (P < 0.05) compared with those of the Thr-S and Pair-F groups. However, Thr-D and Pair-F treatments did not affect (P > 0.05) total lipids and cholesterol concentrations in liver.
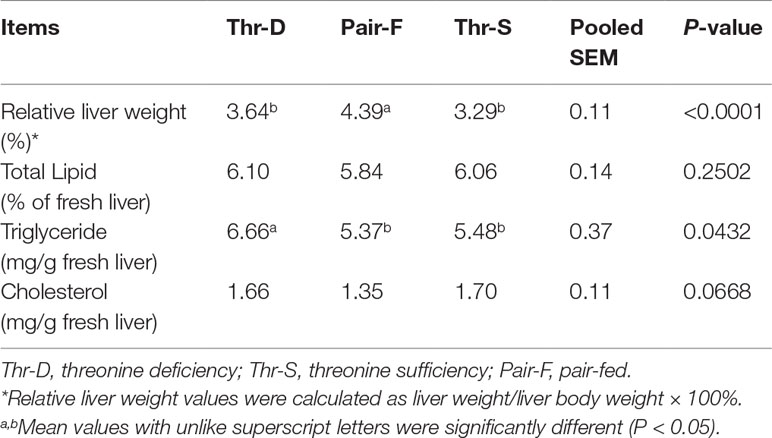
Table 3 Effects of threonine deficiency and feed restriction on liver lipids of ducks at 21 day of age.
Plasma Parameters
The Pair-F treatment elevated (P < 0.02) the glucose and HDLC concentrations and decreased LDLC (P < 0.001) levels compared with Thr-D and Thr-S diets (Table 4). Thr-D increased (P < 0.001) plasma HDLC concentration compared with the Thr-S diet, but its level was still lower than (P < 0.05) that in the Pair-F group. The Thr-D diet did not affect (P > 0.05) glucose and LDLC concentration compared with Thr-S group diet. Moreover, there were no significant differences in the plasma activities of ALT, AST, and ALP, or the plasma concentrations of CHO and TG between any groups.
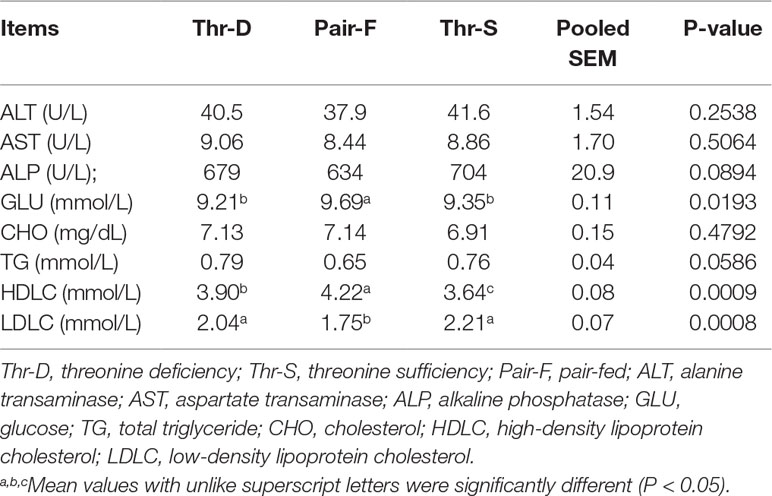
Table 4 Effects of threonine deficiency and feed restriction on plasma parameters of ducks at 21 day of age.
Liver Fatty acid composition
The Thr-D diet increased the hepatic contents of C6:0, C17:1, C18:3n6, C20:0, C20:1n9, and C22:2 and reduced the contents of C18:2n6t and C23:0 compared with the Thr-S diet (Table 5). However, there were no differences in total contents of fatty acid (TFA), saturated fatty acid (SFA), unsaturated fatty acid (USFA), and polyunsaturated fatty acid (PUFA) between Thr-D and Thr-S groups. The Pair-F diet increased the hepatic content of C14:0 and reduced the contents of C15:0, C17:0, C18:0, C20:0, C20:4n6, and C22:0 compared with those of Thr-D and Thr-S groups (P < 0.05). Moreover, the Pair-F group had lower total contents of fatty acid (TFA), saturated fatty acid (SFA), unsaturated fatty acid (USFA), and polyunsaturated fatty acid (PUFA) than those of Thr-D and Thr-S groups.
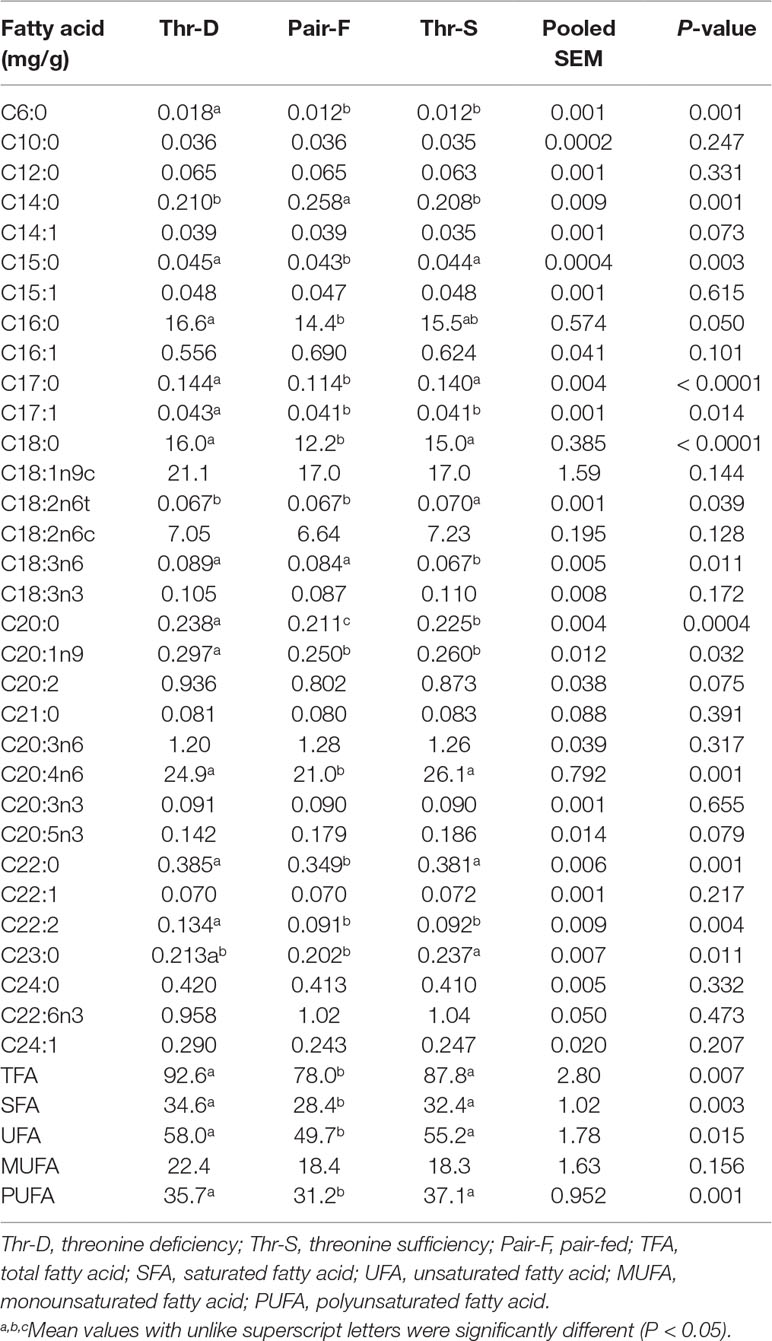
Table 5 Effects of threonine deficiency and feed restriction on hepatic fatty acid composition ducks at 21 day of age.
Hepatic Transcriptome Analysis
To screen differentially expressed genes in liver changed by Thr-D and Pair-F diets, a transcriptome analysis was performed in three individual duck livers from the different groups (Thr-D, Thr-S, and Pair-F). The differential genes were identified using t-test in EdgeR software (|Log2Fold change| > 0.585, P value < 0.05). Compared with the Thr-S group, Thr-D-fed animals had 1,125 differentially expressed genes: 488 were upregulated and 637 were downregulated (Supplementary Table 3A, Figure 1), and Pair-F-fed animals had 1,028 differentially expressed genes: 214 were upregulated and 814 were downregulated (Supplementary Table 3B, Figure 1). Compared with the Pair-F group, 948 genes were differentially expressed in the Thr-D group: 622 were upregulated and 326 were downregulated (Supplementary Table 3C, Figure 1).
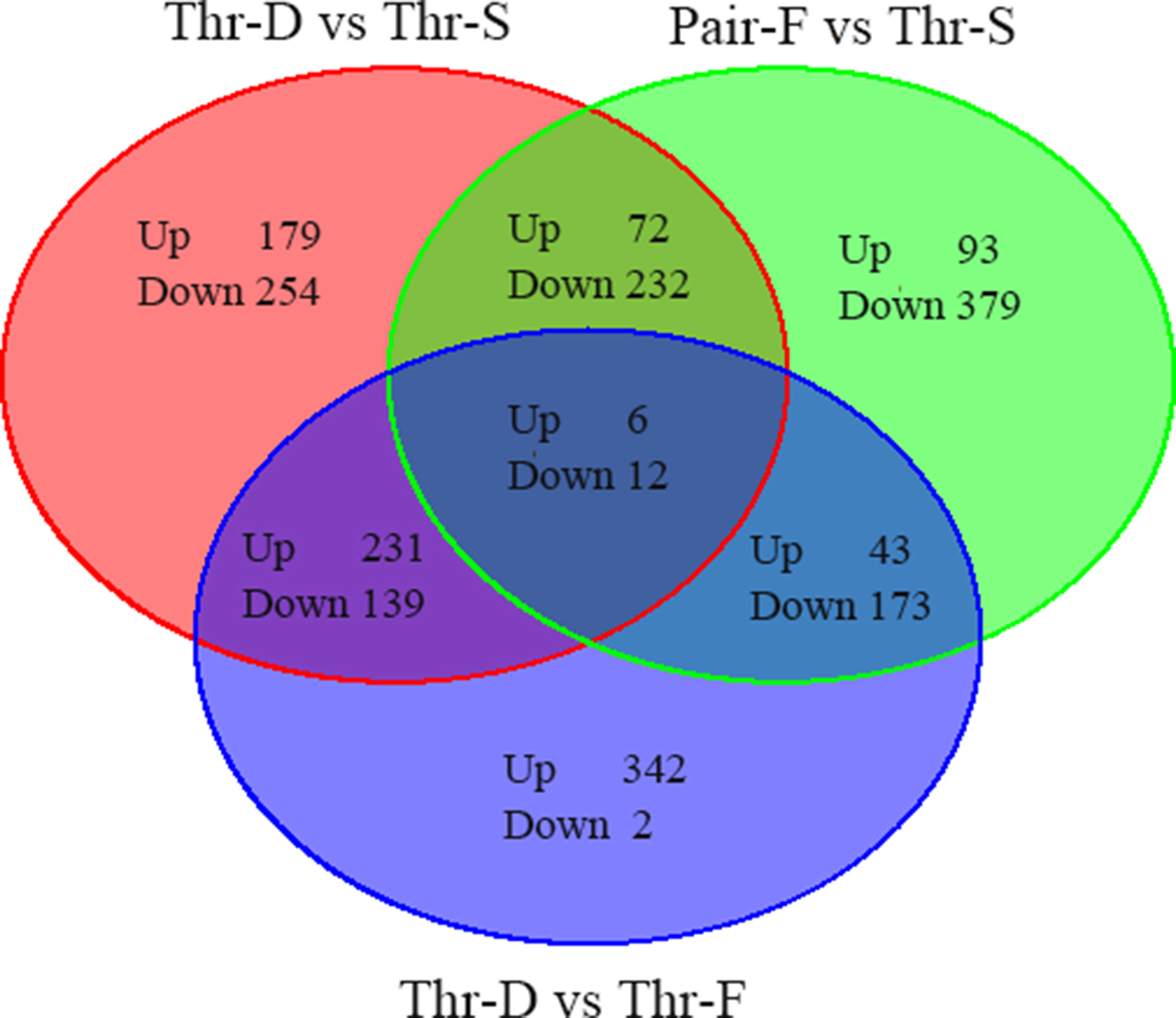
Figure 1 The total differentially expressed genes between groups (Thr-D vs Thr-S, Thr-S vs Pair-F, Pair-F vs Thr-S).
Transcriptome Data Validation by qPCR
The hepatic gene expression patterns were verified using qPCR for 13 genes, which were selected to represent lipid metabolism pathways. The expression levels of 11 out of 13 tested genes were similar to those detected in the transcriptomic analysis (n = 6). The exceptions were FADS1 and ACADSB (Figure 2).
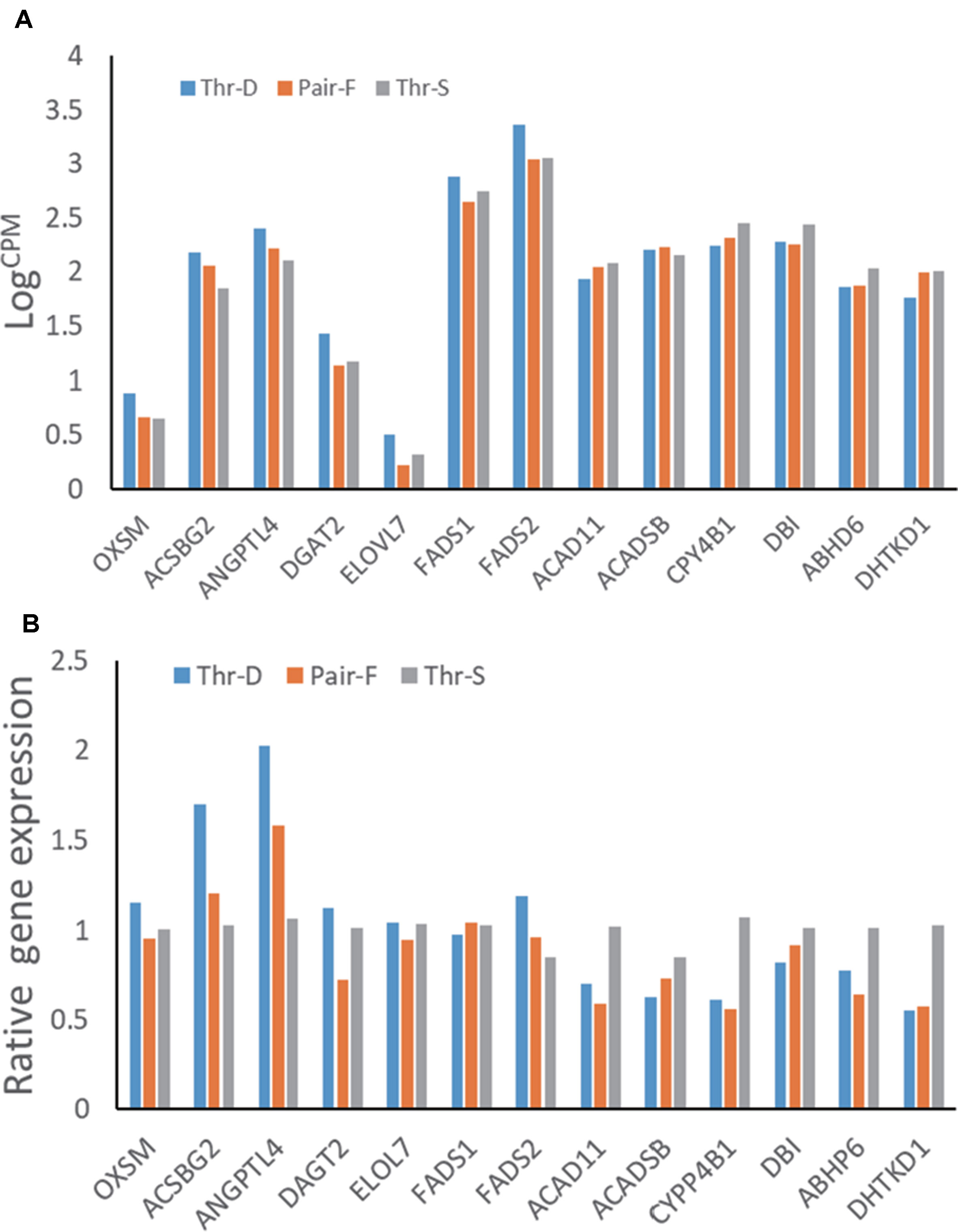
Figure 2 Gene expressions from transcriptome data (A) and qRT-PCR (B) of hepatic gene expressions of 21-day-old ducks from threonine defciency (Thr-D), threonine suffciency (Thr-S), and pair-fed (Pair-F). FADS2, fatty acid desaturase 2; ACSBG2, acyl-CoA synthetase bubblegum family member 2; OXSM, 3-oxoacyl-ACP synthase; ELOVL7, ELOVL fatty acid elongase 7; FADS1, atty acid desaturase 1; DBI, diazepam binding inhibitor, acyl-CoA binding protein; DGAT2, diacylglycerol O-acyltransferase 2; ABHD6, abhydrolase domain containing 6; ACADSB, acyl-CoA dehydrogenase, short/branched chain; ACAD11, acyl-CoA dehydrogenase family member 11; CYP4B1, cytochrome P450 family 4 subfamily B member 1; DHTK1, dehydrogenase E1 and transketolase domain containing 1; ANGPTL4, angiopoietin like 4. Data are means ± SEM (n = 6). a,bMean values with unlike superscript letters were significantly different (P < 0.05).
Gene Ontology and KEGG Pathway Analysis of Differential Genes
To identify biological processes and KEGG pathways, the differentially expressed genes were decoded into their protein sequences. This resulted in 829, 682, and 733 protein sequences in Thr-D vs Thr-S, Thr-S vs Pair-F, and Pair-F vs Thr-S comparisons, respectively. An enrichment test was applied to search for significantly overrepresented GO terms (P value < 0.05) and KEGG pathways (P value < 0.05). The GO term enrichment showed that more genes related to lipid processes and molecular function were enriched in both Thr-D vs Thr-S and Thr-S vs Pair-F comparisons than in Pair-F vs Thr-S (Figure 3). Some KEGG pathways were also related to signal transduction, immunity, hormones, lipid, and amino acid metabolism (Figure 4).
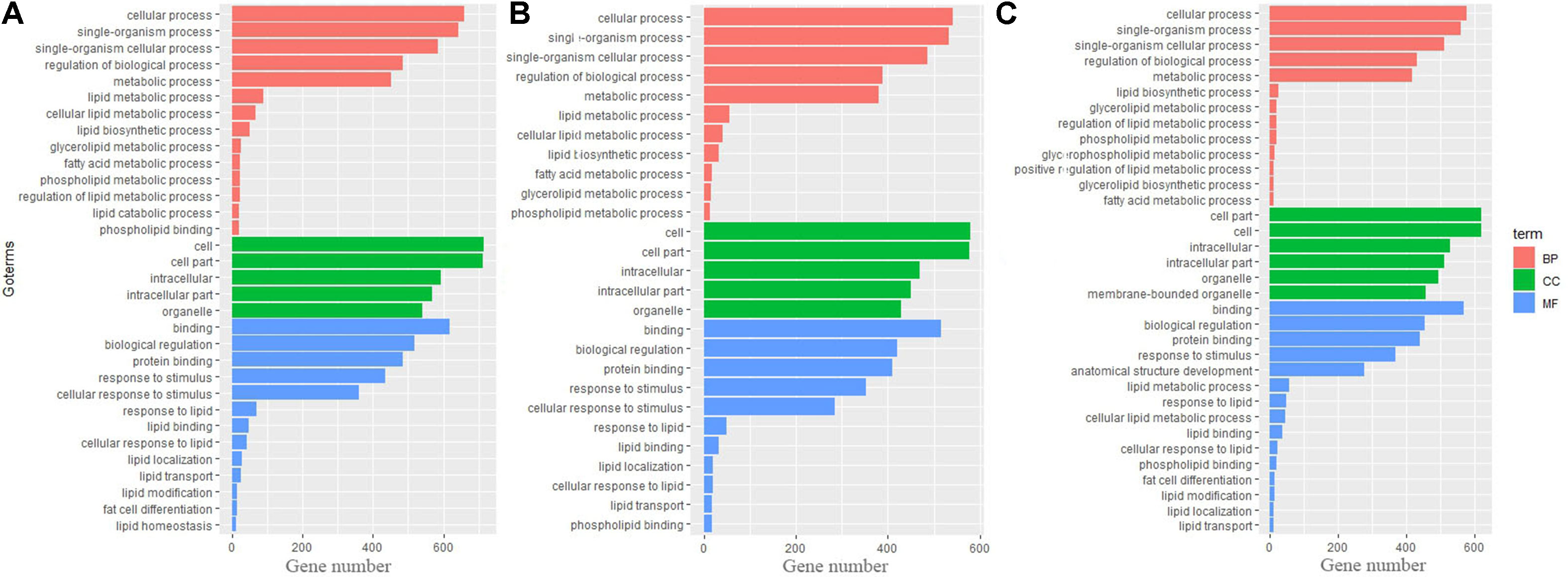
Figure 3 The GO terms involved in lipid metabolism enriched by differentially expressed genes (A, Thr-D vs Thr-S; B, Thr-D vs Pair-F; C, Pair-F vs Thr-D)
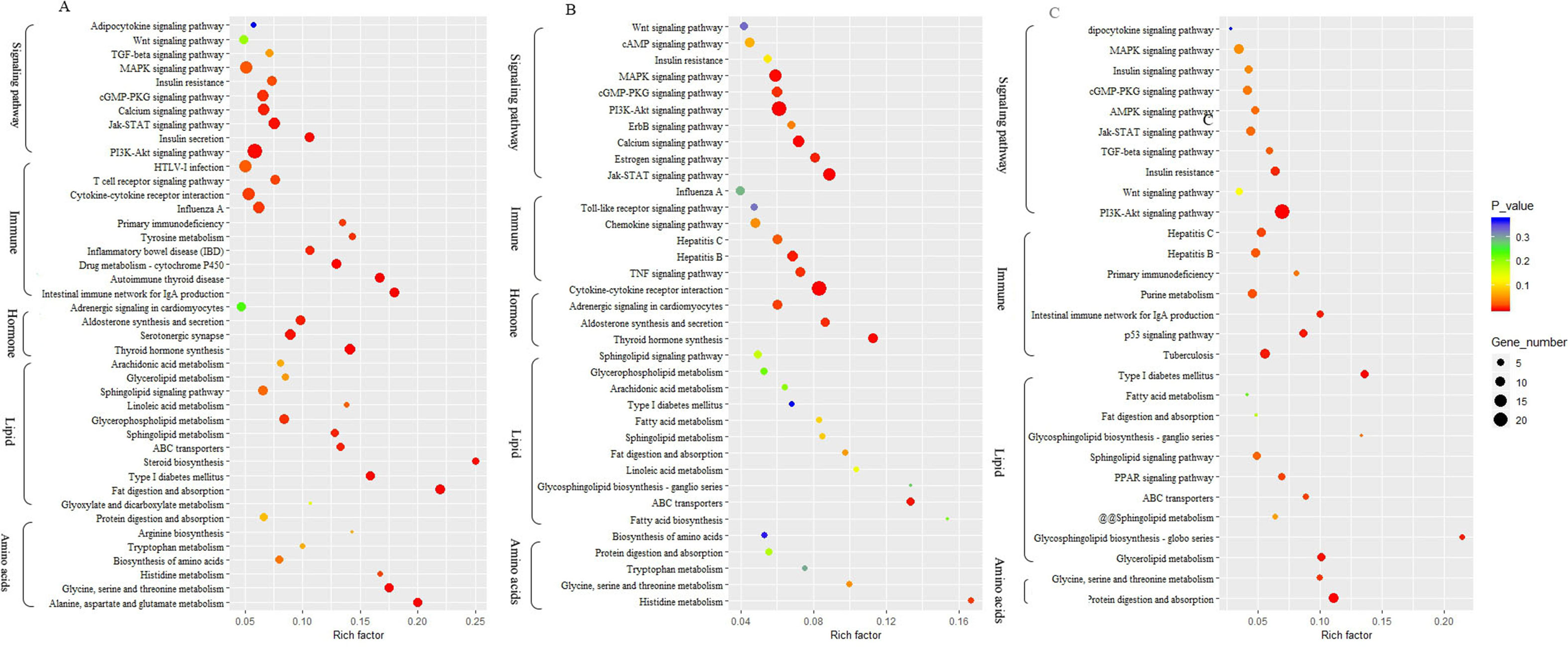
Figure 4 The KEGG pathway involved in lipid metabolism enriched by differentially expressed genes (A, Thr-D vs Thr-S; B, Thr-D vs Pair-F; A, Pair-F vs Thr-D)
Discussion
Previous studies from our laboratory showed that dietary Thr deficiency increases hepatic triglyceride concentration and reduces the abdominal fat levels (Jiang et al., 2017; Jiang et al., 2019). In the present study, there were a reduction in growth and abdominal fat percentage and an increase in hepatic triglyceride concentration of ducks during dietary Thr deficiency (Tables 1, 2, and 3). Similar to those previous studies, we also observed a reduction in feed intake in the present study, which would affect physiological responses. Long-term (4–12 weeks) feed restriction reduces the concentration of ghrelin and increases the concentration of corticosterone in the plasma of turkeys (Vizcarra et al., 2018). In chickens, ghrelin activates the expression of corticotropin-releasing hormone and β-adrenergic receptor (Zendehdel and Hassanpour, 2014). In mammals, adrenaline can stimulate lipolysis by interacting with β-adrenergic receptor or inhibit lipolysis by interacting with α2-adrenergic receptor (Lafontan and Berlan, 1993; Robidoux et al., 2004). In addition, the reduction in feed intake reduces lipid deposition in cattle (Basarab et al., 2003). Therefore, the decreased feed intake itself observed in the present study might affect the hepatic lipid deposition in ducks.
Therefore, we assessed the role of feed restriction to distinguish its effects on hepatic triglyceride levels from those of Thr deficiency. By implementing feed restriction in the Pair-F group, we could assure that the feed intake of ducks in the Thr-D was similar to that of the Pair-F group. However, the growth performance of ducks in the Thr-D group was lower than that in the Pair-F group (Table 1), whereas ducks fed with the Thr-D diet increased their hepatic triglyceride concentration compared with those in the Pair-F group; Pair-F and Thr-S groups had similar hepatic triglyceride concentrations (Table 4). This indicates that low Thr intake directly increases hepatic triglyceride accumulation in ducks. These results differ from previous studies using broilers, in which feed restriction tends to reduce fat content in breast and thigh muscles (Wang et al., 2010) and reduce hepatic triglyceride content (Yang et al., 2010b). In lambs, feed restriction reduces total fatty acid, saturated fatty acid, and unsaturated fatty acid levels in the longissimus thoracis muscle (de Araújo et al., 2017). In the present study, the Pair-F group had increased hepatic content of C14:0 and reduced contents of C15:0, C17:0, C18:0, C20:0, C20:4n6, and C22:0, as well as reduced total fatty acid, saturated fatty acid, and unsaturated fatty acid contents, compared with Thr-D and Thr-S groups. The Thr-D group had increased hepatic fatty acid content (C6:0, C17:1, C18:3n6, C20:0, C20:1n9, and C22:2) compared with the Thr-S group. Ducks fed with the Pair-F diet had reduced hepatic fatty acid, whereas those fed with the Thr-D diet had increased hepatic triglyceride content. In addition, although there was a clear accumulation of triglycerides in the liver, its biological functions were not affected by Thr deficiency, as the plasmatic activities of AST, ALT, and ALP were unaffected by Thr-D and Pair-F diets. Nevertheless, Thr-D and Pair-F diets changed the concentration of GLU, HDLC, and LDLC in plasma, which indicates that Thr deficiency and feed restriction affect lipid metabolism.
In order to determine the underlying molecular changes caused by dietary Thr deficiency, we had previously assessed the expression of several genes involved in fatty acid synthesis and oxidation and triglyceride transport (Jiang et al., 2019). Unfortunately, the underlying mechanism of triglyceride accumulation caused by dietary Thr deficiency was still unclear after that study because of the large number of genes involved in the process. Therefore, here, we screened differentially expressed genes in response to dietary Thr deficiency using RNA sequencing techniques. It was observed that the lots of genes involved in amino acid, immune, energy, steroid, and lipid metabolism were differentially expressed.
Amino Acid Biosynthesis
In the present study, 29 differentially expressed genes were associated with amino acid metabolism in the comparison of the Thr-D group against the Thr-S group, of which nine genes were upregulated and 20 genes were downregulated (Supplementary Table 4A). When compared with the Pair-F group, the Thr-D group presented nine upregulated genes and nine downregulated gene expression involved in amino acid metabolism (Supplementary Table 4B). The Pair-F diet affected the expression of 14 genes compared with the Thr-S diet, of which four genes were upregulated and 11 genes were downregulated (Supplementary Table 4C). Dynamic research about amino acid networks showed that Thr have positive effects on methionine, valine, isoleucine, leucine, asparagine, glutamic acid, tyrosine, cysteine, and a negative effect on serine pathways (Shikata et al., 2007; Shikata et al., 2010). A previous study from our laboratory showed that dietary deficiency reduces the plasmatic concentration of methionine, cysteine, lysine, arginine, isoleucine, valine, histidine, and phenylalanine and increases that of serine and alanine (Jiang et al., 2017). In the present study, the differentially expressed genes included those associated with alanine, histidine, aspartate, glutamate, glycine, serine, threonine, and tryptophan metabolism, and most of these genes were downregulated by the Thr-D diet (Supplementary Table 4A). In addition, genes involved in amino acid biosynthesis were downregulated by threonine deprivation (Supplementary Table 5A). These results indicate that amino acid biosynthesis decreased, whereas amino acid degradation increased in the liver of ducks fed with the Thr-D diet.
Immunity
Threonine plays an important role in immune responses, as it affects the production of intestinal IgA in laying hens and pigs (Azzam et al., 2011; Zhang et al., 2017) and serum IgY in ducks (Zhang et al., 2014). In the present study, the enrichment analysis revealed that 57 genes were related to immune responses, including T cell and Toll-like receptor signaling in the comparison of the Thr-D against the Thr-S group (Supplementary Table 5A). The comparison between the Thr-D and the Pair-F groups resulted in 45 differentially expressed genes associated with immune responses (Supplementary Table 5B). Only 32 genes involved in immune responses were differentially expressed between Pair-F and Thr-S groups (Supplementary Table 5C). T cells play a key role in cell-mediated immunity and T cell receptor activation, and they enhance a number of signaling cascades that determine cell fate by regulating cytokine production, cell survival, proliferation, and differentiation (Burbach et al., 2007). We found that seven genes related to the T cell receptor signaling were downregulated, whereas only one was upregulated in the liver of animals fed with the Thr-D diet. This indicates that T cell immunity was repressed by threonine deprivation.
Toll-like receptors (TLRs) are key components of innate and adaptive immunities. They control host immune responses against pathogens through recognition of molecular patterns that are specific to microorganisms. In the present study, five genes associated with Toll-like receptor signaling were upregulated, and one gene was downregulated by threonine restriction. The nucleotide-sensing TLR3 is activated by double-stranded viral RNA, which is a sign of viral infection. Its activation leads to NF-κB induction, IRF3 nuclear translocation, cytokine secretion, and the inflammatory response via TRIF/TICAM1 (Oshiumi et al., 2003; Johnsen et al., 2006). Toll-like receptor 5 (TLR5) participates in the innate immune response to microbial agents (e.g., bacterial flagellin) by activating NF-κB and promoting cytokine secretion and the inflammatory response (Hayashi et al., 2001). In the present study, TLR3 and TLR5 were upregulated by the Thr-D diet compared with the Thr-S diet. Moreover, their downstream gene, MAP3K8, was also upregulated. In addition, compared with the Pair-F group, the Thr-D group upregulated five gene expression related to Toll-like receptor signaling. Genes affected by the Pair-F diet were not enriched in genes associated with TLR signaling and T cell receptor signaling. Taken together, our results show that the Thr-D diet regulates the TLR signaling by affecting the expression of genes related to both the activation of TLR signaling and its downstream effectors.
Energy Metabolism
Previous studies reported that dietary Thr deficiency reduces hepatic cell respiration in rats (Ross-Inta et al., 2009). In the present study, the Thr-D diet had increased expressions of enolase 2 (ENO2) and hexokinase domain containing 1 (HKDC1) and reduced the expression of dehydrogenase E1 and transketolase domain containing 1 (DHTKD1) and 3-hydroxymethyl-3-methylglutaryl-CoA lyase (HMGCL) genes compared with the that of Thr-S group (Table 6). One of those genes, HKDC1, encodes hexokinase, which phosphorylates hexoses (Wolf et al., 2011; Guo et al., 2015). Enolase, which catalyses the production of phosphoenolpyruvate, is encoded by the ENO2 gene (Marangos and Schmechel, 1987). Glycolysis supplies glycerol 3-phoshate for triglyceride synthesis and acyl-CoA for fatty acid synthesis or tricarboxylic acid (TCA). In that regard, DHTKD1 is a key gene for the TCA cycle, which codes the enzyme that converts 2-oxoglutarate to succinyl-CoA, generating ATP (Danhauser et al., 2012). A previous study showed that dietary Thr deficiency decreases liver mitochondrial coupling, leading to a reduction in ATP production (Ross-Inta et al., 2009). Another key metabolic pathway, ketogenesis, depends on the activity of HMGCL enzyme, which catalyses acetoacetate synthesis in the liver from 3-hydroxy-3-methylglutaryl-CoA (Tuinstra and Miziorko, 2003). Acetoacetate is transported to extrahepatic tissues (e.g., the brain) as energy source. In addition, the Thr-D diet induced the expressions of acyl-CoA synthetase short-chain family member 1 (ACSS1) and 3-hydroxybutyrate dehydrogenase 1 (BDH1) genes compared with that of the Pair-F group (Table 6). The protein encoded by ACSS1 converts acetate to acetyl-CoA, used in the TCA cycle to produce energy and electron carriers, whereas BDH1 enzyme catalyses the interconversion of acetoacetate to 3-hydroxybutyrate (Churchill et al., 1992). 3-Hydroxybutyrate is absorbed by target tissues and converted back to acetoacetate by the same enzyme (Newman and Verdin, 2014). Acetoacetyl-CoA can be converted to two acetyl-CoA molecules, and then, they can be oxidized and produced ATP in TCA (Fukao et al., 2004). The Thr-D group had increased expressions of ENO2 and HKDC1 and reduced expression of DHTKD1 compared with the Pair-P group (Table 6). However, there were no differences in the expression of these genes between Pair-F and Thr-S groups. Therefore, threonine deprivation increases glycolysis and affects ATP production and ketogenesis (Figure 5).
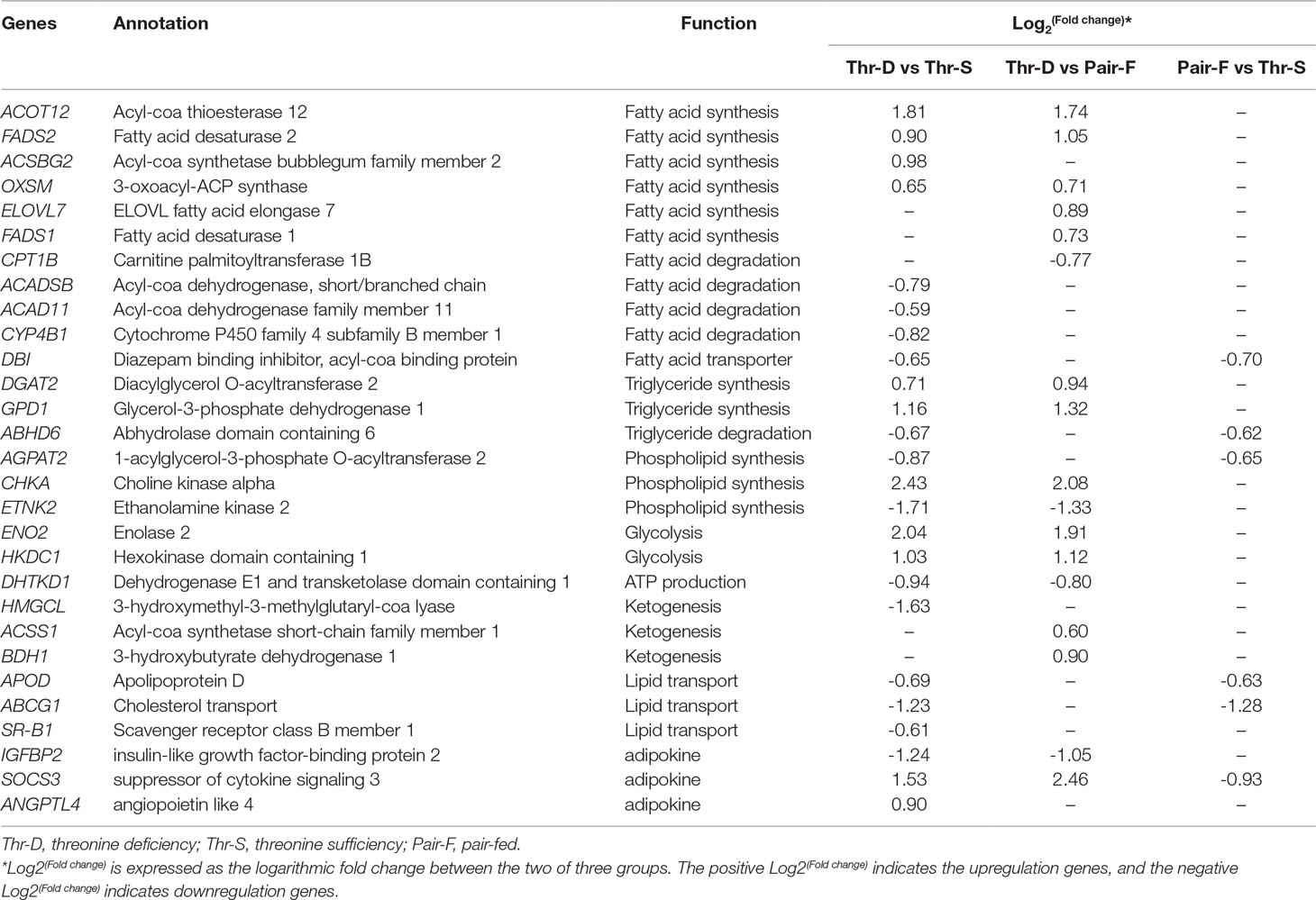
Table 6 Differentially expressed genes in liver involved in lipid metabolism on day 21 of ducks caused by threonine deficiency.
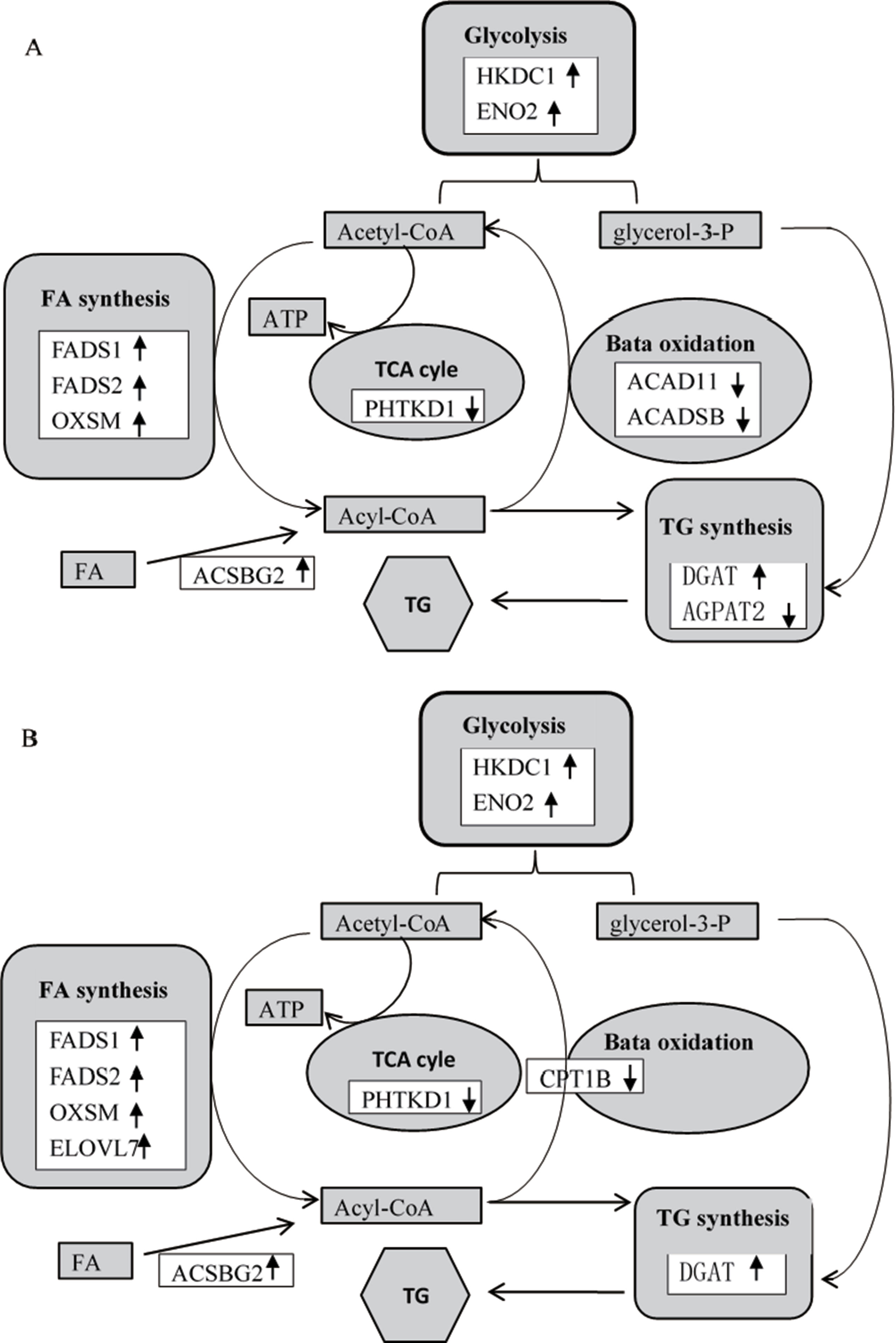
Figure 5 Differentially expressed genes (A, Thr-D vs Thr-S; B, Thr-D vs Pair-F) involved in glycolysis, fatty acid synthesis and beta oxidation, triglyceride synthesis, and tricarboxylic acid (TCA) cycle. The black up arrows at right indicate increased gene levels in response to threonine deficiency, and the black down arrows at right indicate reduced gene levels in response to threonine deficiency. HKDC1, hexokinase domain containing 1; ENO2, enolase 2; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; OXSM, 3-oxoacyl-ACP synthase; ACADSB, acyl-CoA dehydrogenase, short/branched chain; ACAD11, acyl-CoA dehydrogenase family member 11; ACSBG2, acyl-CoA synthetase bubblegum family member 2; AGPAT2, 1-acylglycerol-3-phosphate O-acyltransferase 2; DGAT2, diacylglycerol O-acyltransferase 2; DHTKD1, dehydrogenase E1 and transketolase domain containing 1; ELOVL7, ELOVL fatty acid elongase 7; CPT1B, carnitine palmitoyltransferase 1B.
Lipid Metabolism
In the present study, 64, 31, and 30 genes that were related to lipid metabolism were differentially expressed between Thr-D and Thr-S, Thr-D and Pair-F, and Pair-F and Thr-S groups, respectively (Supplementary Table 4A, B, C). Among those, genes involved in fatty acid and triglyceride synthesis were upregulated, whereas genes related to fatty acid and triglyceride transport and degradation were downregulated by either Thr-D or Pair-F diets. The gene upregulated by the Thr-D diet included 3-oxoacyl-ACP synthase (OXSM), very long chain fatty acids protein 7 (ELOVL7), and long chain fatty acyl-CoA ligase (ACSBG2), which are involved in de novo synthesis of fatty acids (Zhang et al., 2005; Ohno et al., 2010; Naganuma et al., 2011). However, acyl-CoA dehydrogenase, short/branched chain (ACADSB), acyl-CoA dehydrogenase family member 11 (ACAD11), and cytochrome P450 (CYP4B) were downregulated by the Thr-D diet. These genes are involved in fatty acid β-oxidation and ω-oxidation (Vockley et al., 2000; He et al., 2011; Hsu et al., 2017; Roellecke et al., 2017; Scott, 2017). The Thr-D diet downregulated the expression of carnitine palmitoyltransferase 1B (CPT1B) compared with the Pair-F diet. The product of this gene is a protein that transports long chain acyl-CoA molecules from cytoplasm into mitochondria and thus controls a key regulatory step of β-oxidation (Kerner and Hoppel, 2000). In addition, Thr-D and Pair-F diets downregulated the expression of acyl-CoA binding protein (DBI), apolipoprotein D (APOD), and microsomal triglyceride transfer protein (MTTP). These genes participate in fatty acid and triglyceride transport (Rasmussen et al., 1994; Faergeman and Knudsen, 1997; Xie et al., 2006; Walsh et al., 2015). Based on the aforementioned mRNA results, it would be expected that the fatty acid content in Thr-D and Pair-F groups should be higher than that in the Thr-S group. However, we observed similar total fatty acid levels between Thr-D and Thr-S groups. In Thr-D and Pair-F groups, only the levels of C22:2, C20:1n9, C18:3n6, C18:2n6t, and C17:1 were higher than those in the liver of animals fed with the Thr-S diet. Moreover, hepatic triglyceride content in the Thr-D group was higher than that in the Thr-S group, but both groups had similar total lipids contents, which indicated that dietary Thr deficiency affected the hepatic lipid compositions. However, it was still unclear and need further experiments to prove it. Therefore, it was speculated that the synthesis of triglycerides is favored during dietary Thr deficiency. The expression of diacylglycerol O-acyltransferase 2 (DGAT2) was upregulated by the Thr-D diet compared with those by the other two dietary treatments. The protein coded by this gene binds diacylglycerol to long-chain fatty acid acyl-CoA in the final reaction of triglyceride biosynthesis (Agarwal et al., 2011; McFie et al., 2011; Qi et al., 2012). On the other hand, the Pair-F diet did not change the expression of DGAT2 compared with the Thr-S group diet. This observation is consistent with that of similar levels of total lipids between the Thr-S and Pair-F groups.
Steroid Biosynthesis
In the present study, the Thr-D diet upregulated the expression of 7-dehydrocholesterol r eductase (DHCR7), methylsterol monooxygenase 1 (MSMO1), NAD(P) dependent steroid dehydrogenase-like, lanosterol 14-alpha demethylase, squalene epoxidase (SQLE), and class I histocompatibility antigen compared with the Thr-S group diet. These genes are involved in steroid biosynthesis. To the best of our knowledge, there are no reports on the effects of dietary amino acid levels on the expression of genes related to steroid biosynthesis. However, feed restriction downregulates the expression of such genes in pigs and rats (Selman et al., 2006; Lkhagvadorj et al., 2009; Lkhagvadorj et al., 2010). In contrast, we found that the Pair-F diet did not change the expression of genes related to steroid biosynthesis compared with the Thr-S group diet. The discrepancy between the present study and previous studies might be due to species-specific characteristics.
Effects of Thr on Adipocytokines
Adipocytokines play key roles in the regulation of lipid metabolism. In the present study, several adipocytokine factors involved in lipid metabolism were differentially expressed, including SOCS3, IGFBP2, and ANGPTL4. These genes have diverse functions, for example, SOCS3 is a negative regulator of insulin signaling in skeletal muscle, adipose tissue, and the liver (Shi et al., 2004; Ueki et al., 2004; Yang et al., 2012), whereas it is also a negative regulator of leptin in the hypothalamus (Bjorbaek et al., 1999). The overexpression of SOCS3 increases insulin and leptin resistance (Yang et al., 2012), which in turn increases hepatic lipid accumulation (Liu et al., 2014). In addition, ANGPTL4 is expressed in the adipose tissue, liver, and placenta (Kersten et al., 2000; Kim et al., 2000; Yoon et al., 2000), where it regulates lipid metabolism primarily by inhibiting lipoprotein lipase activity (Ge et al., 2004; Köster et al., 2005) and promotes triglyceride uptake. The upregulation of ANGPTL4 occurs in response to palmitic, oleic, arachidonic, and eicosapentaenoic acids (Gonzalez-Muniesa et al., 2011). Overexpression of ANGPTL4 in mice increases the concentrations of triglycerides and cholesterol in plasma but does not increase body fat mass (Köster et al., 2005). Moreover, the overexpression of ANGPL4 increases hepatic fat accumulation in mice (Xu et al., 2005). IGF binding protein 2 (IGFBP2) is expressed by several tissues, including the liver and white adipocytes (Gosteli-Peter et al., 1994), where it contributes to the prevention of diet-induced fat accumulation (Wheatcroft et al., 2007). Overexpression of IGFBP2 reduces body fat accumulation in mice fed with a high-fat diet (Kammel et al., 2016) and decreases liver triglyceride deposition in ob/ob mice (Hedbacker et al., 2010). In the present study, the Thr-D diet downregulated IGFBP2 and upregulated ANGPTL4 and SOCS3 in the liver of Pekin ducks, which agrees with the increased triglyceride deposition in the liver of these animals.
Effects of Thr on Pathway Related to Lipid Metabolism
In addition, we found that several cellular regulatory pathways involved in lipid metabolism were differentially expressed in Thr-D and Pair-F groups, such as PI3K-Akt, Jak-STAT, cGMP-PKG, and Wnt signaling pathway. Apoptosis-related pathways, such as PI3K/AKT-TOR, cAMP/PKA/CREB, and LKB1/AMPK-FOXO, regulate methionine-induced changes in hepatic lipid metabolism in fish and mammals (Gao et al., 2018). Signal transduction in the Jak-STAT pathway has been demonstrated to regulate lipid metabolism in mice (Xu et al., 2013; Shi et al., 2014). For instance, di-(2-ethylhexyl) phthalate reduces lipid hydrolysis and promotes triglyceride accumulation by regulating the activation state of the Jak-STAT pathway in the liver and adipose tissue in rats (Jia et al., 2016) and magnesium-induced reduction in hepatic lipid deposition in yellow catfish (Wei et al., 2017). In the present study, the Thr-D diet downregulated the expression of signal transducer and activator of transcription 4 (STAT4) and upregulated the expression of suppressor of cytokine signaling 3 (SOCS3), whereas the Pair-F diet did not change the expression of STAT4 but downregulated the expression of SOCS3 compared with the Thr-S group diet. Cytokines activate the JAK-STAT signaling pathway by binding to its receptor, leading to the expression of SOCS (Krebs and Hilton, 2000). The upregulated SOCS protein acts through a feedback mechanism to inhibit the JAK-STAT signaling pathway in the cytosol (Miao et al., 2006). Therefore, in Pekin ducks, the high expression of SOCS3 might inhibit that of STAT4 in the liver of animals fed with the Thr-D diet.
Activation of PI3K/AKT might lead to the expression of Fas cell surface death receptor and Sterol regulatory element-binding proteins (Jeon and Osborne, 2012; Liu et al., 2016). On the other hand, inhibition of PI3K/AKT impairs the suppression of very-low-density lipoprotein assembly and insulin secretion by regulating the secretion and degradation of apolipoprotein B and the gene expression of hepatic MTTP (Sidiropoulos et al., 2007). Moreover, inhibition of PI3K/AKT/mTOR pathway reduces hepatic lipid accumulation by increasing fatty acid oxidation and VLDL assembly and secretion in geese (Liu et al., 2016). In the present study, the Thr-D diet upregulated eight genes and downregulated 12 genes involved in the PI3K-AKT signaling pathway, as well as downregulated the expression of MTTP, compared with the Thr-S group diet. Feed restriction in the Pair-F group upregulated 15 genes and downregulated 59 genes in PI3K-AKT signaling pathway, when compared with that in the Thr-S group. Thus, it is clear that both Thr-D and Pair-F diets changed the expression of genes that compose the PI3K-AKT signaling pathway, but there were differences between Thr-D and Pair-F groups.
In summary, both Thr-D and Pair-F diets affected hepatic lipid metabolism in Pekin ducks. However, the molecular mechanisms underlying lipid metabolism changes differed between Thr-D and Pair-F groups. Animals in the Pair-F group had reduced hepatic individual fatty acid contents, whereas ducks in the Thr-D group had a similar fatty acid profile when compared with those in the Thr-S group. However, the Thr-D diet increased the accumulation of hepatic triglycerides, and the Pair-F diet had no effect on hepatic triglyceride accumulation. Transcriptome analysis showed that the Thr-D diet upregulated the expression of genes related to fatty acid and triglyceride synthesis and downregulated genes related to fatty acid oxidation and triglyceride transport. This indicates that threonine deprivation promoted the entrance of fatty acids into the triglyceride pathway. Both Thr-D and Pair-F diets induced regulatory changes involved in lipid metabolism that might be controlled by PI3K-AKT, Jak-STAT, and adipocytokine signaling pathways. The role of these signaling pathways in the regulation of lipid metabolism during dietary threonine deprivation warrants further research.
Data Availability
The datasets generated for this study can be found in NCBI. The number of BioProject is PRJNA530027, and the data can be visited at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA530027.
Ethics Statement
The study was approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS20160322, IAS-CAAS, Beijing, China) and performed in accordance with the guidelines. Ethical approval on animal survival was given by the animal ethics committee of IAS-CAAS.
Author Contributions
SH conceived and coordinated the study. YJ performed the study, was involved in all aspects of analysis, and drafted the manuscript. MX and SH were involved in experimental design. MX performed data analysis. JT, WF, and JX performed the sample analysis. GC participated in editing the manuscript. SH had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the earmarked fund for China Agriculture Research System (CARS-42) and the science and technology innovation project of Chinese Academy of Agricultural Sciences (CXGC-IAS-09).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00710/full#supplementary-material
References
Agarwal, A. K., Sukumaran, S., Cortes, V. A., Tunison, K., Mizrachi, D., Sankella, S., et al. (2011). Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J. Biol. Chem. 286, 37676–37691. doi: 10.1074/jbc.M111.250449
Azzam, M. M., Zou, X. T., Dong, X. Y., Xie, P. (2011). Effect of supplemental L-threonine on mucin 2 gene expression and intestine mucosal immune and digestive enzymes activities of laying hens in environments with high temperature and humidity. Poult. Sci. 90, 2251–2256. doi: 10.3382/ps.2011-01574
Basarab, J. A., Price, M. A., Aalhus, J. L., Okine, E. K., Snelling, W. M., Lyle, K. L. (2003). Residual feed intake and body composition in young growing cattle. Can. J. Anim. Sci. 83, 189–204. doi: 10.4141/A02-065
Bjorbaek, C., El-Haschimi, K., Frantz, J. D., Flier, J. S. (1999). The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 274, 30059–30065. doi: 10.1074/jbc.274.42.30059
Browning, J. D., Horton, J. D. (2004). Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152. doi: 10.1172/JCI200422422
Burbach, B. J., Medeiros, R. B., Mueller, K. L., Shimizu, Y. (2007). T-cell receptor signaling to integrins. Immunol. Rev. 218, 65–81. doi: 10.1111/j.1600-065X.2007.00527.x
Chalasani, N., Younossi, Z., Lavine, J. E., Diehl, A. M., Brunt, E. M., Cusi, K., et al. (2012). The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609. doi: 10.1053/j.gastro.2012.04.001
Churchill, P., Hempel, J., Romovacek, H., Zhang, W. W., Brennan, M., Churchill, S. (1992). Primary structure of rat liver D-beta-hydroxybutyrate dehydrogenase from cDNA and protein analyses: a short-chain alcohol dehydrogenase. Biochemistry 31, 3793–3799. doi: 10.1021/bi00130a009
Ciftci, I., Ceylan, N. (2004). Effects of dietary threonine and crude protein on growth performance, carcase and meat composition of broiler chickens. Br. Poult. Sci. 45, 280–289. doi: 10.1080/00071660410001715894
Cohen, J. C., Horton, J. D., Hobbs, H. H. (2011). Human fatty liver disease: old questions and new insights. Science 332, 1519–1523. doi: 10.1126/science.1204265
Danhauser, K., Sauer, S. W., Haack, T. B., Wieland, T., Staufner, C., Graf, E., et al. (2012). DHTKD1 mutations cause 2-aminoadipic and 2-oxoadipic aciduria. Am. J. Hum. Genet. 91, 1082–1087. doi: 10.1016/j.ajhg.2012.10.006
de Araújo, T. L. A. C., Pereira, E. S., Mizubuti, I. Y., Campos, A. C. N., Pereira, M. W. F., Heinzen, E. L., et al. (2017). Effects of quantitative feed restriction and sex on carcass traits, meat quality and meat lipid profile of Morada Nova lambs. J. Anim. Sci. Biotechnol. 8, 46. doi: 10.1186/s40104-017-0175-3
Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., et al. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. doi: 10.1093/bioinformatics/bts635
Faergeman, N. J., Knudsen, J. (1997). Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323, 1–12. doi: 10.1042/bj3230001
Folch, J., Lees, M., Sloane-Stanley, G. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Fukao, T., Lopaschuk, G. D., Mitchell, G. A. (2004). Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fatty Acids 70, 243–251. doi: 10.1016/j.plefa.2003.11.001
Gao, Y., Hogstrand, C., Li, D., Pan, Y., Luo, Z. (2018). Upstream regulators of apoptosis mediates methionine-induced changes of lipid metabolism.%A Song YF. Cell. Signal. 51, 176–190. doi: 10.1016/j.cellsig.2018.08.005
Ge, H., Yang, G., Huang, L., Motola, D. L., Pourbahrami, T., Li, C. (2004). Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 279, 2038–2045. doi: 10.1074/jbc.M307583200
Gonzalez-Muniesa, P., de Oliveira, C., de Heredia, F. P., Thompson, M. P., Trayhurn, P. (2011). Fatty Acids and Hypoxia Stimulate the Expression and Secretion of the Adipokine ANGPTL4 (Angiopoietin-Like Protein 4/Fasting-Induced Adipose Factor) by Human Adipocytes. J. Nutrigenet. Nutrigenomics 4, 146–153. doi: 10.1159/000327774
Gosteli-Peter, M. A., Winterhalter, K. H., Schmid, C., Froesch, E. R., Zapf, J. (1994). Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology 135, 2558–2567. doi: 10.1210/endo.135.6.7527334
Guo, C., Ludvik, A. E., Arlotto, M. E., Hayes, M. G., Armstrong, L. L., Scholtens, D. M., et al. (2015). Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat. Commun. 6, 6069. doi: 10.1038/ncomms7069
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., et al. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
He, M., Pei, Z., Mohsen, A. W., Watkins, P., Murdoch, G., Van Veldhoven, P. P., et al. (2011). Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol. Genet. Metab. 102, 418–429. doi: 10.1016/j.ymgme.2010.12.005
Hedbacker, K., Birsoy, K., Wysocki, R. W., Asilmaz, E., Ahima, R. S., Farooqi, I. S., et al. (2010). Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 11, 11–22. doi: 10.1016/j.cmet.2009.11.007
Hsu, M., Baer, B., Rettie, A., Johnson, E. (2017). The Crystal Structure of Cytochrome P450 4B1 (CYP4B1) Monooxygenase Complexed with Octane Discloses Several Structural Adaptations for ω-Hydroxylation. J. Biol. Chem. 292, 5610–5621. doi: 10.1074/jbc.M117.775494
Janssen, I., Katzmarzyk, P. T., Boyce, W. F., Vereecken, C., Mulvihill, C., Roberts, C., et al. (2005). Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes. Rev. 6, 123–132. doi: 10.1111/j.1467-789X.2005.00176.x
Jeon, T. I., Osborne, T. F. (2012). SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 23, 65–72. doi: 10.1016/j.tem.2011.10.004
Jia, Y., Liu, T., Zhou, L., Zhu, J., Wu, J., Sun, D., et al. (2016). Effects of Di-(2-ethylhexyl) Phthalate on Lipid Metabolism by the JAK/STAT Pathway in Rats. Int. J. Environ. Res. Public Health 13, 1085. doi: 10.3390/ijerph13111085
Jiang, Y., Liao, X. D., Xie, M., Tang, J., Qiao, S. Y., Wen, Z. G., et al. (2019). Dietary threonine supplementation improves hepatic lipid metabolism of Pekin ducks. Anim. Prod. Sci. 59, 673–680. doi: 10.1071/AN17633
Jiang, Y., Tang, J., Xie, M., Wen, Z. G., Qiao, S. Y., Hou, S. S. (2017). Threonine supplementation reduces dietary protein and improves lipid metabolism in Pekin ducks. Br. Poult. Sci. 58, 687–693. doi: 10.1080/00071668.2017.1363871
Jiang, Y., Uzma, M., Tang, T., Wen, Z. G., Hou, S. S., Huang, W., et al. (2016). Effects of dietary protein on threonine requirements of Pekin ducks from hatch to 21 days of age. Anim. Feed Sci. Technol. 217, 95–99. doi: 10.1016/j.anifeedsci.2016.04.010
Johnsen, I. B., Nguyen, T. T., Ringdal, M., Tryggestad, A. M., Bakke, O., Lien, E., et al. (2006). Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 25, 3335–3346. doi: 10.1038/sj.emboj.7601222
Kammel, A., Saussenthaler, S., Jahnert, M., Jonas, W., Stirm, L., Hoeflich, A., et al. (2016). Early hypermethylation of hepatic Igfbp2 results in its reduced expression preceding fatty liver in mice. Hum. Mol. Genet. 25, 2588–2599. doi: 10.1093/hmg/ddw121
Kerner, J., Hoppel, C. (2000). Fatty acid import into mitochondria. Biochim. Biophys. Acta 1486, 1–17. doi: 10.1016/S1388-1981(00)00044-5
Kersten, S., Mandard, S., Tan, N. S., Escher, P., Metzger, D., Chambon, P., et al. (2000). Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28488–28493. doi: 10.1074/jbc.M004029200
Kidd, M. T., Kerr, B. J. (1996). L-threonine for poultry: a review. J. Appl. Poult. Res. 5, 358–367. doi: 10.1093/japr/5.4.358
Kim, I., Kim, H. G., Kim, H., Kim, H. H., Park, S. K., Uhm, C. S., et al. (2000). Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem. J. 346 Pt 3, 603–610. doi: 10.1042/bj3460603
Köster, A., Chao, Y. B., Mosior, M., Ford, A., Gonzalez-DeWhitt, P. A., Hale, J. E., et al. (2005). Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146, 4943–4950. doi: 10.1210/en.2005-0476
Krebs, D. L., Hilton, D. J. (2000). SOCS: physiological suppressors of cytokine signaling. J. Cell Sci. 113 (Pt 16), 2813–2819. doi: 10.1023/A:1005536305526
Lafontan, M., Berlan, M. (1993). Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lipid Res. 34, 1057–1091.
Lazo, M., Clark, J. M. (2008). The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin. Liver Dis. 28, 339–350. doi: 10.1055/s-0028-1091978
Liu, D. D., Han, C. C., Wan, H. F., He, F., Xu, H. Y., Wei, S. H., et al. (2016). Effects of inhibiting PI3K-Akt-mTOR pathway on lipid metabolism homeostasis in goose primary hepatocytes. Animal 10, 1319–1327. doi: 10.1017/S1751731116000380
Liu, Z., Gan, L., Yang, X., Zhang, Z., Sun, C. (2014). Hydrodynamic tail vein injection of SOCS3 eukaryotic expression vector in vivo promoted liver lipid metabolism and hepatocyte apoptosis in mouse. Biochem. Cell Biol. 92, 119–125. doi: 10.1139/bcb-2013-0117
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lkhagvadorj, S., Qu, L., Cai, W., Couture, O. P., Barb, C. R., Hausman, G. J., et al. (2009). Microarray gene expression profiles of fasting induced changes in liver and adipose tissues of pigs expressing the melanocortin-4 receptor D298N variant. Physiol. Genomics 38, 98–111. doi: 10.1152/physiolgenomics.90372.2008
Lkhagvadorj, S., Qu, L., Cai, W., Couture, O. P., Barb, C. R., Hausman, G. J., et al. (2010). Gene expression profiling of the short-term adaptive response to acute caloric restriction in liver and adipose tissues of pigs differing in feed efficiency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R494–R507. doi: 10.1152/ajpregu.00632.2009
Loomba, R., Sanyal, A. J. (2013). The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690. doi: 10.1038/nrgastro.2013.171
Marangos, P. J., Schmechel, D. E. (1987). Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu. Rev. Neurosci. 10, 269–295. doi: 10.1146/annurev.ne.10.030187.001413
McFie, P. J., Banman, S. L., Kary, S., Stone, S. J. (2011). Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J. Biol. Chem. 286, 28235–28246. doi: 10.1074/jbc.M111.256008
Methfessel, A. H., Mudambi, S., Harper, A. E., Falcone, A. B. (1964). Biochemical changes in fatty liver induced by choline or threonine deficiency. Ii. Various hepatic enzymic activities during the development of fatty livers in rats. Arch. Biochem. Biophys. 104, 360–368. doi: 10.1016/0003-9861(64)90476-X
Miao, T., Wu, D., Zhang, Y., Bo, X., Subang, M. C., Wang, P., et al. (2006). Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J. Neurosci. 26, 9512–9519. doi: 10.1523/JNEUROSCI.2160-06.2006
Naganuma, T., Sato, Y., Sassa, T., Ohno, Y., Kihara, A. (2011). Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Lett. 585, 3337–3341. doi: 10.1016/j.febslet.2011.09.024
Newman, J. C., Verdin, E. (2014). Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 25, 42–52. doi: 10.1016/j.tem.2013.09.002
Ohno, Y., Suto, S., Yamanaka, M., Mizutani, Y., Mitsutake, S., Igarashi, Y., et al. (2010). ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 18439–18444. doi: 10.1073/pnas.1005572107
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T., Seya, T. (2003). TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4, 161–167. doi: 10.1038/ni886
Park, H. S., Han, J. H., Choi, K. M., Kim, S. M. (2005). Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am. J. Clin. Nutr. 82, 1046–1051. doi: 10.1093/ajcn/82.5.1046
Qi, J., Lang, W., Geisler, J. G., Wang, P., Petrounia, I., Mai, S., et al. (2012). The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J. Lipid Res. 53, 1106–1116. doi: 10.1194/jlr.M020156
Rangel-Lugo, M., Su, C. L., Austic, R. E. (1994). Threonine requirement and threonine imbalance in broiler chickens. Poult. Sci. 73, 670–681. doi: 10.3382/ps.0730670
Rasmussen, J. T., Færgeman, N. J., Kristiansen, K., Knudsen, J. (1994). Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for β-oxidation and glycerolipid synthesis. Biochem. J. 299, 165–170. doi: 10.1042/bj2990165
Rector, R. S., Thyfault, J. P., Wei, Y., Ibdah, J. A. (2008). Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J. Gastroenterol. 14, 185–192. doi: 10.3748/wjg.14.185
Robidoux, J., Martin, T. L., Collins, S. (2004). Beta-adrenergic receptors and regulation of energy expenditure: a family affair. Annu. Rev. Pharmacol. Toxicol. 44, 297–323. doi: 10.1146/annurev.pharmtox.44.101802.121659
Robinson, M. D., McCarthy, D. J., Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. doi: 10.1093/bioinformatics/btp616
Roellecke, K., Jager, V. D., Gyurov, V. H., Kowalski, J. P., Mielke, S., Rettie, A. E., et al. (2017). Ligand characterization of CYP4B1 isoforms modified for high-level expression in Escherichia coli and HepG2 cells. Protein Eng. Des. Sel. 30, 205–216. doi: 10.1093/protein/gzw075
Ross-Inta, C. M., Zhang, Y. F., Almendares, A., Giulivi, C. (2009). Threonine-deficient diets induced changes in hepatic bioenergetics. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1130-G1139. doi: 10.1152/ajpgi.90545.2008
Scott, E. (2017). ω- versus (ω-1)-hydroxylation: cytochrome P450 4B1 sterics make the call. J. Biol. Chem. 292, 5622–5623. doi: 10.1074/jbc.H117.775494
Selman, C., Kerrison, N. D., Cooray, A., Piper, M. D., Lingard, S. J., Barton, R. H., et al. (2006). Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol. Genomics 27, 187–200. doi: 10.1152/physiolgenomics.00084.2006
Shi, H., Tzameli, I., Bjorbaek, C., Flier, J. S. (2004). Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J. Biol. Chem. 279, 34733–34740. doi: 10.1074/jbc.M403886200
Shi, S. Y., Luk, C. T., Brunt, J. J., Sivasubramaniyam, T., Lu, S. Y., Schroer, S. A., et al. (2014). Adipocyte-specific deficiency of Janus kinase (JAK) 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing. Diabetologia 57, 1016–1026. doi: 10.1007/s00125-014-3185-0
Shikata, N., Maki, Y., Nakatsui, M., Mori, M., Noguchi, Y., Yoshida, S., et al. (2010). Determining important regulatory relations of amino acids from dynamic network analysis of plasma amino acids. Amino Acids 38, 179–187. doi: 10.1007/s00726-008-0226-3
Shikata, N., Maki, Y., Noguchi, Y., Mori, M., Hanai, T., Takahashi, M., et al. (2007). Multi-layered network structure of amino acid (AA) metabolism characterized by each essential AA-deficient condition. Amino Acids 33, 113–121. doi: 10.1007/s00726-006-0412-0
Sidiropoulos, K. G., Meshkani, R., Avramoglu-Kohen, R., Adeli, K. (2007). Insulin inhibition of apolipoprotein B mRNA translation is mediated via the PI-3 kinase/mTOR signaling cascade but does not involve internal ribosomal entry site (IRES) initiation. Arch. Biochem. Biophys. 465, 380–388. doi: 10.1016/j.abb.2007.06.029
Thiex, N. J., Manson, H., Anderson, S., Persson, J.-Å. (2002). Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. J. AOAC Int. 85, 309–317. doi: 10.1080/09637480220132184
Tuinstra, R. L., Miziorko, H. M. (2003). Investigation of conserved acidic residues in 3-hydroxy-3-methylglutaryl-CoA lyase: implications for human disease and for functional roles in a family of related proteins. J. Biol. Chem. 278, 37092–37098. doi: 10.1074/jbc.M304472200
Ueki, K., Kondo, T., Kahn, C. R. (2004). Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell. Biol. 24, 5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3 (7), 0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034
Vizcarra, F. R., Verghese, M., Vizcarra, J. A. (2018). Effect of short- and long-term feed restriction on ghrelin concentrations in turkeys. Poult. Sci. 97, 2183–2188. doi: 10.3382/ps/pey039
Vockley, J., Mohsen al, W. A., Binzak, B., Willard, J., Fauq, A. (2000). Mammalian branched-chain acyl-CoA dehydrogenases: molecular cloning and characterization of recombinant enzymes. Methods Enzymol. 324, 241–258. doi: 10.1016/S0076-6879(00)24236-5
Walsh, M. T., Iqbal, J., Josekutty, J., Soh, J., Di Leo, E., Özaydin, E., et al. (2015). Novel Abetalipoproteinemia Missense Mutation Highlights the Importance of the N-terminal β-barrel in microsomal triglyceride transfer protein function. Circ. Cardiovasc. Genet. 8, 677–687. doi: 10.1161/CIRCGENETICS.115.001106
Wang, X. J., Lin, H., Song, Z. G., Jiao, H. C. (2010). Dexamethasone facilitates lipid accumulation and mild feed restriction improves fatty acids oxidation in skeletal muscle of broiler chicks (Gallus gallus domesticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 151, 447–454. doi: 10.1016/j.cbpc.2010.01.010
Wei, C. C., Wu, K., Gao, Y., Zhang, L. H., Li, D. D., Luo, Z. (2017). Magnesium Reduces hepatic lipid accumulation in yellow catfish (Pelteobagrus fulvidraco) and modulates Lipogenesis and Lipolysis via PPARA, JAK-STAT, and AMPK pathways in hepatocytes. J. Nutr. 147, 1070–1078. doi: 10.3945/jn.116.245852
Wheatcroft, S. B., Kearney, M. T., Shah, A. M., Ezzat, V. A., Miell, J. R., Modo, M., et al. (2007). IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes 56, 285–294. doi: 10.2337/db06-0436
Wolf, A., Agnihotri, S., Micallef, J., Mukherjee, J., Sabha, N., Cairns, R., et al. (2011). Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 208, 313. doi: 10.1084/jem.20101470
Wu, J., Mao, X., Tao, C., Luo, J., Wei, L. (2006). KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 34, W720-W724. doi: 10.1093/nar/gkl167
Xie, C., Mao, X., Huang, J., Ding, Y., Wu, J., Dong, S., et al. (2011). KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39, 316–322. doi: 10.1093/nar/gkr483
Xie, M., Zhang, L., Wen, Z. G., Tang, J., Huang, W., Hou, S. S. (2014). Threonine requirement of White Pekin ducks from hatch to 21 d of age. Br. Poult. Sci. 55, 553–557. doi: 10.1080/00071668.2014.929638
Xie, Y., Newberry, E. P., Young, S. G., Robine, S., Hamilton, R. L., Wong, J. S., et al. (2006). Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281, 4075–4086. doi: 10.1074/jbc.M510622200
Xu, A. M., Lam, M. C., Chan, K. W., Wang, Y., Zhang, J. L., Hoo, R. L. C., et al. (2005). Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. U.S.A. 102, 6086–6091. doi: 10.1073/pnas.0408452102
Xu, D., Yin, C., Wang, S., Xiao, Y. (2013). JAK-STAT in lipid metabolism of adipocytes. JAKSTAT 2, e27203. doi: 10.4161/jkst.27203
Yang, X., Guo, Y., Yan, J., Zhang, J., Song, T., Rocheford, T., et al. (2010a). Major and minor QTL and epistasis contribute to fatty acid compositions and oil concentration in high-oil maize. Theor. Appl. Genet. 120, 665–678. doi: 10.1007/s00122-009-1184-1
Yang, X., Zhuang, J., Rao, K., Li, X., Zhao, R. (2010b). Effect of early feed restriction on hepatic lipid metabolism and expression of lipogenic genes in broiler chickens. Res. Vet. Sci. 89, 438–444. doi: 10.1016/j.rvsc.2010.04.003
Yang, Z., Hulver, M., McMillan, R. P., Cai, L., Kershaw, E. E., Yu, L., et al. (2012). Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS One 7, e47493. doi: 10.1371/journal.pone.0047493
Yoon, J. C., Chickering, T. W., Rosen, E. D., Dussault, B., Qin, Y., Soukas, A., et al. (2000). Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20, 5343–5349. doi: 10.1128/MCB.20.14.5343-5349.2000
Zendehdel, M., Hassanpour, S. (2014). Ghrelin-induced hypophagia is mediated by the β 2 adrenergic receptor in chicken. J. Physiol. Sci. 64, 383–391. doi: 10.1007/s12576-014-0330-y
Zhang, L., Joshi, A. K., Hofmann, J., Schweizer, E., Smith, S. (2005). Cloning, expression, and characterization of the human mitochondrial beta-ketoacyl synthase. Complementation of the yeast CEM1 knock-out strain. J. Biol. Chem. 280, 12422–12429. doi: 10.1074/jbc.M413686200
Zhang, Q., Chen, X., Eicher, S., Ajuwon, K., Applegate, T. (2017). Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 96, 3043–3051. doi: 10.3382/ps/pex111
Zhang, Q., Xu, L., Doster, A., Murdoch, R., Cotter, P., Gardner, A., et al. (2014). Dietary threonine requirement of Pekin ducks from 15 to 35 days of age based on performance, yield, serum natural antibodies, and intestinal mucin secretion. Poult. Sci. 93, 1972–1980. doi: 10.3382/ps.2013-03819
Keywords: threonine deficiency, triglyceride accumulation, fatty acid oxidation, energy metabolism, fatty acid profile, Pekin duck
Citation: Jiang Y, Xie M, Fan W, Xue J, Zhou Z, Tang J, Chen G and Hou S (2019) Transcriptome Analysis Reveals Differential Expression of Genes Regulating Hepatic Triglyceride Metabolism in Pekin Ducks During Dietary Threonine Deficiency. Front. Genet. 10:710. doi: 10.3389/fgene.2019.00710
Received: 17 March 2019; Accepted: 04 July 2019;
Published: 02 August 2019.
Edited by:
Andrea Masotti, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Miklos Mézes, Szent István University, HungaryWimal Pathmasiri, University of North Carolina at Chapel Hill, United States
Copyright © 2019 Jiang, Xie, Fan, Xue, Zhou, Tang, Chen and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ShuiSheng Hou, aG91c3NAMjYzLm5ldA==
 Yong Jiang
Yong Jiang Ming Xie1
Ming Xie1 Jing Tang
Jing Tang Guohong Chen
Guohong Chen